Abstract
BACKGROUND
Childhood cardiovascular risk factors predict subclinical adult cardiovascular disease, but links to clinical events are unclear.
METHODS
In a prospective cohort study involving participants in the International Childhood Cardiovascular Cohort (i3C) Consortium, we evaluated whether childhood risk factors (at the ages of 3 to 19 years) were associated with cardiovascular events in adulthood after a mean follow-up of 35 years. Body-mass index, systolic blood pressure, total cholesterol level, triglyceride level, and youth smoking were analyzed with the use of i3C-derived age- and sex-specific z scores and with a combined-risk z score that was calculated as the unweighted mean of the five risk z scores. An algebraically comparable adult combined-risk z score (before any cardiovascular event) was analyzed jointly with the childhood risk factors. Study outcomes were fatal cardiovascular events and fatal or nonfatal cardiovascular events, and analyses were performed after multiple imputation with the use of proportional-hazards regression.
RESULTS
In the analysis of 319 fatal cardiovascular events that occurred among 38,589 participants (49.7% male and 15.0% Black; mean [±SD] age at childhood visits, 11.8±3.1 years), the hazard ratios for a fatal cardiovascular event in adulthood ranged from 1.30 (95% confidence interval [CI], 1.14 to 1.47) per unit increase in the z score for total cholesterol level to 1.61 (95% CI, 1.21 to 2.13) for youth smoking (yes vs. no). The hazard ratio for a fatal cardiovascular event with respect to the combined-risk z score was 2.71 (95% CI, 2.23 to 3.29) per unit increase. The hazard ratios and their 95% confidence intervals in the analyses of fatal cardiovascular events were similar to those in the analyses of 779 fatal or nonfatal cardiovascular events that occurred among 20,656 participants who could be evaluated for this outcome. In the analysis of 115 fatal cardiovascular events that occurred in a subgroup of 13,401 participants (31.0±5.6 years of age at the adult measurement) who had data on adult risk factors, the adjusted hazard ratio with respect to the childhood combined-risk z score was 3.54 (95% CI, 2.57 to 4.87) per unit increase, and the mutually adjusted hazard ratio with respect to the change in the combined-risk z score from childhood to adulthood was 2.88 (95% CI, 2.06 to 4.05) per unit increase. The results were similar in the analysis of 524 fatal or nonfatal cardiovascular events.
CONCLUSIONS
In this prospective cohort study, childhood risk factors and the change in the combined-risk z score between childhood and adulthood were associated with cardiovascular events in midlife. (Funded by the National Institutes of Health.)
Prevention of cardiovascular disease remains a major public health issue, with well-documented associations between cardiovascular risk factors in adulthood and cardiovascular events.1 Despite the interest in childhood risk factors and subsequent adult cardiovascular disease, as recently reviewed,2,3 findings from longitudinal studies that begin with the evaluation of childhood risk factors have generally been restricted to associations with subclinical disease in adulthood. The possibility of extending the findings to include associations with adult cardiovascular events has been hampered by a lack of cohorts with available comprehensive childhood data on anthropometric measures, blood pressure, and laboratory values and with follow-up conducted up to ages at which cardiovascular events become prevalent.
The International Childhood Cardiovascular Cohort (i3C) Consortium4,5 includes seven cohorts in Australia, Finland, and the United States, in which data on cardiovascular risk factors from early childhood through adolescence have been collected and adult cardiovascular events have been adjudicated. In the current study, we used these data to examine the development of cardiovascular disease over the life course and test our hypothesis that traditional cardiovascular risk factors in childhood are associated with the subsequent development of adult cardiovascular events. Details on the study hypotheses are provided in Section S1 in Supplementary Appendix 1, available with the full text of this article at NEJM.org.
METHODS
STUDY DESIGN AND OVERSIGHT
A total of 42,324 participants 3 to 19 years of age were enrolled in the seven i3C Consortium cohorts from the 1970s through the 1990s; of these participants, 40,648 had identifying information for follow-up and were included in the sampling frame (Fig. S1 in Supplementary Appendix 1).4 This study was approved by the institutional review board at each site of the seven cohorts. Written parental permission and oral assent by the participant were obtained for childhood visits, written informed consent was obtained from the participant for in-person adult visits, and oral consent under waiver of documentation of consent was obtained for the recent follow-up questionnaire. Full details of the study methods are provided in Section S2.
Our study focused on the five risk factors most often evaluated in childhood and adolescence: the body-mass index, systolic blood pressure, total cholesterol level, triglyceride level, and youth smoking. Triglyceride levels were transformed by means of the natural logarithm (ln[triglycerides]). Data on the cardiovascular risk factors were harmonized across the seven cohorts into a single database (114,476 visits, with 1 to 19 visits per participant). Because independent protocols, with variable schedules for clinic visits that were conducted at various participant ages, were used for each cohort, not every study measure was assessed in every cohort, in every participant within a cohort, or in every participant at every age.5 Age, sex, parent-reported race (which was updated if the participant was seen in adulthood), height, weight, and systolic blood pressure (measured by mercury sphygmomanometry) were assessed prospectively at the clinic visits; fasting levels of plasma or serum cholesterol and triglycerides were measured by means of standard methods.6 The education levels of the parents and participants were obtained at childhood and adult visits.
From 2015 through 2019, the i3C Consortium investigators conducted a coordinated study to locate and survey participants and search national death indexes for the participants who were not located (Figs. S1 and S2).5 Fatal cardiovascular events in all the cohorts were classified according to the coded causes of death in the International Classification of Diseases (ICD), versions 9 and 10 (Table S1). Finnish participants were followed for nonfatal cardiovascular events through December 31, 2017, with the use of the Finnish national medical registry, and the events were classified according to the same version of the ICD that was used for the classification of deaths. U.S. and Australian adult participants who had been successfully located reported any cardiovascular event that had occurred (Tables S2 and S3), and medical records were requested for adjudication of the participant reports. The medical records were reviewed by a physician committee that was unaware of the study data from the participants, and each reported event was classified as a confirmed cardiovascular event, not a cardiovascular event, or not possible to adjudicate (Section S2B). Nonfatal cardiovascular events included the first instance of adjudicated myocardial infarction, stroke, transient ischemic attack, ischemic heart failure, angina, peripheral artery disease, carotid intervention, abdominal aortic aneurysm, or coronary revascularization.
