Abstract
Background
Intrauterine growth restriction (IUGR) is a major inducer of higher morbidity and mortality in the pig industry and catch-up growth (CUG) before weanling could significantly restore this negative influence. But there was limited knowledge about the underlying mechanism of CUG occurrence.
Methods
Eighty litters of newborn piglets were divided into normal birth weight (NBW) and IUGR groups according to birth weight. At 26 d, those piglets with IUGR but over average body weight of eighty litters of weaned piglets were considered as CUG, and the piglets with IUGR still below average body weight were considered as NCUG. This study was conducted to systemically compare the intestinal difference among NBW, CUG and NCUG weaned piglets considering the crucial role of the intestine for piglet growth.
Results
The results indicated that the mRNA expression of nutrients (amino acids, glucose, and fatty acids) transporters, and mitochondrial electron transport chain (ETC) I were upregulated in CUG piglets’ gut with improved morphology compared with those NCUG, as well as the ratio of P-AMPK/AMPK protein expression which is the indicator of energy metabolism. Meanwhile, CUG piglet’s gut showed higher antioxidative capacity with increased SOD and GSH-Px activity, decreased MDA levels, as well as higher mRNA expressions of Nrf2, Keap1, SOD, and GSH-Px. Furthermore, inflammatory parameters including TNF-α, IL-1β, IL-6, and IL-12 factors, and the activation of MAPK and NF-κB signaling pathways were significantly elevated in the NCUG intestine, while the protein expression of ZO-1, Occludin and Claudin-1 was reduced. The alpha diversity of fecal microbiota was higher in CUG piglets in contrast with NCUG piglets, and the increased beneficial bacteria and decreased pathogenic bacteria was also observed in CUG piglets.
Conclusions
CUG piglet’s intestine showed comprehensive restoration including higher nutrients transport, energy metabolism, antioxidant capacity, and intestinal physical barrier, while lower oxidative stress, inflammatory response, and pathogenic microbiota.
Keywords: Antioxidative capacity, Catch-up growth, Gut, Intrauterine growth retardation, Piglets
Background
Intrauterine growth restriction (IUGR) is defined as the limited growth and development of mammalian fetus and organs during pregnancy and born with smaller body weight [1]. It has been well established that IUGR fetus are usually accompanied by comprehensive negative postnatal outcomes and metabolic disorders in various species [1–3]. As multiparous animals, pigs are most prone to IUGR and its occurrence has been reported up to 15%–20%, which might continue to raise due to the bigger litter size attributed to the improvement of breeding science nowadays [1]. The weaning morbidity of piglets with IUGR was about 11%, which was significantly higher than that of piglets without IUGR, causing huge economic loss to animal husbandry [1, 4]. Catch-up growth (CUG) refers to the body’s rapid growth after a period of growth inhibition, which has been observed in a portion of IUGR newborns in their early postnatal life [5, 6]. Previous studies have shown that CUG infants are 65% less likely to be hospitalized in the first 20 months of birth and 75% less likely to die at age 6 than those infants still with smaller body weight at that time, which was a considerable benefit for human health [7]. Limited results in pigs also showed that early postnatal CUG has similar morbidity and growth potential as normal birth weight (NBW) piglets at birth during growth and fattening period [8], suggesting that the importance of CUG in pigs should be addressed especially before weanling to reduce economic loss caused by IUGR in swine production.
Gut plays a crucial role in nutrient digestion and absorption directly contributing to piglet growth, and its abnormal development is closely associated with high diarrhea and mortality rates in weaned piglets, as well as impaired overall health and productive performance [9]. It has been reported that newborn IUGR pigs have significantly lower intestinal weight compared with that of NBW piglets [10], and this stunned intestinal development subsequently continued during the whole later life in the absence of CUG [11–13]. Besides, IUGR has shown negative effects on the expression of proteins involved in the absorption, digestion, and transportation of nutrients and is continuously impaired during lactation [14, 15]. To date, a large number of studies explored the mechanism of impaired intestinal development in IUGR pigs from abroad aspects, including immune and inflammatory system, cellular apoptosis signal transduction, protein synthesis, as well as microbial diversity [1, 15, 16], which provide great help to understand the difference between NBW and IUGR piglets and better take care of IUGR piglets to reduce their mortality and morbidity. However, to our knowledge, there were very limited investigations focusing on the underlying mechanisms about CUG of piglets, which is of importance and significance for pig industry to explore new strategies to improve productive performance by increasing the occurrence rate of this beneficial phenomenon.
Hence, we used different growth patterns IUGR (CUG and NCUG before weaning) and NBW piglets as animal models in the present study to systematically compare the intestinal difference in morphology, barrier function, antioxidant status, inflammation levels, nutrient transport, energy metabolism, and fecal microbiome. Moreover, considering that most previous studies mainly focused on duodenum and jejunum function in IUGR piglets [2, 17, 18], but paid little attention in the ileum. The ileum has recently been reported closely related to viral intestinal diseases and has attracted more and more attention. Therefore, three segments of intestine including duodenum, jejunum and ileum were all collected in current study for analysis due to their different physiological function. This study may also shed light on the detailed understanding of gut development and adaption for CUG infants.
Materials and methods
Animals and experimental design
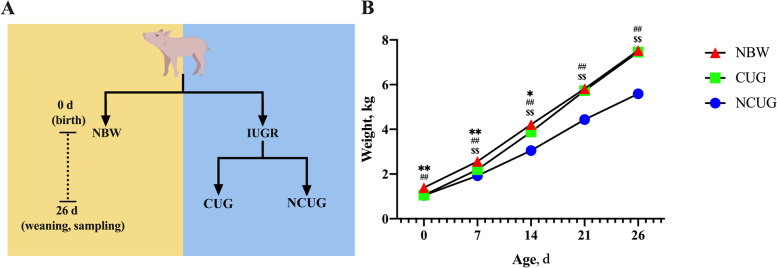
All animal procedures were carried out in accordance with the Guidelines for Care and Use of Laboratory Animals of South China Agricultural University and approved by the Animal Ethics Committee of South China Agricultural University (No. 20110107–1, Guangzhou, China). During gestation, 80 healthy pregnant sows (Landrace × Yorkshire) were preselected according to similar due dates and parity (2–3). The newborn piglets were weighed immediately after delivery. IUGR piglets were defined as the average birth weight was 2 standard deviations below the average birth weight of the total population, and NBW piglets are defined as the birth within 0.5 standard deviations of the average birth weight of the total population. According to the birth weight, 55 IUGR piglets and 28 NBW piglets weighing more than 1.2 kg were selected to participate in this study. 55 IUGR piglets were separated from NBW piglets and fostered to 6 sows with similar body conditions during lactation. All the piglets were weighed and measured weekly to track their growth pattern. On the weaning day (26 d), 14 IUGR piglets with weaning weight exceeding the average body weight of 80 litters of weaned piglets were divided into the CUG group, and 33 IUGR piglets with weaning weight not exceeding the average body weight of 80 litters of weaned piglets were divided into the NCUG group (Fig. 1A).
Fig. 1.
Experiment design and the establishment of CUG model. A Schematic representation of the experimental design. B Body weight from birth to postnatal 26 d of NBW, CUG and NCUG piglets. NBW, normal birth weight. IUGR, intrauterine growth retardation. CUG, catch-up growth. NCUG, not catch-up growth. P < 0.05, *NBW vs. CUG-IUGR. P < 0.01, **NBW vs. CUG, ##NBW vs. NCUG, $$CUG vs. NCUG
Sample collection
At age of 7 days, all IUGR piglets were included in this study to collect feces by separating them one by one into a clean crate, then administered rectal stimulation with a sterile swab, and collected feces at least 5 g directly into a sterile centrifuge tube. After weaning, 3 weaned piglets were randomly selected from CUG and NCUG piglets to perform 16S rRNA sequencing analysis for their feces samples collected at the age of 7 days. At age of 26 days, 6 weaned piglets were randomly selected from each group of NBW, CUG, and NCUG groups for the slaughter experiment. After intravenous injection of 100 mg/kg lethal dose of sodium pentobarbital, the abdominal cavity was opened. The duodenum was considered as 10 cm away from the gastric pylorus, the jejunum was considered as 40 cm away from the cecum, and the ileum was considered as 10 cm away from the cecum [19]. About 1 cm long samples were collected at the middle part of the duodenum, jejunum, and ileum and then fast stored in 4% paraformaldehyde fix solution for subsequent morphological observation. The active component of 4% paraformaldehyde, using 0.1 mol/L phosphoric acid buffer as solvent, pH 7.0–7.5 at 25 °C. At the same time, each intestinal segment about 2 cm was opened longitudinally, and the intestinal contents were rinsed three times using cold normal saline, then quickly frozen in liquid nitrogen for mRNA and protein expression analysis. The time from euthanasia to complete sampling was controlled at about 30 min for each piglet.
Intestinal morphology
Duodenum, jejunum, and ileum samples were stored in 4% paraformaldehyde fix solution for morphological analysis. Standard paraffin embedding procedures and standard hematoxylin and eosin staining protocols were used. In simple terms, intestinal tissues were dehydrated in a series of ethanol diluents, washed with xylene, and then embedded in paraffin wax. Paraffin samples were cut into 5 μm sections and stained with hematoxylin and eosin. Two discontinuous sections were selected from each tissue, and 5 representative villi and their associated crypt were selected from each section. Villi height (VH) and crypt depth (CD) were viewed on the light microscope and measured using an image processing and analysis system (NIS-Elements Viewer, Tokyo, Japan).
Real time quantitative PCR
The total RNA was isolated from duodenum, jejunum and ileum using the Tissue RNA Purification kit PLUS (EZB-RN001-plus, EZBioscience, Roseville, MN, USA) followed the manufacturer’s instruction. The extracted RNA quality and concentration were evaluated with 1.0% agarose gel electrophoresis (130 V, 18 min), and the absorption ratio of RNA (A260/A280) for all RNA samples was greater than 1.8 and less than 2.3 by using a spectrophotometer. The cDNA synthesis was performed by the RNA reverse transcription reaction with the Color Reverse Transcription Kit (A0010CGQ, EZBioscience, Roseville, MN, USA), according to the manufacturer’s protocol. Real time PCR was conducted using ABI Prism 7500 sequence detection system (Applied Biosystems, Carlsbad, CA, USA) with a reaction volume of 20 μL. The PCR reaction scheme includes initial denaturation one cycle at 95 °C for 2 min, amplification forty cycles at 95 °C for 15 s, 60 °C for 30 s. The relative target gene expression levels were determined based on the quantification approach (2−ΔΔCt method), with β-actin acting as the housekeeping gene to normalize all mRNA levels. The total primers used are presented in Table 1 [20–22].
