Abstract
Simple Summary
Anoikis is a programmed cell death process resulting from the loss of interaction between cells and the extracellular matrix. Therefore, it is necessary to overcome anoikis when tumor cells acquire metastatic potential. In lung cancer, the composition of the extracellular matrix, cell adhesion-related membrane proteins, cytoskeletal regulators, and epithelial–mesenchymal transition are involved in the process of anoikis, and the initiation of apoptosis signals is a critical step in anoikis. Inversely, activation of growth signals counteracts anoikis. This review summarizes the regulators of lung cancer-related anoikis and explores potential drug applications targeting anoikis.
Abstract
Tumor metastasis occurs in lung cancer, resulting in tumor progression and therapy failure. Anoikis is a mechanism of apoptosis that combats tumor metastasis; it inhibits the escape of tumor cells from the native extracellular matrix to other organs. Deciphering the regulators and mechanisms of anoikis in cancer metastasis is urgently needed to treat lung cancer. Several natural and synthetic products exhibit the pro-anoikis potential in lung cancer cells and in vivo models. These products include artonin E, imperatorin, oroxylin A, lupalbigenin, sulforaphane, renieramycin M, avicequinone B, and carbenoxolone. This review summarizes the current understanding of the molecular mechanisms of anoikis regulation and relevant regulators involved in lung cancer metastasis and discusses the therapeutic potential of targeting anoikis in the treatment of lung cancer metastasis.
Keywords: lung cancer, anoikis, apoptosis, neoplasm metastasis, molecular mechanism therapy
1. Introduction
Lung cancer is one of the most malignant tumors worldwide, characterized by high morbidity and mortality [1]. The outcomes for patients with metastatic lung cancer are poor, with a 5-year survival rate of <5% [2]. Tumor metastasis is a complex multistep process, including migration from cancer tissue, intravasation, survival in the circulatory system, extravasation, homing, and metastatic colonization [3,4,5]. To finish the initial migration step, tumor cells must interrupt cell–cell and cell–stromal interactions, become flexible, and acquire plasticity to navigate the tumor stroma mechanically [3]. However, migrating tumor cells are then confronted with a survival challenge resulting from the loss of cell adhesion. Extracellular matrix (ECM) adhesion plays an essential role in cell differentiation, proliferation, and motility [6]. Normal cells can only grow and differentiate in the correct environment within the tissue, and they eliminate themselves by apoptosis in abnormal environments.
Anoikis (Greek for “homeless”) is apoptosis induced by loss of cell adhesion to the ECM or inappropriate cell adhesion [7]. Differentiated cells of multicellular organisms grow in appropriate tissue environments, and when cells are lost or leave their native environment, apoptosis signal transduction occurs. Anoikis occurs in malignancies, cardiovascular disease, diabetes, and infectious diseases; it includes metastatic detachment of tumor cells, cardiomyocyte detachment in heart failure, and endothelial cell detachment; other examples are hyperglycemia in diabetic microangiopathy and disruption of ECM-mediated cell loss of adhesion in infectious diseases [8,9,10]. Cancer cells escape tumor tissue and overcome anoikis to adapt to uncontrolled growth elsewhere in tumor metastasis. Tumor cells can require resistance to anoikis; this escape protects them in the lymphatic and circulatory systems [11,12]. Anoikis resistance is critical for tumor progression and metastasis, and tumor cells can escape from anoikis in several ways [13]. Therefore, we reviewed animal and cell models of anoikis in lung cancer and relevant transmembrane proteins and intracellular regulators. Then natural and synthetic products that promote anoikis were summarized.
2. Methodology
A literature search was performed in PubMed and Google scholar from January 1990 to September 2022. The search term was “anoikis”. A secondary search was conducted by screening the list of articles that met the inclusion criteria. The keywords were “lung cancer” OR “lung adenocarcinoma” OR “non-small-cell lung cancer” OR “small-cell lung cancer”. The obtained 362 articles were screened, and 79 duplicate articles, 23 review articles, and 113 irrelevant studies were removed. One hundred forty-seven relevant studies were included after reading the abstract. Among them, 101 were related to the mechanism of anoikis, and 46 were related to therapies for anoikis in lung cancer. Finally, we organized the tables, wrote the text, and created figures to summarize mechanisms and therapies of anoikis-associated lung cancer metastasis according to the SANRA [14].
