Abstract
Background
A high-fat diet (HFD) induced perturbation of gut microbiota is a major contributory factor to promote the pathophysiology of HFD-associated metabolic syndrome. The HFD could also increase the susceptibility to the microbial infections warranting the use of antibiotics which are independently capable of impacting both gut microbiota and metabolic syndrome. Further, the usage of antibiotics in individuals consuming HFD can impact mitochondrial function that can be associated with an elevated risk of chronic conditions like inflammatory bowel disease (IBD). Despite this high propensity to infections in individuals on HFD, the link between duration of HFD and antibiotic treatment, and its impact on diversity of the gut microbiome and features of metabolic syndrome is not well established. In this study, we have addressed these knowledge gaps by examining how the gut microbiota profile changes in HFD-fed mice receiving antibiotic intervention in the form of amoxicillin. We also determine whether antibiotic treatment in HFD-fed mice may adversely impact the ability of immune cells to clear microbial infections.
Methods and Results
We have subjected mice to HFD and chow diet (CD) for 3 weeks, and a subset of these mice on both diets received antibiotic intervention in the form of amoxicillin in the 3rd week. Body weight and food intake were recorded for 3 weeks. After 21 days, all animals were weighted and sacrificed. Subsequently, these animals were evaluated for basic haemato-biochemical and histopathological attributes. We used 16S rRNA sequencing followed by bioinformatics analysis to determine changes in gut microbiota in these mice. We observed that a HFD, even for a short-duration, could successfully induce the partial pathophysiology typical of a metabolic syndrome, and substantially modulated the gut microbiota in mice. The short course of amoxicillin treatment to HFD-fed mice resulted in beneficial effects by significantly reducing fasting blood glucose and skewing the number of thrombocytes towards a normal range. Remarkably, we observed a significant remodelling of gut microbiota in amoxicillin-treated HFD-fed mice. Importantly, some gut microbes associated with improved insulin sensitivity and recovery from metabolic syndrome only appeared in amoxicillin-treated HFD-fed mice reinforcing the beneficial effects of antibiotic treatment in the HFD-associated metabolic syndrome. Moreover, we also observed the presence of gut-microbiota unique to amoxicillin-treated HFD-fed mice that are also known to improve the pathophysiology associated with metabolic syndrome. However, both CD-fed as well as HFD-fed mice receiving antibiotics showed an increase in intestinal pathogens as is typically observed for antibiotic treatment. Importantly though, infection studies with S. aureus and A. baumannii, revealed that macrophages isolated from amoxicillin-treated HFD-fed mice are comparable to those isolated from mice receiving only HFD or CD in terms of susceptibility, and progression of microbial infection. This finding clearly indicated that amoxicillin treatment does not introduce any additional deficits in the ability of macrophages to combat microbial infections.
Conclusions
Our results showed that amoxicillin treatment in HFD-fed mice exert a beneficial influence on the pathophysiological attributes of metabolic syndrome which correlates with a significant remodelling of gut microbiota. A novel observation was the increase in microbes known to improve insulin sensitivity following amoxicillin treatment during short-term intake of HFD. Even though there is a minor increase in gut-resistant intestinal pathogens in amoxicillin-treated groups, there is no adverse impact on macrophages with respect to their susceptibility and ability to control infections. Taken together, this study provides a proof of principle for the exploration of amoxicillin treatment as a potential therapy in the people affected with metabolic syndrome.
Keywords: High-fat diet, Gut microbiota, Metabolic syndrome, Pathogens, Amoxicillin, Immune cells
Background
High-fat diet (HFD) consumption can have serious adverse effects on human metabolic and immune health. The HFD sparks chronic low-grade systemic inflammation that can modulate gut microbiota by increasing endotoxins, circulating free fatty acids, and inflammatory mediators, which may impede the homeostasis in many organs [1–3]. Several studies have shown that long-term consumption of a high-fat diet modulates mice’s gut microbiota composition and functionality. Further, a high-fat diet develops the pathophysiology of metabolic disorders by dysbiosis of gut microbiota [4–7]. Individuals with HFD-associated metabolic syndrome are often susceptible to microbial infections. This may warrant the use of antibiotics that can further alter the microbiota resulting in dysregulation of host immune homeostasis, poor nutrient digestion, and increased risk to opportunistic pathogens owing to a reduced intestinal colonization resistance [8, 9].
However, very little knowledge is available as to how antibiotic treatment in the backdrop of HFD may impact gut microbiota, haemato-biochemical parameters and histopathological changes in an organism. This knowledge is especially relevant now as the modern diet is often rich in fat, and antibiotics are administered during infections to treat patients. However, how antibiotic treatment potentially impacts resident microbiota and macrophages function to play a promoting or inhibitory role affecting the physiology and health of a person on HFD remains unknown.
In this study, we addressed these gaps by performing experiments wherein we provided HFD to mice for 3-weeks and subjected these mice to amoxicillin treatment during the 3rd week to mimic a typical course of antibiotics. The choice of amoxicillin as the preferred antibiotic in this study was based on it being the most common antibiotic used in primary healthcare settings [10]. We first showed that short-term intake of HFD for 3-weeks induces partial physiopathology of metabolic syndrome by disruption of homeostasis of gut microbiota. By using 16S rRNA high-throughput sequencing, we examined the changes in the caecal microbiota and discussed how changes in microbiota composition may promote or suppress the features associated with metabolic pathophysiology in the presence of amoxicillin. We also scored for the changes in haemato-biochemical profile, and histo-pathological analysis on vital organs, namely the heart, liver and kidney in these mice. Finally, we also demonstrated that amoxicillin treatment does not adversely affect the functional capacity of macrophages of clearing infection in the HFD-fed mice. Collectively, our study provides strong evidence to explore amoxicillin-mediated modulation of gut-microbiota as a potential intervention for improving the health of people affected with metabolic syndrome.
Results
Effects of HFD and amoxicillin treatment in HFD-fed mice on body weight and feed intake
As shown in Figure 1, mice fed on HFD for 3 weeks did not show any significant differences in weight when compared to CD-fed mice. Interestingly, amount of feed consumed was similar in all groups including those receiving amoxicillin as shown in Figure 2. Likewise, we also did not observe significant weight differences between HFD-fed mice and amoxicillin-treated HFD-fed mice. Also, there were no significant differences in the water intake among different groups.
Fig. 1.
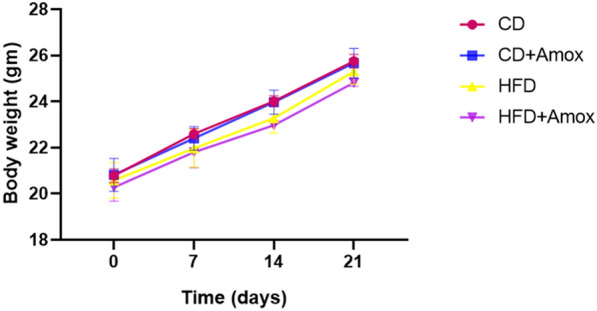
Effect of high-fat diet (3 weeks) and amoxicillin treatment on body weight of mice. As shown by the data, no significant difference in body weight of mice was observed among different groups. Data represent mean ± SD; N = 6 mice/group, One-way ANOVA followed by Bonferroni post-hoc test (* p < 0.05, ** p < 0.01, *** p < 0.001). CD: Standard-chow diet for 3 weeks; CD + Amox: Standard-chow diet for 3 weeks and subjected to amoxicillin treatment in 3rd week; HFD: High-fat diet for 3 weeks; HFD + Amox: High-fat diet for 3 weeks and subjected to amoxicillin treatment in 3rd week
Fig. 2.
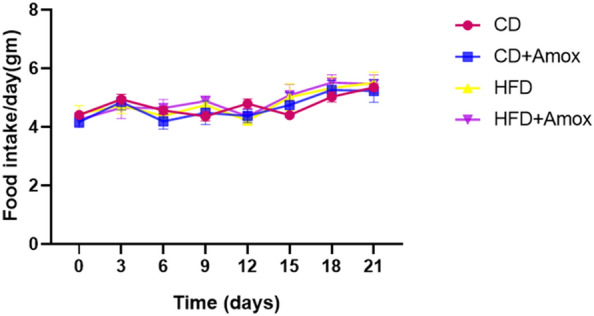
Daily feed intake of mice subjected to high-fat diet and amoxicillin treatment. As shown by the data, no significant difference on daily feed intake of mice was observed among different groups. Data represents mean ± SD; N = 6 mice/group, One-way ANOVA followed by Bonferroni post-hoc test (* p < 0.05, ** p < 0.01, *** p < 0.001). CD: Standard- chow diet for 3 weeks; CD + Amox: Standard- chow diet for 3 weeks and subjected to amoxicillin treatment in 3rd week; HFD: High-fat diet for 3 weeks; HFD + Amox: High-fat diet for 3 weeks and subjected to amoxicillin treatment in 3rd week
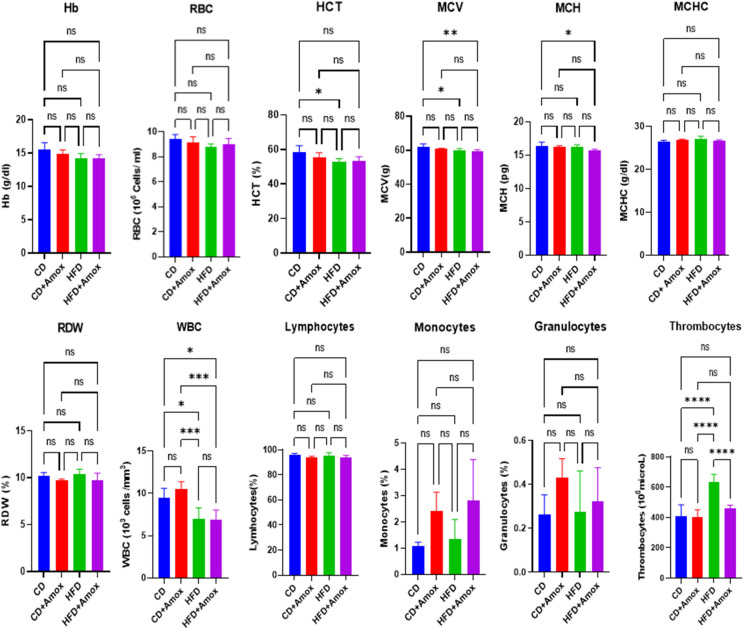
Amoxicillin treatment restores the HFD-promoted thrombocytosis
We next evaluated the impact of the HFD and amoxicillin on haematological parameters (Figure 3 and Table 1). Compared to CD, in HFD, we observed a slight decline in the value of haemoglobin (Hb), red blood cells (RBC), mean corpuscular haemoglobin (MCH), and a significant reduction in haematocrit (HCT, p = 0.022), mean corpuscular volume (MCV, p = 0.02189) and white blood cells (WBCs, p = 0.0128). Moreover, HFD-treated mice significantly increased thrombocytes (p < 0.0001) compared to the CD group. On the other hand, amoxicillin-treated HFD-fed mice could decrease the thrombocytes (p < 0.0001) significantly towards normal range without any significant alterations in HCT and MCV values. Nonetheless, we did not observe any significant change in haematological parameters in the mice belonging to CD + Amox group or CD group alone. Based on this data, it is clear that amoxicillin treatment in CD-fed mice did not affect thrombocyte value while it exerted a positive effect on restoring their number towards normalcy in the HFD-fed mice.
Fig. 3 .
Effect of high-fat diet (3 weeks) and amoxicillin treatment on haematological parameters in mice. HFD treatment for 3 weeks in mice induced the depressive effect on thrombocytes. Amoxicillin treatment in HFD mice in the 3rd week caused restoration of the thrombocytes towards the normal range. Values are mean ± SD for 6 samples in each group. Statistically significance of differences was evaluated by One-way ANOVA followed by Bonferroni post-hoc test (* p < 0.05, ** p < 0.01, **** p < 0.0001). CD: Standard-chow diet for 3 weeks; CD + Amox: Standard-chow diet for 3 weeks and subjected to amoxicillin treatment in 3rd week; HFD: High-fat diet for 3 weeks; HFD + Amox: High-fat diet for 3 weeks and subjected to amoxicillin treatment in 3rd week
Table 1.
