Abstract
A whole-blood model was used to evaluate the effects of temperature and anticoagulant on the expression of activation markers HLA-DR and CD11b on peripheral leukocytes. Venous blood, anticoagulated with either EDTA or heparin, was obtained from six healthy blood donors and 13 hospitalized patients (8 human immunodeficiency virus type 1-seropositive individuals with concurrent pulmonary tuberculosis and 5 patients with pneumonia). A preliminary evaluation was carried out with whole blood from two of the normal donors, and cells were stained immediately for HLA-DR and CD11b markers or stained after incubation at room temperature or 37°C for 18 h with or without the addition of the cytokines gamma interferon (IFN-γ), granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ plus GM-CSF, tumor necrosis factor beta, or interleukin-6. Of the cytokines tested, the combination of IFN-γ and GM-CSF had the most pronounced modulation of marker expression on polymorphonuclear neutrophils (PMN), in particular, HLA-DR expression, which required induction for its detection. These cytokines were therefore used in further evaluations that considered the above-mentioned effects in the presence of disease. Results indicated that the expression of activation markers on PMN and lymphocytes in whole blood are influenced by the temperature of incubation and the choice of anticoagulant and the effects noted were dependent on (i) the particular cell surface marker, (ii) the cell type being studied, and (iii) the presence or absence of disease. It is therefore recommended that ex vivo whole-blood models for evaluating phenotype or immune function be carefully evaluated for the above-mentioned effects.
Polymorphonuclear neutrophils (PMN) are the first cells to migrate to the site of infection in response to invading pathogens. The interaction of PMN with cells and antigens is mediated by an important group of adhesion molecules, belonging to the integrin family, known as the CD11-CD18 antigen complex. These molecules are composed of three heterodimers with a common β-subunit (CD18) noncovalently linked to each of three α-subunits, CD11a (also known as LFA-1), CD11b (also known as MAC-1 or CR3), and CD11c (also known as CR4). These receptors contribute to PMN functions such as chemotaxis, adhesion to endothelium, phagocytosis, and antibody-dependent cellular cytotoxicity (for a review, see reference 2). Significant intracellular pools of CD11b-CD18 are present in the secondary and tertiary granules in circulating PMN; therefore, they can increase their surface expression of CD11b within minutes by severalfold upon activation.
Mature PMN were originally thought to play only a passive role in inflammation, through phagocytosis and the release of cytotoxic compounds (26). It was subsequently shown, however, that these cells have the ability to synthesize a number of proteins—including surface receptors CR1 (32, 33), CR3 (33), and FcR (22, 36, 37)—and a number of cytokines—such as interleukin-1 (IL-1) (28, 41), IL-6 (6, 31), IL-8 (5, 40), transforming growth factor β1 (12, 18), and tumor necrosis factor alpha (TNF-α) (9, 20). Furthermore, they can be induced to express class II molecules by granulocyte-macrophage colony-stimulating factor (GM-CSF), gamma interferon (IFN-γ), and IL-3 (17). In addition to possessing protein-synthesizing capacity, PMN are also responsive to a number of cytokines. Cytokines such as GM-CSF can have both direct and potentiating effects on neutrophil function, while others such as IFN-γ, TNF, and IL-6 enhance responses to other stimuli, a process called priming. GM-CSF enhances antibody-dependent cellular cytotoxicity (27, 42), primes PMN for chemotaxis and respiratory burst in response to formyl-Met-Leu-Phe (44), and increases phagocytosis of bacteria (14). Its direct effects include the induction of other cytokines by PMN (6, 24, 25) and inhibition of PMN migration (16). IFN-γ upregulates FcγRI in PMN (35, 36), while IL-6 primes PMN for the generation of oxygen radicals by formyl-Met-Leu-Phe (21).
Data from a number of studies have shown that antigen expression on the surface of PMN is easily altered by procedures used for their purification (4, 13, 43). The whole-blood model therefore presents an ideal system for the analysis of cell phenotype and immune function. Current knowledge on the effects of anticoagulants, temperature, and cytokines on cell surface antigen expression in whole blood is limited. Of interest to us has been the effect of human immunodeficiency virus type 1 (HIV-1) disease and pulmonary tuberculosis on PMN phenotype and function (29, 30, 38), and we have utilized both whole blood and isolated PMN, depending on the analysis in question. It was, in part, through studies of this nature that we noticed the effects of anticoagulants and standing time on the modulation of particular markers. The present study therefore compares the effects of both the temperature of incubation and the choice of anticoagulant on the surface expression of two activation markers, HLA-DR and CD11b, on whole-blood PMN in the presence of cytokines known to prime PMN function. Furthermore, in order to determine if the effects found were cell type specific, we included for comparison the evaluation of these same markers on whole-blood lymphocytes. These evaluations were carried out in both the presence and absence of disease.
MATERIALS AND METHODS
Reagents.
Recombinant cytokines IFN-γ, GM-CSF, TNF-β, and IL-6 were obtained from Boehringer Mannheim (Mannheim, Germany). Phycoerythrin (PE)-conjugated mouse antibodies against human HLA-DR and CD11b, mouse isotype control antibody immunoglobulin G2a-PE, fluorescein isothiocyanate (FITC)-conjugated mouse anti-CD14 (CD14-FITC), and FACS lysing solution (10× concentrate) were from Becton Dickinson (San Jose, Calif.). The cell fixative was 1.5% (vol/vol) formaldehyde (Merck, Darmstadt, Germany) with 2% (wt/vol) bovine serum albumin (Sigma Chemical Co., St. Louis, Mo.).
Study subjects.
