Abstract
BACKGROUND
It has been reported that 10% of all pregnancies are complicated by a hypertensive disorder of pregnancy. Previous research has shown that moderate-vigorous intensity exercise has a positive effect on maternal resting blood pressure. A research gap, however, exists related to how different types of exercise (resistance, aerobic, combined resistance and aerobic) affect maternal blood pressure. Most of the previous studies solely focused on aerobic exercise.
OBJECTIVE
The aim of this study was to examine the effects of exercise types on maternal blood pressure throughout pregnancy.
STUDY DESIGN
This study employed a secondary analysis using data from a randomized controlled prenatal exercise intervention trial. This study utilized 3 exercise intervention groups (aerobic, resistance, combination) and compared the results with those of a nonexercize control group. Participants completed 3 50-minute sessions weekly from 16 weeks of gestation until delivery. Maternal vital signs and physical measurements such as systolic blood pressure, diastolic blood pressure, and heart rate were measured every 4 weeks throughout the intervention period. Between-group mean differences in maternal measurements were assessed using Pearson's chi-square tests for continuous (age, prepregnancy body mass index, heart rate, systolic blood pressure, diastolic blood pressure, pulse pressure) variables. For gravida, exact Wilcox 2-sample tests were performed to determine between-group differences in mean values. Hierarchical linear growth curves were used to estimate maternal trajectories of systolic blood pressure and diastolic blood pressure from 16 weeks to 36 weeks’ gestation in each of the 4 groups (aerobic, combination, control, and resistance).
RESULTS
There were no differences among the groups in maternal age or prepregnancy body mass index. Controlling for maternal body mass index, the lowest significant systolic blood pressure curve was noted throughout the pregnancy for women who participated in resistance exercise, followed by women in the aerobic exercise group all relative to the no exercise control group. At 36 weeks’ gestation, the systolic blood pressure was lower in the resistance group by 12.17 mm Hg (P<.001) and in the aerobic group by 7.90 mm Hg (P<.001) relative to controls. No significant change in systolic blood pressure was noted in the combination group in comparison with controls at 36 weeks’ gestation. Similarly, we demonstrated a significantly lower linear growth curve in diastolic blood pressure that was maintained throughout pregnancy in any exercise type relative to controls. After controlling for maternal body mass index, all 3 exercise types (combination, resistance, and aerobic) significantly predicted a similar decrease in diastolic blood pressure that was maintained throughout pregnancy. At 36 weeks’ gestation, the diastolic blood pressure was lower in the aerobic group by 7.30 mm Hg (P<.01), in the combination group by 6.43 mm Hg (P<.05), and in the resistance group relative to controls.
CONCLUSION
Overall, all exercise types were beneficial in lowering maternal resting blood pressure throughout pregnancy. Resistance training was noted to be the most beneficial in improving systolic blood pressure, followed by aerobic exercise. All 3 exercise groups were noted to improve diastolic blood pressure equally. Further research needs to be done to determine if either resistance or aerobic exercise throughout pregnancy decreases the risk for hypertensive disorders of pregnancy and the associated morbidity and mortality.
Key words: aerobic, blood pressure, exercise, pregnancy, resistance
AJOG Global Reports at a Glance.
Why was this study conducted?
Previous data have shown that exercise decreases the risk of hypertensive disorders during pregnancy, but little research has been done regarding which exercise regimen has the greatest effect.
Key findings
Aerobic, resistance, and combined exercise regimens have similar benefits on diastolic blood pressure. Aerobic and resistance exercise regimens are shown to have more positive effects on systolic blood pressure.
What does this add to what is known?
This study demonstrated the effect of different types of exercises on improving maternal blood pressure throughout pregnancy.
Introduction
Hypertensive disorders complicate about 10% of all pregnancies and are associated with 16% of maternal deaths in the United States.1 Recent data show that a prepregnancy body mass index (BMI) of >30 kg/m2, advanced maternal age (>35 years), and both gestational and pregestational diabetes increase a patient's risk of being diagnosed with a hypertensive disorder of pregnancy (HDP).2
Consistent research demonstrates that exercise during pregnancy elicits numerous maternal health benefits, including a decrease in preterm birth and low birthweight infants, an increase in vaginal deliveries, and a variety of cardiovascular benefits including a decrease in systolic (SBP) and diastolic blood pressure (DBP).3 For example, research demonstrates that regular aerobic exercise performed during weeks 12 through 20 of pregnancy significantly improved SBP and DBP and proteinuria among women with preeclampsia.4 Furthermore, women with a history of preeclampsia who participated in either moderate intensity walking or light intensity stretching and breathing showed lower incidences of preeclampsia (22% and 40%, respectively) than nonactive controls.5 Another study showed similar data with a 19% lower odds of gestational hypertension among women who participated in aerobic exercise than among those who did not.6
Research has shown that aerobic training decreases BP through a reduction in systemic vascular resistance with no change in cardiac output.1,3 The individuals participating in aerobic exercise had a reduction in the activity of the autonomic nervous system, leading to lower BP and peripheral vascular resistance as evidenced by lower norepinephrine levels and an overall decrease in heart rate.1 Research has shown that resistance exercise lowers BP through a decrease in systemic vascular resistance as well.1,3 However, the mechanism behind these changes remain unclear because the autonomic nervous system does not seem to be involved as evidenced by the unchanged levels of epinephrine and norepinephrine after training.1 Studies performed on nonpregnant patients have shown significant improvements in SBP and DBP with both aerobic and resistance training individually.5 However, these same improvements were not noted in studies looking at combination (aerobic and resistance) training in hypertensive older adults.7 In the pregnant population, no studies, to date, have directly compared the 3 different types of exercise on maternal cardiovascular adaptation.
