Abstract
BACKGROUND
Maternal death rates remain high in many low- and middle-income countries. Hypertensive disorders of pregnancy account for 18% of maternal mortality in Ghana. The maternal near-miss approach was designed to evaluate severe (acute) complications in pregnancy, which is useful to detect potential areas for clinical care improvement.
OBJECTIVE
This study aimed (1) to determine the incidence of severe maternal complications, maternal near-miss cases, and mortality cases associated with hypertensive disorders of pregnancy remote from term and (2) to assess the health system's performance indicators for the management of hypertensive disorders of pregnancy remote from term in middle-income country referral hospitals.
STUDY DESIGN
This study was nested in the ongoing Severe Preeclampsia adverse Outcome Triage study, a multicenter observational cohort study, and included women recruited from December 1, 2017, to May 31, 2020, from 5 referral hospitals in Ghana. Women aged >16 years, admitted to the hospital with hypertensive disorders of pregnancy, with gestational age between 26 and 34 weeks were eligible. Near miss was defined according to the World Health Organization and sub-Saharan African near-miss criteria. Descriptive statistics of pregnancy and maternal and perinatal outcomes up to 6 weeks after delivery of women with severe maternal outcomes were presented for maternal deaths and maternal near-miss casigurees and compared with that of women without severe maternal outcomes. The health system's maternal and perinatal performance indicators were calculated.
RESULTS
Overall, 447 women with hypertensive disorders of pregnancy were included in the analyses with a mean maternal age of 32 (±5.8) years and mean gestational age at recruitment of 30.5 (±2.4) weeks. Of these patients, 46 (10%) had gestational hypertension, 338 (76%) had preeclampsia, and 63 (14%) had eclampsia. There were 148 near-miss cases (33.1%) and 12 maternal deaths (2.7%). Severe maternal outcomes constituted complications from severe preeclampsia (80/160 [50%]) and eclampsia (63/160 [39.4%]). Concerning organ dysfunction, hematologic and respiratory dysfunctions constituted 59/160 [38.6%] and 23/160 [14.8%] respectively. Nearly all women had a cesarean delivery (347/447 [84%] and 140/160 [93%] in the severe maternal outcome group) and delivered prematurely (83%, with 178/379 [93%] at <32 weeks of gestation). Stillbirth and neonatal deaths occurred in 63 of 455 women (14%) and 81 of 392 women (19%), respectively, constituting a stillbirth ratio of 161 per 1000 live births and neonatal mortality rate of 207 per 1000 live births as there were 392 live births in this cohort. Overall, the intensive care unit admission rate was 12.7% (n=52/409); moreover, 45 of 52 women (86.5%) admitted to the intensive care unit had severe maternal outcomes. The maternal death ratio was 3100 per 100,000 live births, the maternal near-miss–to–mortality ratio was 12.3, and the mortality index was 8%.
CONCLUSION
Maternal near miss and maternal and perinatal mortalities were common in women with hypertensive disorders of pregnancy remote from term in referral hospitals in Ghana. Providing appropriate patient-centered and multidisciplinary quality care for these women is crucial in improving pregnancy outcomes. Context-tailored interventions should be considered in the clinical management of complications associated with hypertensive disorders of pregnancy in resource-limited settings. Further research on interventions to improve timely referral and reduce in-hospital delays in care provision is recommended to facilitate emergency care services for women with hypertensive emergencies.
Key words: eclampsia, Ghana, hypertensive disorders of pregnancy, low- and middle-income countries, maternal mortality, maternal near miss, preeclampsia, severe maternal morbidity
AJOG Global Reports at a Glance.
Why was this study conducted?
Hypertensive disorders of pregnancy (HDP) are highly prevalent and an important cause of severe morbidity, long-term health impact, and maternal and perinatal deaths. Near-miss studies are clinically useful in assessing potential areas for improvement of maternal healthcare.
Key findings
Maternal near-miss (MNM) and mortality cases associated with HDP at <34 weeks of gestation were high in referral hospitals in Ghana. The ratio of MNM events to maternal deaths (MDs) was 12.3 to 1.0, with a mortality index of 8%. This indicated substantial substandard care for women with HDP.
What does this add to what is known?
This study has presented data on a large prospective cohort of women with HDP remote from term in a low-resource setting and has shown the importance of improving healthcare quality for these women with a higher risk of severe complications in resource-limited settings to reducing MD rates.
Introduction
Hypertensive disorders of pregnancy (HDP), such as gestational hypertension, preeclampsia, and eclampsia, are the most common medical complication encountered during pregnancy, affecting approximately 10% of pregnancies.1,2 Worldwide, HDP are an important cause of death, severe morbidity, and long-term adverse health outcomes among mothers and their neonates.3,4 Low- and middle-income countries are disproportionately affected.5 In Ghana, where the maternal mortality ratio (MMR) was estimated to be 308 deaths per 100,000 live births in 2017,6 approximately 18% of maternal mortality cases were caused by eclampsia and preeclampsia.7
Improving maternal health and reducing death rates have been on the global agenda for decades, including the Sustainable Development Goals. Evaluation of maternal near miss (MNM) is a recommended strategy to identify and analyze factors leading to adverse maternal outcomes.8 MNM is defined as a woman who nearly died but survived a complication that occurred during pregnancy, childbirth, or within 42 days of termination of pregnancy.9 There is evidence that women who experience severe acute complications in pregnancy share many pathologic and circumstantial factors with women who experience mortality. Thus, the evaluation of MNM allows for cross-case comparisons to identify care and contextual factors with reduced sentiments of blame, within maternal care improvement cycles.10
To improve uniformity in MNM studies, standardized methods for study setup and classification of the criteria were provided in 2011 by the World Health Organization (WHO).9 However, several studies have locally adapted or proposed new criteria because of the unavailability of some of the recommended clinical parameters in low-resource settings (eg, the arterial oxygen partial pressure to fractional inspired oxygen [PaO2/FiO2] or arterial blood gas analyses [pH and lactate]).11, 12, 13, 14, 15, 16, 17 Although this adaptation might improve the identification of MNM cases locally, the cross-setting comparison is reduced.10
Analyzing high-risk pregnancies with high mortality and near-miss rates, such as HDP in early pregnancy (<34 weeks of gestation), is clinically useful to create awareness about quality-of-care issues and detect potential areas for improving maternal healthcare. Therefore, this study aimed to assess pregnancy outcomes in near-miss cases associated with HDP and evaluate the maternal and perinatal health system's performance indicators of quality of care.
