Abstract
Interactions between B cells and CD4+ T cells play a central role in the development of Type 1 Diabetes (T1D). Two helper cell subsets, follicular (Tfh) and peripheral (Tph) helper T cells, are increased in patients with T1D but their role in driving B cell autoimmunity is undefined. We used a personalized immune (PI) mouse model to generate human immune systems de novo from hematopoietic stem cells (HSCs) of patients with T1D or from healthy controls (HCs). Both groups developed Tfh and Tph-like cells, and those with T1D-derived immune systems demonstrated increased numbers of Tph-like and Tfh cells compared to HC-derived PI mice. T1D-derived immune systems included increased proportions of unconventional memory CD27−IgD− B cells and reduced proportions of naïve B cells compared to HC PI mice, resembling changes reported for patients with systemic lupus erythematosus. Our findings suggest that T1D HSCs are genetically programmed to produce increased proportions of T cells that promote the development of unconventional, possibly autoreactive memory B cells. PI mice provide an avenue for further understanding of the immune abnormalities that drive autoantibody pathogenesis and T1D.
Keywords: Type 1 diabetes, T helper follicular cell, T peripheral helper cell, B cell, Autoreactive, Autoantibodies, Personalized immune mice, Human immune system mice, Hematopoietic stem cells, Autoimmunity
1. Introduction
Type 1 Diabetes (T1D) is a T cell-driven autoimmune disorder that includes a likely role for B cells, either as antigen-presenting cells to T cells or by producing autoantibodies [1-3]. Self-reactive B cells in T1D patients demonstrate failure to achieve self-tolerance already in the bone marrow and produce autoantibodies due to perturbations in the germinal center response [2-4]. B cell maturation into autoantibody producing plasma cells largely requires cognate CD4+ T cell help within the germinal center and the inflammatory site [5,6]. Such CD4+ T helper cells include follicular (Tfh) and peripheral (Tph) helper T cells [7,8], which express ICOS, PD-1, IL-21, CXCL13 and MAF. Tfh cells act in the germinal center and express CXCR5, while Tph cells act in the inflammatory site and express CCR2, CCR5 and CXCR3 [7-9]. Alterations in these subsets have been observed in non-obese diabetic (NOD) mice [10,11] and circulating Tfh and Tph cells were increased close to the time of diagnosis of T1D in children with multiple autoantibodies and in children with established T1D [7,8]. However, the genetic versus environmental drivers of these abnormalities remain poorly defined.
The NOD mouse model shares many features with human T1D [12], but the relevance of this mouse model is limited due to species differences and the genetic heterogeneity of patients with T1D, which contrasts to the single, uniform genotype of inbred NOD mice. The analysis of early, genetically predetermined immunoregulatory abnormalities that drive T1D in humans is a challenging task [13]. We have developed a unique human immune system (HIS) mouse model that allows the study of human immune development from adult hematopoietic stem cells (HSCs). In this “Personalized Immune (PI)” mouse model, NSG mice are reconstituted with fetal human thymus tissues and partially HLA-matched CD34+ cells from adult bone marrow (BM) donors, permitting the study of the role of genetic predispositions in driving human immunological abnormalities that result in T1D [14]. Using this model, we obtained evidence for defective central B-cell tolerance in PI mice derived from HSCs of T1D patients [15], recapitulating observations that have been made in T1D patients [16]. We also identified increased proportions of memory T cells in T1D-derived compared to healthy control (HC)-derived PI mice [14].
Here, we demonstrate increased numbers of the Tfh and Tph-like memory cell subsets in T1D compared to HC HSC-derived PI mice, demonstrating an additional recapitulation of immunological abnormalities described in patients with T1D [7,8]. Tfh and Tph-like cells expressed markers associated with B cell helper function and inflammatory activity, including ICOS, CXCR3, and CCR2, and increased proportions of unconventional memory CD27−IgD− B cells were detected. These results suggest that abnormalities in Tfh and Tph-like cells of T1D patients that are genetically programmed may drive the development of autoreactive/unconventional B cells in this disease.
