Abstract
A competitive ELISA method is described for the measurement of total antibodies to the capsular polysaccharide of Haemophilus influenzae type b (HibCPS) in human sera. The competitive method showed an excellent correlation to the radioantigen binding assay (RABA, or Farr assay) and improved correlation of sera with low titers with respect to the more conventional noncompetitive method. Overestimation of samples in the low concentration range was no longer observed with the competitive ELISA method. The free HibCPS competition allowed us to eliminate the day-to-day background variation typical of some sera; thus, only values representing the true anti-HibCPS response were determined. The use of precoated microplates, which could be stored up to 8 months, greatly improved the speed of the procedure. An overall correlation coefficient of 0.9660 was found when 407 serum samples with a wide variety of anti-HibCPS antibody levels were tested with the competitive ELISA and RABA. The regression line was very close to the ideal line, with a slope of 1.0045 and an intercept of −0.1996. A subset of 96 serum samples representative of all pre- and postimmunization samples was used to compare the competitive ELISA with a previously described ELISA method. The competitive method performed in two laboratories in different countries showed a better correlation with the RABA. The correlation factors were 0.9770 and 0.9816, respectively, while a factor of 0.9547 was found with the previously described noncompetitive procedure, which was better for this method than previously reported (r = 0.917). Therefore, the competitive ELISA is proposed for the assay of anti-HibCPS titers in sera from vaccinated subjects.
Haemophilus influenzae type b (Hib) has been a leading cause of bacterial meningitis among infants and young children worldwide. The organism also causes other invasive infections, including epiglottitis, cellulitis, pneumonia, pericarditis, arthritis, bacteremia, empyema, and osteomyelitis (5, 19). Antibodies to the capsular polysaccharide of Hib (HibCPS) protect against invasive disease from this organism (15, 18).
Serum antibodies to HibCPS have been quantitatively determined by using the radioantigen binding assay (RABA) technique described by Farr in 1958 (6), modified for specificity and labelling (2, 5, 12). The concentration of serum anti-HibCPS antibody sufficient to confer protection is not known (10). Estimates have varied from concentrations of 0.1 μg/ml to concentrations of 1.0 μg/ml (1, 11, 14, 17). Because of qualitative differences in antibody functions attributable to a combination of differences in isotype and avidity (1, 11), precise estimates are probably not possible. However, vaccinated subjects are considered protected when a level of 1.0 μg of anti-HibCPS antibodies per ml is found (1, 11), although the use of conjugate Hib vaccines able to elicit a T-cell-dependent immune response may lower this limit in the future because of their ability to prime for memory serum antibody responses, as recently suggested by Kayhty (10).
In 1990, Phipps et al. proposed an enzyme-linked immunosorbent assay (ELISA) measurement of total immunoglobulin (Ig) to HibCPS that correlated well with RABA results (16). This ELISA procedure, although unable to resolve the dependence of the assay on antibody avidity (3), was an improvement in terms of the feasibility of assaying large numbers of serum samples, while avoiding the use of radioisotopes. However, in our hands, this assay showed a high variability in serum antibody background levels. Therefore, we developed and standardized an improved ELISA measuring total specific Ig levels with a competitive assay, in which the specific binding to HibCPS was measured in each sample by subtraction of the uninhibited fraction after addition of a saturating amount of soluble HibCPS. Here, we describe the competitive ELISA method for quantitative measurement of serum antibodies to HibCPS.
MATERIALS AND METHODS
Preparation of conjugated HibCPS.
A procedure to prepare human serum albumin-HibCPS conjugate (HSA-Hib) was developed and standardized to provide antigen to coat microtiter plates. Twenty percent (wt/vol) HSA (Sclavo S.p.A., Siena, Italy) was first characterized for protein content according to the method of Lowry et al. (13) and then was characterized for amino group content (8). Fifty milliliters of an aqueous solution of 500 mg of HibCPS (40% [wt/wt] ribose content; lot 12/89; CHIRON S.p.A., Siena, Italy), corresponding to 1,335 μmol in ribose was added to 0.4 M NaIO4 at a ribose/NaIO4 molar ratio of 8. The mixture was maintained for 20 min in the dark at room temperature and then stored at 4°C. The content of ribose and the aldehyde groups was determined according to conventional colorimetric assays (4, 9). A volume containing 100 μmol of the aldehyde groups was added, while being stirred, to a volume of the HSA solution equivalent to 50 μmol of amino groups (about 50 to 60 mg of protein) and a volume of a 1 M pyridineborane (PyBH3) solution in methanol corresponding to 1,310 μmol (molar ratios: CHO-NH2 = 2, PyBH3-CHO = 13, and PyBH3-NH2 = 26). The mixture was continuously stirred overnight at room temperature. (NH4)2SO4 was added to a final concentration of 22.5% (wt/vol), and chromatography was performed with a phenyl-Sepharose (26 by 10 mm) column previously equilibrated with 10 mM phosphate buffer (pH 7.2) containing 22.5% (wt/vol) (NH4)2SO4. The column was washed with about 10 volumes of equilibration buffer, and then the conjugate was eluted with 10 mM phosphate buffer (pH 7.2). The elution profile was monitored at 280 nm, and 2-ml fractions were collected. The respective fractions of the first peak (washing) and of the second peak (elution) were pooled and assayed for ribose and protein content. The elution peak solution also was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (7.5% polyacrylamide) under reducing conditions and silver staining in order to exclude the presence of free albumin. The solution containing the elution peak was then divided into aliquots and stored at −20°C.