Materials and Methods
Study setting and design
This analysis was nested within the ongoing Severe Preeclampsia adverse Outcome Triage (SPOT) study, a multicenter observational prospective cohort study in Ghana, which aims to validate previously developed risk prediction models for the management of women with preeclampsia and other HDP.18,19 Of note, 4 major referral hospitals in the Greater Accra Region (Greater Accra Regional Hospital [Ridge Hospital], Korle-Bu Teaching Hospital, La General Hospital, and Tema General Hospital) and 1 hospital in the Eastern Region of Ghana (Koforidua Regional Hospital) were selected on the basis of their large patient volume and infrastructure to conduct this study. The total number of deliveries in these facilities exceeds 30,000 annually, and all hospitals have neonatal intensive care units (NICUs). Moreover, HDP are a leading cause of maternal morbidities in these facilities and account for 18% of all maternal mortalities in the country.7
Women aged ≥16 years with a diagnosis of preeclampsia or another HDP (definitions are provided in Supplementary A) at a gestational age between 26 and 34 weeks admitted to any 1 of the participating facilities were eligible for participation in the SPOT study. The exclusion criteria were spontaneous active labor at admission and occurrence of any of the adverse maternal outcomes before meeting the inclusion criteria or collecting the independent variables. In this analysis, all women who were recruited between December 1, 2017, and May 31, 2020, were included.
Maternal death and maternal near-miss classification
Women who died during admission or within the follow-up period of 6 weeks because of pregnancy-related complications were classified as maternal deaths (MDs). In all surviving women, those who met the WHO or sub-Saharan African (SSA) MNM criteria were considered MNM. Supplementary B provides an overview of the criteria. This approach was chosen because of the appropriateness of SSA in this context.20 The fulfillment of at least 1 criterion was enough to consider a woman as MNM.9,17 The SSA criterion on severe complications of abortion was not applicable as only pregnant women with a gestational age >26 weeks were considered eligible. Several other criteria were not included because of (1) limited access to laboratory tests (ie, pH or lactate), (2) nonrecording of observations that were not commonly documented in medical files (ie, acute cyanosis, gasping, or jaundice), and (3) other data that were not included in the case report forms of the SPOT study (ie, respiratory rates, urine production, loss of consciousness, cardiopulmonary resuscitation, or severe malaria).
Definitions of clinical conditions and diseases that were included as maternal outcomes (eg, severe postpartum hemorrhage and severe preeclampsia) followed WHO MNM guideline definitions (Supplementary A).9 Intensive care unit (ICU) was defined as a ward where mechanical ventilation and administration of continuous vasoactive drugs were possible. This included an extended stay at the postoperative recovery room >6 hours, considering the limited availability of actual ICU departments.21 Body mass index was calculated on the basis of height in meters and weight in kilogram at first booking in antenatal care (ANC).
All MNM cases and MDs conjointly were categorized as “severe maternal outcomes” (SMOs). Women who did not experience MD or near miss were considered as the comparison group.
Data on near-miss cases, MDs, SMO cases, stillbirths, and neonatal mortality cases were presented as ratio per 1000 live births. The MNM mortality ratio (=MNM cases/MDs), mortality index (=MDs/SMO cases × 100%), and ICU admission rate (which is equal to the number of women admitted to the ICU/all included women) in total and among SMO cases were calculated to assess complexity and performance of care. All ratios are listed in Supplementary A.9
Data sources and measurement
Trained research assistants prospectively collected data from medical records supplemented by face-to-face interview of the women to complete the information that were not initially obtained from the medical records, using standardized data collection forms designed for the SPOT study. Information regarding sociodemographic characteristics (eg, ethnicity, religion, marital status, and the highest level of education), medical history, obstetrical history (especially previous pregnancy complications), and information regarding current pregnancy and ANC services provided were recorded within 24 hours after admission. Symptoms and clinical signs of organ dysfunction were documented at the time of admission and daily during hospitalization. When delivery occurred during admission, circumstances of delivery, required interventions and maternal and neonatal outcomes were recorded. In case of discharge before delivery or data collection completion, information regarding pregnancy outcomes was collected at follow-up. Data of any readmissions before the end of pregnancy were added to the study file. Late maternal complications and neonatal outcomes were obtained at follow-up, during a routine visit, 6 weeks after delivery. All available data at the time of analysis were considered for this study.
Statistical analysis
Baseline characteristics and maternal and pregnancy outcomes for all women with HDP were presented using descriptive statistics for women without SMOs, women with SMOs, near-miss cases, and MDs. Categorical variables were presented as frequency (percentage), whereas continuous variables were presented as mean (standard deviation) and transformed into categorical groups when necessary. P values were calculated using the chi-square, Fisher exact, or unpaired 2-samples Wilcoxon test. Outcomes among the 5 study sites were compared using stratified analyses. Missing values and inconsistent data were cross-checked, source documents consulted, and missing data were excluded in the analyses. All analyses were executed using R statistics (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria).
Ethical approval
The SPOT study protocol was approved by the Ghana Health Service Ethical Review Committee (protocol ID GHSERC-GHSERC015/09/17) and the Ethical and Protocol Review Committee of the College of Health Sciences, University of Ghana (protocol ID GHSERC-CHS-EtM.4-P1.2/2017-2018). All participants gave their written informed consent.
Results
Maternal near miss and maternal deaths
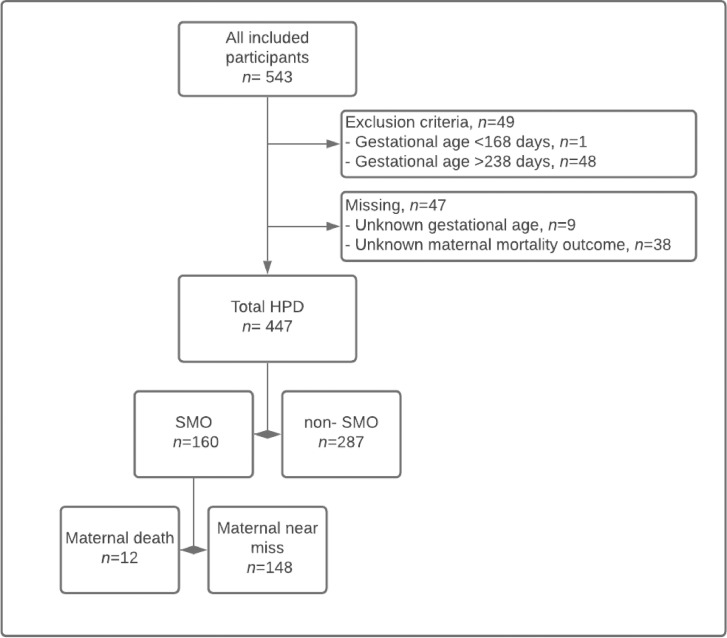
A total of 543 women were included in the SPOT study at the start of this analysis. However, 49 patients did not meet the inclusion criterion regarding gestational age at admission (ie, 48 with gestational age >34 weeks and 1 with gestational age <26 weeks) and were subsequently excluded from the initial study population. An additional group of 47 patients was excluded because of missing values (gestational age [n=9] and maternal mortality outcome [n=38]), resulting in a total of 447 women with HDP included (82%) in the final analysis (Figure).
Figure.
Flowchart
HDP, hypertensive disorders of pregnancy; SMO, severe maternal outcome.