If antihypertensive effects can be shown in a normotensive population of pregnant women, then the effects are likely to benefit pregnant women with HDPs. Thus, the aim of this study was to further investigate the effects of different exercise regimens on maternal BP throughout pregnancy. We hypothesized that aerobic and resistance training individually, but not combined, will lead to improvements in both maternal SBP and DBP throughout pregnancy when compared with these measurements in nonexercizers (control group).
Materials and Methods
Study participants
A post hoc analysis was conducted on a randomized, blinded, prospective study focused on the influence of exercise type on infant and maternal outcomes throughout pregnancy. The primary focus for this study was to determine the influence of different types of maternal exercise on offspring outcomes (ie, morphometric, neuromotor). All protocols were approved by the East Carolina University institutional review board. Women who were enrolled in the study met the following criteria: clearance from a healthcare provider to participate in physical activity; aged between 18 and 40 years; prepregnancy BMI of 18.5 to 39.9 kg/m2; singleton pregnancy; gestational age ≤16 weeks; and no current alcohol, tobacco, or medication use. Criteria for exclusion included smoking, preexisting diabetes mellitus, hypertension, cardiovascular disease, and comorbidities known to affect fetal growth and well-being such as systemic lupus erythematosus.
Randomization
Following study enrollment, participants completed a submaximal exercise treadmill test to determine individual aerobic capacity and calculate specific target heart rate ranges for moderate intensity exercise training. Peak oxygen consumption (VO2peak) was estimated via the modified Balke protocol previously validated and replicated for pregnant women by Mottola et al.8 After completing this test, participants were randomized via computerized sequencing (GraphPad Software, SanDiego, CA) to aerobic, resistance, combination (aerobic and resistance), or a nonexercizing, stretching and breathing comparison group.
Exercise protocol
All participants were supervised by trained exercise instructors at the university facilities and followed a standard protocol. All sessions started at 16 weeks’ gestation and were performed 3 times weekly until delivery. All participants’ sessions began with a 5-minute warm-up, 50 minutes of their randomized group activity, and ended with a 5-minute cool-down. The aerobic training group completed moderate intensity (40% to 59% VO2peak) training on treadmills, ellipticals, recumbent bicycles, rowing, and/or stair-stepping equipment. To maintain the appropriate HR zone, speed and grade were adjusted on the treadmill and resistance and speed levels were adjusted on the elliptical and recumbent bike. The resistance training group completed sessions consisting of 2 to 3 sets of 15 repetitions of each exercise at a moderate intensity (28). Seated machines (Cybex) (leg extension, leg curl, shoulder press, chest press, triceps extension, latissimus dorsi pull down), dumbbells (biceps curls, lateral shoulder raises, front shoulder raises), resistance bands/dumbbells, exercise balls, benches, and/or mats were used. The combination training group performed half of the aerobic protocol and half of the resistance protocol exercises in 5 circuits lasting 4.5 to 5 minutes each. For the combination group, resistance exercises were performed in 15 repetitions (same exercises and equipment as resistance training group). For the combination group, the aerobic exercises were performed on the same equipment as the aerobic group. Moderate intensity was monitored in the same way as for the aerobic group. The control group performed stretching, breathing, and flexibility exercises for the duration of the session. Stretches targeted major muscle groups, breathing exercises combined stretches and breathing (30, 31), and flexibility exercises consisted of stretches with controlled breathing. Low intensity was confirmed during sessions using HR (<40% VO2peak). To ensure that the proper intensity was achieved during sessions, the Borg scale rating of perceived exertion (26, 29) and the “talk test” were used. HR monitoring (Polar FS2C) ensured that appropriate target HR ranges were maintained; target HR zones that have been validated for pregnant women were utilized (27). Supervised exercise and stretching and breathing sessions took place at 1 of 2 university-affiliated gyms.
Maternal measurements
Maternal demographic and pregnancy-related characteristics including age, gravida, race and ethnicity, prepregnancy weight, and height were abstracted from prescreening eligibility questionnaires and verified using the electronic health records; presence or absence of HDPs (eg preeclampsia, gestational hypertension) were recorded from electronic health records. Height was also measured in inches to the nearest 0.25 inch and converted to meters on a standard stadiometer. Prepregnancy BMI was calculated by dividing the participant self-reported prepregnancy weight by the product of height squared (kg/m2). Prepregnancy BMI was used as a control in the hierarchical growth curves.
Maternal physical measurements such as BP and HR were measured every 4 weeks throughout the intervention period starting at 16 weeks’ gestation. Maternal resting BP and sitting heart rate HR were assessed in a sitting position. The BP was recorded using a manual sphygmomanometer at the brachial artery just proximal to the elbow (arm straight or in mild elbow flexion) with an appropriately sized cuff. All measures were performed by trained research staff before each exercise session. Pulse pressure (PP) was calculated as the difference between DBP and SBP.
