Abstract
BACKGROUND
Worldwide, hypertensive disorders of pregnancy are a serious complication of pregnancy, and contribute to poor maternal and neonatal outcomes. The most significant consequences of hypertensive disorders of pregnancy are observed in sub-Saharan Africa, where neonatal outcomes have not been fully described. Understanding relationships between maternal disease severity and neonatal outcomes can guide patient counseling and allow the targeting of limited resources to the most at-risk neonates.
OBJECTIVE
To describe and compare neonatal outcomes in pregnancies complicated by preeclampsia with severe features and eclampsia.
STUDY DESIGN
This study is a secondary analysis of data collected as part of a randomized controlled trial at the Korle-Bu Teaching Hospital in Ghana. Participants were adult pregnant women with preeclampsia with severe features or eclampsia and their neonates. Data include prospectively collected medical and obstetrical history, intrapartum events, and neonatal outcomes. The main outcome of this secondary analysis was a composite of poor neonatal outcomes, defined as 1 or more of the following: stillbirth, very low birthweight (<1500 g), 5-minute Apgar score <7, neonatal intensive care unit admission, or a live birth with a subsequent death before discharge.
RESULTS
Median gestational age at delivery was 36.6 weeks (interquartile range, 33.3–38.9). Median birthweight was 2.3 kg (interquartile range, 1.6–3.0), with 227 (19.0%) birthweights <1500 g. There were 162 neonates (15.5%) with an Apgar score <7 at 5 minutes and 144 (11.9%) were stillbirths. Of live births, half (n=524, 50.3%) were admitted to the neonatal intensive care unit and 7.9% (n=91) died before discharge. A composite of poor neonatal outcomes was experienced by 58.2% (n=707) of neonates and was twice as likely with a maternal diagnosis of eclampsia (odds ratio, 1.91; P=.04). For each additional week of gestational age, the probability of a poor neonatal outcome was reduced by 39% (odds ratio, 0.61; P<.0001).
CONCLUSION
Poor neonatal outcomes were experienced by more than half of pregnancies complicated by preeclampsia with severe features or eclampsia. Even after controlling for gestational age, pregnancies complicated by eclampsia were twice as likely to have poor neonatal outcomes.
Key words: high blood pressure in pregnancy, high-risk pregnancy, hypertension in pregnancy, hypertensive disorders of pregnancy, low- and middle-income countries, perinatal outcome, pregnancy complication, stillbirth
AJOG Global Reports at a Glance.
Why was this study conducted?
This study explored differences in neonatal outcomes between pregnancies complicated by preeclampsia and those complicated by eclampsia in sub-Saharan Africa, where neonatal outcomes have not been fully described.
Key findings
A composite of poor neonatal outcomes was experienced by 58.2% (n=707) of neonates born to pregnancies complicated by preeclampsia or eclampsia, and was twice as likely with a maternal diagnosis of eclampsia (odds ratio, 1.91; P=.04).
What does this add to what is known?
Pregnancies complicated by eclampsia were twice as likely to have poor neonatal outcomes compared with those complicated by preeclampsia, even after controlling for gestational age.
Introduction
Worldwide, hypertensive disorders of pregnancy are a serious complication of pregnancy, and contribute to poor maternal and neonatal outcomes.1, 2, 3, 4 The incidence of preeclampsia and eclampsia is higher in low- and middle-income countries (LMIC),3 where associated morbidities are also more significant.3,5 Global estimates of rates of eclampsia range from 0.1% in Europe to up to 4% in sub-Saharan Africa.2,6 Fatality rates of eclampsia similarly vary widely, from 0% to 2% in high-income countries to 18% in low-income countries.7 In Ghana, where this study was conducted, incidence of hypertensive disorders of pregnancy is estimated at 7.6%,8,9 and institutional reports from 2 major tertiary-level hospitals suggest that hypertensive disorders have overtaken hemorrhage as the leading cause of maternal mortality.8
Pregnancies complicated by preeclampsia and eclampsia are prone to poor neonatal outcomes because of preterm delivery, impaired uteroplacental perfusion, and maternal–fetal hypoxia during seizures. In LMIC, capacity for neonatal support is limited, increasing morbidity and mortality for vulnerable neonates.6 Previous studies have reported high rates of stillbirth, neonatal death, low birthweight, and neonatal intensive care unit (NICU) admission in pregnancies complicated by preeclampsia and eclampsia.6,10, 11, 12 Some studies suggest that these poor neonatal outcomes can be explained by preterm gestational age alone,1 whereas others find persistent differences relative to uncomplicated pregnancies even when controlling for gestational age and birthweight.12,13
Few studies have explored differences in neonatal outcomes between preeclampsia- and eclampsia-complicated pregnancies.1,10,12 The worst outcomes from hypertensive disorders of pregnancy are observed in sub-Saharan Africa. In this population, neonatal outcomes have not been fully described. Understanding the relationship between maternal disease severity and neonatal outcomes can guide patient counseling and allow the targeting of limited resources to the most at-risk neonates. Therefore, our study aims to describe and compare neonatal outcomes in pregnancies complicated by preeclampsia with severe features and eclampsia at the Korle-Bu Teaching Hospital (KBTH) in Ghana.
