Abstract
Gynecologic surgery carries a known risk of injury to the urinary tract, especially in the presence of risk factors. Injury to the bladder, particularly a mechanical injury, is more common than injury to the ureter. Urinary tract injuries occur in 0.3% to 0.8% of all gynecologic procedures, and injuries to the bladder occur in 0.05% to 0.66% of such surgeries. The risk of bladder injury increases in hysterectomy procedures. Most research studies have cited occurrence of bladder injuries to be 1.0% to 1.8% in laparoscopically assisted vaginal hysterectomies and vaginal hysterectomies. Despite its frequency, there is limited research on best practices for bladder injury repair. The authors performed a literature search through the PubMed database using the terms “bladder anatomy,” “bladder injury,” “bladder repair,” “cystotomy,” “routine cystoscopy,” and “vesicovaginal fistula.” This review uses gynecologic and trauma literature and discusses prevention, recognition, types of iatrogenic bladder injuries, their clinical significance, current guidelines on bladder injury repair, and the expected follow-up care, and concludes by identifying areas for further research.
Key words: anatomy, bladder injury, cystogram, gynecologic surgery, urinary sling
Bladder anatomy
The bladder is a hollow muscular organ situated posterior to the pubic symphysis and anterior to the cervicovaginal junction that expands into the abdominal cavity when full. The superior and part of the posterior surfaces of the bladder are covered by peritoneum. The inferior portion and inferolateral sides of the bladder are covered by endopelvic fascia.2 There are 4 anatomic parts to the bladder: apex or dome, body, fundus, and neck.2 The trigone is a triangular area in the body between the 2 ureteric orifices (Figures 1). The superior and inferior vesical arteries provide the primary blood supply to the bladder.2 The detrusor muscle is the primary muscle of the bladder, composed of transitional epithelium or “urothelium.” The detrusor is innervated by muscarinic receptors under parasympathetic control and beta-adrenergic receptors under sympathetic control.2 As an empty bladder fills up, the sympathetic response is stimulated on the detrusor and internal sphincter via the hypogastric nerves, which causes them to respectively relax and constrict.2 To allow micturition, the parasympathetic response contracts the detrusor muscle and relaxes the internal sphincter.2
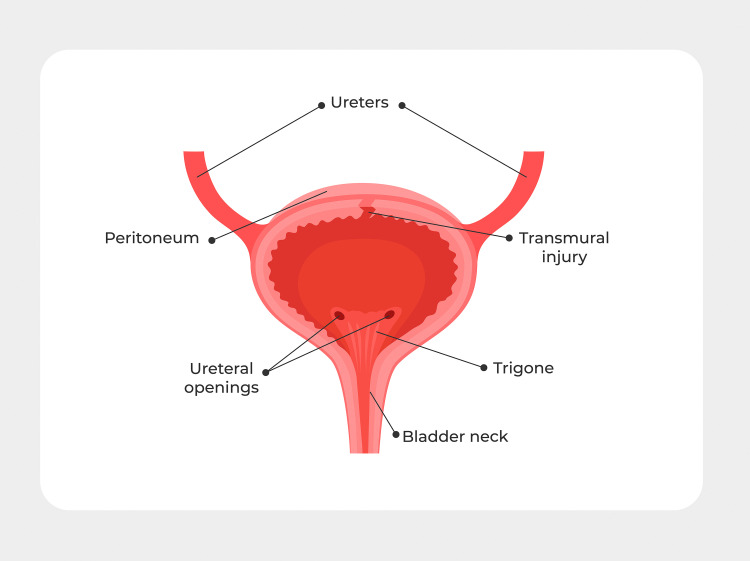
Figure 1.
Basic bladder anatomy
The peritoneum covers the dome of the bladder. The trigone is the area of the bladder contains the 2 ureteral openings and is the area most frequently injured in gynecologic surgery.
Zelivianskaia. Best practices for repair of iatrogenic bladder injury. Am J Obstet Gynecol Glob Rep 2022.