Statistical analysis
Between-group mean differences in maternal descriptive characteristics and cardiovascular factors were assessed by Pearson chi-square tests for continuous (age, prepregnancy BMI, heart rate, SBP, DBP, PP) variables. For race, a chi-square analysis was done to determine between-group differences among White, African American, and other minority races (Asian, Pacific Islander, Hispanic, and mixed). For gravida, exact Wilcox 2-sample tests were performed to determine between-group differences in mean values.
Hierarchical linear growth curves were subsequently used to estimate the maternal trajectories for SBP and DBP from 16 weeks to 36 weeks of gestation in each of the 4 groups (aerobic, combination, control, and resistance). A hierarchical linear growth curve is advantageous for longitudinal data and allows for examination of fixed (prepregnancy BMI) and random (BP) effects, strengthening an already powerful randomized controlled trial (RCT) design. For both SBP and DBP, unconditional models were first tested with random intercepts and random slopes. Baseline SBP and DBP levels were included as the baseline for their respective models. Time-invariant variables of group type and maternal prepregnancy BMI (or fixed effects) were included in the second model. Following hierarchical linear growth curve models, post hoc contrast analyses were conducted to compare the exercise groups with the control group at each time point without needing to rotate the original comparison and re-run the model numerous times. Analysis for 36-week outcomes indicated that with an alpha level of 0.05 and 80% confidence, we would need a total sample of 80 (20 per group) to detect differences in SBP and a total sample of 116 (29 per group) to detect differences in DBP. All analyses were performed in Stata (version 16.1) (StataCorp, College Station, TX).
Results
Descriptive statistics
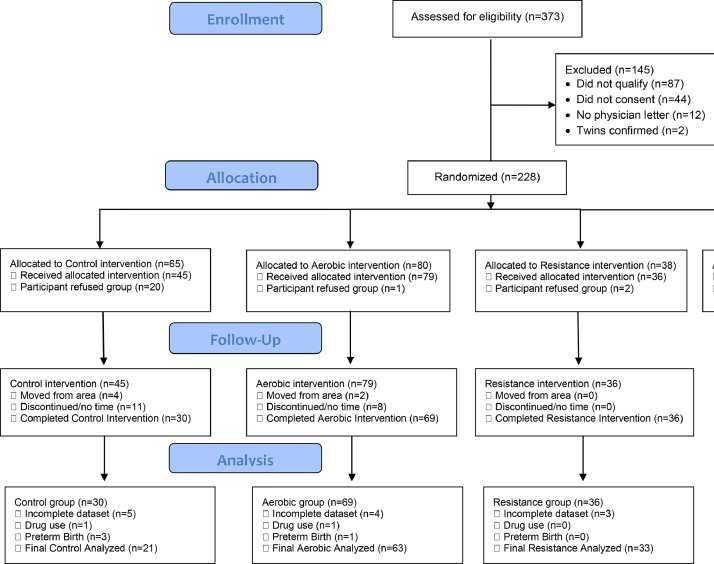
We had 373 pregnant women who expressed interest in the study. A total of 87 participants were excluded because they did not meet the inclusion criteria, 44 were excluded for not consenting to the proposed exercise regimen, 12 did not obtain a physician clearance letter, and 2 participants were confirmed to have a multifetal pregnancy. After excluding these 145 participants (Figure 1), our final analysis included 154 participants who were enrolled and then randomized to 1 of the 4 groups (control, combination, aerobic, or resistance). On average, pregnant women in the current study were 30 (standard deviation [SD], 3.87) years old, overweight (BMI, 25.57±4.43;) and had 1 previous pregnancy (SD, 0.81). Participants in the aerobic and no exercise control groups were similar in age, gravida, race and ethnicity, and prepregnancy BMI (Table 1). The study population was diverse with 21% of participants self-reporting as a minority race or ethnicity group (Table 1). A total of 10.4% of participants self-reported as African American and 10.4% self-reported as other (Asian, Hispanic, Pacific Islander, mixed race). Baseline (16 week) values for SBP, PP, and HR were similar among groups before the intervention (Table 1). The control group had a significantly higher incidence of HDPs, higher DBP at 16 weeks’ gestation, and lower compliance at delivery than all the exercise groups (Table 1).
Figure 1.
Recruitment and enrollment of pregnant participants
The CONSORT diagram describes the number of participants from the beginning of recruitment to the analysis stage of the study.
CONSORT, Consolidated Standards of Reporting Trials.
Murphy. Influence of exercise type on maternal blood pressure. Am J Obstet Gynecol Glob Rep 2022.
Table 1.