Drechsel. Maternal near-miss and hypertensive disorders of pregnancy. Am J Obstet Gynecol Glob Rep 2022.
In addition, 12 women died during pregnancy or within 6 weeks after delivery, resulting in a maternal mortality incidence of 2.7% (12/447). Moreover, 148 cases were classified as MNM (33.1%) (69 fulfilled both SSA and WHO criteria and 79 fulfilled only SSA criteria). Of the MNM cases, 138 (93%) met the clinical criteria, 41 (28%) met the laboratory criteria, and 14 (9%) met the management-based criteria (Table 1). The most common fulfilled MNM criteria were failure to form clots (ie, bedside clotting time of >7 minutes; 35/148 [45.5%]), eclampsia (60/148 [40.5%]), and/or severe preeclampsia with ICU admission (39/148 [26.4%]).
Table 1.
Type of criteria (clinical, laboratory, and management) in maternal near miss cases
| MNM criteria | MNM (SSA), n (%) | MNM (WHO), n (%) | MNM (combined), n (%) | Maternal death, n (%) |
|---|---|---|---|---|
| n=148 | n=69 | n=148 | n=12 | |
| Clinical criteria | 138 (93) | 35 (51) | 138 (93) | 7 (58) |
| Failure to form clots | 35 (24) | 35 (51) | 35 (24) | 2 (17) |
| Stroke | 0 | 0 | 0 | 0 |
| Eclampsia | 60 (41) | NA | 60 (41) | 3 (25) |
| Ruptured uterus | 1 (1) | NA | 1 (1) | 0 |
| Sepsis or severe systemic infection | 0 | NA | 0 | 1 (8) |
| Pulmonary edema | 3 (2) | NA | 3 (2) | 1 (8) |
| Severe preeclampsia with ICU admission | 39 (26) | NA | 39 (26) | 0 |
| Laboratory criteria | 36 (24) | 41 (59) | 41 (28) | 4 (33) |
| Oxygen saturation<90% for >60 min | 17 (11) | 17 (25) | 17 (11) | 2 (16) |
| PaO2/FiO2<200 mm Hg | NA | 5 (7) | 5 (3) | 0 |
| Creatinine level≥300 μmol/L or ≥3.5 mg/dL | 6 (4) | 6 (9) | 6 (4) | 1 (8) |
| Bilirubin level>100 μmol/L or >6.0 mg/dL | NA | 0 | 0 | 1 (8) |
| Acute thrombocytopenia (<50,000 platelets/mL) | 13 (9) | 13 (19) | 13 (9) | 0 |
| Management-based criteria | 14 (9) | 3 (4) | 14 (9) | 4 (33) |
| Use of continuous vasoactive drugs | NA | 0 | 0 | 1 (8) |
| Hysterectomy following infection or hemorrhage | 0 | 0 | 0 | 0 |
| Transfusion of ≥2 (SSA) or ≥5 (WHO) units of blood or red cells | 11 (7) | 0 | 11 (7) | 2 (17) |
| Intubation and ventilation not related to anesthesia | 3 (2) | 3 (4) | 3 (2) | 0 |
| Dialysis for acute renal failure | NA | 0 | 0 | 1 (8) |
| Laparotomy other than cesarean delivery | 0 | NA | 0 | 0 |
ICU, intensive care unit; MNM, maternal near miss; NA, not applicable; SSA, sub-Saharan African; WHO, World Health Organization.
Drechsel. Maternal near-miss and hypertensive disorders of pregnancy. Am J Obstet Gynecol Glob Rep 2022.
Sociodemographic and obstetrical characteristics
Baseline characteristics are summarized in Table 2. The mean age was 32 (±5.8) years among women with HDP, and 256 of 447 women (70%) were between 30 and 40 years old. Of the 447 women, 226 (51.8%) belonged to the Akan ethnic group, 401 (89.6%) were Christians, 347 were married (79.4%), and 278 (63.8%) completed secondary education and 100 (22.9%) completed tertiary education; moreover, 404 of 447 women were employed.
Table 2.
Maternal sociodemographic and obstetrical characteristics of women with hypertensive disorders of pregnancy
| Maternal variable | Total HDP, n (%) | Missing, n (%) | Non-SMOs, n (%) | SMOs, n (%) | P value | Maternal deaths, n (%) | Maternal near miss, n (%) | P value |
|---|---|---|---|---|---|---|---|---|
| n=447 | n=287 | n=160 | n=12 | n=148 | ||||
| Sociodemographic factors | ||||||||
| Maternal age (y) | ||||||||
| Mean age (±SD) | 32.0±5.8 | 12 (2.7) | 32.2±5.5 | 31.7±6.2 | 0.52 | 31.3±6.1 | 31.7±6.2 | .86 |
| <20 | 13 (3.0) | 5 (1.8) | 8 (5.1) | 0.21 | 1 (8.3) | 7 (4.8) | .86 | |
| 20–30 | 126 (29.0) | 82 (29.6) | 44 (27.8) | 2 (16.7) | 42 (28.8) | |||
| 30–40 | 256 (58.9) | 166 (59.9) | 90 (57.0) | 8 (66.7) | 82 (56.2) | |||
| >40 | 40 (9.2) | 24 (8.7) | 16 (10.1) | 1 (8.3) | 15 (10.3) | |||
| Marital status | 10 (2.2) | 0.99 | .80 | |||||
| Single | 84 (19.2) | 53 (18.9) | 31 (19.7) | 3 (25.0) | 28 (19.3) | |||
| In a relationship | 6 (1.4) | 4 (1.4) | 2 (1.3) | 0 | 2 (1.4) | |||
| Married | 347 (79.4) | 223 (79.6) | 124 (79.0) | 9 (75.0) | 115 (79.3) | |||
| Education | 11 (2.5) | 0.06 | <.05 | |||||
| No education | 19 (4.4) | 13 (4.7) | 6 (3.8) | 2 (16.7) | 4 (2.7) | |||
| Primary | 39 (8.9) | 32 (11.5) | 7 (4.4) | 2 (16.7) | 5 (3.4) | |||
| Secondary | 278 (63.8) | 166 (59.7) | 112 (70.9) | 8 (66.7) | 104 (71.2) | |||
| Tertiary | 100 (22.9) | 67 (24.1) | 33 (20.9) | 0 | 33 (22.6) | |||
| Religion | 4 (0.9) | 0.41 | 1.000 | |||||
| Christianity | 397 (89.6) | 253 (89.4) | 144 (90.0) | 11 (91.7) | 133 (89.9) | |||
