Abstract
BACKGROUND
The syndromic approach is a simple and affordable strategy for the management of sexually transmitted infections in countries with low-resource settings. However, because of the lack of specificity and accuracy, the risk of overuse and misuse of antibiotics is very high. Here, we proposed a more specific and accurate algorithm compared with the current algorithm used for syndromic case management of 3 common sexually transmitted pathogens and compared its precision with laboratory-based tests.
OBJECTIVE
This study aimed to report a comparative account of the accuracy of existing syndromic case management guidelines followed in mainstream hospitals, for taking care of patients with nonviral sexually transmitted infections, concerning an approach involving an alternative algorithm formulated in our laboratory followed by polymerase chain reaction testing.
STUDY DESIGN
This was an observational study that compared the data between 2 categories based on diagnostics accuracy and treatment. In category I, symptoms of infection were scored on the basis of the existing National AIDS Control Organization and National AIDS Control Programme guidelines, and patients were treated before testing by polymerase chain reaction. In category II, patients were recruited on the basis of the National AIDS Control Organization and National AIDS Control Programme guidelines with additional alternative syndromic case management parameters. All samples were tested by polymerase chain reaction for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis and clinically correlated before giving the treatment.
RESULTS
In category I, among 646 women with symptomatic infection, only 46 (7.82%) tested positive by polymerase chain reaction assay for at least 1 of the pathogens, and 600 (92.87%) tested negative for infection by any of the 3 pathogens. The total estimated percentages of the overuse and misuse of antibiotics were 92.87% and 8.69%, respectively. Correct and complete treatment based on laboratory outcome compared with National AIDS Control Programme guidelines was 42 of 46 (91.30%). The estimated overuse of azithromycin and cefixime (Gray Kit) was 29.69%, the estimated overuse of a combination of doxycycline, cefixime, and metronidazole (Yellow Kit) was 29.87%, and the estimated overuse of a combination of doxycycline, cefixime, metronidazole, and azithromycin (Gray with Yellow Kit) was 11.45%. In category II, wherein patients were treated using an alternative syndromic approach and polymerase chain reaction diagnostics, 243 of 319 patients (76.15%) were infected with either of the pathogens (Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis), whereas 76 of 319 patients (23.82%) were negative for any of the 3 pathogens. Among 243 patients with infection, 99 of 243 (40.74%) were infected with a single pathogen, whereas 144 of 243 (59.20%) were coinfected. Of 144 coinfected patients, the percentage of Chlamydia trachomatis + Neisseria gonorrhoeae infection was the highest (51.38%), followed by coinfection with all 3 pathogens (30%). Coinfection with Chlamydia trachomatis + Trichomonas vaginalis was 9.72%, and coinfection with Neisseria gonorrhoeae + Trichomonas vaginalis was 9.03%. The estimated overuse of antibiotics was found to be 23.82% only.
CONCLUSION
The proposed alternative strategies of syndromic case management can reduce the percentage of misuse and overuse of antibiotics from 92.87% to 23.82%. Moreover, syndromic case management alone was insufficient for disease management.
Key words: alternative approach, antibiotics treatment, nonviral sexually transmitted infections, polymerase chain reaction, syndromic case management
AJOG Global Reports at a Glance.
Why was this study conducted?
The existing syndromic case management (SCM) approach as described in the National AIDS Control Organization-National AIDS Control Programme (NACO-NACP) guidelines for managing sexually transmitted infections has been compared with an alternative SCM approach coupled with a polymerase chain reaction test for the management of patients. The alternative approach has been found to give promising results in terms of accurate diagnosis, keeping the focus on 3 common sexually transmitted pathogens.
Key findings
This study reflected that the existing management guidelines need some revision as it is prone to misdiagnosis and consequently overuse and misuse of antibiotics ultimately leading to antibiotic resistance. The findings of this study can significantly reduce the risk of antibiotic resistance and provide better diagnoses if translated into practice for clinical management, thereby reducing the time of recovery in patients.
What does this add to what is known?
The NACO-NACP guidelines have been challenged time and again because of their lack of clinical specificity. Aside from highlighting the error-prone nature of the existing SCM, the current study provides additional parameters that improve syndromic disease management as validated by nucleic acid-based diagnosis.