STATISTICAL ANALYSIS
Full details of the statistical methods are provided in Section S3 and in the statistical analysis plan in Supplementary Appendix 2, available at NEJM.org. Because of the potential for bias due to loss to follow-up, fatal cardiovascular events were analyzed separately from the composite outcome of fatal or nonfatal cardiovascular events. There were 319 fatal cardiovascular events among the 38,589 participants (95% of the sampling frame) who could be classified as alive and located, deceased with known cause, or searched for and not found in the death indexes and thus presumed to be alive. The analysis of fatal or nonfatal cardiovascular events included 779 adjudicated nonfatal events and 784 imputed nonfatal events (the mean number across imputations) for persons who were not located or who reported a cardiovascular event that was not possible to be adjudicated. Among 13,401 participants with adult measurements before any cardiovascular event, there were 115 fatal cardiovascular events and a mean of 524 fatal or nonfatal events (406 observed) across imputations.
Because of age-related developmental changes, childhood risk factors at each visit were normalized to z scores within the i3C Consortium, which were calculated with the mean values (with standard deviations) of the study variables, stratified according to age and sex (Section S3A and Tables S4 and S5). The resulting i3C-derived z scores for each participant were then averaged across their childhood and adolescent measurements (obtained at the ages of 3 to 19 years) to obtain a single mean z score of childhood risk per person. The classification of youth smoking was based on reports by the participants during childhood,7 augmented by adult recall of the smoking initiation date, and was analyzed as a dichotomous variable (yes vs. no). An a priori combined-risk z score was calculated as the unweighted mean of the z scores of the four childhood risk factors plus youth smoking, which was included in the calculation as either 2 (a high-risk value in terms of z score units) for smoking or 0 (average risk) for nonsmoking. The use of this combined-risk z score addresses our hypothesis that all five risk factors predict future events, without the estimation of risk-factor weights. Individual risk factors and the combined-risk z score were analyzed as continuous measures. In addition, we examined childhood risk factors using thresholds for standard clinical categories,8–10 dividing the clinically normal category into low-normal and high-normal groups (Table S6). Adult combined-risk z scores were calculated with the same algebraic procedures and risk factors as those used for the childhood combined-risk z scores (Section S3B and Tables S7 and S8).
All primary analyses were performed after multiple imputation of missing values by means of chained equations with fully conditional specification (10 replications) in PC-SAS software (version 9.4, SAS Institute); data were assumed to be missing at random.11 Imputation was conducted in three phases with the use of subsampling methods (Section S3C and Table S9).12 In phase 1, multiple imputation was applied for missing data on childhood risk factors and events among 38,589 participants; in phase 2, for nonfatal events that could not be adjudicated among 1360 participants who reported a nonfatal event; and in phase 3, for missing ages at which the imputed event occurred among 779 adjudicated events and a mean of 784 imputed events. All proportional-hazards regression analyses were conducted with the use of adult age as the time axis and noncardiovascular mortality as a competing risk13 and were adjusted for sex, race, cohort indicator, mean childhood age at and mean calendar year of childhood measurement, and parental education level. The widths of the 95% confidence intervals were not adjusted for multiple comparisons.
Adequacy of the linearity assumption was visualized with the use of restricted cubic splines and by examining categories of z score units in widths of 0.5 with extreme higher and lower categories open-ended. The proportionality assumption was assessed with the addition of the interaction term between the risk factor and age transformed by the natural logarithm (risk factor★ln[age]). When hazards varied according to participant age during follow-up, we present hazard ratios for events in participants younger than the median age of 47.7 years or 47.7 years of age or older. Interactions with sex, race, and age group of childhood measurement (3 to 11 years vs. 12 to 19 years) were estimated. We examined the predictive power of the childhood combined-risk z score, accounting for adult risk factors using three analytic models, one in which the adult combined-risk z score was considered alone, one in which the childhood combined-risk z score was paired with the adult combined-risk z score, and one in which the childhood combined-risk z score was paired with the change in combined-risk z score between childhood and adulthood.
RESULTS
PARTICIPANTS
A total of 38,589 participants were included in the overall sample; 19,168 (49.7%) were male, 5792 (15.0%) were Black, and the mean (±SD) age at which the participant was seen during childhood was 11.8±3.1 years (Table 1). The mean age of the participants at the time of their cardiovascular event was 47.0±8.0 years. Participants with cardiovascular events were older, more likely to be male, and had a lower parental and personal education level than those without cardiovascular events. Correlations among childhood risk factors ranged from −0.002 to 0.35 (Section S4 and Table S10), and within-person correlations among childhood, adolescence, and adulthood ranged from 0.40 to 0.84 (Tables S11 and S12). The mean combined-risk z score was 0.16±0.49 (Table S13).
Table 1.
Demographic Characteristics of the Participants in the Analysis Sample, Stratified According to Cardiovascular Outcome.*
| Characteristic† | Analysis Sample | Fatal Cardiovascular Events | Fatal or Nonfatal Cardiovascular Events‡ | ||