2. Materials and methods
2.1. Animals
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) pig cytokine transgenic (pct) mice [17] that produce porcine hematopoietic cytokines (interleukin-3, granulocyte-macrophage colony-stimulating factor and stem cell factor; see Supplemental Materials online) were obtained from The Jackson Laboratory and bred in our animal facility.
2.2. Human tissue transplantation and generation of PI mice
Six- to twelve-week-old female mice were sub-lethally irradiated (1.2 Gy) with an X-irradiator (RS-2000, Rad Source Technologies, Inc.). After mechanical agitation to dislodge residual thymocytes, 1 mm3 thawed human fetal thymus fragments were implanted beneath the kidney capsule. One to 2.6 × 105 adult BM CD34+ cells were injected IV as described [14]. 400 μg of anti-human CD2 monoclonal antibody Lo-CD2 (produced at Bio X Cell, Inc) were injected intraperitoneally [14], on days 0, 7, and 14. Human HC and T1D BM donors shared T1D-associated class II HLA DR3, DR4, and/or DQ8 alleles. Information relative to the donors' HLA-type is provided in Supplementary Table 1.
2.3. Flow cytometry
Human T and B cell subsets were quantified in single cell suspensions of recipient splenocytes 20 weeks post-transplantation. Cells were stained using the fluorochrome-labeled mAbs listed in the online Supplemental Material. Flow cytometry was performed on an Aurora Cytek (Fremont, California) and analyzed with FlowJo (Tree Star, USA) software.
2.4. ELISA of IgM and IgG concentration
Enzyme-linked immunosorbent (ELISA) assays were used to quantify human antibody levels in serum of PI mice as indicated in the online Supplementary Material.
2.5. Statistical analysis
Statistical analyses and comparisons were performed with Graph-Pad Prism 8.0 (GraphPad Software). All data are expressed as average ± standard error of mean. Non-parametric tests were used for analysis. Comparisons between two groups were performed using Mann Whitney U test. Comparisons among matched values were performed using Friedman test with Dunn's post-test for multiple comparisons. Spearman's correlation coefficient was computed to study the strength of correlation between quantitative variables.
2.6. Data and resource availability
The data sets generated during or analyzed during the current study are available from the corresponding author upon reasonable request.
3. Results
3.1. Detection and phenotypic characterization of Tfh and Tph-like cells in PI mice
To analyze the phenotype of memory T cells in PI mice, we investigated the presence of Tfh and Tph-like cells (T cell gating strategy in Supplementary Fig. 1A). We stained splenocytes with CXCR5 and PD-1 to identify PD-1+CXCR5+ Tfh and PD-1+CXCR5− Tph-like cells (Supplementary Fig. 2A). We detected both Tfh and Tph-like cells among the memory CD4+CD45RA− T cell compartment from mice reconstituted with adult HSCs (Supplementary Fig. 2A) (Tfh cells: mean 2.35% ± 2.28 SD; Tph-like cells: mean 32.0 ± 14.96 SD; n = 16 PI mice). In contrast to CD45RA+CCR7+ naïve T cells, these cells expressed markers associated with B cell helper function and inflammatory function, including ICOS, CXCR3, and CCR2 (Supplementary Fig. 2B-D). The B helper function-associated costimulatory marker ICOS tended to be expressed at higher levels on Tfh cells compared to CD45RA+CCR7+ Tn cells, but expression levels on Tph-like did not achieve significant differences from Tn or Tfh (Supplementary Fig. 2B). Moreover, Tfh and Tph-like cells differed in the expression of inflammatory chemokine receptors, as Tph-like but not Tfh cells exhibited increased expression of CXCR3 and CCR2 compared to Tn cells (Supplementary Fig. 2C). In summary, these data demonstrate the presence of putative Tfh and Tph-like cells in the spleens of PI mice.