Maternal demographics by group at enrollment
| Maternal measures | Full sample (N=154) | Control (n=21) | Aerobic (n=63) | Resistance (n=33) | Combination (n=37) | P value |
|---|---|---|---|---|---|---|
| Age (y) |
30.47 (3.87) | 29.4±3.77 | 30.78±3.79 | 31.6±3.73 | 29.7±3.35 | .11 |
| Prepregnancy BMI (kg/m2) |
25.57 (4.43) | 26.8±4.03 | 24.8±4.47 | 26.3±4.71 | 26.1±4.19 | .21 |
| Gravidaa | 1.90 (1.05) | 2.27 (1–5) | 1.88 (1–5) | 2.15 (1–5) | 1.7 (1–7) | .16 |
| Race and ethnicity | .46 | |||||
| White | 122 (79) | 16 (76) | 49 (78) | 28 (85) | 29 (78) | |
| African- American |
16 (10) | 4 (19) | 8 (13) | 1 (3) | 3 (8) | |
| Other | 16 (10) | 1 (5) | 6 (10) | 4 (12) | 5 (14) | |
| Resting SBP (mm Hg) |
102.64 (9.69) | 109.9±14.7 | 103.9±10.1 | 102.0±10.6 | 104.3±10.8 | .08 |
| Resting DBP (mm Hg) |
58.06 (7.47) | 66.8±9.0 | 59.8±8.8 | 58.8±8.4 | 59.4±7.6 | .01 |
| Resting pulse pressure (mm Hg) | — | 43.1±10.0 | 44.0±7.2 | 43.1±7.8 | 46.4±8.8 | .32 |
| Resting HR (bpm) | 86.69 (10.57) | 93.0±20.4 | 87.0±12.3 | 84.6±7.6 | 85.0±11.9 | .15 |
| Prevalence of HDP | 14 (9) | 6 (29) | 3 (5) | 3 (9) | 2 (5) | .03 |
| Compliance (%) | — | 52.5±45.8 | 80.6±16.3b | 79.5±14.2b | 83.3±11.5b | <.0001 |
Data are reported as mean (standard deviation) or number (percentage).
BMI, body mass index; DBP, diastolic blood pressure; HDP, hypertensive disorders of pregnancy; HR, heart rate; SBP, systolic blood pressure.
Because of a non-normal distribution, Mann Whitney U tests were performed and the values were expressed as median (minimum–maximum);
Indicates significant difference (P<.0001) from control.
Murphy. Influence of exercise type on maternal blood pressure. Am J Obstet Gynecol Glob Rep 2022.
Systolic blood pressure
The results from an analysis of variance (ANOVA) show a significant improvement in SBP at 36 weeks’ gestation among women enrolled in any exercise program than the control group (Table 2). At 36 weeks’ gestation, women enrolled in the resistance exercise group had the most significant decrease in SBP, followed by women in the aerobic exercise group (Table 3).
Table 2.
Group membership predicts blood pressure at 36 weeks’ gestation
| Unstandardized coefficient (SE) |
||
|---|---|---|
| Group type | Diastolic BP | Systolic BP |
| Exercise | −6.82 (2.45)a | −8.44 (3.37)b |
| Constant | 66.04 (2.27)c | 110.73 (3.09)c |
BP, blood pressure; SE, standard error.
Indicates a significance of P<.01;
Indicates a significance of P<.05;
Indicates a significance of P<.001.
Murphy. Influence of exercise type on maternal blood pressure. Am J Obstet Gynecol Glob Rep 2022.
Table 3.
Prenatal exercise type predicts blood pressure at 36 weeks’ gestation
| Unstandardized coefficient (SE) |
||
|---|---|---|
| Group type | Diastolic BP | Systolic BP |
| Aerobic | −7.30 (2.66)a | −7.90 (3.63)b |
| Combination | −6.43 (2.89)b | −6.84 (3.94)c |
| Resistance | −6.16 (3.21) | −12.17 (4.30)a |
| Constant | 66.04 (2.27)d | 110.73 (3.09)d |
BP, blood pressure; SE, standard error.
Indicates a significance of P<.01;
Indicates a significance of P<.05;
Indicates a significance of P<.10;
Indicates a significance of P<.001.
Murphy. Influence of exercise type on maternal blood pressure. Am J Obstet Gynecol Glob Rep 2022.
Hierarchical linear model
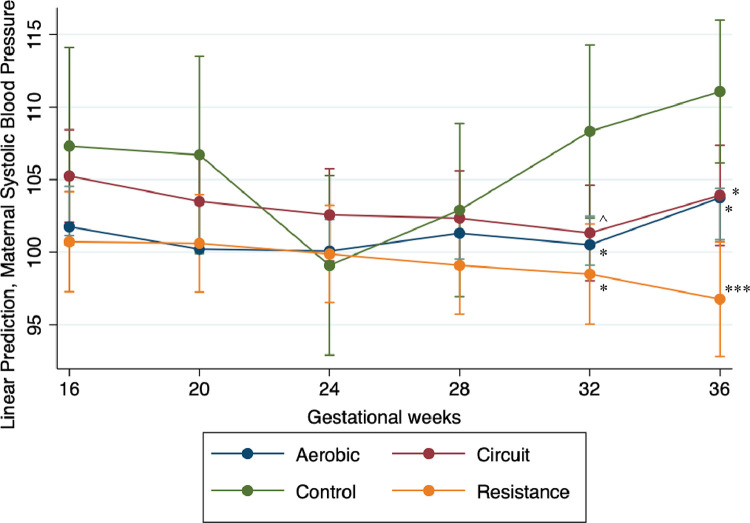
More specifically, throughout pregnancy, all 3 exercise groups showed a similar trajectory (Figure 2) with the greatest decrease in SBP seen in the resistance exercise group, followed by women in the aerobic exercise group, and then by women in the combination group. Results from the final model suggest that significantly lower SBP values were recorded for women in all the exercise groups when compared with women in the control group, with the most significant reduction recorded for women in the resistance exercise group (Table 4). Post hoc contrast analyses revealed a significant difference between exercise types during later pregnancy. At 36 weeks’ gestation, participants who engaged in resistance exercise had an SBP that was significantly lower than participants in the aerobic (b, −6.62; mean difference [MD], 2.49; P<.001) or combination (b, −6.48; MD, 2.72; P<.01) exercise groups. Overall, participants in the resistance group reported a mean SBP of approximately 6.5 mm Hg lower than participants in the aerobic or combination groups and a mean of 13 mm Hg lower than participants in the control group.
