Abstract
A broad antibody panel was used for immunophenotyping of human immunodeficiency virus type 1 (HIV-1)-infected patients who were long-term nonprogressors (LTNP). The LTNP were compared with patients in the early phase of infection and patients who had progressed to advanced immunodeficiency. Changes in CD8+ subset distribution were observed mainly at acquisition of HIV-1 infection, whereas CD4+ subset changes appeared during progression of HIV-1 infection. The decreasing levels of CD4+ cells were characterized by an increasing frequency of cells expressing the activation markers HLA-Dr and CD45RO but not the CD28 surface antigen. The LTNP exhibited significant changes compared to HIV-negative patients in almost all markers. Compared to patients in the early phase of infection, the only difference was a relatively lower frequency of CD4+ cells expressing CD26 among the LTNP. The results show that HIV-1-infected persons who have no signs of immunodeficiency despite many years of infection have an immunophenotypic pattern that is substantially different from that of noninfected persons. Despite the long duration of infection, the LTNP exhibit a pattern similar to that of newly infected persons, with the exception of lower expression of CD26 on CD4+ cells.
In recent years, a growing interest has been directed towards individuals who do not develop immunodeficiency in spite of long-term human immunodeficiency virus type 1 (HIV-1) infection (5, 9, 10). These long-term nonprogressors (LTNP), estimated to be less than 5% of all HIV-1-infected persons, have been studied from several aspects i.e., HLA genotyping (8, 13), intricate molecular virology (21), descriptive immunology (12), and various lifestyle practices (2). It has also been advocated that LTNP have no specific characteristics but merely reflect the far-lower end of the normal distribution curve (14). Hitherto, no specific parameters have been identified to distinguish this key group of patients, in whom HIV-1 seems to fail to induce immunodeficiency.
Immunological studies of LTNP have dealt mainly with longitudinal patterns of CD4+ lymphocytes (2, 10, 12, 16). The CD8+ lymphocyte pattern has been studied in relation to cytokine production (20). Detailed knowledge of the expression of cell surface molecules on both the CD4+ and CD8+ cells of this patient group is still limited. Our objective was to study and characterize the T-cell phenotype of LTNP in comparison to those of newly infected patients, patients who had progressed to AIDS, and HIV-negative blood donors.
MATERIALS AND METHODS
Selection of patients.
The Department of Infectious Diseases, Karolinska Institutet, at Huddinge University Hospital is the largest HIV center in Sweden and cares for approximately 600 patients. The three major transmission groups (intravenous drug users [IVDU], homosexual men, and patients with heterosexual transmission [mainly immigrants from areas where HIV infection is endemic]) are equally represented. From May 1994 to June 1995, we included all patients fulfilling the following criteria. (i) LTNP had a CD4+ cell count maintained above 500/μl after more than 8 years of HIV-1 infection. (ii) Early-infection patients had a HIV-1 diagnosis within the previous 24 months and a CD4+ cell count of over 500/μl (early phase of infection). These patients had documented seroconversion. The median length of the infection was 11.5 months (range, 5 to 23 months). (iii) Progressors had a CD4+ cell count of less than 100/μl, a diagnosis of AIDS and more than 8 years of HIV-1 infection. These patients had fallen below 500 CD4+ cells/μl at a median of 108 months (range, 78 to 123 months) before inclusion in the study. (iv) Controls were HIV-negative blood donors. The study was approved by the ethics committee of Huddinge Hospital.
T-cell determinations.
CD4+ T cells were enumerated by standard procedures (3). In short, peripheral blood samples were processed by using a direct two-color immunofluorescence whole-blood lysis method. The monoclonal antibody panel used for each specimen is shown in Table 1. For each tube, at least 2,500 lymphocytes were acquired and analyzed by using a FACScan flow cytometer and SimulSET software (Becton Dickinson, Hägersten, Sweden). The distribution of subsets among CD4+ and CD8+ cells was analyzed for CD4+ bright and CD8+ bright cell populations, respectively, to avoid inclusion of monocytes or NK cells.
TABLE 1.