3.2. Increased splenic Tfh and Tph-like cells in T1D compared to HC immune systems
We previously demonstrated that mice reconstituted with HSCs from T1D patients did not develop insulitis or diabetes, despite robust immune reconstitution from BM HSCs [14]. However, we had detected an increased proportion of memory T cells in the peripheral blood of the T1D-derived compared to the HC PI mice, which had a predominantly naïve T cell phenotype [14]. Here, the percentages of memory T cells among CD4 cells tended to be higher in spleens of T1D-derived than HC-derived animals, but these differences did not achieve statistical significance (data not shown). While CD4+ T cell percentages were similar, total CD4+ T cell numbers were greater in T1D HSC-derived compared to HC HSC-derived spleens, reflecting increases in memory-type but not in naïve-type cells in T1D-PI mice (Fig. 1A).
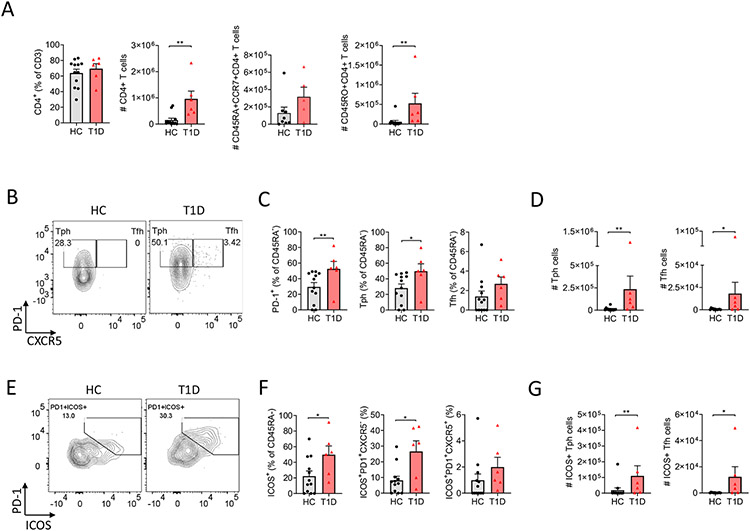
Fig. 1.
Analysis of memory CD4+ T cells, Tph-like and Tfh cells in spleens of T1D-derived PI mice. (A) CD4+ T cells and absolute counts of CD4+ T cells, CD45RA + CCR7+ T cells and CD45RO + CD4+ T cells from of HC (n = 12) and T1D (n = 6) PI mice of 4 independent experiments. (B) Representative staining for Tfh and Tph-like cells gated on memory CD4+ T cells. (C) Frequency of PD-1+, Tph-like and Tfh cells among memory CD4+ T cells. (D) Representative staining for double positive PD-1+ and ICOS+ within memory CD4+ T cells. (E) Frequencies of ICOS+, ICOS+PD1+ Tph-like and ICOS+PD1+ Tfh cells within memory CD4+ T cells. (F) absolute numbers for Tph-like and Tfh cells. (G) Absolute numbers of ICOS+ Tph-like and ICOS+ Tfh cells. For (A), Friedman test was performed and corrected using Dunn's multiple comparisons test. For (B-C-D-E-F-G), nonparametric Mann-Whitney test was used. Data are represented as mean ± SEM. *P < .05 and **P < .005 (statistically significant).
Circulating Tfh and Tph cells are increased in children at risk for T1D and in those with recent onset T1D [7,8]. To determine whether or not these differences were genetically programmed and HSC-intrinsic to T1D immune systems, we compared the frequencies of Tfh and Tph-like cells in the spleens of PI mice generated with T1D or HC HSC (n = 4 HC and 3 T1D donors, n = 12 HC mice and 6 TID mice). Increased percentages of CD45RA− CD4+ T cells expressed PD-1 (Fig. 1B-C) and ICOS (Fig. 1E-F) in T1D HSC-derived spleens compared to HC HSC-derived spleens, reflecting greater percentages and absolute numbers of Tph-like cells and greater numbers of Tfh cells in T1D compared to HC HSC-derived spleens (Fig. 1B-C-D) (Tph-like: mean 26.39% ± 18.45 SD for HC PI mice and 48.75% ± 23.07 SD for T1D PI mice; Tfh: mean 1.51% ± 1.99 SD for HC PI mice and 2.22% ± 1.82 for T1D PI mice). We also assessed ICOS expression as a marker of activated Tfh and Tph-like cells. Spleens from T1D HSC-derived mice also demonstrated increased percentages of total ICOS+ and ICOS+PD-1+ Tph-like and increased absolute numbers of ICOS+ Tph-like and Tfh cells compared to HC HSC-derived mice (Fig. 1E-F-G) (T cell gating strategy in Supplementary Fig. 2A; examples of staining for PD-1 and ICOS in Supplementary Fig. 3A). The frequencies and absolute numbers of Tfh cells correlated with those of Tph-like cells (Supplementary Fig. 3B and data not shown). Collectively, our data provide evidence for a genetically-determined tendency for CD4+ T cells derived from human T1D HSC to generate higher numbers of activated Tfh and Tph-like cells than HC HSCs.