Introduction
Every day, >1 million sexually transmitted infections (STIs) occur worldwide because of several sexually transmitted diseases. According to a report by the World Health Organization (WHO) in 2019, approximately 376 million people are affected every year by one or more of the 4 STIs, namely, Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis, and Treponema pallidum, and more than 1 million STIs are acquired every day worldwide.1,2 The infection caused by these pathogens constitutes one-third of the reproductive and sex-related health problems among women aged 15 to 44 years old, and in some cases, STIs can have serious reproductive health consequences beyond the immediate impact of the infection itself (eg, infertility or mother-to-child transmission). The risk of adverse birth outcomes, including stillbirths and newborn deaths associated with STIs, are very high.3 In India, STIs constitute important known public health problems that cause morbidity and severe complications, such as cancer of the cervix, spontaneous abortions, premature births, low birthweight, or infertility.4 Per previously reported epidemiologic and biologic evidence, patients with STI have a significantly higher chance of acquiring and transmitting HIV.5,6 In the current scenario, overuse and misuse of antibiotics in STI are not new; the emergence of antimicrobial or multidrug resistance is well reported by several study groups worldwide.1,2,7, 8, 9 The prevalence of multidrug-resistant infections varies widely across different regions of the world, but some of the highest levels of infection have been found in low-income, middle-income, and underdeveloped countries.10,11 The reasons are complex and include poor quality of health services, high burden of disease, and lack of accessibility of accurate and confirmed diagnostic assays, regulatory oversight and overuse of antibiotics, inappropriate dosing, and lack of knowledge about the risks of microbial resistance. In low-income and developing countries, screening and treatment of symptomatic STI are based on syndromic case management (SCM) because of the unavailability of inexpensive point-of-care diagnostic assays.12,13 In asymptomatic cases, these are completely ignored and untreated. The effectiveness of the SCM-based studies in low-resource settings has shown that the specificity of established syndromic algorithms is below 50%.10, 11, 12, 13, 14, 15, 16 Correspondingly, low positive predictive values of syndromic algorithms lead to substantial overtreatment with antibiotics, resulting in an increase in average cost per true case treated.17, 18, 19, 20, 21, 22, 23, 24 The WHO report on antimicrobial resistance (AMR) emphasized the increasing threat of other pathogens developing resistance.25 The lack of unavailability of suitable models for vaccine development poses an important challenge. Therefore, the unavailability of the vaccine against these STIs is another challenge for the management of infection.26, 27, 28
In India, because of limited resources, the treatment of STIs is based on subjective judgment using SCM under the National AIDS Control Programme (NACP) and Reproductive and Child Health of the National Rural Health Mission. This study was undertaken with a specific objective of developing a better approach to identify specific causative agents of STI using SCM and polymerase chain reaction (PCR)-based diagnosis and to highlight the misuse and overuse of antibiotics using SCM.
Materials and Methods
Ethics statements
This study was conducted per the institutional ethical guidelines and approval from the Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India (IEC/VMCC/SJH/PROJECT/FEB/2017/681 and number 47-11-EC/30/51), and Dr. B.R. Ambedkar Center for Biomedical Research, University of Delhi, New Delhi, India (Eth. No.F. 50-2/Eth.Com/ACBR/17/255 and ACBR number F-50-2/Eth.com/ACBR/11/2107). Informed written consent from all participants involved in the study was obtained. All patients were treated using National AIDS Control Organization and National Aids Control Programme III (NACO-NACP III) STI or respiratory tract infection (RTI) kits based on NACO guidelines (http://www.naco.gov.in/sites/default/files/National%20RTI%20STI%20technical%20guidelines%20Sep2014_0.pdf).
The recruitment of the subjects
Among 3239 patients who visited the outpatient department (OPD) of gynecology of the hospitals during the study period, women who reported vaginal discharge, cervical discharge, lower abdominal pain (LAP), or pelvic inflammatory disease (PID) were recruited in the study under 2 groups: category I (n=646) and category II (n=437).
Inclusion criteria
Patients recruited in the study were in the age group of 18 to ≥60 years with one or more signs and symptoms of STI. All the symptoms were noted by the attending clinician during the patient examination, such as (1) color of the vaginal discharge (white, green, or brown), frothy discharge, and odor of discharge; (2) vulval itching; (3) edema or erythema; (4) pruritus or genital ulcers; (5) colpitis macularis (strawberry cervix) by punctate hemorrhages; (6) dysuria or frequency of urination; (7) pain during intercourse; (8) urinary tract infection; (9) soreness; (10) vaginitis; (11) presence of amines, vaginal leukocytosis, vulvar erythema, or purulent with white blood cells; (12) cervicitis; (13) frequency of micturition or burning and pain on micturition; (14) physiological changes; (15) lower back pain; (16) genital ulceration; (17) abnormal growth or mass in the genital area; (18) LAP; (19) inguinal lymphadenopathy; (20) dyspareunia; (21) perianal pain; (22) anal discharge; and (23) pharyngitis.
Exclusion criteria
Patients in the following category were not recruited: (1) age <18 years, (2) Rh isoimmunization, (3) use of antibiotics in the preceding 2 weeks, (4) pregnancy, and (5) unmarried females. To confirm nonpregnancy, every patient coming to the OPD of gynecology was asked about her last menstrual period and urine pregnancy test.
Enrollment and selection of patients
Patient consent was taken, and the inclusion and exclusion criteria were followed in enrolling and selecting the patients attending the gynecology outpatient clinic. Follow-up of the patients could not be included in this study because of the small sample size of patients who gave consent.