Materials and Methods
This study is a secondary analysis of data collected as part of a randomized controlled trial evaluating the impact of magnesium sulfate regimens on maternal seizure rates among women with preeclampsia with severe features and eclampsia.14,15 All participants received magnesium sulfate. Apart from the duration of magnesium sulfate therapy, all other care was provided according to standard obstetrical practice. Decisions about administration of antihypertensives, fetal surveillance, and timing and mode of delivery were all made by the on-call obstetrical providers. Ethical approval was granted by the scientific and technical committee of the KBTH (KBTH-IRB 00096/2018) and the University of Michigan Institutional Review Board (HUM00139104).
The study site was the KBTH, Ghana's largest tertiary-care hospital located in the capital city of Accra. Study participants were adult pregnant women admitted to KBTH with a diagnosis of preeclampsia with severe features or eclampsia.16 All adult pregnant women admitted to KBTH's maternity ward were screened for an inclusion diagnosis of preeclampsia with severe features or eclampsia, and all women with a qualifying diagnosis were invited to participate. The sampling strategy, detailed inclusion criteria, and sample size determination have been described in the previously published study protocol14 and randomized controlled trial manuscript.15 The recruitment flowchart is shown in the Supplemental Figure. Written informed consent was obtained from all participants.
Data collection was conducted between October 2018 and November 2020. Data, including medical and obstetrical history, were extracted from participants’ medical records. During their intrapartum hospitalization, clinical information was prospectively collected, including mode and timing of delivery, gestational age at delivery, birthweight, delivery outcome, NICU admission, Apgar score, and status at discharge. All neonates were followed up until discharge from the hospital.
The main outcome of this secondary analysis was a composite of poor neonatal outcomes, defined as 1 or more of the following: stillbirth, very low birthweight (<1500 g), 5-minute Apgar score <7, NICU admission, or a live birth with a subsequent death before discharge. Secondary outcomes included the individual components of the poor neonatal outcome composite.
Analysis was done using SAS, version 9.4 (SAS Institute Inc, Cary, NC). A composite of poor neonatal outcomes was created, as defined previously. Normality of all continuous variables was determined by assessing skewness and kurtosis, and using the Shapiro–Wilk test. Demographic and obstetrical characteristics were described for the total population, using median (interquartile range) and proportions. Bivariate analysis was performed to compare demographic and obstetrical history characteristics between women with a diagnosis of preeclampsia with severe features and those with a diagnosis of eclampsia, using Wilcoxon signed-rank, chi square, and Fisher exact tests, as appropriate. Next, neonatal outcomes were described for the total population and compared between the 2 groups. To account for multiple gestations, the denominator for neonatal variables was all births.
Bivariate analysis was performed to identify potential predictors of the composite of poor neonatal outcomes. Factors significant in bivariate analysis and other clinically relevant factors were considered candidates for inclusion in a multivariable logistic regression model. Separate models were run for the composite poor neonatal outcome and each of the 4 elements of the composite. Models were adjusted for age and parity. To examine the relationship between poor neonatal outcomes across gestational age, we reported the results of the logistic regression models as predicted probabilities. All calculated P values were 2-sided and a P value <.05 was considered statistically significant.
Results
Among 1176 total participants, median age was 31.0 years and 29.2% (n=343) were of advanced maternal age, defined as an age of ≥35 years (Table 1). Median parity was 1.0, with 376 (32.0%) nulliparous and 46 (4.3%) grand multiparous women. Regarding relevant medical history, median prepregnancy body mass index (BMI) was 30.1 and 32.7% (n=279) had a diagnosis of chronic hypertension. Most pregnancies were singleton (n=1086; 94.3%), and two-thirds were delivered by cesarean delivery (n=767; 66.4%). Of the 1176 participants, 1060 (90.1%) had a diagnosis of preeclampsia with severe features, and 116 (9.9%) had eclampsia. Complete maternal and antenatal care data are included in the Supplemental Table. Participants with eclampsia were younger, more likely to be nulliparous, had lower BMI, were less likely to have chronic hypertension, and were more likely to have twins compared with participants with preeclampsia.
Table 1.
