Abstract
Discovered in the 1920’s, polyhydroxyalkanoates (PHA) are a naturally occurring class of biopolyesters that have long been touted as a renewable, biodegradable plastic alternative. Demand for sustainable products and over a half century of research have led to moderate commercial success of PHA. Yet, these materials are not pervasive. Therefore, an important question to address is, “what is the barrier that prevents widespread application of these materials?” PHA can be made from an incredibly diverse class of monomers that incorporate both simple and complex organic acids. Herein, we provide an updated list of unique PHA monomers that are substrates for a PHA polymerase. Unfortunately, most unique monomers are incorporated only after feeding a structurally related feedstock to a PHA accumulating bacterium. Therefore, we put forward an argument that research must now turn to developing feedstock-independent, synthetic pathways to produce an increased diversity of PHAs capable of competing with petroleum-derived plastics.
Keywords: polyhydroxyalkanoate, synthetic biology, bioplastic, metabolism, biotechnology, sustainability
1. Introduction
Polyhydroxyalkanoates (PHA) are a class of renewable, biodegradable polyesters: renewable because their synthesis involves biological conversion of sugars or other biomass derived feedstocks to a polymer that accumulates inside a cell; biodegradable because native producers can depolymerize and metabolize PHA – even if the material is encountered outside the cell (Tokiwa and Calabia, 2004). PHA exist as a way for the cell to store carbon and energy when the availability of non-carbon nutrients limits growth. Since the discovery of PHA in the 1920’s, it has been postulated that more than 150 unique monomers have been incorporated into a PHA polymer. Varying the monomer composition impacts the material properties of the polyester and provides handles for further functionalization in specialty applications. Therefore, one focus of PHA research has been to identify substrates for PHA polymerase, usually through feeding studies, and subsequent characterization of the resulting material. The broad specificity of PHA polymerases is both impressive in its ability to accept more than 150 hydroxy acids, and appealing from an industrial perspective. Specifically, the opportunity to capitalize on a versatile platform for generating a series of renewable, biodegradable plastics that could supplement and eventually replace petroleum derived counterparts has been the perpetual promise of PHA since the first commercial enterprises in the 1980’s. In the intervening time, a number of ventures have emerged around the globe [recently reviewed here: (Chen, 2009)] and yet, PHA are not a pervasive material in daily life.
The most promising, near-term commercial applications of PHA are in the medical, biomaterials and pharmaceutical industries where premium prices are easily commanded or in other niche markets where there is a demand for biodegradability. For example, Metabolix, a US-based bioplastics company, has had commercial success in incorporating Mirel™ (PHA) into biodegradable gardening containers and premium, eco-friendly beach toys. On the other hand, the realization of PHA as a replacement for non-biodegradable, petroleum derived plastics still seems to be a ways off due to the premium (~$0.75/lb) over comparable, renewably sourced polymers and even larger premiums compared to traditional plastics (Table 1). Metabolix’s Mirel™ is marketed at $2.25-$2.75/lb, whereas polypropylene, a plastic with comparable material properties, is available at $0.75/lb. Oil price volatility and increasing demand for sustainable alternatives have continued to motivate researchers to improve PHA production processes despite past obstacles to commercialization. While many non-PHA renewable plastics are being developed (Harracksingh, 2012; Sudesh and Iwata, 2008), PHA biochemistry offers flexibility to pursue a wide range of material properties from the same platform.
Table 1.
Prices for a selection of common polymers derived from both traditional (petrochemical) and alternative (biological) routes. Pricing data was acquired from ICIS Chemical Business and are listed as of the date of the publication.
| Material | price ($/lb) | Date of Publication |
|---|---|---|
| PVC | 0.45 | 8/27/2012 |
| PET | 0.59–0.85 | 2/14/2011 |
| PP | 0.74 | 8/28/2012 |
| LDPE | 0.77 | 8/27/2012 |
| PLA | 0.85–1.25 | 8/27/2012 |
| PS | 1.00 | 2/14/2011 |
| Starch-based biodegradable plastics | 1.50–2.20 | 2/14/2011 |
| BASF Ecoflex | 2.00 | 2/14/2011 |
| PLA/PBS | 2.00–2.50 | 2/27/2012 |
| PHA (Mirel) | 2.25–2.75 | 2/14/2011 |
This review will provide a brief summary of the range of monomers that have been incorporated into PHA granules and suggest future research directions that leverage the promising field of synthetic biology to increase the viability of PHA as a petrochemical plastic alternative. Our strategy for enhancing PHA production via synthetic biology is based upon the following logic:
More than 150 monomers can be incorporated into PHA, providing routes to a wide range of material properties that would be useful in high-value applications.
The cost of related feedstocks that are fed to PHA accumulating organisms to make non-traditional PHA precludes the economic viability of these materials.
Synthetic biology can and has been used to assemble novel metabolic pathways inside cells for producing high-value molecules relevant to chemical and polymer synthesis.
If metabolic pathways that link unrelated feedstocks (e.g., glucose) to high-value PHA monomers are assembled, then PHA production costs could be greatly reduced.
For these reasons, we posit that research should turn towards developing feedstock-independent, synthetic pathways for producing an increased diversity of PHA monomers that when polymerized produce materials capable of competing with traditional, petroleum-derived plastics.
2. Diversity of PHA monomers
In 1995, the review “Diversity of Bacterial Polyhydroxyalkanoic Acids,” detailed the known monomer constituents of this class of polyester materials (Steinbüchel and Valentin, 1995). At the time of publication, a striking 91 unique monomers had been characterized as substrates for a PHA polymerase. Since then, it has been postulated that more than 150 unique PHA monomers have been described in the literature (Steinbüchel and Lutke-Eversloh, 2003). Confirming this claim, we have identified an additional 64 monomers that have been described in the PHA literature bringing the current total to 155 unique monomers (Fig. 1, 2 and supplementary materials). Of the 64 unique monomers described since the 1995 review, many are closely related to prior classes of known PHA monomers, but a handful of new functional groups were successfully incorporated. These include dimethyl substituted carbons, terminal methyl- and fluorophenoxy, thiophenoxy, oxo (keto) and acetylthioester groups (Fig. 3). Additionally, an expanded number of 4-hydroxy and methyl substituted PHA monomers have been added to the list of unique monomers.
Fig. 1.
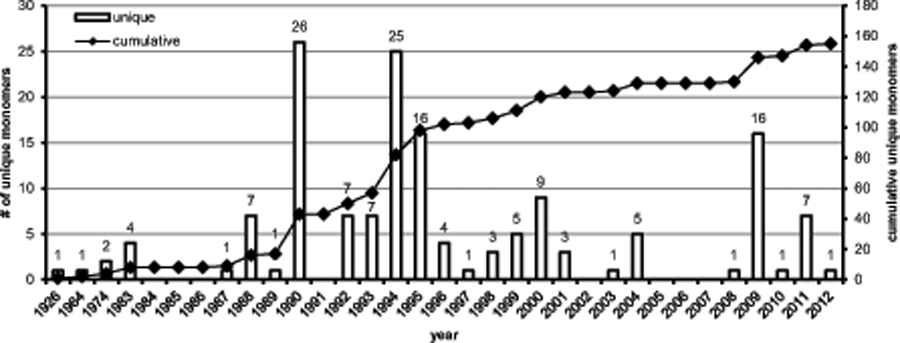
Unique PHA monomers discovered since 1926. Bars indicate number of unique PHA monomers discovered in a given year while points indicate the cumulative total number of unique monomers. Note that the horizontal axis is non-linear for 1926–1983 and linear from 1983–2012. See Fig. S1 for a detailed list of monomers.
Fig. 2.