Table 1.
Primer sequences used in Real-time PCR
| Genes | Accession | Forward primer(5’ → 3’) | Reverse primer (5’ → 3’) |
|---|---|---|---|
| LAT1 | NM_003486 | GCCCATTGTCACCATCATC | GAGCCCACAAAGAAAAGC |
| CAT1 | NW_003611328.1 | GCCTGAGAGCAAGACCAAAC | GCCGTAGCCGAAGTAGATGA |
| EAAC1 | NM_001164649.1 | GTTCCTGATTGCCGGGAAGA | ATGGCGAATCGGAAAGGGTT |
| PepT1 | AY180903.1 | AGCATCTTCTTCATCGTG GTCAA | GTCTTGAACTTCCCCAGCCA |
| SGLT1 | NM_001164021 | CATCATCGTCCTGGTCGTC | CATCATCGTCCTGGTCGTC |
| GLUT2 | EF140874 | GTCCAGAAAGCCCAAGAT ACC | GTGACATCATCACTTCCTCTGAG |
| CD36 | NM_001083931.1 | GGCAACAGACGTGATCTATGAC | AGCGGCTGGCTGAAAACT |
| FATP4 | XM_003353676.1 | AGCCGCATCCTGTCCTTT | GACATCCTTGGCGATCTTTT |
| NDUF A1 | XM_003135339.4 | GCTTCCGGGGAAGGAATCAA | CCGGGGAGAATTTCGAACCA |
| NDUF A6 | NM_001185178.1 | TCTCAGAGCCTTGCATGTCG | AAGCCATCCAGCATCGTACC |
| NDUF A13 | NM_001244646.1 | ATGAAGGATGTGCCGGACTG | CCATAGGTGGCGCTGAGAAT |
| NDUF B1 | XM_003482306.3 | TGCCTTCCGGAACAAGAGTC | GCAATTCAGCCACAGCCTTT |
| Nrf2 | NM_001114671.1 | GACAAACCGCCTCAACTCAG | GTCTCCACGTCGTAGCGTTC |
| Keap1 | XM_021076667.1 | CGTGGAGACAGAAACGTGGA | CAATCTGCTTCCGACAGGGT |
| SOD | NM_001190422.1 | AAGGCCGTGTGTGTGCTGAA | GATCACCTTCAGCCAGTCCTTT |
| GSH-Px | NM_214201.1 | CCTCAAGTACGTCCGACCAG | GTGAGCATTTGCGCCATTCA |
| TNF-α | NM_214022.1 | CCACCAACGTTTTCCTCACT | TAGTCGGGCAGGTTGATCTC |
| IL-1β | NM_214055.1 | CCAAAGAGGGACATGGAGAA | TTATATCTTGGCGGCCTTTG |
| IL-6 | NM_001252429.1 | TGGCTACTGCCTTCCCTACC | AGAGCCTGCATCAGCTCAGT |
| IL-12 | NC_010458.4 | CAACCCTGTGCCTTAGCAGT | AGAGCCTGCATCAGCTCAGT |
| MAPK3 | XM_021088019.1 | CAGTCTCTGCCCTCCAAGAC | GGGTAGATCATCCAGCTCCA |
| MAPK8 | XM_001929166.6 | TGGATGAAAGGGAACACACA | ATGATGACGATGGATGCTGA |
| MAPK14 | XM_021091323.1 | CCCTGAGGTTCTAGCGAAGA | TCTCATCGTAGGGCTCTGCT |
| NFKB1 | NM_001048232.1 | CTCGCACAAGGAGACATGAA | ACTCAGCCGGAAGGCATTAT |
| β-actin | XM_021086047.1 | TGCGGGACATCAAGGAGAAG | AGTTGAAGGTGGTCTCGTG |
Western blot
Total protein was extracted from the duodenal, jejunal and ileal tissues and homogenized by adding a mixture of RIPA lysis buffer (P0013B, Beyotime, Shanghai, China) containing protease inhibitor PMSF (ST506, Beyotime, Shanghai, China). The protein concentration in supernatant was measured using a BCA Protein Assay Kit (P0010, Beyotime, Shanghai, China) after the centrifuge. Thereafter, the equal quantities of protein (25 μg) were separated by 10% SDS-PAGE gels and electrically transferred onto polyvinylidene difluoride (PVDF) membranes. After that, the membranes were blocked with 5% skimmed milk for 2 h at room temperature and then incubated with the primary antibody against Claudin-1 (1:1000, ab129119, Abcam, Cambridge, UK), ZO-1(1:1000, 21773–1-AP, Proteintech, Wuhan, China), Occludin (1:1000, 27260–1-AP, Proteintech, Wuhan, China), P-JNK (1:1000, 4688S, Cell Signaling Technology, Boston, MA, USA), JNK (1:1000, 9252S, Cell Signaling Technology, Boston, MA, USA), P-NF-κB (1:1000, 3033S, Cell Signaling Technology, Boston, MA, USA), NF-κB (1:1000, 10745–1-AP, Proteintech, Wuhan, China), P-AMPK(1:1000, 2535S, Cell Signaling Technology, Boston, MA, USA), AMPK (1:1000, 2432S, Cell Signaling Technology, Boston, MA, USA) and β-actin (1:2000, bs-0061R, Bioss, Beijing, China) overnight at 4 °C. Then, the membranes were washed by Tris buffered saline Tween and incubated with a corresponding secondary antibody (1:50,000, 511203, ZenBio, Chengdu, China) for 1.5 h at room temperature, followed by visualizing the target bands using an enhanced chemiluminescence kit (P1020, Applygen, Beijing, China) using the ImageQuant LAS 4000 mini system.
Antioxidant status
The duodenal, jejunal and ileal tissues were homogenized with saline solution (1:4, weight:volume) and centrifuged at 3000 × g for 15 min. According to the kit instructions, the supernatants were diluted to the optimal concentration for detecting the activities of glutathione peroxidase (GSH-PX), reduced glutathione (GSH), superoxide dismutase (SOD), total antioxidant capacity (T-AOC) and malonaldehyde (MDA) by commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
16S rRNA Sequencing
Total genomic DNA was extracted from samples by CTAB method [23]. The concentration and purity of DNA were monitored on 1% agarose gels. V3-V4 variable regions of 16S rRNA genes were amplified with primers 341F (5’-CCTAYGGGRBGCASCAG-3’) and 806R (5’- GGACTACNNGGGTATCTAAT-3’). 15 μL Phusion® High-Fidelity PCR Master Mix (New England Biolabs, Beijing, China) was used for PCR reaction. The primers of 341F and 806R mentioned above were 2 μmol/L, and the template DNA was about 10 ng. The thermal cycles include initial denaturation at 98 °C for 1 min, denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, and extension at 72 °C for 30 s. Finally, 72 °C for 5 min. An equal amount of 1× loading buffer was mixed with PCR products and electrophoresis was performed on 2% agarose gel. PCR products were mixed at an isodensity ratio. The mixed PCR products were purified using the Qiagen Gel Extraction Kit (Qiagen, Dusseldorf, Germany). Sequencing libraries were generated by TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, San Diego, California, USA) according to manufacturer's recommendations and index codes were added. The library quality was evaluated on the Qubit@ 2.0 Fluorometer (Thermo Scientific, Waltham, MA, USA) and Agilent Bioanalyzer 2100 system. Finally, the library was sequenced on an Illumina Nova Seq platform to obtain 250 bp paired-end reads. Paired-end reads were performed on samples based on their unique barcodes and truncated by truncating barcodes and primer sequences. Paired-end reads were spliced using the FLASH software to obtain Raw Tags. Date filtration and noise reduction were performed on DADA2 module of QIME software (Version 1.9.1), then the ASVs and their feature table are obtained. The obtained ASVs were annotated for species, and finally the species information of each ASV was obtained.
Statistical analysis
All data in the experiment except microbial part were analyzed by one-way ANOVA using SPSS 22.0 (IBM Inc., Armonk, New York, USA) to determine whether significant differences among the groups. The data were expressed by means ± SEM. Correlations were evaluated by Pearson correlation analysis of the Euclidean distance using GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA). P < 0.05 was considered a significant difference and highly significant when P < 0.01.
Alpha diversity, including Chao1, Shannon, and Simpson was used to analyze the complexity of species diversity. All alpha diversity indices in our samples were calculated with QIIME (Version 1.7.0) and displayed with R software (Version 2.15.3). Beta diversity analysis was used to assess differences of samples in species complexity, and beta diversity on weighted_unifrac was performed using QIIME software (Version 1.9.1). Principal coordinate analysis (PCoA) was performed to get principal coordinates and visualize from complex, multidimensional data. T test statistical algorithm was used to analyze differences. Linear discriminant analysis (LDA) was used to identify the bacterial groups in each group by LEfSe.
Results
Establishment of piglet models with different growth patterns
The birth weight of IUGR piglets including CUG and NCUG piglets was significantly lower than that NBW piglets (P < 0.01), and there was no difference between CUG piglets and NBW piglets at the weanling day (26 d), indicating that CUG and NCUG models were successfully established (Fig. 1B).