| Islam | 46 (10.4) | 30 (10.6) | 16 (10.0) | 1 (8.3) | 15 (10.1) | |||
| Employment | 9 (2.0) | 0.43 | 1.000 | |||||
| Yes | 404 (92.2) | 263 (93.3) | 141 (90.4) | 11 (91.7) | 130 (90.3) | |||
| Student | 5 (1.1) | 3 (1.1) | 2 (1.3) | 0 | 2 (1.4) | |||
| No | 29 (6.6) | 16 (5.7) | 13 (8.3) | 1 (8.3) | 12 (8.3) | |||
| Ethnicity | 11 (2.5) | 0.22 | .70 | |||||
| Akan | 226 (51.8) | 134 (47.9) | 92 (59.0) | 7 (63.6) | 85 (58.6) | |||
| Ewe | 66 (15.1) | 47 (16.8) | 19 (12.2) | 1 (9.1) | 18 (12.4) | |||
| Ga | 80 (18.3) | 57 (20.4) | 23 (14.7) | 2 (18.2) | 21 (14.5) | |||
| Northern | 59 (13.5) | 40 (14.3) | 19 (12.2) | 1 (9.1) | 18 (12.4) | |||
| Other | 5 (1.1) | 2 (0.7) | 3 (1.9) | 0 | 3 (2.1) | |||
| BMI (kg/m2)a | ||||||||
| Mean BMI (±SD) | 30.3±7.5 | 157 (35.1) | 30.8±7.8 | 29.4±6.9 | .12 | 28.8±4.1 | 29.4±7.1 | .97 |
| Underweight (<18.5) | 4 (1.4) | 1 (0.5) | 3 (3.1) | .18 | 0 | 3 (3.3) | .47 | |
| Normal (18.5–25.0) | 53 (18.3) | 36 (18.6) | 17 (17.7) | 0 | 17 (18.9) | |||
| Overweight (25.0–30.0) | 94 (32.4) | 61 (31.4) | 33 (34.4) | 4 (66.7) | 29 (32.2) | |||
| Obese (>30.0) | 139 (47.9) | 96 (49.5) | 43 (44.8) | 2 (33.3) | 41 (45.6) | |||
| BP on admission (mm Hg) | ||||||||
| Mean systolic BP (±SD) | 156.0±26.9 | 9 (2.0) | 157.0±25.8 | 155.0±29.0 | .32 | 174.0±23.2 | 153.0±28.9 | .01 |
| Mean diastolic BP (±SD) | 98.0±19.6 | 12 (2.7) | 99.0±18.3 | 97.0±21.9 | .41 | 109.0±39.4 | 96.0±21.0 | .27 |
| Medical history | ||||||||
| Preexisting hypertension | 96 (27.1) | 93 (20.8) | 70 (29.9) | 26 (21.7) | <.05 | 5 (41.7) | 21 (19.4) | .01 |
| Sickle cell disease | 7 (1.6) | 8 (1.8) | 5 (1.8) | 2 (1.3) | .84 | 0 | 2 (1.4) | 1.000 |
| Malaria | 69 (15.7) | 9 (2.0) | 42 (14.9) | 27 (17.0) | .53 | 3 (25.0) | 24 (16.3) | .09 |
| Urinary tract infections | 36 (8.2) | 10 (2.2) | 25 (8.9) | 11 (6.9) | .64 | 0 | 11 (7.5) | .36 |
| Diabetes mellitus | 14 (3.2) | 7 (1.6) | 11 (3.9) | 3 (1.9) | .30 | 0 | 3 (2.0) | 1.000 |
| Obstetrical history | ||||||||
| Parity | 16 (3.6) | .06 | .07 | |||||
| Nulliparous | 124 (28.8) | 77 (28.2) | 47 (29.7) | 4 (36.4) | 43 (29.3) | |||
| Multiparous (1–3) | 264 (61.3) | 174 (63.7) | 90 (57.0) | 7 (63.6) | 83 (56.5) | |||
| Multiparous (>4) | 43 (10.0) | 22 (8.1) | 21 (13.3) | 0 | 21 (14.3) | |||
| HDP (in multiparous women) | .69 | 1.000 | ||||||
| Gestational hypertension | 60 (19.5) | 0 | 37 (18.9) | 23 (20.7) | 1 (14.3) | 22 (21.2) | ||
| Preeclampsia | 14 (4.6) | 0 | 9 (4.6) | 5 (4.5) | 0 | 5 (4.8) | ||
| Eclampsia | 1 (0.3) | 0 | 0 | 1 (0.9) | 0 | 1 (1.0) | ||
| Current pregnancy | ||||||||
| Number of fetuses | 33 (7.4) | <.05 | 1.000 | |||||
| Singleton | 391 (94.4) | 258 (93.5) | 133 (96.4) | 10 (100.0) | 123 (96.1) | |||
| Multiple | 23 (5.5) | 18 (6.5) | 5 (3.6) | 0 | 5 (3.9) | |||
| Smoking in current pregnancy | 3 (0.7) | 3 (0.7) | 2 (0.7) | 1 (0.6) | .57 | 0 | 1 (0.7) | 1.000 |
| Number of antenatal visits | 36 (8.1) | .22 | .48 | |||||
| <4 | 142 (34.5) | 84 (31.7) | 58 (39.7) | 3 (30.0) | 55 (40.4) | |||
| ≥4 | 269 (65.5) | 181 (68.3) | 88 (60.3) | 7 (70.0) | 81 (59.6) | |||
| Mean GA at admission (wk) (±SD) | 30.5±2.4 | 0 | 30.6±2.5 | 30.4±2.3 | .82 | 30.2±2.1 | 30.5±2.4 | .50 |
| HDP | 0 | <.05 | .55 | |||||
| Gestational hypertension | 46 (10.3) | 45 (15.7) | 1 (0.6) | 0 | 1 (0.7) | |||
| Preeclampsia | 338 (75.6) | 242 (84.3) | 96 (60.0) | 9 (75.0) | 87 (58.8) | |||
| Eclampsia | 63 (14.1) | 0 | 63 (39.4) | 3 (25.0) | 60 (40.5) |
P values were calculated using the chi-squared, Fisher exact, or unpaired 2-samples Wilcoxon tests.
BMI, body mass index; BP, blood pressure; GA, gestational age; HDP, hypertensive disorders of pregnancy; SD, standard deviation; SMO, severe maternal outcome.
Based on the calculation of weight in kilograms during the first booking in antenatal care, divided by squared body length in meters.
Drechsel. Maternal near-miss and hypertensive disorders of pregnancy. Am J Obstet Gynecol Glob Rep 2022.
Women were on average 30.5 (±2.4) weeks pregnant when admitted with hypertension (46/447 [10.3%]), preeclampsia (338/447 [75.6%]), or eclampsia (63/447 [14.1%]). Nearly all pregnancies (391/447 [94.7%]) were singleton pregnancies.
Compared with women without SMOs, women with SMOs were often younger (<20 years; 5.1% vs 1.8%) or older (>40 years; 10.1% vs 9.7%), had a higher unemployment rate (8.3% vs 5.7%), had slightly higher frequencies of grand multiparity (13.3% vs 8.1%), and had <4 ANC visits (39.7% vs 31.7%), all not statistically significant. All women with eclampsia and 28.4% of all women with preeclampsia were included in the SMO group.
