Abstract
The modification of physical activity (PA) on the metabolic status in relation to atrial fibrillation (AF) in obesity remains unknown. We aimed to investigate the independent and joint associations of metabolic status and PA with the risk of AF in obese population. Based on the data from UK Biobank study, we used Cox proportional hazards models for analyses. Metabolic status was categorized into metabolically healthy obesity (MHO) and metabolically unhealthy obesity (MUO). PA was categorized into four groups according to the level of moderate-to-vigorous PA (MVPA): none, low, medium, and high. A total of 119,424 obese participants were included for analyses. MHO was significantly associated with a 35% reduced AF risk compared with MUO (HR = 0.65, 95% CI: 0.57–0.73). No significant modification of PA on AF risk among individuals with MHO was found. Among the MUO participants, individuals with medium and high PA had significantly lower AF risk compared with no MVPA (HR = 0.84, 95% CI: 0.74–0.95, and HR = 0.87, 95% CI: 0.78–0.96 for medium and high PA, respectively). As the severity of MUO increased, the modification of PA on AF risk was elevated accordingly. To conclude, MHO was significantly associated with a reduced risk of AF when compared with MUO in obese participants. PA could significantly modify the relationship between metabolic status and risk of AF among MUO participants, with particular benefits of PA associated with the reduced AF risk as the MUO severity elevated.
Supplementary information
The online version contains supplementary material available at 10.1186/s12933-022-01644-z.
Keywords: Atrial fibrillation, Obesity, Metabolic status, Physical activity
Introduction
Atrial fibrillation (AF) is a major cause of morbidity and mortality worldwide, including a five-fold increased risk of stroke and a substantial healthcare burden [1–3]. There were a large number of AF cases undetected and untreated, posing a severe challenge to stroke prevention especially in the aging population [4]. Therefore, predicting the onset of AF and identifying strategies for AF prevention are of significant importance for the public health. Previous studies have demonstrated that obesity is an important contributing factor for developing AF [5–8]. Based on various metabolic features including blood pressure, glucose tolerance, and lipid profile, obesity can be categorized into two categories: metabolically healthy obesity (MHO) and metabolically unhealthy obesity (MUO) [9–11]. One nationwide study in Asia suggested that metabolically unhealthy patients had significantly higher AF risk when compared with a metabolically healthy population [12]. However, evidence of metabolic status in relation to AF among obese individuals remains scarce. Hence, the relationship between metabolic status and AF risk in the obese population requires further exploration [13].
Physical activity (PA) is a major modifiable factor for cardiovascular disease [14–16]. Moreover, some observational studies have suggested a positive association between PA and the risk of AF onset [17–19]. Nevertheless, previous reports did not consider the modification of PA on the association between metabolic status and AF risk. This is important given the focus on integrated care management of AF in contemporary guidelines, including attention to comorbidities and lifestyle factors [20–22].
In this study, we aimed to investigate the independent and joint associations of metabolic status and PA with the risk of AF in obese population using the data from the nationwide United Kingdom (UK) Biobank study.
Methods
Study participants
The UK Biobank covers more than 500,000 participants with middle and old age recruited between 2006 and 2010. All participants signed written informed consent, and completed touch-screen questionnaires, interviews with trained medical personnel and physical measurements. Details of the study design and data collection have been previously reported [23].
There were 122,244 obese participants at baseline, among whom 2,820 individuals were excluded because they had AF at baseline or a history of AF. We used the information from self-reported illness and operation history, disease diagnosis codes linkage of international classification of diseases ninth (ICD-9) and 10th (ICD-10) revisions, and operative procedure codes (OPCS) to identify baseline AF (STable 1). Overall, a total of 119,424 participants were included for the present analysis (SFigure 1). All participants were followed up from baseline until an AF diagnosis, death, or the censoring date (March 2017 for England and October 2016 for Scotland), whichever came first.
Outcomes
Our primary outcome was incident AF, and the secondary outcome was AF mortality. In the UK Biobank, information on incident AF was evaluated by linkage with hospital in-patient data and death registry records. Specifically, the incident AF was identified by using the combination of disease diagnosis codes linkage of ICD-9 and ICD-10, OPCS, and death registry records during follow-up. We used the death registry records to document the AF mortality, in which the AF was the primary or secondary cause of death. STable 1 displays the codes used for the identification of incident AF and AF mortality.
Exposures
The information on exposures was obtained at baseline. ‘Metabolically healthy’ indicated none of the metabolic disorders, and ‘metabolically unhealthy’ indicated at least one of the metabolic disorders, where metabolic disorders included hypertension, hypercholesterolemia, and diabetes [24, 25].
Participants who met at least one of the following criteria were deemed to be hypertensive: systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, taking anti-hypertensive medications, a self-reported history of hypertension or ICD-10 code I10-I15 and ICD-9 code 401 and 403. Self-reported history of high cholesterol, taking medications, or ICD-10 code E780 and ICD-9 code 2720 was the criteria for hypercholesterolemia. Diabetes mellitus was defined as hospital records of diabetes at baseline based on ICD-10 code E10-E14 and ICD-9 code 250 or taking medications or a self-reported history of type 1 or type 2 diabetes. The 119,424 obese participants were categorized into MHO and MUO.
PA information, including the frequency and duration of activities, was collected at baseline with the short form International Physical Activity Questionnaire (IPAQ) [26]. PA was presented as metabolic equivalent task-(MET)-mins per week computed by multiplying the MET score by the weekly duration of activity. In line with public health guidelines, PA was categorized into four groups according to the level of moderate-to-vigorous PA (MVPA): none (0 MET-mins per week for MVPA), low (< 600 MET-mins per week), medium (600–1200 MET-mins per week), and high (≥ 1200 MET-mins per week) [26, 27].