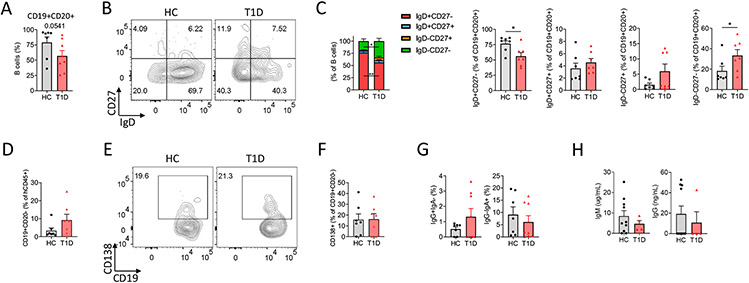
Fig. 2.
Characterization of B cell subsets in spleens of T1D and HC PI mice. (A) Frequency of total CD19+CD20+ B cells in HC (n = 7) and T1D (n = 8) PI mice from 2 independent experiments. (B) Representative staining and (C) aggregate summary of naïve CD27−IgD+, memoiy CD27+IgD−, unswitched CD27+IgD+ and unconventional memory CD27−IgD− B cells. (D) Frequency of CD19+CD20− cells among total human CD45+ cells. (E) Representative staining and (F) analysis of CD138+CD19+CD20− plasma cell frequencies. (G) Frequencies of IgG+IgA− and IgG−IgA+ B cells are shown. (H) Serum IgM and IgG levels in HC (n = 10) and T1D (n = 4) PI mice. Nonparametric Mann-Whitney test was used to calculate *P < .05 and **P < .005 (statistically significant). Data are represented as mean ± SEM.
3.3. Increased autoimmunity-associated splenic B cells in T1D immune systems
We assessed the B cell compartment for alterations that might be associated with the increased number of Tfh and Tph-like cells in T1D compared with HC PI mice (B cell gating strategy is provided in Supplementary Fig. 4A). No significant differences in the percentages of splenic B cells were detected (Fig. 2A) between the groups, consistent with our previous report, in which increased B cells were detected only in the peripheral blood and not the spleen of T1D compared to HC HSC-derived PI mice [15]. However, splenic B cells of T1D PI mice contained reduced proportions of naïve B cells and increased proportions of unconventional memory CD27−IgD− B cells compared to HC PI mice (Fig. 2B-C).
Activated B cells differentiate from naïve B cells to antibody-secreting cells (ASC), including short-lived plasmablasts (PB) and non-proliferating plasma cells (PC), which can produce autoantibodies. While there was a trend toward increased CD19+CD20− cells in T1D compared to HC HSC-derived PI mice (Fig. 2D), this did not achieve significance. There were no differences in the percentage of CD138+ PCs between HC and T1D PI mice (Fig. 2E-F). Variable proportions of T1D and HC PI mouse CD19+CD20+ B cells expressed IgG and IgA (Fig. 2G), indicating that class-switching occurred in both groups. IgM and IgG could be detected in sera of some T1D and HC PI mice (IgG antibodies deteceted in 1/4 T1D PI mice and 4/10 HC PI mice), but there were no significant differences between the two groups (Fig. 2H), consistent with our previous report [15].
