Abstract
The interaction between the macrophage and Mycobacterium tuberculosis is mediated by a variety of macrophage membrane-associated proteins. Complement receptors have been implicated in the adherence of M. tuberculosis to macrophages. In the present work, the adherence and/or ingestion of M. tuberculosis H37Rv to human monocyte-derived macrophages (MDM) from patients with tuberculosis (TB) and healthy controls was measured by microscopical examination, [3H]uracil incorporation, and CFU. The adherence and/or ingestion was enhanced by fresh serum and inhibited by heat inactivation, EDTA treatment, and anti-CR1 and anti-CR3 antibodies. Comparison of MDM from TB patients and healthy controls showed that the former exhibited a significantly decreased capacity to adhere and/or ingest M. tuberculosis, as determined by the number of CFU and 3H incorporation. The expression of CR1 (CD35) and CR3 (CD11b/CD18) on MDM from TB patients and healthy controls, as determined by flow cytometry, did not show significant differences. These results suggest that the lower ingestion of M. tuberculosis by MDM from TB patients is not due to defects in complement receptors, and therefore, there might be other molecules involved in the adherence and/or ingestion process that render MDM from TB patients ingest less mycobacteria than those from healthy controls.
The infectious process by intracellular pathogens is very complex, and it initially involves the adherence of the microorganisms to the surfaces of phagocytic cells. Adherence and phagocytosis are increased in the presence of several serum proteins that act as opsonins (4, 10) and by extracellular matrix proteins (15).
The complement system is composed of a group of serum proteins and their corresponding receptors located on the surfaces of many cells including phagocytes (4). There are at least four complement receptors (CRs) (4). Complement receptor type 1 (CR1) (CD35 or C3b/C4b receptor) binds mainly C3b/C4b, whereas complement receptor type 3 (CR3) (CD11b/CD18 or iC3b receptor) binds iC3b (10). Complement receptor type 4 (CR4) (CD11c/CD18) also binds iC3b (10). CR1, CR3, and CR4 have been implicated as mediators of adherence of mycobacteria to mononuclear phagocytes (for recent reviews, see references 8 and 21). Schlesinger et al. (22, 23) using monoclonal antibodies against CR1 and CR3 showed a significant reduction in the adherence of Mycobacterium tuberculosis and Mycobacterium leprae to human monocyte-derived macrophages (MDM). It is noteworthy that both CR1 and CR3 were equally involved in the adherence of M. tuberculosis, while M. leprae adherence was mainly mediated by CR3 (22, 23). In addition to CRs, there are other receptors involved in the adherence of M. tuberculosis to MDM, such as mannose receptors (20) and class A scavenger receptor (32), and it is possible that CD14, which serves as lipopolysaccharide receptor, may also act as M. tuberculosis receptor by interacting with the mycobacterial wall-associated lipoarabinomannam (LAM) (17, 26).
Surprisingly little is known about the capacity of macrophages from patients with tuberculosis (TB) compared to that of healthy controls to adhere and/or ingest mycobacteria and how the process could relate with the pathogenesis of the disease. In this report, we present evidence that MDM from TB patients exhibit decreased adherence and/or ingestion of M. tuberculosis compared to MDM from healthy controls.
MATERIALS AND METHODS
Subjects studied.
Patients with clinically and bacteriologically diagnosed TB were recruited from Hospital La María, Medellín, Colombia. All patients were under antituberculous treatment for less than 1 month. One patient had meningeal TB and another had renal TB, both without clinical pulmonary compromise. No patients were receiving an immunosuppressive drug. Blood hemoglobin ranged from 11.8 to 14.8 g/dl. Volunteer healthy donors were also studied as controls for the different experiments. All subjects studied were human immunodeficiency virus negative. Participants were informed of the objectives of the study and voluntarily agreed to participate in it.
Mycobacteria.
M. tuberculosis H37Rv was grown in Proskauer-Boek liquid medium (31), collected, and maintained at −70°C in RPMI 1640 (Gibco BRL, Grand Island, N.Y.) containing 30% glycerine and 10% fetal bovine serum (Gibco BRL). The number of bacteria was determined by plating serial dilutions onto petri dishes containing Middlebrook 7H10 solid medium (5) (Becton Dickinson Microbiology Systems, Cockeysville, Md.). For all the experiments described herein, a single batch of mycobacteria was used.