Sera.
The correlation between the ELISA and RABA was done by assaying 407 serum samples collected pre- or postvaccination from children who had participated in clinical trials with three conjugated Hib vaccines (VAXEM-Hib [Chiron], HibTITER [Lederle-Praxis], or ACT-Hib [Pasteur-Merieux]). Assays also were done with pre- and postvaccination serum samples from 28 adults (13.2%) who received a plain HibCPS investigational vaccine. The sera were collected prior to immunization (93 samples [22.9%]) and at different times after immunization—post-1 (8 samples [2.0%]), pre-3 (21 samples [5.2%]), post-3 (111 samples [27.3%]), pre-4 (56 samples [13.2%]), and post-4 (62 samples [15.2%])—according to different clinical protocols. This variety of sera ensured a broad range of antibody concentrations elicited by different vaccines. All sera were stored at −80°C in small aliquots to avoid repeated freeze-thawing.
A subset of 96 serum samples of the panel consisting of 26 preimmunization samples (27.1%), 26 post-3 samples (27.1%), 19 pre-4 samples (19.8%), and 25 post-4 samples (26.0%) was used for further comparison with the Phipps’ ELISA as described by Phipps et al. in 1990 (16). Hib serum lot 1983, with an assigned value of 70 μg/ml, was obtained from Carl Frash (Center for Biological Evaluation and Research [CBER], Food and Drug Administration) and used as a reference serum. Serum pools A110 (90 μg/ml), A143 (1.7 μg/ml), A144 (49.1 μg/ml), A146 (13.1 μg/ml), and A148 (6.8 μg/ml) were kindly provided by Dan Granoff (Chiron Vaccines, Emeryville, Calif., and Children’s Hospital Oakland Research Center, Oakland, Calif.) and used as internal standard (A110) and quality control sera.
RABA.
All sera were analyzed by RABA to determine the total anti-HibCPS antibody concentration. The general procedure of Robbins et al. was followed (17). 125I-labelled HibCPS was obtained according to the chloramine-T method (7) by labelling a HibCPS-tyramine conjugate to conform to the specifications established in 1987 by Carl Frash. Purified HibCPS from strain Eagan was fractionated on a Sepharose CL-2B (Pharmacia, Uppsala, Sweden) column, and fractions in the 0.3- to 0.7-kDa range were pooled to exclude the very-high-molecular-weight outer membrane protein-containing material. The purity of the radiolabelled antigen was assayed for binding to rabbit serum prepared against a capsular-deficient variant (S-2) of strain Eagan (average of 3.8% binding). The antigen concentration was estimated in RABA by competition with cold HibCPS at concentrations ranging from 7.8 to 2,000 ng/ml in the presence of an antibody concentration of 0.875 μg/ml. The antigen concentration used in the RABA was about 50 to 60 ng/ml. Antibody concentrations of test sera were determined by comparison of binding with that of the standard CBER reference serum (70 μg/ml). For calculation, we used the average of values determined by serum dilutions within the 15 to 80% binding range.
Competitive ELISA.
Phosphate-buffered saline (PBS) was prepared with sterile, nonpyrogenic water. HSA-Hib conjugate was diluted in PBS (pH 7.4) to a concentration of 1.0 μg/ml (based on saccharide concentration). One hundred-microliter aliquots of this solution were dispensed into the wells of polystyrene microtiter plates (Maxisorp, Nunc, Roskilde, Denmark). After overnight incubation at 4°C, plates were washed with PBS (pH 7.4) containing 0.01% (vol/vol) Tween 20. Wells were overcoated with 250 μl of a 1% (wt/vol) gelatin solution in PBS (pH 7.4) for 3 h at 37°C. After washing, 250 μl of a fixative solution (saline containing 4% [wt/vol] polyvinylpyrrolidone and 10% [wt/vol] saccharose) was added to each well. After 2 h of incubation at room temperature, the fixative solution was removed from the wells, and the plates were dried overnight at room temperature. Dried plates, hermetically sealed in plastic bags, could be stored for up to 8 months.
All sera were plated in duplicate. Each plate contained reference serum, quality control sera, and sample sera diluted in a mixture of 10 mM phosphate buffer and 150 mM NaCl (pH 7.4) containing 0.05% (vol/vol) Tween 20, and 1% (wt/vol) bovine serum albumin (PBS-BSA). Each plate contained eight replicates of PBS-BSA (50 μl) to determine general nonspecific binding. The reference serum was diluted in duplicate from 0.250 to 0.0019 μg/ml (final dilutions; 50 μl); there were 4 internal quality control serum samples (50 μl) and 15 serum samples at four fourfold serial dilutions (50 μl). Fifty microliters of PBS-BSA was then added to the first wells (final dilutions of quality control and sample sera were 1:50, 1:200, 1:800, 1:3,200). Fifty microliters of a 100-μg/ml HibCPS solution (final dilution 50 μg/ml) in PBS-BSA was added to the duplicate wells. Plates were then incubated for 3 h at 37°C (or overnight at 4°C). After incubation, the wells were washed three times with a mixture of 10 mM phosphate, 150 mM NaCl, (pH 7.4), and 0.05% Tween 20 (PBS-Tween).