|---|---|---|---|---|---|
| Participants with No Event | Participants with Event | Participants with No Event | Participants with Event | ||
| Participants — no./total no. (%) | 38,589/38,589 (100) | 38,270/38,589 (99.2) | 319/38,589 (0.8) | 19,877/20,656 (96.2) | 779/20,656 (3.8) |
| Sex — no./total no. (%) | |||||
| Male | 19,168/38,589 (49.7) | 18,941/19,168 (98.8) | 227/19,168 (1.2) | 8,972/9,474 (94.7) | 502/9,474 (5.3) |
| Female | 19,421/38,589 (50.3) | 19,329/19,421 (99.5) | 92/19,421 (0.5) | 10,905/11,182 (97.5) | 277/11,182 (2.5) |
| Race — no./total no. (%)§ | |||||
| Black | 5,792/38,589 (15.0) | 5,731/5,792 (98.9) | 61/5,792 (1.1) | 2,486/2,592 (95.9) | 106/2,592 (4.1) |
| White or other than Black orWhite | 32,797/38,589 (85.0) | 32,539/32,797 (99.2) | 258/32,797 (0.8) | 17,391/18,064 (96.3) | 673/18,064 (3.7) |
| Cohort, country, years of childhood visits — no./total no. (%) | |||||
| Bogalusa Heart Study, U.S., 1973–1994 | 11,737/38,589 (30.4) | 11,623/11,737 (99.0) | 114/11,737 (1.0) | 4,058/4,253 (95.4) | 195/4,253 (4.6) |
| Childhood Determinants of Adult Health, AUS, 1985 | 8,426/38,589 (21.8) | 8,414/8,426 (99.9) | 12/8,426 (0.1) | 3,103/3,130 (99.1) | 27/3,130 (0.9) |
| Minnesota Childhood Cardiovascular Cohorts, U.S., 1978–1996 | 2,032/38,589 (5.3) | 2,023/2,032 (99.6) | 9/2,032 (0.4) | 1,218/1,232 (98.9) | 14/1,232 (1.1) |
| Muscatine Study, U.S., 1970–1981 | 9,842/38,589 (25.5) | 9,699/9,842 (98.5) | 143/9,842 (1.5) | 6,577/6,978 (94.3) | 401/6,978 (5.7) |
| NHLBI Growth and Health Study, U.S., 1987–1996 | 820/38,589 (2.1) | 818/820 (99.8) | 2/820 (0.2) | 509/513 (99.2) | 4/513 (0.8) |
| Princeton Lipid Research Study, U.S., 1973–1978 | 2,201/38,589 (5.7) | 2,173/2,201 (98.7) | 28/2,201 (1.3) | 954/1,019 (93.6) | 65/1,019 (6.4) |
| Cardiovascular Risk in Young Finns Study, FIN, 1980–1986 | 3,531/38,589 (9.2) | 3,520/3,531 (99.7) | 11/3,531 (0.3) | 3,458/3,531 (97.9) | 73/3,531 (2.1) |
| Birth year | 1969.6+7.4 | 1969.7+7.4 | 1963.8+6.2 | 1968.3+7.6 | 1963.0+5.9 |
| Mean calendaryearofchildhood visit¶ | 1981.5+6.3 | 1981.5+6.3 | 1976.7+4.8 | 1980.9+6.4 | 1976.3+4.6 |
| Mean age at childhood visits — yr‖ | 11.8+3.1 | 11.8+3.1 | 12.9+2.9 | 12.3+3.1 | 13.3+2.8 |
| Attainable age in 2016 — yr | 46.4+7.4 | 46.3+7.4 | 52.2+6.2 | 47.4+7.6 | 53.0+5.9 |
| Age at time of event — yr** | NA | NA | 47.0+8.0 | NA | 47.1+7.4 |
| Parental education level — no./total no. (%) | |||||
| Less than high school degree | 5,051/23,401 (21.6) | 5,011/5,051 (99.2) | 40/5,051 (0.8) | 3,284/3,397 (96.7) | 113/3,397 (3.3) |
| High school degree | 7,501/23,401 (32.1) | 7,426/7,501 (99.0) | 75/7,501 (1.0) | 4,303/4,504 (95.5) | 201/4,504 (4.5) |
| Higherthan high school degree but no college degree | 5,104/23,401 (21.8) | 5,080/5,104 (99.5) | 24/5,104 (0.5) | 3,261/3,340 (97.6) | 79/3,340 (2.4) |
| College degree or higher | 5,745/23,401 (24.6) | 5,727/5,745 (99.7) | 18/5,745 (0.3) | 3,664/3,728 (98.3) | 64/3,728 (1.7) |
| Participant education level — no./total no. (%) | |||||
| Less than high school degree | 1,469/21,473 (6.8) | 1,460/1,469 (99.4) | 9/1,469 (0.6) | 835/887 (94.1) | 52/887 (5.9) |
| High school degree | 4,952/21,473 (23.1) | 4,910/4,952 (99.2) | 42/4,952 (0.8) | 4,114/4,292 (95.9) | 178/4,292 (4.1) |
| Higher than high school degree but no college degree | 7,088/21,473 (33.0) | 7,074/7,088 (99.8) | 14/7,088 (0.2) | 6,021/6,185 (97.3) | 164/6,185 (2.7) |
| College degree | 5,187/21,473 (24.2) | 5,173/5,187 (99.7) | 14/5,187 (0.3) | 4,495/4,592 (97.9) | 97/4,592 (2.1) |
| Higher than college degree | 2,777/21,473 (12.9) | 2,774/2,777 (99.9) | 3/2,777 (0.1) | 2,501/2,534 (98.7) | 33/2,534 (1.3) |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding. AUS denotes Australia, FIN Finland, NA not applicable, and NHLBI National Heart, Lung, and Blood Institute.
There were no missing data for birth year, mean calendar year of childhood visits, mean age at childhood visits, or attainable age in 2016.
Data are presented for the 20,656 participants who could be evaluated for fatal or nonfatal cardiovascular events. A comparison between observed and imputed values is provided in Table S26 in Supplementary Appendix 1. Nonfatal cardiovascular events included the first instance of adjudicated myocardial infarction, stroke, transient ischemic attack, ischemic heart failure, angina, peripheral artery disease, carotid intervention, abdominal aortic aneurysm, or coronary revascularization.
Race was reported by the parent and was updated if the participant was seen in adulthood.
The mean calendar year of childhood visits was the mean calendar year across all available childhood visits by the participant from the age of 3 to 19 years.
The mean age at childhood visits was the mean age of the participant across all available childhood visits from the age of 3 to 19 years.
Age at time of event was assessed among 319 participants who had a fatal event and among 779 participants who had a fatal or nonfatal event.
ADULT CARDIOVASCULAR EVENTS
Hazard ratios for a fatal cardiovascular event in adulthood with respect to the risk-factor z scores (Table 2) ranged from 1.30 (95% confidence interval [CI], 1.14 to 1.47) per unit increase in the z score for the total cholesterol level to 1.61 (95% CI, 1.21 to 2.13) for youth smoking (yes vs. no). The hazard ratio for a fatal cardiovascular event in adulthood with respect to the combined-risk z score was 2.71 (95% CI, 2.23 to 3.29) per unit increase, and the hazard ratio for a fatal or nonfatal cardiovascular event in adulthood was 2.75 (95% CI, 2.48 to 3.06) per unit increase. The hazard ratio with respect to the combined-risk z score showed some attenuation for fatal or nonfatal events at older adult ages (Fig. 1 and Table S14). None of the interaction terms of childhood age group (3 to 11 years vs. 12 to 19 years), race, or sex were notable (Tables S15, S16, and S17). The childhood risk score was also positively associated with total mortality (Table S18).
Table 2.