Other independent variables
Covariates of consideration included age (in years), sex (males and females), body mass index (BMI; in kg/m2), smoking status (current, previous or never), alcohol drinking status (current, previous or never), employment status (unemployment, retired, employed in night shift work, employed in day shift work, or employed not in shift work), income (< £ 18,000, £ 18,000 - £ 30,999, £ 31,000 - £ 51,999, £ 52,000 - £ 100,000, or > £ 100,000), sleep scores (the number of healthy sleep characteristics: not usual insomnia, no frequent daytime sleepiness, no habitual snoring, morning chronotype, and adequate sleep duration), socioeconomic status (TDI: Townsend deprivation index), vegetable and fruit intake, sedentary behavior (in hours), mental health issues (yes or no) and cardiovascular disease (yes or no) [26, 28, 29].
Statistical analyses
We performed descriptive analysis for continuous variables (mean and standard deviation [SD]) and categorical variables (counts and percentages). Chi-square test and two independent t-tests were conducted for the baseline characteristics by metabolic status.
Cox proportional hazards models were used to investigate the independent and joint associations of metabolic status and PA with the risk of AF in obese participants. The results were quantified as hazard ratios (HRs) and 95% confidence intervals (CIs). We used MUO, no MVPA, or combination of both as reference. Results of the age- and sex-adjusted model and the fully adjusted model were shown. The latter was obtained after adjusting for age, sex, body mass index, smoking status, alcohol drinking status, employment status, income, sleep scores, socioeconomic status, vegetable and fruit intake, sedentary behavior, mental health issues and cardiovascular disease. We also conducted subgroup analyses and added interaction terms in the model to explore whether there were differences in joint associations of metabolic status and PA with the risk of AF, including sex (males versus females), and age (< 65 versus ≥ 65 years) groups.
In an exploratory analysis, we further explored the modification of PA in different metabolic statuses including MHO status and three MUO statuses: mild (with only one of the metabolic disorders), moderate (with two of the metabolic disorders), and severe (with all of three metabolic disorders). Furthermore, we performed additional analyses for modification of metabolic status and PA with AF risk among obese individuals, where metabolic status is the exposure of interest and PA is the potential modifier [30].
We conducted a sensitivity analysis by using multiple imputation techniques for the missing data of PA to assess the joint associations. As another sensitivity analysis, we examined the joint associations by using a different definition of the metabolic status. Participants with ≥ 2 of the following criteria were considered as MUO: (1) systolic blood pressure ≥ 130 mmHg, diastolic blood pressure ≥ 85 mmHg, or taking anti-hypertensive medications, (2) triglycerides ≥ 1.7 mmol/L or taking lipid-lowering drugs, (3) fasting glucose ≥ 5.6 mmol/L or taking medications for diabetes, and (4) HDL-cholesterol < 1.0 mmol/L for male and < 1.3 mmol/L for female [31]. Furthermore, we performed another post hoc sensitivity analysis by adjusting for age, sex, height, C-reactive protein, white blood cell counts, smoking status, alcohol drinking status, income, sleep scores, mental health issues, employment status, socioeconomic status, vegetable and fruit intake, sedentary behavior, and cardiovascular disease.
All the statistical analyses were conducted in SAS software version 9.4 and R software version 4.1.1.
Results
A total of 119,424 obese participants were included for analyses, with a median follow-up of 8.1 years. The description of participants’ baseline characteristics by metabolic status was summarized in Table 1. Participants with MUO (77%) were older, less likely to be physically active, poorer, and more sedentary compared with MHO participants. A significantly higher BMI was found in participants with MUO. There were more males, smokers in participants with MUO, while MHO individuals had higher sleeping scores, fewer mental health issues, and less cardiovascular disease.