Figure 2.
Systolic blood pressure throughout gestation within different maternal exercise types
The systolic blood pressure of pregnant women from 16 to 36 weeks’ gestation at 4-week intervals during the pregnancy. The aerobic group is indicated by the blue line and markers, the resistance group is indicated by the yellow line and markers, the red line and markers indicate the combination group, and the controls are indicated by the green line and markers. Significance values are in comparison with the control at the same gestational time period. Note: Triple asterisks represent P<.000; double asterisks represent P<.001; single asterisk represents P<.01; circumflex accent represents P<.10.
Murphy. Influence of exercise type on maternal blood pressure. Am J Obstet Gynecol Glob Rep 2022.
Table 4.
Hierarchical linear models predicting maternal systolic blood pressure
| Unstandardized coefficient (SE) |
||
|---|---|---|
| Variables | Model 1 | Model 2 |
| Gestational wk (16 wk as control group) | ||
| 20 wk | −1.11 (0.73) | −0.60 (3.46) |
| 24 wk | −2.05 (0.73)a | −8.21 (3.26)b |
| 28 wk | −1.60 (0.77)b | −4.40 (3.20) |
| 32 wk | −1.96 (0.85)b | 1.01 (3.20) |
| 36 wk | 0.48 (0.95) | 3.76 (3.12) |
| Exercise type (reference: control group) | ||
| Aerobic | −5.55 (3.72) | |
| Combination | −2.06 (3.83) | |
| Resistance | −6.58 (3.89) | |
| Prepregnancy BMI | 0.65 (0.16)c | |
| Hypertensive disorders of pregnancy | 11.40 (3.35)b | |
| Constant | 103.09 (0.97)c | 88.17 (5.48)c |
| Gestational wk × exercise type | ||
| Reference: 16 wk × control | ||
| 16 wk × aerobic | −5.00 (3.79) | |
| 16 wk × combination | −1.87 (3.90) | |
| 16 wk × resistance | −5.68 (3.96) | |
| Reference: 20 wk × control | ||
| 20 wk × aerobic | −5.96 (3.75) | |
| 20 wk × combination | −3.02 (3.90) | |
| 20 wk × resistance | −5.18 (3.94) | |
| Reference: 24 wk × control | ||
| 24 wk × aerobic | −1.51 (3.47) | |
| 24 wk × combination | −3.64 (3.62) | |
| 24 wk × resistance | 1.68 (3.66) | |
| Reference: 28 wk × control | ||
| 28 wk × aerobic | −1.10 (3.36) | |
| 28 wk × combination | −0.40 (3.54) | |
| 28 wk × resistance | −2.97 (3.57) | |
| Reference: 32 wk × control | ||
| 32 wk × aerobic | −7.33 (3.39)b | |
| 32 wk × combination | −6.83 (3.54)d | |
| 32 wk × resistance | −8.98 (3.59)b | |
| Reference: 36 wk × control | ||
| 36 wk × aerobic | −6.73 (2.94)b | |
| 36 wk × combination | −6.86 (3.15)b | |
| 36 wk × resistance | −13.35 (3.30)c | |
Coefficients with different subscripts differed significantly from each other (P<.05) when compared in post hoc, contrast models that rotated the reference category. Coefficients with the same subscript did not differ significantly from each other.
BMI, body mass index; SE, standard error.
Indicates a significance of P<.01;
Indicates a significance of P<.05;
Indicates a significance of P<.001;
Indicates a significance of P<.10.
Murphy. Influence of exercise type on maternal blood pressure. Am J Obstet Gynecol Glob Rep 2022.
Diastolic blood pressure
The results from an ANOVA demonstrate a significant decrease in DBP at 36 weeks’ gestation in women participating in exercise at the recommended levels when compared with women in the nonexercizing control group (Table 2). Specifically, aerobic exercise had the most significant effect on DBP at 36 weeks’ gestation, followed by combination (aerobic and resistance) training although post hoc analyses showed that the exercise groups are not significantly different from each other.