Intestinal morphology
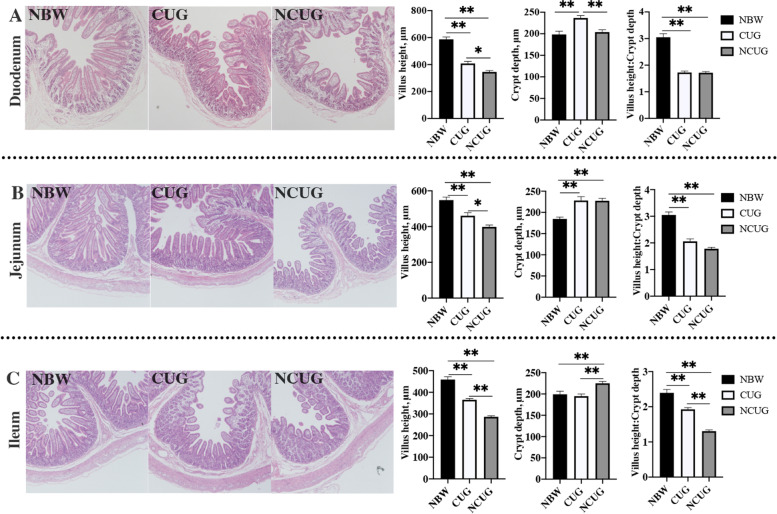
Obvious decreases in VH and VH/CD ratio in duodenum, jejunum, and ileum of CUG and NCUG piglets were observed compared with the NBW (Fig. 2; P < 0.05). In contrast with NBW piglets, the CD was significantly increased in duodenum of CUG piglets, jejunum of CUG and NCUG piglets, and ileum of NCUG piglets (P < 0.05). Simultaneously, higher VH in the duodenum, jejunum and ileum was observed, as well as lower CD in ileum, but higher CD in duodenum and VH/CD ratio in ileum of CUG piglets compared with NCUG counterparts (P < 0.05). The VH/CD ratio in the duodenum and jejunum were similar between the CUG and NCUG groups (P > 0.05).
Fig. 2.
Intestinal morphology of NBW, CUG and NCUG piglets on 26 d. A Duodenal morphology, villus height, crypt depth, ratio of villus height to crypt depth. B Jejunal morphology, villus height, crypt depth, ratio of villus height to crypt depth. C Ileal morphology, villus height, crypt depth, ratio of villus height to crypt depth. Dates are presented as means ± SEM (n = 6). NBW, normal birth weight. CUG, catch-up growth, NCUG, not catch-up growth. *P < 0.05; **P < 0.01
Intestinal nutrients transport mRNA expression
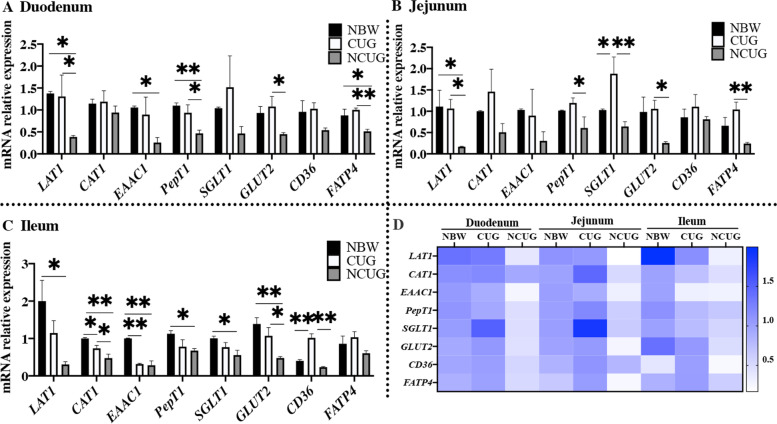
Gut plays a crucial role in nutrient transport, which processes are mainly regulated by specific amino acids transporters, glucose transporters and fatty acids transporters located in intestine. At weaning (26 d), compared to NBW group, piglets with NCUG had a significantly lower expression of duodenal LAT1, EAAC1, PepT1 and FATP4, jejunal LAT1, ileal LAT1, CAT1, EAAC1, PepT1, SGLT1 and GLUT2 (Fig. 3A, B, C; P < 0.05). CUG piglets had significantly higher expression of SGLT1 in the jejunum, significantly lower expression of CAT1, EAAC1 in the ileum, and significantly higher expression of CD36 in the ileum compared with NBW piglets (P < 0.05). Specifically, CUG group showed higher mRNA expression levels of duodenal and jejunal LAT1, PepT1, GLUT2 and FATP, ileal CAT1, GLUT2 and CD36 in contrast with the NCUG group (P < 0.05).
Fig. 3.
Intestinal nutrition transporters mRNA expression of NBW, CUG and NCUG piglets on 26 d. A The mRNA expression levels of LAT1, CAT1, EAAC1, PepT1, SGLT1, GLUT2, CD36 and FATP4 in the duodenum. B The mRNA expression levels of LAT1, CAT1, EAAC1, PepT1, SGLT1, GLUT2, CD36 and FATP4 in the jejunum. C The mRNA expression levels of LAT1, CAT1, EAAC1, PepT1, SGLT1, GLUT2, CD36 and FATP4 in the ileum. D Heat map comparison of mRNA expression levels of nutrition transporters in NBW, CUG and NCUG. Dates are presented as means ± SEM (n = 4). NBW, normal birth weight. CUG, catch-up growth. NCUG, not catch-up growth. *P < 0.05; **P < 0.01
The mRNA and proteins associated with energy metabolism
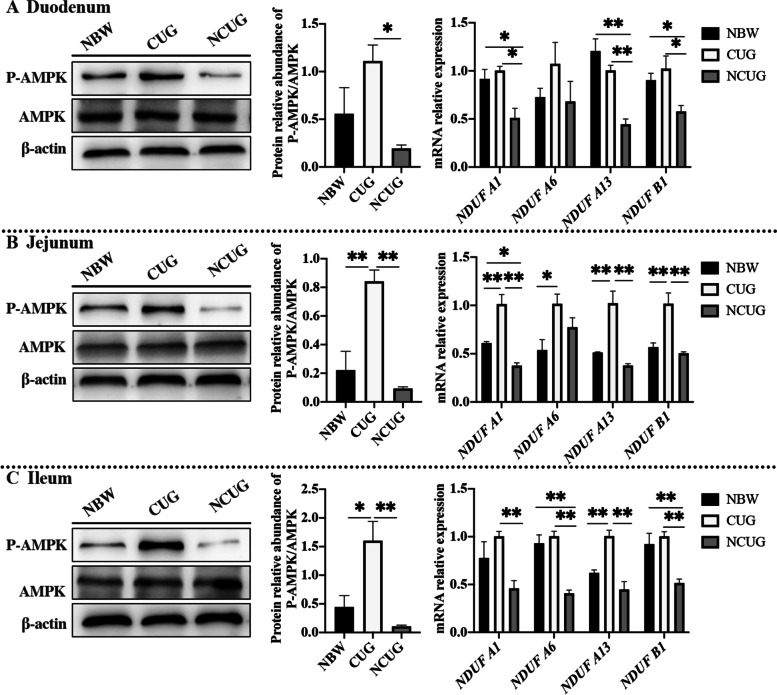
To detect the function of mitochondria and AMPK in energy metabolism, the mRNA expression of mitochondrial electron transport chain (ETC) I and the protein expression of P-AMPK/AMPK were detected. The data indicated lower mRNA expression levels of NDUF A1, NDUF A13 and NDUF B1 in the duodenum, NDUF A1 in the jejunum, NDUF A6, and NDUF B1 in the ileum of the NCUG group in comparison with the NBW group (Fig. 4; P < 0.05). Meantime, higher mRNA expression levels of NDUF A1, NDUF A6, NDUF A13, NDUF B1 in the jejunum, NDUF A13 in the ileum of CUG piglets than the NBW piglets were also observed (P < 0.05). The mRNA expression levels of duodenal and jejunal NDUF A1, NDUF A13, NDUF B1, ileal NDUF A1, NDUF A6, NDUF A13, NDUF B1 were higher in CUG compared with the NCUG group (P < 0.05). Additionally, compared to NBW group, CUG group has higher protein expression of P-AMPK/AMPK in the jejunum and ileum (P < 0.05). The protein expression of P-AMPK/AMPK was upregulated in the duodenum, jejunum, and ileum of the CUG piglets compared to the NCUG piglets (P < 0.05).
Fig. 4.
The intestinal mRNA and proteins expression associated with energy metabolism of NBW, CUG and NCUG piglets on 26 d. A Duodenal protein expression of P-AMPK/AMPK and mRNA expression of mitochondrial electron transport chain I, including NDUF A1, NDUF A6, NDUF A13 and NDUF B1. B Jejunal protein expression of P-AMPK/AMPK and mRNA expression of mitochondrial electron transport chain I, including NDUF A1, NDUF A6, NDUF A13 and NDUF B1. C Ileal protein expression of P-AMPK/AMPK and mRNA expression of mitochondrial electron transport chain I, including NDUF A1, NDUF A6, NDUF A13 and NDUF B1. Dates are presented as means ± SEM (n = 3 for protein expression; n = 4 for mRNA expression). NBW, normal birth weight. CUG, catch-up growth. NCUG, not catch-up growth. *P < 0.05; **P < 0.01
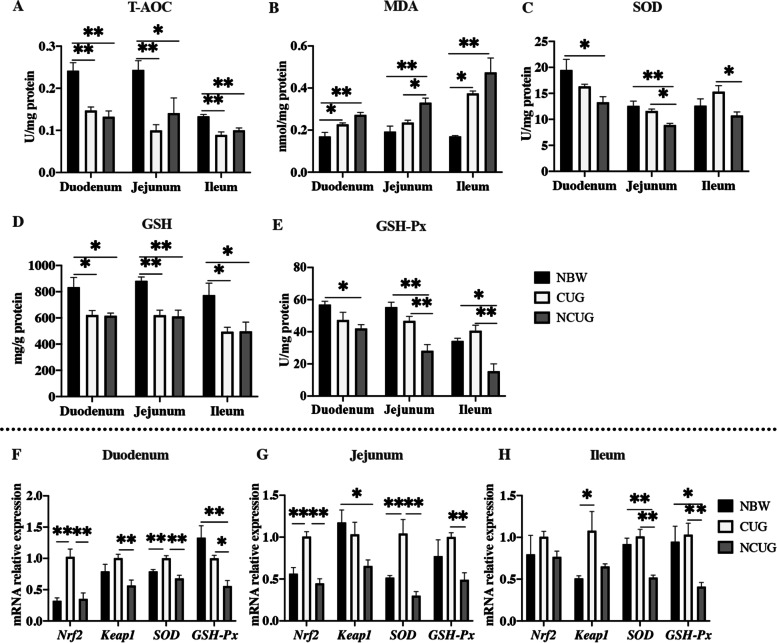
Intestinal redox status
The results obtained on the status of redox in the intestinal mucosa are presented in Fig. 5. Compared to the NBW group, T-AOC and GSH activity were decreased in the duodenum, jejunum and ileum of both CUG and NCUG piglets. Meantime, in contrast with NBW piglets, MDA concentration was increased in the duodenum and ileum of CUG piglets, MDA concentration was also increased in the duodenum, jejunum and ileum of NCUG piglets, SOD activity was decreased in the duodenum and jejunum of NCUG piglets, GSH-Px activity was decreased in the duodenum, jejunum and ileum of NCUG piglets (P < 0.05). SOD and GSH-Px in CUG group had no difference with NBW group (P > 0.05). Nevertheless, compared to the NCUG group, the SOD activity and GSH-Px content were higher in the jejunum and ileum of CUG group (P < 0.05). MDA of jejunum in CUG group was lower in comparison with the NCUG group (P < 0.05).