3. Regulators and Mechanism of Anoikis in Lung Cancer
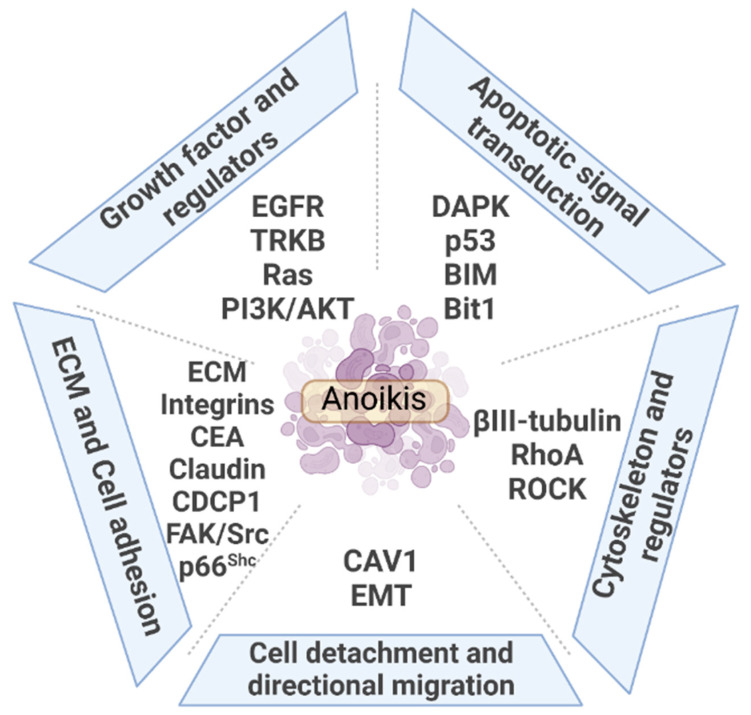
The present work reviews the regulation of lung cancer on anoikis, including extrinsic and intrinsic regulators, which mainly includes five aspects provided in Figure 1. Anoikis originates from changes in the external environment triggered by cell detachment from the primary site. ECM components and cell adhesion-related membrane proteins act as sensors to transmit cell signals intracellularly. Regulatory proteins of cell adhesion and cytoskeleton, p66shc and Ras homolog family A (RhoA), activate cells to initiate apoptosis. Activation of apoptosis signal transduction is an executor of anoikis. By contrast, proteins that increase cell adhesion, tight junction biological processes, and cytoskeletal proteins are influential factors of anoikis. Proliferation signals help tumor cells escape anoikis. The epithelial–mesenchymal transition (EMT) process causes loss of cell adhesion, accompanied by proteins such as caveolin 1 (CAV1) that promote cell directional migration and ultimately mediate anoikis resistance. The role of the above five aspects on anoikis in lung cancer metastasis is discussed in detail (Table 1).
Figure 1.
Overview of the regulation of anoikis in lung cancer metastasis.
3.1. ECM and Cell Adhesion
3.1.1. ECM
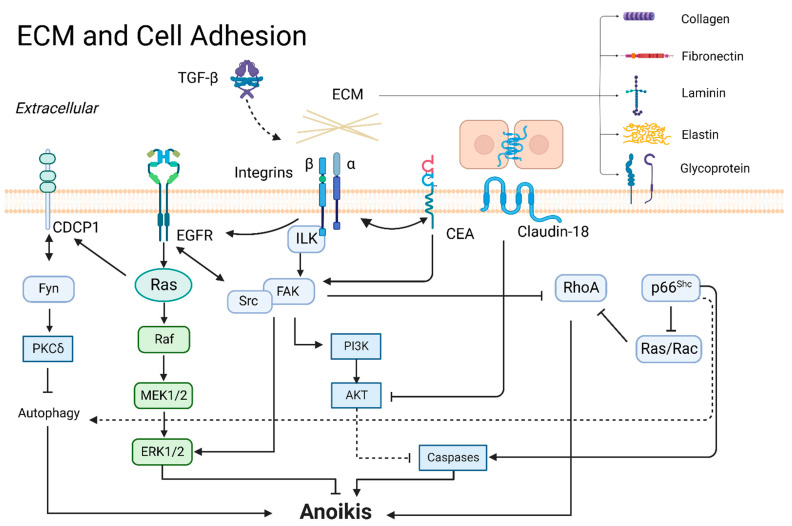
ECM comprises fibronectin, laminins, collagens, elastin, and several other glycoproteins. They bind with cell adhesion receptors to form a three-dimensional macromolecular network, which regulates various cell functions, including survival, growth, morphology, and migration [15]. Tumor cells undergo anoikis due to loss or inappropriate cell adhesion to ECM [16]. Acquisition of anchorage-independent survival requires tumor cells survival when detached from the ECM matrix [17,18]. In different artificially mimicked ECM compositions, A549 cells exhibited different sensitivity to doxorubicin and anoikis in vitro. Downregulation of focal adhesion kinase (FAK) signaling is accompanied by anoikis sensitization [18]. As for ECM components, fibronectin is upregulated for cell aggregate formation during cell detachment, which enhances anoikis resistance in lung cancer. Fibronectin knockdown decreases glycoproteins, including desmoglein-2, desmocollin-2/3, and plakoglobin, to increase anoikis [19]. Laminin 5 expression is significantly correlated with advancement along the alveolar wall growth pattern and metastasis in lung adenocarcinoma. Laminin 5 activates integrin/FAK signaling to induce anoikis resistance in several lung adenocarcinoma cell lines suspended in soft agar-coated dishes [20]. Liu et al. showed that collagen XVII cooperated with laminin 5 to activate FAK and mediate suspension survival. The authors found that activation of PP2A/STAT3 promotes collagen XVII-induced suspension survival, which determined anoikis resistance and initial metastasis in A549 cells and malignant lung cancer pleural effusion mouse models [21]. Highly expressed collagen IV