Hierarchical linear model
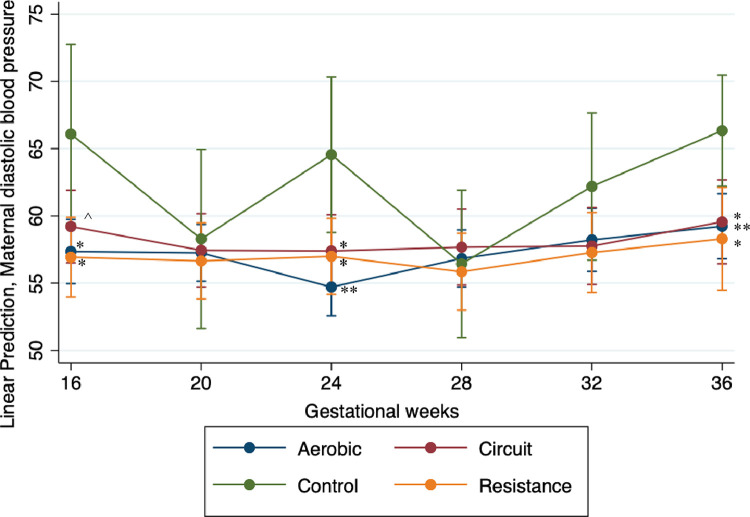
Throughout pregnancy, all 3 exercise groups showed different overall trajectories with the most significant decrease in DBP observed for the aerobic group, followed by the combination group, and then the resistance exercise group (overlapping at times) when compared with the control group (Figure 3). Results from the final model demonstrate that being in the aerobic or resistance exercise groups predicted lowered DBP values (Table 5). Post hoc contrast analyses showed that, overall, there was no statistical difference among any of the exercise groups. However, the post hoc contrast analyses also revealed significant differences in the DBP between women in the control group and women in the exercise groups at 16 weeks, 24 weeks, and 36 weeks’ gestation (Table 5, Figure 3).
Figure 3.
Maternal DBP throughout gestation within maternal exercise groups
The DBP of pregnant women from 16 to 36 weeks’ gestation at 4-week intervals during the pregnancy. The aerobic group is indicated by the blue line and markers, the resistance group is indicated by the yellow line and markers, the red line and markers indicate the combination group, and the controls are indicated by the green line and markers. Significance values are in comparison with the control at the same gestational time period.
Note: Triple asterisks represent P<.000; double asterisks represent P<.001; single asterisk represents P<.01; circumflex accent represents P<.10.
DBP, diastolic blood pressure.
Murphy. Influence of exercise type on maternal blood pressure. Am J Obstet Gynecol Glob Rep 2022.
Table 5.
Hierarchical linear models predicting diastolic maternal blood pressure
| Unstandardized coefficient (SE) |
||
|---|---|---|
| Variables | Model 1 | Model 2 |
| Gestational wk (reference: 16 wk) | ||
| 20 wk | −1.08 (0.85) | −7.80 (4.09)a |
| 24 wk | −1.76 (0.86)b | −1.54 (3.85) |
| 28 wk | −1.65 (0.88) | −9.65 (3.77)b |
| 32 wk | −0.30 (0.94) | −3.90 (3.77) |
| 36 wk | 1.50 (1.02) | 0.25 (3.56) |
| Exercise type (reference: control group) | ||
| Aerobic | −8.74 (3.61)b | |
| Combination | −6.88 (3.66)a | |
| Resistance | −9.16 (3.72)b | |
| Prepregnancy BMI | 0.28 (0.11)b | |
| Hypertensive disorders of pregnancy | 8.45 (2.33)c | |
| Constant | 58.57 (0.84)c | 58.71 (4.41)c |
| Gestational wk × exercise type | ||
| Reference: 16 wk × control | ||
| 16 wk × aerobic | −8.50 (3.65)b | |
| 16 wk × combination | −6.91 (3.71)a | |
| 16 wk × resistance | −8.68 (3.77)b | |
| Reference: 20 wk × control | ||
| 20 wk × aerobic | −0.84 (3.61) | |
| 20 wk × combination | −0.88 (3.72) | |
| 20 wk × resistance | −1.13 (3.74) | |
| Reference: 24 wk × control | ||
| 24 wk × aerobic | −9.55 (3.19)d | |
| 24 wk × combination | −7.15 (3.31)b | |
| 24 wk × resistance | −7.00 (3.34)b | |
| Reference: 28 wk × control | ||
| 28 wk × aerobic | −0.64 (3.05) | |
| 28 wk × combination | −1.27 (3.20) | |
| 28 wk × resistance | −0.13 (3.21) | |
| Reference: 32 wk × control | ||
| 32 wk × aerobic | −3.78 (3.09) | |
| 32 wk × combination | −4.41 (3.21) | |
| 32 wk × resistance | −4.44 (3.24) | |
| Reference: 36 wk × control | ||
| 36 wk × aerobic | −6.72 (2.49)d | |
| 36 wk × combination | −6.55 (2.69)b | |
| 36 wk × resistance | −7.41 (2.92)b | |
Coefficients with different subscripts differed significantly from each other (P<.05) when compared in post hoc, contrast models that rotated the reference category. Coefficients with the same subscript did not differ significantly from each other.
BMI, body mass index; SE, standard error.