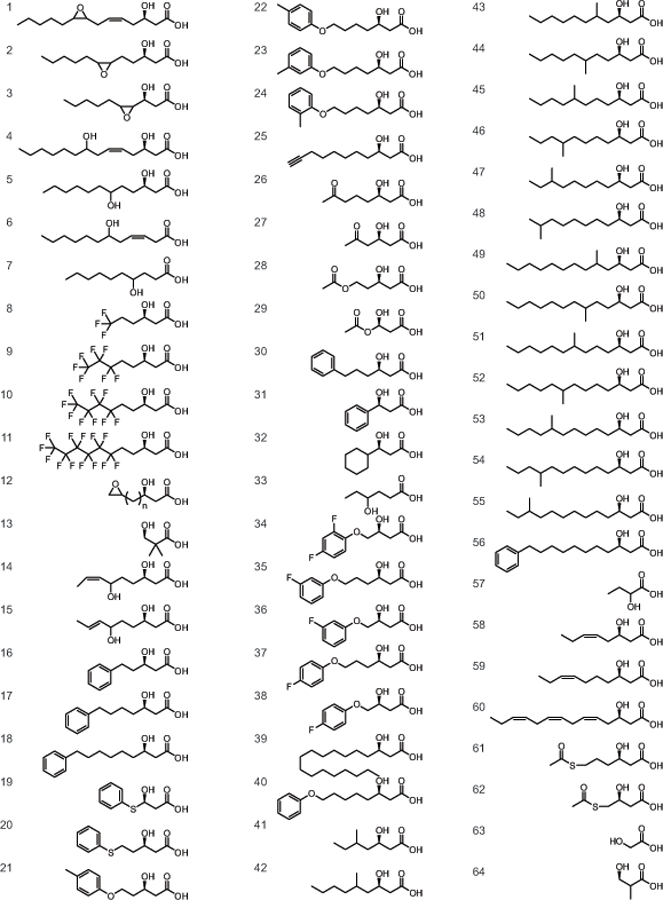
Structures of unique monomers incorporated into PHA since 1995.
Fig. 3.
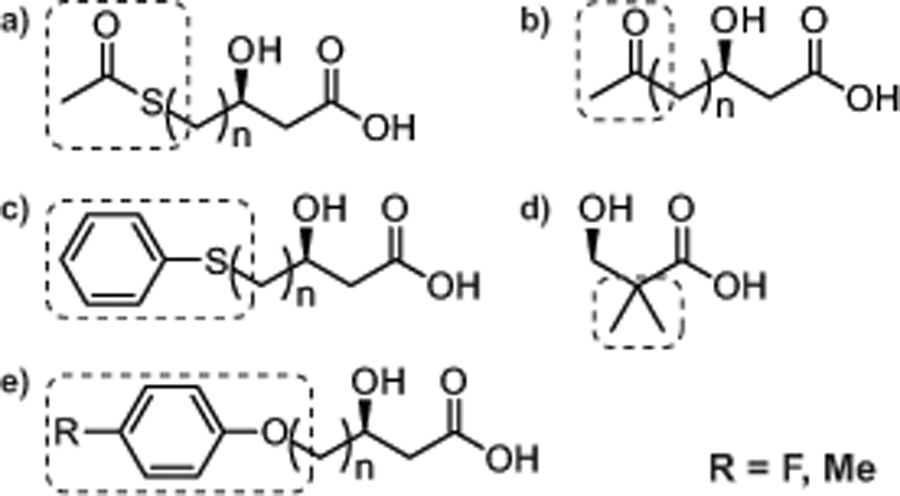
New categories of unique PHA monomers described since 1995 including (a) acetylthioester (b) oxo (keto) (c) thiophenoxy (d) dimethyl substituted carbons and (e) terminal methyl- and fluorophenoxy groups where R = F, Me (para-substitution shown as an example). See Fig. S1 for monomer structures.
Of the 155 unique monomers that have been incorporated into PHA, a relatively small subset has been produced from an unrelated feedstock (carbon source). Here, a related feedstock is defined as any starting material that is structurally related to the resulting monomer and especially the resulting side-chain/pendant group. In many cases feeding of exotic feedstocks will be the only feasible route to producing the desired PHA monomer. However, the costs associated with synthesizing, purifying, and feeding complex precursors to a PHA producer will significantly limit economic viability – especially in situations where traditional chemistry can be used to make similar polyesters (Table 2). Conversely, there are examples where existing biochemical pathways can be metabolically engineered to produce desirable monomers without the need of feeding related substrates. For example, feeding of lipids to PHA accumulating bacteria is one route to produce mcl-PHA (see section 4.3). These compounds could also be produced by engineering fatty acid metabolism to synthesize the lipids de novo. Considering the value of plant oils and the corresponding demand for their use in biodiesel and oleochemical production, it will be more economical to use an unrelated feedstock. Thus, adopting a synthetic biology approach for constructing and engineering pathways to novel PHA monomers could lead to the development of processes for manufacturing higher-value PHA at low cost.
Table 2.
Prices for a selection of common feedstocks associated with polymer synthesis. Pricing data was acquired from ICIS Chemical Business and are listed as of the date of the publication.
| Feedstock | price ($/lb) | Date of Publication |
|---|---|---|
| glucose | 0.20 | 9/19/2012 |
| stearic | 0.30 | 8/31/2009 |
| glycerin (low grade) | 0.40 | 3/26/2012 |
| ethanol | 0.42 | 10/03/2011 |
| naptha | 0.42 | 8/15/2012 |
| oleic | 0.60 | 8/31/2009 |
| glycerin (pharmaceutical grade) | 0.65 | 3/26/2012 |
| butanediol | 0.98 | 8/27/2012 |
| propionate | 1.00 | 3/30/2009 |
3. Synthetic biology for PHA economy
3.1. Why synthetic biology?
The field of “synthetic biology” has various definitions among its practitioners, but many view it as an extension of recombinant DNA technology in which complex systems are constructed inside a living organism to confer a desired ability. The systems built by synthetic biologists range in function from genetic circuits (Nandagopal and Elowitz, 2011), to biosensors (Xie et al., 2011), to microbe-based chemical factories (Keasling, 2010b). A growing application of synthetic biology is transplantation of a biological trait from a complex system to a more facile model organism. For example, many natural products have attractive medicinal properties but are made by exotic species that are difficult if not impossible to cultivate.
The synthetic biology approach begins with the realization that these compounds are made via metabolic pathways that can be recapitulated in a different host. One of the most cited examples of this strategy is the production of artemisinin, an anti-malarial isoprenoid, natively produced in sweet wormwood (Artemisia annua) (Westfall et al., 2012). While artemisinin could be produced by extraction from crops of A. annua, crop yields and other process variability contributed to costs that were too high for targeted patients in developing nations. After several years of research, a strain of Saccharomyces cerevisiae was shown to produce g L−1 titers of the drug (Ro et al., 2006; Westfall et al., 2012). With the help of Amyris Biotechnologies and the Institute for One World Health, the process has been commercialized by Sanofi-Aventis (Keasling, 2010a).
3.2. A synthetic biology approach to PHA biosynthesis
Synthetic biology has been applied to the production of fuels, medicines, commodity chemicals, and plastics - PHA included (Keasling, 2010b). While a number of synthetic biology approaches have been explored for PHA biosynthesis we propose that making PHA from an unrelated carbon source can be viewed as a three piece puzzle (Fig. 4). First, one must identify a (ideally flexible) metabolic pathway that leads to a defined composition of organic acids. Second, the resulting molecules must be activated via coenzyme A (CoA) ligation and modified (where necessary) to exhibit a hydroxyl group with the required chirality. Third, the monomers must be presented to a PHA synthase capable of catalyzing polymerization. When suitable side-chains (e.g., a terminal alkene) are present, a fourth, post-synthesis step involving chemical functionalization is conceivable (in addition to blending PHA with other polymers) to achieve the desired material properties and process economics. The remainder of the review will examine each of these steps in more detail.
Fig. 4.