Six healthy volunteers (normal donors [ND]) and 13 individuals hospitalized with either pneumonia (n = 5) or coinfection with HIV-1 and Mycobacterium tuberculosis (n = 8) were recruited for whole-blood evaluations.
Whole-blood cultures.
Venous blood was collected into 5-ml glass Vacutainer tubes (12 by 75 mm) (Becton Dickinson) containing either 71.5 USP units of heparin or 15% (wt/vol) EDTA. The blood was dispensed in 150-μl aliquots into Falcon polystyrene tubes (12 by 75 mm) and incubated at room temperature (RT) and 37°C for 18 h with or without the addition of cytokines. A range of cytokines were added to whole-blood aliquots from two of the ND: IFN-γ (100 U/ml), GM-CSF (100 U/ml), IFN-γ plus GM-CSF (100 U/ml each), TNF-β (2.5 ng/ml), and IL-6 (100 U/ml). Whole blood from the 6 ND and 13 diseased patients were treated as described above but only stimulated with a combination of IFN-γ and GM-CSF. Untreated controls received phosphate-buffered saline (PBS). One sample of each treatment was stained with antibody immediately, providing a baseline (time zero) control.
Flow cytometric analysis of whole-blood leukocytes.
Two-color staining was performed on 50 μl of each of the whole-blood samples with 5 μl of immunoglobulin G2a-PE, HLA-DR–PE, or CD11b-PE together with 5 μl of CD14-FITC and incubating at RT for 20 min. Erythrocytes were lysed with FACS lysing solution, washed with PBS, and resuspended in 200 μl of cell fixative. A Becton Dickinson FACSort with a 488-nm-wavelength argon laser was used for all analyses. The instrument was calibrated and standardized between each analysis by using Calibrite beads (Becton Dickinson). Granulocyte and lymphocyte populations were identified based on their forward and side scatter properties, and monocytes were excluded by gating out high-CD14-fluorescing cells. The data were analyzed by using Cellquest software (version 1.0; Becton Dickinson) and expressed as the percentage of cells expressing the particular antigen (cursor M1 was placed on the isotype control antibody fluorescence histogram such that <2% fluorescence occurred to its right; marker-specific fluorescence was measured as the shift in fluorescence within M1 as set on the control, minus the percentage value obtained for the control) and the fluorescence intensity or mean channel shift (mean channel number of the sample fluorescence minus the mean channel number of the isotype control antibody).
Statistical analysis.
Differences between treatments in the ND group (n = 6) and diseased group (n = 13) were determined by the nonparametric Mann-Whitney U test (Statgraphics software; STSC, Inc., Rockville, Md.).
RESULTS
Effects of incubation temperature and cytokines on HLA-DR expression on peripheral leukocytes.
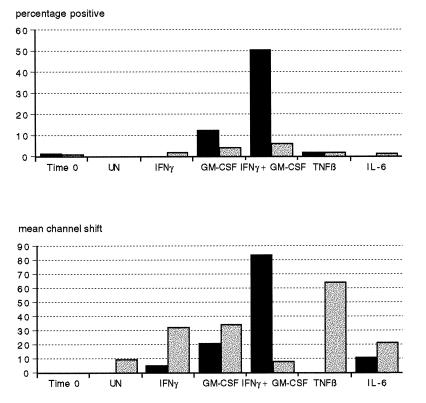
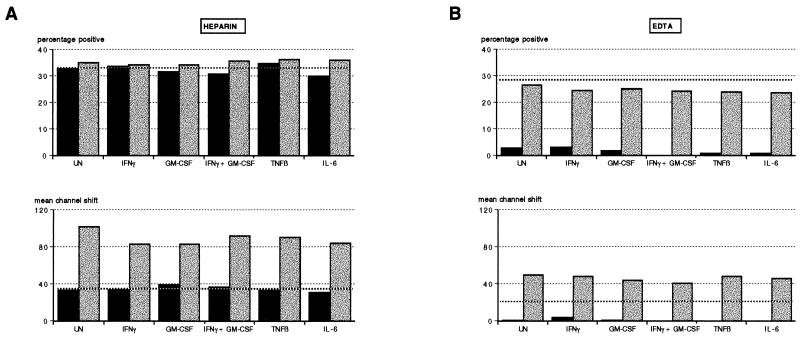
Whole-blood cultures from two donors were incubated for 18 h at RT and 37°C in the presence of cytokines IFN-γ, GM-CSF, IFN-γ plus GM-CSF, TNF-β, and IL-6. There was no modulation of HLA-DR expression on PMN by any of the cytokines at RT (data not shown). Figure 1 shows the effect of incubation at 37°C in the presence of the various cytokines on the percentage of PMN expressing HLA-DR and on the shift in fluorescence intensity (mean channel shift). In the presence of heparin, there was an increase in HLA-DR expression owing to IFN-γ (fluorescence intensity only), to GM-CSF, and to a synergistic effect on both these cytokines. On the other hand, cytokine-treated EDTA cultures showed small but demonstrable increases in the proportions of HLA-DR-expressing PMN in the presence of these cytokines, with the magnitude of induction being donor dependent. Fluorescence intensity was, however, increased independently by IFN-γ and GM-CSF when used alone but not when these cytokines were added in combination. TNF-β and IL-6 did not alter the proportion of PMN expressing HLA-DR in heparin or EDTA cultures but did increase their intensity of fluorescence in the presence of EDTA.
FIG. 1.