Table 1.
Descriptions of baseline characteristics for the participants and by their metabolic status
| Total (n = 119,424) |
MUO (n = 92,195) |
MHO (n = 27,229) |
P-value | |
|---|---|---|---|---|
| Age, mean (SD), y | 56.7 (7.9) | 57.9 (7.5) | 52.8 (7.8) | < 0.01 |
| Male, n (%) | 55,958 (46.9) | 45,845 (49.7) | 10,113 (37.1) | < 0.01 |
| Body mass index, mean (SD), kg/m2 | 33.9 (3.9) | 34.1 (4.0) | 33.3 (3.3) | < 0.01 |
| Physical activity, n (%) | ||||
| No MVPA | 18,225 (15.3) | 14,362 (15.6) | 3,893 (14.3) | < 0.01 |
| Low PA | 26,229 (22.0) | 19,871 (21.6) | 6,358 (23.4) | < 0.01 |
| Medium PA | 14,426 (12.1) | 10,996 (11.9) | 3,430 (12.6) | < 0.01 |
| High PA | 32,982 (27.6) | 25,226 (27.4) | 7,756 (28.5) | < 0.01 |
| Smoking status, n (%) | ||||
| Current smoker | 11,659 (9.8) | 8,418 (9.1) | 3,241 (11.9) | < 0.01 |
| Previous smoker | 46,109 (38.6) | 37,097 (40.2) | 9,012 (33.1) | < 0.01 |
| Never | 60,883 (51.0) | 46,067 (50.0) | 14,816 (54.4) | < 0.01 |
| Alcohol drinking status, n (%) | ||||
| Current drinker | 106,901 (89.5) | 82,320 (89.3) | 24,581 (90.3) | < 0.01 |
| Previous drinker | 5,461 (4.6) | 4,353 (4.7) | 1,108 (4.1) | < 0.01 |
| Never | 6,689 (5.6) | 5,231 (5.7) | 1,458 (5.4) | < 0.01 |
| Employment status, n (%) | ||||
| Unemployment | 9,075 (7.6) | 7,366 (8.0) | 1,709 (6.3) | < 0.01 |
| Retired | 39,431 (33.0) | 34,485 (37.4) | 4,946 (18.2) | < 0.01 |
| Employed in night shift work | 3,170 (2.7) | 2,220 (2.4) | 950 (3.5) | < 0.01 |
| Employed in day shift work | 5,063 (4.2) | 3,571 (3.9) | 1,492 (5.5) | < 0.01 |
| Employed not in shift work | 57,398 (48.1) | 40,812 (44.3) | 16,586 (60.9) | < 0.01 |
| Income, n (%) | ||||
| < £ 18,000 | 27,955 (23.4) | 23,026 (25.0) | 4,929 (18.1) | < 0.01 |
| £ 18,000 - £ 30,999 | 26,288 (22.0) | 20,670 (22.4) | 5,618 (20.6) | < 0.01 |
| £ 31,000 - £ 51,999 | 24,859 (20.8) | 18,304 (19.9) | 6,555 (24.1) | < 0.01 |
| £ 52,000 - £ 100,000 | 17,086 (14.3) | 11,957 (13.0) | 5,129 (18.8) | < 0.01 |
| > £ 100,000 | 3,610 (3.0) | 2,521 (2.7) | 1,089 (4.0) | < 0.01 |
| Healthy sleep score, n (%) | ||||
| 0 - 1 | 7,307 (6.1) | 5,869 (6.4) | 1,438 (5.3) | < 0.01 |
| 2 - 3 | 61,100 (51.2) | 47,683 (51.7) | 13,417 (49.3) | < 0.01 |
| 4 - 5 | 51,017 (42.7) | 38,643 (41.9) | 12,374 (45.4) | < 0.01 |
| TDI, mean (SD) | -0.8 (3.3) | -0.8 (3.3) | -0.8 (3.3) | 0.39 |
| Vegetable and fruit intake, mean (SD) | 4.6 (3.1) | 4.6 (3.0) | 4.6 (3.2) | 0.8 |
| Sedentary behavior, mean (SD), hour/day | 5.6 (2.7) | 5.6 (2.7) | 5.4 (2.7) | < 0.01 |
| Mental health issue, n (%) | 45,200 (37.8) | 34,389 (37.3) | 10,811 (39.7) | < 0.01 |
| Cardiovascular disease, n (%) | 12,077 (10.1) | 9,909 (10.7) | 2,168 (8.0) | < 0.01 |
| Diabetes, n (%) | 16,249 (13.6) | 16,249 (17.6) | -* | -* |
| Hypertension, n (%) | 87,339 (73.1) | 87,339 (94.7) | -* | -* |
| Hypercholesterolemia, n (%) | 33,865 (28.4) | 33,865 (36.7) | -* | -* |
MUO, metabolically unhealthy obesity; MHO, metabolically healthy obesity; MVPA, moderate-to-vigorous physical activity; PA, physical activity; TDI, Townsend deprivation index
* Not available
During follow-up, a total of 5,506 incident AF events and 117 AF deaths were observed. There were 16 AF events occurred within the first two weeks after patients received a heart valve surgery, demonstrating that the majority of AF events were not post-operation related. Independent associations of metabolic status and PA with incident AF were demonstrated in STable 2 (for the age- and sex-adjusted model) and Table 2 (for the fully adjusted model). MHO was significantly associated with a 35% reduced risk of incident AF compared with MUO (HR = 0.65, 95% CI: 0.57–0.73) (Table 2). Compared with no MVPA, medium and high PA were significantly associated with decreased risk of incident AF (HR = 0.84, 95% CI: 0.75–0.95, and HR = 0.88, 95% CI: 0.80–0.97 for medium and high PA, respectively) (Table 2). There was no significant association found for AF mortality. SFigure 2 shows the independent associations of all other variables with incident AF in the fully adjusted model.
Table 2.