Patient characteristics by maternal diagnosis of preeclampsia and eclampsia
| Characteristic | Total (n=1176) | Preeclampsia(n=1060) | Eclampsia(n=116) | P value |
|---|---|---|---|---|
| Age, y | 31.0 (27.0–35.0) | 31.0 (27.0–36.0) | 27.0 (23.0–33.0) | <.001a,b |
| Age, group (y) | <.001ac | |||
| <20 | 30 (2.6) | 19 (1.8) | 11 (9.5) | |
| 21–34 | 803 (68.3) | 713 (67.3) | 90 (77.6) | |
| ≥35 | 343 (29.2) | 328 (30.9) | 15 (12.9) | |
| Parity, number | 1.0 (0.0–2.0) | 1.0 (0.0–3.0) | 1.0 (0.0–2.0) | .00a,b |
| Parity, group | .02ac | |||
| Nulliparous | 376 (32.0) | 329 (31.0) | 47 (41.2) | |
| Primiparous | 283 (24.1) | 251 (23.7) | 32 (28.1) | |
| Multiparous (2–4) | 464 (39.5) | 434 (40.9) | 30 (26.3) | |
| Grand multiparity (5+) | 51 (4.3) | 46 (4.3) | 5 (4.4) | |
| Prepregnancy BMI, kg/m2 | 30.1 (25.3–35.6) | 30.6 (25.7–35.9) | 26.5 (23.3–31.6) | <.001a,b |
| Chronic hypertension | 279 (32.7) | 272 (25.7) | 7 (6.0) | <.001ac |
| Mode of delivery | .75c | |||
| Vaginal delivery | 386 (33.5) | 350 (33.6) | 36 (32.1) | |
| Cesarean delivery | 767 (66.5) | 691 (66.4) | 76 (67.9) | |
| Number of gestations | .01ac | |||
| Singleton | 1086 (94.3) | 989 (94.8) | 97 (89.0) | |
| Twins | 66 (5.7) | 54 (5.2) | 12 (11.0) |
Denominator is all pregnancies, n=1176.
Data presented as number (percentage) or median (interquartile range) and compared with the chi square test unless otherwise specified.
BMI, body mass index.
Significant at p < 0.05
Comparisons between preeclampsia with severe features and eclampsia tested using the Wilcoxon signed-rank test
Comparisons between preeclampsia with severe features and eclampsia tested using the Fisher exact test.
Lawrence. Neonatal outcomes in pregnancies complicated by preeclampsia and eclampsia. Am J Obstet Gynecol Glob Rep 2022.
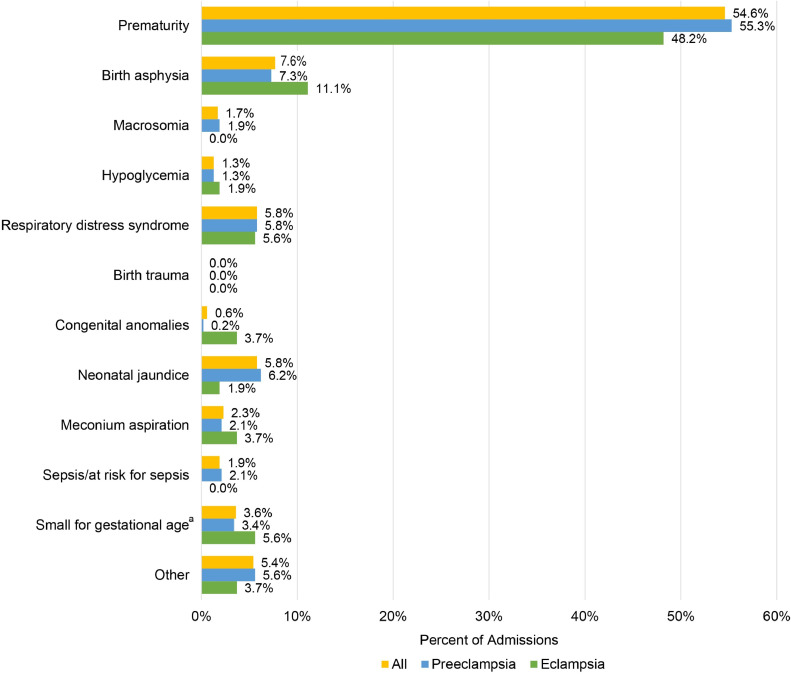
There were 1218 neonates born to the 1176 women included in our study (Table 2). Median gestational age at delivery was 36.6 weeks and 11.9% (n=144) were stillbirths. Median birthweight was 2.3 kg, and 19.0% (n=227) of neonates had a birthweight <1500 g. Among live births, median Apgar scores at 1 and 5 minutes were 7.0 and 8.0, respectively, and half of the live-born neonates (n=524; 50.3%) were admitted to the NICU. The most common reasons for NICU admission were prematurity (n=286; 54.6%) and birth asphyxia (n=40; 7.6%) (Figure 1). Among the neonates admitted to the NICU, median length of stay was 8.0 days. Of all neonates born alive, 7.9% (n=91) died before discharge, with a median survival of 2.0 days (Table 2). Overall, 79.6% (n=917) of neonates were alive at time of discharge, and 20.4% (n=235) were either stillborn or died between delivery and discharge. The composite of poor neonatal outcomes was observed in 58.2% (n=707) of all neonates. In the unadjusted bivariate comparison (Table 2), stillbirth, lower Apgar scores at 1 and 5 minutes, and the poor neonatal outcome composite were more often observed in neonates born to women with eclampsia.