Before each experiment, a vial of M. tuberculosis was thawed and incubated in phosphate-buffered saline (0.15 M, pH 7.2) (PBS) containing 50% (vol/vol) fresh pooled human serum (PHS), obtained from seven tuberculin-skin-test-negative healthy subjects, for 20 min at 37°C to opsonize mycobacteria. To disrupt the bacterial clumps, the suspension was passed through a 26-gauge tuberculin syringe at least 20 times without bubble formation as previously described (25). The number of CFU after thawing was 70 to 75% of the original counts.
Isolation of MNC.
Fifty-milliliter samples of venous blood were poured into Erlenmeyer flasks containing 15 to 20, 2-mm-diameter glass beads. Defibrination was done by gentle shaking for 10 min until a clot formed. Defibrinated blood was collected and centrifuged at 900 × g for 10 min at room temperature. The buffy coat was recovered and diluted 1:3 with PBS and centrifuged (3:1 [vol/vol]) on Histopaque (Sigma Chemical Co., St. Louis, Mo.). The fraction containing the mononuclear cells (MNC) was recovered and washed twice with PBS. The viability was determined by trypan blue exclusion and was always ≥95%. The percentage of monocytes was determined by Wright’s and nonspecific α-naphthyl-acetate esterase (Sigma) stains of smears obtained by cytocentrifugation of the MNC at 40 × g for 5 min. Monocytes were adjusted to 106/ml in RPMI 1640 (Gibco) (pH 7.2) containing 25 mM HEPES and l-glutamine without serum and antibiotics and cultured under conditions described below for specific experiments.
Quantification of associated AFB per cell.
The effect of in vitro maturation of monocytes and the optimal dose of infectious inocula was determined by light microscopy examination. Two hundred microliters of the MNC suspension (approximately 2 × 105 monocytes) was dropped into each 15-mm-diameter, round coverslip (Nunc, Inc., Napersville, Ill.) placed in 24-flat-bottom-well plates (Nunc). After 30 to 60 min at 37°C and 5% CO2, the volume of each well was adjusted to 1 ml with RPMI 1640. After 24 h of culture, PHS inactivated by being heated at 56°C for 30 min (HI-PHS) was added to a final concentration of 5% and the plates were incubated for 1 to 6 days to allow differentiation of monocytes into macrophages (MDM). The day of the experiment, the nonadherent cells were washed by immersing the coverslips seven or eight times in PBS prewarmed at 37°C. Coverslips were placed again in 24-well plates containing 500 μl of RPMI 1640 supplemented with 5% HI-PHS and 100 U of penicillin (Sigma) per ml in each well. Only the confluent monolayers were selected and infected according to the initial number of monocytes. In our hands and by using the method described by Nakagawara and Nathan (14), a maximum of 10% of cells are detached after 1 week of in vitro culture. MDM were infected with opsonized or nonopsonized M. tuberculosis for 2 h at 37°C at different mycobacterium/MDM ratios (1:1, 5:1, and 10:1). Thereafter, the nonadhered bacteria were washed by immersing the coverslips into PBS at 37°C. Then, the coverslips were immersed for 10 min in 10% formaldehyde, and cells were stained with Kinyoun stain (11). The number of MDM with one or more associated acid-fast bacilli (AFB) and the bacterial load per cell were determined by counting 200 cells at a magnification of ×1000 with a light microscope. To assess the bacterial load per cell, we used a previously published scoring method (6). We did not differentiate between adhered or ingested M. tuberculosis in this study.
Measurement of [3H]uracil incorporation by MDM-associated M. tuberculosis.
Fifty thousand monocytes were plated in each well of a 96-flat-bottom-well plate (Nunc) and cultured for 7 days in 200 μl of RPMI 1640. Twenty-four hours after plating, HI-PHS was added to a final concentration of 5%. The day of the experiment, the nonadherent cells were removed by washing the wells three times with 37°C-prewarmed PBS. Then, 200 μl of RPMI 1640 and 5% HI-PHS were added. MDM were infected for 2 h at 37°C with preopsonized M. tuberculosis H37Rv at a mycobacterium/MDM ratio of 5:1. Nonadhered bacteria were washed with prewarmed PBS. Thereafter, MDM with associated mycobacteria were lysed with 200 μl of RPMI 1640 containing 0.2% saponin (Sigma) and supplement 8X (1.6% l-asparagine, 1.6% sodium glutamate, 0.04% ferric ammonium citrate) to allow mycobacterial extracellular growth (18). After lysis, 0.5 μCi of [3H]uracil (specific activity, 50 Ci/mmol; Amersham, Little Chalfont, Buckinghamshire, United Kingdom) was added to each well and the plates were incubated for 7 more days at 37°C. Mycobacteria were harvested on glass-fiber filters with a cell harvester (Inotech Biosystems International, Lansing, Mich.), and the [3H]uracil incorporated by M. tuberculosis was counted in a β-scintillation counter (model 121; LKB-Wallac, Turku, Finland).