Independent and mutually adjusted associations of metabolic status and physical activity with atrial fibrillation*
| AF | AF death | |||
|---|---|---|---|---|
| Exposure* | No. of cases/No. of total participants | HR (95% CI), p-value | No. of cases/No. of total participants | HR (95% CI), p-value |
| Metabolic status | ||||
| MUO | 2,904/54,627 | Ref | 58/54,627 | Ref |
| MHO | 320/16,861 | 0.65 (0.57, 0.73), p < 0.01 | 5/16,861 | 0.67 (0.27, 1.70), p = 0.40 |
| Physical activity | ||||
| No MVPA | 715/13,600 | Ref | 18/13,600 | Ref |
| low | 887/20,727 | 0.91 (0.83, 1.01), p = 0.07 | 17/20,727 | 0.76 (0.39, 1.49), p = 0.43 |
| medium | 456/11,317 | 0.84 (0.75, 0.95), p < 0.01 | 8/11,317 | 0.62 (0.27, 1.44), p = 0.26 |
| high | 1,166/25,844 | 0.88 (0.80, 0.97), p = 0.01 | 20/25,844 | 0.62 (0.32, 1.20), p = 0.16 |
* ‘Metabolically healthy’ indicated none of the metabolic disorders, and ‘metabolically unhealthy’ indicated at least one of the metabolic disorders, where metabolic disorders included hypertension, hypercholesterolemia, and diabetes. Participants who met at least one of the following criteria were deemed to be hypertensive: systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, taking anti-hypertensive medications, a self-reported history of hypertension or ICD-10 code I10-I15 and ICD-9 code 401 and 403. Self-reported history of high cholesterol, taking medications, or ICD-10 code E780 and ICD-9 code 2720 was the criteria for hypercholesterolemia. Diabetes mellitus was defined as hospital records of diabetes at baseline based on ICD-10 code E10-E14 and ICD-9 code 250 or taking medications or a self-reported history of type 1 or type 2 diabetes. In line with public health guidelines, PA was categorized into four groups according to the level of moderate-to-vigorous PA (MVPA): none (0 MET-mins per week for MVPA), low (< 600 MET-mins per week), medium (600 - 1200 MET-mins per week), and high (≥ 1200 MET-mins per week)
* Models adjusted for age, sex, BMI, smoking status, alcohol drinking status, income, sleep scores, mental health issues, employment status, Townsend deprivation index, vegetable and fruit intake, sedentary behavior, cardiovascular disease and mutually adjusted for physical activity or metabolic status as appropriate
AF, atrial fibrillation; MUO, metabolically unhealthy obesity; MHO, metabolically healthy obesity; MVPA, moderate-to-vigorous physical activity; HR, hazard ratio; CI, confidence interval
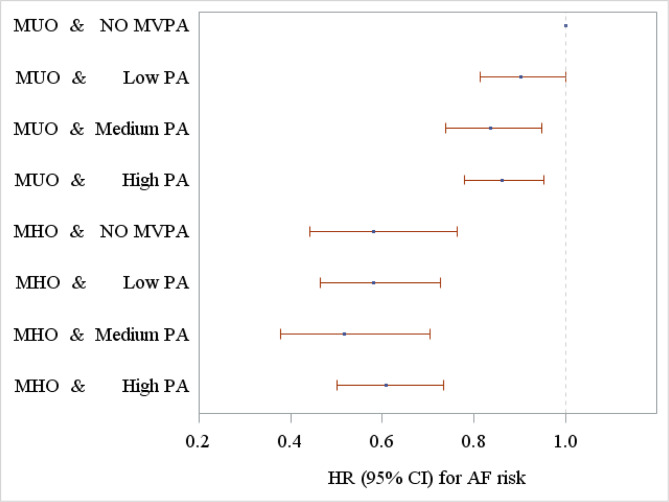
Figure 1 illustrates the joint associations of metabolic status and PA with AF risk in the obese participants, showing the HRs and 95% CIs of different groups defined by metabolic status and PA, taking the MUO and no MVPA as reference. The number of AF events in different groups and the joint associations in different groups defined by metabolic status and physical activity were presented in STable 3. Among the MUO participants, individuals with medium and high PA had significantly lower risk of incident AF compared with no MVPA (HR = 0.84, 95% CI: 0.74–0.95, and HR = 0.87, 95% CI: 0.78–0.96 for medium and high PA, respectively) (STable 3); however the modification effects of the three PA groups when compared with no MVPA did not significantly differ (p = 0.40). No significant modification of PA was found on the AF risk among individuals with MHO (p = 0.88).
Fig. 1.
The joint associations of metabolic status and physical activity with incident atrial fibrillation risk (note- MUO, metabolically unhealthy obesity; MHO, metabolically healthy obesity; MVPA, moderate-to-vigorous physical activity; PA, physical activity; HR, hazard ratio; CI, confidence interval
STable 4 and Table 3 respectively show the independent and joint associations of metabolic status and PA with risk of AF in different subgroups by age and sex. Among participants with MUO, the modification of PA on AF risk was significantly higher in females than that in males (p < 0.01); however there was no significant modification of PA in different age groups (p = 0.85).
Table 3.
Subgroup analyses for the joint associations of metabolic status and physical activity with incident atrial fibrillation risk*
| Sex | Age | |||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | < 65 years | ≥ 65 years | |||||
| Metabolic status & PA | No. of cases/No. of total participants | HR (95% CI), p-value | No. of cases/No. of total participants | HR (95% CI), p-value | No. of cases/No. of total participants | HR (95% CI), p-value | No. of cases/No. of total participants | HR (95% CI), p-value |
| MUO & No MVPA | 439/5,643 | Ref | 219/5,021 | Ref | 415/8,668 | Ref | 243/1,996 | Ref |
| MUO & Low PA | 569/8,133 | 0.97 (0.86, 1.10), p = 0.64 | 232/7,561 | 0.78 (0.65, 0.94), p < 0.01 | 494/12,834 | 0.89 (0.78, 1.01), p = 0.08 | 307/2,860 | 0.92 (0.78, 1.09), p = 0.34 |
| MUO & Medium PA | 294/4,481 | 0.90 (0.78, 1.05), p = 0.18 | 118/4,120 | 0.70 (0.56, 0.88), p < 0.01 | 255/6,875 | 0.85 (0.72, 0.99), p = 0.04 | 157/1,726 | 0.77 (0.63, 0.95), p = 0.01 |