Table 2.
Neonatal outcomes by maternal diagnosis of preeclampsia and eclampsia
| Characteristic | Total (n=1218) | Preeclampsia (n=1097) | Eclampsia (n=121) | P value |
|---|---|---|---|---|
| Gestational age at delivery, wk | 36.6 (33.3–38.9) | 36.6 (33.4–38.9) | 35.3 (32.0–39.1) | .25a |
| Gestational age at delivery, group (wk) | .18 | |||
| <32.0 | 197 (16.7) | 172 (16.0) | 25 (22.9) | |
| 32.0–37.0 | 431 (36.4) | 394 (36.7) | 37 (33.9) | |
| ≥37.0 | 555 (46.9) | 508 (47.3) | 47 (43.1) | |
| Outcome of delivery | .04b | |||
| Live birth | 1065 (88.1) | 968 (88.7) | 97 (82.2) | |
| Stillbirth | 144 (11.9) | 123 (11.3) | 21 (17.8) | |
| Birthweight, g | 2.3 (1.6–3.0) | 2.3 (1.6–3.0) | 2.2 (1.5–2.9) | .19a |
| Birthweight, group (g) | .38 | |||
| <1500 | 227 (19.0) | 200 (18.5) | 27 (23.9) | |
| 1500–2499 | 425 (35.6) | 388 (35.9) | 37 (32.7) | |
| ≥2500 | 542 (45.4) | 493 (45.6) | 49 (43.4) | |
| 1-min Apgar, scorec | 7.0 (6.0–8.0) | 7.0 (6.0–8.0) | 7.0 (5.0–7.0) | .002a,b |
| 5-min Apgar, scorec | 8.0 (7.0–9.0) | 8.0 (7.0–9.0) | 8.0 (7.0–8.0) | .01a,b |
| 5-min Apgar, groupc | .14d | |||
| ≤3 | 22 (2.1) | 20 (2.2) | 1 (1.1) | |
| 4–6 | 140 (13.4) | 122 (12.7) | 18 (20.2) | |
| ≥7 | 887 (84.6) | 817 (85.1) | 70 (78.7) | |
| NICU admissionc | ||||
| Yes | 524 (50.3) | 470 (49.4) | 54 (60.0) | .05b |
| No | 518 (49.7) | 482 (50.6) | 36 (40.0) | |
| Duration of NICU admission, de | 8.0 (3.0–15.0) | 8.0 (3.0–15.0) | 9.0 (4.0–15.0) | .31a |
| Live birth with a subsequent death before discharged | 91 (7.9) | 82 (7.9) | 9 (8.3) | .90 |
| Length of inpatient survival, df | 2.0 (1.0–5.0) | 2.0 (1.0–5.0) | 2.0 (1.0–4.0) | .68a |
| Status of neonate at discharge | .06 | |||
| Alive | 917 (79.6) | 837 (80.3) | 80 (72.7) | |
| Dead | 235 (20.4) | 205 (19.7) | 30 (27.3) | |
| Composite of poor neonatal outcomes | .006b | |||
| Yes | 707 (58.2) | 623 (56.9) | 84 (70.0) | |
| No | 508 (41.8) | 472 (43.1) | 36 (30.0) |
Denominator is all neonates to account for multiple gestations, n=1218.
Data are presented as number (percentage) or median (interquartile range) and compared with the chi square test unless otherwise specified.
NICU, neonatal intensive care unit.
Comparisons between preeclampsia with severe features and eclampsia tested using the Wilcoxon signed-rank test
Significant at p < 0.05
Among live-born infants
Comparisons between preeclampsia with severe features and eclampsia tested using the Fisher exact test
Among live-born infants admitted to the NICU
Among live-born infants who died before discharge.
Lawrence. Neonatal outcomes in pregnancies complicated by preeclampsia and eclampsia. Am J Obstet Gynecol Glob Rep 2022.
Figure 1.
Reasons for neonatal intensive care unit admission
The superscript letter a represents birthweight <10th percentile for gestational age.
Lawrence. Neonatal outcomes in pregnancies complicated by preeclampsia and eclampsia. Am J Obstet Gynecol Glob Rep 2022.