Effects of anticoagulants and cytokines on HLA-DR expression on PMN. Heparin and EDTA whole-blood cultures (solid and stippled bars, respectively) were stained immediately (time zero) or incubated for 18 h at 37°C with either PBS (UN), IFN-γ, GM-CSF, IFN-γ plus GM-CSF, TNF-β, or IL-6 and then stained for HLA-DR as described in Materials and Methods. Results are representative of experiments using two donors and are expressed as the percentage of HLA-DR-positive cells and shift in fluorescence intensity (mean channel shift).
In heparin cultures incubated at 37°C, there was an increase in the percentage and mean channel shift of lymphocytes expressing HLA-DR (Fig. 2A) with further increases dependent on the particular cytokine added. In EDTA cultures (Fig. 2B) the opposite was true in that at 37°C there was a decrease in the intensity of HLA-DR fluorescence with most cytokines, with only marginal alterations in the proportion of HLA-DR-fluorescing lymphocytes. When heparin and EDTA cultures were left at RT for 18 h, with or without the addition of cytokines, fluorescence intensity was increased with only marginal changes in the percentage of positive cells (except IL-6, which showed a substantial increase).
FIG. 2.
Effects of temperature and cytokines on HLA-DR expression on lymphocytes in heparin (A) and EDTA (B) cultures. Whole-blood cultures were incubated at RT or 37°C (stippled and solid bars, respectively) with PBS (UN), IFN-γ, GM-CSF, IFN-γ plus GM-CSF, TNF-β, or IL-6 and then stained for HLA-DR as described in Materials and Methods. Results are representative of experiments using two donors. Values obtained for cultures stained immediately (time zero control) are shown for comparison by boldface dotted lines.
Effects of incubation temperature and cytokines on CD11b expression on peripheral leukocytes.
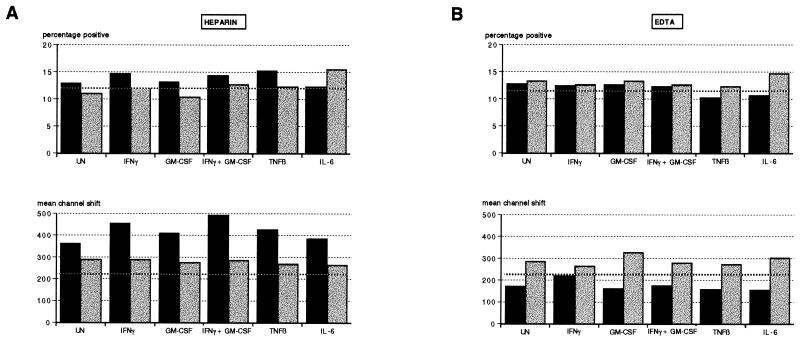
Incubation of cytokine-treated and -untreated blood samples at 37°C as opposed to RT decreased CD11b expression on PMN (Fig. 3). This effect was only marginal in heparin-anticoagulated samples, with a minor reduction in fluorescence intensity (Fig. 3B) and close to 100% of PMN expressing CD11b (Fig. 3A). The down-regulation at 37°C was, however, quite dramatic in EDTA-anticoagulated samples, evidenced as both a reduction in percentage of positive cells (Fig. 3A) and mean channel shift (Fig. 3B). This occurred despite the addition of cytokines known to upregulate CD11b expression on PMN. In the presence of heparin, however, the largest increase in CD11b expression was the result of the addition of both IFN-γ and GM-CSF (Fig. 3B).
FIG. 3.
Effect of incubation temperature on the percentage of PMN expressing CD11b (A) and mean channel shift (B) in cytokine-treated whole-blood cultures. Heparin and EDTA whole-blood cultures were incubated for 18 h at 37°C with either PBS (UN), IFN-γ, GM-CSF, IFN-γ plus GM-CSF, TNF-β, or IL-6 and then stained for CD11b as described in Materials and Methods. (A) The corresponding time zero controls are indicated by boldface dotted lines. Solid bars, 37°C; stippled bars, RT. (B) Vertical solid and dotted lines drawn through the histograms indicate the mean channel numbers corresponding to the untreated controls at 37°C and RT, respectively. For both panels, results are representative of experiments using two donors.
There was no substantial modulation of CD11b expression on lymphocytes at 37°C in heparin cultures (Fig. 4). Incubation at RT for 18 h, however, resulted in an increased intensity of CD11b fluorescence both in heparin and EDTA cultures, although there were small decreases relative to the time zero control in the proportion of positive CD11b lymphocytes in EDTA cultures (Fig. 4). As found for PMN, incubation at 37°C in the presence of EDTA resulted in a marked reduction in CD11b expression (Fig. 4B).
FIG. 4.
Effect of incubation temperature on percentage of CD11b-expressing lymphocytes and their mean channel shift determinations in heparin (A) and EDTA (B) cultures. Heparin and EDTA whole-blood cultures were treated with cytokines as described in the text and incubated at RT or 37°C (stippled and solid bars, respectively). The time zero controls are included for comparison and are indicated by boldface dotted lines.
Temperature and anticoagulant effects on marker expression on whole-blood PMN and lymphocytes in the presence of disease.
In order to determine if effects of anticoagulant and temperature were further altered due to disease, we carried out an evaluation similar to that described above, but compared results from 6 ND and 13 diseased patients. Only the addition of IFN-γ plus GM-CSF was used as a stimulant, as this combination showed the greatest modulation of marker expression, particularly, the expression of HLA-DR on PMN, which, as shown, requires induction for detectable expression. Fresh blood was obtained from the 6 healthy blood donors and from the 13 hospitalized patients, 5 of whom had pneumonia and 8 of whom were diagnosed as HIV-1 seropositive with concurrent pulmonary tuberculosis (HIV/TB patients). HLA-DR and CD11b expression on gated PMN and lymphocytes was determined immediately (time zero), and after 18 h of incubation at RT and at 37°C, and after stimulation in the presence of both IFN-γ and GM-CSF. As there were no significant differences in the expression of either marker between HIV/TB and pneumonia patients, the data were pooled to represent one disease group for comparison with the ND group.