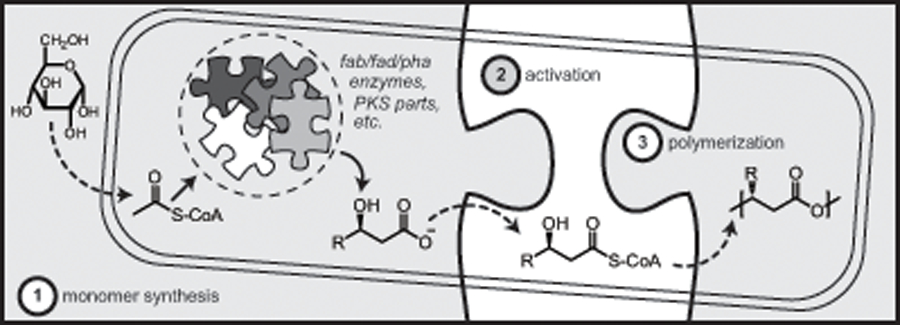
PHA biosynthesis viewed as a three piece puzzle. The first piece consists of feeding an unrelated carbon source such as glucose to a cellular host possessing the necessary parts (metabolic pathways) for the conversion of cellular building blocks to hydroxyalkanoic acid monomers. The second piece is the activation of these monomers with CoA followed by polymerization by a PHA synthase in the third and final piece of the puzzle.
3.3. Challenges faced in engineering PHA metabolism
While this review was written to highlight biological routes to synthesizing PHA monomers from unrelated carbon sources, it should be noted that many production challenges will be faced by synthetic biologists working to commercialize production of novel PHA. An in-depth economic analyses of industrial PHA production was carried out by Van Wegen in the late 1990’s (Van Wegen et al., 1998). Their analysis found that the most sensitive parameters were the recovery strategy and PHA titer (% cell dry weight) followed by choice of media and the corresponding yield. PHA accumulates intracellularly which necessitates purification steps that include cell lysis, clarification, and typically solvent extraction to obtain PHA granules. Synthetic biology strategies (e.g. expression of lytic peptides (You et al., 2004)) could be used to facilitate cell lysis and reduce purification costs. Many native and heterologous producers are cable of PHA accumulation at greater than 70% of the cell dry weight with some strains achieving nearly 90% (Steinbüchel and Füchtenbusch, 1998). Achieving these titers via the pathways described below is a challenge to metabolic engineers. It is worth noting that PHA fermentation is an aerobic process. While the need for aeration can amount to a significant operating expense and aerobic processes are generally less efficient in terms of yield and productivity, many aerobic fermentations have been successfully scaled-up (Weusthuis et al., 2011). Other studies have focused on other factors such as the potential of alternative carbon sources and the design of alternative recovery processes (Castilho et al., 2009; Choi and Lee, 1999; Jacquel et al., 2008; Keshavarz and Roy, 2010; Yang et al., 2011).
4. Monomer Synthesis
4.1. Poly(3-hydroxybutyrate): context for monomers from unrelated feedstocks
To appreciate the elegance of native PHA biosynthesis routes and contextualize routes to PHA from unrelated feedstocks, it is important to begin with polyhydroxybutyrate (PHB). The archetypical and arguably most studied PHA monomer is 3-hydroxybutyric acid (3HB), a four carbon hydroxycarboxylic acid. PHB is a polyester of 3HB that is readily accumulated by a number of organisms as a form of carbon and energy storage. PHB synthesis is conferred by the phaABC genes (Fig. 5) best studied in the bacteria Cupriavidus necator (a.k.a. Ralstonia eutropha) (Madison and Huisman, 1999a). The genes phaA and phaB encode β-ketothiolase (EC 2.3.1.9) and acetoacetyl-CoA reductase (EC 1.1.1.36) for the condensation of two acetyl‐CoA units to acetoacetyl-CoA and subsequent reduction of the newly formed β-keto group to 3‐hydroxybutyryl‐CoA. The short-chain-length (scl) specific PHA polymerase (often referred to as PHB polymerase) (no EC number) (phaC) then incorporates the monomer units into the PHB polymer.
Fig. 5.
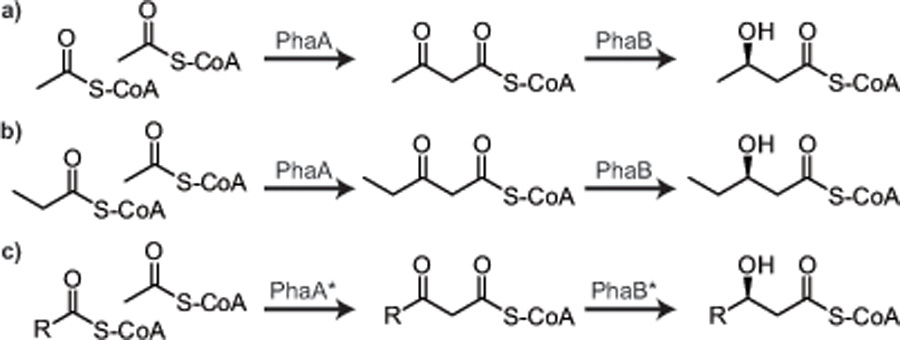
Dedicated pathways for scl-PHA biosynthesis. The pathways for (a) the biosynthesis of polyhydroxybutyrate involves the condensation of two acetyl-CoA by β-ketothiolase (PhaA) and formation of the hydroxyl group by acetoacetyl-CoA reductase (PhaB). Alternatively this metabolic pathway can lead to the formation of (b) hydroxyvalerate monomers or (c) provide hypothetical route to a number of longer chain-length monomers if variants of PhaA and PhaB can be identified or engineered.
The PHB pathway is dedicated to PHA biosynthesis (i.e., not used to make other metabolites) and affords tight control of the resulting monomer composition. One exception is that when propionate is introduced as a feedstock it is possible to produce 3-hydroxyvalerate (3HV), a five carbon hydroxycarboxylic acid monomer. 3HV is produced analogous to 3HB with the key difference being the replacement of one of the two acetyl-CoA units with propionyl-CoA in the initial condensation step carried out by the β-ketothiolase. Many organisms are capable synthesizing a co-polymer of 3HB and 3HV (3HB-co-3HV) when propionate is present. While PHB is brittle (glass transition temperature (Tg) = 4 ºC, melting temperature (Tm) = 175 ºC), incorporation of 3HV yields a copolymer with a slightly lower Tg and Tm depending on the fraction of 3HV incorporated (e.g., for mol% 3HV = 20; Tg ≈ 2 °C, Tm ≈ 171 °C). Because poly(3HB-co-3HV) has properties that lend readily to consumer products such as containers, packaging and films, it was the first commercially produced polymer. However, as mentioned, processes for making poly(3HB-co-3HV) have traditionally involved an external supply of propionic acid.
4.2. 3HV from unrelated feedstocks
The case of 3HV is one example of a focused research effort to identify biosynthetic pathways initiating from an unrelated feedstock. Propionyl-CoA, the activated form of propionic acid can be derived from the amino acids valine, threonine, methionine and isoleucine (Steinbüchel and Lutke-Eversloh, 2003). While valine, isoleucine and methionine catabolism to propionyl-CoA consists of a number of intermediate steps, the threonine pathway passes through a single intermediate, α-ketobutyrate, yielding one molecule each of propionyl-CoA, CO2 and ammonia (Slater et al., 1999). Plant species such as Arabidopsis (Rebeille et al., 2006) and bacteria such as Pseudomonas (Inoue et al., 2003) are additionally capable of methionine catabolism to α-ketobutyrate. Eschenlauer et al., demonstrated that the addition of 1 mM valine or threonine to glucose minimal media resulted in PHA consisting of up to 4% 3HV (Eschenlauer et al., 1996). More recently, an engineered strain of E. coli was shown to be capable of producing either (R)- or (S)-3-hydroxyvaleric acid via threonine catabolism with titers as high as 0.50 g L−1 and 0.31 g L−1, respectively (Tseng et al., 2010).