Next, 100 μl of alkaline phosphatase-conjugated goat IgG–anti-human Ig (IgG, IgM, and IgA) (Sigma Chimica, Gallarate, Milan, Italy) diluted 1:10,000 in PBS-BSA was added to each well. Plates were incubated for 3 h at 37°C and then washed as described above. To prepare the chromogen-substrate solution, p-nitrophenylphosphate tablets (Sigma Chimica) were dissolved in 1 M diethanolamine–0.5 mM MgCl2 (pH 9.8) according to the manufacturer’s instructions. One-hundred-microliter aliquots of the solution were distributed into the wells. After 35 min, the reaction was stopped with 100 μl of 4 N NaOH, and the plates were read at A405 with a reference filter at 595 nm.
The analysis of the positive values (≥0.10 μg/ml) for sera from children (86.8% of the samples) presented a median for specific binding after inhibition of 91.2% with a minimum value of 28.6%, while for sera from adults (13.2% of the samples), the analysis presented a median of 83.55% with a minimum value of 23.26%. The percentage of inhibition was calculated, assuming the optical density (OD) reading in the noninhibited well as 100% of binding, according to the formula % inhibition = 100 − [(ODinhibited well × 100)/ODnoninhibited well]. The higher the percentage was, the more specific the calculated result was. Therefore, as a precaution, low-inhibition samples were considered negative, regardless of their absorbance, when the percentage of specific binding after inhibition obtained by comparison of inhibited and noninhibited wells was lower than 20%, since noninhibitable binding cannot be considered specific.
ELISA specificity.
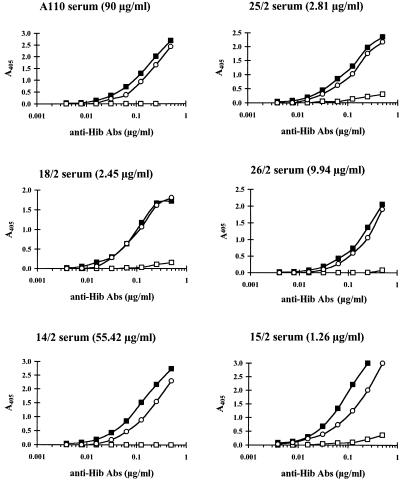
Competition with purified capsular polysaccharide of Neisseria meningitidis serotype C (MenCCPS) was carried out to confirm the specificity of HibCPS inhibition. Six serum samples representative of a wide range of serum titers were serially diluted from 0.250 to 0.0019 μg/ml. The binding was then inhibited by addition of a constant excess of purified HibCPS or MenCCPS (100 μg/ml, final dilution). The first dilutions of the sera were as follows: A110 (90 μg/ml) was diluted 1:360, 14/2 (55.42 μg/ml) was diluted 1:222, 26/2 (9.94 μg/ml) was diluted 1:40, 25/2 (2.81 μg/ml) was diluted 1:11, 18/2 (2.45 μg/ml) was diluted 1:10, and 15/2 (1.26 μg/ml) was diluted 1:5. It should be underlined that the sera showing low titers were diluted at dilutions lower than the first serum dilution in the assay (1:50) to obtain the inhibition curve.
Data selection and statistical analysis.
A titration curve was obtained for each serum sample by plotting the absorbance values as a function of the logarithm of the reciprocal of the serum dilution. Sample concentrations were determined by using only absorbance values in the linear portion of the standard curve (OD of 0.050 to the reading plateau of an OD of about 2.600). To calculate serum anti-HibCPS antibody concentrations, absorbance values in wells containing serum dilutions incubated with soluble HibCPS were subtracted as background from the corresponding values of the wells in which sera were diluted with buffer alone. Only sera with absorbance values which were inhibited at least 20% by soluble HibCPS and giving a difference between noninhibited and inhibited A405 values of ≥0.050 were considered positive. Antibody concentrations in sera were calculated from the standard curve and were expressed in micrograms per milliliter.
Noncompetitive ELISA results were obtained from the same data generated with competitive ELISA, but with the average absorbance of the buffer subtracted as background instead of the absorbance of the inhibited serum sample. At a 1:50 dilution of serum, the ELISA sensitivity limit in undiluted sera corresponded to 0.10 μg/ml. For data analysis, samples with an antibody concentration of <0.10 μg/ml were assigned 50% of the minimum (0.05 μg/ml). Logarithmically transformed values of antibody concentrations were used for determination of the correlation coefficients between the different assay methods.
Comparison of competitive ELISA and Phipps’ ELISA.
The Phipps’ ELISA was performed as described previously (16). Two minor differences were the starting dilution of the samples, which was 1:20, and the fact that the alkaline phosphatase-conjugated polyspecific goat anti-human Ig was purchased from Caltag (San Francisco, Calif.). The main differences between the two procedures are summarized in Table 1 and seem to be the incubation time, buffer, and temperature, in addition to the materials used. For the comparison, the panel of 96 serum samples described above was used.
TABLE 1.
Major differences between the competitive ELISA used in this study and Phipps’ ELISA
| Type of ELISA | Type of plate | Coating | Coating time | Overcoating and fixing | Serum binding buffer | Serum dilution | Serum incubation time | Conjugate | Conjugate incubation time |
|---|---|---|---|---|---|---|---|---|---|
| Competitive | MaxiSorp (Nunc) | HSA-Hib | Overnight at 4°C | Yes | PBS–1% BSA–0.05% Tween 20 | 4-fold serial 1/50–1/3,200 | 3 h at 37°C | Alkaline phosphatase-conjugated goat IgG–anti-human Ig (IgG, IgM, and IgA) (Sigma), dilution 1/10,000 | 3 h at 37°C |
| Phipps’ | PolySorp (Nunc) | HbHOA | 90 min at 37°C | No | PBS–0.3% Tween 20–0.01 M EDTA | 2-fold serial 1/20–1/5,120 | 1 h at room temp | Alkaline phosphatase-conjugated goat IgG–anti-human polyspecific Ig (Caltag), dilution 1/2,000 | 1 h at room temp |
RESULTS AND DISCUSSION
HSA-Hib conjugate and plate coating.