Effect of diet including HFD used in this study on various haematological parameters
| Parameters | Groups | Significance (p value) | |||||
|---|---|---|---|---|---|---|---|
| CD | CD + A | HFD | HFD + A | CD vs CD + A | CD vs HFD | HFD vs HFD + A | |
| Hb (g/dl) | 15.46 ± 1.1 | 14.83 ± 0.6 | 14.26 ± 0.6 | 14.16 ± 0.5 | NS | NS | NS |
| RBC (106 cells/ml) | 9.40 ± 0.3 | 9.1 ± 0.4 | 8.77 ± 0.24 | 8.98 ± 0.4 | NS | NS | NS |
| HCT (%) | 58.23 ± 3.8 | 55.23 ± 2.8 | 52.56 ± 2.1 | 53.46 ± 2.2 | NS | 0.0226 | NS |
| MCV (g) | 61.9 ± 1.8 | 60.76 ± 0.3 | 60.0 ± 1.04 | 59.6 ± 0.7 | NS | 0.0218 | NS |
| MCH (pg) | 16.36 ± 0.5 | 16.26 ± 0.1 | 16.2 ± 0.3 | 15.7 ± 0.2 | NS | NS | NS |
| MCHC(g/dl) | 26.5 ± 0.2 | 26.83 ± 0.2 | 27.1 ± 0.6 | 26.6 ± 0.2 | NS | NS | NS |
| RDW (%) | 10.2 ± 0.34 | 9.72 ± 0.11 | 10.3 ± 0.57 | 9.7 ± 0.78 | NS | NS | NS |
| WBC (106 cells/ml) | 9.41 ± 1.1 | 10.52 ± 0.8 | 6.95 ± 1.3 | 6.92 ± 1.1 | NS | 0.0128 | NS |
| Lymphocytes (106 cells/ml) | 96.3 ± 0.7 | 93.6 ± 1.3 | 95.0 ± 2.7 | 93.6 ± 1.9 | NS | NS | NS |
| Monocytes (%) | 1.06 ± 0.1 | 2.4 ± 0.7 | 1.33 ± 0.7 | 2.8 ± 1.5 | NS | NS | NS |
| Granulocytes (%) | 0.26 ± 0.08 | 0.43 ± 0.08 | 0.27 ± 0.1 | 0.32 ± 0.1 | NS | NS | NS |
| Thrombocytes (103/microL) | 407.6 ± 76.2 | 400.3 ± 50.5 | 630.6 ± 55.08 | 459 ± 21.9 | NS | < 0.0001 | < 0.0001 |
Values are expressed as the mean ± SD (n = 6). Data were analysed by One-way ANOVA followed by Bonferroni post-hoc test, p ≤ 0.05, NS = not significant, CD: Standard-chow diet for 3 weeks; CD + A: Standard-chow diet for 3 weeks and subjected to amoxicillin treatment (50 mg/kg body weight) in 3rd week; HFD: High-fat diet for 3 weeks (73% energy from fat); HFD + A: High-fat diet for 3 weeks and subjected to amoxicillin treatment (50 mg/kg body weight) in 3rd week
Amoxicillin treatment moderates the elevated blood glucose level associated with HFD
Analysis of biochemical parameters (Figure 4 and Table 2) show that levels of Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), and creatinine values are not significantly different in mice receiving standard CD or short-term HFD. However, we did observe a significant increase in total cholesterol and glucose (p < 0.0001) levels in the HFD-fed mice vis a vis mice fed on normal CD. Further, amoxicillin treatment of HFD- fed mice did not significantly change levels of triglycerides, cholesterol and urea as compared to mice fed only on HFD. Interestingly, amoxicillin treatment significantly reduced glucose levels in HFD-fed mice (p < 0.0001). Further, amoxicillin also improved other biochemical parameters of blood and restored levels of liver enzyme AST (p < 0.0001) in mice on a HFD. Interestingly, the mice administered standard CD and receiving amoxicillin treatment showed a significant reduction in the levels of cholesterol, triglycerides and urea as compared to the only CD group. For an inter-group comparison between CD and HFD-fed, we did not observe any significant difference in ALT and creatinine concentration. Thus, our data suggest that amoxicillin treatment in HFD-fed mice improves levels of blood glucose and AST suggesting a possible beneficial impact on metabolic pathophysiology.
Fig. 4.
Effect of high-fat diet (3 weeks) and amoxicillin treatment on biochemical parameters in mice. HFD treatment for 3 weeks in mice showed a significant increase in total cholesterol and glucose and a significant decrease in triglycerides. Amoxicillin treatment in HFD mice in the 3rd week caused restoration of the blood glucose level towards the normal range. Values are mean ± SD; N = 6 mice/group. Statistical significance of differences was evaluated by One-way ANOVA followed by Bonferroni post-hoc test (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001). CD: Standard-chow diet for 3 weeks; CD + Amox: Standard-chow diet for 3 weeks and subjected to amoxicillin treatment in 3rd week; HFD: High-fat diet for 3 weeks; HFD + Amox: High-fat diet for 3 weeks and subjected to amoxicillin treatment in 3rd week
Table 2.
Effect of diet including HFD used in this study on various biochemical parameters
| Parameters | Groups | Significance (p value) | |||||
|---|---|---|---|---|---|---|---|
| CD | CD + A | HFD | HFD + A | CD vs CD + A | CD vs HFD | HFD vs HFD + A | |
| Cholesterol (mg/dl) | 71.49 ± 3.6 | 52.7 ± 0.3 | 127.39 ± 5.5 | 135.08 ± 3.1 | < 0.0001 | < 0.0001 | NS |
| Triglycerides(mg/dl) | 122.99 ± 7.5 | 95.44 ± 0.9 | 92.79 ± 7.1 | 102.41 ± 9.8 | < 0.0001 | < 0.0001 | NS |
| ALT (IU) | 56.80 ± 4.5 | 48.04 ± 0.5 | 53.12 ± 2.1 | 38.82 ± 6.3 | NS | NS | 0.0015 |
| AST (IU) | 74.47 ± 4.8 | 75.89 ± 1.6 | 83.08 ± 2.49 | 59.61 ± 6.07 | NS | NS | < 0.0001 |
| Creatinine (mg/dl) | 0.36 ± 0.06 | 0.37 ± 0.01 | 0.33 ± 0.005 | 0.30 ± 0.01 | NS | NS | NS |
| Urea (mg/dl) | 72.21 ± 4.5 | 66.15 ± 0.2 | 59.57 ± 1.9 | 57.20 ± 2.97 | 0.0029 | < 0.0001 | NS |
| Glucose (mg/dl) | 219.80 ± 13.08 | 231.54 ± 0.89 | 340.04 ± 14.7 | 290.77 ± 19.8 | NS | < 0.0001 | < 0.0001 |
Values are expressed as the mean ± SD (n = 6). Data were analyzed by One-way ANOVA followed by Bonferroni post-hoc test, p ≤ 0.05, NS= not significant, CD: Standard-chow diet for 3 weeks; CD + A: Standard-chow diet for 3 weeks and subjected to amoxicillin treatment (50 mg/kg body weight) in 3rd week; HFD: High-fat diet for 3 weeks (73% energy from fat); HFD + A: High-fat diet for 3 weeks and subjected to amoxicillin treatment (50 mg/kg body weight) in 3rd week
Amoxicillin treatment decreases HFD induced richness and diversity of specific gut bacteria
Considering an improved glucose profile observed in HFD-fed mice treated with amoxicillin, we posited that these metabolic changes may be indicative of the changes in the gut microbiome associated with pathophysiology of metabolic syndrome. For this purpose, we next investigated how amoxicillin treatment with or without HFD can impact the diversity of gut microbiota. Towards this end, we have performed sequencing of 16S rRNA and obtained a total of 11,69,822 high-quality sequences, with an average of 2,92,455 reads per sample. Using a similarity threshold of 97%, we obtained 22,980 as the total number of operating taxonomic units OTUs (Figure 5 and Table 3). The Shannon–Wiener curve, indicating the adequacy of sequencing depth for all samples, achieved a plateau (Figure 5), suggesting that the sequencing has sufficient depth to capture species abundance present in the samples. Next, we determined diversity using Chao1 (richness of species index), Shannon (rare species index), Simpson (common species index) indices, and number of observed species in each group are summarized in Table 3. Both Chao1 and Shannon indices have the highest values in the HFD-fed mice which were significantly reduced following amoxicillin treatment [p=0.0002 for Chao1, and p<0.0001 for Shannon index]. Similarly, amoxicillin treatment in CD-fed mice significantly reduced value of Chao1 (p<0.0002) and Shannon indices (p<0.0001).We did not observe a statistically significant difference for the Simpson index among all the four groups. From this data, we concluded that HFD increases the number of specific gut bacteria, while amoxicillin treatment depletes these bacteria.
Fig. 5.
Microbiota diversity in mice subjected to high-fat diet (3 weeks) and amoxicillin treatment. Rarefaction curves (a), and Shannon–Wiener curve (b). The plateuing observed in Shannon–Wiener curve suggested that the number of OTUs was sufficient to capture the authentic bacterial communities in each sample. CD: Standard-chow diet for 3 weeks; CD + Amox: Standard-chow diet for 3 weeks and subjected to amoxicillin treatment in 3rd week; HFD: High-fat diet for 3 weeks; HFD + Amox: High-fat diet for 3 weeks and subjected to amoxicillin treatment in 3rd week
Table 3.
Effect of diet on alpha diversity of gut microbiota in mice
| Alpha diversity indices | Groups | Significance (p value) | |||||
|---|---|---|---|---|---|---|---|
| CD | CD + A | HFD | HFD + A | CD vs CD + A | CD vs HFD | HFD vs HFD + A | |
| OTUs | 6468.7 ± 568.6 | 4871.6 ± 678.5 | 6543.4 ± 634.1 | 5104.7 ± 686.5 | 0.0013 | NS | 0.0053 |
| Chao1 | 8682.7 ± 42.69 | 7152.3 ± 74.4 | 9354.4 ± 651.4 | 7500.5 ± 46.09 | < 0.0001 | 0.0229 | 0.0002 |
| Shannon | 6.99 ± 0.0132 | 5.63 ± 0.020 | 8.19 ± 0.055 | 6.39 ± 0.018 | < 0.0001 | < 0.0001 | < 0.0001 |
| Simpson | 0.933 ± 1.110 | 0.912 ± 0.00 | 0.979 ± 0.00 | 0.935 ± 0.00 | NS | NS | NS |
Values are expressed as the mean ± SD (n = 6). Data were analyzed by One-way ANOVA followed by Bonferroni post-hoc test, p≤ 0.05, NS =not significant, CD: Standard-chow diet for 3 weeks; CD + A: Standard-chow diet for 3 weeks and subjected to amoxicillin treatment (50 mg/kg body weight) in 3rd week; HFD: High-fat diet for 3 weeks (73% energy from fat); HFD + A: High-fat diet for 3 weeks and subjected to amoxicillin treatment (50 mg/kg body weight) in 3rd week
Amoxicillin treatment reshapes the gut microbiota associated with improved pathophysiology of metabolic syndrome
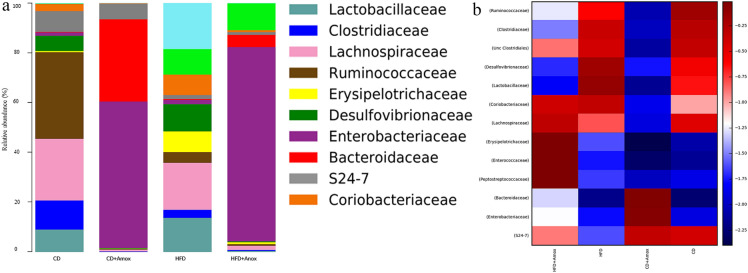
After determining the richness and diversity associated with various treatment groups, we next determined and analyzed the relative abundance of the microbiota at the phylum level (Figure 6 and Table 4). Our analysis revealed that consumption of HFD in mice caused a significant increase in the Firmicutes to Bacteroidetes ratio (F/B ratio) compared to the CD group (15.46 ± 0.361 to 48.35 ± 0.449, p < 0.0001). Interestingly, amoxicillin treatment in HFD mice significantly reduced the F/B ratio (48.35 ± 0.449 to 19.30 ± 0.394, p < 0.0001) as compared to the group receiving HFD only. Similarly, amoxicillin treatment of mice fed on CD also showed a significantly decreased F/B ratio (0.05 ± 0.022, p < 0.0001) as compared to the group on CD only. Values are expressed as the mean ± SD.
Fig. 6.
Bar chart (a) depicts the effect of high-fat diet (3 weeks) and amoxicillin treatment on the relative abundance of the most represented bacterial taxa at the phylum level for each sample. The x coordinate represents the name of the groups. The y coordinate represents the relative abundance. HFD treatment for 3 weeks in mice showed a significant increase of Firmicutes to Bacteroidetes (F/B) ratio and Proteobacteria. Amoxicillin treatment in HFD mice in the 3rd week caused a significant decrease in the Firmicutes to Bacteroidetes (F/B) ratio and Proteobacteria. The analysis of color heat map (b) illustrates the abundance of top 32 mean bacterial community taxa assigned to phyla. The color intensity in each sample is normalized to represent its relative ratio in the four groups. The color scale of dark red and dark blue represents the higher and lower relative abundances of bacterial communities, respectively. CD: Standard- chow diet for 3 weeks, CD + Amox: Standard- chow diet for 3 weeks and subjected to amoxicillin treatment in 3rd week; HFD: High-fat diet for 3 weeks; HFD + Amox: High-fat diet for 3 weeks and subjected to amoxicillin treatment in 3rd week
Table 4 .