Determination of macrophage-associated M. tuberculosis by CFU.
Three 5-μl droplets were obtained from the lysate before the addition of [3H]uracil and plated on petri dishes containing Middlebrook 7H10 agar medium (Becton Dickinson). The petri dishes were incubated for 7 days at 37°C, and the microcolonies were counted microscopically by using a calibrated ocular lens.
Inhibition of the adherence and/or ingestion of M. tuberculosis by MDM.
To determine the type of serum opsonins, M. tuberculosis was incubated for 20 min at 37°C with HI-PHS or with serum containing 20 mM EDTA (Sigma) before addition to MDM. In parallel, 7-day-cultured MDM from healthy individuals were incubated for 30 min with monoclonal antibodies against either CR1 (clone E11) (15 μg/ml) or CR3 (clone ICRF44,44) (10 μg/ml) (both from Pharmingen, San Diego, Calif.) or both or an irrelevant mouse anti-human immunoglobulin G1 (IgG1) (Pharmingen) as the isotype control. MDM were infected for 2 h at a mycobacterium/MDM ratio of 5:1. Thereafter, MDM were lysed and CFU were determined as described above. To establish the role played by the mannose receptor in mediating the adherence of opsonized bacteria to human macrophages, MDM were preincubated for 60 min with different doses (10−5 to 10−1 M) of α-methyl mannoside (α-MM) (Sigma) and then infected at a mycobacterium/MDM ratio of 5:1 as described above. The percentage of MDM with associated mycobacteria and the bacterial load per cell were determined by light microscopy.
Quantification of CR1 and CR3 by flow cytometry.
MDM were obtained as described above. After 7 days of culture, 106/ml MDM were incubated with 20 μg of a monoclonal mouse anti-human CR1 (clone E11) IgG1 (Pharmingen) per ml for 30 min at 4°C. The cells were washed and incubated at 4°C for 30 min with a fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse IgG1 (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). For CR3, the cells were incubated with 10 μl of a phycoerythrin-conjugated monoclonal mouse IgG1 anti-human CR3 (Leu15) (Becton Dickinson) for 20 min. The cells were washed, and the positive fluorescence was determined by flow cytometry (FACSort; Becton Dickinson) by comparison with the respective isotype control antibody (Becton Dickinson). Results are shown as percentage of positive cells, mean of median net fluorescence intensity, and total fluorescence from the product of the two former variables.
Statistical analyses.
Comparisons between healthy controls and TB patients were done by the unpaired Student t test. One- and two-way analysis of variance (ANOVA) were used to compare the results obtained with different treatments. Correlation analysis was used to compare the results of the different techniques. All statistical analyses were performed with Prism 2 software (GraphPad Software, San Diego, Calif.).
RESULTS
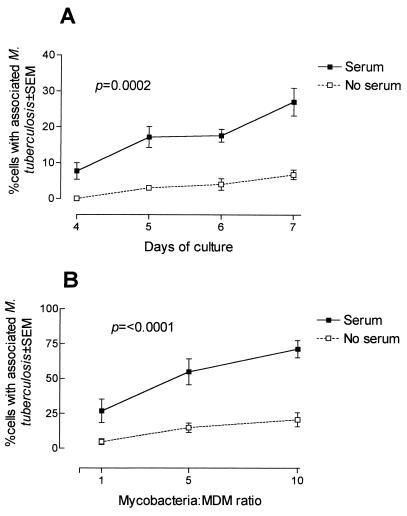
To determine the effect of mycobacterial opsonization and time of culture of human MDM on the adherence of mycobacteria, MDM were cultured for different periods of time and incubated for 2 h with opsonized or nonopsonized mycobacteria at a mycobacterium/MDM ratio of 1:1 (Fig. 1A). The percentage of cells with associated AFB was significantly higher (P = 0.0002) with opsonized microorganisms than with nonopsonized mycobacteria at all times of MDM culture studied. In the case of opsonized M. tuberculosis, there was a significant, time-dependent increase (P < 0.0001) in the percentage of MDM with associated M. tuberculosis from 7.7% ± 2.3% with MDM cultured for 4 days to 27.1% ± 3.9% at day 7, an increase of 284%. Based on these results, the next experiments were done with 7-day-cultured MDM.
FIG. 1.