4. Discussion
We report here that PI mice reconstituted with adult bone marrow HSCs and fetal thymic grafts develop B-helper Tfh and Tph-like cells in the spleen. This study provides novel evidence for the maturation of naïve CD4 T cells to the Tfh and Tph-like stage in human immune system mice. The Tfh and Tph-like cells appear to be functional, as B cell class switching and IgG production was evident in the mice. Importantly, we provide evidence that T1D patient HSCs are genetically programmed to enhance or accelerate the development of these B cell helper CD4+ T cell subsets, providing novel insight into the underlying autoimmune processes that lead to autoantibody production and T1D pathogenesis.
T-cell help for B cells is essential for class-switched high-affinity antibody responses and B-cell memory. Human B cells from several types of HIS mice have been reported to go through the first stages of development from pro-B, through pre-B and to immature B cells in the bone marrow [18]. However, human B cells in HIS mice have shown severe limitations in class switching and affinity maturation in response to pathogens or immunization with antigens [19,20]. B cells in HIS mice have been reported to be predominantly immature and at best transitional, with a high percentage expressing CD5 and CD10 [20,21]. A role for T cells in promoting B cell maturation was demonstrated in HIS mice constructed from cord blood (CB) CD34+ cells, in which the development or adoptive transfer of autologous CB T cells produced this effect [22]. In HIS mice constructed with human fetal thymus and fetal liver HSCs, long-term human cell reconstitution (greater than 20 weeks, when T cell reconstitution was well-established) was associated with development of mature follicular B cells [19]. The robust HLA-restricted T cell reconstitution provided by the combination of human thymus tissue and partly HLA-matched adult BM HSCs may enhance the maturation of B cells that coexist long-term with the autologous T cells.
Tfh and Tph can promote B cell differentiation in an IL-21-dependent manner, and expansions of both cell types have been detected in peripheral blood in rheumatoid arthritis [9,23], systemic lupus erythematosus [24,25] and T1D [7,8]. Our results demonstrate that mice reconstituted with BM from T1D donors have greater absolute numbers of CD4+ T cells, memory CD4+ cells, Tfh and Tph cells compared to those reconstituted with HC-derived BM. Of note, the T1D BM-derived PI mice do not develop diabetes and we have not detected infiltration of human T immune cells in the murine islets of these mice [14], demonstrating that the increased Tfh/Tph phenotype is not a consequence of T1D. Consistently, the expansion of Tfh and Tph-like cells precedes disease development in humans destined to develop T1D [7,8].
Unconventional memory B cell frequency was increased, while the frequency of naïve B cells was reduced in spleens of T1D HSC-derived mice compared to those reconstituted with HC HSCs. It has been suggested that unconventional memory CD27−IgD− B cells can be either exhausted memory B cells that downregulate CD27, or are the product of a defective germinal center reaction in which CD27, a marker of memory B cells, fails to upregulate appropriately [26]. B cells in patients with systemic lupus erythematosus [27] and rheumatoid arthritis [28] show downregulation of CD27 and IgD expression. B cells lacking CD27 and IgD can be detected in heathy individuals at low levels within peripheral blood and tonsils [27,29] but are expanded in peripheral blood of elderly patients [29].
We previously reported that T1D HSC-derived PI mice generated increased numbers of polyreactive/autoreactive B cell clones (reactive against double-stranded DNA, insulin, and lipopolysaccharide) compared to HC HSC-derived PI mice, indicating defects in central B-cell tolerance [15], and recapitulating a defect reported in patients with T1D [30] and other autoimmune diseases [31]. It is possible that these cells differentiate into the unconventional memory B cells that are enriched in PI mice. Studies in NOD mice indicate that autoreactive B cells escape anergy and migrate from the peripheral blood into pancreatic lymph nodes, where they present self-antigens to CD4+ helper T cells [32,33] and a similar pathway may contribute to the increased Tfh and Tph expressing markers of B helper function, including PD-1 [34] and ICOS [35], in T1D- compared to HC HSC-derived PI mice.