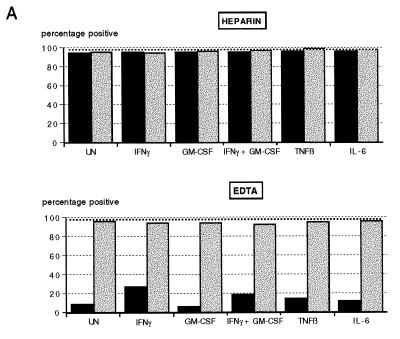
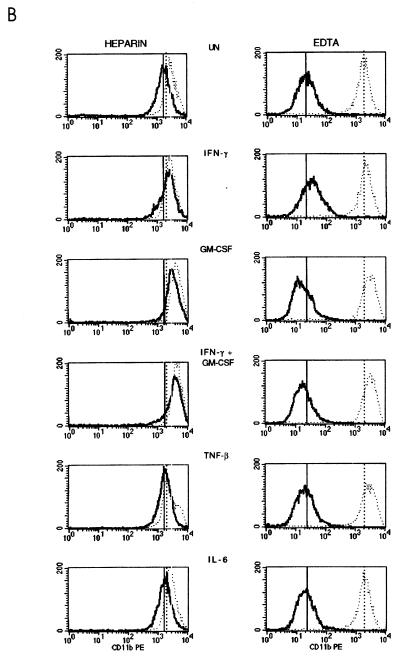
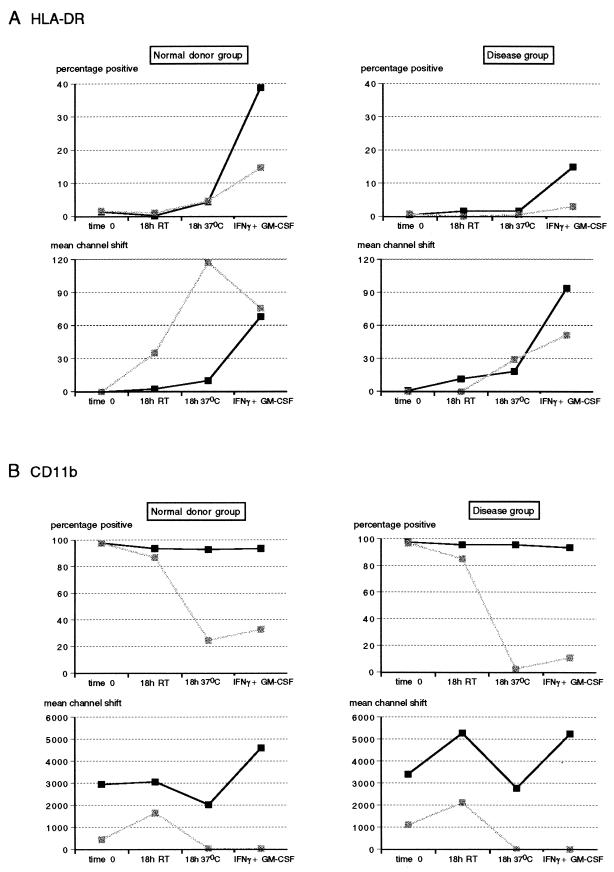
In the presence of EDTA there were no significant changes in percentage of HLA-DR expressing PMN with regard to the different treatments in either the ND group or the disease group (P > 0.05) (Fig. 5A). However, heparin-treated whole blood showed a significant increase in HLA-DR expression in the presence of IFN-γ plus GM-CSF above that of EDTA-anticoagulated blood in both the ND and disease groups (P < 0.05), with the ability to be induced, however, being less in the disease group. HLA-DR expression increased only when blood was incubated at 37°C, with a wide variation in the fluorescence intensities obtained in the presence of EDTA in the ND group (Fig. 5A). The median value obtained for EDTA cultures at 37°C, although severalfold higher, was therefore not significantly different from that obtained for the corresponding heparin cultures (P > 0.05). Overall, fluorescence intensities obtained in both groups showed no significant difference between the two anticoagulants with any of the treatments.
FIG. 5.
Effects of anticoagulants and temperature on HLA-DR (A) and CD11b (B) expression on whole-blood PMN from healthy blood donors (n = 6) and diseased individuals (n = 13). Whole-blood samples anticoagulated with either heparin or EDTA (solid and stippled squares, respectively) were stained for surface expression of HLA-DR and CD11b immediately (time zero), after an 18-h incubation at RT, and after an 18-h incubation at 37°C with or without the addition of IFN-γ plus GM-CSF. PMN were gated, and results are expressed as percentage of positive cells and fluorescence intensity (mean channel shift).
CD11b expression on PMN, on the other hand, was markedly reduced in the presence of EDTA (Fig. 5B). The proportion of CD11b-positive cells in heparin blood cultures remained close to 100% in both the ND and disease groups, irrespective of the incubation conditions. Although differences were small, a standing time of 18 h, whether at RT or 37°C, significantly reduced the proportion of CD11b-expressing PMN compared to that of blood being stained immediately (P < 0.05). Both the proportion of CD11b-positive PMN and their fluorescence intensities were significantly reduced in the presence of EDTA when blood was held for 18 h at RT or 37°C (P < 0.05). Although there was a trend toward decreased intensity of CD11b expression at time zero in EDTA-anticoagulated blood, this was only significant in the disease group (P < 0.001). Whereas stimulation in the presence of IFN-γ and GM-CSF did not significantly alter the proportion of CD11b-positive PMN (P > 0.05), there was a significant increase in the intensity of fluorescence in heparin cultures only (P < 0.01) in both ND and disease groups. Furthermore, CD11b expression in the disease group was more adversely affected by incubation at 37°C in EDTA than was that of the ND group.