Three lots of HSA-Hib conjugate were prepared. As summarized in Table 2, the results were consistent. Therefore, the lots were pooled. The activity of the pooled lot was tested against that of a previous lot by comparing standard reference serum curves and medium-titer (6.5 μg/ml) serum dilution curves in an ELISA. A t test showed that the difference between the mean values of the two groups was not great enough to exclude the possibility that the difference was just due to random sampling variability. No statistically significant difference between the groups analyzed was found. In fact, statistical analysis gives t = −0.133, with an associated P = 0.896 for the standard reference serum curves, and t = −0.019, with an associated P = 0.985 for the medium-titer serum curves. The same results were also obtained with a nonparametric analysis according to the Mann-Whitney rank-sum test.
TABLE 2.
HSA-Hib conjugate characteristics and consistency
| HSA-Hib lot no. | Amt of:
|
% Recovery | Oligo-CPS/protein ratio (%) | |
|---|---|---|---|---|
| Ribose (μmol/ml) | Protein (mg/ml) | |||
| 150694 | 17.12 | 192.9 | 82.4 | 22.18 |
| 200694 | 19.48 | 218.5 | 82.4 | 22.28 |
| 220694 | 21.30 | 212.9 | 83.6 | 25.01 |
| 240694 (pool) | 18.93 | 214.2 | 22.09 | |
The final lot gave a total amount of 133 mg (in protein) of HSA-Hib conjugate that was stored in aliquots at −20°C. The HSA-Hib conjugate was stable for at least 8 months at 4°C and could be frozen at −20°C for prolonged storage, since at least four freeze-thawing cycles did not affect antigen stability.
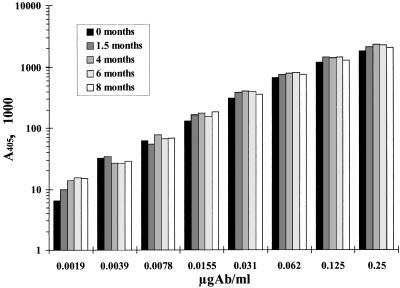
HSA-Hib conjugate-coated plates prepared as described in Materials and Methods were stored for up to 8 months at 4°C in sealed plastic bags. Plates were tested simultaneously after different storage times: when freshly prepared (0 months) and at 1.5, 4, 6, and 8 months. Coated plates were proved stable by the consistency of a standard curve with A110 serum in the different plates, as depicted in Fig. 1. The HSA-Hib conjugate was also successfully tested for equivalence against Phipp’s HbHOA conjugate (data not shown).
FIG. 1.
Stability of microtiter plates coated with HSA-Hib conjugate. Results represent the consistency of the anti-HibCPS total Ig standard curve in plates stored at 4°C for different times. Ab, antibody.
ELISA specificity.
The use of competitive ELISA ensured the specificity of antibody measured for each serum sample. Competition with purified MenCCPS was carried out in parallel to that with HibCPS to confirm the specificity of HibCPS inhibition; the results are shown in Fig. 2. The curves obtained with the six diluted serum samples representative of a wide range of serum titers showed that the excess concentrations of the specific Haemophilus polysaccharide were able to completely inhibit the specific binding in all cases, while the noncorrelated Neisseria polysaccharide did not interfere with the specific antibody binding to the plate wells.
FIG. 2.
Specificity of competitive ELISA for total anti-Hib Igs. Results are shown for sample serum dilution curves in the presence of buffer (solid squares), HibCPS (open squares), and MenCCPS (open circles). Abs, antibodies.
ELISA results.
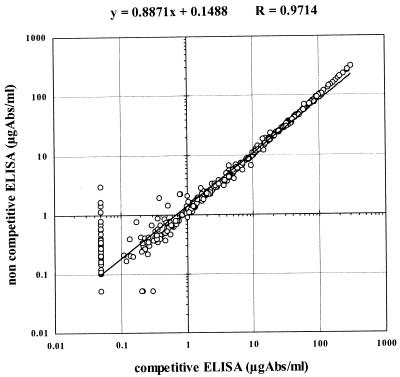
With sera containing low (<2.0 μg/ml) antibody concentrations, day-to-day variability and background variability were observed with the conventional indirect ELISA. Therefore, a specific binding inhibition with purified HibCPS was evaluated. Figure 3 shows the correlation between competitive and noncompetitive ELISA results. The overall correlation was very good (r = 0.9714; n = 407). This correlation was even better (r = 0.9973; n = 232) when calculated for samples at ≥2.0 μg/ml, but decreased when calculated for samples at <2.0 μg/ml (r = 0.7618; n = 175).
FIG. 3.
Correlation of competitive versus noncompetitive ELISA results. Low-titer values resulted in overestimation by the noncompetitive assay, thus causing possible false-positive results in vaccinated individuals. Abs, antibodies.