(A) Effect of opsonization and time of culture on the adherence and/or ingestion of M. tuberculosis H37Rv by human MDM. MDM cultured for 4 to 7 days were infected for 2 h with M. tuberculosis H37Rv preopsonized (solid line) or not (broken line) with fresh PHS at a mycobacterium/MDM ratio of 1:1. Nonadhered bacteria were washed, and coverslips were stained with Kinyoun stain. The results show the means ± standard errors of the means (SEMs) of the percentage of MDM with one or more associated AFB of three different experiments. The differences between opsonized and nonopsonized bacteria are significant (P = 0.0002 by two-way ANOVA). (B) Effect of the dose of inoculum on the adherence and/or ingestion of M. tuberculosis H37Rv by human MDM. Seven-day-cultured MDM obtained from six healthy donors were infected for 2 h with M. tuberculosis H37Rv opsonized (solid line) or nonopsonized (broken line) with fresh PHS at different mycobacterium/MDM ratios. Nonadhered bacteria were washed, and cells were stained with Kinyoun stain. Results are shown as means ± SEMs of the percentage of MDM with one or more associated AFB of six different experiments (P = <0.0001 by two-way ANOVA).
The adherence and/or ingestion of mycobacteria by MDM was dose dependent (Fig. 1B). The percentage of MDM with associated M. tuberculosis under nonopsonizing conditions was lower than under opsonized conditions and was independent of the dose used (P < 0.0001). In the case of opsonized mycobacteria, there was a dose-dependent increase in the percentage of MDM-adhering and/or ingesting bacteria, reaching 71.4% ± 6.2% at a mycobacterium/MDM ratio of 10:1.
In the experiments designed to define the characteristics of the opsonins involved in the adherence and/or ingestion of mycobacteria by MDM (Table 1), it was found that in the absence of serum, there was a reduction of 42 to 75% in the number of CFU/milliliter compared to bacteria opsonized with fresh serum, confirming the microscopical observation described above. Heat inactivation of the serum resulted in a decrease of 35 to 66% in the number of CFU/milliliter recovered from the lysates of MDM from that of mycobacteria opsonized with fresh serum. Similar reductions were observed with EDTA treatment. Incubation with monoclonal anti-CR1 caused a reduction of 45 to 58% in the number of CFU/milliliter. Monoclonal antibody against CR3 caused reductions of 49 and 41% in the number of CFU recovered in two of the subjects studied. When anti-CR1 and anti-CR3 were used together, the inhibitory effect increased to 61% in subject 1 and to 68% in subject 2. The use of an isotype antibody control did not affect the number of CFU/milliliter recovered.
TABLE 1.
Effects of heat inactivation and EDTA treatment of serum and CR1 and/or CR3 blockade on the adherence and/or ingestion of M. tuberculosis H37Rv by MDMa
| Opsonization condition | % Reduction in no. of mycobacteria from:
|
||||
|---|---|---|---|---|---|
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | |
| Medium alone | 69 | 45 | 42 | 52 | 75 |
| HI-PHS | 66 | 55 | 59 | 35 | 64 |
| EDTA (20 mM) | 65 | 77 | NTb | NT | NT |
| Anti-CR1 (15 μg/ml) | 49 | 46 | 45 | 49 | 58 |
| Anti-CR3 (10 μg/ml) | 49 | 41 | NT | NT | NT |
| Anti-CR1 and anti-CR3 | 61 | 68 | NT | NT | NT |
| IgG1 isotype control | NT | NT | 5 | −5.6 | 9 |
Seven-day-old MDM were treated for 30 min with anti-CR1 (15 μg/ml), anti-CR3 (10 μg/ml), or both, or IgG1 isotype-matched control and then exposed for 2 h to M. tuberculosis nonopsonized or opsonized with either EDTA-treated fresh serum or heat-inactivated serum. Infections were done at a mycobacterium/MDM ratio of 5:1. MDM were lysed as described in Materials and Methods, and the numbers of CFU were determined on Middlebrook 7H10 agar plates. The numbers of M. tuberculosis were 1.6 × 105, 1.1 × 105, 1.67 × 105, 1.08 × 105, and 2.01 × 105 CFU/ml for subjects 1 to 5, respectively.
NT, not tested.
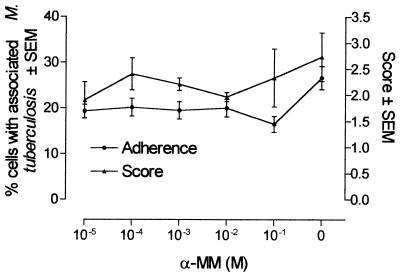
Since the adherence of mycobacteria to MDM could also be mediated by other membrane molecules, including mannose receptors (20), we used different concentrations of α-MM trying to block these receptors and therefore the adherence of mycobacteria. At the doses used, α-MM had no significant effect on the adherence of opsonized M. tuberculosis to MDM, as detected either by the percentage of cells with adhered mycobacteria or by the score of the bacterial load per cell (Fig. 2).