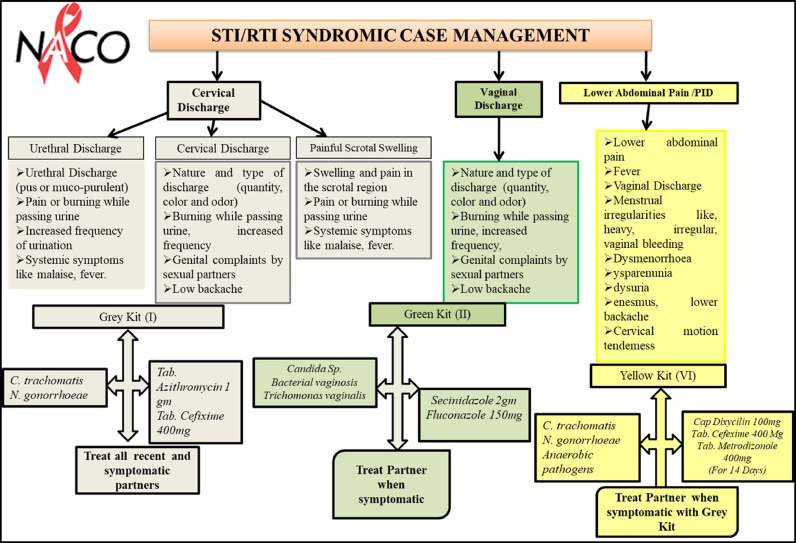
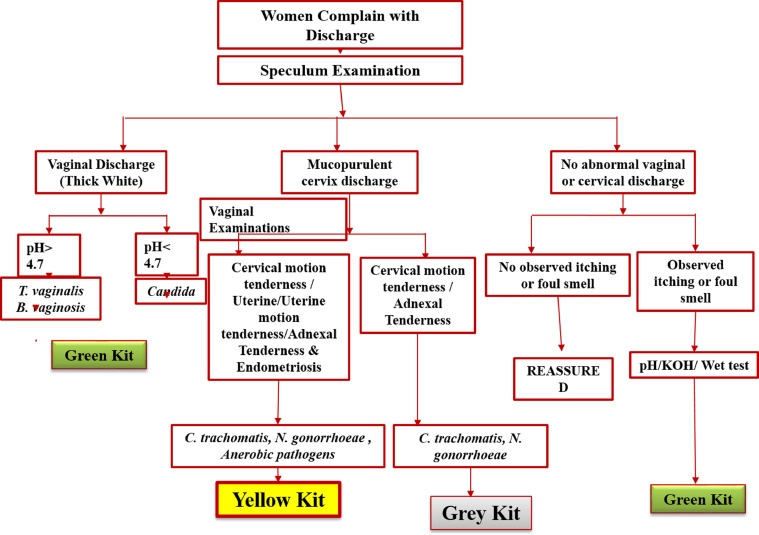
Patients in category I were screened per NACP guidelines (http://www.naco.gov.in/sites/default/files/National%20RTI%20STI%20technical%20guidelines%20Sep2014_0.pdf). The guidelines detail comprehensive STI or RTI SCM and refer to a set of signs and symptoms to define a syndrome that prompts the treatment for all putative causative agents in the absence of diagnostic tests (Figure 1). According to the NACP guidelines, essential steps for SCM of STI or RTI were taken care of by the attending clinician, such as history taking and clinical examination (genital, oral, and anorectal examinations apart from general examinations). Appropriate syndromic diagnosis per syndromic flowcharts was made in the absence of minimal laboratory tests, and kits were used for early and effective treatment, preferably single dose (Figure 1). After SCM-based screening with the inclusion criteria, samples were collected and genomic DNA (gDNA) was isolated before testing by PCR-based assay to check for the presence of C trachomatis, N gonorrhoeae, and T vaginalis infections (category II). In addition to NACP guidelines (speculum examination, vaginal discharge [thick white], urethral discharge, and mucopurulent cervix discharge), the color, pH, consistency, and odor of the vaginal or cervical discharge were also taken into consideration (Figure 3). Furthermore, other characteristics, such as cervical motion tenderness and/or uterine motion tenderness,29 adnexal tenderness, and endometriosis, were considered to predict infections of C trachomatis, N gonorrhoeae, and/or anaerobic pathogens. All samples were tested using PCR for the presence of C trachomatis, N gonorrhoeae, and T vaginalis infection.
Figure 1.
Management and treatment using NACP-II guidelines for symptomatic patients (category I)
NACP, National AIDS Control Programme; PID, pelvic inflammatory disease.
Sonkar. Use of additional parameters to improve the syndromic case management of nonviral sexually transmitted infections. Am J Obstet Gynecol Glob Rep 2022.
Figure 3.
Proposed alternative algorithm for the management of category-II patients
Sonkar. Use of additional parameters to improve the syndromic case management of nonviral sexually transmitted infections. Am J Obstet Gynecol Glob Rep 2022.
Sample collection, processing, and polymerase chain reaction amplification
Samples were collected from the cervix or vagina per NACP guidelines, by the resident doctor on duty (senior resident, MBBS, MS) after documenting the symptoms described above. All patients who gave the consent and were suspected of infection by C trachomatis, N gonorrhoeae, and T vaginalis per the guidelines used were recruited in the study to avoid any bias in the sample collection. The collected clinical specimens were transported to the research laboratory at room temperature as dry swabs within 1 to 2 hours and stored at −20°C until use. Total gDNA was isolated, and PCR amplification was performed by the PhD student and/or postdoctoral fellow according to the in-house–developed method published earlier.12,13,30, 31, 32, 33, 34 In both categories (I and II), PCR tests were conducted after sample collection. However, in category II, only PCR was performed before giving the treatment. Briefly, vaginal or cervical discharge dry swab samples were collected and placed in the empty vial and transported to the laboratory at ambient temperature and were kept frozen at −20°C until use. Each swab was incubated in phosphate-buffered saline (PBS, 1 mL) for 10 minutes at 4°C, mixed by vortexing thoroughly to disperse the sample and then the cotton was squeezed. The sample (400 μL) was centrifuged at 11,000 × g at 4°C for 10 minutes, and the cell pellet was suspended in 40 μL of PBS followed by centrifugation at 11,000 × g at 4°C for 5 minutes. Total gDNA was isolated and was stored at 4°C until further use as template DNA for PCR assay and clinical evaluation. For PCR diagnosis and amplification, a set of primers specific to the genes of different microorganisms with corresponding amplicon size and previously used references is listed in the Table 1. PCR amplification was carried out in 25 µL volume containing 1X Taq DNA polymerase buffer (50 mM KCl, 10 mM Tris-HCl pH 8.3, 1.5 mM MgCl2), 200 µM each of the 4 deoxynucleoside triphosphate (Merck, Darmstadt, Germany), 5 pmol of forward and reverse primer each, 5 µl of total gDNA isolated from the clinical sample, and 1.0 U of Taq DNA Polymerase (Genei Laboratories Pvt Ltd, Bangalore, India). Each set of PCR assays included a negative control (sterile water instead of DNA template) and positive control (1 ng purified gDNA isolated from C trachomatis, N gonorrhoeae, and T vaginalis cultures). Amplification was performed in thermal cycler (iCycler; Bio-Rad Laboratories, Inc, Hercules, CA) using different primer sets per their reported protocols in the reference papers (Table 1). The amplicons were analyzed by agarose gel electrophoresis (2%) in Tris-acetate-EDTA buffer containing 0.5 μg/mL of ethidium bromide. After electrophoresis, the DNA bands were visualized on an ultraviolet transilluminator (Aplegen, Inc, Pleasanton, CA).