Fig. 5.
Intestinal antioxidant status and the mRNA expression of Nrf2 pathway in NBW, CUG and NCUG piglets on 26 d. A–E The activities of T-AOC, MDA, SOD, GSH and GSH-Px of duodenum, jejunum and ileum.F The mRNA expression levels of Nrf2, Keap1, SOD, GSH-Px in the duodenum. G The mRNA expression levels of Nrf2, Keap1, SOD, GSH-Px in the jejunum. H The mRNA expression levels of Nrf2, Keap1, SOD, GSH-Px in the ileum. Dates are presented as means ± SEM (n = 4). NBW, normal birth weight. CUG, catch-up growth. NCUG, not catch-up growth. *P < 0.05; **P < 0.01
To further reveal the molecular mechanism of CUG in regulating antioxidant capacity in the intestinal mucosa, mRNA expressions of Nrf2 pathway (Nrf2, Keap1, SOD and GSH-Px) were determined, as shown in Fig. 5F, G, H. Compared to the NBW group, NCUG showed lower mRNA expression of duodenal GSH-Px, jejunal Keap1, ileal SOD and GSH-Px, CUG showed higher mRNA expression of duodenal and jejunal Nrf2 and SOD, ileal Keap1 (P < 0.05). We observed a higher mRNA expression of duodenal Nrf2, Keap1, SOD, GSH-Px, jejunal Nrf2, SOD, GSH-Px, ileal SOD and GSH-Px in CUG compared with the NCUG (P < 0.05).
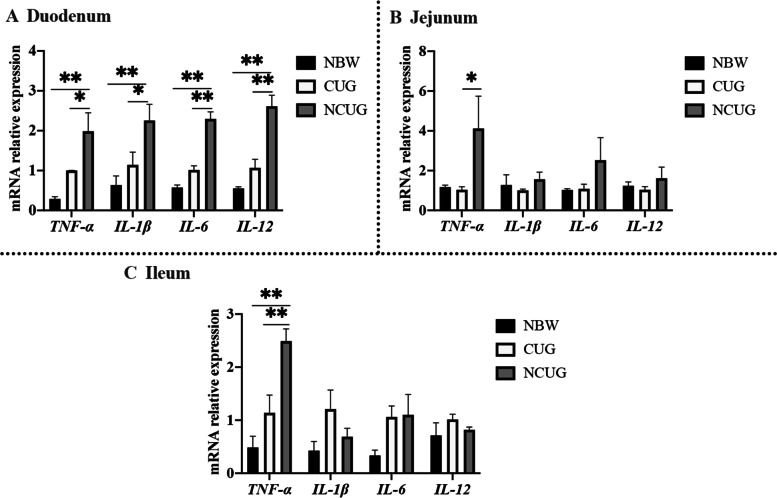
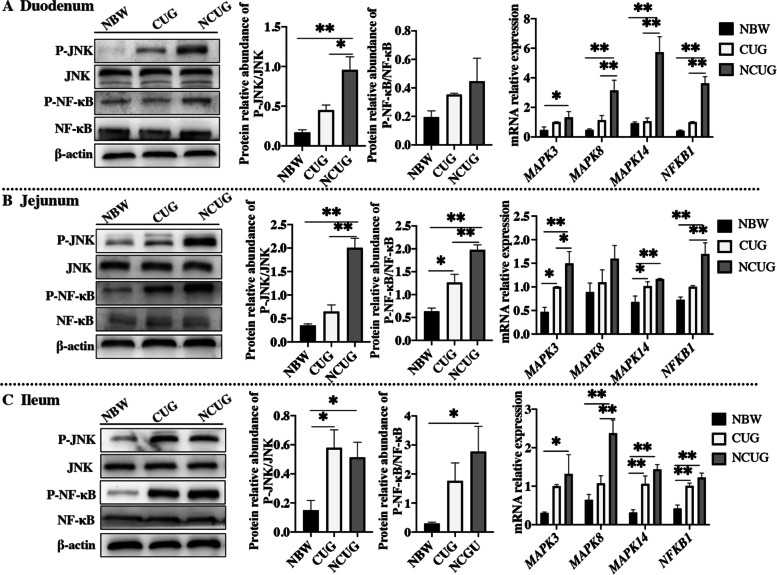
Intestinal inflammation status
As shown in Fig. 6, NCUG groups exhibited significantly increased the mRNA expression of TNF-α, IL-1β, IL-6 and IL-12 in duodenum, TNF-α in ileum than the NBW group (P < 0.05). There were no differences in TNF-α, IL-1β, IL-6 and IL-12 in duodenum, jejunum and ileum between CUG and NBW piglets (P > 0.05). Compared to NCUG piglets, CUG indicated lower mRNA expression of duodenal TNF-α, IL-1β, IL-6 and IL-12, jejunal and ileal TNF-α (P < 0.05). In addition, western blot analysis revealed that the NCUG piglets showed higher ratio of duodenal P-JNK/JNK, jejunal and ileal P-JNK/JNK and P-NF-κB/NF-κB compared with NBW piglets (Fig. 7; P < 0.05). Also, CUG piglets showed higher ratio of jejunal P-NF-κB/NF-κB and ileal P-JNK/JNK compared with NBW piglets (P < 0.05). Meanwhile, the CUG piglets showed lower protein levels of duodenal P-JNK/JNK, jejunal P-JNK/JNK and P-NF-κB/NF-κB compared with the NCUG piglets (P < 0.05). Similarly, in contrast to NBW piglets, the mRNA expression of duodenal and ileal MAPK3, MAPK8, MAPK14 and NFKB1, jejunal MAPK3 and NFKB1 were upregulated in NCUG, while the mRNA expression of jejunal MAPK3 and MAPK14, ileal MAPK14 and NFKB1 were upregulated in CUG (Fig. 7; P < 0.05). Moreover, CUG showed markedly downregulated mRNA levels of MAPK8, MAPK14, and NFKB1 in the duodenum, MAPK3 and NFKB1 in the jejunum, and MAPK8 in the ileum than that in the NCUG group (P < 0.05).
Fig. 6.
The mRNA expression of intestinal inflammation status of NBW, CUG and NCUG piglets on 26 d. A The mRNA expression levels of TNF-α, IL-1β, IL-6 and IL-12 in the duodenum. B The mRNA expression levels of TNF-α, IL-1β, IL-6 and IL-12 in the jejunum. C The mRNA expression levels of TNF-α, IL-1β, IL-6 and IL-12 in the ileum. Dates are presented as means ± SEM (n = 4). NBW, normal birth weight. CUG, catch-up growth. NCUG, not catch-up growth. *P < 0.05; **P < 0.01
Fig. 7.
The intestinal protein and mRNA expression of NF-κB and MAPK signaling pathways of NBW, CUG and NCUG piglets on 26 d. A Protein expression of P-JNK/JNK and P-NF-κB/NF-κB, mRNA expression of MAPK3, MAPK8, MAPK14 and NFKB1 in the duodenum. B Protein expression of P-JNK/JNK and P-NF-κB/NF-κB, mRNA expression of MAPK3, MAPK8, MAPK14 and NFKB1 in the jejunum. C Protein expression of P-JNK/JNK and P-NF-κB/NF-κB, mRNA expression of MAPK3, MAPK8, MAPK14 and NFKB1 in the ileum. Dates are presented as means ± SEM (n = 3 for protein expression; n = 4 for mRNA expression). NBW, normal birth weight. CUG, catch-up growth. NCUG, not catch-up growth. *P < 0.05; **P < 0.01
Intestinal tight junction proteins levels
Western blot analysis showed that the protein levels of duodenal, jejunal and ileal Occludin, Claudin-1, and ZO-1 were decreased in NCUG piglets compared with the NBW piglets (Fig. 8; P < 0.05). CUG piglets decreased the protein levels of duodenal Occludin, Claudin-1, ZO-1, ileal Occludin compared with the NBW piglets (P < 0.05). Further, in contrast to NCUG piglets, no significant CUG action for Occludin, Claudin-1 was observed in the duodenum and jejunum (P > 0.05), but evident higher protein expression levels of ZO-1 were observed in the duodenum and ileum of CUG piglets (P < 0.05).
Fig. 8.