There also exists the possibility to feed alternative, low cost carbon sources for the production of PHA monomers. For example, levulinic acid is readily derived from lignocellulosic biomass via acid treatment and is a promising feedstock for producing chemicals (Alonso et al., 2010). Bacteria such as Pseudomonas putida and Cupriavidus necator are capable of PHA incorporating 3HB, 3HV and 4-hydroxyvaleric acid (4HV) monomers into PHA when fed levulinic acid (Jaremko and Yu, 2011; Martin and Prather, 2009). A proposed pathway for levulinic acid catabolism that includes 4- and 3-hydroxyvaleryl-CoA is based on the PHA composition observed in these cells.
4.3. Medium-chain-length (mcl)-PHA biosynthesis
PHA are classified based on the carbon chain-length of the monomers that make up the polymer itself. PHA consisting of 3–5 carbon monomers such as 3HB and 3HV are considered scl-PHA, while PHA incorporating monomers with a chain-length ≥ 6 carbons are considered medium-chain-length or mcl-PHA. Most mcl-PHA are derived from fatty acid metabolism whereby intermediates of either fatty acid biosynthesis or β-oxidation can be scavenged and incorporated into a polyester (Fig. 6). These pathways work in iterative cycles. Therefore, the resulting PHA is a heteropolymer consisting of monomers ranging from 6 to 14 carbons in length. Several enzymes have been shown to link fatty acid metabolism to mcl-PHA biosynthesis including enoyl-CoA hydratase (PhaJ, EC 4.2.1.119), (R)-3-hydrocyacyl-acyl-carrier protein (ACP) thioesterase (PhaG, EC 3.1.2.-), and mcl-PHA polymerases (PhaC1), which tend to have a broad specificity for monomers between 6 and 14 carbons. Recently, an acyl-CoA ligase from P. putida (PP_0763) was shown to act on (R)-3-hydrocyacids derived from fatty acid biosynthesis via (R)-3-hydrocyacyl-ACP thioesterase (Wang et al., 2012a). By co-expressing a thioesterase, CoA-ligase and an mcl-PHA polymerase, up to 400 mg L−1 mcl-PHA heteropolymer could be synthesized from glucose as a sole carbon source.
Fig. 6.
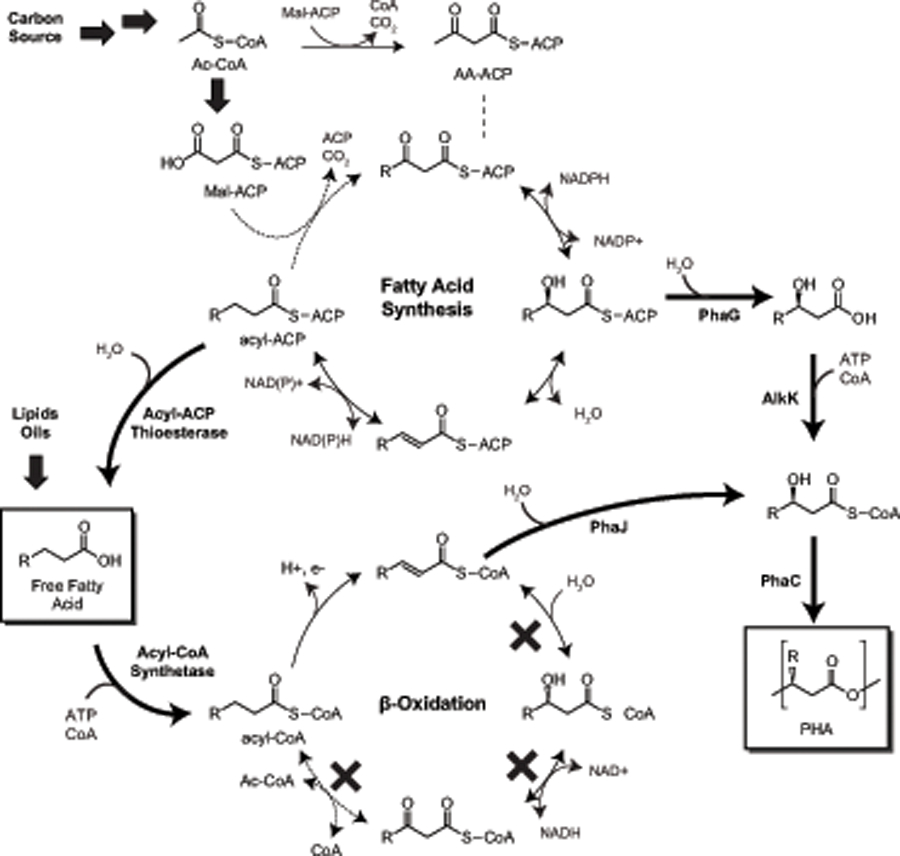
Routes to mcl-PHA monomer biosynthesis via fatty acid biosynthesis and β-oxidation starting from the central metabolite acetyl-CoA. Key enzymes are bolded to indicate steps that convert intermediates of fatty acid metabolism to hydroxyalkanoates. ACP = acyl-carrier protein. Block arrows represent multiple enzymatic reactions.
4.4. Manipulation of β-oxidation for mcl-PHA homopolymer
Early attempts at increasing the production of PHA via β-oxidation intermediates involved single gene deletions – usually in (S)-specific enoyl-CoA hydratase (FadB) – or by addition of inhibitory molecules such as acrylic acid (Fiedler et al., 2000), salicylic acid (Choi et al., 2009), 2-bromooctanoic acid and 4-pentenoic acid (Lee et al., 2001) which inhibit β-oxidation enzymes. Functional inactivation of the transcriptional regulator of β-oxidation (FadR) has also been implemented to increase flux through this pathway (Rhie and Dennis, 1995). Recently, a number of labs have focused on the production of mcl-PHA homopolymers in both Pseudomonas putida and E. coli. These have generally been feeding strategies wherein a single chain-length fatty acid has been fed to a β-oxidation impaired strain expressing PHA biosynthesis genes. In Pseudomonas, homopolymer synthesis was achieved through inactivation of six genes involved in native fatty acid degradation and one gene associated with native PHA metabolism (Liu et al., 2011). When supplied with either C10 or C14 fatty acids, C10 or C14 homopolymer was observed. Additional work in Pseudomonas demonstrated production of C4-C9 homopolymers when the corresponding fatty acid was supplied exogenously (Wang et al., 2011). A similar approach was implemented in E. coli expressing a broad activity polymerase whereby PHA homopolymer was synthesized from fatty acids with a chain-length of 4 to 14 carbons (Tappel et al., 2012).
4.5. Combining fatty acid biosynthesis and beta-oxidation to produce mcl-PHA
Approaches to producing mcl-PHA from unrelated carbon sources have also been explored. A popular strategy is to use native or heterologously expressed thioesterases to generate a pool of free fatty acid for incorporation into a heterogeneous polyester. Two thioesterases from E. coli, the broad specificity thioesterase II (TesB) (Chung et al., 2009) and a multifunctional thioesterase (TesA) (Qiu et al., 2005) have been targeted for PHA biosynthesis as well as a plant thioesterase from the California Bay Laurel (BTE/FatB) (Rehm and Steinbuchel, 2001). Recently, our lab has combined the β-oxidation manipulation and thioesterase based approaches in a single strain for the production of a PHA with a composition matching the fatty acid profile generated by the thioesterase (Agnew et al., 2012). This strategy offers an opportunity to produce a PHA with a defined composition simply by tuning the specificity of the thioesterase (Yuan et al., 1995) or by identifying an existing thioesterase with a desirable profile (Cantu et al., 2010).