Table 1.
List of primer sets specific for different pathogens used in the study
| Serial number | Microorganisms | Gene | Primers sequences | References |
|---|---|---|---|---|
| 1 | Chlamydia trachomatis | gyrA | C2 5′TGATGCTAGGGACGGATTAAAACC3′ | 30,33,34 |
| C5 5′TTCCCCTAAATTATGCGGTGGAA3’ | ||||
| 2 | Neisseria gonorrhoeae | Orf1 | Orf1 F 5′CAACTATTCCCGATTGCGA3′ | 31,32,34 |
| R 5′GTTATACAGCTTCGCCTGAA3′ | ||||
| 3 | Trichomonas vaginalis | pfoB | F 5′CAAAGTCAACATGGCTATGAT3 | 12,13,33,34 |
| R 5′GAAGACCTGTGTGGATGGATGT3′ |
Sonkar. Use of additional parameters to improve the syndromic case management of nonviral sexually transmitted infections. Am J Obstet Gynecol Glob Rep 2022.
Data analysis
A computational statistical test, Stata (version 12; StataCorp, College Station, TX), was used to analyze the accuracy of the treatments in an observational study, including distribution of infection-specific treatment in symptomatic women.
Results
Comparison of existing guidelines for syndromic case management with polymerase chain reaction diagnostics for category I suggests overuse or misuse of antibiotics
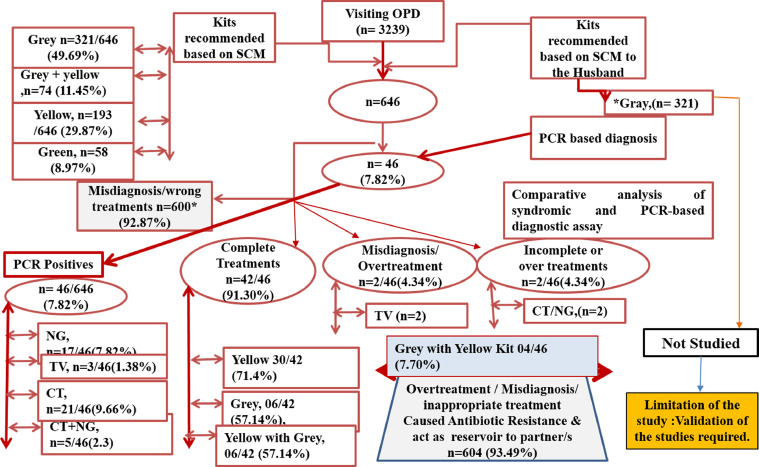
In category I, a total of 646 patients were considered positive based on SCM and were given antibiotic treatment as follows: 321 of 646 (49.69%), 193 of 646 (29.87%), 58 of 646 (8.97%), and 74 of 646 (11.45%) were treated using the Gray Kit, Yellow Kit, Green Kit, and Grey + Yellow Kit, respectively (Figure 1). Among 646 patients (category I), 46 (7.82%) with maternal cardiovascular disease and LAP, tested positive for C trachomatis, N gonorrhoeae, and T vaginalis, whereas 600 (92.87%) were negative for any of the 3 pathogens as determined by PCR-based assay. However, the possibility of all these clinical samples infected or coinfected with pathogens other than C trachomatis, N gonorrhoeae, and T vaginalis cannot be ruled out (Figure 2). Based on the observed concordance of results between SCM and PCR-based specific detection of the pathogens, 42 of 46 patients (91.30%) were given the correct treatment. However, 4 of 46 samples (7.70%) that were further analyzed showed that 2 samples were positive for C trachomatis and T vaginalis but were treated for C trachomatis and N gonorrhoeae per SCM (incorrect use of antibiotics), whereas the other 2 samples were positive only for T vaginalis but were treated for all 3 pathogens (C trachomatis, N gonorrhoeae, and T vaginalis) based on SCM (overuse of antibiotics).
Figure 2.
Management of category-I patients using NACP-II guidelines and its validation by PCR
CT, Chlamydia trachomatis; NACP, National AIDS Control Programme; NG, Neisseria gonorrhoeae; OPD, outpatient department; PCR, polymerase chain reaction; SCM, syndromic case management; STI, sexually transmitted infection; TV, Trichomonas vaginalis.