Prevention and diagnosis of bladder injury
Injury to the bladder can occur at several points in gynecologic surgery. The bladder is in danger during lysis of adhesions, bladder dissection in all routes of hysterectomy, or entry into the anterior cul-de-sac in a vaginal hysterectomy.1 Bladder injury can also occur when using a suprapubic incision for trocar placement or tissue extraction. The authors always place the suprapubic trocar under direct visualization to decrease the risk of penetrating bladder injury. Via a similar mechanism, the bladder can be injured in obstetrical procedures, such as cesarean deliveries. A retrospective analysis compared a Pfannenstiel incision with vertical midline subumbilical incision and demonstrated that the latter involved a significantly higher risk of bladder injury (P<.0001; odds ratio, 6.7; 95% confidence interval, 2.6–16.5).3 Bladder injuries can be characterized as intra- or extraperitoneal, as further described below.
Best practices for injury prevention include using sharp, rather than blunt, dissection, backfilling the bladder, and cephalad traction on a uterine manipulator. Sharp dissection rather than blunt tearing of tissues avoids distortion of anatomic planes.1 Ensuring that the bladder is drained can reduce injury during a cesarean delivery. Retrograde filling of the bladder with either normal saline or sterile milk during laparoscopic or vaginal hysterectomy can more clearly delineate the borders of the bladder. The dissection of the vesicouterine peritoneum and cephalad traction on a uterine manipulator help mobilize the bladder away from the uterine arteries, allowing ligation to be accomplished safely.1 Lastly, electrosurgical energy should be cautiously used around the bladder because thermal spread can lead to delayed injury. Additional mechanisms of injury include sharp instrumentation, perforation, or placement of suture into the bladder.
Cystoscopy can be used to diagnose bladder injury intraoperatively. Because of the low incidence rates of ureter and bladder injury, most studies show an absolute, but not a statistically significant, increase in injury recognition with routine cystoscopy after hysterectomy.4 Furthermore, most bladder injuries are recognized during surgery. One systematic review reported that 84% and 94% of bladder injuries were identified intraoperatively without and with routine cystoscopy, respectively, and the difference was not statistically significant.5 When examining the detection of ureteral injuries specifically, cystoscopy has a sensitivity and specificity of 94.4% and 95.5%, respectively.6 Given that cystoscopy detects nearly all unrecognized bladder and ureteral injuries (another study found a 97.4% combined detection rate) and is likely cost-effective, the American Association of Gynecologic Laparoscopists4 recommends that surgeons and institutions “consider the routine implementation” of cystoscopy with hysterectomy.4 Several agents can be used to help assess patency during cystoscopy, including dextrose, phenazopyridine, sodium fluorescein, and indigo carmine.6 All these agents have a high success rate.6 The specific cost-effectiveness and adverse effects of each agent are outside the scope of this review.
Types of bladder injury and clinical impact of each type
The bladder is one of the weakest organs in relation to tissue strength. Despite the thin bladder wall, the bladder is an incredibly resilient organ with rapid gain in tissue strength after injury and subsequent repair. There are multiple types of mechanical bladder injury based on whether the injury is extra- or intraperitoneal and the injury size. Iatrogenic injuries to the bladder are staged by the American Association for the Surgery of Trauma injury severity scale as follows:
-
•
Grade 1: contusion, intramural hematoma, or partial thickness laceration
-
•
Grade 2: extraperitoneal bladder wall laceration <2 cm
-
•
Grade 3: extraperitoneal >2 cm or intraperitoneal <2 cm bladder wall laceration
-
•
Grade 4: intraperitoneal bladder wall laceration >2 cm
-
•
Grade 5: intra- or extraperitoneal bladder wall laceration involving the trigone or bladder neck1