Hazard Ratios for Adult Cardiovascular Events According to Childhood, Adult, or Childhood plus Adult Risk Scores.*
| Variable | Hazard Ratio (95% Ci)† | |
|---|---|---|
| Fatal Event | Fatal or Nonfatal Event | |
| Childhood risk factor | ||
| Youth smoking: yes vs. no | 1.61 (1.21–2.13) | 1.70 (1.49–1.93) |
| z Score for body-mass index | 1.44 (1.33–1.57) | 1.45 (1.38–1.53)‡ |
| z Score for systolic blood pressure | 1.34 (1.19–1.50) | 1.33 (1.24–1.42) |
| z Score for ln(triglycerides) | 1.50 (1.33–1.70) | 1.45 (1.34–1.56) |
| z Score for total cholesterol level | 1.30 (1.14–1.47) | 1.31 (1.22–1.42) |
| Combined-risk z score§ | 2.71 (2.23–3.29) | 2.75 (2.48–3.06)‡ |
| Subgroup with risk factors evaluated in adulthood | ||
| Adult combined-risk z score§ | 3.20 (2.46–4.17) | 2.88 (2.47–3.35)‡ |
| Childhood plus adult combined-risk z scored¶ | ||
| Childhood combined-risk z score | 1.23 (0.83–1.82) | 1.25 (1.03–1.52) |
| Adult combined-risk z score | 2.88 (2.06–4.05) | 2.58 (2.15–3.09) |
| Childhood combined-risk z score plus change in combined-risk z score from childhood to adulthood¶ | ||
| Childhood combined-risk z score | 3.54 (2.57–4.87) | 3.21 (2.69–3.85)‡ |
| Change in combined-risk z score from childhood to adulthood | 2.88 (2.06–4.05) | 2.58 (2.15–3.09) |
All models were adjusted for sex, Black race, cohort, mean age at and calendar year of childhood visits, and parental education level. Hazard ratios and confidence intervals were based on data imputed to represent all 38,589 childhood participants. The imputation was based on 319 fatal cardiovascular events that occurred among 38,589 participants and 779 observed fatal or nonfatal cardiovascular events that occurred among 20,656 participants. The widths of the confidence intervals were not adjusted for multiple comparisons. ln(triglycerides) denotes the triglyceride level transformed by means of the natural logarithm.
Hazard ratios are reported per unit increase in the z score for the risk factor, except for the hazard ratio with respect to youth smoking, which is reported for the comparison of “yes” and “no.”
In the testing of the proportionality assumption, which was performed with the addition of the interaction term between the risk factor and age (transformed by the natural logarithm), the 95% confidence interval excluded 1, which means that the proportional-hazards assumption might have been violated. A separate analysis of early as compared with late events is provided in Section S4 and Table S14 in Supplementary Appendix 1.
Hazard ratios and confidence intervals were based on data imputed to represent all 13,401 participants in whom both childhood and adult risk-factor data were available. The imputation was based on 115 fatal cardiovascular events in 13,401 participants and 406 observed fatal or nonfatal cardiovascular events in 11,156 participants. The combined-risk z score is the mean of the z scores for body-mass index, systolic blood pressure, ln(triglycerides), and total cholesterol, with the youth smoking dichotomous variables coded as 0 z score units for no and 2 z score units for yes. The combined-risk z score was calculated consistently in childhood and adulthood.
These two models are alternate ways of expressing the same information in a regression model through rearrangement of the terms of the model. Algebraically, the adult minus child term in the second model is the same as the adult term in the first, whereas the child term in the second model is the same as the child term plus the adult term in the first (Section S3C).
Figure 1. Hazard Ratios for Cardiovascular Events at Younger and Older Ages.
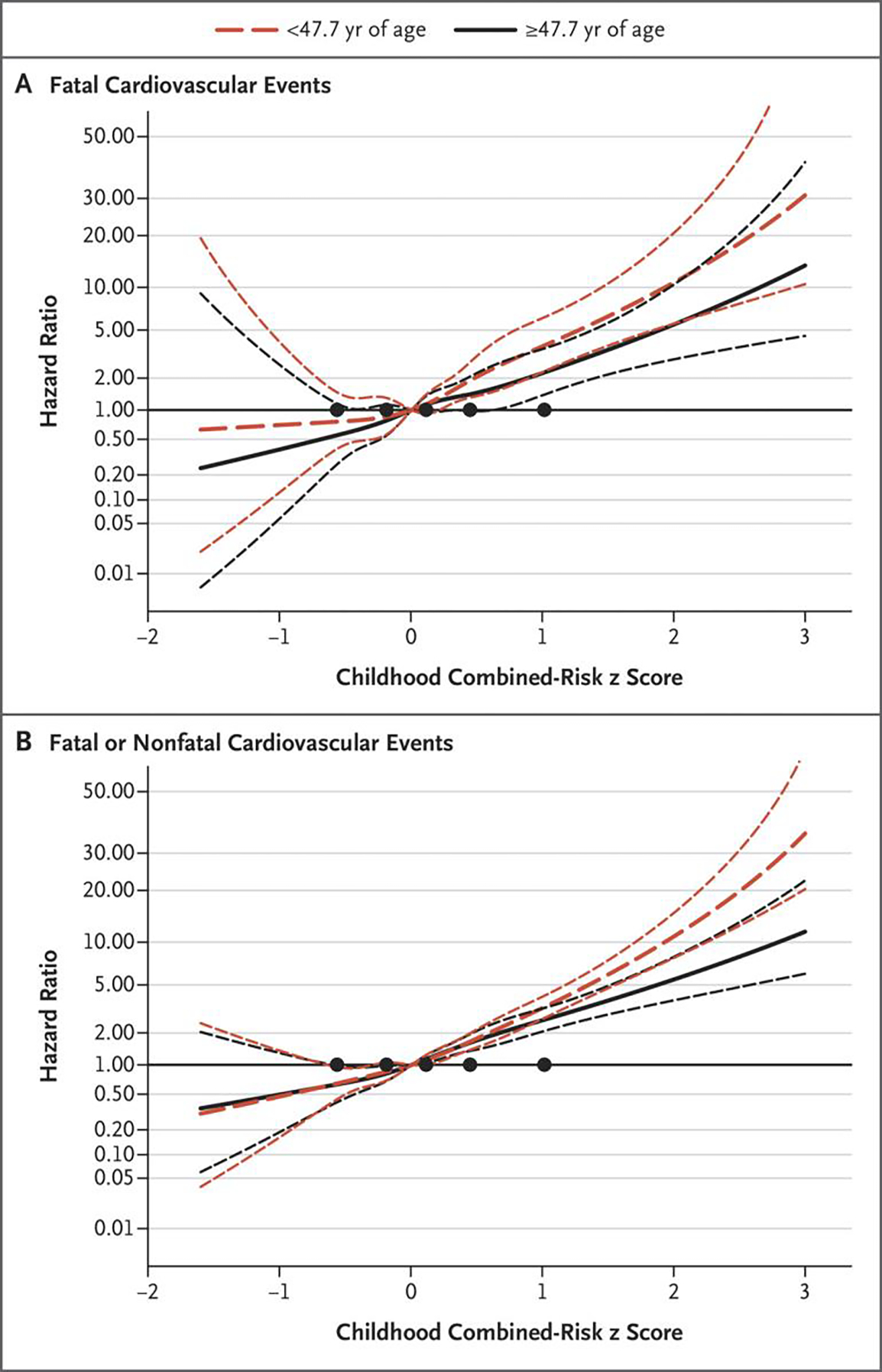
Panel A shows the hazard ratios for fatal cardiovascular events, and Panel B shows the hazard ratios for fatal or nonfatal cardiovascular events. The spline of the hazard ratio is presented on a logarithmic scale across the distribution of the childhood combined-risk z scores, with 95% confidence intervals (shorter dashed lines). Younger age (<47.7 years) includes all the participants, among whom there were 157 fatal events and a mean of 797 fatal or nonfatal events across imputations. The older age group includes only the participants who were followed and had no event or had events at or after 47.7 years of age (a total of 18,352 participants, among whom 162 had a fatal cardiovascular event and 1049 either had a fatal event due to other causes or were not followed past the age of 47.6 years; the 17,141 remaining participants had a mean of 766 fatal or nonfatal events across imputations). The black circles indicate knots placed at the 5th, 25th, 50th, 75th, and 95th percentiles of the combined-risk z score.