Indicates a significance of P<.10;
Indicates a significance of P<.05;
Indicates a significance of P<.001;
Indicates a significance of P<.01.
Murphy. Influence of exercise type on maternal blood pressure. Am J Obstet Gynecol Glob Rep 2022.
Overall, the results from this study demonstrate an overall significant decrease in both SBP (Figure 2) and DBP (Figure 3) throughout gestation in women enrolled in an exercise program than in participants who did not exercise (controls).
Discussion
Principal findings
We hypothesized that aerobic and resistance training individually, but not combined, will lead to improvements in both maternal SBP and DBP throughout gestation when compared with these measures in nonexercizers (control group). The main findings of the study were that (1) resistance and aerobic exercises individually had the greatest effect on SBP, (2) throughout the course of the pregnancy, all 3 types of exercises decreased DBP equally, and (3) prepregnancy BMI was a significant predictor of the overall trend in both SBP and DBP throughout the pregnancy.
Results
The results of this study suggest that any form of exercise, whether resistance, aerobic, or combination training, is effective in reducing BP throughout pregnancy. A retrospective study performed in 1989 by Marcoux et al9 evaluated the benefits of exercise in terms of its effect on BP. Marcoux et al9 showed that self-reported leisure-time physical activity, whether light, moderate, or high intensity, significantly decreased the risk for preeclampsia.9 In 2000, Yeo et al10 performed an RCT that showed that a 10-week program of moderate intensity aerobic exercise significantly decreased both SBP and DBP in women with HDPs. Although there seems to be a benefit to light intensity exercise as opposed to a sedentary lifestyle during pregnancy,11,12 existing research demonstrates that light to moderate exercises have a more significant effect on HDPs than exercise combined with another intervention (eg, lifestyle counseling).6 This study demonstrated the effect of different types of exercises on improving maternal BP throughout pregnancy.
Interestingly, resistance and aerobic exercises individually were shown to influence maternal BP, whereas for women in the combination exercise group, there was no significant effect on SBP at 36 weeks’ gestation. This could possibly be because of our protocol that specified a transition between aerobic and resistance exercise every 4.5 to 5 minutes, which decreased the time specifically devoted to each. It may be that a combination protocol with just a half split between the types of exercises and only 1 transition would yield significant findings. However, the finding that the average exercising HR was still in the target HR zones suggests that this may not be the case. Potentially, there may not be 1 single type of exercise that is superior to others in terms of maternal cardiovascular adaptations. Furthermore, all exercise groups had an SBP and a DBP that were >5.6 mm Hg lower than the control participants; although these are not all statistically significant, previous research demonstrates that decreases in SBP and DBP of >2 mm Hg significantly attenuated cardiovascular disease risk in hypertensive and normotensive people and thus our results are considered clinically meaningful differences.13, 14, 15
Our study also showed that prepregnancy BMI was a significant predictor of the overall trend in both SBP and DBP throughout the pregnancy. The participants with a higher BMI at the beginning of the study were shown to have significantly higher BPs throughout pregnancy. This has been supported in a previous study that showed that a higher prepregnancy BMI in addition to a greater gestational weight gain was associated with an overall higher SBP and DBP.16 The overall trend demonstrated a significant decrease in BP in participants assigned to an exercise group regardless of BMI.
Research implications
Future research should focus on replicating the effects found in this study in women with high-risk pregnancies including those with preexisting diabetes, chronic hypertension, or obesity. To best understand the effectiveness of prenatal exercise type, future research could include measurements of pro- and anti-inflammatory markers and determinations of the associations between these markers and maternal BP throughout pregnancy.
Clinical strengths and limitations
Notably, there are a few strengths of this study. Although the focus of this study was on infant outcomes related to different types of exercises during pregnancy, this secondary analysis was also based on a response to the different types of exercise regimens during pregnancy and thus a strength is the RCT design. This study was a secondary analysis of an RCT that examined the effects of supervised exercise types during pregnancy on maternal cardiovascular measures. Lastly, the repeated measurements collected throughout gestation to track the steady changes in maternal cardiovascular health support the stable physiological changes that occur throughout pregnancy. In addition to strengths, we acknowledge limitations. First, our sample size was relatively small. Second, BPs were taken during a regular visit that fit participants’ schedules and thus were taken at different times of the day; although, there was a standard rest time to allow for a resting measure, the differences in the circadian rhythm at different times in the day may lead to slight changes in the resting BP measurements. Although we had participants with a range of BMIs including BMIs categorized as overweight and obese, our sample of pregnant women all had healthy singleton pregnancies free from most pregnancy complications. Because many women have obesity or other medical complications that could increase their risk for HDPs, further research is needed to ensure the generalizability of these results. Although we had a diverse population, only a few had a history of HDPs. All the control participants with an HDP diagnosis could not complete all the measurements, whereas women with an HDP diagnosis in any of the exercise groups exhibited more mild symptoms only in late pregnancy and could complete the study. This presents a bias in the data; thus, further research needs to be done and should be focused on those women with HDPs. It is possible that a greater effect would be seen in women at risk for or with a diagnosed HDP. The participants in this study also had access to professional trainers who led them through their assigned workouts. Because of financial and time constraints, this workout regimen may not be possible for the average pregnant woman.