FIG. 2.
Effect of α-MM on the adherence and/or ingestion of opsonized M. tuberculosis to human MDM. Cells were cultured on round, 15-mm-diameter coverslips. MDM cultured for 7 days were incubated with different doses of α-MM for 1 h at 37°C and then infected for 2 h at a mycobacterium/MDM ratio of 5:1 with M. tuberculosis H37Rv preopsonized for 20 min with fresh PHS. Nonadhered bacteria were washed as described in Materials and Methods, and the cells were stained with Kinyoun stain. Results are shown as means ± SEMs of the percentage of MDM with one or more associated AFB in three different experiments.
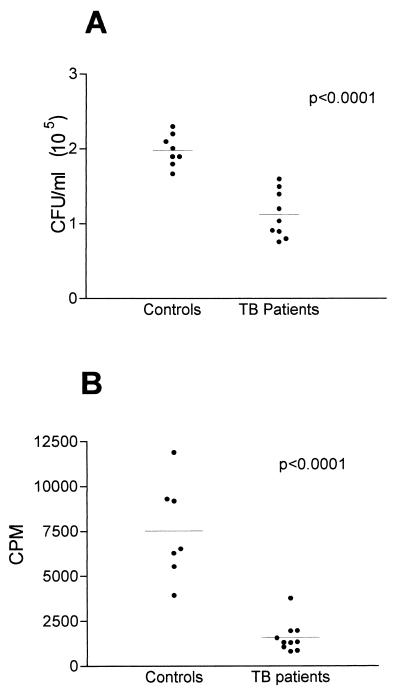
Analysis of the adherence and/or ingestion of M. tuberculosis to MDM from both TB patients and healthy controls by counting the CFU (Fig. 3A) showed that MDM from TB patients had significantly fewer associated M. tuberculosis than MDM from healthy controls, with values of (1.1 ± 0.1) × 105 and 2.0 ± 0.1/ml × 105, respectively (P < 0.0001). The difference between controls and TB patients was also demonstrated by [3H]uracil incorporation (Fig. 3B). Cultures from controls exhibited a mean of 7,526 ± 1,030 cpm, while the incorporation in cultures from TB patients was 1,590 ± 272 cpm (P < 0.0001). The determination of the adhesion and/or ingestion of M. tuberculosis by counting the CFU and counts per minute showed a significant correlation (r = 0.81, P = 0.0004).
FIG. 3.
Adherence and/or ingestion of M. tuberculosis H37Rv by MDM from healthy controls and patients with TB. Seven-day-cultured MDM from healthy controls and TB patients were infected for 2 h with M. tuberculosis preopsonized for 20 min with fresh PHS at a 5:1 mycobacterium/MDM ratio. Nonadhered bacteria were washed, and MDM were lysed. (A) CFU were determined by plating the lysate onto Middlebrook 7H10 agar. (B) MDM were lysed, and M. tuberculosis H37Rv organisms were pulsed with 0.5 μCi of [3H]uracil per well. Seven days later, cultures were collected and the counts per minute were counted by liquid scintillation. Each dot represents the mean for three samples from each subject.
Since the differences observed between healthy controls and TB patients could be due to differences in the number of CR1 or CR3 expressed on the membranes of MDM, we determined the expression of these molecules by flow cytometry. As shown in Table 2, we did not found significant differences in the percentage of CR1- or CR3-positive cells when we compared MDM from TB patients and healthy controls. It is also shown in Table 2 that the mean of median fluorescence intensity for CR1 in the group of TB patients was 333.7 ± 26, while in healthy controls, it was 366.5 ± 46, showing no significant difference. In the case of CR3 expression, the mean of median fluorescence intensity was 632.9 ± 40 and 695 ± 22.15 for TB patients and healthy controls, respectively, with no significant differences between the values for the two groups. Moreover, when we compared the total expression of these molecules on the membranes of MDM from TB patients and healthy controls, we did not find any significant differences (Table 2). Thus, there was no significant differences in the two groups in the expression of CR1 and CR3 present on the membranes of MDM.
TABLE 2.