Effect of diet on the distribution of gut microbiota in mice at phylum level
| Phylum | Relative abundance (%) | Significance (p value) | |||||
|---|---|---|---|---|---|---|---|
| CD | CD + A | HFD | HFD + A | CD vs CD + A | CD vs HFD | HFD vs HFD + A | |
| Firmicutes | 80.4 ± 0.396 | 1.80 ± 0.132 | 67.70 ± 0.467 | 73.70 ± 0.440 | < 0.0001 | < 0.0001 | < 0.0001 |
| Proteobacteria | 7.50 ± 0.897 | 56.30 ± 0.496 | 13.80 ± 0.344 | 9.20 ± 0.289 | < 0.0001 | < 0.0001 | < 0.0001 |
| Bacteroidetes | 5.20 ± 0.233 | 34.00 ± 0.473 | 1.40 ± 0.117 | 3.80 ± 0.191 | < 0.0001 | < 0.0001 | < 0.0001 |
| Actinobacteria | 3.80 ± 0.191 | 3.60 ± 0.186 | 9.60 ± 0.294 | 9.10 ± 0.287 | NS | < 0.0001 | 0.0143 |
| Verrucomicrobia | 0.20 ± 0.044 | 0.40 ± 0.063 | 0.30 ± 0.054 | 0.30 ± 0.054 | < 0.0001 | NS | NS |
| TM7 | 0.10 ± 0.031 | 0.10 ± 0.031 | 3.30 ± 0.178 | 0.10 ± 0.031 | NS | < 0.0001 | < 0.0001 |
| Acidobacteria | 0.30 ± 0.054 | 0.50 ± 0.070 | 0.60 ± 0.077 | 0.60 ± 0.077 | 0.0264 | < 0.0001 | NS |
| Cyanobacteria | 0.50 ± 0.070 | 0.90 ± 0.094 | 0.30 ± 0.054 | 0.40 ± 0.063 | < 0.0001 | 0.0002 | 0.0435 |
| F/B ratio | 15.46 ± 0.361 | 0.05 ± 0.022 | 48.35 ± 0.449 | 19.30 ± 0.394 | < 0.0001 | < 0.0001 | < 0.0001 |
Values are expressed as the mean ± SD (n = 6). Data were analyzed by One-way ANOVA followed by Bonferroni post-hoc test, p ≤ 0.05, NS= not significant, CD: standard- chow diet for 3 weeks; CD + A: Standard- chow diet for 3 weeks and subjected to amoxicillin treatment (50 mg/kg body weight) in 3rd week; HFD: high-fat diet for 3 weeks (73% energy from fat); HFD + A: High-fat diet for 3 weeks and subjected to amoxicillin treatment (50 mg/kg body weight) in 3rd week
We further observed that the relative abundance of Proteobacteria was significantly higher in HFD-fed mice compared to CD-fed mice (p < 0.0001). However, amoxicillin treatment in HFD-fed mice resulted in a significant decrease of Proteobacteria compared to the HFD group. In contrast, amoxicillin- treated CD group has a significantly increased relative abundance of Proteobacteria compared to the CD group (p < 0.0001). A balance of the F/B ratio is a widely accepted indicator of health that influences gut homeostasis. Various research studies have shown that the increased F/B ratio is associated with obesity while the decrease in this ratio is connected to dysbiosis [11–13]. Further, Proteobacteria is considered the most variable species that contribute to dysbiosis, while body leanness is positively correlated with Bacteroidetes [11, 14].
Taken together, analysis of the data in this study highlighted the presence of 45, 53, 63, and 61 bacterial families in the CD, CD + Amox, HFD and HFD + Amox groups, respectively (Figure 7 and Table 5). Compared to CD group, we observed a significant reduction of Lachnospiraceae and S24-7 family (p < 0.0001) in the HFD group. However, amoxicillin treatment in HFD-fed mice caused a significant increase of Lachnospiraceae and S24-7 (p < 0.0001), while in CD group, amoxicillin treatment led to a significant decrease of Lachnospiraceae, and an increase of S24-7 (p < 0.0001) compared to CD group only. Lachnospiraceae produces beneficial metabolites for the host such as short-chain fatty acids (SCFAs) especially propionate, and can provide energy for the maintenance of other gut microbes while supporting the development of host epithelial cells [15]. On the other hand, reduction in S24-7 is associated with a decrease in butyrate production that may induce metabolic endotoxemia and various other metabolic disorders [4]. Further, Enterobacteriaceae and Bacteroidaceae were significantly higher in the amoxicillin-treated CD group as compared to CD group alone. The Enterobacteriaceae and Bacteroidaceae are also usually associated with acute infective processes [16]. Likewise, we also observed a significant increase in Erysipelotrichaceae in HFD-fed mice compared to CD-fed mice. Erysipelotrichaceae abundance in HFD-fed mice was further amplified following amoxicillin treatment. Erysipelotrichaceae flourishing following broad-spectrum antibiotic treatment has also been observed previously [17].
Fig. 7.
Bar chart (a) depicting the effect of high-fat diet (3 weeks) and amoxicillin treatment on the relative abundance of the most represented bacterial taxa at the family level for each sample. HFD treatment for 3 weeks in mice showed a significant decrease of Lachnospiraceae and S24-7. Amoxicillin treatment in HFD mice in 3rd week caused a significant increase in Lachnospiraceae and S24-7. The analysis of the color heat map (b) illustrates the mean abuandances of top 13 bacterial community taxa assigned to family. The color intensity in each sample is normalized to represent its relative ratio in the four groups. The color scale of dark red and dark blue represents the higher and lower relative abundances of bacterial communities, respectively. CD: Standard- chow diet for 3 weeks; CD + Amox: Standard- chow diet for 3 weeks and subjected to amoxicillin treatment in 3rd week; HFD: High-fat diet for 3 weeks; HFD + Amox: High-fat diet for 3 weeks and subjected to amoxicillin treatment in 3rd week
Table 5 .
Effect of diet on distribution of gut microbiota of mice at family level
| Family | Relative abundance (%) | Significance (Adjusted p value) | |||||
|---|---|---|---|---|---|---|---|
| CD | CD + A | HFD | HFD + A | CD vs CD + A | CD vs HFD | HFD vs HFD + A | |
| Enterococcaceae | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.1 ± 0.031 | 3.1 ± 0.173 | NS | NS | < 0.0001 |
| Lactobacillaceae | 4.5 ± 0.207 | 0.1 ± 0.031 | 15.2 ± 0.359 | 0.3 ± 0.054 | < 0.0001 | < 0.0001 | < 0.0001 |
| Unc Clostridiales | 39.0. ± 0.487 | 0.0 ± 0.000 | 32.6 ± 0.468 | 11.2 ± 0.315 | < 0.0001 | < 0.0001 | < 0.0001 |
| Clostridiaceae | 5.9 ± 0.235 | 0.1 ± 0.031 | 4.9 ± 0.222 | 0.3 ± 0.054 | < 0.0001 | < 0.0001 | < 0.0001 |
| Lachnospiraceae | 12.4 ± 0.329 | 0.4 ± 0.063 | 5.7 ± 0.231 | 17.7 ± 0.381 | < 0.0001 | < 0.0001 | < 0.0001 |
| Peptostreptococcaceae | 0.1 ± 0.031 | 0.0 ± 0.000 | 0.1 ± 0.031 | 4.7 ± 0.211 | < 0.0001 | NS | < 0.0001 |
| Ruminococcaceae | 17.4 ± 0.379 | 0.2 ± 0.044 | 6.3 ± 0.242 | 1.4 ± 0.117 | < 0.0001 | < 0.0001 | < 0.0001 |
| Erysipelotrichaceae | 0.3 ± 0.054 | 0.1 ± 0.031 | 0.8 ± 0.089 | 33.5 ± 0.471 | < 0.0001 | < 0.0215 | < 0.0001 |
| Desulfovibrionaceae | 3.0 ± 0.170 | 0.2 ± 0.044 | 7.5 ± 0.263 | 0.2 ± 0.141 | < 0.0001 | < 0.0001 | < 0.0001 |
| Enterobacteriaceae | 0.7 ± 0.083 | 47.3 ± 0.499 | 0.9 ± 0.094 | 3.3 ± 0.178 | < 0.0001 | 0.0442 | < 0.0001 |
| Bacteroidaceae | 0.2 ± 0.044 | 26.7 ± 0.442 | 0.2 ± 0.044 | 1.4 ± 0.117 | < 0.0001 | NS | < 0.0001 |
| S24-7 | 4.1 ± 0.198 | 4.9 ± 0.215 | 0.3 ± 0.054 | 1.4 ± 0.117 | < 0.0001 | < 0.0001 | < 0.0001 |
| Coriobacteriaceae | 1.4 ± 0.117 | 0.2 ± 0.044 | 6.7 ± 0.250 | 5.7 ± 0.231 | < 0.0001 | < 0.0001 | < 0.0001 |
Values are expressed as the mean ± SD (n = 6). Data were analyzed by One-way ANOVA followed by Bonferroni post-hoc test, p ≤ 0.05, NS= not significant, CD: Standard- chow diet for 3 weeks; CD + A: Standard- chow diet for 3 weeks and subjected to amoxicillin treatment (50 mg/kg body weight) in 3rd week; HFD: high-fat diet for 3 weeks (73% energy from fat); HFD + A: High-fat diet for 3 weeks and subjected to amoxicillin treatment (50 mg/kg body weight) in 3rd week. Unc=Unculturable
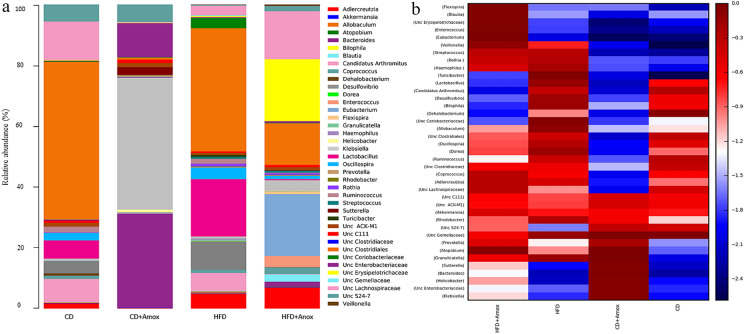
At the genus level, a total of 21, 26, 38, and 37 genera (Figure 8 and Table 6) were observed in CD, CD + Amox, HFD, and HFD + Amox groups, respectively. Further, the relative abundance of microbiota at the species level is shown in Table 7. Data analysis revealed a significant increase in the relative abundance of Lactobacillus, Turicibacter, Desulfovibrio, Oscillospira, Allobaculum, Streptococcus, Granulicatella, and Rothia (p < 0.0001) in HFD-fed mice as compared to CD-fed mice (Table 6). Most of the Lactobacillus strains nourish gut health by secreting anti-microbial substances that stop pathogens from colonizing the gut [18]. HFD-fed mice had a significant reduction in Lactobacillus reuteri (p < 0.0001) that is helpful in maintaining the integrity of the gut epithelial barrier and prevents the growth of pathogens in the intestine by secreting anti-microbial substances such as reuteri [18, 19].
Fig. 8.
Bar chart (a) depicting effect of high-fat diet (3 weeks) and amoxicillin treatment on the relative abundance of the most represented bacterial taxa at the genus level for each sample. Amoxicillin treatment in the 3rd week in the HFD group showed a significant rise in the relative abundance of Blautia, Coprococcus, Bacteroides, Akkermansia and a decreased in Granulicatella, Lactobacillus, Streptococcus, Turicibacter, Desulfovibrio, Oscillospira, and Allobaculum. The analysis of the color heat map (b) illustrates the mean abundances of top 39 bacterial community taxa assigned to genera. The color intensity in each sample is normalized to represent its relative ratio in the four groups. The color scale of dark red and dark blue represents the higher and lower relative abundances of bacterial communities, respectively. CD: Standard—chow diet for 3 weeks; CD + Amox: Standard—chow diet for 3 weeks and subjected to amoxicillin treatment in 3rd week; HFD: High-fat diet for 3 weeks; HFD + Amox: High-fat diet for 3 weeks and subjected to amoxicillin treatment in 3rd week
Table 6 .