facilitated liver metastasis in lung cancer patients. Burnier et al. investigated lung-metastasizing M-27 cells and found that collagen IV silencing enhanced anoikis through the integrin α2/FAK axis in vitro and reduced liver metastasis by inoculation of tumor cells into the intrasplenic/portal system in vivo [22]. These findings suggest that upregulation of ECM components can mediate cell survival and re-adhesion to promote metastasis of tumor cells, primarily through integrin/FAK. A summary is presented in Figure 2.
Figure 2.
ECM and cell adhesion in anoikis.
3.1.2. Integrins
Integrins are a family of cell surface heterodimeric receptors consisting of noncovalently linked α- and β-subunits that determine the receptor affinity for ECM. Integrin-mediated tumor stroma sensing, stiffening, and remodeling are critical steps in cancer progression that support tumor invasion, acquiring tumor stem cell properties, and drug resistance [23]. Integrins help metastatic cancer cells facilitate anchorage-independent survival and resist anoikis [24,25], and abnormal integrin expression contributes to several cancers’ metastasis [26]. In addition, integrin signaling was stimulated by growth factors to induce crosstalk. Integrin β3 was activated by transforming growth factor-β1 (TGF-β1) to increase the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor resistance, then EMT and anoikis resistance was observed in lung cancer cell lines [27]. Several studies have proven that FAK was a major downstream effector resisting anoikis through the Src or ERK pathway [20,22,25]. Furthermore, membrane proteins carcinoembryonic antigen (CEA) and cellular retinoic acid binding protein 2 (CRABP2) engage in integrin signaling to suppress anoikis [28]. Wu et al. found that CRABP2 promoted integrin β1/FAK/ERK signaling and inhibited anoikis. Overexpressed CRABP2 increased lymph node and liver metastasis through in vivo tail vein injection of lung cancer cell models [25].
3.1.3. CEA
CEA family members are involved in the biological function of intercellular adhesion [29]. First, Camacho-Leal et al. found that the expression of CEA is closely related to integrins, and deleting the CEA functional domain weakened the cross-linking of integrin α5β1 and cell adhesion to fibronectin, which led to anoikis [28]. Subsequently, antibody-mediated CEA deletion attenuated specific binding to integrin α5β1. The co-clustered CEA and integrin α5β1 activated integrin-linked kinase (ILK), AKT, and ERK/mitogen-activated protein kinase (MAPK) pathways to impair anoikis [30]. Furthermore, CEA reduces anoikis through the downregulation of the intrinsic cell death pathway, and the inactivation of caspase-9 and -3 [31]. The above studies demonstrate the effect of CEA on anoikis resistance. In addition, CEA-related cell adhesion molecule 6 (CEACAM6) is upregulated in lung adenocarcinoma patients with poor outcomes. Homophilic interactions of CEACAM6 between lung cancer cells and the tumor microenvironment could inhibit anoikis through Src/FAK pathway activation [32].