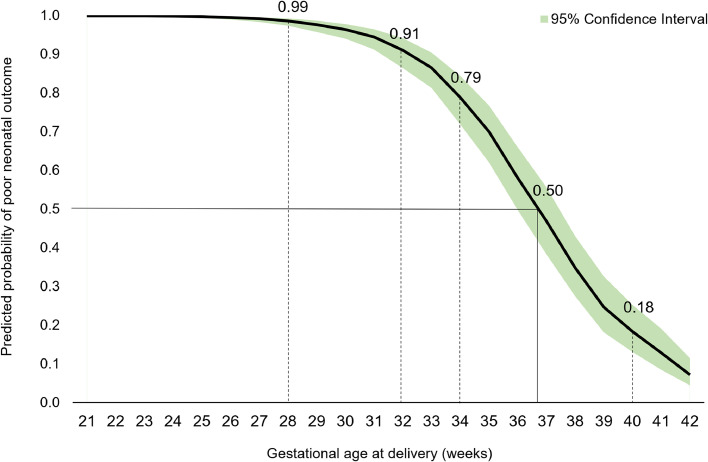
Table 3 demonstrates unadjusted and adjusted odds ratios (ORs) for the impact of a diagnosis of eclampsia on the composite poor neonatal outcome and each element of the composite. Compared with neonates born to women with preeclampsia, neonates of pregnancies complicated by eclampsia had approximately twice the odds of a poor neonatal outcome (adjusted OR, 1.91; 95% confidence interval, 1.03–3.54; P=.04). For each additional week of gestational age starting at 24 weeks, the chance of a poor neonatal outcome was reduced by 39% (OR, 0.61; P<.001). Figure 2 demonstrates probability of the poor neonatal outcome composite by gestational age, with progressively improved outcomes with increasing gestational age. The probability of a poor neonatal outcome was 99% at 28 weeks, 79% at 34 weeks, and 18% at 40 weeks, indicating a 50% chance of a poor neonatal outcome at 36.7 weeks. BMI and mode of delivery were not significant predictors of the composite poor neonatal outcome in this population of women.
Table 3.
Impact of maternal diagnosis of eclampsia (vs preeclampsia) on neonatal outcomes
| Neonatal outcome | Unadjusted odds ratio | Adjusted odds ratio | 95% confidence interval | P value |
|---|---|---|---|---|
| Composite poor neonatal outcome | 1.91 | 1.91 | 1.03–3.54 | .04a |
| Stillbirth | 1.56 | 1.57 | 0.78–3.17 | .21 |
| Birthweight <1500 g | 1.51 | 0.85 | 0.33–2.18 | .74 |
| 5-min Apgar <7 | 1.88 | 1.81 | 1.05–3.14 | .03a |
| NICU admission | 1.47 | 1.13 | 0.67–1.90 | .66 |
| Live birth with a subsequent death before discharge | 1.15 | 0.61 | 0.23–1.63 | .32 |
All models adjusted for age, parity, body mass index, mode of delivery, and gestational age at delivery.
NICU, neonatal intensive care unit.
Significant at p < 0.05.
Lawrence. Neonatal outcomes in pregnancies complicated by preeclampsia and eclampsia. Am J Obstet Gynecol Glob Rep 2022.
Figure 2.
Probability of poor neonatal outcome by gestational age
Lawrence. Neonatal outcomes in pregnancies complicated by preeclampsia and eclampsia. Am J Obstet Gynecol Glob Rep 2022.
Table 4 demonstrates adjusted ORs for all variables significantly associated with the composite poor neonatal outcome and each element of the composite, including maternal diagnosis (preeclampsia vs eclampsia), BMI, mode of delivery, and gestational age. Variables significantly associated with at least 1 secondary outcome are described in further text.
Table 4.
Clinical predictors of poor neonatal outcomes
| Predictor | Adjusted odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Composite of poor neonatal outcomes | |||
| Eclampsia (compared with preeclampsia) | 1.91 | 1.03–3.54 | .04 |
| Gestational age at delivery, wk | 0.61 | 0.57–0.65 | <.001a |
| Cesarean delivery (compared with vaginal) | 1.13 | 0.81–1.57 | .48 |
| Prepregnancy BMI, kg/m2 | 0.99 | 0.97–1.01 | .26 |
| Stillbirth | |||
| Eclampsia (compared with preeclampsia) | 1.57 | 0.78–3.17 | .21 |
| Gestational age at delivery, wk | 0.77 | 0.73–0.81 | <.001a |
| Cesarean delivery (compared with vaginal) | 0.18 | 0.11–0.27 | <.001 |
| Prepregnancy BMI, kg/m2 | 0.99 | 0.96–1.02 | .49 |
| Birthweight <1500 g | |||
| Eclampsia (compared with preeclampsia) | 0.85 | 0.33–2.18 | .74 |
| Gestational age at delivery, wk | 0.43 | 0.38–0.48 | <.001a |
| Cesarean delivery (compared with vaginal) | 0.75 | 0.39–1.43 | .38 |
| Prepregnancy BMI, kg/m2 | 0.96 | 0.92–0.99 | .02a |
| 5-min Apgar <7 | |||
| Eclampsia (compared with preeclampsia) | 1.81 | 1.05–3.14 | .03a |
| Gestational age at delivery, wk | 0.77 | 0.74–0.80 | <.001a |
| Cesarean delivery (compared with vaginal) | 0.47 | 0.33–0.66 | <.001a |
| Prepregnancy BMI, kg/m2 | 0.98 | 0.96–1.01 | .11 |
| NICU admission | |||
| Eclampsia (compared with preeclampsia) | 1.13 | 0.67–1.90 | .66 |
| Gestational age at delivery, wk | 0.82 | 0.79–0.85 | <.001a |
| Cesarean delivery (compared with vaginal) | 3.81 | 2.76–5.26 | <.001a |
| Prepregnancy BMI, kg/m2 | 0.99 | 0.97–1.01 | .24 |
| Live birth with a subsequent death before discharge | |||
| Eclampsia (compared with preeclampsia) | 0.61 | 0.23–1.63 | .32 |
| Gestational age at delivery, wk | 0.76 | 0.71–0.81 | <.001a |
| Cesarean delivery (compared with vaginal) | 4.15 | 1.96–8.77 | <.001a |
| Prepregnancy BMI, kg/m2 | 1.00 | 0.97–1.03 | .95 |
All models adjusted for age and parity.