4.6. Megasynthase engineering
While fatty acid metabolism produces acyl-chains with the essential hydroxyl group needed for polymerization, the pathways result in a limited set of side chains (i.e., saturated, mono-unsaturated, or modified by addition of a methyl-branch or cyclopropane ring) when incorporated into PHA. An attractive possibility for producing a wider range of monomers lies in exploiting polyketide synthases (PKS) which use many of the same chemistries as fatty acid biosynthesis to produce highly substituted acyl-chains. PKS are large, modular enzymes that catalyze the assembly of complex natural products through consecutive condensation and reduction reactions (Sattely et al., 2008; Weissman and Leadlay, 2005). The molecular machines responsible for type I polyketide biosynthesis, often called megasynthases, function in a modular fashion similar to assembly lines seen in classical manufacturing processes (Sattely et al., 2008). A polyketide’s carbon backbone is assembled and modified in a stepwise fashion. At each stage, a different enzymatic domain operates on a growing acyl-chain, performing monomer addition, reduction, and chemical modification until the final product is released. The order of domains, (i.e., the sequence of the protein) dictates the structure of the final compound. Therefore, the ability to fine-tune these modifications through manipulation at the enzyme level has long been a research and drug discovery goal (Sherman, 2005). As such, efforts to understand the logic behind polyketide synthesis and to engineer novel polyketides have progressed greatly in the past decade (Wong and Khosla, 2012).
Given this better understanding, we hypothesize that megasynthase engineering offers a flexible path to PHA monomers. A majority of the “newly discovered” monomers as well as many of the originally described 91 monomers could be made via a PKS strategy. PKS incorporate a broad range of chemical building blocks both as starter and extender units (Chan et al., 2006; Chan et al., 2009; Moore and Hertweck, 2002). In our proposed strategy, the starter unit determines the structure of the PHA side-chain terminus. Known PKS starter units possess hydroxyl, amino, (hydroxy and amino substituted) phenyl, (hydroxy substituted) hexyl, pyrrole and branched carbon substituents. Incorporation of reactive groups into the PHA side chains via PKS starter units would enable subsequent functionalization of the polymer. Further diversity can be introduced in the form of methyl, hydroxyl and amino moieties at the α-carbon of each PKS extender unit. Lastly, the PKS reductive cycle manipulates the β-carbon of each acyl-chain generating keto, hydroxyl (including the correct stereochemistry needed for polymerization by a PHA synthase), enoyl, and methylene variants. While many monomers could be produced by a PKS strategy, each megasynthase will synthesize a defined molecule based on the domain architecture of the enzyme. This means that the troubles encountered in producing a homopolymer via the iterative fatty acid pathway can be avoided.
Previous work with common PKS enzymes has demonstrated the possibility of truncation to produce an enzyme that is capable of producing small polyketides (Oliynyk et al., 1996). For example, a derivative of the 6-deoxyerythronolide B (DEBS) PKS has been created which incorporates only the loading domain and first extension module (module 1) artificially fused to the DEBS thioesterase (TE) (which is normally part of module 6) (Bohm et al., 1998; Ostergaard et al., 2002). This diketide synthase has been shown to produce 2‐methyl‐3‐hydroxybutyrate and 2‐methyl‐3‐hydroxyvalerate products in appreciable quantities – both of which are monomers that have previously been identified as substrates for a PHA polymerase (Fig. 7c). Fusing the TE at the end of the second extension module has been shown to produce a triketide “mini‐lactone” construct as well (Cortes et al., 1995). Further investigation has demonstrated that it is possible to not only exchange domains within a single PKS but also between PKS’s from distinct organisms to create novel polyketides (Oliynyk et al., 1996). While research continues as to why certain combinations of loading and extensions modules work better than others, recent study has begun to shed light on interactions between PKS modules. This research is paving the way for the successful design of tailor‐made polyketide products, many of which could be incorporated into PHA (Buchholz et al., 2009).
Fig. 7.
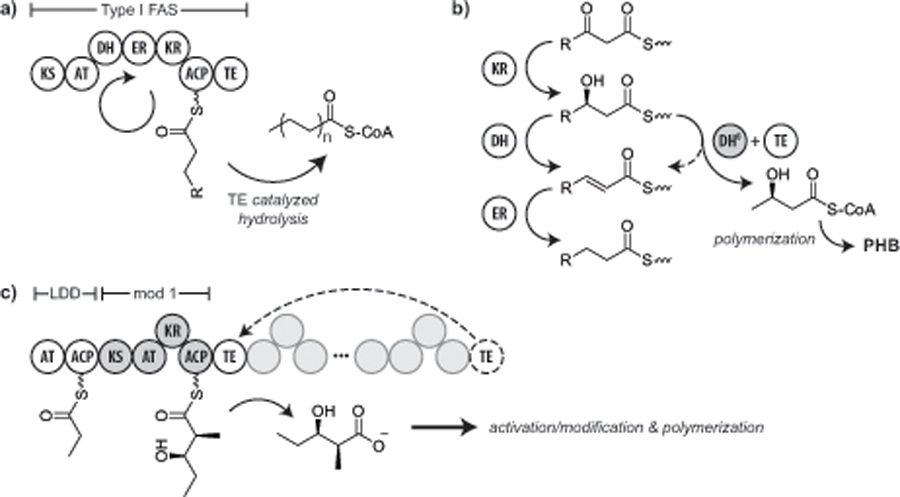
Schematic of megasynthase modification strategies. (a) The native Type I FAS catalyzes the formation of long chain fatty acids. (b) Mutation of the DH domain results in loss of dehydrogenase activity and converts the FAS to a 3-hydroxybutyryl-CoA synthase. (c) A hybrid Type I PKS derived from the DEBS loading domain, module I and terminal thioesterase domains catalyzes the formation of the diketide 3-hydroxy-2-methylvalerate. The products of the engineered FAS and PKS are substrates for PHA biosynthesis. KS = ketosynthase, AT = acyltransferase, DH = dehydratase, ER = enoylreductase, KR = ketoreductase, ACP = acyl-carrier protein, TE = thioesterase.
While no one has demonstrated the use of a PKS to produce PHA monomers, the strategy has been applied to the highly similar Type I fatty acid synthases (FAS) (Fig. 7a,b). Researchers mutated a rat Type I FAS dehydration domain that in theory would lead to the accumulation of 3-hydroxybutyl-adduct on the acyl-carrier protein domain. Thioesterase activity would generate 3HB that could be activated for polymerization. When the mutant FAS was co-expressed in insect cells with a PHA synthase, PHB production was established (Williams et al., 1996). While PHB is not a unique PHA, and this route is overly complex compared to the classical PhaAB pathway, the strategy demonstrated that it is possible to use megasynthases to produce PHA monomers.
5. Conversion of organic acids to PHA monomers
5.1. Generation of hydroxyacids with required stereospecificity
Stereospecificity is critical to PHA polymerization. PHA synthases requires the (R) stereoisomer in the case of a 3-hydroxyalkanoate. While most PHA acquire the correct stereochemistry after activation to the CoA thioester, there is also the possibility to achieve this modification prior to CoA activation. Conveniently, ketoreductases used by fatty acid biosynthesis and many PKS pathways generate (R)-hydroxyacyl-ACP intermediates that are substrates for hydroxyacyl-ACP thioesterases such as PhaG. Thioesterase cleavage generates the corresponding free acid that can be activated for polymerization by CoA ligation. In contrast, β-oxidation intermediates (which are already activated as CoA thioesters) are substrates for ketoacyl-CoA reductase (FabG) and enoyl-CoA hydratase (PhaJ). These reactions incorporate the correct (R) stereochemistry. Alternatively, the native PHB pathway enzymes (PhaA, PhaB) could be mutated to permit elongation (with acetyl-CoA) and ketoreduction (of the ketone at the beta position) of organic acids missing the requisite hydroxyl group.