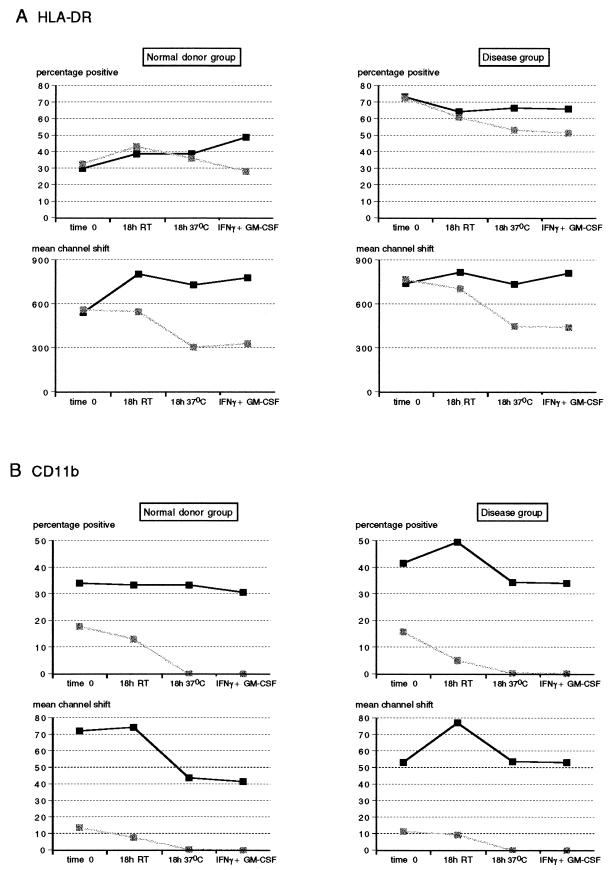
Compared to granulocytes, a greater proportion of lymphocytes expressed HLA-DR with no apparent modulation of this expression by cytokines IFN-γ and GM-CSF. In the disease group, HLA-DR expression on lymphocytes was significantly higher than that found for control individuals. There were no significant differences in the proportion of lymphocytes expressing HLA-DR (P > 0.05) in heparin and EDTA cultures or between any of the treatments (Fig. 6A). However, the intensity of HLA-DR expression was substantially reduced (P < 0.05) when cultures were incubated at the higher temperature in EDTA (Fig. 6A). In the disease group, there was a significant decrease in the proportion of lymphocytes expressing HLA-DR when EDTA cultures were held for 18 h, irrespective of incubation temperature (P < 0.05), compared to their time zero control, but these independently were not significantly different from the corresponding treatment in heparin cultures (P > 0.05). As for the ND group, mean channel shift values were significantly reduced in EDTA cultures at the higher incubation temperature relative to the corresponding values obtained for heparin cultures (P < 0.05).
FIG. 6.
Effects of anticoagulants and temperature on HLA-DR (A) and CD11b (B) expression on whole-blood lymphocytes from healthy blood donors (n = 6) and diseased individuals (n = 13). Whole-blood samples anticoagulated with either heparin or EDTA (solid and stippled squares, respectively) were stained for surface expression of HLA-DR and CD11b immediately (time zero), after an 18-h incubation at RT, and after an 18-h incubation at 37°C with or without the addition of IFN-γ plus GM-CSF. Lymphocytes were gated, and results are expressed as percentage of cells expressing HLA-DR and fluorescence intensity (mean channel shift).
As for PMN, CD11b expression on lymphocytes was markedly reduced in EDTA cultures as opposed to heparin cultures, with this effect being most pronounced at the elevated temperature (P < 0.05) (Fig. 6B). Whereas the percentage of CD11b-positive lymphocytes in the disease group was significantly greater than that in the ND group (P < 0.05), these differences were no longer apparent at the higher incubation temperature, showing a greater modulation of this marker in the presence of disease. On the other hand, the intensity of CD11b fluorescence decreased substantially in heparin cultures of the ND group when the incubation temperature was 37°C (P < 0.05). This was modulated differently in diseased patients in that CD11b fluorescence was significantly increased after standing at RT for 18 h (P < 0.05) but returned to mean channel shift values similar to time zero controls when incubated at 37°C. As for HLA-DR expression on lymphocytes, there was no modulation of CD11b expression by IFN-γ and GM-CSF (P > 0.05).
Both the proportion of HLA-DR-expressing lymphocytes and the intensity of their fluorescence were significantly higher than the corresponding values obtained for PMN, even though comparable proportions of PMN could be induced to express HLA-DR, but with low fluorescence intensity. The reciprocal situation occurred with CD11b expression, during which both proportions of CD11b-expressing cells and fluorescence intensities were significantly less in lymphocytes than in PMN. A substantially reduced ability to induce HLA-DR on the surface of PMN was noted in the disease group compared to the ND group, as was a significant increase in CD11b fluorescence intensity (P < 0.05). In addition, the proportions of both HLA-DR- and CD11b-expressing lymphocytes were significantly elevated in the presence of disease (P < 0.05).
DISCUSSION
Human whole blood has provided a convenient ex vivo model for the monitoring of leukocyte receptor expression (11, 23), cytokine production (1, 3, 7), and lipid mediator synthesis (15, 39), as well as for functional assays such as PMN oxidative burst (10, 11, 34, 38) and phagocytosis (34, 38).