Educational levels were relatively lower in MD cases than in MNM cases (16.7% with no education and 16.7% with primary education in MD cases vs 2.7% with no education and 3.4% with primary education in MNM cases). In addition, blood pressure (BP) on admission was higher in MD cases than in MNM cases (mean systolic BP 174 [±23] vs 153 [±29] mm Hg and mean diastolic BP 109 [±39] vs 96 [±21] mm Hg). Finally, the percentage of women with preexisting hypertension was higher in MD cases than in MNM cases (41% vs 19%). All cases of MD had a singleton pregnancy.
Maternal and pregnancy outcomes
Results regarding maternal outcomes are presented in Table 3. The most prevalent severe complications were preeclampsia with severe features (249/447 [55.7%]) and eclampsia (63/447 [14.1%]). All cases of MD had either 1 of 2 diagnoses (preeclampsia [9/12 (75%)] or eclampsia [3/12 (25%)]). All women presenting with organ dysfunction were included in the SMO group; the most prevalent outcomes were respiratory and hematologic dysfunctions. Compared with women without SMOs, women with SMOs seemed to require blood products more frequently (22/160 [15.0%] in the SMO group vs 3/287 [1.2%] in the non-SMO group) and were more frequently admitted to the ICU (31% in the SMO group vs 2.7% in the non-SMO group). Complications, such as sepsis, cardiovascular dysfunction, and hepatic dysfunction, occurred once, and all these complications resulted in maternal mortality (mortality index was 100% for each complication).
Table 3.
Maternal and pregnancy outcomes
| Outcome | Total HDP, n (%) | Missing, n (%) | Non-SMOs, n (%) | SMOs, n (%) | Maternal deaths, n (%) | Maternal near miss, n (%) | Mortality index |
|---|---|---|---|---|---|---|---|
| n=447 | n=287 | n=160 | n=12 | n=148 | |||
| Maternal outcome | |||||||
| Severe complications | |||||||
| Severe postpartum hemorrhage | 13 (3.4) | 66 (14.8) | 7 (2.8) | 6 (4.5) | 0 | 6 (4.9) | 0.0 |
| Severe preeclampsia | 249 (55.7) | 0 | 169 (58.9) | 80 (50.0) | 9 (75.0) | 71 (48.0) | 11.3 |
| Eclampsia | 63 (14.1) | 0 | 0 | 63 (39.4) | 3 (25.0) | 60 (40.5) | 4.8 |
| Sepsis or severe systemic infection | 1 (0.3) | 50 (11.2) | 0 | 1 (0.7) | 1 (9.1) | 0 | 100.0 |
| Uterine rupture | 1 (0.2) | 0 | 0 | 1 (0.6) | 0 | 1 (0.7) | 0.0 |
| Critical interventions | |||||||
| Use of blood products | 25 (6.3) | 48 (10.7) | 3 (1.2) | 22 (15.0) | 3 (30.0) | 19 (13.9) | 13.6 |
| Laparotomy | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| Admission to the intensive care unit | 52 (12.7) | 38 (8.5) | 7 (2.7) | 45 (31.0) | 1 (9.1) | 44 (32.8) | 2.2 |
| Organ dysfunction | |||||||
| Cardiovascular dysfunction | 1 (0.2) | 24 (5.4) | 0 | 1 (0.6) | 1 (9.1) | 0 | 100.0 |
| Respiratory dysfunction | 23 (5.4) | 24 (5.4) | 0 | 23 (14.8) | 2 (18.2) | 21 (14.6) | 8.7 |
| Renal dysfunction | 7 (1.6) | 9 (2.0) | 0 | 7 (4.4) | 1 (9.1) | 6 (4.1) | 14.3 |
| Coagulation or hematologic dysfunction | 59 (14.0) | 26 (5.8) | 0 | 59 (38.6) | 3 (27.3) | 56 (39.4) | 5.1 |
| Hepatic dysfunction | 1 (0.3) | 102 (22.8) | 0 | 1 (0.8) | 1 (9.1) | 0 | 100.0 |
| Neurologic dysfunction | 0 | 23 (5.1) | 0 | 0 | 0 | 0 | NA |
| Uterine dysfunction or hysterectomy | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| Other maternal outcomes | |||||||
| Pulmonary edema | 4 (1.0) | 31 (6.9) | 0 | 4 (2.6) | 1 (9.1) | 3 (2.1) | 25.0 |
| Pregnancy outcome | |||||||
| Delivery | |||||||
| First admission-delivery interval (d), mean (±SD) | 15.0±20.9 | 19 (4.3) | 19±22.8 | 8.9±15.6 | 5.3±7.8 | 9.2±15.9 | NA |
| Mode of delivery | 36 (8.1) | NA | |||||
| Spontaneous vaginal delivery | 9 (2.2) | 7 (2.7) | 2 (1.3) | 0 | 2 (1.4) | ||
| Induced vaginal delivery | 55 (13.4) | 46 (17.7) | 9 (6.0) | 2 (22.2) | 7 (4.9) | ||
| Elective cesarean delivery | 30 (7.3) | 24 (9.2) | 6 (4.0) | 0 | 6 (4.2) | ||
| Emergency cesarean delivery | 317 (77.1) | 183 (70.4) | 134 (88.7) | 7 (77.8) | 127 (89.4) | ||
| Cesarean delivery ratea | (84) | 36 (8.1) | (80) | (93) | (78) | (94) | |
| Obstetrical outcome (in all neonates) | n=455 | n=294 | n=161 | n=12 | n=149 | ||
| Mean GA (wk) (±SD) | 33±3.3 | 14 (3.1) | 33±3.4 | 32±2.84 | 30±1.67 | 32±2.87 | NA |
| Prematurity | 379 (83.3) | 14 (3.1) | 230 (78.2) | 149 (92.5) | 9 (75.0) | 140 (94.0) | NA |
| <28 | 34 (9.0) | 22 (9.6) | 12 (8.1) | 0 | 12 (8.6) | ||
| 28–32 | 144 (38.0) | 76 (33.0) | 68 (45.6) | 2 (22.2) | 61 (43.6) | ||
| 32–37 | 201 (53.0) | 132 (57.4) | 69 (46.3) | 7 (77.8) | 67 (47.9) | ||
| Stillbirths | 63 (13.8) | 0 | 41 (13.9) | 22 (13.7) | 5 (41.7) | 17 (11.4) | NA |
| Neonatal outcome (in live births) | n=392 | n=253 | n=139 | n=7 | n=132 | ||
| Mean birthweight (g) (±SD) | 1795±821.4 | 34 (8.7) | 1936±857.4 | 1547±690 | 1743±1556 | 1539±645 | NA |
| NICU admission | 308 (70.2) | 8 (2.0) | 179 (64.2) | 129 (80.6) | 6 (50.0) | 123 (83.1) | NA |
| Low Apgar score (<7) | NA | ||||||
| 1 min | 215 (51.6) | 30 (7.7) | 121 (42.2) | 94 (58.8) | 3 (25.0) | 91 (61.5) | |
| 5 min | 122 (29.3) | 31 (7.9) | 68 (23.7) | 54 (33.8) | 1 (8.3) | 53 (35.8) | |
| Newborn deathsb | 81 (18.7) | 14 (3.6) | 41 (14.9) | 40 (25.3) | 6 (50.0) | 34 (23.3) | NA |
GA, gestational age; HDP, hypertensive disorders of pregnancy; NA, not applicable; NICU, neonatal intensive care unit; SD, standard deviation; SMO, severe maternal outcome.