3.1.4. Claudins and Occludin
Claudins and occludin are the essential components of the tight junctions, which establish the paracellular barrier. Serglycin interacted with CD44-enhanced claudin-1 expression to promote vimentin expression, EMT, and anoikis resistance [33]. Conversely, claudin-18 promoted cell death and anoikis by inhibiting phosphorylated 3-phosphoinositide-dependent protein kinase-1 (PDK1) and pAKT levels in several lung cancer cell lines [34]. Occludin is an integral membrane linker protein. The thyroid transcription factor 1 (TTF-1) enhances the promoter of occludin and mediated anoikis [35].
3.1.5. CUB-Domain-Containing Protein 1 (CDCP1)
CDCP1 is an Src family kinase-binding phosphoprotein implicated in promoting metastasis via anoikis inhibition [36,37]. Phosphorylation of CDCP1 enhanced anchorage-independent growth of A549, PC14, H520, and H322 in a colony formation assay on soft agar. A549 cells with knockdown CDCP1 injected into the tail veins of a mouse model were found to have fewer metastatic nodules [36]. Regarding the mechanism of CDCP1 against anoikis, a few studies have proven that Ras activated CDCP1; subsequently, Tyr734 of CDCP1 bound to Fyn and was phosphorylated to activate protein kinase Cδ (PKCδ) and inhibit autophagy, helping tumor cells to escape apoptosis [36,38,39].
3.1.6. FAK
FAK is a ubiquitously expressed non-receptor tyrosine kinase that regulates cellular functions from embryonic development to wound healing, cell adhesion, cell migration, and angiogenesis [40,41]. For cell adhesion, FAK is recruited by integrins that form a dual kinase complex. The complex responds to signals from ECM components, including fibronectin and collagen [20,22,25]. FAK modulates both cell death- and survival-related pathways to resist anoikis. On the one hand, FAK binds to Src or directly activates the phosphatidylinositide-3-kinase (PI3K)/AKT, ERK/MAPK, and p38/MAPK pathways for survival during cell detachment [22,25,42]. On the other hand, FAK upregulates the phosphorylation of p190RhoGAP and inhibits RhoA-triggered pro-apoptotic phosphorylation of BCL-2-interacting mediator of cell death (BIM) to escape from anoikis [10]. Olfactomedin III is highly expressed in anoikis-resistant lung cancer cell lines and promotes the phosphorylation of FAK to keep procaspase-3 from activation [43]. Conversely, apoptosis signals inhibit FAK. X-linked ectodermal dysplasia receptor (XEDAR) was an essential effector downstream of p53 and negatively regulated FAK to induce anoikis [44].
3.1.7. Tyrosine-Protein Kinase Src
Cellular functions of Src kinases are involved in the cell cycle, proliferation, differentiation, adhesion, and angiogenesis [45]. These functions are mediated by Src kinases acting as membrane adhesion molecular switches, linking various extracellular signals to intracellular signaling pathways [46]. Src phosphorylation creates anoikis resistance and causes lung cancer cells to “float” in lymph nodes [47]. Src is a membrane-attached molecule regulated by several pathways to attenuate anoikis. FAK recruits Src to inhibit anoikis as previously mentioned [42]. It is also reported that Pyk2 appeared to be the critical downstream effector of Src and induced metastasis in lung cells [48]. Furthermore, Wei et al. found that Src Tyr418 phosphorylation triggered phosphorylation of p130Cas and caused anchorage-independent growth and metastasis in lung cancer [49].
Some regulators counteract anoikis through Src, including zinc finger protein 2 (ZIC2) and growth factor receptors. ZIC2 promoted tumorigenesis and anoikis resistance of non-small-cell lung cancer (NSCLC) by FAK/Src signaling, verified in NSCLC patients [50]. Platelet-derived growth factor receptor (PDGFR) increased the phosphorylation of Src Tyr419 and induced metastasis in lung cells [48]. In addition, EGFR mediated phosphorylation of c-Src to attenuate anoikis [51]. Family with sequence similarity 188 member B (FAM188B, a deubiquitinase) prevented degradation in phospho-EGFR, phospho-Src, and phospho-ERK to inhibit anoikis in lung cancer cells [52].