The study measures in adulthood were evaluated in the participants at a mean age of 31.0±5.6 years. In the analyses involving participants who had data on the study measures in both childhood and adulthood (Table S19), the adult combined-risk z score was associated with adult cardiovascular events, both alone and when paired with the childhood combined-risk z score (Table 2). The childhood combined-risk z score, when paired with the adult combined-risk z score, was attenuated and remained independently associated only with fatal or nonfatal cardiovascular events. In the analysis including the childhood combined-risk z score and the change in the combined-risk z score from childhood to adulthood, both predictors were associated with fatal cardiovascular events and fatal or nonfatal cardiovascular events, with the hazard ratio lower for the prediction of events at older adults ages than at younger adult ages. Between 30% and 50% of the participants in each quartile of childhood combined-risk z score remained in the same quartile of combined-risk z score in adulthood (Table S20).
RISK-FACTOR CATEGORIES AND THRESHOLDS
With the use of “no” (for youth smoking), low-normal (for body-mass index and systolic blood pressure), and low-acceptable (for triglyceride level and total cholesterol level) as references in the standard clinical categories currently used for the risk factors, the gradient of the hazard ratio for cardiovascular events, whether fatal only or fatal or nonfatal, was apparent across the clinical categories for each risk factor (Table 3). There was a higher risk observed not only among the participants in the highest category of the risk-factor level but also — in the analysis of fatal or nonfatal events — among those in the high-normal or high-acceptable categories for the body-mass index, systolic blood pressure, and triglyceride level. The gradient of risk across the categories of the combined-risk z score was steeper than the gradient of risk across the categories of any of the individual risk factors (Table S21); among the participants with a combined-risk z score of 0 or greater (23,103 of 38,589 [59.9%]), the risk of adult cardiovascular events was 2 to 9 times as high as the risk among those in the lowest z score category (a z score of less than −0.5), with the risk increasing with age in the life-table analysis (Fig. 2 and Figs. S3 through S6). Several sensitivity analyses were conducted to evaluate the effect of loss to follow-up (Section S5 and Tables S22 through S25), the reasonableness of the imputation results (Tables S26 and S27), and the differences among the cohorts (Figs. S7 and S8 and Tables S28 and S29), which did not materially change the findings.
Table 3.
Hazard Ratios for Adult Cardiovascular Events According to Clinical Categories of Childhood Risk-Factor Levels.*
| Risk Factor | Fatal Cardiovascular Events | Fatal or Nonfatal Cardiovascular Events | ||||
|---|---|---|---|---|---|---|
| Participants | Participants with Event | Hazard Ratio (95% Cl)† | Participants | Participants with Event | Hazard Ratio (95% Cl)† | |
| no./total no. (%) | no./total no. (%) | |||||
| Youth smoking | ||||||
| No | 15,922/25,471 (62.5) | 75/15,922 (0.5) | Reference | 12,301/19,520 (63.0) | 283/12,301 (2.3) | Reference |
| Yes | 9,549/25,471 (37.5) | 82/9,549 (0.9) | 1.61 (1.21–2.13) | 7,219/19,520 (37.0) | 327/7,219 (4.5) | 1.70 (1.49–1.93) |
| Body-mass index | ||||||
| Low-normal | 14,857/38,451 (38.6) | 96/14,857 (0.6) | Reference | 8,048/20,599 (39.1) | 237/8,048 (2.9) | Reference |
| High-normal | 16,055/38,451 (41.8) | 107/16,055 (0.7) | 1.01 (0.77–1.33) | 8,688/20,599 (42.2) | 280/8,688 (3.2) | 1.19 (1.01–1.41) |
| Overweight | 4,720/38,451 (12.3) | 49/4,720 (1.0) | 1.61 (1.14–2.27) | 2,423/20,599 (11.8) | 134/2,423 (5.5) | 1.92 (1.62–2.27) |
| Obese | 2,819/38,451 (7.3) | 61/2,819 (2.2) | 3.34 (2.42—4.60) | 1,440/20,599 (7.0) | 117/1,440 (8.1) | 3.39 (2.73—4.21) |
| Systolic blood pressure | ||||||
| Low-normal | 17,458/32,339 (54.0) | 125/17,458 (0.7) | Reference | 8,879/18,288 (48.6) | 288/8,879 (3.2) | Reference |
| High-normal | 12,709/32,339 (39.3) | 141/12,709 (1.1) | 1.59 (1.24–2.04) | 7,942/18,288 (43.4) | 346/7,942 (4.4) | 1.46 (1.26–1.69) |
| Prehypertension | 1,009/32,339 (3.1) | 16/1,009 (1.6) | 2.24 (1.31–3.35) | 666/18,288 (3.6) | 67/666 (10.1) | 1.99 (1.48–2.67) |
| Hypertension | 1,163/32,339 (3.6) | 20/1,163 (1.7) | 2.04 (1.24–3.35) | 801/18,288 (4.4) | 67/801 (8.4) | 2.31 (1.74–3.07) |
| Triglyceride level | ||||||
| Low-acceptable | 6,209/29,221 (21.2) | 37/6,209 (0.6) | Reference | 3,226/16,674 (19.3) | 92/3,226 (2.9) | Reference |
| High-acceptable | 14,045/29,221 (48.1) | 127/14,045 (0.9) | 1.42 (0.84–2.39) | 8,175/16,674 (49.0) | 326/8,175 (4.0) | 1.34 (1.05–1.70) |
| Borderline-high | 5,680/29,221 (19.4) | 67/5,680 (1.2) | 1.91 (1.17–3.12) | 3,396/16,674 (20.4) | 174/3,396 (5.1) | 1.69 (1.28–2.24) |
| High | 3,287/29,221 (11.2) | 62/3,287 (1.9) | 2.75 (1.71—4.42) | 1,877/16,674 (11.3) | 135/1,877 (7.2) | 2.47 (1.89–3.24) |
| Total cholesterol level | ||||||
| Low-acceptable | 5,413/29,495 (18.4) | 55/5,413 (1.0) | Reference | 2,888/16,819 (17.2) | 130/2,888 (4.5) | Reference |
| High-acceptable | 12,289/29,495 (41.7) | 121/12,289 (1.0) | 1.12 (0.81–1.54) | 6,557/16,819 (39.0) | 284/6,557 (4.3) | 1.15 (0.94–1.40) |
| Borderline-high | 7,925/29,495 (26.9) | 79/7,925 (1.0) | 1.43 (1.01–2.03) | 4,615/16,819 (27.4) | 200/4,615 (4.3) | 1.50 (1.23–1.83) |
| High | 3,868/29,495 (13.1) | 39/3,868 (1.0) | 2.20 (1.44–3.37) | 2,759/16,819 (16.4) | 114/2,759 (4.1) | 2.13 (1.60–2.83) |
The values for the number/total number (percent) of participants and of participants with an event were derived from observed data; hazard ratios and confidence intervals were based on imputed data. For fatal or nonfatal events, data are presented from the 20,656 participants whose event status could be ascertained.