| MUO & High PA | 721/11,628 | 0.85 (0.75, 0.96), p = 0.01 | 312/8,040 | 0.89 (0.75, 1.07), p = 0.21 | 633/15,245 | 0.89 (0.79, 1.02), p = 0.08 | 400/4,423 | 0.77 (0.65, 0.91), p < 0.01 |
| MHO & No MVPA | 29/1,079 | 0.56 (0.39, 0.82), p < 0.01 | 28/1,857 | 0.62 (0.42, 0.92), p = 0.02 | 48/2,777 | 0.50 (0.37, 0.67), p < 0.01 | 9/159 | 0.52 (0.27 1.01), p = 0.05 |
| MHO & Low PA | 48/1,785 | 0.59 (0.44, 0.80), p < 0.01 | 38/3,248 | 0.52 (0.37, 0.74), p < 0.01 | 65/4,710 | 0.44 (0.34, 0.57), p < 0.01 | 21/323 | 0.64 (0.41, 1.00), p = 0.05 |
| MHO & Medium PA | 23/1,034 | 0.52 (0.34, 0.79), p < 0.01 | 21/1,682 | 0.53 (0.34, 0.83), p < 0.01 | 36/2,522 | 0.45 (0.32, 0.63), p < 0.01 | 8/194 | 0.40 (0.20, 0.81), p = 0.01 |
| MHO & High PA | 84/2,878 | 0.63 (0.50, 0.80), p < 0.01 | 49/3,298 | 0.56 (0.41, 0.77), p < 0.01 | 99/5,651 | 0.49(0.39, 0.61), p < 0.01 | 34/525 | 0.59 (0.41, 0.84), p < 0.01 |
*Models adjusted for age, sex, body mass index, smoking status, alcohol drinking status, income, sleep scores, mental health issues, employment status, Townsend deprivation index, vegetable and fruit intake, sedentary behavior, and cardiovascular disease
MUO, metabolically unhealthy obesity; MHO, metabolically healthy obesity; MVPA, moderate-to-vigorous physical activity; PA, physical activity; HR, hazard ratio; CI, confidence interval
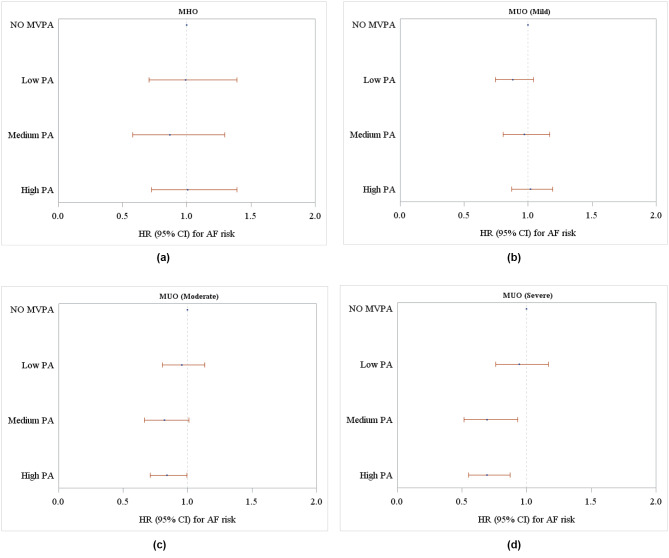
In the exploratory analyses, there was a significant modification of PA on the risk of AF among the participants with severe MUO: HR = 0.70, 95% CI: 0.52–0.94 and HR = 0.70, 95% CI: 0.55–0.88 for medium and high PA when compared with no MVPA (Fig. 2d and STable 5). As the severity of MUO increased, the modification of PA on incident AF risk was elevated accordingly (p for trend < 0.01; STable 5).
Fig. 2.
Associations between physical activity and incident atrial fibrillation risk stratified by different metabolic status: a for MHO; b for mild MUO, c for moderate MUO, d for severe MUO. (note- Metabolically unhealthy status was categorized into: mild (with only one of the metabolic disorders), moderate (with two of the metabolic disorders), severe (with all of three metabolic disorders). Models were adjusted for age, sex, body mass index, smoking status, alcohol drinking status, income, sleep scores, mental health issues, employment status, Townsend deprivation index, vegetable and fruit intake, sedentary behavior, and cardiovascular disease. MUO, metabolically unhealthy obesity; MHO, metabolically healthy obesity; MVPA, moderate-to-vigorous physical activity; PA, physical activity; HR, hazard ratio; CI, confidence interval)
Non-significantly additive or multiplicative modification was found in the whole obese population, as shown in STable 6. The sensitivity analyses yielded similar results to the main findings (STable 7–9).
Discussion
In this study, our principal findings were as follows: (i) among obese participants, both metabolically healthy status and PA (medium and high PA) were significantly associated with reduced risk of incident AF as expected compared with MUO and no MVPA, respectively; (ii) individuals with medium and high PA had a lower risk of incident AF, especially for the medium PA; (iii) among the MUO participants, individuals with medium and high PA had significantly lower risk of AF compared with no MVPA, while there was no significant risk modification by PA on AF risk among individuals with MHO; and (iv) with worsening of MUO (mild, moderate, and severe), the risk reduction of PA on AF risk was elevated accordingly.
There were 77% of participants with MUO in our study (Table 1), broadly similar to the study by Cao et al. (73%) [32]. A nationwide study conducted in Korea with an average follow-up of 7.5 years reported AF incidence rates for MHO and MUO groups (1.10 and 2.88 per 1,000 person-years respectively), and also showed the adjusted HR for MHO and MUO groups (1.30, 95% CI: 1.14–1.48; 1.71, 95% CI: 1.56–1.86, respectively) when compared with metabolically healthy non-obese group [28]. Their results suggested that individuals with MUO had a higher risk of incident AF than the remaining obese participants, consistent with our study. However, the relationship between MUO and AF has been debated. Another study conducted in Norway presented the similar AF risks for the MHO and MUO groups (adjusted HR = 1.6, 95% CI: 1.2–2.1; adjusted HR = 1.6, 95% CI: 1.3–1.9, respectively) compared with the metabolically healthy non-obese group, while the crude HRs showed a lower AF risk for MUO group (crude HR = 1.7, 95% CI: 1.3–2.1) than that for the MHO group (crude HR = 2.4, 95% CI: 2.1–2.8) [29]. Nevertheless, the majority of previous studies regarded the metabolically healthy non-obese individuals as the reference group, rather than the MHO participants. In this study, we investigated AF risk in the MUO group compared with that in the MHO counterpart. Individuals with MUO have impaired vascular function, inflammation and fibrosis at the cellular level, as well as remodeling of the left atrium, left ventricle, and arterial wall, which eventually results in a high risk of developing AF [33–36].
