Abstract
Peritoneal dialysis effluent (PDE) contains a low-molecular-weight solute that will activate and prime the NADPH oxidase of human neutrophils via a phospholipase A2 (PLA2)-dependent mechanism. Since the products of PLA2 are known to activate and prime the oxidase we have investigated their role in the dialysis effluent-mediated activation and priming of human neutrophils. NADPH oxidase activity of PDE-primed and -unprimed neutrophils was measured by lucigenin-enhanced chemiluminescence in the presence of known inhibitors of the arachidonic acid cascade. Incubation of neutrophils with the nonselective PLA2 inhibitor quinacrine (0 to 100 μM) reduced oxidase activity in both primed and unprimed cells. Furthermore, primed cells were more sensitive to the action of quinacrine than were unprimed cells. We were unable to determine the relative roles of secretory PLA2 (sPLA2) and cytosolic PLA2 (cPLA2) since the selective sPLA2 inhibitor scalaradial (0 to 100 μM) inhibited oxidase activity in both groups of cells by similar degrees, while the specific cPLA2 inhibitor AACO-CF3 (0 to 50 μM) failed to affect activity in either group. Inhibition of platelet-activating factor (PAF), cycloxygenase, and 5-lipoxygenase-activating protein by hexanolamino-PAF (0 to 25 μM), flurbiprofen (0 to 25 μM), and MK886 (0 to 5 μM), respectively, had no effect upon oxidase activity. However, the direct inhibition of 5-lipoxygenase by caffeic acid or lipoxin A4 resulted in a similar concentration-dependent attenuation of oxidase activity in both primed and unprimed cells. Leukotriene B4 (LTB4) release from primed neutrophils was comparable to that from unprimed cells with the exception of phorbol myristate acetate-stimulated cells, which released fivefold more LTB4 than control. Taken together, these results suggest that it is arachidonic acid per se, and not its metabolites, that is important in priming of the neutrophil NADPH oxidase by dialysis effluent.
Recurrent bacterial peritonitis is a major cause of morbidity in patients undergoing continuous ambulatory peritoneal dialysis (CAPD). Polymorphonuclear leukocytes (PMN) are the predominant defense cells mobilized during an episode of infection; however, the intracellular killing mechanisms of these cells are impaired in the presence of peritoneal dialysis effluent (PDE) (18, 19). Paradoxically, PDE augments the release of secondary granules from PMN (11), causes low-level activation of the NADPH oxidase, and primes the latter response to both soluble and particulate stimuli (10). Subsequent investigation into the PMN signalling pathways affected by PDE suggest that both priming and activation involve phospholipase A2 (PLA2) (28). The main products of PLA2 activation in PMN are arachidonic acid (AA) and, if phosphatidylcholine is the substrate, platelet-activating factor (PAF). AA may be further metabolized by 5-lipoxygenase (5-LO) to yield leukotriene B4 (LTB4) or by cycloxygenase (COX) to yield prostaglandin. This latter reaction is thought to be minimal in PMN. Both PAF and LTB4 are well recognized as being potent inflammatory mediators, and both are capable of priming (13, 22) and activating the NADPH oxidase (1, 46). Furthermore, AA is able to directly activate oxidase in cell (17) and cell-free systems (44) and to prime the response to subsequent stimulation by fMLP (40). We have therefore investigated the roles of PAF and AA and its metabolites in the priming and activation of the NADPH oxidase by PDE.
MATERIALS AND METHODS
Reagents.
Lucigenin, bovine serum albumin (BSA; fraction V, low endotoxin), quinacrine (PLA2 inhibitor), flurbiprofen (COX inhibitor), and caffeic acid (lipoxygenase inhibitor) were obtained from Sigma Chemical Company Ltd., Poole, Dorset, United Kingdom. [5,6,8,9,11,12,14,15-3H]AA was obtained from Amersham International plc, Buckingham, United Kingdom. Lipoxin A4 (LTB4 receptor antagonist), hexanolamino-PAF (PAF receptor antagonist), arachidonyl trifluromethyl ketone (AACO-CF3) (cytosolic PLA2 [cPLA2] inhibitor) and scalaradial (secretory PLA2 [sPLA2] inhibitor) were obtained from Cascade Biochemical, Reading, Berkshire, United Kingdom. MK886 (5-LO-activating protein [FLAP] inhibitor) was a generous gift from A. W. Ford-Hutchingson, Merck, Frosst Centre, Quebec, Canada.
Preparation of PMN.
Human peripheral blood PMN were prepared by standard methods (4). Fresh venous blood was added to EDTA (dipotassium salt) to a final concentration of 3.5 mM. Erythrocytes were sedimented on dextran, and the leukocyte-rich plasma was further purified over a Ficoll gradient (lymphocyte separation medium; Flow Laboratories, Herts, United Kingdom). The PMN-rich pellet was subjected to hypotonic lysis to remove the remaining erythrocytes and was washed twice in phosphate-buffered saline (PBS; pH 7.4). Purification of cells by this method routinely gave preparations of >99% viability as assessed by trypan blue exclusion and of >97% purity as assessed by examination of stained cytospin preparations. Cells were counted in a hemocytometer and were suspended at a concentration of 107 ml−1 for lucigenin-enhanced chemiluminescence or 108 ml−1 for LTB4 determination by enzyme-linked immunosorbent assay (ELISA) (R & D Systems, Abingdon, Oxon, United Kingdom).
PDE.