The frequencies of splenic Tph-like cells correlated with those of splenic Tfh cells in T1D and HC PI mice, suggesting a common developmental relationship between these two CD4+ T cell subsets. Indeed, it has been suggested that Tph may be precursors of Tfh [36], and T cell receptor and RNA-seq analyses of T cells from lymph nodes of patients with HIV previously demonstrated that Tph are clonally related to Tfh cells and exhibit a common epigenetic phenotype [37]. High dimensional analyses of circulating Tph and Tfh cells from individuals with rheumatoid arthritis [9] and T1D [7] have identified shared markers associated with B cell helper function e.g., PD-1, ICOS, IL-21, and distinct migratory features, e.g., expression of CXCR5 for Tfh cells, and CCR2 for Tph cells, supporting the hypothesis that Tph and Tfh cells may be clonally related.
A limitation of our research is that it is mostly observational and does not focus on islet antigen-specific T cells, which are thought to be involved in T1D pathogenesis and the production of islet autoantibodies [38,39]. Kenefeck et al., have demonstrated that islet-specific conventional T cells from pancreatic lymph nodes of DO1110 RIP-mOVA mice have a typical Tfh gene signature, with elevated levels of CXCR5, PD-1 and IL-21 [40]. Thus, future studies will need to address the direct role of human islet-specific Tph and Tfh cells and their impact on peripheral B cell tolerance in T1D PI mice. Our insulin-reactive TCR transgenic model [41,42] could be useful in dissecting this pathway.
Taken together, this study demonstrates that PI mice provide an avenue to study the role that Tfh and Tph cells play in human autoimmune diseases such as T1D prior to disease onset. More precisely, it: (a) provides the first evidence for the presence of splenic Tfh and Tph-like cells in HIS mice; (b) shows that Tph-like and Tfh cells are increased in TID immune systems in an HSC-intrinsic manner that is likely genetically-driven; (c) shows that there is an abnormal distribution of B cell subsets with an increased autoimmunity-associated phenotype in T1D immune systems and that these changes are HSC-intrinsic and likely genetically-driven. These results demonstrate the power of the PI mouse model to manifest genetically-predetermined immunoregulatory abnormalities in their de novo-developed immune systems, thereby providing novel opportunities to unravel the pathways by which pathological interactions between B cells, CD4+ T helper cells and other human immune cells can drive disease development.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health (NIH) grants #P01AI045897, #UC4DK104207, #U01DK123559, #R01DK103585 and NIAID #R21AI14682802. These studies used the resources of the Diabetes Research Center Flow Core Facility funded in part through Center Grant #P30DK063608, including LSRII instrument acquired with support from award #S10RR027050.
Dr. Megan Sykes is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Declaration of Competing Interest
The authors declare that they have no conflict of interests.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2022.109048.
References
- [1].Atkinson MA, Eisenbarth GS, Michels AW, Type 1 diabetes, Lancet 383 (2014) 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fousteri G, Ippolito E, Ahmed R, Hamad A. Rahim, Beta-cell specific autoantibodies: are they just an indicator of type 1 diabetes? Curr. Diabetes Rev 13 (2017) 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hinman RM, Cambier JC, Role of B lymphocytes in the pathogenesis of type 1 diabetes, Curr. Diabet. Rep 14 (2014) 1–7. [DOI] [PubMed] [Google Scholar]
- [4].Jeker LT, Bour-Jordan H, Bluestone JA, Breakdown in peripheral tolerance in type 1 diabetes in mice and humans, Cold Spring Harb. Perspect Med 2 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Suurmond J, Diamond B, Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity, J. Clin. Invest 1–5 (2015) 2194–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rubin SJS, Bloom MS, Robinson WH, B cell checkpoints in autoimmune rheumatic diseases. Nature reviews, Rheumatology. 15 (2019) 303–315. [DOI] [PubMed] [Google Scholar]