Extraperitoneal injuries can be further classified as having complex features, which are defined as:
-
•
Open pelvic fracture with exposed bone within the bladder lumen
-
•
Concurrent rectal or vaginal injury to prevent subsequent fistula formation to the bladder
-
•
Bladder neck injury
-
•
Persistent hematuria as a consequence of the bladder injury, with clots interfering with adequate bladder drainage (Figures 2 and Table 1)7
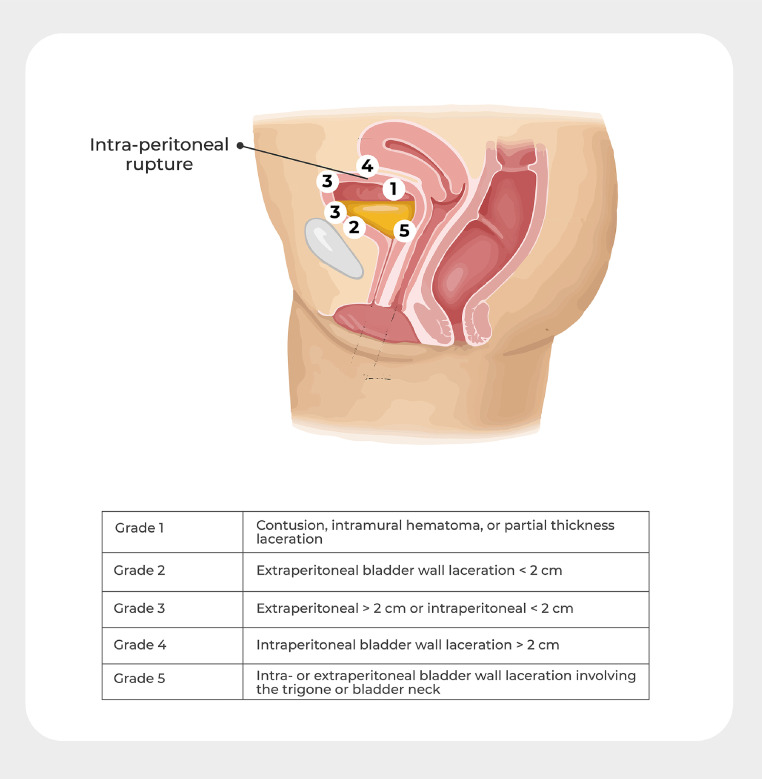
Figure 2.
Types of bladder injury
Zelivianskaia. Best practices for repair of iatrogenic bladder injury. Am J Obstet Gynecol Glob Rep 2022.
Table 1.
Types of bladder injury
| Grade 1 | contusion, intramural hematoma, or partial thickness laceration |
| Grade 2 | extraperitoneal bladder wall laceration <2 cm |
| Grade 3 | extraperitoneal >2 cm or intraperitoneal <2 cm bladder wall laceration |
| Grade 4 | intraperitoneal bladder wall laceration >2 cm |
| Grade 5 | intra- or extraperitoneal bladder wall laceration involving the trigone or bladder neck |
Zelivianskaia. Best practices for repair of iatrogenic bladder injury. Am J Obstet Gynecol Glob Rep 2022.
The above classification is accepted by various organizations, including the American Urological Association (AUA) and the European Association of Urology (EAU), to aid with categorizing repair guidelines. Most guidelines noted here are published by these societies or resulted from independent research studies, as cited.
Bladder injuries of grade 1 or 2 should be managed with prolonged drainage with indwelling urethral catheter for at least 7 to 14 days, and some studies recommend up to 3 weeks. Grade 1 and 2 injuries are not managed surgically.7,8 Bladder injuries of grade ≥3 require surgical management.8,9 Intraperitoneal injuries are generally more significant and involve higher risk of complications than extraperitoneal injuries.
Various factors can increase the risk of bladder injury. In obstetrics, risk factors for bladder injury during cesarean delivery include previous cesarean delivery, adhesions, emergent cesarean delivery, and cesarean delivery performed at the time of the second stage of labor and/or after failed instrumentation.3 In both obstetrics and gynecologic surgery, risk factors include coexisting medical conditions that can lead to adhesions, such as endometriosis and Crohn's disease, and previous abdominal surgery, including cesarean delivery.10 Complications of intraperitoneal bladder injury include uroascites and infection leading to urosepsis. Complications of extraperitoneal bladder injury managed conservatively include urethral stricture and bladder hyperreflexia. Both types of injuries can lead to persistent hematuria, infection, and incontinence.
Pathophysiology of wound healing
After mechanical bladder injury, the bladder epithelium, known as the urothelium, takes a shorter amount of time to heal than other organ types. The force needed for tissue disruption approaches that of unwounded tissue in 14 to 21 days after wounding in animal models.11 The urothelium is covered by a smooth muscle layer called the lamina propria, followed by the covering serosa layer called the adventitia.8 Wound healing has 3 general stages: inflammation, new tissue formation and proliferation, and remodeling. Bladder wound healing relies primarily on proliferation and migration of epithelial cells to wound edges, without a significant inflammatory phase.8 Animal studies have suggested that bladder injury heals in as little as 2 to 10 days because of rapid increase in urothelial turnover rate and exponential upregulation.8 However, the time frame for this process varies by magnitude and depth of injury, and there is no consensus on a more specific time frame. Furthermore, animal studies use different in vitro and in vivo models to study wound healing pathophysiology, and the lack of uniformity makes comparison of studies challenging. In conclusion, bladder wound healing after mechanical injury follows a somewhat different process and occurs in a shorter amount of time compared with other types of epithelia wound healing, but the exact mechanism and duration of this process is still unknown.