Six samples of PDE (1.36%, wt/vol, glucose) (Dianeal; Baxter Travenol Inc., Chicago, Ill.) were obtained from patients receiving CAPD after an intraperitoneal dwell of 4 h. All patients had been established on CAPD for more than 2 months, were not suffering infection, and had not received antibiotic therapy over the preceding 4 weeks. PDE was stored at −70°C until required. Before use PDE was filtered through a 0.2-μm-pore-size membrane and the pH was adjusted to 7.4 by the addition of HEPES to give a final concentration of 20 mM. The concentrations of creatinine and urea in PDE were determined by autoanalysis. The concentrations of protein endotoxin, and tumor necrosis factor alpha (TNF-α) and interleukin 1β (IL-1β) were determined by the method of Lowry et al. (29), the limulus amoebocyte lysate assay (Sigma Diagnostic Kit, Sigma Chemical Company) and ELISA, respectively.
Determination of inhibitor toxicity.
The toxicity of the inhibitors and vehicle (ethanol) upon PMN over a 30-min period was determined by ATP bioluminescence (7) and confirmed by trypan blue exclusion. The ability of antagonists to scavenge superoxide anions was determined by using a xanthine-xanthine oxidase cell-free system (9).
Determination of AA release.
PMN (107 ml−1) were incubated with 1 μCi of [3H]AA ml−1 at 37°C for 60 min in PBS (without Ca2+)–0.1% (wt/vol) BSA. Cells were washed three times in PBS and were suspended at a concentration of 4 × 107 ml−1. Under these conditions the percentage of label incorporated into PMN was 31.33% ± 6.67% (mean ± standard error of the mean [SEM], n = 6). The reaction mixture (200 μl) contained PBS (pH 7.4), 1 mM CaCl2, 0.7 mM MgCl2, 0.1% (wt/vol) BSA, inhibitor and/or vehicle, and PMN to give a final concentration of 4 × 106 ml−1 (corresponding to 260,188 ± 23,981 cpm [n = 6]). PDEs were used at a concentration of 50% (vol/vol). The mixture was preincubated at 37°C for 10 min before the reaction was initiated by the addition of 1 μM fMLP. After 20 min the reaction was terminated by the addition of 500 μl of ice-cold physiological saline, and PMN were sedimented by centrifugation (12,000 × g for 15 s). The amounts of [3H]AA in the pellet and supernatant were determined by liquid scintillation counting.
Determination of superoxide anion generation.
Superoxide generation by PMN was determined by lucigenin-enhanced chemiluminescence in a plate-reading luminometer (Lumiscan; Labsystems, Basinstoke, United Kingdom). The reaction mixture (200 μl) contained 25 μM lucigenin, PBS (pH 7.4), 1 mM CaCl2, 0.7 mM MgCl2, 0.1% (wt/vol) BSA, inhibitor and/or vehicle, and PMN to give a final concentration of 106 ml−1. PDEs were used at a concentration of 50% vol/vol. The mixture was preincubated at 37°C for 10 min before the reaction was initiated by the addition of stimulus. fMLP, phorbol myristate acetate (PMA), and preopsonized Staphylococcus epidermidis were added to give final concentrations of 1 μM, 10 ng ml−1, and 2 × 107 ml−1, respectively. Superoxide anion formation was taken as the integral of superoxide dismutase-inhibitible light output over the initial 30 min of the reaction.
Determination of LTB4 generation.
The generation of LTB4 by PMN was determined by ELISA (R&D Systems). The reaction mixture (250 μl) contained PBS (pH 7.4), 1 mM CaCl2, 0.7 mM MgCl2, 0.1% (wt/vol) BSA, 50% (vol/vol) PDE, and PMN to give a final concentration of 107 ml−1. The mixture was preincubated at 37°C for 10 min before the reaction was initiated by the addition of stimulus. fMLP, PMA, and preopsonized S. epidermidis were added to give final concentrations of 1 μM, 10 ng ml−1, and 2 × 108 ml−1, respectively. The reaction was terminated by the addition of 13 μl of ice-cold citric acid (0.035M) to reduce the pH to 5.5. The reaction mixture was spun at 400 × g and 4°C for 10 min. The supernatants were removed and stored at −80°C before the assay for LTB4.
Statistical analysis.
All data are given as means ± SEMs. Analysis was performed using the Mann-Whitney U test calculated by the computer multifunction statistics library package NWAStatpak (Northwest Analytical Inc., Portland, Oreg.).
RESULTS
The biochemical profiles (means ± SEMs) of the six dialysis effluents employed in this study were as follows: protein, 3.18 ± 0.37 mg ml−1 (range, 2.25 to 4.90 mg ml−1); endotoxin, 71.25 ± 9.22 pg ml−1 (range, 57 to 114 pg ml−1); urea, 18.18 ± 1.56 mM (range, 14.0 to 25.4 mM); and creatinine, 900 ± 70 μM (range, 707 to 1290 μM). Levels of TNF-α and IL-1β were below the level of detection by ELISA.
Effects of inhibitors upon NADPH oxidase activity.
When concentrations of ethanol in the reaction mixture exceeded 0.1% (vol/vol) (ca. 20 mM) a significant reduction in PMN chemiluminescence was observed (50% inhibitory concentration [IC50] = 0.5% [vol/vol]). This inhibitory effect of ethanol was not affected by the presence of PDE and was not due to cell death since the release of ATP from PMN was constant. In subsequent experiments the final concentration of ethanol (used as the vehicle for inhibitors) did not exceed 0.05% (vol/vol). The release of ATP from PMN when incubated with all concentrations of inhibitors was comparable to the release observed from cells in buffer, suggesting that at the concentrations routinely used these compounds did not result in cell death (data not shown). The oxygen radical scavenging ability of the inhibitors employed in this study was assessed in a xanthine-xanthine oxidase cell-free system. At the concentrations used these compounds did not affect measurement of cell-free-generated superoxide (data not shown).
Phospholipase A2 inhibitors.