Monoclonal antibody panel used and cell types enumerated
| Monoclonal antibodiesa | Cell type enumerated |
|---|---|
| CD45/CD14 | Lymphocytes (% in gating region) |
| IgG1/IgG2 | Isotype-matched control |
| CD3/CD4 | CD4+ T cells |
| CD3/CD8 | CD8+ T cells |
| CD4/HLA-Dr | Activated CD4+ cells |
| CD4/CD45RO | Memory CD4+ cells |
| CD4/CD26 | CD4+ cells reactive against recall antigens |
| CD4/CD28 | CD4+ cells expressing CD28 costimulatory molecule |
| CD8/CD45RO | Memory CD8+ cells |
| CD8/CD26 | CD8+ cells reactive against recall antigens |
| CD8/CD28 | Cytotoxic T-cell precursors |
| CD8/CD38 | Activated CD8+ cells |
| CD8/CD57 | Cytotoxic (MHC-nonrestricted) CD8+ cells |
All monoclonal antibodies were manufactured by Becton Dickinson, except CD4/CD26, CD4/CD28, and CD8/CD26 (Coulter). IgG, immunoglobulin G.
MHC, major histocompatibility complex.
HIV-1 RNA analysis.
HIV-1 RNA levels were determined by commercial kits as recommended by the manufacturer (Amplicor HIV-1 Monitor test, version 2.0; Roche Diagnostic Systems, Inc., Branchburg, N.J.). If the viral load was found to be below 500 copies/ml, the sample was reexamined by using the ultrasensitive Amplicor HIV-1 Monitor test (detection limit, 50 copies/ml).
Statistical analysis.
Differences between groups were tested initially with the Kruskal-Wallis test. Thereafter, if a statistical difference between the groups was found for an antigen, differences between the individual categories of study subjects were analyzed by the Mann-Whitney U test.
RESULTS
Thirty-four HIV-infected persons were included in this study. The median (range) ages of the nine patients in the early stage of infection, the 15 LTNP, the 10 progressors, and the 12 blood donors were as follows: 33 (28 to 55), 38 (33 to 49), 38 (30 to 53), and 49 (27 to 63) years. The corresponding CD4+ cell counts were 700 (550 to 990), 670 (520 to 1,280), and 15 (10 to 80) cells/μl.
None of the LTNP or the patients in the early phase of infection received antiretroviral treatment. Six of the 10 progressors had received zidovudine (AZT) treatment for a median of 26 (range, 12 to 42) months, but only 1 patient had ongoing AZT monotherapy. One patient had been treated alternatly with AZT and didanosine for 27 months at the time of the study (Table 2). Statistically significant differences in the number of HIV RNA copies per milliliter were found between the LTNP (median, 5,000; range, <50 to 162,000), the patients in the early phase of infection (median, 46,800; median, 2,540 to >750,000), and the progressors (median, 142,000; range, 4,900 to >750,000) (P < 0.003).
TABLE 2.
Clinical characteristics of study subjects
| Group (n) | CDCa classification | Median (range) age (yr) | No. of:
|
No. treated with:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Males | Females | IVDU | Heterosexuals | Homosexuals | AZT | AZT-ddIb | |||
| LTNP (15) | A: 1 | 38 (33–49) | 11 | 1 | 12 | 3 | 0 | 0 | |
| Early infection (9) | A: 1 | 33 (28–55) | 5 | 4 | 2 | 3 | 4 | 0 | 0 |
| Progressors (10) | C: 3 | 38 (30–52) | 9 | 1 | 4 | 6 | 6 | 1 | |
| Controls (12) | 49 (27–63) | 12 | |||||||
CDC, Centers for Disease Control and Prevention.
ddI, dideoxyinosine.
T-cell phenotype patterns at various clinical stages.