Effect of diet on mice gut microbiota at genus level
| Genus | Relative abundance at genus level (%) | Significance (adjusted p value) | |||||
|---|---|---|---|---|---|---|---|
| CD | CD + A | HFD | HFD + A | CD vs CD + A | CD vs HFD | HFD vs HFD + A | |
| Unc Gemellaceae | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.2 ± 0.044 | 0.01 ± 0.009 | NS | < 0.0001 | < 0.0001 |
| Granulicatella | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.2 ± 0.044 | 0.1 ± 0.031 | NS | < 0.0001 | < 0.0001 |
| Enterococcus | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.1 ± 0.031 | 3.0 ± 0.170 | NS | NS | < 0.0001 |
| Lactobacillus | 4.5 ± 0.207 | 0.1 ± 0.031 | 15.2 ± 0.359 | 0.3 ± 0.054 | < 0.0001 | < 0.0001 | < 0.0001 |
| Streptococcus | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.6 ± 0.077 | 0.5 ± 0.070 | NS | < 0.0001 | 0.0341 |
| Turicibacter | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.5 ± 0.070 | 0.0 ± 0.000 | NS | < 0.0001 | < 0.0001 |
| Unc Clostridiales | 39.1 ± 0.489 | 0.6 ± 0.077 | 32.7 ± 0.469 | 11.3 ± 0.316 | < 0.0001 | < 0.0001 | < 0.0001 |
| Unc Clostridiaceae | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.2 ± 0.044 | NS | NS | < 0.0001 |
| Candidatus Arthromitus | 5.7 ± 0.232 | 0.1 ± 0.031 | 4.8 ± 0.213 | 0.1 ± 0.031 | < 0.0001 | < 0.0001 | < 0.0001 |
| Dehalobacterium | 0.6 ± 0.077 | 0.0 ± 0.000 | 0.1 ± 0.031 | 0.0 ± 0.000 | < 0.0001 | < 0.0001 | 0.0077 |
| Unc Lachnospiraceae | 9.5 ± 0.293 | 0.3 ± 0.054 | 2.5 ± 0.156 | 12.8 ± 0.334 | < 0.0001 | < 0.0001 | < 0.0001 |
| Blautia | 0.1 ± 0.031 | 0.0 ± 0.000 | 0.1 ± 0.031 | 2.0 ± 0.140 | < 0.0001 | NS | < 0.0001 |
| Coprococcus | 0.8 ± 0.089 | 0.0 ± 0.000 | 0.8 ± 0.089 | 1.6 ± 0.239 | < 0.0001 | NS | < 0.0001 |
| Dorea | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.2 ± 0.044 | 0.0 ± 0.000 | NS | < 0.0001 | < 0.0001 |
| Oscillospira | 1.8 ± 0.132 | 0.1 ± 0.031 | 3.1 ± 0.173 | 0.8 ± 0.089 | < 0.0001 | < 0.0001 | < 0.0001 |
| Ruminococcus | 1.9 ± 0.136 | 0.0 ± 0.000 | 0.6 ± 0.077 | 0.2 ± 0.044 | < 0.0001 | < 0.0001 | < 0.0001 |
| Veillonella | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.1 ± 0.031 | 0.4 ± 0.063 | NS | 0.0020 | < 0.0001 |
| Unc Erysipelotrichaceae | 0.2 ± 0.044 | 0.1 ± 0.031 | 0.3 ± 0.054 | 16.6 ± 0.372 | 0.0019 | NS | < 0.0001 |
| Allobaculum | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.4 ± 0.063 | 0.0 ± 0.000 | NS | < 0.0001 | < 0.0001 |
| Eubacterium | 0.1 ± 0.031 | 0.1 ± 0.031 | 0.2 ± 0.044 | 16.6 ± 0.372 | NS | NS | < 0.0001 |
| Rhodobacter | 0.0 ± 0.000 | 0.1 ± 0.031 | 0.3 ± 0.054 | 0.1 ± 0.031 | 0.0019 | < 0.0001 | < 0.0001 |
| Sutterella | 0.0 ± 0.00 | 2.2 ± 0.146 | 0.0 ± 0.000 | 0.2 ± 0.044 | < 0.0001 | NS | < 0.0001 |
| Bilophila | 0.0 + 0.000 | 0.0 ± 0.000 | 0.1 ± 0.031 | 0.0 ± 0.00 | NS | < 0.0001 | < 0.0001 |
| Desulfovibrio | 3.0 ± 0.170 | 0.2 ± 0.044 | 7.4 ± 0.261 | 0.2 ± 0.044 | < 0.0001 | < 0.0001 | < 0.0001 |
| Flexispira | 0.0 ± 000 | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.7 ± .083 | NS | NS | < 0.0001 |
| Helicobacter | 0.0 ± 000 | 0.8 ± 0.089 | 0.0 ± 0.000 | 0.1 ± 0.031 | < 0.0001 | NS | < 0.0001 |
| Unc Enterobacteriaceae | 0.2 ± 0.044 | 9.7 ± 0.295 | 0.2 ± 0.044 | 0.6 ± 0.077 | < 0.0001 | NS | < 0.0001 |
| Klebsiella | 0.5 ± 0.070 | 37.5 ± 0.484 | 0.6 ± 0.077 | 2.7 ± 0.162 | < 0.0001 | NS | < 0.0001 |
| Haemophilus | 0.0 ± 000 | 0.0 ± 000 | 0.3 ± 0.054 | 0.1 ± 0.031 | NS | < 0.0001 | < 0.0001 |
| Bacteroides | 0.2 ± 0.044 | 26.7 ± 0.442 | 0.2 ± 0.044 | 1.4 ± 0.117 | < 0.0001 | NS | < 0.0001 |
| Prevotella | 0.0 ± 0.00 | 0.5 ± 0.070 | 0.1 ± 0.031 | 0.3 ± 0.054 | < 0.0001 | < 0.0001 | < 0.0001 |
| Unc S24-7 | 4.1 ± 0.115 | 4.9 ± 0.215 | 0.3 ± 0.054 | 1.4 ± 0.117 | < 0.0001 | < 0.0001 | < 0.0001 |
| Unc C111 | 0.7 ± 0.083 | 1.0 ± 0.099 | 0.4 ± 0.063 | 0.7 ± 0.083 | 0.0002 | < 0.0001 | < 0.0001 |
| Unc ACK-M1 | 0.8 ± 0.089 | 1.2 ± 0.108 | 0.5 ± 0.070 | 0.7 ± 0.083 | < 0.0001 | < 0.0001 | < 0.0001 |
| Rothia | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.7 ± 0.083 | 0.6 ± 0.077 | NS | < 0.0001 | NS |
| Unc Coriobacteriaceae | 0.1 ± 0.031 | 0.0 ± 0.000 | 2.7 ± 0.162 | 0.1 ± 0.031 | < 0.0001 | < 0.0001 | < 0.0001 |
| Adlercreutzia | 1.3 ± 0.113 | 0.1 ± 0.031 | 3.9 ± 0.193 | 5.6 ± 0.229 | < 0.0001 | < 0.0001 | < 0.0001 |
| Atopobium | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.1 ± 0.031 | NS | NS | < 0.0001 |
| Akkermansia | 0.0 ± 0.000 | 0.10 ± 0.031 | 0.0 ± 0.000 | 0.1 ± 0.031 | < 0.0001 | NS | < 0.0001 |
Values are expressed as the mean ± SD (n = 6). Data were analyzed by One-way ANOVA followed by Bonferroni post-hoc test, p ≤ 0.05, NS= not significant, CD: Standard- chow diet for 3 weeks; CD + A: Standard- chow diet for 3 weeks and subjected to amoxicillin treatment (50 mg/kg body weight) in 3rd week; HFD: high-fat diet for 3 weeks (73% energy from fat); HFD + A: High-fat diet for 3 weeks and subjected to amoxicillin treatment (50 mg/kg body weight) in 3rd week. Unc=Unculturable
Table 7 .
Effect of diet on mice gut microbiota at species level
| Species | Relative abundance (%) | Significance (adjusted p value) | |||||
|---|---|---|---|---|---|---|---|
| CD | CD + A | HFD | HFD + A | CD vs CD + A | CD vs HFD | HFD vs HFD + A | |
| Enterococcus casseliflavus | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.0 ± 0.000 | 2.6 ± 0.159 | NS | NS | < 0.0001 |
| Lactobacillus reuteri | 0.2 ± 0.044 | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.0 ± 0.000 | < 0.0001 | < 0.0001 | NS |
| Blautia producta | 0.1 ± 0.039 | 0.0 ± 0.000 | 0.1 ± 0.039 | 1.8 ± .0.132 | < 0.0001 | NS | < 0.0001 |
| [Ruminococcus] gnavus | 1.1 ± 0.104 | 0.1 ± 0.039 | 1.9 ± 0.136 | 0.1 ± 0.039 | < 0.0001 | < 0.0001 | < 0.0001 |
| Veillonella dispar | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.1 ± 0.039 | 0.4 ± 0.063 | NS | 0.0031 | < 0.0001 |
| Eubacterium dolichum | 0.1 ± 0.039 | 0.0 ± 0.000 | 0.2 ± 0.044 | 16.6 ± 0.372 | < 0.0001 | NS | < 0.0001 |
| Brevundimonas diminuta | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.1 ± 0.039 | 0.1 ± 0.039 | NS | 0.0002 | NS |
| Haemophilus Parainfluenzae | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.3 ± 0.054 | 0.1 ± 0.039 | NS | < 0.0001 | 0.0013 |
| Bacteroides acidifaciens | 0.1 ± 0.039 | 18.1 ± 0.385 | 0.1 ± 0.039 | 0.2 ± 0.044 | < 0.0001 | NS | 0.0021 |
| Bacteroides uniformis | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.3 ± 0.054 | NS | NS | < 0.0001 |
| Prevotella melaninogenica | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.0 ± 0.000 | 0.3 ± 0.054 | NS | NS | < 0.0001 |
| Rothia mucilaginosa | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.7 ± 0.083 | 0.50 ± 0.070 | NS | < 0.0001 | 0.0002 |
| Akkermansia muciniphila | 0.0 ± 0.00 | 0.1 ± 0.039 | 0.0 ± 0.00 | 0.10 ± 0.039 | < 0.0001 | NS | < 0.0001 |
Values are expressed as the mean ± SD (n = 6). Data were analyzed by One-way ANOVA followed by Bonferroni post-hoc test p ≤ 0.05, NS= not significant, CD: standard- chow diet for 3 weeks; CD + A: Standard- chow diet for 3 weeks and subjected to amoxicillin treatment (50 mg/kg body weight) in 3rd week; HFD: high-fat diet for 3 weeks (73% energy from fat); HFD + A: High-fat diet for 3 weeks and subjected to amoxicillin treatment (50 mg/kg body weight) in 3rd week
Lactobacillus is one of the most predominant genus in patients with Type 2 diabetes and Zucker Diabetic Fatty (ZDF) rats, and plays an active role in the development of chronic inflammation of diabetes and contributes to insulin resistance [20]. Desulfovibrio on the contrary is believed to contribute to the impairment of gut barrier and promotes insulin resistance [21, 22]. There is a positive correlation of Oscillospira with fasting serum insulin, and with a reduction in mRNA expression of Zonula occludens-1 (ZO-1) that prevent leakage of solutes from gut [22, 23]. Streptococcus can ferment sugars to yield lactic acid linked to the development of metabolic diseases [24]. The enrichment of Granulicatella is associated with obesity [25]. Allobaculum plays a crucial role in lipid deposition by repressing the Lipoprotein lipase (LPL) enzyme by expressing Angiopoietin-related protein (ANGPTL4) [26]. Rothia is known for its versatile metabolic capacities and provides growth substrates to Gram-negative non-mucus-degrading bacteria including Pseudomonas aeruginosa [27]. Amoxicillin treatment in HFD-fed mice showed a significant reduction of Lactobacillus, Turicibacter, Desulfovibrio, Oscillospira, Allobaculum, Streptococcus, Granulicatella (p < 0.0001) and a slight decrease of Rothia compared to the HFD-fed mice. The amoxicillin-treated CD-fed mice showed a significant decrease of Lactobacillus (p < 0.0001), Oscillospira (p < 0.0001) and Desulfovibrio (p < 0.0001), when compared to the CD-fed mice.
Interestingly, amoxicillin-treated HFD mice showed a significant increase in the relative abundance of Blautia, Coprococcus, Sutterella, Bacteroides and, Akkermansia as compared to mice receiving HFD, and CD diets. However, amoxicillin treatment in CD-fed mice caused a significant decrease in the relative abundance of Blautia and Coprococcus (p < 0.0001), and a significant increase in Akkermansia (p < 0.0001) compared to the CD-fed mice. In our study, we also observed a substantial increase in Bacteroides acidifaciens, Bacteroides uniformis, and Akkermansia muciniphila (p < 0.0001) in amoxicillin- treated HFD-fed mice compared to HFD-fed mice only. Likewise, amoxicillin treatment in CD-fed mice also increased the abundance of Bacteroides acidifaciens and Akkermansia muciniphila (p < 0.0001) compared to the mice receiving only CD. Importantly, Blautia has a negative correlation with the fasting plasma glucose levels and haemoglobin A1C (HbA1c) levels [28]. Further, Blautia also plays an important role in repressing the colonization of pathogenic bacteria in the intestine [29]. Similarly, Coprococcus is shown to negatively correlate with hypertension, body mass index (BMI), and body fat percentage [30, 31]. Bacteroides acidifaciens and Bacteroides uniformis induce Glucagon-like peptide-1 (GLP-1) activation by using the G-protein-coupled bile acid receptor (TGR5) through bile acids, taurine, and cholate that counters obesity and increases insulin sensitivity in mice [32]. Akkermansia muciniphila decreases the expansion of inflammation markers in adipose tissue, lipid metabolism and improves insulin sensitivity [33].
In this study, we also observed a slight rise in Enterococcus and a significant increase in Eubacterium, Klebsiella and Prevotella (Table 6) in HFD-fed mice compared to the CD-fed mice (p < 0.0001). Amoxicillin treatment of HFD-fed mice could not diminish this effect as substantiated by an abundance of Enterococcus, Eubacterium, Klebsiella and Prevotella (p < 0.0001) in these mice. Similarly, amoxicillin treatment in CD-fed mice also showed a substantial rise in Klebsiella, Prevotella, and Helicobacter compared to mice on CD only (p < 0.0001). We also observed a considerable increase in Enterococcus casseliflavus and Prevotella melaninogenica in amoxicillin-treated HFD mice compared to mice receiving HFD alone, or CD alone (p < 0.0001).