5.2. CoA activation
A monomer unit cannot be incorporated unless covalently linked to a CoA. Novel PHA biosynthesis pathways will require this additional step to generate thioesters from organic acids (e.g., PKS release their products via hydrolysis). Conveniently, there are several enzymes capable of activating acid monomers for polymerization. CoA transferases can move CoA from acetyl-CoA (or other thioester donor) to a desired organic acid. Examples are found in short chain fatty acid catabolic pathways (e.g., AtoDA). Alternatively, CoA ligases such as AlkK use ATP to activate organic acids prior to formation of the thioester bond with free CoA (Satoh et al., 2005). These enzymes are classified based on the substrate they act upon, however most show relaxed specificity by acting on additional substrates – although usually with reduced activity (Satoh et al., 2005). Substrate flexibility has also been engineered through the use of rational design to expand the substrate range for CoA ligases (Wu et al., 2007). Alternatively, it may be possible to cleave acyl-chains from PKS ACP domains using CoA instead of water. This has been shown via site directed mutagenesis of a Rat type II TE which conferred acyltransferase capabilities and resulted in CoA‐products (Witkowski et al., 1994).
6. Polymerization
The final step in PHA biosynthesis involves PHA polymerase (PhaC) catalyzing the formation of an ester bond between the terminal carboxylic acid group from one monomer with the (usually β-) hydroxyl group from a second monomer (Fig. 3). PHA polymerases have activity on a broad range of substrates and can be classified in one of several ways including substrate specificity, the architecture of the enzyme and the nucleotide/amino acid sequence. In terms of activity for a given monomer, PHA polymerases are generally biased toward either scl-PHA or mcl-PHA. The scl-PHA polymerases tend to be specific for the polymerization of 3HB, although they have activity towards 3 and 5 carbon monomers as well. The mcl-PHA polymerases, on the other hand, have a broader activity and catalyze the polymerization of 6–14 carbon monomers. Polymerization of longer chain-length monomers including C16 and C18 has also been demonstrated (Lee et al., 1995; Singh and Mallick, 2008).
There is an abundance of PHA synthase gene sequences and substrate specificity data in the literature (Rehm, 2003, 2007; Rehm and Steinbuchel, 1999). Bioinformatic and structural comparisons of these enzymes will likely lead to structure-function relationships that can be used to identify synthases for novel PHA monomers. Alternatively, random mutagenesis and rational re-design approaches have been used to improve the kinetics of polymerases (Nomura and Taguchi, 2007). Due to the difficulty associated with the crystallization of these proteins, techniques such as error-prone PCR, gene shuffling, and saturation of key amino acids have been employed. For example, site-directed mutagenesis of the PHA synthase (phaC) from C. necator was demonstrated to increase the substrate tolerance of the enzyme (Tsuge et al., 2004). Similarly, engineering of mcl-PHA polymerase PhaC1 from Pseudomonas sp. 61–3 conferred the enzyme with the ability to polymerize both scl- and mcl-PHA (Takase et al., 2003). More recently, truncations of two PhaC from C. necator and P. aeruginosa and subsequent recombination to form hybrid polymerases resulted in altered product specificity (Wang et al., 2012b). Even with engineered polymerases, co-feeding of substrates is sometimes necessary to incorporate particular monomers into PHA granules. For example, in an attempt to incorporate monomers with fluorinated side-chains, feeding a single, fluorinated precursor resulted in no polymer accumulation (Kim et al., 1996). Upon co-feeding of nonanoic acid, various fluorinated monomers were successfully incorporated into a polymer.
A final consideration is the extent of polymerization. The material properties of plastics, PHA included, are often dependent on the molecular weight of the polymer. In this regard, PHA have the advantage of high molecular weights and low dispersities that lend to commericial applications. When produced from glucose, PHB is characterized by weight-average molecular weights (MW) of 500–1,000 kDa and melting temperatures of 170–180°C (Madison and Huisman, 1999b). Recent studies have also demonstrated production of PHB with MW values exceeding 6,000 kDa and dispersities less than 2 (Hiroe et al., 2012).
7. Conclusions
In summary, the scientific framework is in place for the construction of metabolically engineered pathways for synthesizing custom PHA monomers from unrelated carbon sources. While a large number of monomers have been successfully incorporated into PHA granules, the target price of commercial PHA limits the economic viability of these materials when derived from feedstocks more expensive than glucose or glycerol. Synthetic biology has been used to construct metabolic pathways for producing a wide range of commodity chemicals including unnatural molecules. We hypothesize that synthetic biology can be similarly used to engineer metabolic pathways from renewable feedstocks to PHA monomers. If successfully incorporated, novel monomers will have a significant impact on the material properties of the resulting polyester. We anticipate that this approach will increase the industrial relevance and commercial viability of PHA in the polymer marketplace.
Supplementary Material
8. Acknowledgments
The authors would like to acknowledge David Sherman, Michael Thomas, and Laura Schmidli for their contributions. This work was funded by the Wisconsin Alumni Research Foundation, the National Science Foundation (CBET-1149678), and by the DOE Great Lakes Bioenergy Research Center (DOE BER Office of Sciences DE-FC02–07ER64494). Daniel E. Agnew is the recipient of a National Institutes of Health Biotechnology Training Program Fellowship (NIH 5 T32 GM08349).
Abbreviations:
- PHA
polyhydroxyalkanoate
- PHB
polyhydroxybutyrate
- 3HB
3-hydroxybutyrate
- scl
short-chain-length
- 3HV
3-hydroxyvalerate
- 4HV
4-hydroxyvalerate
- mcl
medium-chain-length
- BTE
California bay laurel thioesterase
- PKS
polyketide synthase
- FAS
fatty acid synthase
- ACP
acyl-carrier protein
- CoA
coenzyme A
12. References
- Agnew DE, Stevermer AK, Youngquist JT, Pfleger BF, 2012. Engineering Escherichia coli for production of C12-C14 polyhydroxyalkanoate from glucose. Metabolic Engineering (In Press). [DOI] [PMC free article] [PubMed]
- Alonso DM, Bond JQ, Dumesic JA, 2010. Catalytic conversion of biomass to biofuels. Green Chemistry 12, 1493–1513. [Google Scholar]
- Bohm I, Holzbaur IE, Hanefeld U, Cortes J, Staunton J, Leadlay PF, 1998. Engineering of a minimal modular polyketide synthase, and targeted alteration of the stereospecificity of polyketide chain extension. Chemistry & Biology 5, 407–412. [DOI] [PubMed] [Google Scholar]
- Buchholz TJ, Geders TW, Bartley FL, Reynolds KA, Smith JL, Sherman DH, 2009. Structural Basis for Binding Specificity between Subclasses of Modular Polyketide Synthase Docking Domains. Acs Chemical Biology 4, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu DC, Chen Y, Reilly PJ, 2010. Thioesterases: A new perspective based on their primary and tertiary structures. Protein Science 19, 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho LR, Mitchel DA, Freire DMG, 2009. Production of polyhydroxyalkanoates (PHAs) from waste materials and by-products by submerged and solid-state fermentation. Bioresource Technology 100, 5996–6009. [DOI] [PubMed] [Google Scholar]