All models were adjusted for sex, Black race, cohort, mean age at and calendar year of childhood measurement, and parental education level. The widths of the confidence intervals were not adjusted for multiple comparisons.
Figure 2. (facing page). Cumulative Risk of Cardiovascular Events According to Categories of the Combined-Risk z Score and Age.
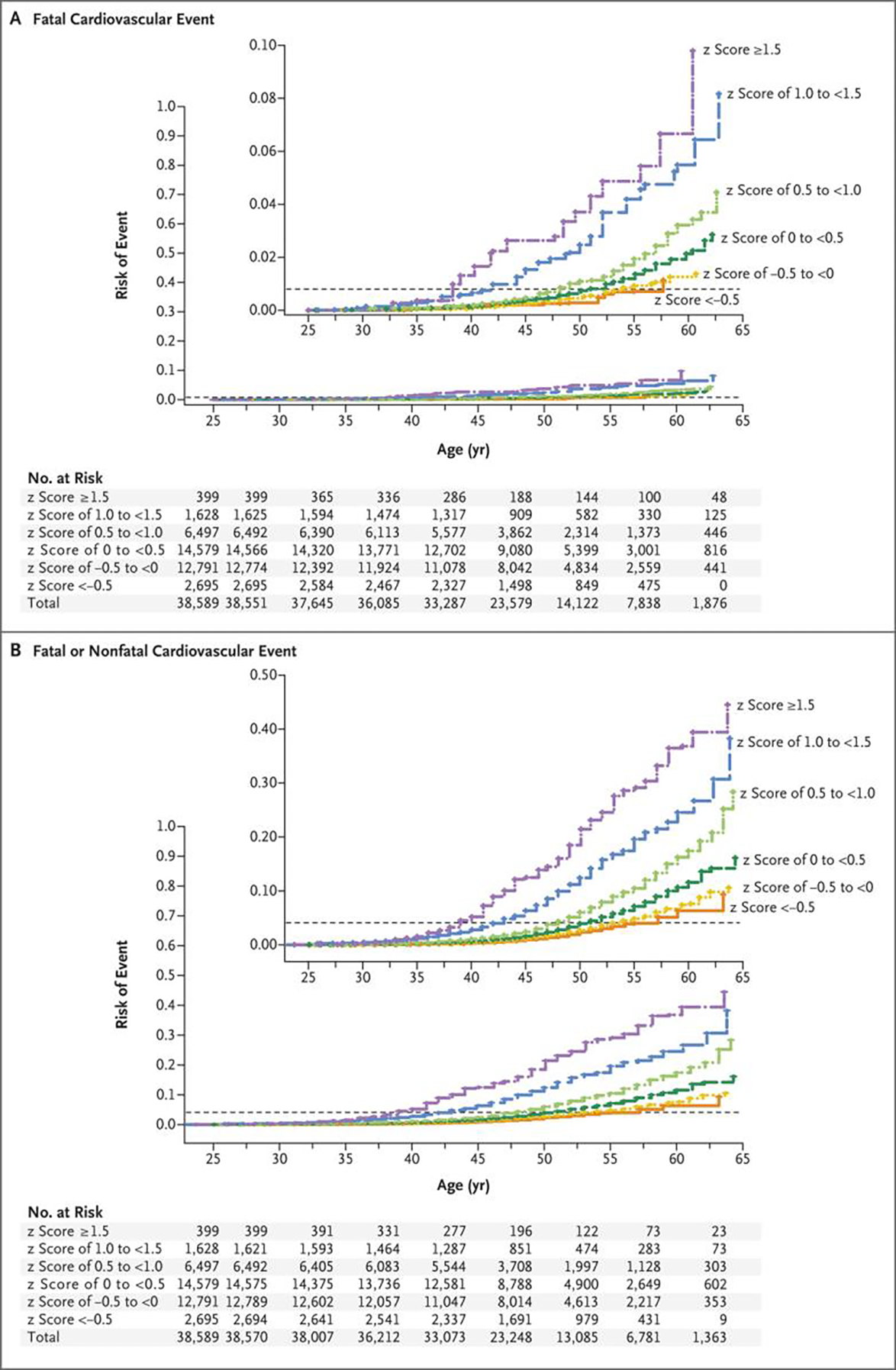
The cumulative risk of cardiovascular events is shown as the estimated probability according to the combined-risk z score and age (Kaplan–Meier method). The horizontal dashed line in each panel indicates the overall risk of the event. Panel A shows the hazard ratios for fatal cardiovascular events, and Panel B the hazard ratios for fatal or nonfatal cardiovascular events. All participants were included in these analyses; there were 319 fatal events and a mean of 1563 fatal or nonfatal events across imputations. In each panel, the inset shows the same data on an enlarged y axis. The columns of tabular data below the graphs correspond to the following ages (in years): 25.0, 27.5, 32.5, 37.5, 42.5, 47.5, 52.5, 57.5, and 62.5.
DISCUSSION
The current study, with its large sample and use of prospective data on five traditional cardiovascular risk factors (body-mass index, total cholesterol level, triglyceride level, systolic blood pressure, and youth smoking) from childhood to adulthood, showed comprehensive associations between the levels of these childhood risk factors, individually and in combination, and the development of incident adult cardiovascular events beginning as early as 40 years of age. Cardiovascular events in children are rare,14 but autopsies have shown pervasive histologic atherosclerotic lesions of the aorta and coronary arteries in young persons that were associated with dyslipidemia, elevated blood pressure, and smoking.15,16 Data from the Coronary Artery Risk Development in Young Adults (CARDIA) study have shown a relation between the Framingham risk score and cardiovascular events among young adults followed for 20 years,17 but studies linking childhood risk factors to adult events have been lacking.