In the present study, the expression of two antigens, HLA-DR and CD11b, on the surface of peripheral leukocytes was analyzed in whole-blood cultures. These two phenotypic markers were chosen for study for two reasons: (i) they are both activation markers and hence are much more sensitive to changes in blood that could occur ex vivo, and (ii) they are expressed very differently on PMN and lymphocytes and can therefore indicate if a particular compartment is differentially modulated. With the use of cytokines selected on the basis of their capacity to prime PMN responses, it was possible to show modulation of the respective activation markers owing to both the temperature of incubation and the choice of anticoagulant added. It has been reported by Gosselin et al. (17) that the capacity to express high levels of HLA-DR on PMN is donor dependent. Of the cytokines they tested, only GM-CSF, IFN-γ, and IL-3 induced class II expression on purified PMN. In this study, the greatest inducible increase of HLA-DR on PMN was in heparin-anticoagulated blood incubated in the presence of IFN-γ plus GM-CSF at 37°C. This occurred within 18 h, a period much shorter than the induction times required for isolated PMN, whose viability is well maintained under well-optimized conditions (17). CD11b up-regulation on these cells was also maximal in the presence of these two cytokines at RT. This combination of cytokines was therefore chosen to further evaluate anticoagulant and temperature effects in whole blood in the presence of disease. The disease group, which consisted of HIV/TB patients and patients with pneumonia, showed no significant differences in expression of either marker on PMN or lymphocytes and was therefore analyzed as one group for comparison with the ND group.
Our findings that CD11b expression was lower on PMN at 37°C than at RT while the opposite was true for HLA-DR suggest that these surface antigens are differentially stimulated. The same was true for expression of these markers on lymphocytes, except that the expression of HLA-DR was not significantly increased at 37°C, further indicating differential regulation of this marker based on cell type. This indication was further supported by the fact that, unlike their effects on PMN, the addition of both IFN-γ and GM-CSF did not upregulate the expression of HLA-DR and CD11b on lymphocytes. Furthermore, EDTA as an anticoagulant had drastic effects on CD11b expression whereas HLA-DR expression was less affected, indicating that this effect is somewhat selective for the antigen.
Although there were changes in expression of both markers in diseased patients relative to that found for ND, in general the patterns of modulation were similar. The down-regulation of CD11b in EDTA cultures was, however, more severe than that found for ND cultures. In addition, a greater tendency toward a fluctuation in expression levels was evident in whole blood of diseased patients held for 18 h at RT. Patterns of modulation based on different treatments were similar between ND and diseased patients but differed between the two anticoagulants. Therefore, the choice of anticoagulant had a marked influence on activation marker expression, as did an increase in temperature to 37°C.
One possible explanation for the maintenance of high CD11b expression in heparin cultures at 37°C but not in EDTA cultures is that a cytokine such as IL-8 is produced in the presence of heparin at levels approximately 20-fold-higher than those in the presence of EDTA (40a). A major effect of IL-8 is the up-regulation of CD11b on PMN (8). Anticoagulation of whole blood by EDTA or by heparin is achieved by chelating calcium ions and by combining with antithrombin III to remove thrombin, respectively (19). It may therefore be the specific stage at which inhibition of coagulation occurs that has a significant effect on whether factors such as cytokines affect phenotypic expression ex vivo.
In summary, our results indicate that the expression of activation markers on PMN and lymphocytes in whole blood are influenced by factors such as the time and temperature of incubation and the choice of anticoagulant. In addition, responses to these factors were also dependent on the particular marker being analyzed, the cell type of interest, and to a lesser extent the presence of disease. These findings have relevance with respect to specimen handling for clinical flow cytometric monitoring of cell surface markers, in which specimens do not always reach the clinical laboratory in a timely fashion and specimens may be subjected to elevated temperatures. In addition, levels of various cytokines are known to be raised in a number of disease states, which can in turn contribute to phenotypic changes ex vivo. As use of the whole-blood culture model is gaining in popularity as a system simulating the in vivo situation more closely than fractionated leukocytes and one that avoids the problems associated with activation due to cell isolation procedures, it is important to be aware of the effects of additives such as anticoagulants and factors such as incubation temperature and time on cell phenotype and function.
ACKNOWLEDGMENTS
This work was supported by the Poliomyelitis Research Foundation and Medical Research Council of South Africa.