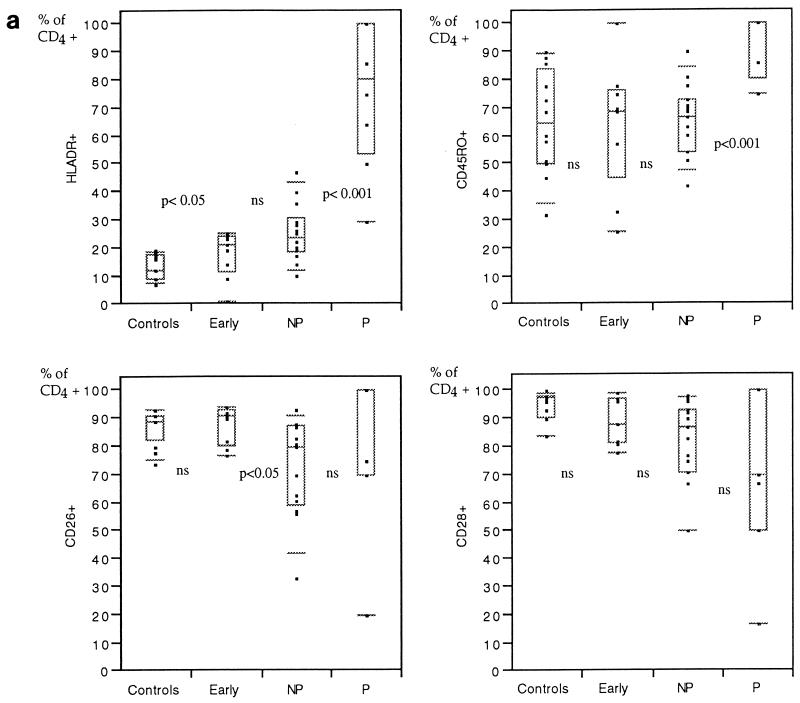
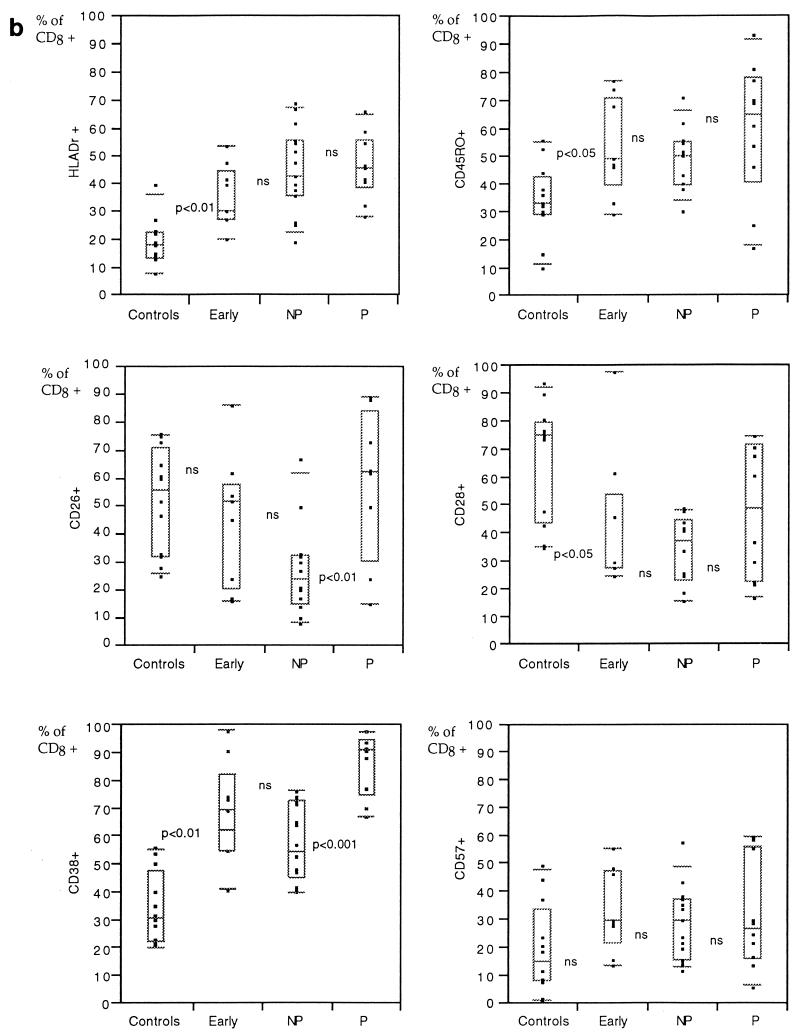
In patients in the early phase of infection, an increased proportion of CD4+ cells expressed HLA-Dr, whereas the other markers tested (CD45RO, CD26, and CD28) were not significantly different from those of the HIV-negative controls (Fig. 1a). The main immunophenotype pattern changes were found in the CD8+ subsets, with increased percentages of cells expressing markers associated with activation, i.e., HLA-Dr, CD38, or memory populations (CD45RO) (Fig. 1b).
FIG. 1.
Percentages of CD4+ (a) or CD8+ (b) cells expressing different surface molecules in peripheral blood from HIV-negative controls in comparison with individuals with less than 2 years of HIV-1 infection and CD4+ counts of more than 500/μl (early infection), those with more than 8 years without symptoms and CD4+ counts above 500/μl (nonprogressors [NP]), and those who have progressed to AIDS (progressors [P]). The results are presented as box plots: the limits of the boxes represent the 25th and 75th percentiles. Additional symbols indicate the 10th and 90th percentiles (bars) and individual results (closed squares). Levels of significance of differences between adjacent groups as measured by the Mann-Whitney U test are indicated (ns, not significant). NP, LTNP.