3.1.8. p66Shc
p66Shc is an SHC adapter protein 1 (SHC1) gene-encoding protein, localized to focal adhesions to permit anchorage-dependent growth and promote anoikis function as a tumor metastasis suppressor [53]. Expression of p66Shc in lung cancer samples correlated with good outcomes and anoikis [54]. p66Shc promotes anoikis in lung cancer through the perception of cell attachment, inhibition of proliferation, and promotion of apoptosis. p66Shc restrains Ras and Rac1 hyperactivation and increases RhoA activation to require focal adhesion and restore anoikis [55,56]. Typically, p66Shc did not affect cell death in adherent cells. During cell detachment, p66Shc translocated into mitochondria and bound to cytochrome c to release and cause death [55]. On the other hand, in the absence of adhesion conditions, p66Shc induced autophagy and activated apoptosis-associated protein cleavage of caspase-7 and poly (ADP-ribose) polymerase (PARP) by inhibiting phosphorylation of ERK1/2 [57,58]. DNA methylation in promoter-mediated epigenetic repression of p66Shc increased cancer cell survival and tumor progression [59]. Aiolos is an Ikaros zinc finger family member associated with epigenetic modifications [60]. Aiolos acts as an oncogene to disrupt enhancer–promoter interactions of p66Shc to inhibit transcription [61]. Aiolos also silences the zinc finger transcription factor PR domain containing 1 (PRDM1), resulting in anoikis resistance [62]. Similarly, zinc-finger E-box binding protein 1 (ZEB1) repressed the p66Shc promoter. Furthermore, ZEB1 potentiates EMT and blocks anoikis by increasing vimentin and decreasing E-cadherin and β-catenin [63].
3.2. Growth Factors and Regulators
3.2.1. EGFR
EGFR is a transmembrane protein that transduces intracellular growth factor signals, promotes tumor proliferation and metastasis, and is an essential target of current lung cancer therapy. In the absence of cell adhesion, normal cells lose expression of EGFR and induce apoptosis; by contrast, tumor cells do not exhibit loss of EGFR during detachment [64]. In growth signaling, EGFR acquires survival and anoikis resistance by activating Ras, ERK, and PI3K/AKT pathways [51,65,66]. EGFR has crosstalk through cell-adhering proteins or inhibits apoptosis signaling to inhibit anoikis. EGFR and integrins cooperatively inhibited anoikis by downregulating the BIM during cell detachment from the ECM [27,64]. NADPH oxidase 4 (NOX4) is a reactive oxygen species (ROS)-generating enzyme. NOX4 increased the activation of EGFR through ROS generation; a soft agar colony assay showed that si-NOX4 and si-EGFR attenuated Src expression and enhanced anoikis [51]. Regulators that mediate EGFR protein degradation are also involved in the regulation of anoikis. E3-ubiquitin ligase c-Cbl is an essential ligand for EGFR degradation. CCN family protein 2 (CCN2) bound EGFR and recruited E3-ubiquitin ligase for EGFR ubiquitination and degradation [66]. FAM188B prevented EGFR from degrading to cause lung cancer cells to re-adhere to the ECM [52].
3.2.2. Neurotrophic Tyrosine Kinase Receptor 2 (NTRK2/TrkB)
TrkB is a neurotrophin that serves as a growth factor regulating embryonic stem cells and promoting tumorigenesis in cancer cells. TrkB is upregulated in anoikis-resistant lung-derived tumor cells isolated from malignant ascites [67]. TrkB activates EMT, tumorigenesis, and lung metastasis and attenuates anoikis via the twist/snail axis in breast and lung cancer [68]. The expression of TrkB and E-cadherin had an inverse relationship in a panel of lung adenocarcinoma samples [69]. Cancer-derived TrkB mutations partly altered the functional characterization of the protein. TrkBT695I and TrkBD751N in colon cancer-derived mutants showed less activity than wild-type TrkB, and the function of TrkBL138F in lung cancer and TrkBP507L in breast cancer was indistinguishable from wild-type [70].