Repair techniques
As outlined above, large or complex intra- and extraperitoneal injuries require surgical management.7 Adequate visualization is particularly important when starting the surgical repair. If the injury occurred during laparoscopic surgery, the repair can either be accomplished using intracorporeal suturing laparoscopically or via an exploratory laparotomy. Visual inspection is the most reliable method of assuring bladder integrity.12 The most common sutures used for bladder repair are polyglactin or poliglecaprone absorbable sutures, which are well-suited given the minimal tissue reaction they cause and their absorption by 21 days.5,11,14,15 Plain catgut sutures lead to an even lower amount of bladder tissue reaction but last for a longer amount of time than polyglactin or poliglecaprone, which is unnecessary given the speed of bladder tissue healing.13 Silk and Mersilene sutures produce the greatest tissue reaction and are not recommended for bladder repair.5,13 Bladder defects can be closed in 1 or 2 layers using a running nonlocked stitch, or with interrupted sutures, although there is much variation in closure techniques (Figures 3).16,17 There is very limited research comparing suture types and mechanism of repair in patients, which is a limitation of these guidelines.
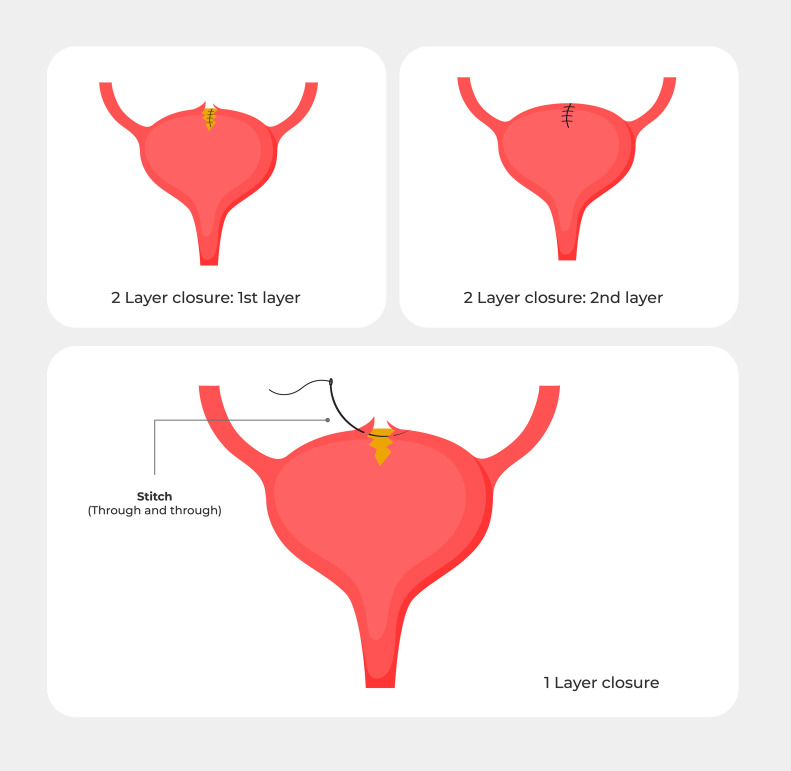
Figure 3.
Various ways of repairing a bladder injury
The injury can either be repaired in 2 separate layers or in a single layer. If a 2-layer closure is used, the bladder should be backfilled after the first layer to test for a watertight closure.
Zelivianskaia. Best practices for repair of iatrogenic bladder injury. Am J Obstet Gynecol Glob Rep 2022.
Recent studies have started to explore barbed suture for bladder repair because its use increases in laparoscopic surgery. Several ex vivo studies demonstrated no difference in mean leak pressure of sheep bladders closed with barbed vs nonbarbed suture.18,19 In vivo studies in humans are limited, but a meta-analysis in the urologic literature indicated barbed suture had an equivalent postoperative leakage rate and other surgical outcomes in urethrovesical anastomosis.20 A recent study examined laparoscopic cystotomy closure using 1 or 2 layers of barbed suture in 14 patients without major complications.18 This study suggested that barbed suture is also an acceptable material for bladder repair in gynecologic surgery, but was limited by a small sample size and single-site design.