- [7].Ekman I, Ihantola EL, Viisanen T, Rao DA, Näntö-Salonen K, Knip M, et al. , Circulating CXCR5–PD-1hi peripheral T helper cells are associated with progression to type 1 diabetes, Diabetologia. 62 (2019) 1681–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Viisanen T, Ihantola EL, Näntö-Salonen K, Hyöty H, Nurminen N, Selvenius J, et al. , Circulating CXCR5+PD-1+ICOS+follicular T helper cells are increased close to the diagnosis of type 1 diabetes in children with multiple autoantibodies, Diabetes. 66 (2017) 437–447. [DOI] [PubMed] [Google Scholar]
- [9].Rao DA, Gurish MF, Marshall JL, Slowikowski K, Fonseka CY, Liu Y, et al. , Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis, Nature. 542 (2017) 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vecchione A, Di Fonte R, Gerosa J, Jofra T, Cicalese MP, Napoleone V, et al. , Reduced PD-1 expression on circulating follicular and conventional FOXP3+ Treg cells in children with new onset type 1 diabetes and autoantibody-positive at-risk children, Clin. Immunol 211 (2020). [DOI] [PubMed] [Google Scholar]
- [11].Xu X, Shen M, Zhao R, Cai Y, Jiang H, Shen Z, et al. , Follicular regulatory T cells are associated with β-cell autoimmunity and the development of type 1 diabetes, J. Clin. Endocrinol. Metab 104 (September) (2019) 4199–4213. [DOI] [PubMed] [Google Scholar]
- [12].Chen YG, Mathews CE, Driver JP, The role of NOD mice in type 1 diabetes research: lessons from the past and recommendations for the future, Front. Endocrinol. (Lausanne) 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zeng D, Bridge between type 1 diabetes in mouse and man, Proc. Natl. Acad. Sci. U. S. A 114 (2017) 10821–10823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kalscheuer H, Danzl N, Onoe T, Faust T, Winchester R, Goland R, et al. , A model for personalized in vivo analysis of human immune responsiveness, Sci. Transl. Med 4 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Borsotti C, Danzl NM, Nauman G, Hölzl MA, French C, Chavez E, et al. , HSC extrinsic sex-related and intrinsic autoimmune disease–related human B-cell variation is recapitulated in humanized mice, Blood Adv. 1 (2017) 2007–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Menard L, Saadoun D, Isnardi I, Ng YS, Meyers G, Massad C, et al. , The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans, J. Clin. Invest 121 (2011) 3635–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen AM, Zhou Y, Swenson K, Sachs DH, Sykes M, Yang YG, Porcine stem cell engraftment and seeding of murine thymus with class II+ cells in mice expressing porcine cytokines: toward tolerance induction across discordant xenogeneic barriers, Transplantation. 69 (2000) 2484–2490. [DOI] [PubMed] [Google Scholar]
- [18].Manz MG, Di Santo JP, Renaissance for mouse models of human hematopoiesis and immunobiology, Nat. Immunol 10 (2009) 1039–1042. [DOI] [PubMed] [Google Scholar]
- [19].Seung E, Tager AM, Humoral immunity in humanized mice: a work in progress, J. Infect. Dis 208 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vuyyuru R, Patton J, Manser T, Human immune system mice: current potential and limitations for translational research on human antibody responses, Immunol. Res 51 (2011) 257–266. [DOI] [PubMed] [Google Scholar]
- [21].Chen Q, He F, Kwang J, Chan JKY, Chen J, GM-CSF and IL-4 stimulate antibody responses in humanized mice by promoting T, B, and dendritic cell maturation, J. Immunol 189 (2012) 5223–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lang J, Kelly M, Freed BM, McCarter MD, Kedl RM, Torres RM, et al. , Studies of lymphocyte reconstitution in a humanized mouse model reveal a requirement of T cells for human B cell maturation, J. Immunol 190 (2013) 2090–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ma J, Zhu C, Ma B, Tian J, Baidoo SE, Mao C, et al. , Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis, Clin. Dev. Immunol 2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bocharnikov AV, Keegan J, Wacleche VS, Cao Y, Fonseka CY, Wang G, et al. , PD-1hiCXCR5- T peripheral helper cells promote B cell responses in lupus via MAF and IL-21, JCI Insight. 