- Chan YA, Boyne MT, Podevels AM, Klimowicz AK, Handelsman J, Kelleher NL, Thomas MG, 2006. Hydroxymalonyl-acyl carrier protein (ACP) and aminomalonyl-ACP are two additional type I polyketide synthase extender units. Proceedings of the National Academy of Sciences of the United States of America 103, 14349–14354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YA, Podevels AM, Kevany BM, Thomas MG, 2009. Biosynthesis of polyketide synthase extender units. Natural Product Reports 26, 90–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G-Q, 2009. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chemical Society Reviews 38, 2434–2446. [DOI] [PubMed] [Google Scholar]
- Choi JI, Lee SY, 1999. Efficient and economical recovery of poly(3-hydroxybutyrate) from recombinant Escherichia coli by simple digestion with chemicals. Biotechnology and Bioengineering 62, 546–553. [DOI] [PubMed] [Google Scholar]
- Choi MH, Xu J, Rho JK, Shim JH, Yoon SC, 2009. Shifting of the Distribution of Aromatic Monomer-Units in Polyhydroxyalkanoic Acid to Longer Units by Salicylic Acid in Pseudomonas fluorescens BM07 Grown With Mixtures of Fructose and 11-Phenoxyundecanoic Acid. Biotechnology and Bioengineering 102, 1209–1221. [DOI] [PubMed] [Google Scholar]
- Chung A, Liu Q, Ouyang SP, Wu Q, Chen GQ, 2009. Microbial production of 3-hydroxydodecanoic acid by pha operon and fadBA knockout mutant of Pseudomonas putida KT2442 harboring tesB gene. Applied Microbiology and Biotechnology 83, 513–519. [DOI] [PubMed] [Google Scholar]
- Cortes J, Wiesmann KEH, Roberts GA, Brown MJB, Staunton J, Leadlay PF, 1995. Repositioning of a Domain in a Modular Polyketide Synthase to Promote Specific Chain Cleavage. Science 268, 1487–1489. [DOI] [PubMed] [Google Scholar]
- Eschenlauer AC, Stoup SK, Srienc F, Somers DA, 1996. Production of heteropolymeric polyhydroxyalkanoate in Escherichia coli from a single carbon source. International Journal of Biological Macromolecules 19, 121–130. [DOI] [PubMed] [Google Scholar]
- Fiedler S, Steinbuchel A, Rehm BHA, 2000. PhaG-mediated synthesis of poly(3-hydroxyalkanoates) consisting of medium-chain-length constituents from nonrelated carbon sources in recombinant Pseudomonas fragi. Applied and Environmental Microbiology 66, 2117–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harracksingh RIA, 2012. Bioplastics near commercialization. ICIS Chemical Business 282, 32–33. [Google Scholar]
- Hiroe A, Tsuge K, Nomura CT, Itaya M, Tsuge T, 2012. Rearrangement of Gene Order in the phaCAB Operon Leads to Effective Production of Ultrahigh-Molecular-Weight Poly[(R)-3-Hydroxybutyrate] in Genetically Engineered Escherichia coli. Applied and Environmental Microbiology 78, 3177–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Nishito A, Eriguchi S, Tamura T, Inagaki K, Tanaka H, 2003. Purification and substrate characterization of alpha-ketobutyrate decarboxylase from Pseudomonas putida. Journal of Molecular Catalysis B-Enzymatic 23, 265–271. [Google Scholar]
- Jacquel N, Lo CW, Wei YH, Wu HS, Wang SS, 2008. Isolation and purification of bacterial poly (3-hydroxyalkanoates). Biochemical Engineering Journal 39, 15–27. [Google Scholar]
- Jaremko M, Yu J, 2011. The initial metabolic conversion of levulinic acid in Cupriavidus necator. Journal of Biotechnology 155, 293–298. [DOI] [PubMed] [Google Scholar]
- Keasling, 2010a. Microbial artemisinin plant online by late 2011. Chemistry & Industry, 12–12.
- Keasling JD, 2010b. Manufacturing Molecules Through Metabolic Engineering. Science 330, 1355–1358. [DOI] [PubMed] [Google Scholar]
- Keshavarz T, Roy I, 2010. Polyhydroxyalkanoates: bioplastics with a green agenda. Current Opinion in Microbiology 13, 321–326. [DOI] [PubMed] [Google Scholar]
- Kim O, Gross RA, Hammar WJ, Newmark RA, 1996. Microbial synthesis of poly(beta-hydroxyalkanoates) containing fluorinated side-chain substituents. Macromolecules 29, 4572–4581. [Google Scholar]
- Lee EY, Jendrossek D, Schirmer A, Choi CY, Steinbuchel A, 1995. Biosynthesis of copolyesters consisting of 3-hydroxybutyric acid and medium-chain length 3-hydroxyalkanoic acids from 1,3-butanediol or from 3-hydroxybutyrate by Pseudomonas sp A33. Applied Microbiology and Biotechnology 42, 901–909. [Google Scholar]
- Lee HJ, Choi MH, Kim TU, Yoon SC, 2001. Accumulation of polyhydroxyalkanoic acid containing large amounts of unsaturated monomers in Pseudomonas fluorescens BM07 utilizing saccharides and its inhibition by 2-bromooctanoic acid. Applied and Environmental Microbiology 67, 4963–4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Luo G, Zhou XR, Chen G-Q, 2011. Biosynthesis of poly(3-hydroxydecanoate) and 3-hydroxydodecanoate dominating polyhydroxyalkanoates by beta-oxidation pathway inhibited Pseudomonas putida. Metabolic Engineering 13, 11–17. [DOI] [PubMed] [Google Scholar]
- Madison LL, Huisman GW, 1999a. Metabolic Engineering of Poly(3-Hydroxyalkanoates): From DNA to Plastic. Microbiology and Molecular Biology Reviews 63, 21–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison LL, Huisman GW, 1999b. Metabolic engineering of poly(3-hydroxyalkanoates): From DNA to plastic. Microbiology and Molecular Biology Reviews 63, 21-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CH, Prather KLJ, 2009. High-titer production of monomeric hydroxyvalerates from levulinic acid in Pseudomonas putida. Journal of Biotechnology 139, 61–67. [DOI] [PubMed] [Google Scholar]
- Moore BS, Hertweck C, 2002. Biosynthesis and attachment of novel bacterial polyketide synthase starter units. Natural Product Reports 19, 70–99. [DOI] [PubMed] [Google Scholar]
- Nandagopal N, Elowitz MB, 2011. Synthetic Biology: Integrated Gene Circuits. Science 333, 1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura CT, Taguchi S, 2007. PHA synthase engineering toward superbiocatalysts for custom-made biopolymers. Applied Microbiology and Biotechnology 73, 969–979. [DOI] [PubMed] [Google Scholar]
- Oliynyk M, Brown MJB, Cortes J, Staunton J, Leadlay PF, 1996. A hybrid modular polyketide synthase obtained by domain swapping. Chemistry & Biology 3, 833–839. [DOI] [PubMed] [Google Scholar]
- Ostergaard LH, Kellenberger L, Cortes J, Roddis MP, Deacon M, Staunton J, Leadlay PF, 2002. Stereochemistry of catalysis by the ketoreductase activity in the first extension module of the erythromycin polyketide synthase. Biochemistry 41, 2719–2726. [DOI] [PubMed] [Google Scholar]
- Qiu YZ, Han J, Guo JJ, Chen GQ, 2005. Production of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from gluconate and glucose by recombinant Aeromonas hydrophila and Pseudomonas putida. Biotechnology Letters 27, 1381–1386. [DOI] [PubMed] [Google Scholar]
- Rebeille F, Jabrin S, Bligny R, Loizeau K, Gambonnet B, Van Wilder V, Douce R, Ravanel S, 2006. Methionine catabolism in Arabidopsis cells is initiated by a gamma-cleavage process and leads to S-methylcysteine and isoleucine syntheses. Proceedings of the National Academy of Sciences of the United States of America 103, 15687–15692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm BHA, 2003. Polyester synthases: natural catalysts for plastics. Biochemical Journal 376, 15–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm BHA, 2007. Biogenesis of microbial polyhydroxyalkanoate granules: a platform technology for the production of tailor-made bioparticles. Current Issues in Molecular Biology 9, 41–62. [PubMed] [Google Scholar]