Traditional cardiovascular risk factors have been evaluated in childhood because of their presumed association with the occurrence of adult cardiovascular events.2 Each risk factor was related to adult cardiovascular events in our study, and the combination of the risk factors into a mean risk score, similar in concept to the Framingham score, resulted in a stronger association than any single risk factor; among 38,589 participants, the 59.9% who had a combined-risk z score of 0 or greater, corresponding to the risk-factor level of an average child, were at increased risk for cardiovascular events, as compared with those who were in the lowest z score category (a z score of less than −0.5). Risk factors during childhood (3 to 11 years of age) and adolescence (12 to 19 years of age) were similarly related to adult cardiovascular events, as were risk factors according to sex and racial groups. Hazard ratios for fatal or nonfatal events with respect to the childhood combined-risk z score decreased as adult age increased.
We evaluated cardiovascular events in relation to the standard clinical categories currently used for childhood risk factors and found that children in the highest category of risk (e.g., overweight or obese body-mass index and prehypertensive or hypertensive blood-pressure levels) had a markedly higher risk of adult cardiovascular events, as expected. However, most children who were at excess risk for the development of adult cardiovascular events were in the middle to lower categories of the combined-risk z score. Previous longitudinal studies involving the i3C Consortium also showed an association of the midrange of childhood risk-factor levels with the development of adult hypertension6 and adult diabetes.18
Because of the rarity of cardiovascular disease in childhood, there continue to be questions about the merits of evaluating cardiovascular risk factors in childhood as opposed to adulthood, when subclinical and clinical disease are prevalent.19 In the model including both childhood and adult combined-risk z scores, the adult combined-risk z score was a strong predictor of adult events, and the childhood combined-risk z score was seemingly attenuated. Such attenuation suggests that childhood risk factors predicted adult events principally because they tracked to adult values. However, a complementary analysis20 showed that both the childhood combined-risk z score and the change in combined-risk z score between childhood and adulthood were important in predicting the risk of adult events. From the perspective of prevention, both childhood risk-factor levels and the path to risk in adulthood appear to be informative. Thus, we posit that assessment of cardiovascular risk should begin in childhood, and a reduction in risk-factor levels between childhood and adulthood may have the potential to lower the incidence of premature cardiovascular disease.
Our study raises important questions about broader childhood strategies for reducing the risk of premature cardiovascular disease. Rather than a sole focus on a medical approach of identifying children with elevated risk-factor levels, the current results would suggest that an equally relevant focus on public health strategies for maintaining ideal cardiovascular health in all children is warranted.21 Previous studies showed that persons with lifelong genetic exposure to low levels of low-density lipoprotein cholesterol and systolic blood pressure tend to have a lower risk of cardiovascular disease,22 and recently published results from the Special Turku Coronary Risk Factor Intervention Project (STRIP) study showed beneficial effects on risk factors over a period of 26 years after dietary counseling that began in infancy and continued throughout childhood.23 Furthermore, comprehensive public health efforts in Finland over the past 40 years have led to positive lifestyle changes, a decline in major risk-factor levels, and dramatic reductions in cardiovascular-related mortality.24,25 In acknowledgment of the difficulty of individual behavioral change, we think our findings suggest a need for stronger public health programs for children.
Strengths of our study include the large sample, the broad age range of childhood participants, adjudication of medical records, and a mean follow-up of children and adolescents of 35 years. The evidence that pooling the data from the separate cohorts was an appropriate strategy was that the risk of adult cardiovascular events before the age of 40 years was similarly low in all seven cohorts and that the adjusted hazard ratios with respect to each risk factor were similar across the four oldest cohorts.
Our study also has certain limitations. First, because 46.5% of the sample could not be located to ascertain nonfatal cardiovascular events, loss to follow-up presented a potential response bias. We addressed this issue through two analytic approaches. We determined vital status and cause of death in 95% of the original participants. By limiting the initial analysis to fatal cardiovascular events in that nearly complete sample, we found the expected relationships with the childhood risk factors. Next, we used multiple imputation to assess the association of childhood risk factors with adult fatal or nonfatal cardiovascular events in all participants. With respect to this end point, we relied heavily on the imputation model in the assessment of the findings. The primary function of the multiple imputation was to reinstate the data on all study variables in the analysis of the full sample distribution, as compared with analysis restricted to participants who were located or found to be dead. These two approaches yielded similar results; thus, bias resulting from loss to follow-up was unlikely to have affected our findings. Second, the generalizability of the current study was restricted by the limited number of participants from non-White groups, a factor that reflects the decades-old recruitment of the cohorts (Table S30). Black participants represented 15% of the analysis sample and 21% of the participants from the United States, which is a higher percentage than that in the U.S. population. However, the current study was not specifically powered to detect racial differences, did not include many Hispanic participants, and focused on the experience of high-income countries. Third, we would posit that our unweighted and straightforward combined-risk z score facilitates comparison of childhood and adult risk but may not improve risk prediction across the life course.
This prospective cohort study showed that the cardiovascular risk factors of body-mass index, systolic blood pressure, total cholesterol level, triglyceride level, and youth smoking, particularly in combination beginning in early childhood, were associated with adult cardiovascular events and death from cardiovascular causes before the age of 60 years.
Supplementary Material
Acknowledgments
Supported by a grant (NIH HL121230) from the National Institutes of Health. Historical funding sources for cohorts in the i3C Consortium are listed in Table S31.
APPENDIX
The authors’ full names and academic degrees are as follows: David R. Jacobs, Jr., Ph.D., Jessica G. Woo, Ph.D., Alan R. Sinaiko, M.D., Stephen R. Daniels, M.D., Ph.D., Johanna Ikonen, M.S., Markus Juonala, M.D., Ph.D., Noora Kartiosuo, M.S., Terho Lehtimäki, M.D., Ph.D., Costan G. Magnussen, Ph.D., Jorma S.A. Viikari, M.D., Ph.D., Nanhua Zhang, Ph.D., Lydia A. Bazzano, M.D., Ph.D., Trudy L. Burns, Ph.D., M.P.H., Ronald J. Prineas, M.B., B.S., Ph.D., Julia Steinberger, M.D., Elaine M. Urbina, M.D., Alison J. Venn, Ph.D., Olli T. Raitakari, M.D., Ph.D., and Terence Dwyer, M.D., M.B., B.S., M.P.H.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
D.R. Jacobs, Jr., Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis
J.G. Woo, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center. Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati.
A.R. Sinaiko, Department of Pediatrics, University of Minnesota Medical School, University of Minnesota, Minneapolis
S.R. Daniels, Department of Pediatrics, University of Colorado School of Medicine, and Anschutz Medical Campus, Children’s Hospital Colorado, Aurora
J. Ikonen, Center for Population Health Research. Research Center of Applied and Preventive Cardiovascular Medicine. Center for Population Health Research, Turku University Hospital, Turku, Finland.