The LTNP displayed a significantly lower proportion of CD26-expressing cells within the CD4+ T-cell population than newly infected individuals (Fig. 1a; Table 3). A similar tendency, although not statistically significant, was found for CD8+ cells (Fig. 1b; Table 3). Significant differences were found between the LTNP and the HIV-negative persons for all but two markers (Table 3).
TABLE 3.
Comparison of T-cell subset percentages in LTNP, individuals in the early phase of infection, and progressors
| Marker(s) |
P value
|
||
|---|---|---|---|
| HIV nega vs LTNP | Earlyb vs LTNP | Pc vs LTNP | |
| CD3+ | <0.01 | NSd | <0.05 |
| CD4+ | <0.001 | NS | <0.001 |
| CD8+ | <0.001 | NS | NS |
| CD4+ HLA-Dr+ | <0.001 | NS | <0.001 |
| CD4+ CD45RO+ | NS | NS | <0.001 |
| CD4+ CD26+ | <0.01 | <0.02 | NS |
| CD4+ CD28+ | <0.01 | NS | NS |
| CD8+ HLA-Dr+ | <0.0001 | NS | NS |
| CD8+ CD45RO+ | <0.05 | NS | NS |
| CD8+ CD26+ | <0.01 | NS | <0.05 |
| CD8+ CD28+ | <0.001 | NS | NS |
| CD8+ CD38+ | <0.001 | NS | <0.001 |
| CD8+ CD57+ | NS | NS | NS |
HIV neg, HIV-negative controls.
Early, individuals in the early phase of infection.
P, progressors with AIDS.
NS, no significant difference.
The patients who had developed AIDS showed an increased percentage of CD4+ cells positively stained by antibodies to HLA-Dr and CD45RO but not by antibodies to CD28. Similarly, the proportion of CD8+ T cells expressing CD38 was significantly higher in the progressors.
DISCUSSION
A broad immunophenotyping panel was used to define the distribution of functional subpopulations in LTNP, compared to patients in other stages of HIV-1 infection and to HIV-negative controls. Since we included all consecutive patients fulfilling the inclusion criteria, we were not able to balance the transmission categories recruited to the different groups. The LTNP were predominately IVDU. These patients were all rehabilitated. It has recently been reported that IVDU with HIV-1 infection survive longer than homosexual men (15). In contrast, among the recently infected patients and among the progressors, more than 60% of the patients were sexually infected. These conditions could be a possible source of bias, although we believe that the influence on our results is not major.
In the present study, we observed changes in immunological markers that could be linked to the acquisition and progression of HIV-1 infection. We studied the proportions and not the concentrations of the different markers within the CD4 subsets, and the results are therefore independent of the CD4 cell count.
Firstly, the most pronounced changes (besides the general decrease in CD4+ cell level) after acquisition of HIV-1 infection were found within the CD8+ subsets. In early HIV-1 infection, a significantly increased frequency of CD8+ cells expressed markers for activated cells (CD38 and HLA-Dr) and memory cells (CD45RO) but lacked expression of CD28. Like other investigators, we found that progression of HIV-1 infection was paralleled by an increased frequency of CD38-expressing CD8+ cells.
As a second major pattern, we found that progression of HIV-1 infection was characterized by changes in the CD4+ cell subsets. Thus, an increased relative frequency of activated memory cells expressing HLA-Dr and CD45RO were observed, while the fraction of CD4+ cells expressing CD28 was decreasing. This finding is in accordance with what has been reported by other investigators (16).
The LTNP had been infected for more than 8 years without developing any immunodeficiency. Despite that, they differed substantially from the HIV-negative subjects in almost all cell surface markers. Differences were also found between the LTNP and the progressing patients in some of the CD4+ and CD8+ subpopulation markers. In contrast, the LTNP displayed a general distribution pattern of CD4+ and CD8+ subpopulations that did not distinguish them from the patients in the early phase of HIV-1 infection, with the exception of one marker, CD4+ CD26+.