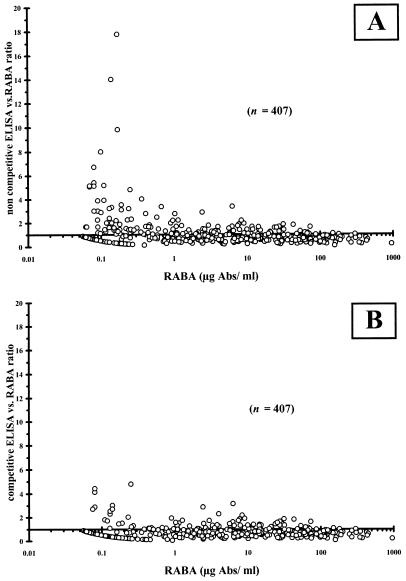
Antibody concentrations in low-titer samples were often overestimated when the noncompetitive assay was used, which is illustrated in Fig. 4. This is also illustrated by comparison of the geometric means. The geometric means for samples with concentrations ≥2.0 μg/ml were 13.36 μg/ml for the competitive assay versus 14.05 μg/ml for the noncompetitive one, while for samples with concentrations <2.0 μg/ml, they amounted to 0.16 and 0.28 μg/ml, respectively. Discrepancies in the results from low-titer sera with antibody concentrations of <1.0 μg/ml, as determined by RABA, are greater with the noncompetitive ELISA (Fig. 4A) than with the competitive ELISA (Fig. 4B). As a result, the percentages of samples with antibody concentrations >1.0 μg/ml were overestimated after analysis with the noncompetitive ELISA, as shown in Table 3 for preimmune sera, especially for adults. Thus, the noncompetitive ELISA might overestimate the percentage of subjects with an antibody concentration >1.0 μg/ml.
FIG. 4.
Scatter plots of noncompetitive (A) and competitive (B) ELISA/RABA value ratios versus RABA values. The wider dispersion in the low data range of 0.1 to 2 μg/ml found with noncompetitive ELISA (A) was largely reduced with the competitive assay (B). Abs, antibodies.
TABLE 3.
Comparison of RABA and ELISA results with sample sera
| Group | No. (%) of samples (n = 407) | % of samples with titer resulta
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RABA
|
ELISA
|
|||||||||
| Competitive
|
Noncompetitive
|
|||||||||
| >0.15 | >1.0 | GMT | >0.15 | >1.0 | GMT | >0.15 | >1.0 | GMT | ||
| Infants | ||||||||||
| Preimmunization | 93 (22.9) | 55.9 | 9.7 | 0.19 | 34.4 | 8.6 | 0.11 | 49.5 | 10.8 | 0.16 |
| Post-1 | 8 (2.0) | 100 | 100 | 6.57 | 100 | 87.5 | 7.00 | 100 | 87.5 | 7.83 |
| Pre-3 | 21 (5.2) | 71.4 | 42.9 | 0.72 | 66.7 | 23.8 | 0.42 | 90.5 | 28.6 | 0.72 |
| Post-3 | 111 (27.3) | 91.0 | 82.9 | 7.70 | 89.2 | 82.0 | 5.65 | 94.6 | 81.1 | 6.40 |
| Pre-4 | 56 (13.8) | 98.2 | 80.4 | 2.91 | 92.9 | 71.4 | 1.68 | 98.2 | 78.6 | 2.10 |
| Post-4 | 62 (15.2) | 100 | 100 | 64.3 | 100 | 100 | 34.5 | 100 | 100 | 35.1 |
| Adults | ||||||||||
| Preimmunization | 28 (6.9) | 82.1 | 42.9 | 1.11 | 75.0 | 46.4 | 0.81 | 100 | 64.3 | 2.19 |
| Post-2 | 28 (6.9) | 100 | 92.9 | 9.41 | 92.9 | 89.3 | 6.29 | 100 | 96.4 | 8.74 |
>0.15, percentage of serum samples with a titer >0.15 μg of specific Igs per ml (prevaccination protection); >1.0, percentage of serum samples with a titer >1.0 μg of specific Igs per ml (postvaccination protection); GMT, geometric mean of the calculated titers.
Reproducibility.
The standard curve, quality control sera, three negative samples, and three low-concentration samples were used to determine the consistency of the assay results from day to day and with different operators. These sera were measured by competitive ELISA on three different days by two different operators testing four replicates of each point of the standard curve, six replicates of each quality control point, and four replicates of each sample serum dilution. The results, expressed as percentages of coefficients of variation (%CVs), were calculated by the absorbances read for each point and are summarized in Tables 4 to 7.
TABLE 4.
Standard curve reproducibility of the ELISA used in this study with reference serum
| Run | Operator | No. of replicates/run | No. of points/run | Within run
|
Between run
|
||
|---|---|---|---|---|---|---|---|
| %CVa | %CVb | %CVa | %CVb | ||||
| Series 1 | 1 | 4 | 8 | 6.8 | 7.6 | ||
| 1 | 8.0 | 10 | |||||
| 2 | 7.8 | 8.1 | |||||
| 3 | 4.5 | 4.8 | |||||
| Series 2 | 2 | 4 | 8 | 9.6 | 13.9 | ||
| 1 | 6.4 | 6.9 | |||||
| 2 | 10.2 | 15.3 | |||||
| 3 | 11.2 | 17.7 | |||||
Average of the individual point %CVs calculated by the absorbancy of each point (overall %CV, 8.1).
Average of the individual point %CVs calculated by the absorbancy of each point after background (buffer) subtraction (overall %CV, 10.6).
TABLE 7.