Sonkar. Use of additional parameters to improve the syndromic case management of nonviral sexually transmitted infections. Am J Obstet Gynecol Glob Rep 2022.
Based on these observations, specific complete treatments given to the patients in category I were 30 of 42 (71.4%), 06 of 42 (57.14%), and 06 of 42 (57.14%) for the Yellow Kit, Gray Kit, and Yellow with Gray Kit, respectively, whereas incomplete treatment and overtreatment was 04 of 46 (7.70%) for Gray Kit and Yellow with Grey Kit recommended for other symptomatic women (Figure 2).
Unfortunately, based on PCR results, 600 of 646 patients (92.87%) were negative for all 3 pathogens but treated for the same based on SCM. It may be possible that these patients were infected with pathogens other than C trachomatis, N gonorrhoeae, and T vaginalis. Based on PCR results, patients were overtreated with different kits, namely, Gray (321/646 [49.69%]), Grey + Yellow (74/646 [11.45%]), Yellow (193/646 [29.87%]), and Green (58/646 [8.97%]) (Figure 2).
Use of additional parameters for syndromic case management resulted in better concordance of syndromic management with polymerase chain reaction (categories II)
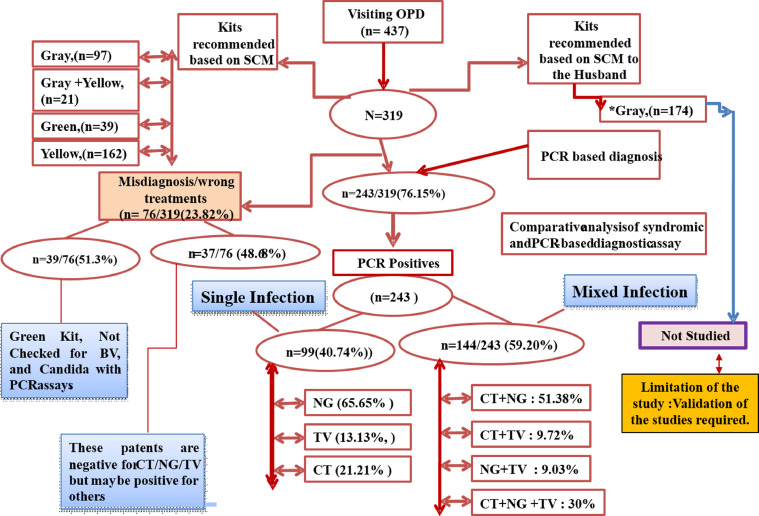
In category II, an alternative approach was used to identify, treat, and manage the 3 pathogens (Figure 3). Of 437 suspected patients who visited the OPD of gynecology from 2017 to 2018, 319 patients were enrolled in the studies. Of 319 patients, 243 (76.15%) were infected with one or more of the 3 pathogens and were recommended treatment of STI after confirming the infection status using the PCR method. Only 76 patients (23.82%) were negative for any of the 3 pathogens by PCR but were scored positive using alternative SCM (Figure 4). Among 243 patients with infection, 99 (40.74%) were infected with a single pathogen, whereas 144 (59.20%) were coinfected. Based on these results, the burden of C trachomatis, N gonorrhoeae, and T vaginalis infections was scored 13.13%, 65.65%, and 21.21%, respectively (Figure 4). Of 144 patients with mixed infection of coinfection, the percentage of C trachomatis + N gonorrhoeae infection was the highest (51.38%), followed by coinfection with all 3 pathogens (30%). Coinfection with C trachomatis + T vaginalis was 9.72%, and coinfection with N gonorrhoeae + T vaginalis was 9.03% (Figure 4).
Figure 4.
Management of category-II patients using alternative algorithm and its validation by PCR
CT, Chlamydia trachomatis; NG, Neisseria gonorrhoeae; OPD, outpatient department; PCR, polymerase chain reaction; SCM, syndromic case management; STI, sexually transmitted infection; TV, Trichomonas vaginalis.
Sonkar. Use of additional parameters to improve the syndromic case management of nonviral sexually transmitted infections. Am J Obstet Gynecol Glob Rep 2022.
Alternate symptomatic treatment is more accurate as suggested by laboratory diagnostics
In category I, based on standard syndromic management, 646 patients were considered positive for at least 1 of 3 pathogens, and treatment was administered accordingly. However, based on PCR assay only, 46 of 646 patients (7.82%) were found to be positive for at least 1 pathogen, whereas 600 of 646 patients (92.18%) were negative for any of the 3 pathogens. These samples can be positive for other infections, which were not tested in this study. The total estimated percentage rates of the overuse and misuse of antibiotics in these subjects were 92.87% and 8.69%, respectively. The total correct and complete treatment, estimated with laboratory measures and syndromic approach, was 42/46 (91.30%). The overuse of antibiotics estimated for azithromycin and cefixime was 29.69%, for secnidazole and fluconazole was (8.97%), for the combination of doxycycline, cefixime, and metronidazole was 29.87%, and for the combination of doxycycline, cefixime, metronidazole, and azithromycin was 11.45%.