BMI, body mass index; NICU, neonatal intensive care unit.
Significant at p < 0.05.
Lawrence. Neonatal outcomes in pregnancies complicated by preeclampsia and eclampsia. Am J Obstet Gynecol Glob Rep 2022.
Maternal diagnosis: of the 4 individual elements of the composite, eclampsia was associated only with 5-minute Apgar <7 (OR, 1.81; P=.03).
Gestational age at delivery: among all pregnancies, every 1-week increase in gestational age was associated with significantly lower odds of stillbirth (OR, 0.77; P<.001); birthweight <1500 g (OR, 0.43; P<.001); 5-minute Apgar <7 (OR, 0.77; P<.001); NICU admission (OR, 0.82; P<.001); and live birth with a subsequent death before discharge (OR, 0.76; P<.001).
Mode of delivery: compared with vaginal delivery, cesarean delivery was associated with decreased odds of stillbirth (OR, 0.18; P<.001) and 5-minute Apgar <7 (OR, 0.47; P<.001), and 4-fold increased odds of NICU admission (OR, 3.81; P<.001) and live birth with a subsequent death before discharge (OR, 4.15; P<.001). Mode of delivery was not significantly associated with birthweight <1500 g.
Prepregnancy BMI: higher BMI was associated with a decreased risk of birthweight <1500 g (OR, 0.96; P=.02). This was likely driven by a higher proportion of very low BMI (<18.5) in the poor outcome group. BMI was not a significant predictor of stillbirth, 5-minute Apgar <7, NICU admission, or live birth with a subsequent death before discharge.
Discussion
Principal findings
This study of women delivering at a tertiary hospital in Ghana highlights the fact that poor neonatal outcomes are common in pregnancies complicated by preeclampsia and eclampsia. In our population of 1218 babies born to women with preeclampsia with severe features or eclampsia, poor neonatal outcomes were experienced by more than half of all neonates, and these outcomes were twice as likely in women with a diagnosis of eclampsia compared with those with preeclampsia. For every 1-week increase in gestational age at delivery after 24 weeks, there were significantly lower odds of stillbirth, birthweight <1500 g, 5-minute Apgar <7, NICU admission, live birth with a subsequent death before discharge, and the composite of poor neonatal outcomes.
Results
Our neonatal findings are consistent with previous studies that demonstrated an association between hypertensive disorders of pregnancy and preterm delivery, stillbirth, low birthweight, and neonatal death.3,6,12,13 In our study, even after controlling for gestational age, pregnancies complicated by eclampsia were 1.9 times more likely to have a poor neonatal outcome, compared with pregnancies complicated by preeclampsia. A small study in Ecuador compared mild preeclampsia cases with severe cases and found lower Apgar scores and more preterm births, low-birthweight infants, and NICU admissions in severe cases.10 These findings were consistent with 2 American studies from 2000 and 200217,18 and a 2005 Turkish study comparing severe preeclampsia with both mild preeclampsia and chronic hypertension.19 However, a 1999 comparison of severe preeclampsia and hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome concluded that increased neonatal morbidity and mortality associated with HELLP syndrome were likely fully explained by differences in gestational age alone.1
Clinical implications
High rates of prematurity and low Apgar scores—and subsequent need for neonatal resuscitation and support—indicate the importance of facility delivery attended by trained providers. In our study, 47% of neonates were born preterm (<37 weeks) and 17% before 32 weeks’ gestation. Of all live births, 50% were admitted to the NICU for a higher level of care, suggesting that delivery at a tertiary care facility with NICU capabilities may be lifesaving. In neonates born to mothers with eclampsia, despite lower 5-minute Apgar scores, rates of live birth with a subsequent death before discharge were equivalent to those of neonates born to mothers with preeclampsia. This suggests that the poor neonatal outcomes observed in LMICs may be partially mitigated by tertiary-level NICU care, as is provided by KBTH in our study. This is supported by the findings of a multicountry analysis of Demographic and Health Survey data, which suggested that eclampsia is associated with increased risk of early neonatal mortality, but that neonatal mortality decreased with facility delivery.11 In a series of patients with eclampsia in Egypt, prematurity and poor neonatal services were cited as the most common etiology of perinatal death.6
Research implications
Regarding mode of delivery, we demonstrated an interesting, complicated relationship with neonatal outcomes. Compared with vaginal delivery, cesarean delivery was associated with 82% lower odds of stillbirth and 53% lower odds of 5-minute Apgar <7. However, cesarean delivery was conversely associated with 4-fold higher odds of both NICU admission and live birth with a subsequent death before discharge. This association could be explained by cesarean delivery preventing intrapartum demise, but contributing to NICU admission of clinically tenuous neonates who often die in the NICU. Alternatively, healthcare providers would be less likely to recommend cesarean delivery for a known fetal demise, and thus this association could reflect this clinical decision. Given that our study lacks data on the temporal relationship between diagnosis of stillbirth and decision on mode of delivery, additional research is needed to further explore this relationship.