Previous studies have reported that the individuals participating in any MVPA could result in a lower risk of AF [17, 19]. Furthermore, Garnvik et al. found that higher levels of PA were associated with lower AF risk among obese individuals [37]. In the present study, we extended these observations showing that among the MUO participants, individuals with medium and high PA had significantly lower risk of AF, especially the medium PA, while no significant modification of PA was found on AF risk among individuals with MHO. One possible explanation was that PA can strengthen the ability of physiological adjustments including blood flow and vascular function regulation in obesity, which may modify the relationship between metabolic status and AF risk [38]. One study by Lee et al. showed that regardless of the type of metabolic disorder, the metabolic unhealthy status was severer with an increasing number of metabolic disorders [12]. Besides, our study found that with more severe MUO (mild, moderate, and severe), the risk reduction of PA on incident AF risk was elevated accordingly. Less than 40% of obese participants achieved medium or high PA (Table 1). Indeed, obese individuals rarely exercise due to the various complications of obesity including decreased inspiratory muscle strength and restrictive ventilation [39, 40]. However, MVPA can strengthen the ability of physiological adjustments, reduced the risk of cardiovascular disease, and enhance the quality of life [41]. In our analysis, we found the medium PA, but not high PA, had significantly higher modification on the AF risk compared with no MVPA among individuals with MUO. Sanchis-Gomar et al. demonstrated that high level of PA can increase arterial pressure, and stretch of the atrial wall, followed by micro-trauma, inflammation, and fibrosis, which may result in arrhythmogenic and reduce modification effect of PA on the AF risk in participants with MUO [42]. Nevertheless, the modification of medium PA was not significantly higher than low or high PA on AF risk in MUO. One possible reason may be the small sample size or a potential absence of the modification. Therefore, our results should be interpreted with care and caution, requiring further research to validate this relationship.
Through subgroup analysis, among participants with MUO, the modification of PA on AF risk was significantly higher in females than that in males. Wan et al. showed that females reporting PA had lower AF incidence [43]. One possible mechanism was that the appropriate level of PA for males was higher than for females, while a high level of PA may decrease the modification of PA on the AF risk among MUO participants [42–44]. By contrast, we found no significant impact of PA on different age groups among MUO, which may indicate that the PA could modify the AF risk to a similar level across different age strata. Nevertheless, more research is needed to further validate these subgroup findings with an exploratory nature.
Strengths and limitations
This study has some strengths. We categorized the MUO into three groups to further explore the impact of PA on the relationship between MUO and incident AF risk. To our best knowledge, this study is the first attempt to investigate the joint association of metabolic status and PA with AF risk among obese participants. Similar results from sensitivity analyses further supported the robustness of the main findings. While the current guidelines consistently emphasized the importance of AF identification and prevention [20, 45], our study results suggested that participants reporting medium PA were related with lowest AF risk. This may provide some evidence-based data to PA recommendation for the AF prevention specifically in the obese population, regardless of their metabolic status and their severity of MUO.
Several limitations need to be noted. The AF was diagnosed by trained physicians from different hospitals, which may lead to misdiagnosis. Moreover, our results should be interpreted with caution due to possible bias or residual confounding effects that could not be fully precluded in an observational study design. Although we have taken C-reactive protein and white blood cell counts into consideration in the sensitivity analysis, no other variables related to inflammatory status could be assessed due to unavailability of these data. Moreover, left atrial remodeling and left atrial function could be modified by both exercise and metabolic status, which would therefore influence the relationship between risk of AF and metabolic status and PA. Nevertheless, no data on left atrial remodeling and function were available in our current analysis, thereby weakening the strength of our results to an unknown extent. Furthermore, the information on PA was collected by self-report questionnaire, possibly incurring information bias and overestimation of PA due to social desirability. Taking the low response rate at baseline (5.5%) into consideration, the generalizability of our study findings may be compromised [46]. Additionally, in the exploratory analyses we only assessed the severity of MUO based on the number of metabolic disorders at baseline, regardless of the disease severity and management of the individual MUO components, because there was no sufficient information on evaluating these two aspects. Therefore, solely depending on the number of metabolic disorders may not precisely reflect the granularity for the severity of MUO. Moreover, no analyses were performed to explore the changes in or trajectories of metabolic status and PA in relation to risk of AF, due to the limited data available from the study.
Conclusion
MHO was significantly associated with a reduced risk of incident AF compared with MUO in obese participants. PA could significantly modify the relationship between metabolic status and risk of AF among MUO participants, with particular benefits of PA associated with the reduced AF risk as the MUO severity elevated.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the participants and staff of the UK Biobank study for their valuable contributions. This research has been conducted using the UK Biobank Resource under Application Number 63844.
Abbreviations
- AF
Atrial fibrillation.
- MHO
Metabolically healthy obesity.
- PA
Physical activity.
- MVPA
Moderate-to-vigorous physical activity.
- TDI
Townsend deprivation index.
- HR
Hazard ratio.
- CI
Confidence interval.
Author contributions
RW, GYHL and GL: conceived and designed the study. RW, YL, ZY and GL: obtained data, performed analyses and interpretation, and drafted the manuscript. RW, IO, SO and GYHL: provided professional and statistical support, and made critical revisions. All authors read and approved the final manuscript. GYHL and GL shared joint senior authorship.
Funding
This study was funded by the National Natural Science Foundation of China (no. 82103906), and the Science Foundation of Guangdong Second Provincial General Hospital (no. YY2018-002).