4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Choi JY, Ho JHE, Pasoto SG, Bunin V, Kim ST, Carrasco S, et al. , Circulating follicular helper-like T cells in systemic lupus erythematosus: association with disease activity, Arthritis Rheum. 67 (2015) 988–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Centuori SM, Gomes CJ, Kim SS, Putnam CW, Larsen BT, Garland LL, et al. , Double-negative (CD27 - IgD - ) B cells are expanded in NSCLC and inversely correlate with affinity-matured B cell populations, J. Transl. Med 16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, et al. , A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus, J. Immunol 178 (2007) 6624–6633. [DOI] [PubMed] [Google Scholar]
- [28].Mahmood Z, Muhammad K, Schmalzing M, Roll P, Dörner T, Tony HP, CD27-IgD- memory B cells are modulated by in vivo interleukin-6 receptor (IL-6R) blockade in rheumatoid arthritis, Arthritis Res. Ther 17 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Colonna-Romano G, Aquino A, Bulati M, Di Lorenzo G, Listì F, Vitello S, et al. , Memory B cell subpopulations in the aged, in: Rejuvenation Research, 2006. [DOI] [PubMed] [Google Scholar]
- [30].Chamberlain N, Massad C, Oe T, Cantaert T, Herold KC, Meffre E, Rituximab does not reset defective early B cell tolerance checkpoints, J. Clin. Invest 126 (2016) 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kinnunen T, Chamberlain N, Morbach H, Cantaert T, Lynch M, Preston-Hurlburt P, et al. , Specific peripheral B cell tolerance defects in patients with multiple sclerosis, J. Clin. Invest 123 (2013) 2737–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Noorchashm H, Lieu YK, Noorchashm N, Rostami SY, Greeley SA, Schlachterman A, et al. , I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice, J. Immunol 163 (1999) 743–750. [PubMed] [Google Scholar]
- [33].Silveira PA, Johnson E, Chapman HD, Bui T, Tisch RM, Serreze DV, The preferential ability of B lymphocytes to act as diabetogenic APC in NOD mice depends on expression of self-antigen-specific immunoglobulin receptors, Eur. J. Immunol 32 (2002) 3657–3666. [DOI] [PubMed] [Google Scholar]
- [34].Shi J, Hou S, Fang Q, Liu X, Liu X, Qi H, PD-1 controls follicular T helper cell positioning and function, Immunity. 49 (2018) 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Weber JP, Fuhrmann F, Feist RK, Lahmann A, Al Baz MS, Gentz LJ, et al. , ICOS maintains the T follicular helper cell phenotype by down-regulating krüppel-like factor 2, J. Exp. Med 212 (2015) 217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rao DA, T cells that help B cells in chronically inflamed tissues, Front. Immunol 9 (August) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Del Alcazar D, Wang Y, He C, Wendel BS, Del Río-Estrada PM, Lin J, et al. , Mapping the lineage relationship between CXCR5+ and CXCR5− CD4+ T cells in HIV-infected human lymph nodes, Cell Rep. 28 (2019) 3047–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Babon JAB, Denicola ME, Blodgett DM, Crèvecoeur I, Buttrick TS, Maehr R, et al. , Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes, Nat. Med 22 (2016) 1482–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Michels AW, Landry LG, McDaniel KA, Yu L, Campbell-Thompson M, Kwok WW, et al. , Islet-derived CD4 T cells targeting proinsulin in human autoimmune diabetes, Diabetes. 66 (2017) 722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kenefeck R, Wang CJ, Kapadi T, Wardzinski L, Attridge K, Clough LE, et al. , Follicular helper T cell signature in type 1 diabetes, J. Clin. Invest 125 (2015) 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tan S, Li Y, Xia J, Jin CH, Hu Z, Duinkerken G, et al. , Type 1 diabetes induction in humanized mice, Proc. Natl. Acad. Sci. U. S. A 114 (2017) 10954–10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Madley R, Nauman G, Danzl N, Borsotti C, Khosravi Maharlooei M, Li HW, et al. , Negative selection of human T cells recognizing a naturally-expressed tissue-restricted antigen in the human thymus, J. Transl. Autoimmun 3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.