Our results clearly indicate that treatment with amoxicillin reshapes the gut microbiota in both HFD-fed mice as well as in CD-fed mice. Amoxicillin treatment could remodel the gut microbiota to allow inhabitation of beneficial microbes associated with improving the pathophysiology of metabolic syndrome as observed in HFD-fed mice. Nonetheless, amoxicillin treatment could not reduce the gut pathogens appearing in mice receiving HFD.
Amoxicillin treatment in HFD-fed mice has non-significant impact on histopathology of vital organs
To investigate whether antibiotic treatment could negatively impact the vital organs, we performed histopathological analysis of the heart, liver and kidney. We observed that no major histopathological damage occurs in CD-fed mice, HFD-fed mice and mice receiving amoxicillin treatment with these diets (Fig. 9). The heart section of HFD-fed mice exhibited mild congestion of blood vessels (Fig. 3a). Likewise, liver histology also indicated mild congestions in the sinusoidal, central and portal veins in the HFD-fed mice (Fig. 3b). Amoxicillin treatment of these HFD-fed mice showed normal histopathological architecture of the heart, liver and kidneys (Fig. 4a–c). We did not observe any changes in histopathological analysis of CD-fed mice with or without amoxicillin treatment. Overall, albeit promising, these observations are insufficient to conclude a positive effect of amoxicillin treatment on improvement of mild histopathological changes observed in HFD-fed mice.
Fig. 9.
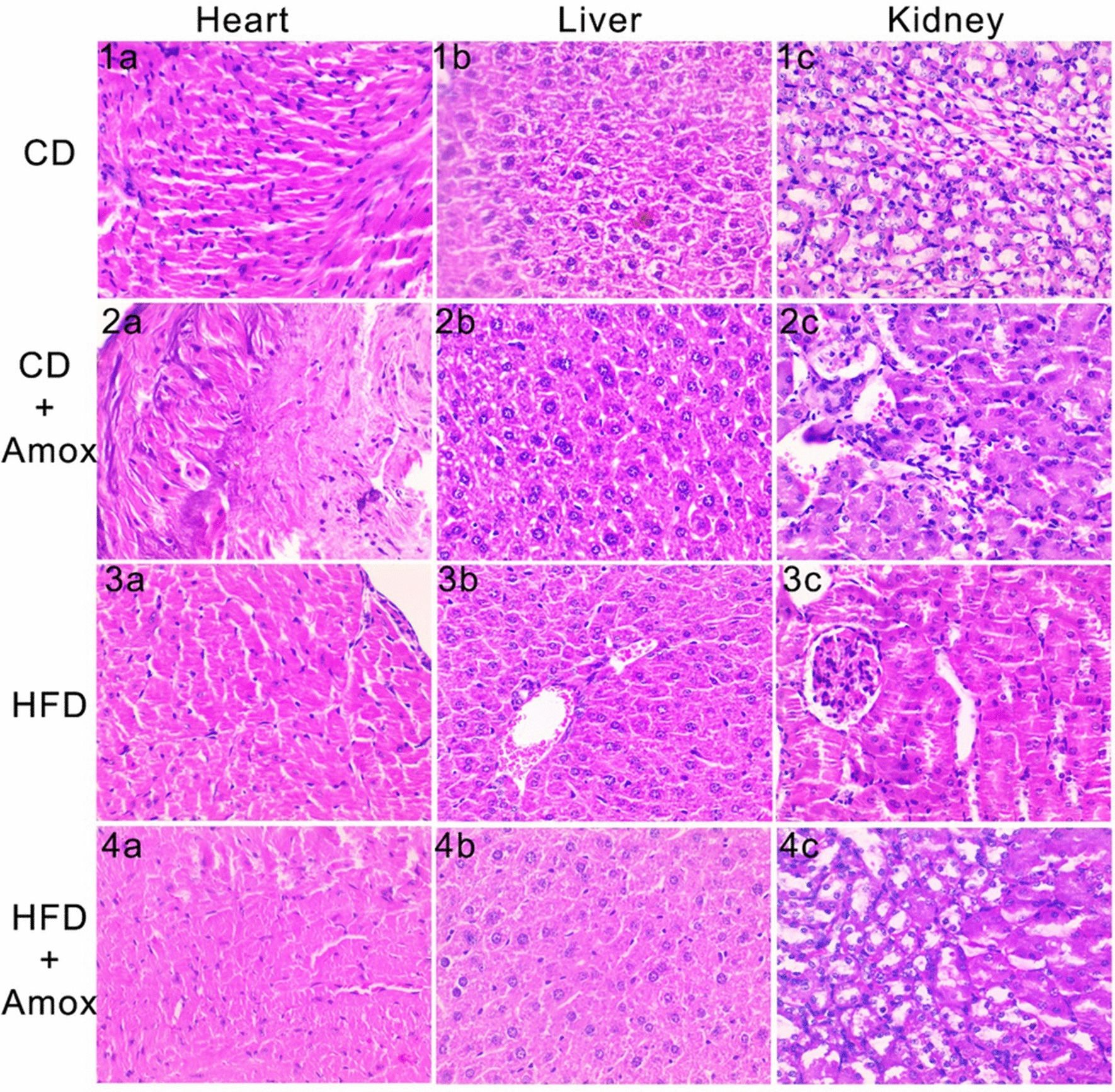
Impact of high-fat diet (3 weeks) and amoxicillin treatment on mice heart, liver, and kidney. The figure represents histopathogical examination of heart, liver and kidney sections by hematoxylin and eosin staining. The heart section of the HFD group showed congestion in the blood vessels (3a). Liver histology in the HFD group (3b) observed mild sinusoidal congestion of the central and portal vein. The kidney section in the HFD group showed normal architecture (3c). The heart (4a) liver (4b) and kidney (4c) histopathological in HFD + Amox showed no sign of morphological changes. CD: Standard- chow diet for 3 weeks; CD + Amox: Standard- chow diet for 3 weeks and subjected to amoxicillin treatment in 3rd week; HFD: High-fat diet for 3 weeks; HFD + Amox: High-fat diet for 3 weeks and subjected to amoxicillin treatment in 3rd week
Amoxicillin treatment in HFD-fed mice does not negatively impact macrophage's ability to clear the infection
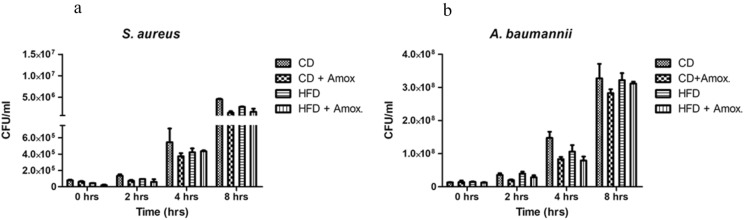
We next wanted to investigate whether treatment with amoxicillin could impact the immune cell function associated with the anti-microbial action. Therefore, we analyzed the effect of amoxicillin treatment on antibacterial activity of primary immune cells i.e., macrophages. For this, we used macrophage infection assays with S. aureus, and A. baumannii, the two major clinically relevant microbial pathogens (Fig. 10). We did not observe any significant difference in the recovery of colony forming units (CFU) from peritoneal macrophages isolated from mice fed on CD, CD + Amox, HFD and HFD + Amox at times 0, 2, 4, and 8 h post-infection. These data clearly show that treatment with amoxicillin did not impair the cell's susceptibility and ability to clear microbial infections.
Fig. 10.
Impact of high-fat diet (3 weeks) and amoxicillin treatment on anti-bacterial activity of macrophages against S. aureus and A. baumannii. CFU recovery from peritoneal macrophages isolated from mice fed on different diets: Data represents mean ± SEM, One-way ANOVA followed by Bonferroni post-hoc test. p ≤ 0.05, NS= not significant. CFU recovery of (a) S. aureus, and (b) A. baumannii at times 0, 2, 4, and 8 h (hrs) post-infection. There is no significant difference in CFU across the groups indicating that amoxicillin treatment does not significantly impair cell’s susceptibility and ability to clear microbial infections. All experiments were performed in triplicates. CD: Standard-chow diet for 3 weeks; CD + Amox: Standard-chow diet for 3 weeks and subjected to amoxicillin treatment in 3rd week; HFD: High-fat diet for 3 weeks; HFD + Amox: High-fat diet for 3 weeks and subjected to amoxicillin treatment in 3rd week
Discussion
Metabolic syndrome induced by HFD has become a major health problem that increases the risk of individuals to many other chronic conditions. Despite the increased propensity to infections in individuals, the link between duration of HFD and antibiotic treatment, and its impact on functionality and diversity of the gut microbiome and features of metabolic syndrome is not well established. In this study, we wish to investigate how antibiotic treatment influences the gut microbiota and other pathophysiological parameters in mice fed on a high-fat diet for a short period. Therefore, we performed a short-term HFD feeding experiment of 3 weeks involving amoxicillin intervention in the 3rd week in a mice model. Firstly, our study has provided a conclusive evidence that HFD, even for a short period, could lead to the reshaping of gut microbiota at the genus and the phylum level that are associated with the development of pathophysiological features typical of metabolic syndrome [34]. Further, we also observed that amoxicillin treatment preferentially improves these pathophysiological attributes associated with metabolic state during consumption of HFD. According to our findings, HFD-fed mice showed an increase in taxonomic diversity and richness, particularly an appearance of specific gut bacteria such as Unc Gemellaceae, Granulicatella, Enterococcus, Streptococcus, Dorea, Turicibacter, Veillonella, Allobaculum, Bilophila, Prevotella, and Rothia. Nonetheless, amoxicillin treatment in HFD-fed mice broadly depleted these specific species namely Gemellaceae, Granulicatella, Streptococcus, Dorea, Turicibacter, Allobaculum, and Rothia. Intrestingly though, amoxicillin treatment of HFD-fed mice resulted in the exclusive appearance of specific gut bacteria such as Unc Clostridiaceae, Sutterella, Flexispira, Helicobacter, Atopobium and Akkermansia. These data show that each dietary group in our study had a distinct gut microbiota composition.
Consistently we observed a significant increase in the F/B ratio associated with pathophysiological features of the obesity- associated metabolic state along with an increase in the relative abundance of Proteobacteria and Actinobacteria in HFD-fed mice [35, 36]. On the other hand, amoxicillin treatment of these HFD-fed mice almost restores the F/B ratio to normal, and comparable to the CD-fed mice with a concomitant decline in Proteobacteria and Actinobacteria. We also observed a significant reduction in Lachnospiraceae, and S24-7 in HFD-fed mice. The depletion of these families are positively associated with disruption of gut epithelial barrier integrity and metabolic endotoxemia [4, 15]. The reduction in the relative abundance of Lachnospiraceae and S24-7 was previously known for long-term HFD. That a 3-weeks HFD is sufficient to reduce the abundance of Lachnospiraceae, and S24-7 is remarkable. However, treatment with amoxicillin in HFD-fed mice increased the relative abundance of Lachnospiraceae and S24-7. Therefore, our results suggest that amoxicillin treatment can be beneficial to moderate the inflammatory state associated with metabolic syndrome rather than further aggravating the pathophysiology of metabolic state observed with other antibiotics [37–39].
At genus level, we observed an abundance of Lactobacillus, Turicibacter, Desulfovibrio, Oscillospira, Allobaculum, Streptococcus, Granulicatella, and Rothia in mice receiving HFD only for 3 weeks. This is consistent with the previous reports on the abundance of these gut bacteria in the long-term high-fat diet [40–43]. This shows that alteration in gut microbiota takes place expeditiously following the changes in dietary habits. Further, most of the above-mentioned gut microbes contribute significantly to the development of pathogenesis of metabolic syndrome and gut inflammatory diseases. The abundance of Lactobacillus and Turicibacter positively correlates with blood glucose levels [19]. Further, several reports have indicated that an abundance of Lactobacillus is critical for the prevalence of obesity and contributes to the development of chronic inflammation in diabetes [44–47] Moreover, Lactobacillus is involved in insulin resistance [48] and impacts enzymatic activity of bile salt hydrolase to disturb lipid and glucose metabolism and contributing to development of Type 2 diabetes [49].
Similarly, the increase of Desulfovibrio and Oscillospira is associated with development of insulin resistance [21–23]. Likewise, enrichment of Streptococcus, Granulicatella, and Allobaculm is associated with metabolic diseases [24–26]. Rothia is known to provide growth substrates to Gram-negative non-mucus-degrading bacteria [27]. The most striking feature in HFD-fed mice was the diminution of Lactobacillus reuteri that prevents the growth of pathogens in the gut [18, 19]. On the contrary, administration of amoxicillin in HFD-fed mice caused a significant decrease in Lactobacillus, Turicibacter, Desulfovibrio, Oscillospira, Streptococcus, Granulicatella., and a slight decrease in Rothia. Therefore, our data suggest that amoxicillin treatment could positively improve the metabolic syndrome pathology by improving the insulin signalling and maintaining intestinal barrier integrity by decreasing the abundance of microbiota linked to obese related metabolic disorders and gut inflammatory diseases.