Data availability
The data can be available on application to the UK Biobank (www.ukbiobank.ac.uk/).
Declarations
Ethics approval and consent to participate
The UK Biobank study was approved by the North West Multicenter Research Ethics Committee. All participants provided written consent before enrolment.
Consent for publication
Not applicable.
Competing interests
GYHL has served as a consultant for Novartis, Bayer/Janssen, Biotronik, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Verseon, and Daiichi-Sankyo and as a speaker for Medtronic, Bayer, BMS/Pfizer, Boehringer Ingelheim, and Daiichi-Sankyo. No fees have been received directly or personally. All other authors have declared no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016;354:i4482. doi: 10.1136/bmj.i4482. [DOI] [PubMed] [Google Scholar]
- 2.Emdin CA, Wong CX, Hsiao AJ, et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ. 2016;532:h7013. doi: 10.1136/bmj.h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J stroke: official J Int Stroke Soc. 2021;16(2):217–21. doi: 10.1177/1747493019897870. [DOI] [PubMed] [Google Scholar]
- 4.Jones NR, Taylor CJ, Hobbs FDR, Bowman L, Casadei B. Screening for atrial fibrillation: a call for evidence. Eur Heart J. 2020;41(10):1075–85. doi: 10.1093/eurheartj/ehz834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Packer M. Heightened risk of intensive rate control in patients with atrial fibrillation who are obese or have type 2 diabetes: A critical review and re-evaluation. J Cardiovasc Electrophys. 2019;30(12):3020–24. doi: 10.1111/jce.14236. [DOI] [PubMed] [Google Scholar]
- 6.Lavie CJ, De Schutter A, Parto P, et al. Obesity and Prevalence of Cardiovascular Diseases and Prognosis-The Obesity Paradox Updated. Prog Cardiovasc Dis. 2016;58(5):537–47. doi: 10.1016/j.pcad.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Tedrow UB, Conen D, Ridker PM, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study) J Am Coll Cardiol. 2010;55(21):2319–27. doi: 10.1016/j.jacc.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med. 2013;159(11):758–69. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- 10.Kim SA, Lim K, Lee JK, Kang D, Shin S. Metabolically healthy obesity and the risk of all-cause and cardiovascular disease mortality in a Korean population: a prospective cohort study. BMJ open. 2021;11(9):e049063. doi: 10.1136/bmjopen-2021-049063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian S, Liu Y, Feng A, Lou K, Dong H. Metabolically healthy obesity and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: a protocol for a systematic review and meta-analysis of prospective studies. BMJ open. 2019;9(10):e032742. doi: 10.1136/bmjopen-2019-032742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SY, Lee SR, Choi EK, et al. Association Between Change in Metabolic Syndrome Status and Risk of Incident Atrial Fibrillation: A Nationwide Population-Based Study. J Am Heart Association. 2021;10(16):e020901. doi: 10.1161/JAHA.121.020901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai SY, Chen HH, Hsu HY, et al. Obesity phenotypes and their relationships with atrial fibrillation. PeerJ. 2021;9:e12342. doi: 10.7717/peerj.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho SS, Dhaliwal SS, Hills AP, Pal S. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health. 2012;12:704. doi: 10.1186/1471-2458-12-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhuo C, Zhao J, Chen M, Lu Y. Physical Activity and Risks of Cardiovascular Diseases: A Mendelian Randomization Study. Front Cardiovasc Med. 2021;8:722154. doi: 10.3389/fcvm.2021.722154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cassidy S, Chau JY, Catt M, Bauman A, Trenell MI. Cross-sectional study of diet, physical activity, television viewing and sleep duration in 233,110 adults from the UK Biobank; the behavioural phenotype of cardiovascular disease and type 2 diabetes. BMJ open. 2016;6(3):e010038. doi: 10.1136/bmjopen-2015-010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunutsor SK, Seidu S, Makikallio TH, Dey RS, Laukkanen JA. Physical activity and risk of atrial fibrillation in the general population: meta-analysis of 23 cohort studies involving about 2 million participants. Eur J Epidemiol. 2021;36(3):259–74. doi: 10.1007/s10654-020-00714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnesen MP, Frodi DM, Haugan KJ, et al. Day-to-day measurement of physical activity and risk of atrial fibrillation. Eur Heart J. 2021;42(38):3979–88. doi: 10.1093/eurheartj/ehab597[. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Neal WT, Bennett A, Singleton MJ, et al. Objectively Measured Physical Activity and the Risk of Atrial Fibrillation (from the REGARDS Study). The American journal of cardiology 2020;128:107 – 12 doi: 10.1016/j.amjcard.2020.05.004[published Online First: Epub Date]. [DOI] [PubMed]
- 20.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 21.Chao TF, Joung B, Takahashi Y, et al. 2021 Focused Update Consensus Guidelines of the Asia Pacific Heart Rhythm Society on Stroke Prevention in Atrial Fibrillation: Executive Summary. Thromb Haemost. 2022;122(1):20–47. doi: 10.1055/s-0041-1739411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potpara TS, Lip GYH, Dagres N, et al. Cohort profile: the ESC EURObservational Research Programme Atrial Fibrillation III (AF III) Registry. European heart journal. Qual care Clin outcomes. 2021;7(3):229–37. doi: 10.1093/ehjqcco/qcaa050. [DOI] [PubMed] [Google Scholar]