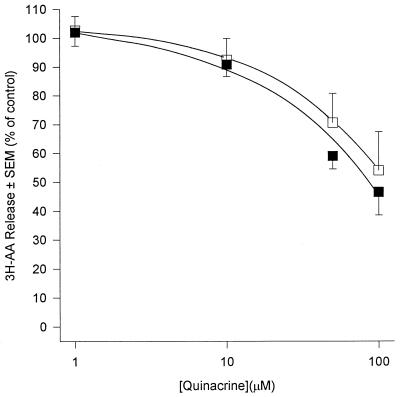
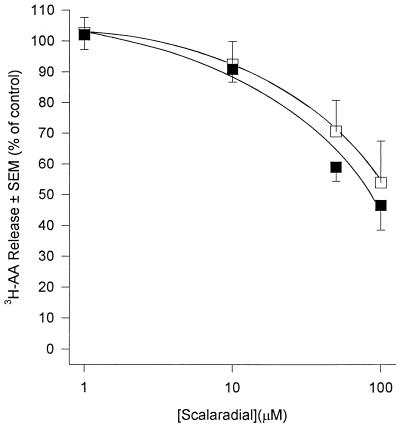
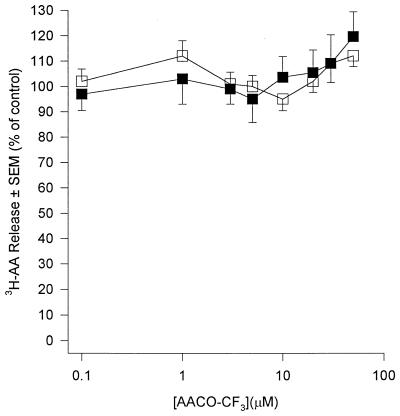
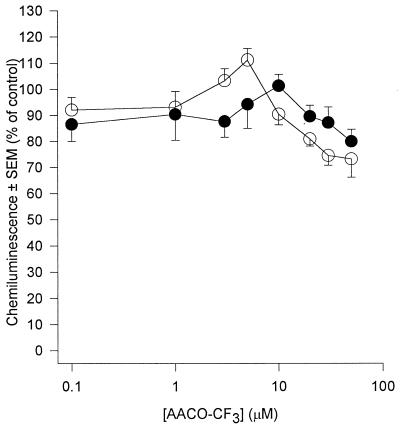
AA generation in human PMN occurs predominantly through the action of PLA2 upon membrane phospholipids. PLA2 acts at the sn-2 position of the glycerol backbone of phospholipids to yield free fatty acid (usually AA) and, if phosphatidylcholine is the substrate, lyso-PAF. Because of the pivotal role that this enzyme plays in AA generation we investigated its role in PDE-mediated priming and activation of human PMN by the use of three structurally distinct PLA2 inhibitors. Quinacrine, a widely used nonselective PLA2 inhibitor (50), inhibited release of AA from fMLP-stimulated PMN with an IC50 of approximately 15 μM (Fig. 1). The compound was a poor inhibitor of basal superoxide generation in unstimulated PMN (IC50 = 100 μM) but was 10-fold more effective if PMN were first primed with PDE (IC50 = 10 μM) (Fig. 2a). The IC50 of quinacrine for receptor-mediated stimuli (fMLP and S. epidermidis) was 20 μM, and this was reduced to 10 μM in PDE-primed cells (Fig. 2b). Quinacrine inhibited superoxide generation by PMA-stimulated cells with a potency which was similar to those of fMLP and S. epidermidis; however, priming of PMN with PDE did not affect this response (data not shown). These observations suggest a role for PLA2 in both the activation and priming of PMN by PDE. In an attempt to further determine the form of PLA2 involved we used scalaradial, which at low concentrations (0.07 μM) is a selective inhibitor of the 14-kDa sPLA2 (31), and AA-COCF3, a trifluoromethyl ketone analogue of AA that acts as a selective, slow, tight-binding inhibitor of the 85-kDa cPLA2 (48). Incubation of PMN with scalaradial (0 to 100 μM) resulted in a concentration-dependent inhibition of both AA release (Fig. 3) and superoxide generation in primed and unprimed cells (IC50s of 8, 8, 40, and 8 μM for basal, fMLP, PMA, and S. epidermidis, respectively) (Fig. 4). Conversely, AACO-CF3 (0 to 50 μM) did not inhibit AA release (Fig. 5) or superoxide generation (Fig. 6) by either primed or unprimed cells under all conditions tested. Indeed at concentrations between 1 and 10 μM superoxide generation was slightly stimulated for all stimuli (Fig. 6).
FIG. 1.
Effect of quinacrine upon AA release from fMLP (1 μM)-challenged PMN. PMN were incubated in buffer (open squares) or PDE (closed squares). Results are expressed as mean (± SEM) percent inhibitions relative to control (no quinacrine) (n = 6, six dialysis effluents upon PMN from six donors). Absolute control values (100%) were 1.90% ± 0.22% and 4.80% ± 0.55% total radioactivity incorporated for unprimed and primed cells, respectively.
FIG. 2.
Effects of quinacrine upon superoxide generation of unstimulated PMN (a) and PMN challenged with 1 μM fMLP (b). PMN were incubated in buffer (open circles) or PDE (closed circles). Results are expressed as mean (± SEM) percent inhibitions relative to control (no quinacrine) (n = 6, six dialysis effluents upon PMN from six donors). Asterisks indicate P values of ≤0.05 (Mann-Whitney U test). Absolute control values (100%) were 28.85 ± 9.40 relative light units (RLU) and 115 ± 23.57 RLU (a) and 1,107 ± 28 RLU and 3,618 ± 153 RLU (b) for unprimed and primed cells, respectively.
FIG. 3.
Effect of scalaradial upon AA release from fMLP (1 μM)-challenged PMN. PMN were incubated in buffer (open squares) or PDE (closed squares). Results are expressed as mean (± SEM) percent inhibitions relative to control (no scalaradial) (n = 6, six dialysis effluents upon PMN from six donors). Absolute control values (100%) were 1.80% ± 0.21% and 3.42% ± 0.37% total radioactivity incorporated for unprimed and primed cells, respectively.