A number of prospective studies have shown that an increased frequency of CD38 expression has a predictive value for incipient progression (1, 6, 11). The CD26 marker has been less studied. Vanham et al. found decreasing concentrations of CD4+ CD26+ cells in the progressing World Health Organization stages (17). In our study, we have assessed the proportion of CD26+ cells among CD4+ cells, since this measurement is independent of the absolute number of all CD4+ cells, and this may have additive prognostic value. The proportion of CD26 expression in the LTNP was lower than in both newly HIV-1-infected patients and HIV-negative controls. CD26 is a 110-kDa T-cell activation antigen that has dipeptidyl peptidase IV activity which cleaves amino-terminal dipeptides with either l-proline or l-alanine at the penultimate position (4). It has been reported that cross-linking of CD26 induced tyrosine phosphorylation of several intracellular proteins with a pattern similar to that seen after T-cell receptor-CD3 stimulation and that CD26 is costimulatory to CD3 signal transduction since co-cross-linking of CD26 and CD3 antigens induced prolonged and increased tyrosine phosphorylation in comparison with CD3 activation alone (7). Although the exact role of the CD26 molecule in T-cell activation has not been demonstrated, one might hypothesize that individuals who do not develop immunodeficiency in spite of long-term HIV-1 infection are selected from persons with a relatively lower tendency to immune activation, as shown by a lower frequency of CD26-expressing CD4+ T cells.
LTNP are a heterogeneous group of patients. Progression may occur even after 10 years of asymptomatic, stable HIV-1 infection. Epidemiological studies have not indicated that the survival curves would be biphasic (19). However, it has been suggested recently that sustained stability in the control of viral replication identifies LTNP as a distinct subgroup among HIV-1-infected individuals (18). Such true nonprogressors could possibly harbor a T-lymphocyte immunophenotype distinguishing them from other asymptomatic HIV-infected individuals with CD4+ cell counts within normal ranges.
The present study has revealed significant differences in some of the cell surface markers between the different categories of HIV-1-infected patients. Furthermore, the viral load also differed substantially among the three categories of HIV-1-infected patients. However, a considerable overlap was seen between the groups and the predictive value of the markers for the prognosis of individual patients seems to be low. The viral load also overlapped between groups. Prospective studies are therefore needed to establish if a smaller fraction of HIV-1-infected individuals with a better long-term prognosis can be identified by a relatively lower frequency of CD26+ CD4+ expression already at the onset of infection.
ACKNOWLEDGMENTS
This work was supported by grants from the Swedish Physicians against AIDS Research Fund and the Claes Grochinsky Research Fund.
REFERENCES
- 1.Bofill M, Mocroft A, Lipman M, Medina E, Borthwick N J, Sabin C A, Timms A, Winter M, Babtista L, Johnson M A, Lee C A, Phillips A N, Janossy G. Increased numbers of primed activated CD8+/CD38+ CD45RO+ T cells predict the decline of CD4+ T cells in HIV-1 infected patients. AIDS. 1996;10:827–834. doi: 10.1097/00002030-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Buchbinder S P, Katz M H, Hessol N A, O’Malley P M, Holmberg S D. Long-term HIV-1-infection without immunologic progression. AIDS. 1994;8:1123–1128. doi: 10.1097/00002030-199408000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Revised guidelines for the performance of CD4+ T-cell determinations in persons with human immunodeficiency virus (HIV) infections. Morbid Mortal Weekly Rep. 1994;43(RR-3):1–21. [PubMed] [Google Scholar]
- 4.Dong R P, Morimoto C. Role of CD26 for CD4 memory T cell function and activation. Hum Cell. 1996;9:153–162. [PubMed] [Google Scholar]
- 5.Easterbrook P J. Non-progression in HIV infection. AIDS. 1994;8:1179–1182. doi: 10.1097/00002030-199408000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Giorgi J V, Liu Z, Hultin L E, Cumberland W G, Hennessey K, Detels R. Elevated levels of CD38+CD8+ T-cells in HIV-infection add to the prognostic value of low CD4+ T cell levels: results of 6 years follow up. J Acquired Immune Defic Syndr. 1993;6:904–912. [PubMed] [Google Scholar]