Next, we did not observe any significant change in the abundance of Blauti, Coprococcus, Sutterella, Bacteroides, and Akkermansia in HFD-fed mice as is also reported for the long-term HFD studies [42, 43, 50–52]. Amoxicillin treatment in HFD-fed mice showed an increase in the relative abundance of Blautia, Coprococcus, Sutterella, Bacteroides and Akkermansia. The increase in the relative abundance of Coprococcus, Blautia, Bacteroides acidifaciens, Bacteroides uniformis, and Akkermansia muciniphila [28–33] was positively correlated with insulin sensitivity and counter of obesity. We also detected Blautia producta in amoxicillin-treated HFD mice which is known to inhibit the growth of pathogens [53]. Taken together, we argue that amoxicillin treatment during HFD can positively influence metabolic syndrome pathophysiology by augmenting the beneficial gut bacteria.
Apart from the beneficial effects of modulating gut microbiota by amoxicillin in HFD-fed mice, we consistently observed an increase in the relative abundance of other gut bacteria such as Erysipelotrichaceae [54], Enterobacteriaceae [24], Bacteroidaceae [51], Enterococcus [21], Eubacterium [24], Klebsiella [6] and Prevotella [11] in HFD-fed mice. Amoxicillin treatment could not diminish their abundance. The potential flourish of Erysipelotrichaceae after broad-spectrum antibiotic treatment has also been observed in earlier long-term HFD studies [55]. However, we observed a decrease of Erysipelotrichaceae in amoxicillin-treated CD groups. Enterobacteriaceae and Bacteroidaceae families are symbionts helping in maintenance of gut health [51]. However, their abundance has also been observed during acute infective processes [16]. We also observed a significant increase in abundance of these families in the amoxicillin-treated CD group. The rise in Eubacterium is associated with critical virulence factors that cause gut inflammation and various human disease states [56–58]. Klebsiella and Prevotella strains also have pathogenic properties that promote inflammatory bowel disease or other inflammatory diseases [59, 60]. It is worth mentioning that we detected Enterococcus casseliflavus and Prevotella melanogenic enrichment which is linked with inflammatory diseases [61, 62]. Thus, we observed that amoxicillin treatment in HFD-fed mice increased few gut intestinal bacterial species including potential pathogens that could impact metabolic syndrome pathology.
Consistently, we observed a significant increase in total cholesterol in HFD-fed mice. Amoxicillin treatment caused a slight but non-significant increase in the cholesterol levels which could be attributed to the increase in expression of hepatic HMG-CoA reductase [63, 64]. Nonetheless, amoxicillin treatment in mice receiving standard CD had significantly reduced cholesterol levels. We noticed a significant decrease in triglycerides (TG) concentration in high-fat diet mice, as is also shown in other studies [65]. This could be attributed to the elevated insulin concentrations that could inhibit hepatic very-low-density lipoprotein (VLDL) secretions and stimulate adipose TG uptake thereby contributing to reduced serum TG levels [65]. On the other hand, amoxicillin treatment in HFD-fed mice did not cause any significant effect on the TG levels but caused a marked decrease in the CD group. Further amoxicillin treatment in HFD-fed mice also restored levels of AST suggesting a beneficial role of amoxicillin in the improvement of liver function.
We next observed that HFD for a short period (> 3 weeks) significantly increased blood glucose levels [66]. We also observed an alteration of gut microbiota in HFD-fed mice that could be directly involved in developing peripheral insulin resistance [66]. It is worth mentioning that amoxicillin-treatment of HFD-fed mice significantly reduced the blood glucose levels indicating its hypoglycaemic effect. Amoxicillin may cause depletion of the microbiome, which in turn could alter glucose homeostasis to affect systemic glucose metabolism via shaping gut microbial communities and regulating gene expression programs in the intestine and liver [67, 68]. The improvement in glucose tolerance following administration of antibiotics has also been observed with vancomycin and could be attributed to the increased abundance of Akkermansia muciniphila. We also observed a significant increase in the relative abundance of Lachnospiraceae, S24-7, Oscillospira, and an increase in the relative abundance of Coprococcus, Blautia, Bacteroides acidifaciens, Bacteroides uniformis and Akkermansia muciniphila, primarily restricted to amoxicillin-treated HFD-fed mice. These specific microbiotas could be involved in the restoration of blood glucose levels in this group.
Based on the above discussion, we believe our findings have an excellent clinical potential as amoxicillin treatment in metabolic syndrome pathophysiology could improve host insulin signalling, leading to a decrease in blood glucose level. There could be a concern about negative effects on the gut microbiota, exerted by antibiotics including reduced species diversity and altered metabolic activity which may lead to antibiotic-associated diarrhoea and recurrent Clostridioides difficile infections [9, 69, 70]. However, increasing reports about the beneficial effects of antibiotic treatment are emerging as well. For example, a blood pressure-lowering effect in a patient with treatment-resistant hypertension is observed when treated with a combination of antibiotics [71]. Therefore, the therapeutic intervention focusing on modulating microbiota by antibiotics is an attractive concept. Studies have also shown that fasting blood glucose and HbA1c levels in mice positively correlates with Lactobacillus abundance [20]. In our study, the reduction in the Lactobacillus abundance may explain improved glucose levels in amoxicillin-treated HFD-fed mice. Similarly, we also observed a significant remodelling of the gut microbiota towards a predominantly beneficial skew, probably contributing to the improved biochemical and physiological outcomes in amoxicillin-treated HFD group. Therefore, our study supports the potential beneficial role of amoxicillin treatment in short-term high-fat diet especially in individuals with diabetes as shown by restoration of blood glucose levels in HFD-fed mice. Nonetheless, further research would be required to explore the detailed mechanisms involving amoxicillin-mediated effects on blood glucose levels.
Long-term HFD studies have shown a significant decrease in haematological parameters [72, 73]. In our study, we observed a slight decline in the value of Hb, RBC, MCH and a considerable reduction in HCT and MCV in HFD-fed mice. Amoxicillin treatment in HFD-fed mice and CD group did not manifest any significant alteration in haematological parameters. Prolonged exposure to HFD (> 4 weeks) induces a depressive effect on thrombocytes in mice [74]. HFD consumption elevates IL-6 and thrombopoietin (TPO) levels that induce platelet production [75]. Remarkably, we observed that amoxicillin treatment caused restoration of the thrombocytes in HFD-fed mice towards the normal range. In contrast, amoxicillin treatment in the CD group did not have any significant effect on the thrombocytes value. Our data suggest that amoxicillin treatment in metabolic pathologies such as HFD could potentially restore thrombocytes towards normal value. Further, we also observed that amoxicillin benefits other blood circulating parameters including restoring levels of liver enzyme AST. Therefore, the possibility of improving liver function and reducing the risk of clotting, stroke or heart attack by amoxicillin appears to be an attractive preposition in individuals consuming HFD.
A short-term HFD intake influences the liver's susceptibility to inflammatory stimuli through the induction of a pro-coagulation state in mice livers [76]. This study observed a mild congestion in the blood vessels of the liver and heart in HFD-fed mice. Consistently, we did not observe significant damage to the kidneys [77]. Amoxicillin-treated HFD-fed mice showed normal heart, liver and kidney architecture. Our data revealed no significant histopathological changes to vital organs including the heart, liver and kidney. It is therefore clear that amoxicillin treatment did not cause any damage to the vital organs.
Next, we also examined the impact of HFD on body weight and food intake. Our results are consistent with the established observations which demonstrate that weight increase is gradual, and it becomes apparent only after 4 weeks of treatment. Typically, only after 16–20 weeks of a high-fat diet, the mice exhibit ~ 20–30% increase in body weight compared to CD-fed mice [78, 79]. Previous studies have shown that rodents of different age groups fed on a long-term HFD display more body weight gain as compared to CD-fed animals [80, 81]. Moreover, the rate of weight gain in adult animals were higher due to their slower metabolic rate as compared to younger animals. However, amoxicillin treatment in HFD-fed mice did not show any significant difference on weight gain. Therefore, in our study, the lack of any significant weight gain in HFD-fed mice could be due to short-duration of HFD and/ or use of young adult mice.
Lastly, we also examined whether amoxicillin treatment in mice may adversely impact the ability of immune cells to clear microbial infections. By using two priority pathogens commonly occurring in hospital patients, namely S. aureus and A. baumannii, we found that amoxicillin treatment administered for a short duration (1-week) did not negatively impact cells' anti-microbial activity. Infection of peritoneal macrophages, one of the major cell types responsible for anti-microbial action, did not reveal a significant difference in the CFU recovery across various groups, neither with S. aureus nor with A. baumannii. There is no adverse impact on macrophages' ability to clear the infection, emphasising the beneficial aspects of amoxicillin in the pathophyiological metabolic state. Thus the minor increase observed in the abundance of gut pathogens in amoxicillin-treated groups could be well taken care of by macrophages and would not be a major concern especially when other gut microbiota involved in improving insulin sensitivity are significantly improved.
Conclusions
In summary, our findings support the idea that amoxicillin treatment in the high-fat diet has potential beneficial impact on biochemical parameters, as indicated by our hematology and serum biochemistry investigations. Furthermore, the gut microbiota undergoes a significant remodelling towards a predominantly beneficial effect, probably contributing to the improved biochemical and physiological outcomes. In addition, there is no adverse impact on macrophages' ability to clear the infection, emphasising the beneficial aspects of an amoxicillin-based regimen for improved health outcomes. Therefore, amoxicillin may reduce the risk of heart attack, stroke or a clot in blood vessels, and promises to be an attractive proposition in individuals consuming HFD. Extrapolation for these findings from animals to humans however will require further research and more advanced studies.
Taken together, we suggest that amoxicillin has a beneficial effect on the pathophysiology of metabolic state during intake of HFD. We believe our study provides a proof-of-principle to support a prophylactic amoxicillin treatment in individuals having a high-fat diet pathophysiology for better biochemical and gut health. Further studies would be required to evaluate long-term effects of antibiotic administration, especially in the context of diabetics.
Methods
Animal Model
According to the NIH guide for the Care and Use of Laboratory Animals (National Research Council, USA), all the experimental procedures were conducted and approved by the Institutional Animal Ethics Committee (IAEC) of All India Institute of Medical Sciences (AIIMS), New Delhi. All mice were maintained at temperature (22 ± 3 °C) and humidity (40–60%) on a 12 h light/dark cycle with free access to drinking water and rodent diet throughout the study. From the same cohort, twenty-four male C57BL/6 J mice of 6 weeks old (initial weight 16–18 g) were allocated into four groups (N = 6 mice/group). The standard chow diet (CD) (10% energy from fat) and high-fat diet (HFD, 73% energy from fat including lard, cholesterol, and veg oil) were fed to CD and HFD groups, respectively for 3 weeks. The formula for the HFD was as follows: lard (380 g /kg), cholesterol (10 g/kg), vegetable oil (60 g/kg), casein 75% (240 g/kg), vitamin mix (10 g/kg), cellulose (50 g/kg), mineral mix (5 g/kg), and starch (200 g/kg). The diet groups were assigned as follows: CD for control, HFD-treated, CD + Amox and HFD + Amox. The amoxicillin treatment was given in the 3rd week at 50 mg/kg body weight, i.e., 0.25 mg/ml in drinking water as per the recommended doses used with mice [82]. The treatment/diet group was CD + Amox for positive control, and HFD + Amox for treated mice. Body weight, water and feed intake was recorded for 3 weeks. After 3 weeks, all animals were euthanized, and samples were collected to observe the effects of amoxicillin on HFD and CD groups.
Blood, Tissue and Caecal samples Collection
After 3 weeks, mice from these groups were allowed to fast overnight, and the blood samples were collected via cardiac puncture as a terminal procedure. Following blood collection, the organs (Heart, Liver and Kidney) were harvested for histopathological examination. Caecal content samples were taken shortly after dissection and immediately frozen in liquid nitrogen and stored at − 80 °C until further use.
Haematology and Serum Biochemistry
Serum biochemistry was performed using serum auto-analyzer Screen Master 3000, Tulip, Alto Santa Cruz, India, then quantifying Coral GPO-PAP kit (CORAL Clinical systems, Goa, India) using manufacturers’ instructions. The haematology analysis was carried out using an automated vet haematology counter (Melet Schloesing Laboratories, Guwahati, India) as per the manufacturer's instruction.
Histopathology
After euthanasia, the liver, kidney and heart tissues were dissected and were fixed overnight in 10% neutral buffer formalin and embedded in paraffin blocks. For haematoxylin–eosin (H & E) staining, tissue sections of 4–5 μm thickness were collected on poly-L-lysine (Sigma) coated slides using a microtome and stained according to standard protocols. The digital images were taken by light microscopy (Olympus CX-29: Olympus Optical Co. Ltd, Tokyo, Japan) and camera (Magnus DC 10).
16S rRNA Gene Sequencing
Caecal samples were sent for next-generation sequencing and genus analysis (DNA Xperts Private Limited, India). According to the manufacturer's instructions, the total genomic DNA of gut microbiota was extracted using Qiagen DNA Stool Mini Kit. DNA concentration and integrity were assessed by fluorometer and agarose gel electrophoresis, respectively. The 16S rRNA gene amplicon sequencing was performed on the Illumina MiSeq according to protocols described by Illumina Miseq high-throughput sequencer guide [83]. The hypervariable region V3- V4 of the bacterial 16S rRNA gene was amplified by using the universal primer 515f, 5'-GTGCCAGCMGCCGCGGTAA-3'; and 806r, 5'-GGACTACHVGGGTWTCTAAT-3' on the Illumina MiSeq platform.