FIG. 4.
Effect of scalaradial upon superoxide generation by fMLP (1 μM)-challenged PMN. PMN were incubated in buffer (open circles) or PDE (closed circles). Results are expressed as mean (± SEM) percent inhibitions relative to control (no scalaradial) (n = 6, six dialysis effluents upon PMN from six donors). Similar profiles were obtained from unstimulated PMN and PMN challenged with S. epidermidis (2 × 107 ml−1) and PMA (10 ng ml−1). Absolute control values (100%) were 503 ± 42 relative light units (RLU) and 2,241 ± 224 RLU for unprimed and primed cells, respectively.
FIG. 5.
Effect of AACO-CF3 upon AA release from fMLP (1 μM)-challenged PMN. PMN were incubated in buffer (open squares) or PDE (closed squares). Results are expressed as mean (± SEM) percent inhibitions relative to control (no AACO-CF3) (n = 6, six dialysis effluents upon PMN from six donors). Absolute control values (100%) were 2.20% ± 0.25% and 4.62% ± 0.19% total radioactivity incorporated for unprimed and primed cells, respectively.
FIG. 6.
Effect of AACO-CF3 upon superoxide generation from fMLP (1 μM)-challenged PMN. PMN were incubated in buffer (open circles) or PDE (closed circles). Results are expressed as mean (± SEM) percent inhibitions relative to (no AACO-CF3) (n = 6, six dialysis effluents upon PMN from six donors). Similar profiles were obtained from unstimulated PMN and PMN challenged with S. epidermidis (2 × 107 ml−1) and PMA (10 ng ml−1). Absolute control values (100%) were 747 ± 83 relative light units (RLU) and 1,842 ± 90 RLU for unprimed and primed cells, respectively.
PAF inhibitor.
The release of AA from phosphatidylcholine by PLA2 is accompanied by the concomitant formation of lyso-PAF, which is subsequently acetylated to generate the bioactive PAF molecule. Since PAF is generated when PMN are activated by many stimuli (36) and will both prime (22) and activate (1) PMN, we investigated its role in PDE-mediated priming with the use of the PAF receptor antagonist hexanolamino-PAF (42). Concentrations of hexanolamino-PAF from 0 to 25 μM had no effect upon either primed or unprimed superoxide anion generation in response to all stimuli tested (unstimulated, fMLP, PMA, and S. epidermidis) (data not shown).
5-LO inhibitors.
5-LO is the predominant route by which AA is metabolized in human PMN. Because of the importance of this enzyme in the AA cascade we chose to use three functionally distinct inhibitors to investigate the role of LTB4 in PDE-primed PMN. The indole-type inhibitor MK886 inhibits FLAP, preventing the translocation of 5-LO from the cytosol to its active sight within the membrane (43). Caffeic acid inhibits LTB4 generation by inactivating the active sight of 5-LO (26), and lipoxin A4 is a reported LTB4 receptor antagonist (6). Incubation of PMN with MK886 (0 to 5 μM) had no effect upon the release of superoxide in response to any of the stimuli used (unstimulated, fMLP, PMA, and S. epidermidis) (data not shown). PMN incubated with caffeic acid (0 to 200 μM) or lipoxin A4 (0 to 5 μM) showed a concentration-dependent decrease in superoxide generation in response to all stimuli. The IC50s of caffeic acid and lipoxin A4 (100 and 3 μM, respectively) were independent of the stimulus employed. Priming PMN with PDE prior to treatment with MK 886, caffeic acid, or lipoxin A4 did not affect the results obtained.
COX inhibitor.
COX is a ubiquitous mammalian enzyme that in PMN demonstrates a Km for AA which is similar to that of lipoxygenase. Activation of COX in PMN results in the formation of prostaglandin E2 and thromboxin B2. We investigated the role of this enzyme in PDE-mediated priming and activation of human PMN with the COX inhibitor flurbiprofen. The generation of superoxide by PMN when unstimulated or stimulated by fMLP, PMA, or S. epidermidis was unaffected by the incubation of cells with the antagonist (0 to 25 μM) (data not shown). Furthermore, priming of PMN by PDE prior to treatment with the inhibitor did not affect the results (data not shown).
ELISA measurement of LTB4.
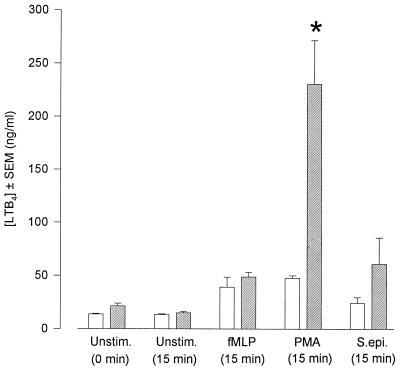
In view of the important role of LTB4 in mediating inflammatory responses, and with the consideration that we achieved various effects upon the generation of superoxide by using three different 5-LO antagonists, we directly quantified LTB4 release from PMN by ELISA. The presence of PDE did not affect the measurement of known amounts of standard LTB4. Furthermore, PDE contained no LTB4 per se (data not shown). The levels of LTB4 released from unstimulated PNM and PMN stimulated by fMLP, PMA, and S. epidermidis were 13.70 ± 0.63, 39.30 ± 9.24, 47.30 ± 2.69, and 24.30 ± 5.35 ng ml−1, respectively (Fig. 7). In the presence of PDE these values remained unchanged with the exception of PMN stimulated by PMA. Under these conditions LTB4 release was increased fivefold to 230.60 ± 41.00 ng ml−1.
FIG. 7.