Strengths and limitations
This study has a number of strengths. First, our study involved a large population of neonates born to pregnancies complicated by preeclampsia with severe features and eclampsia in an LMIC setting. We included key detailed clinical data from antenatal care, throughout intrapartum admission, and until discharge of neonates. We also calculated a composite of poor neonatal outcomes, which may be a more clinically beneficial indicator of health status than individual components of the composite. In addition, the study was conducted at a very busy tertiary obstetrical care center that is supported by a level 3 neonatal intensive care facility and has high rates of hypertensive disorders of pregnancy and maternal complications. This allowed us the unique opportunity of studying a substantial group of pregnancies complicated by eclampsia and varied neonatal outcomes. Despite these strengths, we recognize that generalization of our results may be limited because of our data collection at a single tertiary site in Ghana. In addition, neonatal outcomes were only collected through hospital discharge, with a median of 8 days of follow-up after delivery. Although we recognize that antenatal corticosteroid administration is an important consideration, many participants were referred from other institutions, and referral information on possible administration of corticosteroids was not typically available. Thus, we unfortunately cannot report rates of corticosteroid use. Furthermore, our study does not have comprehensive data on the temporal relationships between timing of diagnosis of stillbirth, onset of labor, and decision on mode of delivery because of both limited referral information and limited documentation at the study site. Thus, relationships between stillbirth and other measured variables should be viewed as associations rather than causal factors. Finally, this paper is a secondary analysis of data collected as part of a randomized controlled trial comparing rates of seizures between 2 durations of magnesium sulfate therapy. There is a potential that different durations of exposure to magnesium sulfate may affect the results presented in this study; however, this is unlikely given the universal exposure to magnesium sulfate. An analysis of neonatal outcomes stratified by duration of magnesium sulfate regimen was described elsewhere.15 The interpretation of our results must be made within the context of these limitations.
Conclusions
Our study explored neonatal outcomes in pregnancies complicated by preeclampsia with severe features and eclampsia in sub-Saharan Africa, where pregnancy complication rates are high. We demonstrated very high rates of poor neonatal outcomes. Worse neonatal outcomes among patients with eclampsia are driven by low Apgar scores, with similar rates of survival at time of hospital discharge, suggesting that capacity for neonatal resuscitation at the study site was lifesaving. Our calculation of predicted probabilities of poor neonatal outcomes based on gestational age can be used to guide selection of personnel and equipment needed for high-risk deliveries and referral of antepartum patients to higher-level facilities. Recognizing that hypertensive disorders of pregnancy are common, that maternal status can rapidly worsen, and that transfer from rural locations may not be timely, our high demonstrated rate of poor neonatal outcomes highlights the need for capacity building for neonatal resuscitation at district hospitals and health centers. Understanding predictors of poor neonatal outcomes allows LMIC health systems and health centers to allocate limited resources and personnel to care for the highest-risk neonates. Additional studies are needed to assess long-term survival, growth, and development outcomes among neonates born to pregnancies complicated by preeclampsia and eclampsia.
Footnotes
From the Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI (Dr Lawrence, Ms Kobernik, and Dr Moyer); Department of Obstetrics and Gynaecology, University of Ghana Medical School, Korle-Bu, Accra, Ghana (Drs Beyuo and Oppong); and Department of Obstetrics and Gynaecology, Korle-Bu Teaching Hospital, Accra, Ghana (Drs Beyuo and Oppong).
The authors report no conflict of interest.
This study received funding from the Rudi Ansbacher Research Award, the Women's Health Leadership Board Innovation Fund, and the Vanderbilt-Emory-Cornell-Duke Global Health Fellowship.
Cite this article as: Lawrence ER, Beyuo T, Kobernik EK, et al. A comparative analysis of neonatal outcomes in pregnancies complicated by preeclampsia and eclampsia in Ghana. Am J Obstet Gynecol Glob Rep 2022;2:100061.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.xagr.2022.100061.