Expression of CR1 and CR3 on MDM from TB patients and healthy controlsa
| CR | Subject | % of positive cells | MFIb | Total expressionc |
|---|---|---|---|---|
| CR1 | TB patients (n = 6) | 70 ± 10.0d | 333.7 ± 26d | 22,840 ± 2,849d |
| Healthy controls (n = 6) | 59 ± 7.0 | 366.5 ± 46 | 21,780 ± 4,213 | |
| CR3 | TB patients (n = 10) | 47 ± 8.0d | 632.9 ± 40d | 31,040 ± 6,075d |
| Healthy controls (n = 16) | 53 ± 6.0 | 695.3 ± 22 | 38,370 ± 5,100 |
Peripheral blood mononuclear cells were cultured for 7 days as described in Materials and Methods. Nonadherent cells were washed, and adherent cells (MDM) were incubated at 4°C for 30 min and recovered by washing with cold PBS. Once recovered, the MDM were counted and incubated with either a monoclonal mouse anti-human CR1 at 4°C for 30 min and then with an FITC-conjugated rat anti-mouse IgG1 or with monoclonal mouse phycoerythrin-conjugated anti-human CR3 IgG1. Cells were incubated for 20 min at room temperature, and the percentage of positive cells and the fluorescence intensity were determined by flow cytometry. Means ± standard errors of the means are shown.
MFI, mean of median fluorescence intensity.
Percentage of positive cells multiplied by MFI.
Not statistically significant compared with the value for healthy controls.
DISCUSSION
The initial contact between intracellular microorganisms and phagocytes can be mediated by opsonic (4, 10) and nonopsonic interactions (16). The former are mediated by either immunoglobulins or C3b/C4b complement fractions that interact with Fc receptors and CR, respectively (4, 10). In our experiments, the role played by serum immunoglobulins was ruled out by opsonizing mycobacteria with pooled serum from healthy, tuberculin-skin-test-negative subjects with no clinical history of tuberculosis. Previous experiments in our laboratory showed that sera of skin-test-negative individuals are negative by an enzyme-linked immunosorbent assay for antimycobacterial IgG and IgM antibodies (13a). The findings that opsonin activity was heat labile and EDTA sensitive and that monoclonal antibodies against CR1 and CR3 significantly reduced the number of CFU recovered from infected MDM, as previously found by microscopical observations (7, 20, 22, 32), are evidence that complement plays an important role in the adherence and/or ingestion of M. tuberculosis. However, total inhibition was not obtained with any of the treatments described, suggesting that other mechanisms of adherence are involved. Mannose receptors are one of the major molecules that mediate nonopsonic interaction of mycobacteria through LAM (20, 24, 28, 32). Although it has been reported (3) that α-MM inhibited the adherence of nonopsonized M. avium to MDM, our finding that α-MM did not affect the adherence and/or ingestion of opsonized M. tuberculosis suggests that in our system, the main adherence was mediated by receptors other than the mannose receptor. Another interesting possibility is CD14, a glycophosphatidylinositol-anchored membrane molecule that can serve as LAM receptor on macrophage membranes (17, 19). It is noteworthy that in body compartments with low levels of opsonins, LAM can interact with CR3 through the mannose residues at a different site than the iC3b-binding site (25). A recent report suggests that class A scavenger receptor can also play an important role in nonopsonic binding of M. tuberculosis to macrophages (32).
MDM from TB patients had a lower capacity to adhere and/or ingest M. tuberculosis compared to those from healthy controls. This difference was observed by CFU results as well as by [3H]uracil incorporation; it must be noted that a high correlation between the two techniques was detected, as previously published (2). There are several possible explanations of the differences between healthy controls and TB patients. Since all patients were receiving antituberculous treatment, it was possible that the drug persisted inside the endocytic vacuoles of MDM, affecting the phagocytosed mycobacteria (5) and reducing the number of live and culturable bacilli. However, it is unlikely that after 7 days in culture there were enough active antimycobacterial compounds within MDM; this scenario is even more unlikely when the interaction of mycobacteria with the MDM was only 2 h. A deactivated state of MDM from TB patients might also explain the differences observed with MDM from healthy controls. M. tuberculosis-infected macrophages or macrophages exposed to LAM produce interleukin 10 (1), which inhibits the production of macrophage-activating cytokines (13, 29). It has been previously reported (12) that monocytes infected with M. tuberculosis H37Ra produced transforming growth factor β1 (TGF-β1) and that treatment of monocytes with TGF-β1 decreased the uptake of M. tuberculosis. Moreover, the spontaneous release of TGF-β was found to be higher in monocyte supernatants from TB patients than in those of healthy controls (27). Thus, it is possible that in our experiments, MDM from TB patients had an increased production of interleukin 10 or TGF-β and consequently reduced monocyte activity. Since MDM were not exposed to lymphocyte-derived cytokines, the diminished capacity of MDM from TB patients to adhere and/or ingest M. tuberculosis may be due to either an acquired defect secondary to the infectious process or a constitutional characteristic of MDM from TB patients.