If a second layer is performed with any type of suture, it can be in an imbricating fashion incorporating the bladder serosa.5 Most cystotomies >2 cm require a 2-layer closure for a watertight seal.21 Checking if the closure is watertight should be performed after the first layer of a 2-layer closure. This is done by gravity filling, which allows water to drain in passively into the bladder with the goal of 300 mL of normal saline, vs pressure filling, which may unnecessarily strain the sutures. These closure techniques are widely used in practice but are based largely on animal models.
Postoperative care: indwelling catheter
After bladder injury repair, the bladder is decompressed with an indwelling catheter for 7 to 14 days, but there is no consensus on the exact duration.16,17,21 As mentioned previously, animal studies suggest that bladder tissue heals in 2 to 10 days, and reepithelialization of bladder mucosa and serosa specifically occurs after 3 to 4 days.1 One randomized trial compared indwelling Foley catheter duration of 10 with that of 14 days following surgical repair of vesicovaginal fistula and did not find a statistically significant difference in cure rates or complications between the 2 groups.22 In a small proportion of cases, the bladder is not completely healed after 14 days and the catheter must be kept in place for as long as 3 weeks. This suggests that in most cases, earlier removal would lead to similar cure rates while reducing the risk of infection, but more research is needed. Prophylactic antibiotics are not required during short-term indwelling catheter use.1
Postoperative care: cystogram
There are mixed recommendations on the necessity of a cystogram before Foley catheter removal. A cystogram is a fluoroscopic study that evaluates the bladder. It is performed by instilling water-soluble contrast through the catheter to a volume of 150 to 300 mL and looking for contrast extravasation.23 Several experts suggest removal of the Foley catheter only after a normal cystogram confirms lack of extravasation.16 Others recommend it before removal after all instances of conservative treatment without surgical intervention, but only in certain complex cases of surgical repair.7 Yet other studies conclude that routine cystogram is not universally recommended unless there was extensive injury.16,24
Multiple studies have demonstrated that following surgical repair of simple intraperitoneal bladder injury, nearly all patients have normal cystograms, but as many as 22% of patients with extraperitoneal bladder injury have a positive cystogram showing extravasation requiring further intervention.1,25 Current guidelines by the AUA and EAU recommend cystography after conservatively treated bladder injuries and surgically repaired extraperitoneal injuries, but do not recommend it after surgical repair of simple intraperitoneal injuries.12 We recommend further research into this area to potentially decrease unnecessary procedures, radiologic exposure, and increased cost to patients.
Bladder injury during sling placement
Urethral sling placement is a particularly high-risk procedure for bladder injury, which complicates between 3% and 9% of procedures.26 The transobturator approach to sling placement decreases the risk, although it has other adverse events, such as greater incidence of groin pain.27 If bladder perforation occurs, trocar removal and reinsertion is recommended.26 There seems to be no difference in postoperative complications or success rates when the sling is correctly replaced, thus the entire procedure does not need to be abandoned.26 Urethral perforation, which is uncommon (0.4%–1%), is a contraindication to maintenance of the sling.26 Intraoperatively, universal cystoscopy is recommended by the EAU after suburethral sling procedures via the retropubic route.12,26
Postoperative care: complications
Bladder injury and repair are associated with several short- and long-term complications. One large study of the American College of Surgeons National Surgical Quality Improvement Program database found that patients who experienced bladder injury and intraoperative repair were more likely to experience urinary tract infection, sepsis, and bleeding.28 Delayed recognition of bladder injury was rare, but patients who experienced delayed repair were noted to have a higher overall complication rate, but not a statistically significant difference in 30-day mortality or morbidity.28
Bladder injury, particularly after gynecologic surgery, can also lead to a vesicovaginal fistula, which is an abnormal communication between the bladder and vagina that leads to leakage of urine from the vagina.29 This is a rare complication with prevalence rates after hysterectomy ranging from 0.2 to 2.2 in 1000 patients.29 It can be diagnosed in an outpatient setting by clinical evaluation, but cystoscopy and imaging studies may be helpful in the assessment.29 Small early-detected and nonmalignant vesicovaginal fistulas may be managed conservatively with transurethral Foley catheter placement for 2 to 8 weeks.29 For larger fistulas or those discovered later in the postoperative course, surgical management may be necessary.29 The details of complex fistula management and the surgical approach are outside the scope of this article.