Microbial Bioinformatics Analysis
The raw data were filtered to capture clean reads by eliminating the adapter pollution and low-quality sequences [84]. By using FLASH (Fast Length Adjustment of Short reads v 1.2.1), the high-quality paired-end reads were combined with tags with an average read length of 252 bp [85]. According to their unique barcode and primer sequences, the reads were assigned to each sample using Mothur software (V1.35.1, http://www.mothur.org). The barcodes as well as primers were removed to get effective Clean Tags. The tags were then clustered as OTU (Operational Taxonomic Unit) with a 97% similarity threshold [86].
Taxonomic classification of the representative OTU was assigned by using three commonly used databases [Greengenes, SILVA and Ribosomal Database Project (RDP)]. Finally, an OTU table and a phylogenetic tree were generated for diversity analysis by calculating Chao1, observed species, Shannon indices and Simpson indices [87].
Bacterial Strain and Culture Condition
This study used two opportunistic pathogens, namely Staphylococcus aureus (MTCC # 1430) and Acinetobacter baumannii (MTCC # 1425) to evaluate the impact of treatment on macrophage health and infection [88]. Briefly, S. aureus and A. baumannii were cultured on LB broth at 37 °C overnight under shaking at 180 rpm [89]. We determined the OD590 of the growing cultures and used this to calculate the multiplicity of infection (MOI) for the experiment.
Microbial infections in Primary Macrophages
Mice peritoneal macrophages (PM) were isolated from the peritoneal cavity as mentioned in our previous studies [90, 91]. RBCs were removed from macrophages by using the RBC lysis buffer followed by washing with 1X PBS. PM were cultured and grown in RPMI 1640 supplemented with 10% fatal bovine serum and penicillin–streptomycin (100 U/mL penicillin, 100 µg/mL streptomycin) at 37 °C in CO2 incubator for 3 days. For infection assays, 5 × 104 cells/well were seeded in a 24-well plate in complete media and cells were allowed to rest for 24 h. After 24 h, macrophages were washed and infected either with S. aureus or with A. baumannii for 2 h with an MOI of 1:3. Subsequently, cells were treated with media containing antibiotics (gentamicin, 200 µg/mL) for 1 hour to eliminate extracellular bacteria. This time post-treatment was considered as time zero. Infected cells were kept for 2, 4 and 8 h in complete media to monitor progress of infection. For CFU enumeration, cells were lysed with 0.025% SDS at required time points and cell lysates were serially diluted and plated on LB agar plates. Plates were incubated at 37 °C overnight followed by CFU enumeration.
Statistical Analysis
GraphPad Prism version 9.2.0 (3.2.0) for Windows, Graph Pad Software, San Diego, California USA, http://www.graphpad.comwas used for the statistical analysis of experimental data. Values are expressed as the mean ± Standard Deviation (SD). Groups were compared by One-way analysis of variance (ANOVA) followed by the Bonferroni post-hoc test for comparisons between multiple groups with a value of p ≤ 0.05 as the cut-off for statistical significance.
Acknowledgements
This work in part by Life Science Research Board (LSRB), DRDO, India (LSRB-375/SH & DD/2020) to VS. The research in the Laboratory of Infection Biology and Translational Research is supported funding to [VS] through HarGobind Khorana Innovative Young Biotechnologist Award (BT/11/IYBA/2018/01), DST-SERB (CRG/2018/004510), DBT (BT/PR39032/ADV/90/285/2020). We greatly appreciate Dr. Perumal Nagarajan and Dr. Surender Yadav (National Institute of Immunology, New Delhi, India), and Dr P.K. Yadav (AIIMS, New Delhi) for guiding experimental and statistical support.
Abbreviations
- CD
Standard chow diet
- HFD
High-fat diet
- CD + Amox
Standard chow diet + Amoxicillin
- HFD + Amox
High-fat diet + Amoxicillin
- Hb
Haemoglobin
- RBC
Red blood cells
- MCH
Mean corpuscular haemoglobin
- HCT
Haematocrit
- MCV
Mean corpuscular volume
- WBC
White blood cells
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- TG
Triglycerides
- LPS
Lipopolysaccharide
- ZO-1
Zonula occludens-1
- ANGPTL4
Angiopoietin-related protein
- LPL
Lipoprotein lipase
- SCFAs
Short-chain fatty acids
- HbA1c
Haemoglobin A1C
- VRE
Vancomycin-resistant enterococci
- GLP-1
Glucagon-like peptide 1
- TGR5 receptor
G-protein-coupled bile acid receptor 5
- IBD
Inflammatory bowel disease
- NEC
Necrotizing enterocolitis
- CRC
Colorectal cancer
- VLDL
Very low-density lipoprotein
- HMG-CoA reductase
3-hydroxy-3-methyl-glutaryl-coenzyme A
- OTUs
Operating taxonomic units
- PCoA
Principal coordinates analysis
- PM
Peritoneal macrophage
- MOI
Multiplicity of infection
- OD
Optical density
- MTTC
Microbial type culture collection and gene bank
- RPMI
Roswell park memorial institute
- CFU
Colony forming unit
- SDS
Sodium dodecyl sulphate
Author contributions
Design and conceptualization: VS and VSR; Performed the experiments: SK and AA, Wrote the manuscript: SK and VS; Data analysis and interpretation: SK, VS; Funding: SK, VS. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The Sequence Read Archive (SRA) submission datasets generated and/or analysed during the current study are submitted to SRA data repository of National Centre for Biotechnology, USA. Submission Id: SUB11233254; Bio Project ID: PRJNA821450. https://submit.ncbi.nlm.nih.gov/subs/bioproject/SUB11233254/overview.
Declarations
Ethics approval
All care, maintenance, and experimental protocols were approved by the Institutional Animal Ethics Committee (IAEC) of All India Institute of Medical Sciences, New Delhi. The infection assays were as per the approved protocols by Institutional Biosafety Committee, AIIMS (IBSC0819/VS).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Suresh Kumar, Email: suresh.kumar@nib.gov.in.
Vikram Saini, Email: vikram@aiims.edu.
References
- 1.Tilg H, Zmora N, Adolph TE, Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol. 2020 doi: 10.1038/s41577-019-0198-4. [DOI] [PubMed] [Google Scholar]
- 2.Duan Y, Zeng L, Zheng C, Song B, Li F, Kong X, Xu K. Inflammatory links between high fat diets and diseases. Front Immunol. 2018 doi: 10.3389/fimmu.2018.02649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guillemot-Legris O, Masquelier J, Everard A, Cani PD, Alhouayek M, Muccioli GG. High-fat diet feeding differentially affects the development of inflammation in the central nervous system. J Neuroinflammation. 2016 doi: 10.1186/s1297401606668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang Y, Khafipour E, Derakhshani H, Sarna LK, Woo CW, Siow YL, O K. Short term high fat diet induces obesity-enhancing changes in mouse gut microbiota that are partially reversed by cessation of the high fat diet. Lipids. 2017 doi: 10.1007/s11745-017-4253-2. [DOI] [PubMed] [Google Scholar]
- 5.Evans CC, LePard KJ, Kwak JW, Stancukas MC, Laskowski S, Dougherty J, Moulton L, Glawe A, Wang Y, Leone V, Antonopoulos DA, Smith D, Chang EB, Ciancio MJ. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS. 2014 doi: 10.1371/journal.pone.0092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniel H, Gholami AM, Berry D, Desmarchelier C, Hahne H, Loh G, Mondot S, Lepage P, Rothballer M, Walker A, Böhm C, Wenning M, Wagner M, Blaut M, Schmitt-Kopplin P, Kuster B, Haller D, Clavel T. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014 doi: 10.1038/ismej.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008 doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol. 2011 doi: 10.1038/nrmicro2536. [DOI] [PubMed] [Google Scholar]
- 9.Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol. 2020 doi: 10.3389/fcimb.2020.572912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akhavan BJ, Khanna NR, Vijhani P. Amoxicillin. Florida: In Treasure StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 11.Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020 doi: 10.3390/microorganisms8111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magne F, Gotteland M, Gauthier L, et al. The firmicutes/bacteroidetes ratio a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020 doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmas V, Pisanu S, Madau V, et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci Rep. 2021 doi: 10.1038/s41598-021-84928-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke SF, Murphy EF, Nilaweera K, et al. The gut microbiota and its relationship to diet and obesity: new insights. Gut Microbes. 2012 doi: 10.4161/gmic.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serino M, Luche E, Gres S, Baylac A, Bergé M, Cenac C, Waget A, Klopp P, Iacovoni J, Klopp C, Mariette J, Bouchez O, Lluch J, Ouarné F, Monsan P, Valet P, Roques C, Amar J, Bouloumié A, Théodorou V, Burcelin R. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut. 2012 doi: 10.1136/gutjnl-2011-301012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King CH, Desai H, Sylvetsky AC, LoTempio J, Ayanyan S, Carrie J, Crandall KA, Fochtman BC, Gasparyan L, Gulzar N, Howell P, Issa N, Krampis K, Mishra L, Morizono H, Pisegna JR, Rao S, Ren Y, Simonyan V, Smith K, VedBrat S, Yao MD, Mazumder R. Baseline human gut microbiota profile in healthy people and standard reporting template. PLoS ONE. 2019 doi: 10.1371/journal.pone.0206484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaakoush NO. Insights into the role of erysipelotrichaceae in the human host. Front Cell Infect Microbiol. 2015 doi: 10.3389/fcimb.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi H, Wang L, Xiong Y, Wen X, Wang Z, Yang X, Gao K, Jiang Z. Effects of barrier function in weaned pigs. J Anim Sci. 2018 doi: 10.1093/jas/sky129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J, Qiao Y, Qi C, Jiang W, Xiao H, Shi Y, Le GW. High-fat-diet-induced obesity is associated with decreased antiinflammatory Lactobacillus reuteri sensitive to oxidative stress in mouse Peyer's patches. Nutrition. 2016 doi: 10.1016/j.nut.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Zhou W, Xu H, Zhan L, Lu X, Zhang L. Dynamic development of fecal microbiome during the progression of diabetes mellitus in zucker diabetic fatty rats. Front Microbiol. 2019 doi: 10.3389/fmicb.2019.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagao-Kitamoto H, Kamada N. Host-microbial cross-talk in inflammatory bowel disease. Immune Netw. 2017 doi: 10.4110/in.2017.17.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam YY, Ha CW, Campbell CR, Mitchell AJ, Dinudom A, Oscarsson J, Cook DI, Hunt NH, Caterson ID, Holmes AJ, Storlien LH. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS ONE. 2012 doi: 10.1371/journal.pone.0034233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q, Xiao X, Li M, Yu M, Ping F, Zheng J, Wang T, Wang X. Vildagliptin increases butyrate-producing bacteria in the gut of diabetic rats. PLoS. 2017 doi: 10.1371/journal.pone.0184735/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He C, Cheng D, Peng C, Li Y, Zhu Y, Lu N. High-fat diet induces dysbiosis of gastric microbiota prior to gut microbiota in association with metabolic disorders in mice. Front Microbiol. 2018 doi: 10.3389/fmicb.2018.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, Cai Q, Zheng W, Steinwandel M, Blot WJ, Shu XO, Long J. 2019 Oral microbiome and obesity in a large study of low-income and African-American populations. J Oral Microbiol. 2019;10(1080/20002297):1650597. doi: 10.1080/20002297.2019.1650597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernández-Hernando C, Suárez Y. ANGPTL4: a multifunctional protein involved in metabolism and vascular homeostasis. Curr Opin Hematol. 2022 doi: 10.1097/MOH.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uranga CC, Arroyo P, Jr, Duggan BM, Gerwick WH, Edlund A. Commensal oral rothia mucilaginosa produces enterobactin, a metal-chelating siderophore. MSystems. 2020 doi: 10.1128/mSystems.00161-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue Ohue-Kitano R, Tsukahara T, Tanaka M, Masuda S, Inoue T, Yamakage H, Kusakabe T, Hasegawa K, Shimatsu A, Satoh-Asahara N. Prediction of functional profiles of gut microbiota from 16S rRNA type 2 diabetic patients. J Clin Biochem Nutr. 2017 doi: 10.3164/jcbn.17-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Mao B, Gu J, Wu J, Cui S, Wang G, Zhao J, Zhang H, Chen W. Blautia-a new functional genus with potential probiotic properties? Gut Microbes. 2021 doi: 10.1080/19490976.2021.1875796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S, Goel R, Kumar A, Qi Y, Lobaton G, Hosaka K, Mohammed M, Handberg EM, Richards EM, Pepine CJ, Raizada MK. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci. 2018 doi: 10.1042/CS20180087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naderpoor N, Mousa A, Gomez-Arango LF, Barrett HL, Dekker Nitert M, de Courten B. Faecal microbiota are related to insulin sensitivity and secretion in overweight or obese adults. J Clin Med. 2019 doi: 10.3390/jcm8040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang JY, Lee YS, Kim Y, Lee SH, Ryu S, Fukuda S, Hase K, Yang CS, Lim HS, Kim MS, Kim HM, Ahn SH, Kwon BE, Ko HJ, Kweon MN. Gut commensal Bacteroide acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017 doi: 10.1038/mi.2016.42. [DOI] [PubMed] [Google Scholar]