REFERENCES
- 1.Allen J N, Herzyk D J, Allen E D, Wewers M D. Human whole blood interleukin-1-beta production—kinetics, cell source, and comparison with TNF-alpha. J Lab Clin Med. 1992;119:538–546. [PubMed] [Google Scholar]
- 2.Arnaout M A. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–1050. [PubMed] [Google Scholar]
- 3.Bayston K, Tomlinson M, Cohen J. In vitro stimulation of TNF-alpha from human whole blood by cell-free supernatants of gram-positive bacteria. Cytokine. 1992;4:397–402. doi: 10.1016/1043-4666(92)90084-5. [DOI] [PubMed] [Google Scholar]
- 4.Berends C, Dijkhuizen B, de Monchy J G R, Gerritsen J, Kaufman H F. Induction of low density and up-regulation of CD11b expression of neutrophils and eosinophils by dextran sedimentation and centrifugation. J Immunol Methods. 1994;167:183–193. doi: 10.1016/0022-1759(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 5.Cassatella M A, Bazzoni F, Ceska M, Ferro I, Baggiolini M, Berton G. IL-8 production by human polymorphonuclear leukocytes. The chemoattractant formyl-methionyl-leucyl-phenylalanine induces the gene expression and release of IL-8 through a pertussis toxin-sensitive pathway. J Immunol. 1992;148:3216–3220. [PubMed] [Google Scholar]
- 6.Cicco N A, Lindemann A, Content J, Vandenbussche P, Lubbert M, Gauss J, Mertelsmann R, Hermann F. Inducible production of interleukin-6 by human polymorphonuclear neutrophils: role of granulocyte-macrophage colony-stimulating factor and tumor necrosis factor-alpha. Blood. 1990;75:2049–2052. [PubMed] [Google Scholar]
- 7.DeForge L E, Remick D G. Kinetics of TNF, IL-6, and IL-8 gene expression in LPS-stimulated whole blood. Biochem Biophys Res Commun. 1991;174:18–24. doi: 10.1016/0006-291x(91)90478-p. [DOI] [PubMed] [Google Scholar]
- 8.Detmers P A, Powell D E, Walz A, Clark-Lewis I, Baggiolini M, Cohn Z A. Differential effects of neutrophil-activating peptide 1/IL-8 and its homologues on leukocyte adhesion and phagocytosis. J Immunol. 1991;147:4211–4217. [PubMed] [Google Scholar]
- 9.Dubravec D B, Spriggs D R, Mannick J A, Rodrick M L. Circulating human peripheral blood granulocytes synthesize and secrete tumor necrosis factor alpha. Proc Natl Acad Sci USA. 1990;87:6758–6761. doi: 10.1073/pnas.87.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elbim C, Bailly S, Chollet-Martin S, Gougerot-Pocidalo M-A. Differential priming effects of proinflammatory cytokines on human neutrophil oxidative burst in response to bacterial N-formyl peptides. Infect Immun. 1994;62:2195–2201. doi: 10.1128/iai.62.6.2195-2201.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elbim C, Chollet-Martin S, Bailly S, Hakim J, Gougerot-Pocidalo M-A. Priming of polymorphonuclear neutrophils by tumor necrosis factor α in whole blood: identification of two polymorphonuclear neutrophil subpopulations in response to formyl-peptides. Blood. 1993;82:633–640. [PubMed] [Google Scholar]
- 12.Fava R A, Olsen N J, Postlethwaite A E, Broadley K N, Davidson J M, Nanney L B, Lucas C, Townes A S. Transforming growth factor beta 1 (TGF-beta 1) induced neutrophil recruitment to synovial tissues: implications for TGF-beta-driven synovial inflammation and hyperplasia. J Exp Med. 1991;173:1121–1132. doi: 10.1084/jem.173.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fearon D T, Collins L A. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol. 1983;130:370–375. [PubMed] [Google Scholar]
- 14.Fleischmann J, Golde D W, Weisbart R H, Gasson J C. Granulocyte-macrophage colony-stimulating factor enhances phagocytosis of bacteria by human neutrophils. Blood. 1986;68:708–711. [PubMed] [Google Scholar]
- 15.Fradin A, Zirrolli J A, Maclouf J, Vausbinder L, Henson P M, Murphy R C. Platelet-activating factor and leukotriene biosynthesis in whole blood. A model for the study of transcellular arachidonate metabolism. J Immunol. 1989;143:3680–3685. [PubMed] [Google Scholar]
- 16.Gasson J C, Weisbart R H, Kaufman S E, Clark S C, Hewick R M, Wong G G, Golde D W. Purified human granulocyte-macrophage colony-stimulating factor: direct action on neutrophils. Science. 1984;226:1339–1342. doi: 10.1126/science.6390681. [DOI] [PubMed] [Google Scholar]
- 17.Gosselin E J, Wardwell K, Rigby W F C, Guyre P M. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor, IFN-γ, and IL-3. J Immunol. 1993;151:1482–1490. [PubMed] [Google Scholar]
- 18.Grotendorst G R, Smale G, Pencev D. Production of transforming growth factor beta by human peripheral blood monocytes and neutrophils. J Cell Physiol. 1989;140:396–402. doi: 10.1002/jcp.1041400226. [DOI] [PubMed] [Google Scholar]
- 19.Guyton A C. Textbook of medical physiology. 8th ed. London, United Kingdom: W. B. Saunders Co.; 1991. pp. 390–399. [Google Scholar]
- 20.Haziot A, Tsuberi B Z, Goyert S M. Neutrophil CD14: biochemical properties and role in the secretion of tumor necrosis factor-alpha in response to lipopolysaccharide. J Immunol. 1993;150:5556–5565. [PubMed] [Google Scholar]
- 21.Kharazmi A, Nielsen H, Rechnitzer C, Bendtzen K. Interleukin 6 primes human neutrophil and monocyte oxidative burst response. Immunol Lett. 1989;21:177–184. doi: 10.1016/0165-2478(89)90056-4. [DOI] [PubMed] [Google Scholar]
- 22.Lanier L L, Ruittenberg J J, Phillips J H. Functional and biochemical analysis of CD16 antigen on natural killer cells and granulocytes. J Immunol. 1988;141:3478–3485. [PubMed] [Google Scholar]