Intestinal tight junction proteins levels of NBW, CUG and NCUG piglets on 26 d. A The protein expression of Occludin, Claudin-1 and ZO-1 in the duodenum. (B) The protein expression of Occludin, Claudin-1 and ZO-1 in the jejunum. (C) The protein expression of Occludin, Claudin-1 and ZO-1 in the ileum. Dates are presented as means ± SEM (n = 3). NBW, normal birth weight. CUG, catch-up growth. NCUG, not catch-up growth. *P < 0.05; **P < 0.01
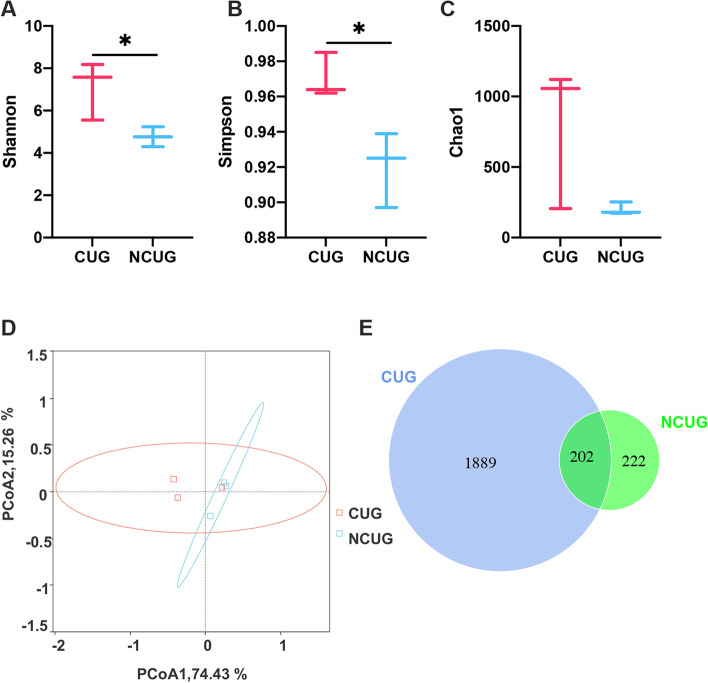
Microbiota populations
16S rRNA gene sequencing technology was used to compare the feces bacterial community composition of different pattern CUG piglets and NCUG piglets. After sequencing, a total of 2091 OTUs were identified from CUG and 424 OTUs were identified from NCUG, 202 shared OTUs out of the total OTUs overlapped between the two groups (Fig. 9E). In this study, the alpha diversity of the feces microbiota expressed by Shannon, Simpson and Chao1 (Fig. 9A, B, C). Compared with the NCUG group, Shannon and Simpson were significantly higher in the CUG group (P < 0.05), while there were no significant differences in Chao1 (P > 0.05). For beta diversity, the PCoA analyses based on weighted_unifrac distance showed that the microbiota there was no obvious tendency to separate CUG from NCUG (Fig. 9D).
Fig. 9.
The shifts in feces alpha and beta diversity of CUG and NCUG piglets on 7 d. A Shannon index of microbiota. B Simpson index of microbiota. C Chao1 index of microbiota. D Principal component analysis (PCoA) scores plot. E Venn diagram for the OTUs. CUG, catch-up growth; NCUG, not catch-up growth. *P < 0.05
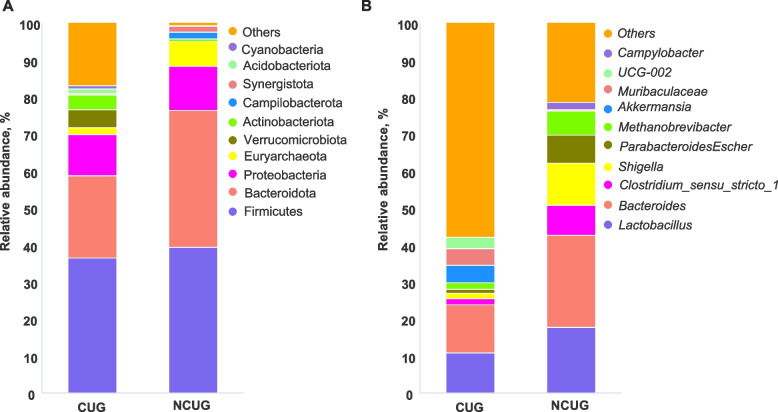
In order to further determine the changes in fecal microbiota composition, the dominant phylum and genus of each group were analyzed. At the phylum level (Fig. 10A), Firmicutes, Bacteroidetes, Proteobacteria and Euryarchaeota predominantly mainly constituted the fecal microbiota of piglets. Compared with the NCUG group, the relative abundance of Firmicutes in the CUG group decreased from 39.30% to 36.4%, and the relative abundance of Bacteroidetes, Proteobacteria and Euryarchaeota decreased from 36.9%, 11.9%, 6.57% to 22.3%, 11.1% and 1.81%, respectively. The ratio of Firmicutes/Bacteroidetes was increased from 1.06% to 1.64% in CUG compared with the NCUG group. At the genus level, a total of 337 bacterial genera were annotated, among which the top 10 were shown in Fig. 10B.
Fig. 10.
The relative abundance of CUG and NCUG feces microbiota community at the phylum and genus level on 7 d. A Relative abundance at the phylum level. B Relative abundance at the genus level. CUG, catch-up growth; NCUG, not catch-up growth
Compared with the NCUG group, the relative abundance of Lactobacillus, Bacteroides, Clostridium_sensu_stricto_1 and Escherichia_Shigella decreased from 17.65%, 24.87%, 8.11%, 11.49% to 10.91%, 12.96%, 1.65% and 1.41%, respectively.
The effect of microbial abundance of each species on the differential effect was evaluated by LDA (LDA threshold > 3.6). The results (Fig. 11A, B) indicated that the fecal microbiota composition was affected by CUG. A higher richness of UCG_002, gut_metagenome, Akkermansiaceae, Verrucomicrobiota, Verrucomicrobiales, Verrucomicrobiae, Ruminococcaceae, Akkermansia, Oscillospiraceae and Oscillospriales in CUG group as well as Escherichia_Shigella, Enterobacteriaceae, Tannerellaceae, Parabacteroides, Campylobacter and Campylobacteraceae in NCUG group were observed in the feces of piglets.
Fig. 11.
Linear discriminant analysis combined effect size (LEfSe) measurement analysis of microbiota in the feces contents of CUG and NCUG piglets on 7 d. A Linear discriminant analysis (LDA) score from phylum to genus level of the fecal microbiota, and the score ≥ 2 means significant. B Cladogram of LEfSe shows taxonomic profiling from the phylum to genus level, the yellow node represents no difference, but other color nodes represent significant difference
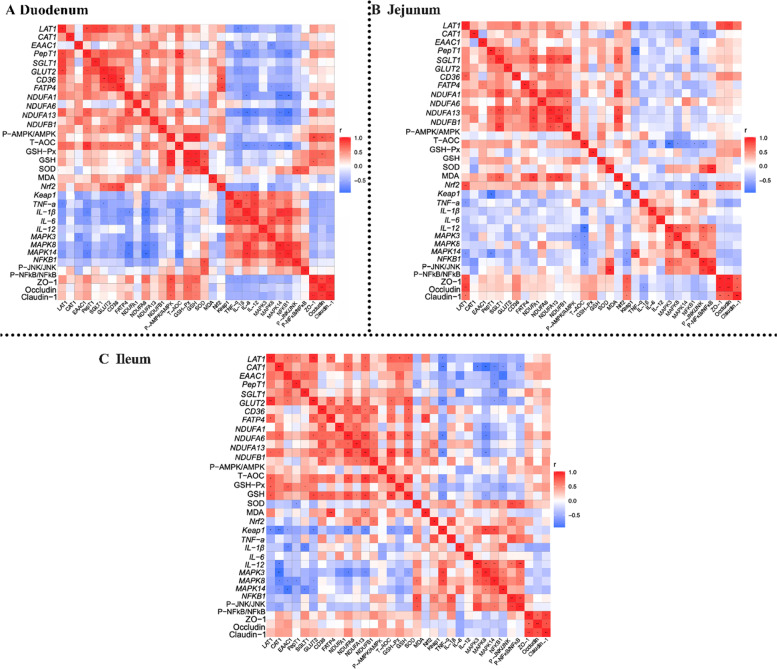
Correlation analysis
A series of correlation analyses between gut nutrient transport, energy metabolism, antioxidant status, inflammatory responses, and gut permeability in piglets was revealed (Fig. 12). In duodenum, nutrient transport was positively correlated with NDUF A1, NDUF A13, NDUF B1, P-AMPK/AMPK and GSH-Px, and negatively correlated with TNF-α, IL-1β, IL-6, IL-12, MAPK8, MAPK14 and NFKB1. Energy metabolism was positively correlated with GSH-Px, and negatively correlated with TNF-α, IL-1β, IL-6, IL-12, MAPK3, MAPK8, MAPK14, NFKB1. Antioxidant status was positively correlated with ZO-1, Occludin and Claudin, and negatively correlated with IL-1β, IL-6, IL-12, MAPK3, MAPK8, MAPK14, NFKB1. Inflammatory responses were negatively correlated with ZO-1. In jejunum, that nutrient transport was positively correlated with NDUF A1, NDUF A13, NDUF B1, P-AMPK/AMPK, Nrf2, ZO-1, Occludin and Claudin-1, and negatively correlated with TNF-α, IL-1β, NFKB1. Energy metabolism was positively correlated with Nrf2. Antioxidant status was negatively correlated with IL-6, MAPK3, MAPK8, MAPK14, NFKB1 and P-JNK/JNK. In ileum, that nutrient transport was positively correlated with NDUF A1, NDUF A6, NDUF A13, NDUF B1, P-AMPK/AMPK, GSH-Px, GSH, SOD, and negatively correlated with TNF-α, IL-6, MAPK3, MAPK8, MAPK14 and NFKB1. Energy metabolism was positively correlated with, GSH-Px, SOD, Nrf2, Keap1, and negatively correlated with TNF-α and MAPK8.
Fig. 12.
Heatmap of Pearson’s correlation coefficients between intestinal nutrition transport, energy metabolism, antioxidant status, immune status, inflammatory pathways and intestinal barrier function. A Heat map of duodenal correlation analysis. B Heat map of jejunal correlation analysis. C Heat map of ileal correlation analysis. In the panel, red with a P < 0.05 represents a significant positive correlation, blue with a P < 0.05 represents a significant negative correlation, and white represents no correlation. *P < 0.05; **P < 0.01
Discussion
It is well known that IUGR has a permanent stunting effect on the postnatal growth of piglets due to accompanied impaired development of momentous organs, which potentially further deteriorate pre-existing impaired growth [24]. The gut plays a vital role in postnatal development of piglets and its abnormal development usually causes feeding intolerance, nutrient absorption problems and necrotizing enterocolitis [25–27], which negatively influence nutrients obtainment and lead to a poorer growth rate of IUGR piglets. Previous studies have shown that both newborn and weaned piglets with IUGR were characterized by damaged intestinal structure, such as decreased VH, increased CD and decreased VH/CD ratio [10, 25, 28]. In our present study, it is not surprising to find the destroyed intestinal morphology in NCUG weanling piglets similar to the previous study, but it is interesting that CUG piglets had much better intestinal structure, almost as good as NBW piglets. Our result indicated that the intestinal development from intrauterine in CUG has been restored during the suckling period, which means the potential for improved ability to get necessary nutrients for growth.