Appendix. Supplementary materials
References
- 1.Abramovici D, Friedman SA, Mercer BM, Audibert F, Kao L, Sibai BM. Neonatal outcome in severe preeclampsia at 24 to 36 weeks’ gestation: does the HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome matter? Am J Obstet Gynecol. 1999;180:221–225. doi: 10.1016/s0002-9378(99)70178-x. [DOI] [PubMed] [Google Scholar]
- 2.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170:1–7. doi: 10.1016/j.ejogrb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. 2011. Available at:https://apps.who.int/iris/bitstream/handle/10665/44703/9789241548335_eng.pdf;jsessionid=476A1D103CE06844B2BD636E94D8E7DD?sequence=1. Accessed February 15, 2022. [PubMed]
- 4.Unwaha EA, Bello FA, Bello OO, Oladokun A. Intravenous magnesium sulfate in the management of severe pre-eclampsia: a randomized study of 12-hour versus 24-hour maintenance dose. Int J Gynaecol Obstet. 2020;149:37–42. doi: 10.1002/ijgo.13082. [DOI] [PubMed] [Google Scholar]
- 5.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Mahran A, Fares H, Elkhateeb R, et al. Risk factors and outcome of patients with eclampsia at a tertiary hospital in Egypt. BMC Pregnancy Childbirth. 2017;17:435. doi: 10.1186/s12884-017-1619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronsmans C, Campbell O. Quantifying the fall in mortality associated with interventions related to hypertensive diseases of pregnancy. BMC Public Health. 2011;11(Suppl3):S8. doi: 10.1186/1471-2458-11-S3-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adu-Bonsaffoh K, Oppong SA, Binlinla G, Obed SA. Maternal deaths attributable to hypertensive disorders in a tertiary hospital in Ghana. Int J Gynaecol Obstet. 2013;123:110–113. doi: 10.1016/j.ijgo.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Acquah-Arhin R, Kwawukuwe E. Trends in eclampsia at Korle Bu Teaching Hospatal, Accra Ghana. Niger J Clin Pract. 2003;6:1–4. [Google Scholar]
- 10.Phoa KY, Chedraui P, Pérez-López FR, et al. Perinatal outcome in singleton pregnancies complicated with preeclampsia and eclampsia in Ecuador. J Obstet Gynaecol. 2016;36:581–584. doi: 10.3109/01443615.2015.1107532. [DOI] [PubMed] [Google Scholar]
- 11.Bellizzi S, Sobel HL, Ali MM. Signs of eclampsia during singleton deliveries and early neonatal mortality in low- and middle-income countries from three WHO regions. Int J Gynaecol Obstet. 2017;139:50–54. doi: 10.1002/ijgo.12262. [DOI] [PubMed] [Google Scholar]
- 12.McKenzie KA, Trotman H. A retrospective study of neonatal outcome in preeclampsia at the University Hospital of the West Indies: a resource-limited setting. J Trop Pediatr. 2019;65:78–83. doi: 10.1093/tropej/fmy014. [DOI] [PubMed] [Google Scholar]
- 13.Omani-Samani R, Ranjbaran M, Amini P, Esmailzadeh A, Sepidarkish M, Almasi-Hashiani A. Adverse maternal and neonatal outcomes in women with preeclampsia in Iran. J Matern Fetal Neonatal Med. 2019;32:212–216. doi: 10.1080/14767058.2017.1376643. [DOI] [PubMed] [Google Scholar]
- 14.Beyuo T, Lawrence E, Langen ES, Oppong SA. Open-labelled randomised controlled trial of 12 hours versus 24 hours modified Pritchard regimen in the management of eclampsia and pre-eclampsia in Ghana (MOPEP Study): study protocol. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-032799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beyuo TK, Lawrence ER, Kobernik EK, Oppong SA. A novel 12-hour versus 24-hour magnesium sulfate regimen in the management of eclampsia and preeclampsia in Ghana (MOPEP study): a randomized controlled trial. Int J Gynaecol Obstet. 2022 doi: 10.1002/ijgo.14181. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.American College of Obstetricians and Gynecologists Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 17.Buchbinder A, Sibai BM, Caritis S, et al. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186:66–71. doi: 10.1067/mob.2002.120080. [DOI] [PubMed] [Google Scholar]
- 18.Hauth JC, Ewell MG, Levine RJ, et al. Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for Preeclampsia Prevention Study Group. Obstet Gynecol. 2000;95:24–28. doi: 10.1016/s0029-7844(99)00462-7. [DOI] [PubMed] [Google Scholar]
- 19.Yücesoy G, Ozkan S, Bodur H, et al. Maternal and perinatal outcome in pregnancies complicated with hypertensive disorder of pregnancy: a seven year experience of a tertiary care center. Arch Gynecol Obstet. 2005;273:43–49. doi: 10.1007/s00404-005-0741-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.