Generation of LTB4 by unstimulated PMN and PMN challenged with fMLP (1 μM), S. epidermidis (2 × 107 ml−1), and PMA (10 ng ml−1). PMN were incubated in buffer (open bars) or PDE (hatched bars). Results are expressed as mean (± SEM) amount of LTB4 released (ng ml−1) (n = 6, six dialysis effluents upon PMN from six donors). The asterisk indicates a P value of ≤0.05 (Mann-Whitney U test).
DISCUSSION
We have previously reported that incubation of PMN with 4-h intraperitoneal dwell, pH-corrected PDE results in low-level activation of the NADPH oxidase and primes this response to receptor-mediated stimuli (10), possibly by an effect upon specific granule release (11). Studies of the signal transduction systems affected by PDE demonstrate a profound activation of PLA2 leading to an increase in the release of AA (28). In human PMN AA can directly activate the NADPH oxidase (17) and cause release of specific granules (23). Furthermore PAF (resulting from the liberation of AA from phosphatidylcholine) and the AA metabolite LTB4 are potent activators and primers of many PMN functions (1, 13, 22, 46). With this in mind we have investigated the role of AA and its metabolites in the priming and activation of human PMN by PDE.
A number of studies have investigated the role of PLA2 in the activation of the NADPH oxidase in human PMN. The fact that superoxide generation in response to many stimuli is reduced upon incubation of cells with PLA2 inhibitors and is restored by the addition of exogenous AA (20, 34, 37) demonstrates the importance of PLA2 in the activation and maintenance of the NADPH oxidase. Using both quinacrine and scalaradial we were able to confirm a role for PLA2 in PMN oxidase activation. Quinacrine reduced both superoxide generation and AA release from PMN in response to all stimuli tested. These data are in agreement with those of others who have demonstrated inhibition of superoxide generation of PMN by quinacrine when challenged with fMLP (37), PMA (20), and immune complexes (37). Our observation that PMN incubated with PDE were more sensitive to quinacrine prompted us to attempt to identify the form of PLA2 activated by PDE by the use of two selective PLA2 inhibitors. Incubation of PMN with scalaradial resulted in a concentration-dependent decrease in superoxide generation and AA release; however, the degrees of inhibition were similar in both primed and unprimed cells. These data would suggest that sPLA2 does play a role in the generation of superoxide; however, since we report no difference between the ICs for the primed and unprimed cells, its role in PDE-mediated priming is questionable. Mayer et al. (32) have reported inhibition of superoxide generation by scalaradial in PMA-stimulated rat alveolar macrophage. In a manner similar to that for PDE, the priming of these macrophage with lipopolysaccharide (LPS) did not affect their results. It is generally accepted that it is cPLA2 that is important in the release of AA from PMN (14). We investigated the role of cPLA2 by using the arachidonyl trifluoromethyl ketone AACO-CF3. AACO-CF3 is a potent inhibitor of cPLA2 in cell-free systems (48). However, in the present study, exposure of PMN to the antagonist failed to inhibit AA release and resulted in a slight stimulation of the NADPH oxidase. These observations may reflect metabolism of the compound by PMN; indeed, recent reports have suggested that AACO-CF3 is reduced to its noninhibitory alcohol upon incubation with many cell types (39).
It is well documented that exogenous PAF can stimulate oxidase activity and prime the response to subsequent stimulation by fMLP (1, 13). More recently, PAF receptor antagonists have been shown to inhibit superoxide production (47). We were, however, unable to demonstrate an effect of the PAF receptor antagonist hexanolamino-PAF upon either primed or unprimed superoxide generation by PMN when challenged with a variety of stimuli. The IC50 of hexanolamino-PAF for superoxide generation in human macrophage is quoted at 52 μM (42). In this study, using ATP bioluminescence as an indicator of cell viability, we observed significant PMN death at concentrations of the antagonist exceeding 25 μM (data not shown). Our results suggest that PAF plays only a limited role in the generation of superoxide anion under the conditions employed in our studies. However, fMLP-stimulated PMN are known to generate PAF (36), and most of this PAF appears to remain within the cell (35). Since PAF may have an autocrine effect (33) and exert its effects by binding to the recently described high-affinity cytosolic PAF receptors (49), the efficiency of hexanolamino-PAF in blocking PAF-mediated responses may be compromised by its inability to penetrate the cell. The major pathway by which AA is metabolized in human PMN is via 5-LO, ultimately leading to the generation of LTB4 (52). LTB4 is released upon challenge of PMN with opsonized particles (5, 38), fMLP, and PMA (45). The inflammatory mediator can directly activate aggregation, Ca2+ mobilization, superoxide generation, and degranulation (46) and prime these responses to stimulation by agents such as fMLP (13). Because of its potential importance in mediating the inflammatory response we used three functionally distinct LTB4 inhibitors to assess its role in PDE-induced priming and activation. In this study we obtained different results using these three inhibitors. Superoxide generation was inhibited with caffeic acid and lipoxin A4 but not with MK886. These observations call into question the target selectivity of some of these antagonists. Recently, lipoxin A4 has been shown to inhibit phosphoinositol hydrolysis in human PMN at concentrations similar to those employed in this study (16). The inhibition of superoxide generation that we report may therefore be a reflection of the inhibition of inositoyl-specific phospholipase C. No effect upon superoxide generation was seen with the FLAP inhibitor MK886. In agreement with others (43) we estimate the IC50 of this compound for ionophore-stimulated LTB4 release from human PMN to be approximately 120 nM (data not shown). However, we observed no reduction in superoxide generation even at concentrations of the antagonist (5 μM) that completely abolished LTB4 release. We confirmed that LTB4 plays little part in priming and activation of PMN by PDE by directly quantifying its release from cells by ELISA. In agreement with others we report that cells stimulated with fMLP, PMA, and Staphylococcus sp. (21, 30, 51) release only low levels of LTB4 in comparison to the level released by ionophore-challenged cells. Only cells primed with PDE and subsequently stimulated by PMA release more LTB4 than their relative controls. This observation may be a reflection of a combination of increased generation of AA (resulting from incubation of PMN with PDE) (28) and a direct activation of 5-LO by PMA. Several studies have reported that exposure of PMN to fresh (unused) dialysates results in a severe reduction in leukotriene release (24, 25). The data in our study confirm their suggestions that this effect is transient and probably the result of the low pH and high osmolality of fresh dialysates.