Cesarean deliveries divided by all deliveries
Neonatal mortality up to 6 weeks after delivery.
Drechsel. Maternal near-miss and hypertensive disorders of pregnancy. Am J Obstet Gynecol Glob Rep 2022.
The mean gestational age at delivery was 32.6 (±3.3) weeks, and 379 of 455 neonates (83%) were premature. The cesarean delivery rate was 84% among study participants (375/447). Neonatal outcomes were known for 455 infants (96%). There were 63 stillbirths (14%). The percentage rates of neonates with an Apgar score below 7 after 1 and 5 minutes were 51.6% and 29.3%, respectively. A total of 81 live births (18.7%) resulted in the death of the neonate within 6 weeks after delivery. Compared with non-SMO cases, SMO cases (especially MDs) seemed to have shorter admission-delivery intervals (8.9±15.6 vs 18.5±22.8 days) and higher (emergency) cesarean delivery rates (93% vs 80%). Neonates among this group were born with a lower gestational age (31.7±2.8 vs 33.1±3.4 weeks), and the prematurity rate was higher (93% vs 78%). The mean birthweight was 1547 (±690) g in the SMO group and 1936 (±857) g in the non-SMO group. The NICU admission rate was high (129/139 [81%]), and eventually, 40 of 139 neonates (25%) born in the SMO group died after delivery.
Maternal and perinatal healthcare indicators
Table 4 presents maternal and perinatal healthcare indicators. The incidence of SMOs was 408 cases per 1000 live births, the MNM ratio was 378 per 1000 live births, and the MD ratio was 3100 per 100,000 live births. The ratio of MNM events to MDs was 12.3 to 1.0 with a mortality index of 8%. Overall, the ICU admission rate was 13%, and 87% of women admitted to ICU had SMOs. Only 1 MD case was admitted to the ICU, which makes the proportion of MDs assisted without ICU 92% (11/12). The stillbirth ratio was 161 per 1000 live births (63/392), and the neonatal mortality rate was 207 per 1000 live births (81/392).
Table 4.
Maternal and perinatal healthcare indicators
| Maternal healthcare indicators | n |
|---|---|
| In the source population | |
| Live births | 392 |
| SMO cases | 160 |
| MDs | 12 |
| MNM cases | 148 |
| Near-miss indicators: complexity of care | |
| SMO ratio (per 1000 live births) | 408 |
| MNM ratio (per 1000 live births) | 378 |
| MD ratio (per 100,000 live births) | 3100 |
| Near-miss indicators: performance | |
| MNM mortality ratio | 12.3 |
| Mortality index | 8% |
| Intensive care use | |
| Total number of women giving birth | 447 |
| ICU admission rate | 13% |
| ICU admission rate among women with SMO | 31% |
| SMO rate among women admitted to the ICU | 87% |
| Proportion of MDs assisted without ICU admission | 92% |
| Perinatal healthcare indicators | |
| In the source population | |
| Live births | 392 |
| Stillbirths | 63 |
| Neonatal deaths | 81 |
| Perinatal health indicators | |
| Stillbirth ratio (per 1000 live births) | 161 |
| Neonatal mortality ratio (per 1000 live births) | 207 |
Supplementary A provides the definitions of the included ratios and indicators.
ICU, intensive care unit; MD, maternal death; MNM, maternal near miss; SMO, severe maternal outcome.
Drechsel. Maternal near-miss and hypertensive disorders of pregnancy. Am J Obstet Gynecol Glob Rep 2022.
Differences among included hospitals
Near-miss indicators and maternal outcomes (ie, severe complications, critical interventions, and organ dysfunction) for each (anonymized) center can be found in Supplementary C. The incidence of MNM, SMO, mortality index, MD ratio, and perinatal mortality differed across hospitals.
Discussion
This multicenter study assessed the SMOs in a large cohort of women with HDP remote from term in Ghana. We observed a high MNM ratio of 408 per 1000 live births with a prevalence of 33%, mortality index of 8%, and near-miss–to–mortality ratio of 12.3:1.0. In addition, high rates of stillbirth and neonatal mortality were observed, and the health system's indicators varied across participating hospitals.
Compared with other near-miss reviews in low-resource settings,11, 12, 13,21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 the reported near-miss ratios were high, and this can be attributed partly to the case mix in this cohort consisting of women with severe HDP remote from term admitted to referral hospitals. A systematic review that included 14 MNM reviews in Africa reported MNM prevalence ranging between 0.05% and 15.00%20 and reflecting the influence of participant selection (eg, only women with HDP vs unselected populations), facilities (eg, only referral hospitals vs smaller facilities), or criteria and definitions used.33 Importantly, considering that severe and early-onset hypertension in pregnancy is generally associated with high MD rates,3,4 the mortality index (8%) and MNMMR (1.0:12.3) that we reported were relatively low but significant and required appropriate interventions for improving care for women with HDP. The mortality index of 8% could suggest that the included healthcare facilities were performing quite well in the management of HDP and the role of the health facility's performance on outcomes is reflected by the substantial differences among the facilities. To optimize pregnancy outcomes, there is a need to improve these indicators of quality of care for women with HDP in the country.
Clinical characteristics were the most frequently fulfilled near-miss criteria. Similar to the observations in previous studies,10,17,20 a large proportion of women with MNM would not have been identified without the expanded SSA near-miss criteria. Although there is a risk of overclassification of MNM with the adapted SSA criteria used compared with the WHO criteria, underclassification (eg, because of context-irrelevant criteria or underregistration) is equally problematic, reflected in the fact that 7 of 12 MDs did not fulfill any criteria.
Severe preeclampsia and eclampsia were the leading conditions associated with SMOs. Affected organ systems were mainly hematologic and respiratory systems. Although in other MNM reviews in low-resource settings, severe hemorrhage and sepsis were often highly prevalent11, 12, 13,23, 24, 25, 26,28,29,32; however, incidences in our cohort were relatively low. These findings could be partly explained by the restrictive inclusion criteria (limited to HDP) and possibly reflective of the referral setting with adequate access to medications and interventions, including timely delivery and active management of the third stage of labor.23 Moreover, this may explain the low sepsis rate (1/447 [0.7%] in women with SMO), despite the very high cesarean delivery rate (88% vs often ±30% in other MNM reviews11,12,16) and associated risk of postpartum maternal infection.34,35
The ICU admission rate among women with SMO in this cohort (31%) was comparable with the rates in other MNM reviews of comparable facilities in Iraq and Rwanda (between 28% and 37%).12,25 The proportion of MDs that was not admitted to the ICU was even higher in this study (92%) than in available literature (46%–50%).12,25 Low ICU admission rates among these women with severe illness could indicate a shortage of ICU beds or difficulty in recognizing deteriorating patients in the absence of sophisticated diagnostics. In a previous MNM study in the largest tertiary hospital in Ghana, the ICU admission rate of 19% was reported among women with SMOs.21 In that study, the definition of admission to the ICU was broadened to include admission to the recovery ward for more than 6 hours because of the frequent unavailability of the ICU for SMO cases. The current study used this extended criterion for similar reasons.