The nutrients uptake is mainly facilitated by their corresponding transporters including amino acids transporters, glucose transporters, and fatty acids transporters distributed aligned on the intestinal epithelium [29]. Nutrient uptake is not only the first step for nutrient absorption, but also the main source of energy and nutrients for intestinal metabolism and development. Accumulating evidence showed that intestinal growth stagnation and dysfunction in piglets with IUGR are accompanied by a series of nutrient transporter changes and disorders of nutrient and energy metabolism [30–32]. It has been reported that mRNA expression of several nutrient transporters including SLC1A1, SLC7A7, SLC7A9, PepT1, FABP4, SLC5A1, and GLUT2 was significantly reduced in the jejunum of IUGR piglets [30], implying that inadequate nutrient uptake is strongly associated with impaired gut development and function. Glucose acts as the primary ATP producer and provides the most energy to intestinal epithelial cells to perform nutrient transportation. In current study, we found that the mRNA expression of GLUT2 and SGLT1, the two major glucose transporters in intestine were upregulated in CUG piglets compared with NCUG piglets, while significantly higher mRNA expression of jejunal SGLT1 in CUG piglets compared with the other two groups. The better uptake capacity of glucose in small intestine of CUG piglets could provide sufficient energy to support intestinal development and ATP dependent nutrient transport, which is the foundation for intestinal function. We also found that the mRNA expression of amino acid transporters, including LAT1, CAT1 and PepT1 were significantly higher in CUG piglets than those of NCUG. This observation implied promoted amino acid obtaining capacity in CUG piglets, which might be a reason for their higher growth rate during lactation since amino acids are precursors of protein synthesis [13]. In addition, the mRNA expression of fatty acid transporters FATP4 and CD36 was also consistently found higher in CUG intestine in this study. Collectively, the higher mRNA expression of nutrient transporters in the intestine of CUG piglets suggested improved nutrients uptake capability potentially providing more substance for accelerated growth.
Intestinal proliferation, renewal, and active transport of nutrients entirely depend on ATP produced by mitochondrial oxidative phosphorylation [33–35]. Mitochondrial dysfunction has been shown to limit energy production resulting in malabsorption and intestinal disorders in IUGR piglets [20, 33, 35, 36]. Mitochondrial complex I is the first and the most important rate-limiting step in the mitochondrial ETC, providing a major proton-motive force that drives ATP synthesis, and its activity is positively correlated with ATP production [37]. A previous study showed complex I of ETC and ATP synthase activities were both decreased in intestine of IUGR piglets [38]. In present study, mRNA expression of ETC complex I was also shown to decrease in NCUG, while no obvious difference between CUG and NBW piglets, suggesting that the characterized energy lack in IUGR intestine has been repaired in CUG, which is also consistent with the enhanced nutrients transporters. AMPK has been widely reported as a critical sensor of cell energy status regulating cell metabolism to maintain energy homeostasis [39]. It is well known that AMPK activation could directly promote ATP production by increasing the activity or expression of proteins involved in catabolism, while switching off biosynthetic pathways to meet cellular energy requirement [30, 40]. Zhang et al. [41] reported that the AMPK signaling activation was inhibited in intestine of weaning IUGR piglets resulting in abnormal energy status and reduced ATP production. In current study, we did not find significant differences in the ratio of P-AMPK/AMPK protein expression between NCUG and NBW piglets’ intestines but observed significant upregulation of jejunum and ileum in the CUG. Hence, these results suggested that activation of intestinal AMPK and ETC complex I in CUG piglets repair intestinal energy metabolism and ensure the supply of ATP required for intestinal nutrient transport, thus promoting the absorption of intestinal nutrients and leading to body recovery in CUG piglets.
Redox imbalance in young animals causes uncontrolled oxidative stress with local and systemic damage, thus reducing the growth performance and increasing the risk of metabolic syndrome in adulthood [42]. Previous studies have observed a deficiency of antioxidative system in IUGR piglets compared with their normal counterparts [43–45]. It has also been pointed out that extensive oxidative stress might be the vital factor leading to intestinal injury in IUGR piglets [45]. In this case, we compared the redox controlling ability among the intestine from three groups of piglets and a significantly higher GSH-Px and SOD content was observed in both the jejunum and ileum in CUG piglets than those NCUG piglets. Meanwhile, the activity of MDA was lower in jejunum of CUG piglets than NCUG piglets. These results illustrate that intestine of CUG piglets has a recovered antioxidant capacity and less oxidative damage. Nrf2 plays an important role in the antioxidant response and inflammation. Under normal conditions, Nrf2 and Keap1 bind stably in the cytoplasm. However, under oxidative stress, Nrf2 is isolated from Keap1 and translocated to the nucleus, where it activates antioxidant gene targets [46]. We found the expressions of several genes involved in Nrf2 signaling pathway such as Nrf2, Keap1, SOD and GSH-Px in the duodenum, jejunum and ileum were significantly higher in CUG intestine than those of NCUG piglets, which may offer a further explanation for the improved antioxidative ability of CUG.
Increased oxidative stress in intestinal tissue can trigger a series of inflammatory responses leading to the overexpression of inflammatory cytokines and eventually severe inflammatory bowel disease [47, 48]. A growing number of studies have shown that oxidative stress activated the NF-κB signal with other related transcription factors to stimulate rapid secretion and accumulation of IL-6, IL-1β, and TNF-α [49]. IUGR piglets have been reported prone to intestinal inflammatory diseases and this situation often involves an increase in pro-inflammatory cytokines and a decrease in anti-inflammatory cytokines [50]. Our dates found that the mRNA abundances of TNF-α, IL-1β, IL-6 and IL-12 were significantly downregulated of CUG group compared with NCUG group. The MAPK subfamily, concluding three major subfamilies, P38, JNK, and ERK, mediate inflammatory induced signal transduction pathways [51]. The NF-κB pathway regulates genes involved in immune and inflammatory processes and its activation could accelerate the release of pro-inflammatory cytokines leading to tissue damage ultimately [52]. In the present study, we observed the expression of MAPK and NF-κB significantly downregulated in CUG piglets compared with NCUG piglets, which further indicates that CUG piglets have lower levels of intestinal inflammation. The decrease of intestinal inflammation in CUG piglets may be due to the higher antioxidant capacity of the intestine, which improves the antiviral ability of the body, thus contributing to the healthy growth of CUG piglets.
The overexpression of pro-inflammatory cytokines could lead to intestinal epithelial cell membrane damage and disruption of intestinal tight junctions, thereby disrupting intestinal permeability [53]. Tight junction structures are momentous parts of the intestinal epithelial barrier system [54]. The integrity of the epithelium will be damaged when the tight junctions are destroyed, leading to entering of pathogens or toxins into the systemic circulation [55]. Evidence has revealed that IUGR piglets have reduced intestinal tight junction protein expression, and intestinal permeability was nearly twice as high as that of NBW piglets, damaging the intestinal physical barrier [43, 56]. Notably, our results showed the decreased protein expression of Occludin, Claudin-1 and ZO-1 in CUG and NCUG piglets. ZO-1 has been upregulated in CUG piglets compared to NCUG piglets, especially in duodenum and ileum, with no effects of treatments on Occludin and Claudin-1. Occludin and Claudin-1 are the main cytoplasmic transmembrane proteins, while ZO-1 is the most important cytoplasmic adaptor protein [55]. Our findings suggest a recovered physical intestinal barrier in CUG piglets around weaning, which can help protect the body from external pathogenic bacteria and toxins and enhance the immune capacity of the body, thus achieving the function of regulating the intestinal health of the body and ensuring the normal growth of the body.
Intestinal microbiota plays an important role in regulating host animal’s immune and physiological functions and numerous researches have shown a high correlation between intestinal microbiota and intestinal barrier function [57]. It has been reported that IUGR alters the intestinal microbiome, with significantly higher levels of Gram-negative bacteria that cause systemic inflammation [58]. Our results showed that microbial Simpson and Shannon indices in alpha diversity were higher in the CUG group. Otherwise, higher alpha diversity is thought to be beneficial for maintaining host intestinal homeostasis. Further analysis by beta diversity of microbiota, the microbial composition in feces of piglets showed that there was no significant difference between CUG and NCUG piglets at 7 d. However, our LEfSe analysis showed that the CUG group had a higher population of the Ruminococcaceae and Ruminococcaceae_UCG_002. Previous studies have shown that the presence of Ruminococcaceae is associated with the maintenance of gut health and the presence of numerous carbohydrate-active enzymes [59]. In addition, the LEfSe proved CUG piglets had a markedly higher relative abundance of the Verrucomicrobiota, Verrucomicrobiales, Verrucomicrobiae, Akkermansiaceae and Akkermansia than NCUG groups. Verrucomicrobiota is regarded as potentially beneficial bacterium in the gut, and in previous studies it was found to be more abundant in the homeostasis of the gut [60, 61]. Akkermansiaceae is the only known species of the phylum Verrucomicrobiota in mammals [62], which can improve intestinal health by adhering to intestinal epithelial cells and enhancing monolayer integrity of intestinal epithelial cells [63]. Higher Oscillospirales and Oscillospiraceae levels were found in CUG piglets, previous research showed that the Oscillospiraceae can produce butyrate and inhibit the growth of pathogenic bacteria in gastrointestinal tract [64]. The Enterobacteriaceae showed a higher population in NCUG piglets in the LEfSe analysis, which was believed to play an important role in developing piglet diarrhea and seriously affect the barrier function of the animal intestinal tract [65]. Meantime, in contrast to CUG piglets, the Campylobacteraceae, Campylobacter and Tannerellaceae were higher in NCUG piglets, which were one of the main causes of diarrhea and can cause acute gastroenteritis [66, 67]. Taken together, these changes suggest that stability of intestinal ecosystem in CUG piglets may be attributed to the higher healthy beneficial bacteria and lower underlying pathogenic microbiota.
Conclusion
In summary, compared with NCUG piglets, intestinal nutrient transport, energy metabolism, antioxidant capacity and intestinal barrier function are better recovered in CUG piglets, while intestinal inflammation and harmful microbiota were mitigated in CUG piglets. This study is the first to investigate the changes in intestinal development function of CUG piglets during weaning and the results also could provide a detailed understanding of intestinal development in infants with CUG due to the limitation of samples obtaining in humans studies.