Flurbiprofen is well recognized as being a selective, potent inhibitor of both COX 1 and COX 11. In this study, concentrations of flurbiprofen of up to 25 μM had no effect upon oxidase activity of PMN when challenged with fMLP, PMA, or S. epidermidis. These observations are in keeping with those of others who report an ibuprofen IC50 of 600 μM for fMLP-induced superoxide generation (37). These observations support the current thinking that although COX and 5-LO have similar Kms for AA, most if not all of the fatty acid generated during cell activation is metabolized by 5-LO (52).
The involvement of PLA2 in primed stimulation is well documented. The enzyme appears to play a role in priming by diacyl- and alkylacylglycarol, inflammatory cytokines, and LPS (2, 3, 8, 12, 15, 27, 41). We were unable to detect appreciable concentrations of cytokines in the fluids employed in this study. Such an observation may not be considered unusual since the patients that we chose were not suffering infection at the time of dialysis. Our observation that only challenge with PMA results in elevated LTB4 release from PDE-primed PMN is reminiscent of LPS priming (14, 15). However, the concentrations of endotoxin in the effluents used were below those required for priming (14), and unlike LPS, PDE-mediated priming of the oxidase is immediate with no requirement for incubation (10).
Taken together, we suggest that these results demonstrate that AA per se, and not its metabolites, is important in priming and activation of the NADPH oxidase by PDE. Further work to support a role for cPLA2 in the generation of AA in PDE-primed human PMN and to study the chemical nature of the molecule(s) responsible for these effects is currently under way in our laboratory.
ACKNOWLEDGMENTS
We thank the Trent Regional Health Authority, Sheffield, United Kingdom, and Baxters Healthcare Corp., Deerfield, Ill., for their support.
REFERENCES
- 1.Bates E J, Harvey D P, Ferrante A. Inhibition of neutrophil respiratory burst and degranulation responses to platelet-activating factor by antagonists WEB 2086, CV 6209 and CV 3988. Int Arch Allergy Immunol. 1992;97:50–56. doi: 10.1159/000236095. [DOI] [PubMed] [Google Scholar]
- 2.Bauldry S A, McCall C E, Cousart S L, Bass D A. TNFα priming of PLA2 activation in human neutrophils—an alternative mechanism of priming. J Immunol. 1991;149:1277–1285. [PubMed] [Google Scholar]
- 3.Bauldry S A, Wykle R L, Bass D A. PLA2 activation in human neutrophils. J Biol Chem. 1988;263:16787–16795. [PubMed] [Google Scholar]
- 4.Boyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Investig. 21(Suppl. 97):77–89. [PubMed]
- 5.Classon H-E, Lundberg U, Malmsten C. Serum coated zymosan stimulates the synthesis of LTB4 in human PMN. Inhibition by cAMP. Biochem Biophys Res Commun. 1981;99:1230–1237. doi: 10.1016/0006-291x(81)90751-8. [DOI] [PubMed] [Google Scholar]
- 6.Conti P, Reale M, Barbacane R C, Panara M R, Bongrazio M. Inhibition of LTB4 in neutrophils by lipoxin A4 and B4. Agents Actions. 1991;32:85–87. doi: 10.1007/BF01983321. [DOI] [PubMed] [Google Scholar]
- 7.Crouch S P M, Kozlowski R, Slater K J, Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Methods. 1993;160:81–88. doi: 10.1016/0022-1759(93)90011-u. [DOI] [PubMed] [Google Scholar]
- 8.Dahinden C A, Zingg J, Maly F E, Weck A L. Leukotriene production in human neutrophils primed by recombinant human GMCSF and stimulated with the complement component C5a and fMLP as second signals. J Exp Med. 1988;167:1281–1293. doi: 10.1084/jem.167.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniels I, Bhatia K S S, Porter C J, Lindsay M A, Morgan A G, Burden R P, Fletcher J. Hydrogen peroxide generation by polymorphonuclear leukocytes exposed to peritoneal dialysis effluent. Clin Diagn Lab Immunol. 1996;3:682–688. doi: 10.1128/cdli.3.6.682-688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniels I, Lindsay M, Porter C, Haynes A P, Fletcher J, Morgan A G. Effect of peritoneal dialysis effluent on superoxide anion production by polymorphonuclear neutrophils. Nephron. 1993;64:382–387. doi: 10.1159/000187358. [DOI] [PubMed] [Google Scholar]
- 11.Daniels I, Crouch S P M, Lindsay M, Morgan A G, Burden R P, Fletcher J. Primary and secondary granule release by polymorphonuclear leukocytes exposed to peritoneal dialysis effluent. Clin Diagn Lab Immunol. 1994;1:227–231. doi: 10.1128/cdli.1.2.227-231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniels R H, Finnen M J, Hill M E, Lackie J M. Recombinant human monocyte IL8 primes NADPH-oxidase and PLA2 activation in human neutrophils. Immunology. 1992;75:157–163. [PMC free article] [PubMed] [Google Scholar]
- 13.Dewald B, Baggiolini M. Activation of NADPH oxidase in human neutrophils. Synergism between fMLP and the neutrophil products PAF and LTB4. Biochem Biophys Res Commun. 1985;128:297–304. doi: 10.1016/0006-291x(85)91678-x. [DOI] [PubMed] [Google Scholar]