Clinical and research implications
The high occurrence of SMOs and adverse perinatal complications associated with HDP has been determined in our study. Most of these were because of substandard care, evidenced by the mortality index of 8%. The near-miss rates and indicators varied among participating centers, and interfacility variation has been observed in other MNM reviews that included multiple facilities.16,36 These interfacility comparisons allow for further understanding of health facility and system-related factors that contribute to poor or good outcomes, including availability and content of local protocols, availability and use of critical interventions or laboratory diagnostics, demographic accessibility or availability of resources, and training and skills of personnel.17 Future studies should consider strategies to optimize the care for women with HDP, including timely referral, regular availability of medications, and laboratory support, and minimize in-hospital delays to facilitate optimal quality of care for women with HDP.
In addition, further research should include the identification, development, and evaluation of context-specific interventions to aid the clinical management of HDP to prevent severe complications, including MNM cases and mortalities. This could include refresher courses for healthcare professionals; these are useful adjuncts in improving the clinical management of HDP. Integrating this within a multidisciplinary clinical audit cycle for all MNM cases to identify treatment gaps or substandard treatment should be the cornerstone for quality-of-care improvement strategies to improve pregnancy outcomes.37,38
Strengths and limitations
The strengths of this study included the large number of women included in this prospective cohort, as most other MNM studies were (retrospective) case-control studies. This resulted in a lower risk of selection bias, the availability of a control group without SMO, and high-quality data for risk factors and adverse outcome incidence. At the same time, as these analyses were nested in and therefore were confined to the eligibility criteria of the SPOT study, we did not include women who presented with near-miss on arrival—a substantial group in sub-Saharan Africa as shown by others.36 The cohort was not purposefully set up for an MNM review, which led to limitations in data availability (eg, unavailability of some near-miss criteria) and generalizability (eg, women with HDP remote from term in nonreferral hospital settings and women with HDP at >34 weeks of gestation).
Conclusions
Women who experienced a hypertensive disorder in their pregnancy remote from term had high levels of SMOs in referral hospitals in Ghana. Our study echoed the applicability concerns of the WHO MNM criteria in low-income settings. Regular review of MNM and maternal mortality cases, as part of a clinical audit for quality improvement system, can advance the quality of healthcare provision, reduce substandard care, and result in better maternal and perinatal outcomes.
Patient and public involvement
The SPOT study consortium includes Action on Preeclampsia Ghana (APECGH), an advocacy organization of survivors of hypertensive disorders in pregnancy. As consortium members, they are involved in meetings and conferences in which research progress is discussed. Research questions and outcomes are informed by their priorities, experience, and preferences, identified either during consortium meetings or through joint public engagement events. This cohort was established before the first contact between SPOT study members and APECGH, and as such, they were not involved in the early design stages of this study and cohort; however, they were involved in subsequent expansions.
This specific study arose from a shared interest to understand the incidence of severe maternal and perinatal outcomes associated with HDP in our study population. APECGH was not involved in the design of this substudy, recruitment of participants, or conduct of the study. They will be involved in the dissemination of the study results to participants and the wider public through their newsletter (layman summary and abstract with link to full article) and public engagement activities (webinars and social media postings).
Acknowledgments
The authors gratefully thank all participating women and involved research assistants. Furthermore, we would like to acknowledge the feedback of the Severe Preeclampsia adverse Outcome Triage studies consortium members on drafts of the manuscript: Prof Robert Davis (PhD), Dr Linda Ahenkorah Fondjo (PhD), Dr Marcus Rijken (MD, PhD), Dr Vincent Boima, Dr Brege de Kok (PhD), Dr George Downward (MD, PhD), and Dr Beth Payne (PhD). In addition, we are grateful to the Bill & Melinda Gates Foundation (grant number OPP1197342), the Dutch Research Council (grant number NWA.1160.18.159), and the University Medical Center Utrecht Global Health Fellowship for funding this project.
Footnotes
K.C.D. and K.A.B. share first authorship.
The authors report no conflict of interest.
This study was supported by the Bill & Melinda Gates Foundation (grant number OPP1197342), the Dutch Research Council (grant number NWA.1160.18.159), and the University Medical Center Utrecht Global Health Fellowship (K.A.B.).
Cite this article as: Drechsel K CE, Adu-Bonsaffoh K, Olde Loohuis KM, et al. Maternal near-miss and mortality associated with hypertensive disorders of pregnancy remote from term: a multicenter observational study in Ghana. Am J Obstet Gynecol Glob Rep 2022;2:100045.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.xagr.2021.100045.