The total estimated percentage of the overuse and misuse of antibiotics in the study In category II patients was only 23.82% (76/319) compared with that of category I patients where it was 92.87% (600/646). This was almost 3-fold of the overuse and misuse of antibiotics. Further analysis of the 76 patients revealed that 39 of 76 women (51.30%) were misdiagnosed because of the lack of correct judgments based on SCM and were recommended secnidazole and fluconazole (Green Kit), which is recommended for bacterial vaginosis and Candida species (not tested by PCR) (Figure 4). In contrast, 37 of 76 women (48.68%) were negative for C trachomatis, N gonorrhoeae, and T vaginalis by PCR but were given treatment based on an alternative algorithm used for SCM. However, these patients may be positive for other STIs, which were not investigated in this study. Of the total patients in category II, only 76 patients (23.82%) were given incorrect antibiotics and were overtreated using alternative SCM approaches (Figure 4). The overuse of antibiotics (azithromycin, cefixime, doxycycline, and metronidazole) in the total samples was observed to be only 23.82% (76/319).
Discussion
Principal findings
The proposed alternative approach for the management of STIs with a focus on C trachomatis, N gonorrhoeae, and T vaginalis was significantly more efficient in treating patients than the existing approach based on the NACO-NACP guidelines, wherein only 23% of misuse and overuse of antibiotics was reported following the formerly stated method compared with the enormously high 92.87% reported with the latter. The limitations of this approach have been well documented and included the inability to detect asymptomatic STIs, the poor positive predictive value of syndromic treatment resulting in the overuse of antibiotics (concerning antibiotic resistance to gonorrhea), the lack of antimicrobial susceptibility testing, and the limited opportunities for routine surveillance.35 In South Africa, syndromic management was implemented in 1995, and the prevalence of STIs remained unchanged.36, 37, 38 Although the prevalence of AMR varies widely across different parts of the world, low- and middle-income countries bear the maximum burden. Unfortunately, a 62% increase in the consumption of antibiotics has arisen in India in the last decade.39,40 A major overuse of antibiotics, azithromycin and cefixime, with an estimate of more than 49% has been reported following category I. With an eye on WHO adding N gonorrhea to the list of superbugs requiring research and development for new antibiotics, this much overuse of the antibiotics currently in practice further raises the urgency.
In category I, we observed that the overuse and misuse of antibiotics were 92.87% and 8.69%, respectively, which shows the insufficiency of the SCM method in disease management. As the symptoms owing to infection with different pathogens are highly overlapping and similar, it may lead to inaccurate diagnosis by SCM. Furthermore, coinfection by ≥2 pathogens can also lead to incorrect judgment owing to mixed symptoms. This can lead to overuse and misuse of antibiotics, resulting in long-term health problems for women because of the development of antibiotic resistance or chronic infection, leading to PID, infertility, ectopic pregnancy, and miscarriage. Moreover, untreated patients serve as a reservoir for spreading the pathogen to their sexual partner or partners. In contrast, using alternate symptoms to recruit patients in category II, the effectiveness of diagnosis with SCM was better as it reduced the misuse and overuse of antibiotics from 92.87% to 23.82 % and thus has the potential to significantly reduce the burden of antibiotic overuse among patients with STI. Given that the alternative approach combined with PCR distinctly differentiates between single infection and coinfections, cases of multiple drug administration can be kept in check, which are believed to be a cause of multiple drug resistance. Hence, the alternative approach mentioned in category II could be a better future strategy for disease management and to check the growing problem of AMR particularly in lower-to-middle-income countries (LMICs).
Clinical and research implications
Antibiotic resistance is a major threat hovering over the human population and is foreseen to only worsen in the coming future unless suitable measures are taken timely. With this perspective, if this alternate approach would be implemented in clinical practices, a great fall is expected to be seen in the fraction of overuse and misuse of antibiotics, thus delaying resistance to the currently used drugs. Aside from this, the proposed alternate approach was a blend of syndromic management and PCR tests; thus, a more accurate and quick treatment can be provided to the patients seeking care without involving coinfections. A significantly reliable patient size was undertaken in both the categories; however, before planning to bring it down into new policies in the healthcare sector, an even larger sample size can be followed, and eventually, the method can be used to benefit society.
Strengths and limitations
The decrease in the number of patients overdosed with antibiotics following our alternate algorithm was very encouraging. Scaling it up to the global level can certainly help reduce the burden of antibiotic resistance to a great extent, especially in LMICs where SCM is still practiced. Incorporation of PCR in the approach further increases confidence in the results and helps in efficiently managing coinfections as well, which are missed on a great deal following existing syndromic management guidelines. In clinical practice, data obtained with different practitioners managing the cases and in large cohorts using the proposed approach can further assert the method.
Conclusion
Antibiotic resistance, a natural consequence of evolutionary selection by bacteria, has been accelerating in recent decades because of the overuse and misuse of antibiotics because of several reasons. The current crisis is a perfect storm of neglected attention to the repeated warning from researchers and clinicians about antibiotic resistance dating to the early 1960S. Our results demonstrated that the prevalence of infection is still significant among female patients visiting the obstetrics and gynecology departments. We further demonstrated the futility of SCM and proposed the use of laboratory-based diagnosis before the patient is given antibiotic treatment. Although the alternate algorithm proposed in the study was more accurate than the standard method, its use should be restricted to only areas with no or poor resources. Moreover, it underpins the need to review the use of SCM for controlling STIs, with large samples and various clinical sites.
Acknowledgments
We gratefully acknowledge the help of Ms Jaya, B.Sc, and Ms Pooja Sharma, B.A., in the collection of clinical samples and update of the patients’ Excel files.