- 14.Doerfler M E, Weiss J, Clark J D, Elsbach P. Bacterial LPS primes human neutrophils for enhanced release of arachidonic acid and causes phosphorylation of an 85-kDa cytosolic PLA2. J Clin Investig. 1994;93:1583–1591. doi: 10.1172/JCI117138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doerfler M E, Danner R L, Shelhamer H J, Parrillo J E. Bacterial LPS primes human neutrophils for enhanced production of LTB4. J Clin Investig. 1987;83:970–977. doi: 10.1172/JCI113983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grandordy B M, Lacroix H, Mavoungou E, Kriliso S, Crea A E G, Spur B W, Lee T H. Lipoxin A4 inhibits phosphoinositide hydrolysis in human neutrophils. Biochem Biophys Res Comm. 1990;167:1022–1029. doi: 10.1016/0006-291x(90)90625-w. [DOI] [PubMed] [Google Scholar]
- 17.Hardy S J, Ferrante A, Poulos A, Robinson B S, Johnson D W, Murray A W. Effect of exogenous fatty acids with greater than 22 carbon atoms (very long chain fatty acids) on superoxide production by human neutrophils. J Immunol. 1994;153:1754–1761. [PubMed] [Google Scholar]
- 18.Harvey D M, Sheppard K J, Morgan A G, Fletcher J. Effect of dialysis fluids on phagocytosis and killing by normal neutrophils. J Clin Microbiol. 1987;25:1424–1427. doi: 10.1128/jcm.25.8.1424-1427.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey D M, Sheppard K J, Morgan A G, Fletcher J. Neutrophil function in patients on continuous ambulatory peritoneal dialysis. Brit J Haematol. 1988;68:273–278. doi: 10.1111/j.1365-2141.1988.tb04202.x. [DOI] [PubMed] [Google Scholar]
- 20.Henderson L M, Chappell J B, Jones O T G. Superoxide generation is inhibited by PLA2 inhibitors. Role for PLA2 in the activation of the NADPH oxidase. Biochem J. 1989;264:249–255. doi: 10.1042/bj2640249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henricks P A J, Van Der Tol M E, Engels F, Nijkamp F P, Verhoef J. Human polymorphonuclear leukocytes release LTB4 during phagocytosis of Staphylococcus aureus. Inflammation. 1986;10:37–47. doi: 10.1007/BF00916039. [DOI] [PubMed] [Google Scholar]
- 22.Ingraham L M, Coates T D, Allen J M, Higgins C P, Baehner R L, Boxer L A. Metabolic, membrane and functional responses of human PMN to PAF. Blood. 1982;59:1259–1266. [PubMed] [Google Scholar]
- 23.Jacobson P B, Schrier D J. Regulation of CD11b/CD18 expression in human neutrophils by phospholipase A2. J Immunol. 1993;151:5639–5652. [PubMed] [Google Scholar]
- 24.Jorres A, Jorres D, Topley N, Gahl G M, Mahiout A. Leukotriene release from peripheral and peritoneal leukocytes following exposure to peritoneal dialysis solutions. Nephrol Dial Transplant. 1991;6:495–501. doi: 10.1093/ndt/6.7.495. [DOI] [PubMed] [Google Scholar]
- 25.Jorres A, Jorres D, Gahl G M, Kessel M, Muller C, Kottgen E, Serke S, Schulz S, Mahiout A. LTB4 and TNF release from leukocytes: effect of peritoneal dialysis. Nephron. 1991;58:276–282. doi: 10.1159/000186436. [DOI] [PubMed] [Google Scholar]
- 26.Koshihara Y, Neichi T, Murota S-I, Lao A-N, Fujimoto Y, Tatsuno T. Caffeic acid is a selective inhibitor for leukotriene biosynthesis. Biochim Biophys Acta. 1984;792:92–97. [PubMed] [Google Scholar]
- 27.Krump E, Borgeat P. Kinetics of 5-LO activation, arachidonic acid release and leukotriene synthesis in human neutrophils: effects of GMCSF. Biochem Biophys Acta. 1994;1213:135–139. doi: 10.1016/0005-2760(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 28.Lindsay M A, Daniels I, Fletcher J. Phospholipases and the activation and priming of neutrophils by peritoneal dialysis effluent. Peritoneal Dial Int. 1997;17:471–479. [PubMed] [Google Scholar]
- 29.Lowry O H, Rosenbrough M L, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Mahadevappa V G, Powell W S. The metabolism of arachidonic and eicosapentaenoic acid in human neutrophils stimulated by A23187 and fMLP. J Cell Biochem. 1989;40:341–352. doi: 10.1002/jcb.240400310. [DOI] [PubMed] [Google Scholar]
- 31.Marshall L A, Winkler J D, Griswold D E, Bolognese B, Roshak A, Sung C-M, Webb E F, Jacobs R. Effects of scalaradial, a type II PLA2 inhibitor, on human neutrophil arachidonic acid mobilization and lipid mediator formation. J Pharmacol Exp Ther. 1994;268:709–717. [PubMed] [Google Scholar]
- 32.Mayer A M S, Brenic S, Stocker R, Glaser K B. Modulation of superoxide generation in in vivo lipopolysaccharide-primed rat alveola macrophage by arachidonic acid and inhibitors of protein kinase C, PLA2, protein serine-threonine phosphatase(s), protein tyrosine kinase(s) and phosphatase(s) J Pharmacol Exp Ther. 1995;274:427–436. [PubMed] [Google Scholar]