Appendix. Supplementary materials
References
- 1.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Steegers EA, von Dadelszen P, Duvekot JJ, Pre-eclampsia Pijnenborg R. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 3.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 4.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–e333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 5.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170:1–7. doi: 10.1016/j.ejogrb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 6.United Nations Population Fund, World Health Organization, UNICEF, World Bank Group, the United Nations Population Division . World Health Organization; Geneva, Switzerland: 2019. Trends in maternal mortality 2000 to 2017: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. [Google Scholar]
- 7.Ghana Statistical Service (GSS) Ghana Health Service (GHS) ICF . GSS, GHS, and ICF; Accra, Ghana: 2018. Ghana maternal health survey 2017. [Google Scholar]
- 8.Lewis G. Beyond the numbers: reviewing maternal deaths and complications to make pregnancy safer. Br Med Bull. 2003;67:27–37. doi: 10.1093/bmb/ldg009. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization, Department of Reproductive Health and Research . 2011. Evaluating the quality of care for severe pregnancy complications.https://www.who.int/reproductivehealth/publications/monitoring/9789241502221/en/ Available at: Accessed August 10, 2021. [Google Scholar]
- 10.Verschueren KJ, Kodan LR, Paidin RR, et al. Applicability of the WHO maternal near-miss tool: a nationwide surveillance study in Suriname. J Glob Health. 2020;10 doi: 10.7189/jogh.10.020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelissen EJ, Mduma E, Ersdal HL, Evjen-Olsen B, van Roosmalen JJ, Stekelenburg J. Maternal near miss and mortality in a rural referral hospital in northern Tanzania: a cross-sectional study. BMC Pregnancy Childbirth. 2013;13:141. doi: 10.1186/1471-2393-13-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalisa R, Rulisa S, van den Akker T, van Roosmalen J. Maternal Near Miss and quality of care in a rural Rwandan hospital. BMC Pregnancy Childbirth. 2016;16:324. doi: 10.1186/s12884-016-1119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldenberg RL, Saleem S, Ali S, et al. Maternal near miss in low-resource areas. Int J Gynaecol Obstet. 2017;138:347–355. doi: 10.1002/ijgo.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tura AK, Stekelenburg J, Scherjon SA, et al. Adaptation of the WHO maternal near miss tool for use in sub-Saharan Africa: an International Delphi study. BMC Pregnancy Childbirth. 2017;17:445. doi: 10.1186/s12884-017-1640-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tura AK, Zwart J, van Roosmalen J, Stekelenburg J, van den Akker T, Scherjon S. Severe maternal outcomes in eastern Ethiopia: application of the adapted maternal near miss tool. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heemelaar S, Kabongo L, Ithindi T, et al. Measuring maternal near-miss in a middle-income country: assessing the use of WHO and sub-Saharan Africa maternal near-miss criteria in Namibia. Glob Health Action. 2019;12 doi: 10.1080/16549716.2019.1646036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tura AK, Trang TL, van den Akker T, et al. Applicability of the WHO maternal near miss tool in sub-Saharan Africa: a systematic review. BMC Pregnancy Childbirth. 2019;19:79. doi: 10.1186/s12884-019-2225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Dadelszen P, Payne B, Li J, et al. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the fullPIERS model. Lancet. 2011;377:219–227. doi: 10.1016/S0140-6736(10)61351-7. [DOI] [PubMed] [Google Scholar]
- 19.Srofenyoh EK et al. on behalf of the SPOT study consortium. Severe Preeclampsia adverse Outcome Triage (SPOT) of women with preeclampsia remote from term: study protocol (Unpublished).
- 20.Tunçalp O, Hindin MJ, Souza JP, Chou D, Say L. The prevalence of maternal near miss: a systematic review. BJOG. 2012;119:653–661. doi: 10.1111/j.1471-0528.2012.03294.x. [DOI] [PubMed] [Google Scholar]
- 21.Tunçalp Ö, Hindin MJ, Adu-Bonsaffoh K, Adanu RM. Assessment of maternal near-miss and quality of care in a hospital-based study in Accra, Ghana. Int J Gynaecol Obstet. 2013;123:58–63. doi: 10.1016/j.ijgo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Adisasmita A, Deviany PE, Nandiaty F, Stanton C, Ronsmans C. Obstetric near miss and deaths in public and private hospitals in Indonesia. BMC Pregnancy Childbirth. 2008;8:10. doi: 10.1186/1471-2393-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lori JR, Starke AE. A critical analysis of maternal morbidity and mortality in Liberia, West Africa. Midwifery. 2012;28:67–72. doi: 10.1016/j.midw.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Ali AA, Khojali A, Okud A, Adam GK, Adam I. Maternal near-miss in a rural hospital in Sudan. BMC Pregnancy Childbirth. 2011;11:48. doi: 10.1186/1471-2393-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jabir M, Abdul-Salam I, Suheil DM, et al. Maternal near miss and quality of maternal health care in Baghdad, Iraq. BMC Pregnancy Childbirth. 2013;13:11. doi: 10.1186/1471-2393-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Akker T, Beltman J, Leyten J, et al. The WHO maternal near miss approach: consequences at Malawian District level. PLoS One. 2013;8:e54805. doi: 10.1371/journal.pone.0054805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Litorp H, Kidanto HL, Rööst M, Abeid M, Nyström L, Essén B. Maternal near-miss and death and their association with caesarean section complications: a cross-sectional study at a university hospital and a regional hospital in Tanzania. BMC Pregnancy Childbirth. 2014;14:244. doi: 10.1186/1471-2393-14-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rulisa S, Umuziranenge I, Small M, van Roosmalen J. Maternal near miss and mortality in a tertiary care hospital in Rwanda. BMC Pregnancy Childbirth. 2015;15:203. doi: 10.1186/s12884-015-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sangeeta G, Leena W, Taru G, Sushma K, Nupur G, Amrita P. Evaluation of severe maternal outcomes to assess quality of maternal health care at a tertiary center. J Obstet Gynaecol India. 2015;65:23–27. doi: 10.1007/s13224-014-0558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mbachu II, Ezeama C, Osuagwu K, Umeononihu OS, Obiannika C, Ezeama N. A cross sectional study of maternal near miss and mortality at a rural tertiary centre in southern nigeria. BMC Pregnancy Childbirth. 2017;17:251. doi: 10.1186/s12884-017-1436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oppong SA, Bakari A, Bell AJ, et al. Incidence, causes and correlates of maternal near-miss morbidity: a multi-centre cross-sectional study. BJOG. 2019;126:755–762. doi: 10.1111/1471-0528.15578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benimana C, Small M, Rulisa S. Preventability of maternal near miss and mortality in Rwanda: a case series from the University Teaching Hospital of Kigali (CHUK) PLoS One. 2018;13 doi: 10.1371/journal.pone.0195711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pembe AB, Hirose A, Alwy Al-Beity F, et al. Rethinking the definition of maternal near-miss in low-income countries using data from 104 health facilities in Tanzania and Uganda. Int J Gynaecol Obstet. 2019;147:389–396. doi: 10.1002/ijgo.12976. [DOI] [PubMed] [Google Scholar]
- 34.Gibbs RS. Clinical risk factors for puerperal infection. Obstet Gynecol. 1980;55 doi: 10.1097/00006250-198003001-00045. 178S–84. [DOI] [PubMed] [Google Scholar]
- 35.Declercq E, Barger M, Cabral HJ, et al. Maternal outcomes associated with planned primary cesarean births compared with planned vaginal births. Obstet Gynecol. 2007;109:669–677. doi: 10.1097/01.AOG.0000255668.20639.40. [DOI] [PubMed] [Google Scholar]
- 36.Filippi V, Ronsmans C, Gohou V, et al. Maternity wards or emergency obstetric rooms? Incidence of near-miss events in African hospitals. Acta Obstet Gynecol Scand. 2005;84:11–16. doi: 10.1111/j.0001-6349.2005.00636.x. [DOI] [PubMed] [Google Scholar]
- 37.Wagaarachchi PT, Graham WJ, Penney GC, McCaw-Binns A, Yeboah Antwi K, Hall MH. Holding up a mirror: changing obstetric practice through criterion-based clinical audit in developing countries. Int J Gynaecol Obstet. 2001;74:119–130. doi: 10.1016/s0020-7292(01)00427-1. [DOI] [PubMed] [Google Scholar]
- 38.Hunyinbo KI, Fawole AO, Sotiloye OS, Otolorin EO. Evaluation of criteria-based clinical audit in improving quality of obstetric care in a developing country hospital. Afr J Reprod Health. 2008;12:59–70. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.