- 33.Dao MC, everard A, aron-wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K, MICRO-Obes Consortium Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity relationship with gut microbiome richness and ecology. Gut. 2016 doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 34.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011 doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin X, Liao W, Li Q, Zhang H, Liu Z, Zheng X, Zheng L, Fen X. Interactions between resveratrol and gut microbiota affect the development of hepatic steatosis: a fecal microbiota transplantation study in high-fat diet mice. J Funct Foods. 2020 doi: 10.3389/fphar.2020.0124967:103883. [DOI] [Google Scholar]
- 36.Kim SJ, Kim SE, Kim AR, Kang S, Park MY, Sung MK. Dietary fat intake and age modulate the composition of the gut microbiota and colonic inflammation in C57BL/6J mice. BMC Microbiol. 2019 doi: 10.1186/s12866-019-1557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lange K, Buerger M, Stallmach A, Bruns T. Effects of antibiotics on gut microbiota. Dig Dis. 2016 doi: 10.1159/000443360. [DOI] [PubMed] [Google Scholar]
- 38.Vrieze A, Out C, Fuentes S, Jonker L, Reuling I, Kootte RS, et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol. 2014 doi: 10.1016/j.jhep.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 39.Yang L, Bajinka O, Jarju PO, et al. The varying effects of antibiotics on gut microbiota. AMB Expr. 2021 doi: 10.1186/s13568-021-01274-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heisel T, Montassier E, Johnson A, Al-Ghalith G, Lin YW, Wei LN, Knights D, Gale CA. High-fat diet changes fungal microbiomes and interkingdom relationships in the murine gut. mSphere. 2017 doi: 10.1128/mSphere.00351-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin H, An Y, Hao F, et al. correlations of fecal metabonomic and microbiomic changes induced by high-fat diet in the pre-obesity state. Sci Rep. 2016 doi: 10.1038/srep21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu S, Qin P, Wang J. High-fat diet alters the intestinal microbiota in streptozotocin-induced type 2 diabetic mice. Microorganisms. 2019 doi: 10.3390/microorganisms7060176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deshpande NG, Saxena J, Pesaresi TG, Carrell CD, Ashby GB, Liao MK, Freeman LR. High fat diet alters gut microbiota but not spatial working memory in early middle-aged Sprague Dawley rats. PLoS ONE. 2019 doi: 10.1371/journal.pone.0217553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature. 2006 doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 45.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. nature. 2006 doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 46.Lagier JC, Million M, Hugon P, Armougom F, Raoult D. Human gut microbiota: repertoire and variations. Front Cell Infect Microbiol. 2012 doi: 10.3389/fcimb.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato M, Dehvari N, Öberg AI, Summers RJ, Hutchinson DS, Bengtsson T. Response to Comment on Sato et al Improving type 2 diabetes through a distinct adrenergic signaling pathway Involving mTORC2 that mediates glucose uptake in skeletal muscle. Diabetes. 2014 doi: 10.2337/db14-1283. [DOI] [PubMed] [Google Scholar]
- 48.Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, Zimmerman LJ. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012 doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012 doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 50.Jang LG, Choi G, Kim SW, Kim BY, Lee S, Park H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: an observational study. J Int Soc Sports Nutr. 2019;16(1):21. doi: 10.1186/s12970-019-0290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh RP, Halaka DA, Hayouka Z, Tirosh O. High-fat diet induced alteration of mice microbiota and the functional ability to utilize fructooligosaccharide for ethanol production. Front Cell Infect Microbiol. 2020 doi: 10.3389/fcimb.2020.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneeberger M, Everard A, Gómez-Valadés A, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015 doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X, Mao B, Gu J, Wu J, Cui S, Wang G, Zhao J, Zhang H, Chen W. Blautia-a newfunctional genus with potential probiotic properties? Gut Microbes. 2021 doi: 10.1080/19490976.2021.1875796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fleissner CK, Huebel N, Abd El-Bary MM, Loh G, Klaus S, Blaut M. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br J Nutr. 2010 doi: 10.1017/S0007114510001303. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Y, Wu J, Li JV, Zhou NY, Tang H, Wang Y. Gut microbiota composition modifies fecal metabolic profiles in mice. J Proteome Res. 2013 doi: 10.1021/pr400263n. [DOI] [PubMed] [Google Scholar]
- 56.Sanchez B, Cobo A, Hidalgo M, Martinez-Rodriguez AM, Prieto I, Galvez A, Martínez-Cañamero M. Influence of the type of diet on the incidence of pathogenicfactors and antibiotic resistance in enterococci isolated from faeces in mice. Int JMol Sci. 2019 doi: 10.3390/ijms20174290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seishima J, Iida N, Kitamura K, et al. Gut-derived enterococcus faecium fromulcerative colitis patients promotes colitis in a genetically susceptible mouse host. Genome Biol. 2019 doi: 10.1186/s13059-019-1879-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mukherjee A, Lordan C, Ross RP, Cotter PD. Gut microbes from the phylogenetically diverse genus eubacterium and their various contributions to gut health. Gut Microbes. 2020 doi: 10.1080/19490976.2020.1802866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pope JL, Yang Y, Newsome RC, Sun W, Sun X, Ukhanova M, Neu J, Issa JP, Mai V, Jobin C. Microbial colonization coordinates the pathogenesis of a klebsiella pneumoniae infant isolate. Sci Rep. 2019 doi: 10.1038/s41598-019-39887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Precup G, Vodnar DC. Gut Prevotella as a possible biomarker of diet and its eubioticversus dysbiotic roles: a comprehensive literature review. Br J Nutr. 2019 doi: 10.1017/S0007114519000680. [DOI] [PubMed] [Google Scholar]
- 61.Klein G. Taxonomy, ecology and antibiotic resistance of enterococci from food and the gastro-intestinal tract. Int J Food Microbiol. 2003 doi: 10.1016/s0168-1605(03)00175-2. [DOI] [PubMed] [Google Scholar]
- 62.Marietta EV, Murray JA, Luckey DH, Jeraldo PR, Lamba A, Patel R, Luthra HS, Mangalam A, Taneja V. Suppression of inflammatory arthritis by human gut-derived prevotella histicola in humanized mice. Arthritis Rheumatol. 2016 doi: 10.1002/art.39785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim MH, Lee EJ, Cheon JM, Nam KJ, Oh TH, Kim KS. Antioxidant andhepatoprotective effects of fermented red ginseng against high fat diet-inducedhyperlipidemia in rats. Lab Animal Res. 2016 doi: 10.5625/lar.2016.32.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rotimi SO, Ojo DA, Talabi OA, Ugbaja RN, Balogun EA, Ademuyiwa O. Amoxillin and pefloxacin induced cholesterogenesis and phospholipidosis in rattissues. Lipids Health Dis. 2015 doi: 10.1186/s12944-015-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo J, Jou W, Gavrilova O, Hall KD. Persistent diet-induced obesity in male C57BL/6 mice resulting from temporary obesigenic diets. PLoS ONE. 2009 doi: 10.1371/journal.pone.0005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Louis XL, Thandapilly SJ, MohanKumar SK, Yu L, Taylor CG, Zahradka P, Netticadan T. Treatment with low-dose resveratrol reverses cardiacimpairment in obese prone but not in obese resistant rats. J Nutr Biochem. 2012 doi: 10.1016/j.jnutbio.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 67.Zarrinpar A, Chaix A, Xu ZZ, Chang MW, Marotz CA, Saghatelian A, Knight R, Panda S. Antibiotic-induced microbiome depletion alters metabolic homeostasis byaffecting gut signaling and colonic metabolism. Nat Commun. 2018 doi: 10.1038/s41467-018-05336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodrigues RR, Greer RL, Dong X, DSouza KN, Gurung M, Wu JY, Morgun A, Shulzhenko N. antibiotic-induced alterations in gut microbiota are associated with changes in glucose metabolism in healthy mice. Front Microbiol. 2017 doi: 10.3389/fmicb.2017.02306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Limaye PB, Renaud HJ, Klaassen CD. Effect of various antibiotics on modulation of intestinal microbiota and bile acid profile in mice. Toxicol Appl Pharmacol. 2014 doi: 10.1016/j.taap.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang L, Bajinka O, Jarju PO, Tan Y, Taal AM, Ozdemir G. The varying effects of antibiotics on gut microbiota. AMB Express. 2021 doi: 10.1186/s13568-021-01274-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qi Y, Aranda JM, Rodriguez V, Raizada MK, Pepine CJ. Impact of antibiotics on arterial blood pressure in a patient with resistant hypertension—a case report. Int J Cardiol. 2015 doi: 10.1016/j.ijcard.2015.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rojas JM, Bolze F, Thorup I, Nowak J, Dalsgaard CM, Skydsgaard M, Berthelsen LO, Keane KA, Søeborg H, Sjögren I, Jensen JT, Fels JJ, Offenberg HK, Andersen LW, Dalgaard M. The effect of diet-induced obesity on toxicological parameters in the polygenic sprague-dawley rat model. Toxicol Pathol. 2018 doi: 10.1177/0192623318803557. [DOI] [PubMed] [Google Scholar]
- 73.Mkandla Z, Mutize T, Dludla PV, Nkambule BB. Impaired glucose tolerance is associated with enhanced platelet-monocyte aggregation in short-term high-fat diet-fed mice. Nutrients. 2019 doi: 10.3390/nu11112695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.PodriniCambridge CEL, Lelliott CJ, Carragher DM, Estabel J, Gerdin AK, Karp NA, Scudamore CL, Ramirez-Solis R, White JK, Sanger Mouse Genetics Project High-fat feeding rapidly induces obesity and lipid derangements in C57BL/6N mice. Mamm Genome. 2013 doi: 10.1007/s00335-013-9456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang M, Lv J, Huang X, Wisniewski T, Zhang W. High-fat diet-induced atherosclerosis promotes neurodegeneration in the triple transgenic (3 × Tg) mouse model of Alzheimer disease associated with chronic platelet activation. Alzheimers Res Ther. 2021 doi: 10.1186/s13195-021-00890-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nanizawa E, Tamaki Y, Sono R, Miyashita R, Hayashi Y, Kanbe A, Ito H, Ishikawa T. Short-term high-fat diet intake leads to exacerbation of concanavalin a-induced liver injury through the induction of procoagulation state. Biochem Biophys Rep. 2020 doi: 10.1016/j.bbrep.2020.100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crinigan C, Calhoun M, Sweaze KL. Short-term high fat intake does not significantly alter markers of renal function or inflammation in young male sprague dawley rats. J Nutr Metab. 2015 doi: 10.1155/2015/157520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang CY, Liao JK. A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol. 2012 doi: 10.1007/978-1-61779-430-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Speakman J, Hambly C, Mitchell S, Krol E. Animal models of obesity. Obes Rev. 2007 doi: 10.1111/j.1467-789X.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 80.Houtkooper RH, Argmann C, Houten SM, Canto C, Jeninga EH, Andreux PA, et al. The metabolic footprint of aging in mice. Sci Rep. 2011 doi: 10.1038/srep00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han J, Nepal P, Odelade A, et al. Front. Nutr. 2021 doi: 10.3389/fnut.2020.591161. [DOI] [Google Scholar]
- 82.Marx JO, Vudathala D, Murphy L, Rankin S, Hankenson FC. Antibiotic administration in the drinking water of mice. J Am Assoc Lab Anim Sci. 2014;53(3):301–306. [PMC free article] [PubMed] [Google Scholar]
- 83.Caporaso J, Lauber C, Walters W, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012 doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fadrosh DW, Ma B, Gajer P, et al. An improved dual-indexing approach formultiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome. 2014 doi: 10.1186/2049-2618-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Magoc T, Salzberg SL. FLASH fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011 doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013 doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 87.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costellon EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley STN, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010 doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gautam K, Negi S, Saini V. 2021 Targeting endogenous gaseous signaling molecules as novel host-directed therapies against tuberculosis infection. Free Radical Res. 2021;10(1080/10715762):1892091. doi: 10.1080/10715762.2021.1892091. [DOI] [PubMed] [Google Scholar]
- 89.Kushwaha N, Negi S, Kumar A, Zangrando E, Kataria R, Saini V. Synthesis, characterization and utility of a series of novel copper (II) complexes as excellent surface disinfectants against nosocomial infections. Dalton Trans. 2021 doi: 10.1039/D1DT00199J50(39):13699-13711. [DOI] [PubMed] [Google Scholar]
- 90.Saini V, Chinta KC, Reddy VP, et al. Hydrogen sulfide stimulates Mycobacterium tuberculosis respiration, growth and pathogenesis. Nat Commun. 2020 doi: 10.1038/s41467-019-14132-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kumar P, Saini K, Saini V, et al. Oxalate alters cellular bioenergetics, redox homeostasis, antibacterial response, and immune response in macrophages. Front Immunol. 2021 doi: 10.3389/fimmu.2021.694865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Sequence Read Archive (SRA) submission datasets generated and/or analysed during the current study are submitted to SRA data repository of National Centre for Biotechnology, USA. Submission Id: SUB11233254; Bio Project ID: PRJNA821450. https://submit.ncbi.nlm.nih.gov/subs/bioproject/SUB11233254/overview.