- 23.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckel N, Li Y, Kuxhaus O, Stefan N, Hu FB, Schulze MB. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90†<257 women (the Nurses’ Health Study): 30 year follow-up from a prospective cohort study. The Lancet Diabetes & Endocrinology. 2018;6(9):714–24. doi: 10.1016/S2213-8587(18)30137-2. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Cao Z, Yang H, Zhang Y, Xu F, Wang Y, Metabolic Healthy Obesity VD, Status, Risk of COVID-19 Aging and disease. 2021;12(1):61–71. doi: 10.14336/AD.2020.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang BH, Duncan MJ, Cistulli PA, Nassar N, Hamer M, Stamatakis E. Sleep and physical activity in relation to all-cause, cardiovascular disease and cancer mortality risk. Br J Sports Med. 2021 doi: 10.1136/bjsports-2021-104046. [DOI] [PubMed] [Google Scholar]
- 27.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 28.Lee H, Choi EK, Lee SH, et al. Atrial fibrillation risk in metabolically healthy obesity: A nationwide population-based study. International journal of cardiology 2017;240:221–27 doi: 10.1016/j.ijcard.2017.03.103[published Online First: Epub Date]. [DOI] [PubMed]
- 29.Feng T, Vegard M, Strand LB, et al. Metabolically Healthy Obesity and Risk for Atrial Fibrillation: The HUNT Study. Obesity. 2019;27(2):332–38. doi: 10.1002/oby.22377. [DOI] [PubMed] [Google Scholar]
- 30.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41(2):514–20. doi: 10.1093/ije/dyr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinnouho GM, Czernichow S, Dugravot A, et al. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study. Eur Heart J. 2015;36(9):551–9. doi: 10.1093/eurheartj/ehu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao Z, Zheng X, Yang H, et al. Association of obesity status and metabolic syndrome with site-specific cancers: a population-based cohort study. Br J Cancer. 2020;123(8):1336–44. doi: 10.1038/s41416-020-1012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Keefe EL, Sturgess JE, O’Keefe JH, Gupta S, Lavie CJ. Prevention and Treatment of Atrial Fibrillation via Risk Factor Modification. Am J Cardiol. 2021;160:46–52. doi: 10.1016/j.amjcard.2021.08.042. [DOI] [PubMed] [Google Scholar]
- 34.Mendys P, Jackson LR, Solimon EZ, Howard G, Ferdinand K. The atrial fibrillation paradox -connecting hypertension to atrial disease and stroke. Am J Prev Cardiol. 2021;8:100284. doi: 10.1016/j.ajpc.2021.100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goudis CA, Korantzopoulos P, Ntalas IV, Kallergis EM, Liu T, Ketikoglou DG. Diabetes mellitus and atrial fibrillation: Pathophysiological mechanisms and potential upstream therapies. International journal of cardiology 2015;184:617 – 22 doi: 10.1016/j.ijcard.2015.03.052[published Online First: Epub Date]. [DOI] [PubMed]
- 36.Zheng Y, Xie Z, Li J, et al. Meta-analysis of metabolic syndrome and its individual components with risk of atrial fibrillation in different populations. BMC Cardiovasc Disord. 2021;21(1):90. doi: 10.1186/s12872-021-01858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garnvik LE, Malmo V, Janszky I, Wisloff U, Loennechen JP, Nes BM. Physical activity modifies the risk of atrial fibrillation in obese individuals: The HUNT3 study. Eur J Prev Cardiol. 2018;25(15):1646–52. doi: 10.1177/2047487318784365. [DOI] [PubMed] [Google Scholar]
- 38.Xu F, Cohen SA, Lofgren IE, Greene GW, Delmonico MJ, Greaney ML. The Association between Physical Activity and Metabolic Syndrome in Older Adults with Obesity. J frailty aging. 2019;8(1):27–32. doi: 10.14283/jfa.2018.34. [DOI] [PubMed] [Google Scholar]
- 39.Mehawej J, J SS, C IK, et al. Factors Associated with Moderate Physical Activity Among Older Adults with Atrial Fibrillation. J Atr fibrillation. 2021;13(5):2454. doi: 10.4022/jafib.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciprandi D, Bertozzi F, Zago M, et al. Study of the association between gait variability and physical activity. European review of aging and physical activity: official journal of the European Group for Research into. Elder Phys Activity. 2017;14:19. doi: 10.1186/s11556-017-0188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramakrishnan R, Doherty A, Smith-Byrne K, et al. Accelerometer measured physical activity and the incidence of cardiovascular disease: Evidence from the UK Biobank cohort study. PLoS Med. 2021;18(1):e1003487. doi: 10.1371/journal.pmed.1003487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchis-Gomar F, Perez-Quilis C, Lippi G, et al. Atrial fibrillation in highly trained endurance athletes - Description of a syndrome. Int J Cardiol. 2017;226:11–20. doi: 10.1016/j.ijcard.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 43.Wan Q, Zhou Y, Zhu W, Liu X. Sex-Specific Exposure-Effect Relationship Between Physical Activity and Incident Atrial Fibrillation in the General Population: A Dose-Response Meta-Analysis of 16 Prospective Studies. Front Cardiovasc Med. 2021;8:710071. doi: 10.3389/fcvm.2021.710071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elliott AD, Linz D, Mishima R, et al. Association between physical activity and risk of incident arrhythmias in 402 406 individuals: evidence from the UK Biobank cohort. Eur Heart J. 2020;41(15):1479–86. doi: 10.1093/eurheartj/ehz897. [DOI] [PubMed] [Google Scholar]
- 45.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125-e51. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 46.Li G, Zhang X, Liu Y, et al. Relationship between glucosamine use and the risk of lung cancer: data from a nationwide prospective cohort study. The European respiratory journal 2021 doi: 10.1183/13993003.01399-2021[published Online First: Epub Date]. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data can be available on application to the UK Biobank (www.ukbiobank.ac.uk/).