Low-titer serum reproducibility for the ELISA used in this study with sera 513a and 516a
| Run | Operator | No. of replicates/run | No. of points/run | Within run
|
Between run
|
||
|---|---|---|---|---|---|---|---|
| %CVa | %CVb | %CVa | %CVb | ||||
| 513a | 1 | 4 | 1 | 10.5 | 23.8 | ||
| 1 | 5.8 | 25.7 | |||||
| 2 | 5.9 | 5.1 | |||||
| 3 | 4.5 | 13.1 | |||||
| 516a | |||||||
| Series 1 | 1 | 4 | 1 | 18.7 | 46.6 | ||
| 1 | 20.4 | 39.8 | |||||
| 2 | 15.9 | 23.6 | |||||
| 3 | 18.3 | 9.9 | |||||
| Series 2 | 30.1 | 34.6 | |||||
| 1 | 18.5 | 21.7 | |||||
| 2 | 18.9 | 22.4 | |||||
| 3 | 11.4 | 6.0 | |||||
Average of the individual point %CVs calculated by the absorbancy of each point.
Average of the individual point %CVs calculated by the absorbancy of each inhibited point.
For the standard curve (Table 4), the %CVs were calculated as the mean of the averages of the absorbances of all of the individual points before and after background (buffer) subtraction. For the quality control (Table 5), negative (Table 6), and low-titer (Table 7) sera, the %CVs were calculated as the averages of the absorbancies of the individual point both in the noninhibited and in the inhibited wells. Good reproducibility of data was observed, and the calculated %CVs were always in an acceptable ELISA range.
TABLE 5.
Quality control serum reproducibility of the ELISA used in this study with sera A143, A144, A146, and A148
| Run | Operator | No. of replicates/run | No. of points/run | Within run
|
Between run
|
Overall
|
|||
|---|---|---|---|---|---|---|---|---|---|
| %CVa | %CVb | %CVa | %CVb | %CVa | %CVb | ||||
| A143 | 25.6 | 26.1 | |||||||
| Series 1 | 1 | 6 | 1 | 17.1 | 24.3 | ||||
| 1 | 13.1 | 19.4 | |||||||
| 2 | 23.6 | 19.9 | |||||||
| 3 | 5.1 | 28.2 | |||||||
| Series 2 | 2 | 6 | 1 | 28.1 | 26.4 | ||||
| 1 | 13.1 | 16.3 | |||||||
| 2 | 38.5 | 17.1 | |||||||
| 3 | 8.4 | 10.9 | |||||||
| A144 | 17.3 | 17.5 | |||||||
| Series 1 | 1 | 6 | 1 | 11.7 | 12.1 | ||||
| 1 | 7.7 | 7.3 | |||||||
| 2 | 5.7 | 11 | |||||||
| 3 | 2.6 | 8.4 | |||||||
| Series 2 | 2 | 6 | 1 | 14.7 | 20.8 | ||||
| 1 | 12.4 | 18.2 | |||||||
| 2 | 11.8 | 17.3 | |||||||
| 3 | 5.7 | 19.8 | |||||||
| A146 | 13.1 | 17.2 | |||||||
| Series 1 | 1 | 6 | 1 | 10.2 | 19.8 | ||||
| 1 | 8.6 | 19.2 | |||||||
| 2 | 10.6 | 7.8 | |||||||
| 3 | 5.0 | 6.1 | |||||||
| Series 2 | 2 | 6 | 1 | 13.6 | 12.7 | ||||
| 1 | 5.4 | 9.4 | |||||||
| 2 | 9.4 | 12.8 | |||||||
| 3 | 5.7 | 15.2 | |||||||
| A148 | 12.3 | 16.8 | |||||||
| Series 1 | 1 | 6 | 1 | 8.4 | 18.4 | ||||
| 1 | 3.1 | 16.3 | |||||||
| 2 | 11.1 | 11 | |||||||
| 3 | 4.6 | 11.9 | |||||||
| Series 2 | 2 | 6 | 1 | 12.3 | 13.2 | ||||
| 1 | 10.0 | 14.4 | |||||||
| 2 | 14.4 | 9 | |||||||
| 3 | 3.7 | 8.8 | |||||||
Average of the individual point %CVs calculated by the absorbancy of each point.
Average of the individual point %CVs calculated by the absorbancy of each inhibited point.
TABLE 6.
Negative serum reproducibility for the ELISA used in this study with sera 501a, 502a, and 401a
| Run | Operator | No. of replicates/run | No. of points/run | Within run
|
Between run
|
||
|---|---|---|---|---|---|---|---|
| %CVa | %CVb | %CVa | %CVb | ||||
| 501a | 1 | 4 | 1 | 9.8 | 11.3 | ||
| 1 | 8.1 | 8.3 | |||||
| 2 | 10.6 | 17.6 | |||||
| 3 | 9.1 | 6.2 | |||||
| 502a | 1 | 4 | 1 | 8.6 | 10.0 | ||
| 1 | 9.8 | 5.3 | |||||
| 2 | 5.7 | 3.3 | |||||
| 3 | 10.8 | 12.3 | |||||
| 401a | 1 | 4 | 1 | 15.3 | 36.2 | ||
| 1 | 7.0 | 12.3 | |||||
| 2 | 25.5 | 49.4 | |||||
| 3 | 12.0 | 23.0 | |||||
Average of the individual point %CVs calculated by the absorbancy of each point.
Average of the individual point %CVs calculated by the absorbancy of each inhibited point.