One interesting possibility is that the differences observed in TB patients and healthy controls in the adherence of opsonized M. tuberculosis were due to variations in the number of CR1 or CR3 molecules expressed on the surfaces of their macrophages. The number of CR1 molecules expressed on the cell surface varies in different cell types (9), and it seems to be genetically regulated by a CR1-linked gene (30). Using flow cytometry, we were unable to demonstrate these differences, which suggests that the deficient adherence and/or ingestion of M. tuberculosis observed in MDM of TB patients is due to abnormalities in receptors other than CR1 or CR3. The fact that none of the patients had advanced anemia suggests that their general clinical status was not responsible for the defective macrophage phagocytic capacity. It remains to be determined whether these abnormalities can be reversed after the antituberculous treatment and whether patients with other diseases, in similar clinical conditions, exhibit similar phagocytic defects. It must be remembered that clinical TB is the final result of many host and mycobacterial factors interacting within particular epidemiological and environmental conditions. The identification of such variables will eventually lead to a better understanding of the disease and to develop more-effective methods to prevent and treat it.
ACKNOWLEDGMENTS
We thank the personnel of the Tuberculosis Control Program of the Hospital La María, Medellín, Colombia, and particularly Maggy E. Muñoz for their help in the recruitment of patients. We thank Luis F. Barrera for critically reviewing the manuscript.
This work was supported by Colciencias (grant 1115-05-024-92).
REFERENCES
- 1.Barnes P F, Chatterjee D, Abrams J S, Lu S, Wang E, Yamamura M, Brennan P J, Modlin R L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992;149:541–547. [PubMed] [Google Scholar]
- 2.Barrera L F, Skamene E, Radzioch D. Assessment of mycobacterial infection and multiplication in macrophages by polymerase chain reaction. J Immunol Methods. 1993;157:91–99. doi: 10.1016/0022-1759(93)90074-h. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez L E, Young L S, Enkel H. Interaction of Mycobacterium avium complex with human macrophages: roles of membrane receptors and serum proteins. Infect Immun. 1991;59:1697–1702. doi: 10.1128/iai.59.5.1697-1702.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown E J. Complement receptors, adhesion, and phagocytosis. Infect Agents Dis. 1992;1:63–70. [PubMed] [Google Scholar]
- 5.Crowle A J, Sbarbaro J A, Judson F N, Douvas G S, May M H. Inhibition by streptomycin of tubercle bacilli within cultured human macrophages. Am Rev Respir Dis. 1984;130:839–844. doi: 10.1164/arrd.1984.130.5.839. [DOI] [PubMed] [Google Scholar]
- 6.Crowle A J, May M H. Replication of lyophilized and cultured BCG in human macrophages. Am Rev Respir Dis. 1983;128:673–679. doi: 10.1164/arrd.1983.128.4.673. [DOI] [PubMed] [Google Scholar]
- 7.Cywes C, Godenir N L, Hoppe H C, Scholle R R, Steyn L M, Kirsch R E, Ehlers M R W. Nonopsonic binding of Mycobacterium tuberculosis to human complement receptor type 3 expressed in Chinese hamster ovary cells. Infect Immun. 1996;64:5373–5383. doi: 10.1128/iai.64.12.5373-5383.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst J D. Macrophage receptors for Mycobacterium tuberculosis. Infect Immun. 1998;66:1277–1281. doi: 10.1128/iai.66.4.1277-1281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fearon D T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980;152:20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank M M, Fries L F. The role of complement in inflammation and phagocytosis. Immunol Today. 1991;12:322–326. doi: 10.1016/0167-5699(91)90009-I. [DOI] [PubMed] [Google Scholar]
- 11.Goodman N L. The mycobacteria. In: Graber C D, editor. Rapid diagnostic methods in medical microbiology. Baltimore, Md: The Williams & Wilkins Co.; 1970. pp. 118–132. [Google Scholar]
- 12.Hirsch C S, Yoneda T, Averill L, Ellner J J, Toossi Z. Enhancement of intracellular growth of Mycobacterium tuberculosis in human monocytes by transforming growth factor-b1. J Infect Dis. 1994;170:1229–1237. doi: 10.1093/infdis/170.5.1229. [DOI] [PubMed] [Google Scholar]
- 13.Lin Y G, Zhang M, Hofman F M, Gong J H, Barnes P F. Absence of a prominent Th2 cytokine response in human tuberculosis. Infect Immun. 1996;64:1351–1356. doi: 10.1128/iai.64.4.1351-1356.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Montoya, F., J. J. Estrada, and L. F. García. Unpublished observations.