- Rehm BHA, Steinbuchel A, 1999. Biochemical and genetic analysis of PHA synthases and other proteins required for PHA synthesis, pp. 3–19. [DOI] [PubMed]
- Rehm BHA, Steinbuchel A, 2001. Heterologous expression of the acyl-acyl carrier protein thioesterase gene from the plant Umbellularia californica mediates polyhydroxyalkanoate biosynthesis in recombinant Escherichia coli. Applied Microbiology and Biotechnology 55, 205–209. [DOI] [PubMed] [Google Scholar]
- Rhie HG, Dennis D, 1995. Role of fadR and atoC(Con) mutations in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) synthesis in recombinant pha(+) Escherichia coli. Applied and Environmental Microbiology 61, 2487–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MCY, Withers ST, Shiba Y, Sarpong R, Keasling JD, 2006. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440, 940–943. [DOI] [PubMed] [Google Scholar]
- Satoh Y, Murakami F, Tajima K, Munekata M, 2005. Enzymatic synthesis of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) with CoA recycling using polyhydroxyalkanoate synthase and acyl-CoA synthetase. Journal of Bioscience and Bioengineering 99, 508–511. [DOI] [PubMed] [Google Scholar]
- Sattely ES, Fischbach MA, Walsh CT, 2008. Total biosynthesis: in vitro reconstitution of polyketide and nonribosomal peptide pathways. Natural Product Reports 25, 757–793. [DOI] [PubMed] [Google Scholar]
- Sherman DH, 2005. The Lego-ization of polyketide biosynthesis. Nat Biotechnol 23, 1083–1084. [DOI] [PubMed] [Google Scholar]
- Singh AK, Mallick N, 2008. Enhanced production of SCL-LCL-PHA co-polymer by sludge-isolated Pseudomonas aeruginosa MTCC 7925. Letters in Applied Microbiology 46, 350–357. [DOI] [PubMed] [Google Scholar]
- Slater S, Mitsky TA, Houmiel KL, Hao M, Reiser SE, Taylor NB, Tran M, Valentin HE, Rodriguez DJ, Stone DA, Padgette SR, Kishore G, Gruys KJ, 1999. Metabolic engineering of Arabidopsis and Brassica for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production. Nature Biotechnology 17, 1011–1016. [DOI] [PubMed] [Google Scholar]
- Steinbüchel A, Füchtenbusch B, 1998. Bacterial and other biological systems for polyester production. Trends in Biotechnology 16, 419–427. [DOI] [PubMed] [Google Scholar]
- Steinbüchel A, Lutke-Eversloh T, 2003. Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochemical Engineering Journal 16, 81–96. [Google Scholar]
- Steinbüchel A, Valentin HE, 1995. Diversity of Bacterial Polyhydroxyalkanoic Acids. Fems Microbiology Letters 128, 219–228. [Google Scholar]
- Sudesh K, Iwata T, 2008. Sustainability of biobased and biodegradable plastics. Clean-Soil Air Water 36, 433–442. [Google Scholar]
- Takase K, Taguchi S, Doi Y, 2003. Enhanced synthesis of poly(3-hydroxybutyrate) in recombinant Escherichia coli by means of error-prone PCR mutagenesis, saturation mutagenesis, and in vitro recombination of the type II polyhydroxyalkanoate synthase gene. Journal of Biochemistry 133, 139–145. [DOI] [PubMed] [Google Scholar]
- Tappel RC, Wang Q, Nomura CT, 2012. Precise control of repeating unit composition in biodegradable poly(3-hydroxyalkanoate) polymers synthesized by Escherichia coli. Journal of bioscience and bioengineering 113, 480–486. [DOI] [PubMed] [Google Scholar]
- Tokiwa Y, Calabia BP, 2004. Degradation of microbial polyesters. Biotechnology Letters 26, 1181–1189. [DOI] [PubMed] [Google Scholar]
- Tseng H-C, Harwell C, Martin C, Prather K, 2010. Biosynthesis of chiral 3-hydroxyvalerate from single propionate-unrelated carbon sources in metabolically engineered E. coli. Microbial Cell Factories 9, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuge T, Saito Y, Kikkawa Y, Hiraishi T, Doi Y, 2004. Biosynthesis and compositional regulation of poly [(3-hydroxybutyrate)-co-(3-hydroxyhexanoate)] in recombinant Ralstonia eutropha expressing mutated polyhydroxyalkanoate synthase genes, pp. 238–242. [DOI] [PubMed]
- Van Wegen RJ, Ling Y, Middelberg APJ, 1998. Industrial production of polyhydroxyalkanoates using Escherichia coli: An economic analysis. Chemical Engineering Research & Design 76, 417–426. [Google Scholar]
- Wang HH, Zhou XR, Liu QA, Chen GQ, 2011. Biosynthesis of polyhydroxyalkanoate homopolymers by Pseudomonas putida. Applied Microbiology and Biotechnology 89, 1497–1507. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tappel RC, Zhu CJ, Nomura CT, 2012a. Development of a New Strategy for Production of Medium-Chain-Length Polyhydroxyalkanoates by Recombinant Escherichia coli via Inexpensive Non-Fatty Acid Feedstocks. Applied and Environmental Microbiology 78, 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Xia Y, Chen Q, Qi Q, 2012b. Incremental truncation of PHA synthases results in altered product specificity. Enzyme and Microbial Technology 50, 293–297. [DOI] [PubMed] [Google Scholar]
- Weissman KJ, Leadlay PF, 2005. Combinatorial biosynthesis of reduced polyketides. Nature Reviews Microbiology 3, 925–936. [DOI] [PubMed] [Google Scholar]
- Westfall PJ, Pitera DJ, Lenihan JR, Eng D, Woolard FX, Regentin R, Horning T, Tsuruta H, Melis DJ, Owens A, Fickes S, Diola D, Benjamin KR, Keasling JD, Leavell MD, McPhee DJ, Renninger NS, Newman JD, Paddon CJ, 2012. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proceedings of the National Academy of Sciences of the United States of America 109, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weusthuis RA, Lamot I, van der Oost J, Sanders JPM, 2011. Microbial production of bulk chemicals: development of anaerobic processes. Trends in Biotechnology 29, 153–158. [DOI] [PubMed] [Google Scholar]
- Williams MD, Rahn JA, Sherman DH, 1996. Production of a polyhydroxyalkanoate biopolymer in insect cells with a modified eucaryotic fatty acid synthase. Applied and Environmental Microbiology 62, 2540–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski A, Witkowska HE, Smith S, 1994. Reengineering the Specificity of a Serine Active-Site Enzyme - 2 Active-Site Mutations Convert a Hydrolase to a Transferase. Journal of Biological Chemistry 269, 379–383. [PubMed] [Google Scholar]
- Wong FT, Khosla C, 2012. Combinatorial biosynthesis of polyketides - a perspective. Current Opinion in Chemical Biology 16, 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Reger AS, Cao J, Gulick AM, Dunaway-Mariano D, 2007. Rational redesign of the 4-chlorobenzoate binding site of 4-chlorobenzoate: Coenzyme A ligase for expanded substrate range. Biochemistry 46, 14487–14499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Wroblewska L, Prochazka L, Weiss R, Benenson Y, 2011. Multi-Input RNAi-Based Logic Circuit for Identification of Specific Cancer Cells. Science 333, 1307–1311. [DOI] [PubMed] [Google Scholar]
- Yang YH, Brigham C, Willis L, Rha C, Sinskey A, 2011. Improved detergent-based recovery of polyhydroxyalkanoates (PHAs). Biotechnology Letters 33, 937–942. [DOI] [PubMed] [Google Scholar]
- You L, Cox RS 3rd, Weiss R, Arnold FH, 2004. Programmed population control by cell-cell communication and regulated killing. Nature 428, 868–871. [DOI] [PubMed] [Google Scholar]
- Yuan L, Voelker TA, Hawkins DJ, 1995. Modification of the substrate-specificity of an acyl-acyl carrier protein thioesterase by protein engineering. Proceedings of the National Academy of Sciences of the United States of America 92, 10639–10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