Conclusion
In summary, our results found that, similar to nongravid adults, all forms of exercise can decrease both resting SBP and DBP. Resistance exercise had the greatest effect on SBP, whereas DBP was affected to the same extent by all types of exercise. An improved understanding of the role of different types of exercise on maternal BP throughout pregnancy may change exercise recommendations during pregnancy. Knowledge of the benefits of specific exercise modes may allow for providers to more easily direct and instruct patients to partake in a particular form of exercise throughout their prenatal course.
Footnotes
The authors report no conflict of interest.
This study received funding from the American Heart Association (under grant numbers #15GRNT24470029 and AHA#18IPA34150006) and from the East Carolina University (internal funding).
Cite this article as: Murphy SE, Johnston CA, Strom C, et al. Influence of exercise type on maternal blood pressure adaptation throughout pregnancy. Am J Obstet Gynecol Glob Rep 2022;2:100023.
References
- 1.Fagard RH. Exercise is good for your blood pressure: effects of endurance training and resistance training. Clin Exp Pharmacol Physiol. 2006;33:853–856. doi: 10.1111/j.1440-1681.2006.04453.x. [DOI] [PubMed] [Google Scholar]
- 2.ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:1. doi: 10.1097/AOG.0000000000003018. [DOI] [PubMed] [Google Scholar]
- 3.Smart NA, Way D, Carlson D, et al. Effects of isometric resistance training on resting blood pressure: individual participant data meta-analysis. J Hypertens. 2019;37:1927–1938. doi: 10.1097/HJH.0000000000002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasawara KT, Burgos CS, Costa ML, E Silva JL. PP046. Adherence to exercise with bicycle during pregnancy in women with risk of preeclampsia. Pregnancy Hypertens. 2012;2:266–267. doi: 10.1016/j.preghy.2012.04.157. [DOI] [PubMed] [Google Scholar]
- 5.Yeo S, Davidge S, Ronis DL, Antonakos CL, Hayashi R, O'Leary S. A comparison of walking versus stretching exercises to reduce the incidence of preeclampsia: a randomized clinical trial. Hypertens Pregnancy. 2008;27:113–130. doi: 10.1080/10641950701826778. [DOI] [PubMed] [Google Scholar]
- 6.Davenport MH, Ruchat SM, Poitras VJ, et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Br J Sports Med. 2018;52:1367–1375. doi: 10.1136/bjsports-2018-099355. [DOI] [PubMed] [Google Scholar]
- 7.Sardeli AV, Griffith GJ, Dos Santos MVMA, Ito MSR, Nadruz W, Chacon-Mikahil MPT. Do baseline blood pressure and type of exercise influence level of reduction induced by training in hypertensive older adults? A meta-analysis of controlled trials. Exp Gerontol. 2020;140 doi: 10.1016/j.exger.2020.111052. [DOI] [PubMed] [Google Scholar]
- 8.Mottola MF, Davenport MH, Brun CR, Inglis SD, Charlesworth S, Sopper MM. VO2peak prediction and exercise prescription for pregnant women. Med Sci Sports Exerc. 2006;38:1389–1395. doi: 10.1249/01.mss.0000228940.09411.9c. [DOI] [PubMed] [Google Scholar]
- 9.Marcoux S, Brisson J, Fabia J. The effect of leisure time physical activity on the risk of pre-eclampsia and gestational hypertension. J Epidemiol Community Health. 1989;43:147–152. doi: 10.1136/jech.43.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeo S, Steele NM, Chang MC, Leclaire SM, Ronis DL, Hayashi R. Effect of exercise on blood pressure in pregnant women with a high risk of gestational hypertensive disorders. J Reprod Med. 2000;45:293–298. [PubMed] [Google Scholar]
- 11.Loprinzi PD, Fitzgerald EM, Woekel E, Cardinal BJ. Association of physical activity and sedentary behavior with biological markers among U.S. pregnant women. J Womens Health (Larchmt) 2013;22:953–958. doi: 10.1089/jwh.2013.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fazzi C, Saunders DH, Linton K, Norman JE, Reynolds RM. Sedentary behaviours during pregnancy: a systematic review. Int J Behav Nutr Phys Act. 2017;14:32. doi: 10.1186/s12966-017-0485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbull F, Blood Pressure Lowering Treatment Trialists’ Collaboration Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 14.Stiller-Moldovan C, Kenno K, McGowan CL. Effects of isometric handgrip training on blood pressure (resting and 24 h ambulatory) and heart rate variability in medicated hypertensive patients. Blood Press Monit. 2012;17:55–61. doi: 10.1097/MBP.0b013e32835136fa. [DOI] [PubMed] [Google Scholar]
- 15.Wong GW, Wright JM. Blood pressure lowering efficacy of nonselective beta-blockers for primary hypertension. Cochrane Database Syst Rev. 2014;2 doi: 10.1002/14651858.CD007452.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser A, Tilling K, Macdonald-Wallis C, et al. Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the Avon Longitudinal Study of Parents and Children (ALSPAC) Am J Clin Nutr. 2011;93:1285–1292. doi: 10.3945/ajcn.110.008326. [DOI] [PMC free article] [PubMed] [Google Scholar]