M. Juonala, Department of Medicine, University of Turku. Division of Medicine, Turku University Hospital, Turku, Finland.
N. Kartiosuo, Center for Population Health Research. Research Center of Applied and Preventive Cardiovascular Medicine. Department of Mathematics and Statistics, University of Turku. Center for Population Health Research, Turku University Hospital, Turku, Finland.
T. Lehtimäki, Department of Clinical Chemistry, Fimlab Laboratories, and the Finnish Cardiovascular Research Center, and the Faculty of Medicine and Health Technology, Tampere University, Tampere, Finland.
C.G. Magnussen, Center for Population Health Research. Research Center of Applied and Preventive Cardiovascular Medicine. Center for Population Health Research, Turku University Hospital, Turku, Finland. Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS, Australia.
J.S.A. Viikari, Department of Medicine, University of Turku. Division of Medicine, Turku University Hospital, Turku, Finland.
N. Zhang, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati.
L.A. Bazzano, School of Public Health and Tropical Medicine, Tulane University, New Orleans
T.L. Burns, Department of Epidemiology, College of Public Health, University of Iowa, Iowa City
R.J. Prineas, Division of Public Health Sciences, Wake Forest School of Medicine, Winston-Salem, NC
J. Steinberger, Department of Pediatrics, University of Minnesota Medical School, University of Minnesota, Minneapolis
E.M. Urbina, Heart Institute, Cincinnati Children’s Hospital Medical Center. Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati.
A.J. Venn, Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS, Australia
O.T. Raitakari, Center for Population Health Research. Research Center of Applied and Preventive Cardiovascular Medicine, University of Turku. Center for Population Health Research. Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, Finland.
T. Dwyer, Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS, Australia. Heart Research Group, Murdoch Children’s Research Institute, Melbourne, VIC, Australia. Nuffield Department of Women’s and Reproductive Health, University of Oxford, Oxford, United Kingdom.
References
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics — 2020 update: a report from the American Heart Association. Circulation 2020;141(9):e139–e596. [DOI] [PubMed] [Google Scholar]
- 2.Pool LR, Aguayo L, Brzezinski M, et al. Childhood risk factors and adulthood cardiovascular disease: a systematic review. J Pediatr 2021;232:118–126.e23. [DOI] [PubMed] [Google Scholar]
- 3.Raitakari O, Pahkala K, Magnussen CG. Prevention of atherosclerosis from childhood. Nat Rev Cardiol 2022. January 5 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 4.Dwyer T, Sun C, Magnussen CG, et al. Cohort profile: the International Childhood Cardiovascular Cohort (i3C) Consortium. Int J Epidemiol 2013;42:86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinaiko AR, Jacobs DR Jr, Woo JG, et al. The International Childhood Cardiovascular Cohort (i3C) Consortium outcomes study of childhood cardiovascular risk factors and adult cardiovascular morbidity and mortality: design and recruitment. Contemp Clin Trials 2018;69:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urbina EM, Khoury PR, Bazzano L, et al. Relation of blood pressure in childhood to self-reported hypertension in adulthood. Hypertension 2019;73:1224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu T, Gall SL, Widome R, et al. Childhood/adolescent smoking and adult smoking and cessation: the International Childhood Cardiovascular Cohort (i3C) Consortium. J Am Heart Assoc 2020;9(7):e014381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 2002;109:45–60. [DOI] [PubMed] [Google Scholar]
- 9.Flynn JT, Falkner BE. New clinical practice guideline for the management of high blood pressure in children and adolescents. Hypertension 2017;70:683–6. [DOI] [PubMed] [Google Scholar]
- 10.National Cholesterol Education Program. Highlights of the report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics 1992;89:495–501. [PubMed] [Google Scholar]
- 11.Raghunathan TE. What do we do with missing data? Some options for analysis of incomplete data. Annu Rev Public Health 2004;25:99–117. [DOI] [PubMed] [Google Scholar]
- 12.Zhang N, Chen H, Elliott MR. Nonrespondent subsample multiple imputation in two-phase sampling for nonresponse. J Off Stat 2016;32:769–85. [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 14.de Ferranti SD, Steinberger J, Ameduri R, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association. Circulation 2019;139(13):e603–e634. [DOI] [PubMed] [Google Scholar]
- 15.Berenson GS, Srinivasan SR, Bao W, Newman WP III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med 1998;338:1650–6. [DOI] [PubMed] [Google Scholar]
- 16.McGill HC Jr, McMahan CA, Gidding SS. Preventing heart disease in the 21st century: implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study. Circulation 2008;117:1216–27. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong AC, Jacobs DR Jr, Gidding SS, et al. Framingham score and LV mass predict events in young adults: CARDIA study. Int J Cardiol 2014;172:350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu T, Jacobs DR Jr, Sinaiko AR, et al. Childhood BMI and fasting glucose and insulin predict adult type 2 diabetes: the International Childhood Cardiovascular Cohort (i3C) Consortium. Diabetes Care 2020;43:2821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krist AH, Davidson KW, Mangione CM, et al. Screening for high blood pressure in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA 2020;324:1878–83. [DOI] [PubMed] [Google Scholar]
- 20.De Stavola BL, Nitsch D, dos Santos Silva I, et al. Statistical issues in life course epidemiology. Am J Epidemiol 2006;163:84–96. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. ‘Best buys’ and other recommended interventions for the prevention and control of noncommunicable diseases: updated (2017) appendix 3 of the global action plan for the prevention and control of noncommunicable diseases 2013–2020 (https://www.who.int/ncds/management/WHO_Appendix_BestBuys.pdf).
- 22.Ference BA, Bhatt DL, Catapano AL, et al. Association of genetic variants related to combined exposure to lower low-density lipoproteins and lower systolic blood pressure with lifetime risk of cardiovascular disease. JAMA 2019;322:138–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pahkala K, Laitinen TT, Niinikoski H, et al. Effects of 20-year infancy-onset dietary counselling on cardiometabolic risk factors in the Special Turku Coronary Risk Factor Intervention Project (STRIP): 6-year post-intervention follow-up. Lancet Child Adolesc Health 2020;4:359–69. [DOI] [PubMed] [Google Scholar]
- 24.Puska P. Fat and heart disease: yes we can make a change — the case of North Karelia (Finland). Ann Nutr Metab 2009;54:Suppl 1:33–8. [DOI] [PubMed] [Google Scholar]
- 25.Salomaa V, Pietilä A, Peltonen M, Kuulasmaa K. Changes in CVD incidence and mortality rates, and life expectancy: North Karelia and national. Glob Heart 2016;11:201–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