Acknowledgements
Not applicable.
Abbreviations
- ANOVA
Analysis of variance
- CUG
Catch-up growth
- CD
Crypt depth
- ETC
Electron transport chain
- GSH-PX
Glutathione peroxidase
- GSH
Reduced glutathione
- NBW
Normal birth weight
- Nrf2
Nuclear factor erythroid 2-related factor
- T-AOC
Total antioxidant capacity
- IUGR
Intrauterine growth restriction
- LDA
Linear discriminant analysis
- LEfSe
The linear discriminant analysis effect size
- MDA
Malonaldehyde
- PCoA
Principal coordinate analysis
- PVDF
Polyvinylidene difluoride
- SOD
Superoxide dismutase
- VH
Villi height
Authors’ contributions
FC and WG conceived and designed the experiments. CC, JW and ZM collected the feces samples and performed the 16 s experiments. CC, CW, XZ and PZ collected the additional tissue samples and performed the molecular experiment. YZ provided the experimental site. CC used software to analyze and statistics the data. CC, NW and FC wrote and revised the article. All author(s) read and approved the final manuscript.
Funding
This study was supported by the Natural Science Foundation of Guangdong Province (2021A1515010944) and the National Natural Science Foundation of China (31402082 and 32272894).
Availability of data and materials
The data analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All animal procedures were handled in accordance with the Guidelines for Care and Use of Laboratory Animals of South China Agricultural University and approved by the Animal Ethics Committee of South China Agricultural University (No. 20110107–1).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Chang Cui, Email: cuichang1998a@163.com.
Caichi Wu, Email: 1102183831@qq.com.
Jun Wang, Email: 2496922883@qq.com.
Ziwei Ma, Email: 937213681@qq.com.
Xiaoyu Zheng, Email: 1935393934@qq.com.
Pengwei Zhu, Email: 542560160@qq.com.
Nuan Wang, Email: lucaswong6@foxmail.com.
Yuhua Zhu, Email: zhuyh1623@163.com.
Wutai Guan, Email: wtguan@scau.edu.cn.
Fang Chen, Email: chenfang1111@scau.edu.cn.
References
- 1.Wu G, Bazer FW, Wallace JM, Spencer TE. Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci. 2006;84(9):2316–2337. doi: 10.2527/jas.2006-156. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe-Asaka T, Hayashi M, Maejima D, Kawai Y, Ohhashi T. From digestion and absorption to innate immunity and health care: water and food intake may contribute to IL-22 in ILC3-dependent mucosal immunity in the jejunum. J Physiol Sci. 2021;71(1):31. doi: 10.1186/s12576-021-00817-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selak MA, Storey BT, Peterside I, Simmons RA. Impaired oxidative phosphorylation in skeletal muscle of intrauterine growth-retarded rats. Am J Physiol Endocrinol Metab. 2003;285(1):E130–E137. doi: 10.1152/ajpendo.00322.2002. [DOI] [PubMed] [Google Scholar]
- 4.Ferenc K, Pietrzak P, Godlewski MM, Piwowarski J, Kiliańczyk R, Guilloteau P, et al. Intrauterine growth retarded piglet as a model for humans–studies on the perinatal development of the gut structure and function. Reprod Biol. 2014;14(1):51–60. doi: 10.1016/j.repbio.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Finkielstain GP, Lui JC, Baron J. Catch-up growth: cellular and molecular mechanisms. World Rev Nutr Diet. 2013;106:100–104. doi: 10.1159/000342535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faienza MF, Brunetti G, Ventura A, D’Aniello M, Pepe T, Giordano P, et al. Nonalcoholic fatty liver disease in prepubertal children born small for gestational age: influence of rapid weight catch-up growth. Horm Res Paediatr. 2013;79(2):103–9. 10.1159/000347217. [DOI] [PubMed]
- 7.Victora CG, Barros FC, Horta BL, Martorell R. Short-term benefits of catch-up growth for small-for-gestational-age infants. Int J Epidemiol. 2001;30(6):1325–1330. doi: 10.1093/ije/30.6.1325. [DOI] [PubMed] [Google Scholar]
- 8.Montoro JC, Manzanilla EG, Sola-Oriol D, Muns R, Gasa J, Clear O, et al. Predicting productive performance in grow-finisher pigs using birth and weaning body weight. Animals-Basel. 2020;10(6):1017. doi: 10.3390/ani10061017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jha R, Fouhse JM, Tiwari UP, Li L, Willing BP. Dietary fiber and intestinal health of monogastric animals. Front Vet Sci. 2019;6:48. doi: 10.3389/fvets.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu RJ, Mellor DJ, Birtles MJ, Reynolds GW, Simpson HV. Impact of intrauterine growth retardation on the gastrointestinal tract and the pancreas in newborn pigs. J Pediatr Gastroenterol Nutr. 1994;18(2):231–240. doi: 10.1097/00005176-199402000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Mickiewicz M, Zabielski R, Grenier B, Le Normand L, Savary G, Holst JJ, et al. Structural and functional development of small intestine in intrauterine growth retarded porcine offspring born to gilts fed diets with differing protein ratios throughout pregnancy. J Physiol Pharmacol. 2012;63(3):225–239. [PubMed] [Google Scholar]
- 12.D’Inca R, Kloareg M, Gras-Le Guen C, Le Huerou-Luron I. Intrauterine growth restriction modifies the developmental pattern of intestinal structure, transcriptomic profile, and bacterial colonization in neonatal pigs. J Nutr. 2010;140(5):925–31. 10.3945/jn.109.116822. [DOI] [PubMed]
- 13.He Q, Ren P, Kong X, Xu W, Tang H, Yin Y, et al. Intrauterine growth restriction alters the metabonome of the serum and jejunum in piglets. Mol Biosyst. 2011;7(7):2147–2155. doi: 10.1039/c1mb05024a. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Chen L, Li D, Yin Y, Wang X, Li P, et al. Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs. J Nutr. 2008;138(1):60–66. doi: 10.1093/jn/138.1.60. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Wu W, Lin G, Li D, Wu G, Wang J. Temporal proteomic analysis reveals continuous impairment of intestinal development in neonatal piglets with intrauterine growth restriction. J Proteome Res. 2010;9(2):924–935. doi: 10.1021/pr900747d. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Zhu Y, Feng C, Lin G, Wu G, Li D, et al. Innate differences and colostrum-induced alterations of jejunal mucosal proteins in piglets with intra-uterine growth restriction. Br J Nutr. 2018;119(7):734–747. doi: 10.1017/S0007114518000375. [DOI] [PubMed] [Google Scholar]
- 17.Patricia JJ, Dhamoon AS. Physiology, Digestion. StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. [PubMed]
- 18.Juste C, Legrand-Defretin V, Corring T, Rerat A. Intestinal absorption of bile acids in the pig. Diges Dis Sci. 1988;33:67–73. 10.1007/BF01536633. [DOI] [PubMed]
- 19.Ren CX, Wang YJ, Lin XF, Song HQ, Zhou QQ, Xu W, et al. A combination of formic acid and monolaurin attenuates enterotoxigenic escherichia coli induced intestinal inflammation in piglets by inhibiting the NF-kappa B/MAPK pathways with modulation of gut microbiota. J Agric Food Chem. 2020;68(14):4155–4165. doi: 10.1021/acs.jafc.0c01414. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Li Y, Su W, Ying Z, Zhou L, Zhang L, et al. Resveratrol attenuates mitochondrial dysfunction in the liver of intrauterine growth retarded suckling piglets by improving mitochondrial biogenesis and redox status. Mol Nutr Food Res. 2017;61(5):1600653. 10.1002/mnfr.201600653. [DOI] [PubMed]
- 21.Xun W, Fu Q, Shi L, Cao T, Jiang H, Ma Z. Resveratrol protects intestinal integrity, alleviates intestinal inflammation and oxidative stress by modulating AhR/Nrf2 pathways in weaned piglets challenged with diquat. Int Immunopharmacol. 2021;99:107989. doi: 10.1016/j.intimp.2021.107989. [DOI] [PubMed] [Google Scholar]
- 22.Yi Q, Liu J, Zhang Y, Qiao H, Chen F, Zhang S, et al. Anethole attenuates enterotoxigenic escherichia coli-Induced intestinal barrier disruption and intestinal inflammation via modification of TLR signaling and intestinal microbiota. Front Microbiol. 2021;12:647242. doi: 10.3389/fmicb.2021.647242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang JN, Zeng ZG, Wang HN, Yang T, Zhang PJ, Li YL, et al. An effective method for isolation of DNA from pig faeces and comparison of five different methods. J Microbiol Meth. 2008;75(3):432–436. doi: 10.1016/j.mimet.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Branca F, Ferrari M. Impact of micronutrient deficiencies on growth: the stunting syndrome. Ann Nutr Metab. 2002;46(Suppl 1):8–17. doi: 10.1159/000066397. [DOI] [PubMed] [Google Scholar]
- 25.Wang T, Huo YJ, Shi F, Xu RJ, Hutz RJ. Effects of intrauterine growth retardation on development of the gastrointestinal tract in neonatal pigs. Biol Neonate. 2005;88(1):66–72. doi: 10.1159/000084645. [DOI] [PubMed] [Google Scholar]
- 26.Shanklin DR, Cooke RJ. Effects of intrauterine growth on intestinal length in the human fetus. Biol Neonate. 1993;64(2–3):76–81. doi: 10.1159/000243974. [DOI] [PubMed] [Google Scholar]
- 27.Lee MJ, Conner EL, Charafeddine L, Woods JR, Jr, Del Priore G. A critical birth weight and other determinants of survival for infants with severe intrauterine growth restriction. Ann N Y Acad Sci. 2001;943:326–339. doi: 10.1111/j.1749-6632.2001.tb03813.x. [DOI] [PubMed] [Google Scholar]
- 28.Han F, Hu L, Xuan Y, Ding X, Luo Y, Bai S, et al. Effects of high nutrient intake on the growth performance, intestinal morphology and immune function of neonatal intra-uterine growth-retarded pigs. Br J Nutr. 2013;110(10):1819–1827. doi: 10.1017/S0007114513001232. [DOI] [PubMed] [Google Scholar]