- 23.Limb G A, Hamblin A S, Wolstencroft R A, Dumonde D C. Selective up-regulation of human granulocyte integrins and complement receptor 1 by cytokines. Immunol. 1991;74:696–702. [PMC free article] [PubMed] [Google Scholar]
- 24.Lindemann A, Riedel D, Oster W, Meuer S C, Blohm D, Mertelsmann R H, Herrmann F. Granulocyte-macrophage colony-stimulating factor induces interleukin 1 production by human polymorphonuclear neutrophils. J Immunol. 1988;149:837–839. [PubMed] [Google Scholar]
- 25.Lindemann A, Riedel D, Oster W, Ziegler-Heitbrock H W L, Mertelsmann R, Herrmann F. Granulocyte-macrophage colony-stimulating factor induces cytokine secretion by polymorphonuclear leukocytes. J Clin Invest. 1989;83:1308–1312. doi: 10.1172/JCI114016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Lloyd A R, Oppenheim J J. Poly’s lament: the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol Today. 1992;13:169–172. doi: 10.1016/0167-5699(92)90121-M. [DOI] [PubMed] [Google Scholar]
- 27.Lopez A F, Williamson D J, Gambie J R. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986;78:1220–1228. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lord P C W, Wilmoth L M G, Mizel S B, McCall C E. Expression of interleukin-1 alpha and beta genes by human blood polymorphonuclear leukocytes. J Clin Invest. 1991;87:1312–1321. doi: 10.1172/JCI115134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meddows-Taylor S, Martin D J, Tiemessen C T. Altered expression of FcγRIII (CD16) on polymorphonuclear neutrophils from individuals with human immunodeficiency virus type 1 disease and pulmonary tuberculosis. Clin Diagn Lab Immunol. 1997;4:789–791. doi: 10.1128/cdli.4.6.789-791.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meddows-Taylor S, Martin D J, Tiemessen C T. Reduced expression of interleukin-8 receptors A and B on polymorphonuclear neutrophils from individuals with human immunodeficiency type 1 disease and pulmonary tuberculosis. J Infect Dis. 1998;177:921–930. doi: 10.1086/515232. [DOI] [PubMed] [Google Scholar]
- 31.Melani C, Mattia G F, Silvani A, Care A, Rivoltini L, Parmiani G, Lolumbo M P. Interleukin-6 expression in human neutrophil and eosinophil peripheral blood granulocytes. Blood. 1993;81:2744–2749. [PubMed] [Google Scholar]
- 32.Moore F D, Jr, Jack R M, Antin J H. Peripheral blood neutrophils in chronically neutropenic patients respond to granulocyte-macrophage colony-stimulating factor with a specific increase in CR1 expression and CR1 transcription. Blood. 1992;79:1667–1671. [PubMed] [Google Scholar]
- 33.Neuman E, Huleatt J W, Jack R M. Granulocyte-macrophage colony-stimulating factor increases synthesis and expression of CR1 and CR3 by human peripheral blood neutrophils. J Immunol. 1990;145:3325–3332. [PubMed] [Google Scholar]
- 34.Perticarari S, Presani G, Banfi E. A new flow cytometric assay for the evaluation of phagocytosis and the oxidative burst in whole blood. J Immunol Methods. 1994;170:117–124. doi: 10.1016/0022-1759(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 35.Perussia B, Dayton E T, Lazarus R, Fanning V, Trinchieri G. Immune interferon induces the receptor for monomeric IgG1 on human monocytic and myeloid cells. J Exp Med. 1983;158:1092–1113. doi: 10.1084/jem.158.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petroni K C, Shen L, Guyre P M. Modulation of human polymorphonuclear leukocytes IgG Fc receptors and Fc receptor-mediated function by IFN-γ and glucocorticoids. J Immunol. 1988;140:3467–3472. [PubMed] [Google Scholar]
- 37.Salmon J E, Browle N L, Edberg J C, Kimberly R P. Fcγ receptor III induces polymerization in human neutrophils and primes phagocytosis mediated by Fcγ receptor II. J Immunol. 1991;146:997–1004. [PubMed] [Google Scholar]
- 38.Shalekoff S, Tiemessen C T, Gray C M, Martin D J. Depressed phagocytosis and oxidative burst in polymorphonuclear leukocytes from individuals with pulmonary tuberculosis with or without human immunodeficiency virus type 1 infection. Clin Diagn Lab Immunol. 1998;5:41–44. doi: 10.1128/cdli.5.1.41-44.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Surette M E, Palmantier R, Gosselin J, Borgeat P. Lipopolysaccharides prime whole human blood and isolated neutrophils for the increased synthesis of 5-lipoxygenase products by enhancing arachidonic acid availability: involvement of the CD14 antigen. J Exp Med. 1993;178:1347–1355. doi: 10.1084/jem.178.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi G W, Andrews D F, Lilly M B, Singer J W, Alderson M R. Effect of granulocyte-macrophage colony-stimulating factor and interleukin-3 on interleukin-8 production by human neutrophils and monocytes. Blood. 1993;81:357–364. [PubMed] [Google Scholar]
- 40a.Tiemessen, C. Unpublished data.
- 41.Tiku K, Tiku M L, Skosey J L. Interleukin 1 production by human polymorphonuclear neutrophils. J Immunol. 1986;136:3677–3685. [PubMed] [Google Scholar]
- 42.Vadas M A, Nicola N A, Metcalf D. Activation of antibody-dependent cell-mediated cytotoxicity of human neutrophils and eosinophils by separate colony-stimulating factors. J Immunol. 1983;130:795–799. [PubMed] [Google Scholar]
- 43.Watson F, Robinson J J, Edwards S W. Neutrophil function in whole blood and after purification—changes in receptor expression, oxidase activity and responsiveness to cytokines. Biosci Rep. 1992;12:123–133. doi: 10.1007/BF02351217. [DOI] [PubMed] [Google Scholar]
- 44.Weisbart R H, Golde D W, Gasson J C. Biosynthetic human GM-CSF modulates the number and affinity of neutrophil f-Met-Leu-Phe receptors. J Immunol. 1986;137:3584–3587. [PubMed] [Google Scholar]