- 14.Nakagawara A, Nathan C F. A simple method for counting adherent cells: application to cultured human monocytes, macrophages and multinucleated giant cells. J Immunol Methods. 1983;56:261–268. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- 15.Newman S L, Tucci M A. Regulation of human monocyte/macrophage function by extracellular matrix. Adherence of monocytes to collagen matrices enhances phagocytosis of opsonized bacteria by activation of complement receptors and enhancement of Fc receptor function. J Clin Invest. 1990;86:703–714. doi: 10.1172/JCI114766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ofek I, Goldhar J, Keisari Y, Sharon N. Nonopsonic phagocytosis of microorganisms. Annu Rev Microbiol. 1995;49:239–276. doi: 10.1146/annurev.mi.49.100195.001323. [DOI] [PubMed] [Google Scholar]
- 17.Pugin J, Heumann D, Tomasz A, Kravchenko V V, Akamatsu Y, Nishijima M, Glauser M P, Tobias P S, Ulevitch R J. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 18.Rook G A W, Steele J, Fraher L, Barker S, Karmali R, O’Riordan J. Vitamin D3, gamma interferon, and control proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57:159–163. [PMC free article] [PubMed] [Google Scholar]
- 19.Savedra R J, Delude R L, Ingalls R R, Fenton M J, Golenbock D T. Mycobacterial lipoarabinomannan recognition requires a receptor that shares components of the endotoxin signalling system. J Immunol. 1996;157:2549–2554. [PubMed] [Google Scholar]
- 20.Schlesinger L S. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- 21.Schlesinger L S. Mycobacterium tuberculosis and the complement system. Trends Microbiol. 1998;6:47–49. doi: 10.1016/S0966-842X(97)01203-1. [DOI] [PubMed] [Google Scholar]
- 22.Schlesinger L S, Bellinger-Kawahara C G, Payne N R, Horwitz M A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- 23.Schlesinger L S, Horwitz M A. Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and IFN-g activation inhibits complement receptor function and phagocytosis of this bacterium. J Immunol. 1991;147:1983–1994. [PubMed] [Google Scholar]
- 24.Schlesinger L S, Hull S R, Kaufman T M. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–4079. [PubMed] [Google Scholar]
- 25.Stokes R W, Haidl I D, Jefferies W A, Speert D P. Mycobacteria-macrophage interactions. Macrophage phenotype determines the nonopsonic binding of Mycobacterium tuberculosis to murine macrophages. J Immunol. 1993;151:7067–7076. [PubMed] [Google Scholar]
- 26.Stokes R W, Speert D P. Lipoarabinomannan inhibits nonopsonic binding of Mycobacterium tuberculosis to murine macrophages. J Immunol. 1995;155:1361–1369. [PubMed] [Google Scholar]
- 27.Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner J J. Enhanced production of TGF-b by blood monocytes from patients with active tuberculosis and presence of TGF-b in tuberculous granulomatous lung lesions. J Immunol. 1995;154:465–473. [PubMed] [Google Scholar]
- 28.Venisse A, Fournié J, Puzo G. Mannosylated lipoarabinomannan interacts with phagocytes. Eur J Biochem. 1995;231:440–447. doi: 10.1111/j.1432-1033.1995.tb20717.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang P, Wu P, Siegel M I, Egan R W, Billah M M. IL-10 inhibits transcription of cytokine genes in human peripheral blood mononuclear cells. J Immunol. 1994;153:811–816. [PubMed] [Google Scholar]
- 30.Wilson J G, Murphy E E, Wong W W, Klickstein L B, Weis J H, Fearon D T. Identification of a restriction fragment length polymorphism by a CR1 cDNA that correlates with the number of CR1 on erythrocytes. J Exp Med. 1986;164:50–59. doi: 10.1084/jem.164.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youmans G P. Tuberculosis. W. B. Philadelphia, Pa: Saunders; 1979. [Google Scholar]
- 32.Zimmerli S, Edwards S, Ernst J D. Selective receptor blockade during phagocytosis does not alter the survival and growth of Mycobacterium tuberculosis in human macrophages. Am J Respir Cell Mol Biol. 1996;15:760–770. doi: 10.1165/ajrcmb.15.6.8969271. [DOI] [PubMed] [Google Scholar]