The reference values of the quality control sera (Table 5) were calculated by using the values obtained from 15 different experiments performed by different operators on different days. The averages of the two replicates of each quality control serum were subtracted from the averages of the corresponding inhibited wells, the resulting absorbancy values were interpolated on the corresponding standard curves, and the values obtained were multiplied by the respective dilution factors and then expressed as micrograms of specific Igs per milliliter. The calculated values were very close to those determined by RABA reported in parentheses as follows). A143 serum gave a mean (±standard deviation) value of 1.05 ± 0.41 μg/ml (1.09 ± 0.32 μg/ml), that for A144 was 55.8 ± 16.8 μg/ml (51.90 ± 10.16 μg/ml), that for A146 was 10.1 ± 3.2 μg/ml (14.40 ± 2.59 μg/ml), and that for A148 was 3.57 ± 1.17 μg/ml (5.06 ± 1.36 μg/ml).
Comparison of competitive ELISA and RABA.
Competitive and noncompetitive ELISA results were compared to the RABA results by analyzing a panel of 407 serum samples which belong to different groups, as shown in Table 3. In Fig. 4, the ratios of noncompetitive (Fig. 4A) and competitive (Fig. 4B) ELISA values versus RABA values are illustrated as a function of calculated RABA antibody concentrations, confirming that the specific binding inhibition allowed a decrease in low-concentration sample overestimation. The overall competitive ELISA versus RABA correlation was good, with a correlation coefficient of 0.9660 (n = 407), indicating that no significant difference between competitive ELISA and RABA was found. The regression line of competitive ELISA versus RABA is y = 1.0045x − 0.1996, which is very close to the ideal line, because it is shown by a slope very close to 1.0 and an intercept close to origin. This correlation was well conserved (r = 0.9251) when calculated for samples with RABA values ≥2.0 μg/ml (n = 232) and lightly decreased (r = 0.7910) when calculated for samples with RABA values <2.0 μg/ml (n = 175).
The reproducibility of the competitive ELISA was also demonstrated with the subset of 96 serum samples being assayed in two laboratories in different countries. This measurement revealed correlation factors of 0.9770 and 0.9816, respectively, compared to those of the RABA. The correlation between the two ELISAs yielded a comparable factor of 0.9734.
Comparison of competitive ELISA and Phipps’ ELISA.
With the same subset of 96 serum samples, the competitive ELISA was also compared with the Phipps’ ELISA (16). This yielded correlation factors of 0.9376 and 0.9516 as found in comparison with the competitive ELISA which was performed in the two laboratories. The correlation factor with the RABA was 0.9547, which is even better than the factor (r = 0.917) reported by Phipps (16). Although the correlation found with the Phipps’ ELISA and RABA is quite good, it is lower than the factors observed with the competitive ELISA and RABA. When the Phipps’ ELISA was performed with the incubation times, buffer, and temperature used in the competitive ELISA, the correlation with the RABA and competitive ELISA improved only slightly. The overestimation of sera in the lower concentration range was still present in the results of the Phipps’ ELISA performed with the change in incubation conditions, as illustrated in Fig. 5.
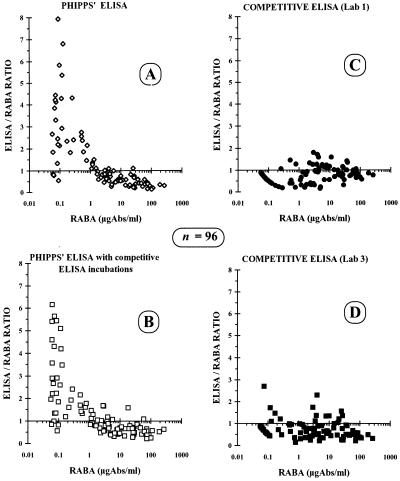
FIG. 5.
Scatter plots of Phipps’ ELISA (A), Phipps’ ELISA with competitive ELISA incubations (B), and competitive ELISA performed in two laboratories (C and D). The wider dispersion in the low data range (0.1 to 2.0 μg/ml) was confirmed with the noncompetitive ELISAs (A and B). The data dispersion was largely reduced with the competitive assay performed in two different laboratories (C and D). Abs, antibodies.
Conclusions.
The effectiveness of vaccination of individuals against Hib is conventionally determined with a RABA. This assay is time-consuming and involves the problems connected with handling radioactivity and waste. In 1990, an ELISA was proposed by Phipps et al. (16) that was clearly an improvement in term of assay feasibility and also paved the way to the replacement of RABA with ELISA for the estimation of anti-HibCPS antibody titers in human sera.
In our hands, human sera were found to give different background values, with day-to-day variability. The noncompetitive ELISA might overestimate the percentage of subjects with antibody titers of >1.0 μg/ml. This fact caused discrepancies in the evaluation of low-titer sera, and although the problem is essentially restricted to preimmune sera, it may not exclude a low response after vaccine administration. In these cases, a marked overestimation was introduced when the conventional indirect (noncompetitive) ELISA was used, because it was clearly shown in the low range of titers with the noncompetitive-competitive ELISA data correlation. To solve this problem, we decided to test the serum samples in duplicate: one well with dilution buffer and, in parallel, one well with dilution buffer containing an excess of purified HibCPS. To determine serum anti-HibCPS antibody concentrations, absorbance values in wells containing serum dilutions incubated with soluble HibCPS were subtracted as specific background from the corresponding values of the wells in which sera were diluted with buffer only. Antibody concentrations of sera were calculated from the standard curve and were expressed in micrograms per milliliter. This approach has been shown to be useful in obtaining a very good correlation between ELISA and RABA values on a panel of sera from different clinical trials representative of a wide range of antibody levels with various isotype and subclass compositions. The correlation of the competitive ELISA with RABA was better than that found for the ELISA from Phipps. Moreover, the overestimation of sera in the lower concentration range was still present when the Phipps’ ELISA was performed under the incubation conditions of the competitive ELISA, indicating that competition with free HibCPS is necessary to avoid this false-positive binding.