- 7.Hegen M, Kameoka J, Dong R P, Schlossman S F, Morimoto C. Cross-linking of CD26 by antibody induces tyrosine phosphorylation and activation of mitogen-activated protein kinase. Immunology. 1997;90:257–264. doi: 10.1046/j.1365-2567.1997.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itescu S, Rose S, Dwyer E, Winchester R. Certain HLA-DR5 and DR6 major histocompatibility complex class II alleles are associated with infection characterized by low viral strain heterogeneity and slow disease progression. Proc Natl Acad Sci USA. 1994;91:1472–1476. doi: 10.1073/pnas.91.24.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy J A. HIV pathogenesis and long term survival. AIDS. 1993;7:1401–1410. doi: 10.1097/00002030-199311000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Lifson A R, Buchbinder S O, Sheppard H W, Mawle A C, Wilber J C, Stanley M, Hart C E, Hessol N A, Holmberg S D. Long-term human immunodeficiency virus infection in asymptomatic homosexual and bisexual men with normal CD4+ lymphocyte counts: immunologic and virologic characteristics. J Infect Dis. 1991;163:959–965. doi: 10.1093/infdis/163.5.959. [DOI] [PubMed] [Google Scholar]
- 11.Mocroft A, Bofill M, Lipman M, Medina E, Borthwick N, Timms A, Batista L, Winter M, Sabin C A, Johnson M, Lee C A, Phillips A, Janossy G. CD8+, CD38+ lymphocyte percent: a useful immunological marker for monitoring HIV-1 infected patients. J Acquired Immune Defic Syndr. 1997;14:158–162. doi: 10.1097/00042560-199702010-00009. [DOI] [PubMed] [Google Scholar]
- 12.Munoz A, Kirby A J, He Y D, Margolick J B, Visscher B R, Rinaldo C R, Kaslow R A, Phair J P. Long-term survivors with HIV-1 infection: incubation period and longitudinal patterns of CD4+ lymphocytes. J Acquired Immune Defic Syndr. 1995;8:496–505. doi: 10.1097/00042560-199504120-00010. [DOI] [PubMed] [Google Scholar]
- 13.Pantaleo G, Menzo S, Vaccarezza M, Graziosi C, Cohen O J, Demarest J F, Montefiori D, Orenstein J M, Fox C, Schrager L K. Studies in subjects with long-term nonprogressive human immuno-deficiency virus infection. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 14.Papadopoulou-Choremi H, Viglis V, Gargalianos P, Kordossis T, Iniotaki-Theodoraki A, Kosmidis J. Downregulation of CD28 surface antigen on CD4+ and CD8+ lymphocytes during HIV-1 infection. J Acquired Immune Defic Syndr. 1994;7:245–253. [PubMed] [Google Scholar]
- 15.Pehrsson P O, Lindbäck S, Lidman C, Gaines H, Giesecke J. Longer survival after HIV infection for injecting drug users than for homosexual men—implications for immunology. AIDS. 1997;11:1007–1010. doi: 10.1097/00002030-199708000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Sheppard H W, Lang W, Ascher M S, Wittinghoff E, Winkelstein W. The characterization of non-progressors: long term HIV-1 infection with stable CD4+ T-cell levels. AIDS. 1993;7:1159–1166. [PubMed] [Google Scholar]
- 17.Vanham G, Kestens L, De Meesters I, Vingerhoets J, Penne G, Vanhoof G, Scharpe S, Heyligen H, Bosmans E, Ceuppens L, Gogase P. Decreased expression of the memory marker CD26 on both CD4+ and CD8+ T lymphocytes of HIV-infected subjects. J Acquired Immune Defic Syndr. 1993;6:749–757. [PubMed] [Google Scholar]
- 18.Vesanen M, Stevens C E, Taylor P E, Rubinstein P, Saksela K. Stability in controlling viral replication identifies long-term nonprogressors as a distinct subgroup among human immunodeficiency virus type 1-infected persons. J Virol. 1996;70:9035–9040. doi: 10.1128/jvi.70.12.9035-9040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss R A. How does HIV cause AIDS? Science. 1993;260:1273–1279. doi: 10.1126/science.8493571. [DOI] [PubMed] [Google Scholar]
- 20.Zanussi S, Simonelli C, D’Andrea M, Caffau C, Clerici M, Tirelli U, De Paoli P. CD8+ lymphocyte phenotype and cytokine production in long-term nonprogressor and progressor patients with HIV-1 infection. Clin Exp Immunol. 1996;105:220–224. doi: 10.1046/j.1365-2249.1996.d01-746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zwart G, van der Hoek L, Valk M, Cornellissen M T E, Baan E, Dekker J, Koot M, Kuiken C L, Goudsmit J. Antibody responses to HIV-1 envelope and gag epitopes in HIV-1 seroconverters: rapid versus slow disease progression. Virology. 1994;201:285–293. doi: 10.1006/viro.1994.1293. [DOI] [PubMed] [Google Scholar]