Footnotes
The funds from the University of Delhi, New Delhi, India (Department of Science and Technology Promotion of University Research and Scientific Excellence grant 2017–2018), given to D.S. for the development of the PCR-based diagnostics and the funds from the Department of Science and Technology, Government of India, given to S.C.S. for the NPDF (PDF/2016/000517) project are acknowledged. G.A. gratefully acknowledges the fellowship from the Indian Council of Medical Research, Government of India. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors report no conflict of interest.
Cite this article as: Sonkar SC, Arora G, Wasnik K, et al. Improved management can be achieved by introducing additional parameters in the syndromic diagnosis of nonviral sexually transmitted infections at low-resource settings. Am J Obstet Gynecol Glob Rep 2022;2:100037.
References
- 1.Rowley J, Vander Hoorn S, Korenromp E, et al. Global and regional estimates of the prevalence and incidence of four curable sexually transmitted infections in 2016. 2019.
- 2.World Health Organization. Report on global sexually transmitted infection surveillance. World Health Organization. 2018. Available at: https://www.who.int/reproductivehealth/publications/stis-surveillance-2018/en/. Accessed March 1, 2021.
- 3.Korenromp EL, Rowley J, Alonso M, et al. Global burden of maternal and congenital syphilis and associated adverse birth outcomes-estimates for 2016 and progress since 2012. PLoS One. 2019;14 doi: 10.1371/journal.pone.0211720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghope P, Sonkar SC, Wasnik K, Mittal P, Saluja D. Prevalence of Chlamydia trachomatis, Neisseria gonorrhoeae and Trichomonas vaginalis infection in pregnant adolescent women and its association with pregnancy outcomes. BMC Infect Dis. 2014;14:E33. [Google Scholar]
- 5.Chun HM, Carpenter RJ, Macalino GE, Crum-Cianflone NF. The role of sexually transmitted infections in HIV-1 progression: a comprehensive review of the literature. J Sex Transm Dis. 2013;2013 doi: 10.1155/2013/176459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro JG, Alcaide ML. High rates of STIs in HIV-infected patients attending an STI clinic. South Med J. 2016;109:1–4. doi: 10.14423/SMJ.0000000000000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghafur A, Mathai D, Muruganathan A, et al. The Chennai Declaration: a roadmap to tackle the challenge of antimicrobial resistance. Indian J Cancer 2013;50:71–3. [DOI] [PubMed]
- 8.Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem. 2014;6:25–64. doi: 10.4137/PMC.S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109:309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krupp K, Madhivanan P. Antibiotic resistance in prevalent bacterial and protozoan sexually transmitted infections. Indian J Sex Transm Dis AIDS. 2015;36:3–8. doi: 10.4103/2589-0557.156680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonkar SC, Wasnik K, Kumar A, et al. Evaluating the utility of syndromic case management for three sexually transmitted infections in women visiting hospitals in Delhi. India. Sci Rep. 2017;7:1465. doi: 10.1038/s41598-017-01422-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonkar SC, Wasnik K, Kumar A, Mittal P, Saluja D. Comparative analysis of syndromic and PCR-based diagnostic assay reveals misdiagnosis/overtreatment for trichomoniasis based on subjective judgment in symptomatic patients. Infect Dis Pover. 2016;5:42. doi: 10.1186/s40249-016-0133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonkar SC, Yadav S, Malla N, et al. Evaluation of DNA based techniques for the diagnosis of human vaginal trichomoniasis in North Indian population. Br Microbiol Res J. 2016;17:1–12. [Google Scholar]
- 14.World Health Organization . World Health Organization; 2001. WHO global strategy for containment of antimicrobial resistance.http://www.who.int/drugresistance/WHO_Global_Strategy_English.pdf?ua=1 Available at: Accessed March 4, 2021. [Google Scholar]
- 15.World Health Organization . World Health Organization; 2012. Global incidence and prevalence of selected curable sexually transmitted infections – 2008.https://www.who.int/reproductivehealth/publications/rtis/stisestimates/en/ Available at: Accessed March 4, 2021. [Google Scholar]
- 16.World Health Organization . World Health Organization; 2015. Global action plan on antimicrobial resistance.http://www.who.int/drugresistance/global_action_plan/en/ Available at: Accessed August 12, 2019. [DOI] [PubMed] [Google Scholar]
- 17.Jamison DT, Breman JG, Measham AR, et al. 2nd ed. The International Bank for Reconstruction and Development/The World Bank; Washington (DC): 2006. Disease control priorities in developing countries. [PubMed] [Google Scholar]
- 18.Tibebu M, Shibabaw A, Medhin G, Kassu A. Neisseria gonorrhoeae non-susceptible to cephalosporins and quinolones in Northwest Ethiopia. BMC Infect Dis. 2013;13:415. doi: 10.1186/1471-2334-13-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen XS, Yin YP, Wei WH, et al. High prevalence of azithromycin resistance to Treponema pallidum in geographically different areas in China. Clin Microbiol Infect. 2013;19:975–979. doi: 10.1111/1469-0691.12098. [DOI] [PubMed] [Google Scholar]