Areas for further study
In conclusion, much is known about bladder composition and healing from animal models alone. Based on this information, there is a consensus on types of bladder injury that need to be repaired surgically vs injury types that can be managed conservatively with an indwelling Foley catheter. Furthermore, there is relative standardization in suture types used for surgical repair. However, more research is needed to determine duration of indwelling Foley catheter and the risk-to-benefit ratio of cystograms before catheter removal. Research on bladder injury is challenging given the small sample size at any one institution, thus further studies should be multiinstitutional and multidisciplinary to gather a large sample size.
Acknowledgments
The authors wish to thank Dr Lee Ann Richter, M.D., for her advice and guidance. The authors also wish to acknowledge the Department of Obstetrics and Gynecology at MedStar Washington Hospital Center for scholarly support.
Footnotes
From the Division of Urogynecology and Minimally Invasive Gynecologic Surgery, Department of Obstetrics and Gynecology, MedStar Washington Hospital Center, Washington, DC (Drs Zelivianskaia, Bradley, and Morozov); and Mission Hospital, Western Carolina Women's Specialty Center, Asheville, NC (Dr Bradley).
The authors report no conflict of interest.
There was no funding source for this article.
Cite this article as: Zelivianskaia AS, Bradley SE, Morozov VV. Best practices for repair of iatrogenic bladder injury. Am J Obstet Gynecol Glob Rep 2022;2:100062.
References
- 1.Glaser LM, Milad MP. Bowel and bladder injury repair and follow-up after gynecologic surgery. Obstet Gynecol. 2019;133:313–322. doi: 10.1097/AOG.0000000000003067. [DOI] [PubMed] [Google Scholar]
- 2.Shermadou ES, Rahman S, Leslie SW. Anatomy, abdomen and pelvis, bladder. 2020. Available at: https://www.ncbi.nlm.nih.gov/books/NBK531465/. Accessed March 1, 2021. [PubMed]
- 3.Tarney CM. Bladder injury during cesarean delivery. Curr Womens Health Rev. 2013;9:70–76. doi: 10.2174/157340480902140102151729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AAGL Advancing Minimally Invasive Gynecology Worldwide AAGL Practice Report: practice guidelines for intraoperative cystoscopy in laparoscopic hysterectomy. J Minim Invasive Gynecol. 2012;19:407–411. doi: 10.1016/j.jmig.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Wong JMK, Bortoletto P, Tolentino J, Jung MJ, Milad MP. Urinary tract injury in gynecologic laparoscopy for benign indication: a systematic review. Obstet Gynecol. 2018;131:100–108. doi: 10.1097/AOG.0000000000002414. [DOI] [PubMed] [Google Scholar]
- 6.Askew AL, Myers ER, Dieter AA. Cost-effectiveness of agents used for evaluation of ureteral patency during intraoperative cystoscopy in gynecologic and urogynecologic surgery. Am J Obstet Gynecol. 2022;226 doi: 10.1016/j.ajog.2021.08.055. 100.e1–6. [DOI] [PubMed] [Google Scholar]
- 7.Morey AF, Brandes S, Dugi DD, 3rd, et al. Urotrauma: AUA guideline. J Urol. 2014;192:327–335. doi: 10.1016/j.juro.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsson P, Chamorro CI, Fossum MA. A review on bladder wound healing after mechanical injury. J Tissue Sci Eng. 2016;7:170. doi: 10.4172/2157-7552.1000170. [DOI] [Google Scholar]
- 9.Marchand TD, Cuadra RH, Ricchiuti DJ. Laparoscopic repair of a traumatic bladder rupture. JSLS. 2012;16:155–158. doi: 10.4293/108680812X13291597716546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibrahim N, Spence AR, Czuzoj-Shulman N, Abenhaim HA. Incidence and risk factors of bladder injury during cesarean delivery: a cohort study. Arch Gynecol Obstet. 2022 doi: 10.1007/s00404-022-06447-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Vaidya B, Chaudhari M, Parmar D, Chaudhari V, Daginawala T, Shah R. Bladder injuries during obstetrical and gynecological surgeries. Int Surg J. 2017;4:2177–2180. [Google Scholar]