- 33.McDonald P P, McColl S R, Braquet P, Borgeat P. Autocrine enhancement of leukotriene synthesis by endogenous LTB4 and platelet activating factor in human neutrophils. Br J Pharmacol. 1994;111:852–860. doi: 10.1111/j.1476-5381.1994.tb14816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muid R E, Twomey B, Dale M M. The effect of inhibition of both diacylglycerol metabolism and PLA2 activity on superoxide generation by human neutrophils. FEBS Lett. 1988;234:235–240. doi: 10.1016/0014-5793(88)81342-5. [DOI] [PubMed] [Google Scholar]
- 35.Muller S, Nigam S. Enhancement of staurosporine of PAF formation in fMLP challenged human PMN is mediated by intracellular PAF binding sites. Biochem Biophys Res Commun. 1992;189:771–776. doi: 10.1016/0006-291x(92)92268-3. [DOI] [PubMed] [Google Scholar]
- 36.Muller S, Nigam S. Arachidonic acid release and platelet-activating factor formation by staurosporine in human neutrophils challenged with n-formyl peptide. Eur J Pharmacol. 1992;218:251–258. doi: 10.1016/0014-2999(92)90176-5. [DOI] [PubMed] [Google Scholar]
- 37.Neal T M, Vissers M C M, Winterbourn C C. Inhibition by nonsteroidal anti-inflammatory drugs of superoxide production and granule enzyme release by polymorphonuclear leukocytes stimulated with immune complexes or fMLP. Biochem Pharmacol. 1987;36:2511–2517. doi: 10.1016/0006-2952(87)90524-7. [DOI] [PubMed] [Google Scholar]
- 38.Palmer R M J, Salmon J A. Release of LTB4 from human neutrophils and its relationship to degranulation induced by fMLP, serum treated zymosan and the ionophore A23187. Immunology. 1983;50:65–73. [PMC free article] [PubMed] [Google Scholar]
- 39.Riendeau D, Guay J, Weech P K, Laliberte F, Yergey J, Li C, Desmarais S, Perrier H, Liu S, Nicoll-Griffith D, Street I P. Arachidonyl trifluoromethyl ketone, a potent inhibitor of 85-kDa PLA2, blocks production of arachidonic acid and 12-hydroxyeicosatetraenoic acid by calcium ionophore-challenged platelets. J Biol Chem. 1994;269:15619–15624. [PubMed] [Google Scholar]
- 40.Roberts P J, Williams S L, Linch D C. The regulation of neutrophil PLA2 by granulocyte-macrophage colony-stimulating factor and its role in priming superoxide production. Br J Haematol. 1996;92:804–814. doi: 10.1046/j.1365-2141.1996.432970.x. [DOI] [PubMed] [Google Scholar]
- 41.Rosenthal M D, Franson R C. Separation of agonist-stimulated arachidonic mobilization from subsequent LTB4 synthesis in human neutrophils: different effects of oleoylacetylglycerol and PMA as priming agents. J Cell Physiol. 1994;160:522–530. doi: 10.1002/jcp.1041600315. [DOI] [PubMed] [Google Scholar]
- 42.Rouis M, Nigon F, Chapman M J. Platelet activating factor is a potent stimulant of the production of active oxygen species by human monocyte-derived macrophages. Biochem Biophys Res Commun. 1988;156:1293–1301. doi: 10.1016/s0006-291x(88)80773-3. [DOI] [PubMed] [Google Scholar]
- 43.Rouzer C A, Ford-Hutchingson A W, Morton H E, Gillard J W. MK886, a potent and specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore-challenged leukocytes. J Biol Chem. 1990;265:1436–1442. [PubMed] [Google Scholar]
- 44.Rubinek T, Levey R. Arachidonic acid increases the activity of the assembled NADPH oxidase in cytoplasmic membranes and endosomes. Biochim Biophys Acta. 1993;1176:51–58. doi: 10.1016/0167-4889(93)90176-p. [DOI] [PubMed] [Google Scholar]
- 45.Salari H, Braquet P, Naccache P, Boregeat P. Characterization of effect of n-fMLP on leukotriene synthesis in human polymorphonuclear leukocytes. Inflammation. 1985;9:127–138. doi: 10.1007/BF00917585. [DOI] [PubMed] [Google Scholar]
- 46.Serhan C N, Radin A, Smolen J E, Korchak H, Samuelson B, Weissmann G. LTB4 is a complete secretagogue in human neutrophils: a kinetic analysis. Biochem Biophys Res Commun. 1982;107:1006–1012. doi: 10.1016/0006-291x(82)90622-2. [DOI] [PubMed] [Google Scholar]
- 47.Steward A G, Harris T. Platelet-activating factor may participate in signal transduction processes in rabbit leukocytes. Lipids. 1991;26:1044–1049. doi: 10.1007/BF02536499. [DOI] [PubMed] [Google Scholar]
- 48.Street I P, Lin H-K, Laliberte F, Ghomashchi F, Wang Z, Perrier H, Tremblay N M, Huang Z, Weech P K, Gelb M H. Slow- and tight-binding inhibitors of the 85-kDa human PLA2. Biochemistry. 1993;32:5935–5940. doi: 10.1021/bi00074a003. [DOI] [PubMed] [Google Scholar]
- 49.Svetlov S, Nigam S. Evidence for the presence of specific high affinity cytosolic binding sites for platelet activating factor in human neutrophils. Biochim Biophys Acta. 1993;190:162–166. doi: 10.1006/bbrc.1993.1025. [DOI] [PubMed] [Google Scholar]
- 50.Vadas P, Pruzanski W. Biology of disease. Role of secretory PLA2 in the pathobiology of disease. Lab Investig. 1986;55:391–404. [PubMed] [Google Scholar]
- 51.Waite M, DeChatelet L R, King L, Shirley P S. Phagocytosis-induced release of arachidonic acid from human neutrophils. Biochem Biophys Res Commun. 1979;90:984–992. doi: 10.1016/0006-291x(79)91924-7. [DOI] [PubMed] [Google Scholar]
- 52.Walsh C E, Waite B M, Thomas M J, DeChatelet L R. Release and metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1981;256:7228–7234. [PubMed] [Google Scholar]