- 20.Kumar SG, Adithan C, Harish BN, Sujatha S, Roy G, Malini A. Antimicrobial resistance in India: a review. J Nat Sci Biol Med. 2013;4:286–291. doi: 10.4103/0976-9668.116970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatia R, Narain JP. The growing challenge of antimicrobial resistance in the South-East Asia Region–are we losing the battle? Indian J Med Res. 2010;132:482–486. doi: 10.4103/0971-5916.73313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganguly NK, Arora NK, Chandy SJ, et al. Rationalizing antibiotic use to limit antibiotic resistance in India. Indian J Med Res. 2011;134:281–294. [PMC free article] [PubMed] [Google Scholar]
- 23.Raghunath D. Emerging antibiotic resistance in bacteria with special reference to India. J Biosci. 2008;33:593–603. doi: 10.1007/s12038-008-0077-9. [DOI] [PubMed] [Google Scholar]
- 24.The Times of India . Times of India; 2014. India becomes world's largest consumer of antibiotics.https://timesofindia.indiatimes.com/india/India-becomes-worlds-largest-consumer-of-antibiotics/articleshow/38251650.cms Available at: Accessed March 5, 2021. [Google Scholar]
- 25.WHO. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates. 2016. Available at: https://www.who.int/bulletin/volumes/97/8/BLT-18-228486-table-T5.html. Accessed March 10, 2021. [DOI] [PMC free article] [PubMed]
- 26.Jain R, Sonkar SC, Chaudhry U, Bala M, Saluja D. In-silico hierarchical approach for the identification of potential universal vaccine candidates (PUVCs) from Neisseria gonorrhoeae. J Theor Biol. 2016;410:36–43. doi: 10.1016/j.jtbi.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Mishra PK, Sonkar SC, Raj SR, Chaudhry U, Saluja D. Functional analysis of hypothetical proteins of Chlamydia trachomatis: a bioinformatics based approach for prioritizing the targets. J Comput Sci Syst Biol. 2013;7 [Google Scholar]
- 28.Patel AL, Mishra PK, Sachdev D, Chaudhary U, Patton DL, Saluja D. Seroprevalence of antibodies against Pkn1, a novel potential immunogen, in Chlamydia trachomatis-infected Macaca nemestrina and human patients. BioMed Res Int. 2014;2014 doi: 10.1155/2014/245483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cortes EG, Adamski JJ. StatPearls Publishing; Treasure Island (FL): 2021. Chandelier sign. [PubMed] [Google Scholar]
- 30.Patel AL, Sachdev D, Nagpal P, et al. Prevalence of Chlamydia infection among women visiting a gynaecology outpatient department: evaluation of an in-house PCR assay for detection of Chlamydia trachomatis. Ann Clin Microbiol Antimicrob. 2010;9:24. doi: 10.1186/1476-0711-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhry U, Saluja D. Detection of Neisseria gonorrhoeae by PCR using orf1 gene as target. Sex Transm Infect. 2002;78:72. doi: 10.1136/sti.78.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sachdev D, Patel AL, Sonkar SC, Kumari I, Saluja D. Diagnosis of Neisseria gonorrhoeae using molecular beacon. BioMed Res Int. 2015;2015 doi: 10.1155/2015/597432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonkar SC, Sachdev D, Mishra PK, Kumar A, Mittal P, Saluja D. A molecular-beacon-based asymmetric PCR assay for easy visualization of amplicons in the diagnosis of trichomoniasis. Biosens Bioelectron. 2016;86:41–47. doi: 10.1016/j.bios.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 34.Sachdev D, Wasnik K, Patel AL, et al. Multi-centric validation of an in-house-developed beacon-based PCR diagnostic assay kit for Chlamydia and Neisseria and portable fluorescence detector. J Med Microbiol. 2018;67:1287–1293. doi: 10.1099/jmm.0.000803. [DOI] [PubMed] [Google Scholar]
- 35.Sharma V, Sonkar SC, Singhal P, et al. Functional impact of allelic variations/haplotypes of TNF-α on reproductive tract infections in Indian women. Sci Rep. 2021;11:627. doi: 10.1038/s41598-020-79963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hathorn E, Dhasmana D, Duley L, Ross JD. The effectiveness of gentamicin in the treatment of Neisseria gonorrhoeae: a systematic review. Syst Rev. 2014;3:104. doi: 10.1186/2046-4053-3-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unemo M, Golparian D, Skogen V, et al. Neisseria gonorrhoeae strain with high-level resistance to spectinomycin due to a novel resistance mechanism (mutated ribosomal protein S5) verified in Norway. Antimicrob Agents Chemother. 2013;57:1057–1061. doi: 10.1128/AAC.01775-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soge OO, Harger D, Schafer S, et al. Emergence of increased azithromycin resistance during unsuccessful treatment of Neisseria gonorrhoeae infection with azithromycin (Portland, OR, 2011) Sex Transm Dis. 2012;39:877–879. doi: 10.1097/OLQ.0b013e3182685d2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Somani J, Bhullar VB, Workowski KA, Farshy CE, Black CM. Multiple drug-resistant Chlamydia trachomatis associated with clinical treatment failure. J Infect Dis. 2000;181:1421–1427. doi: 10.1086/315372. [DOI] [PubMed] [Google Scholar]
- 40.Rice RJ, Bhullar V, Mitchell SH, Bullard J, Knapp JS. Susceptibilities of Chlamydia trachomatis isolates causing uncomplicated female genital tract infections and pelvic inflammatory disease. Antimicrob Agents Chemother. 1995;39:760–762. doi: 10.1128/AAC.39.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]