- 12.Summerton DJ, Kitrey ND, Lumen N, Serafetidinis E, Djakovic N. European Association of Urology. EAU guidelines on iatrogenic trauma. Eur Uro. 2012;62:628–639. doi: 10.1016/j.eururo.2012.05.058. [DOI] [PubMed] [Google Scholar]
- 13.Santucci RA, Bartley JM. Urologic trauma guidelines: a 21st century update. Nat Rev Urol. 2010;7(Sep 7):510–519. doi: 10.1038/nrurol.2010.119. [DOI] [PubMed] [Google Scholar]
- 14.Hastings JC, Van Winkle W, Barker E, Hines D, Nichols W. The effect of suture materials on healing wounds of the bladder. Surg Gynecol Obstet. 1975;140:933–937. [PubMed] [Google Scholar]
- 15.Minas V, Gul N, Aust T, Doyle M, Rowlands D. Urinary tract injuries in laparoscopic gynaecological surgery; prevention, recognition and management. Obstet Gynecol. 2014;16:19–28. [Google Scholar]
- 16.Lee JS, Choe JH, Lee HS, Seo JT. Urologic complications following obstetric and gynecologic surgery. Korean J Urol. 2012;53:795–799. doi: 10.4111/kju.2012.53.11.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis JD. Management of injuries to the urinary and gastrointestinal tract during cesarean section. Obstet Gynecol Clin North Am. 1999;26:469–480. doi: 10.1016/s0889-8545(05)70091-7. [DOI] [PubMed] [Google Scholar]
- 18.Duffy DJ, Duddy HR, Keating S, Gutierrez-Nibeyro SD. Influence of barbed suture on leak pressures after double-layer inverting closure of cystotomy sites in sheep. Vet Surg. 2018;47:902–907. doi: 10.1111/vsu.12935. [DOI] [PubMed] [Google Scholar]
- 19.Montel JS, Duffy DJ, Weng HY, Freeman LJ. Single layer cystotomy closure of excised porcine bladders with barbed versus smooth suture material. Vet Surg. 2017;46:580–586. doi: 10.1111/vsu.12644. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Liu C, Zhang H, et al. The use of unidirectional barbed suture for urethrovesical anastomosis during robot-assisted radical prostatectomy: a systematic review and meta-analysis of efficacy and safety. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chamsy D, King C, Lee T. The use of barbed suture for bladder and bowel repair. J Minim Invasive Gynecol. 2015;22:648–652. doi: 10.1016/j.jmig.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 22.Nardos R, Menber B, Browning A. Outcome of obstetric fistula repair after 10-day versus 14-day Foley catheterization. Int J Gynaecol Obstet. 2012;118:21–23. doi: 10.1016/j.ijgo.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 23.Bell D, Morgan M. “Cystography” Radiopedia. 2022. Available at: https://radiopaedia.org/articles/cystography-1?lang=us. Accessed July 5, 2021.
- 24.Inaba K, Okoye OT, Browder T, et al. Prospective evaluation of the utility of routine postoperative cystogram after traumatic bladder injury. J Trauma Acute Care Surg. 2013;75:1019–1023. doi: 10.1097/TA.0b013e318299b61a. [DOI] [PubMed] [Google Scholar]
- 25.Inaba K, McKenney M, Munera F, et al. Cystogram follow-up in the management of traumatic bladder disruption. J Trauma. 2006;60:23–28. doi: 10.1097/01.ta.0000200096.44452.8a. [DOI] [PubMed] [Google Scholar]
- 26.Sharp HT, Adelman MR. Prevention, Recognition, and management of urologic injuries during gynecologic surgery. Obstet Gynecol. 2016;127:1085–1096. doi: 10.1097/AOG.0000000000001425. [DOI] [PubMed] [Google Scholar]
- 27.Garely AD, Noor N. Diagnosis and surgical treatment of stress urinary incontinence. Obstet Gynecol. 2014;124:1011–1027. doi: 10.1097/AOG.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 28.Cohen AJ, Packiam VT, Nottingham CU, Pariser JJ, Faris SF, Bales GT. Iatrogenic bladder injury: national analysis of 30-day outcomes. Urology. 2016;97:250–256. doi: 10.1016/j.urology.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Medlen H, Barbier H. Vesicovaginal fistula. 2022. Available at: https://www.ncbi.nlm.nih.gov/books/NBK564389/. Accessed April 21, 2022.