- 29.Verrey F, Singer D, Ramadan T, Vuille-dit-Bille RN, Mariotta L, Camargo SM. Kidney amino acid transport. Pflugers Arch. 2009;458(1):53–60. doi: 10.1007/s00424-009-0638-2. [DOI] [PubMed] [Google Scholar]
- 30.Qi M, Wang J, Tan B, Liao S, Long C, Yin Y. Postnatal growth retardation is associated with intestinal mucosa mitochondrial dysfunction and aberrant energy status in piglets. J Cell Mol Med. 2020;24(17):10100–10111. doi: 10.1111/jcmm.15621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W, Blachier F, Fu D, Pan J, Yang H, Guo J, et al. Ontogenic expression of the amino acid transporter b(0,+)AT in suckling Huanjiang piglets: effect of intra-uterine growth restriction. Br J Nutr. 2013;110(5):823–830. doi: 10.1017/s0007114512005843. [DOI] [PubMed] [Google Scholar]
- 32.Wellington MO, Rodrigues LA, Li Q, Dong B, Panisson JC, Yang C, et al. Birth weight and nutrient restriction affect jejunal enzyme activity and gene markers for nutrient transport and intestinal function in piglets. Animals. 2021;11(9):2672. 10.3390/ani11092672. [DOI] [PMC free article] [PubMed]
- 33.Kolli VK, Natarajan K, Isaac B, Selvakumar D, Abraham P. Mitochondrial dysfunction and respiratory chain defects in a rodent model of methotrexate-induced enteritis. Hum Exp Toxicol. 2014;33(10):1051–1065. doi: 10.1177/0960327113515503. [DOI] [PubMed] [Google Scholar]
- 34.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148(6):1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wenchich L, Zeman J, Hansíková H, Plavka R, Sperl W, Houstek J. Mitochondrial energy metabolism in very premature neonates. Biol Neonate. 2002;81(4):229–235. doi: 10.1159/000056753. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Li Y, Hou X, Zhang L, Wang T. Medium-chain TAG improve energy metabolism and mitochondrial biogenesis in the liver of intra-uterine growth-retarded and normal-birth-weight weanling piglets. Br J Nutr. 2016;115(9):1521–1530. doi: 10.1017/S0007114516000404. [DOI] [PubMed] [Google Scholar]
- 37.Senkler J, Senkler M, Braun HP. Structure and function of complex I in animals and plants - a comparative view. Physiol Plant. 2017;161(1):6–15. doi: 10.1111/ppl.12561. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Li Y, Chen Y, Zhang L, Wang T. N-Acetylcysteine protects against intrauterine growth retardation-induced intestinal injury via restoring redox status and mitochondrial function in neonatal piglets. Eur J Nutr. 2019;58(8):3335–3347. doi: 10.1007/s00394-018-1878-8. [DOI] [PubMed] [Google Scholar]
- 39.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, Chen Y, Li Y, Wang T. Protective effect of polydatin on jejunal mucosal integrity, redox status, inflammatory response, and mitochondrial function in intrauterine growth-retarded weanling piglets. Oxid Med Cell Longev. 2020;2020:7178123. doi: 10.1155/2020/7178123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friel JK, Diehl-Jones B, Cockell KA, Chiu A, Rabanni R, Davies SS, et al. Evidence of oxidative stress in relation to feeding type during early life in premature infants. Pediatr Res. 2011;69(2):160–164. doi: 10.1203/PDR.0b013e3182042a07. [DOI] [PubMed] [Google Scholar]
- 43.Tao S, Bai Y, Li T, Li N, Wang J. Original low birth weight deteriorates the hindgut epithelial barrier function in pigs at the growing stage. Faseb j. 2019;33(9):9897–9912. doi: 10.1096/fj.201900204RR. [DOI] [PubMed] [Google Scholar]
- 44.Huang Q, Xu W, Bai KW, He JT, Ahmad H, Zhou L, et al. Protective effects of leucine on redox status and mitochondrial-related gene abundance in the jejunum of intrauterine growth-retarded piglets during early weaning period. Arch Anim Nutr. 2017;71(2):93–107. doi: 10.1080/1745039X.2017.1279712. [DOI] [PubMed] [Google Scholar]
- 45.Su W, Zhang H, Ying Z, Li Y, Zhou L, Wang F, et al. Effects of dietary L-methionine supplementation on intestinal integrity and oxidative status in intrauterine growth-retarded weanling piglets. Eur J Nutr. 2018;57(8):2735–2745. doi: 10.1007/s00394-017-1539-3. [DOI] [PubMed] [Google Scholar]
- 46.Shah SA, Khan M, Jo MH, Jo MG, Amin FU, Kim MO. Melatonin stimulates the SIRT1/Nrf2 signaling pathway counteracting lipopolysaccharide (LPS)-induced oxidative stress to rescue postnatal rat brain. CNS Neurosci Ther. 2017;23(1):33–44. doi: 10.1111/cns.12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem-Biol Interact. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Yin J, Ren W, Liu G, Duan J, Yang G, Wu L, et al. Birth oxidative stress and the development of an antioxidant system in newborn piglets. Free Radical Res. 2013;47(12):1027–1035. doi: 10.3109/10715762.2013.848277. [DOI] [PubMed] [Google Scholar]
- 49.Hu L, Peng X, Chen H, Yan C, Liu Y, Xu Q, et al. Effects of intrauterine growth retardation and Bacillus subtilis PB6 supplementation on growth performance, intestinal development and immune function of piglets during the suckling period. Eur J Nutr. 2017;56(4):1753–1765. doi: 10.1007/s00394-016-1223-z. [DOI] [PubMed] [Google Scholar]
- 50.Niu Y, Zhao Y, He J, Yun Y, Shen M, Gan Z, et al. Dietary dihydroartemisinin supplementation alleviates intestinal inflammatory injury through TLR4/NOD/NF-κB signaling pathway in weaned piglets with intrauterine growth retardation. Anim Nutr. 2021;7(3):667–678. doi: 10.1016/j.aninu.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004;117(Pt 20):4619–4628. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- 52.Li SS, Wu CZ, Zhang BW, Qiu L, Chen W, Yuan YH, et al. Nerve growth factor protects salivary glands from irradiation-induced damage. Life Sci. 2021;265:118748. doi: 10.1016/j.lfs.2020.118748. [DOI] [PubMed] [Google Scholar]
- 53.Kanwar JR, Kanwar RK. Gut health immunomodulatory and anti-inflammatory functions of gut enzyme digested high protein micro-nutrient dietary supplement-enprocal. BMC Immunol. 2009;10:7. doi: 10.1186/1471-2172-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mehandru S, Colombel JF. The intestinal barrier, an arbitrator turned provocateur in IBD. Nat Rev Gastroenterol Hepatol. 2021;18(2):83–84. doi: 10.1038/s41575-020-00399-w. [DOI] [PubMed] [Google Scholar]
- 55.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169(6):1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang W, Degroote J, Van Ginneken C, Van Poucke M, Vergauwen H, Dam TM, et al. Intrauterine growth restriction in neonatal piglets affects small intestinal mucosal permeability and mRNA expression of redox-sensitive genes. FASEB J. 2016;30(2):863–873. doi: 10.1096/fj.15-274779. [DOI] [PubMed] [Google Scholar]
- 57.Guthrie GJ, Aydemir TB, Troche C, Martin AB, Chang SM, Cousins RJ. Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function. Am J Physiol Gastrointest Liver Physiol. 2015;308(3):G171–G178. doi: 10.1152/ajpgi.00021.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiong L, You J, Zhang W, Zhu Q, Blachier F, Yin Y, et al. Intrauterine growth restriction alters growth performance, plasma hormones, and small intestinal microbial communities in growing-finishing pigs. J Anim Sci Biotechnol. 2020;11:86. doi: 10.1186/s40104-020-00490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biddle A, Stewart L, Blanchard J, Leschine S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity. 2013;5(3):627–640. doi: 10.3390/d5030627. [DOI] [Google Scholar]
- 60.Chen KT, Zhao H, Shu LY, Xing HY, Wang C, Lu CP, et al. Effect of resveratrol on intestinal tight junction proteins and the gut microbiome in high-fat diet-fed insulin resistant mice. Int J Food Sci Nutr. 2020;71(8):965–978. doi: 10.1080/09637486.2020.1754351. [DOI] [PubMed] [Google Scholar]
- 61.Zhou JM, Zhang HJ, Wu SG, Qiu K, Fu Y, Qi GH, et al. Supplemental xylooligosaccharide modulates intestinal mucosal barrier and cecal microbiota in laying hens fed oxidized fish oil. Front Microbiol. 2021;12:635333. doi: 10.3389/fmicb.2021.635333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Becken B, Davey L, Middleton DR, Mueller KD, Sharma A, Holmes ZC, et al. Genotypic and phenotypic diversity among human isolates of akkermansia muciniphila. MBio. 2021;12(3):e00478–e521. doi: 10.1128/mBio.00478-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol. 2015;81(11):3655–3662. doi: 10.1128/aem.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van den Abbeele P, Ghyselinck J, Marzorati M, Koch AM, Lambert W, Michiels J, et al. The effect of amino acids on production of SCFA and bCFA by members of the porcine colonic microbiota. Microorganisms. 2022;10(4):762. doi: 10.3390/microorganisms10040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen L, Xu Y, Chen X, Fang C, Zhao L, Chen F. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front Microbiol. 2017;8:1688. doi: 10.3389/fmicb.2017.01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moore JE, Corcoran D, Dooley JSG, Fanning S, Lucey B, Matsuda M, et al. Campylobacter. Vet Res. 2005;36(3):351–382. doi: 10.1051/vetres:2005012. [DOI] [PubMed] [Google Scholar]
- 67.Hernandez M, de Frutos M, Rodriguez-Lazaro D, Lopez-Urrutia L, Quijada NM, Eiros JM. Fecal microbiota of toxigenic clostridioides difficile-associated diarrhea. Front Microbiol. 2019;9:3331. doi: 10.3389/fmicb.2018.03331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed during the current study are available from the corresponding author on reasonable request.