The reliability, ruggedness, and reproducibility of the competitive ELISA and the absence of background, plus the good correlation and regression line with RABA, demonstrate that the proposed competitive ELISA can replace RABA for measuring levels of anti-HibCPS total Ig in vaccinated populations.
ACKNOWLEDGMENTS
We are indebted to Dan Granoff (Chiron Vaccines, Emeryville, Calif., and Children’s Hospital Oakland Research Institute, Oakland, Calif.) and Giuseppe Del Giudice (Chiron S.p.A., Siena, Italy) for helpful discussion and suggestions.
REFERENCES
- 1.Anderson P. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1984;149:1034. doi: 10.1093/infdis/149.6.1034. [DOI] [PubMed] [Google Scholar]
- 2.Anderson P. Intrinsic tritium labelling of the capsular polysaccharide antigen of Haemophilus influenzae type b. J Immunol. 1978;120:866–870. [PubMed] [Google Scholar]
- 3.Anderson P, Insel R A, Porcelli S, Ward J I. Immunochemical variables affecting radioantigen-binding assay of antibody to Haemophilus influenzae type b capsular polysaccharide in children’s sera. J Infect Dis. 1987;156:582–590. doi: 10.1093/infdis/156.4.583. [DOI] [PubMed] [Google Scholar]
- 4.Ashwell G. Colorimetric analysis of sugar. Methods Enzymol. 1957;3:73–105. [Google Scholar]
- 5.Dajani A S, Asmar B I, Thirumoorthi M C. Systemic Haemophilus influenzae disease: an overview. J Pediatr. 1979;14:355–364. doi: 10.1016/s0022-3476(79)80571-5. [DOI] [PubMed] [Google Scholar]
- 6.Farr R S. A quantitative immunochemical measure of the primary interaction between I*BSA and antibody. J Infect Dis. 1958;103:239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- 7.Gruss A D, Spier-Milch I B, Gotschlich E C. A method for a radioimmunoassay using microtiter plates allowing simultaneous determination of antibody to two non cross reactive antigens. Immunochemistry. 1978;15:777–780. doi: 10.1016/0161-5890(78)90108-6. [DOI] [PubMed] [Google Scholar]
- 8.Habeeb A F S A. Determination of free amino groups in proteins by trinitrobenzensulfonic acid. Anal Biochem. 1966;14:328–338. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- 9.Kabat E A, Mayer M M. Experimental immunochemistry. Springfield, Ill: Charles C. Thomas, Publisher; 1967. p. 531. [Google Scholar]
- 10.Kayhty H. Difficulties in establishing a serological correlate of protection after immunization with Haemophilus influenzae conjugate vaccines. Biologicals. 1994;22:397–402. doi: 10.1006/biol.1994.1062. [DOI] [PubMed] [Google Scholar]
- 11.Kayhty H, Peltola H, Karanko V, Makela P H. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983;147:1100. doi: 10.1093/infdis/147.6.1100. [DOI] [PubMed] [Google Scholar]
- 12.Kuo J S, Monji N, Schwalbe R S, McCoy D W. A radioactive antigen-binding assay for the measurement of antibody to Haemophilus influenzae type b capsular polysaccharide. J Immunol Methods. 1981;43:35–47. doi: 10.1016/0022-1759(81)90034-x. [DOI] [PubMed] [Google Scholar]
- 13.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 14.Makela P H, Peltola H, Kayhty H, Jousimics H, Pettay O, Rouslahti E, Sivonen A, Renkonen O V. Polysaccharide vaccines of group A Neisseria meningitidis and Haemophilus influenzae type b: a field trial in Finland. J Infect Dis. 1977;136:843–850. doi: 10.1093/infdis/136.supplement.s43. [DOI] [PubMed] [Google Scholar]
- 15.Peltola H, Kayhty H, Sivonen A, Makela P H. Haemophilus influenzae type b polysaccharide vaccine in children: a double-blind study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics. 1977;60:730–737. [PubMed] [Google Scholar]
- 16.Phipps D C, West J, Eby R, Koster M, Madore D V, Quataert S A. An ELISA employing a Haemophilus influenzae type b oligosaccharide-human serum albumin conjugate correlates with the radioantigen binding assay. J Immunol Methods. 1990;135:121–128. doi: 10.1016/0022-1759(90)90264-v. [DOI] [PubMed] [Google Scholar]
- 17.Robbins J B, Parke J C, Jr, Scheneerson R, Whisnant J K. Quantitative measurement of natural and immunization-induced Haemophilus influenzae type b capsular polysaccharide antibodies. Pediatr Res. 1973;7:103–110. doi: 10.1203/00006450-197303000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Schneerson R, Rodrigues L P, Parke J C, Jr, Robbins J B. Immunity to disease caused by Haemophilus influenzae type b. II. Specificity and some biologic characteristics of natural, infection-acquired and immunization-induced antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Immunol. 1971;107:1081–1089. [PubMed] [Google Scholar]
- 19.Shapiro E D, Ward J I. The epidemiology and prevention of disease caused by Haemophilus influenzae type b. Epidemiol Rev. 1991;13:113–142. doi: 10.1093/oxfordjournals.epirev.a036066. [DOI] [PubMed] [Google Scholar]