3.2.3. Ras
Ras is a small GTPase that includes HRas, KRas, and NRas; these are the most common oncogenes in human cancer [71]. Raf is a downstream effector of Ras, mediating proliferation signaling, including phosphorylation of MEK1/MEK2 and MAPK/ERK1/2 [72]. p66Shc and RhoB restrained Ras hyperactivation to enhance anoikis as mentioned before [56,65,73]. Ras-mutated NSCLC cell lines displayed high CDCP1 expression connected with anoikis resistance. The Ras/ERK/CDCP1 axis represses anoikis in lung cancer [38]. Furthermore, the HRasV12- or KRasV12-induced lung tumorigenesis and anti-anoikis effect required the expression of cisplatin resistance-related protein-9 (CRR9) [74].
3.2.4. PI3K/AKT
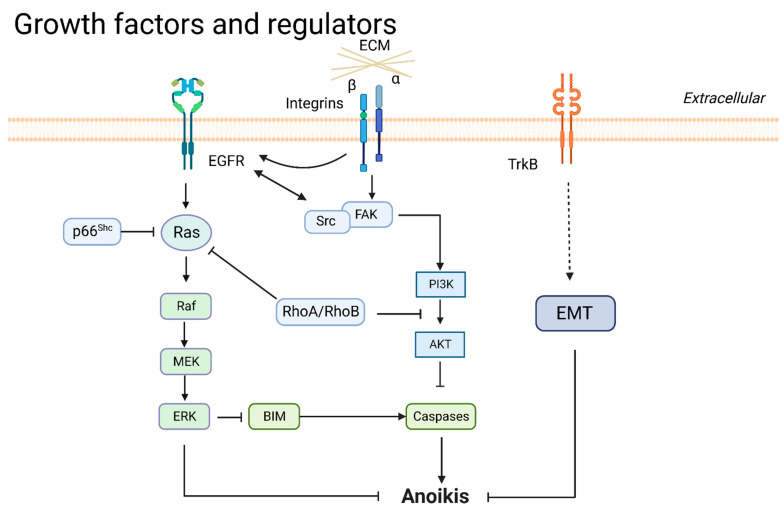
The activation of the PI3K/AKT pathway can cause lung cancer cells’ anoikis resistance [75]. Numerous proteins regulate anoikis resistance through the PI3K/AKT pathway. CEA, TrkB, FAK/Src, and βIII-tubulin promote phosphorylation of AKT for anoikis resistance as mentioned. Then, the activation of PI3K/AKT promotes EMT and inhibits caspases, allowing cells to escape anoikis [30,31,42,70,76,77]. Furthermore, farnesylated AKT1 suppresses anoikis and causes resistance to DNA-reactive agents [78]. Some regulators inhibit anoikis through the PI3K/AKT pathway. Interactions between PI3K and CRR9 activated AKT and BCL-XL accumulation, inducing anoikis resistance [74]. Contactin 1 enhanced the PI3K/AKT pathway, promoting EMT and anoikis resistance [77]. IGF-I-mediated IGF-IR phosphorylation activated downstream AKT to play an anti-apoptotic role [79]. IL-13 receptor subunit alpha-2 (IL13Rα2) activated PI3K/AKT and transcriptional coactivator with PDZ-binding motif, resulting in anoikis resistance. The overexpression of IL13Rα2 was associated with poor outcomes in lung cancer [80]. Noncoding RNA participates in PI3K/AKT regulation. lncRNA VAL binding to vimentin activates AKT, inducing lung cancer anchorage-independent growth and metastasis. lncRNA VAL prevents vimentin from undergoing Trim16 E3 ligase-mediated degradation [81]. A summary of growth factors and regulators is presented in Figure 3.
Figure 3.
Growth factors and regulators in anoikis.
3.3. Cytoskeleton and Regulators
3.3.1. βIII-Tubulin
βIII-tubulin is a microtubule protein constituent of the cytoskeleton. It contributes to tumor metastasis and chemotherapy resistance [82]. βIII-tubulin contributed to anti-anoikis and metastasis in lung cancer. High levels of βIII-tubulin inhibited the phosphatase and tensin homolog deleted on chromosome ten (PTEN) and enhanced phosphorylation of AKT to induce tumor spheroid outgrowth and anoikis resistance in NSCLC cells [76].
3.3.2. Rho and Rho-Associated Kinase (ROCK)
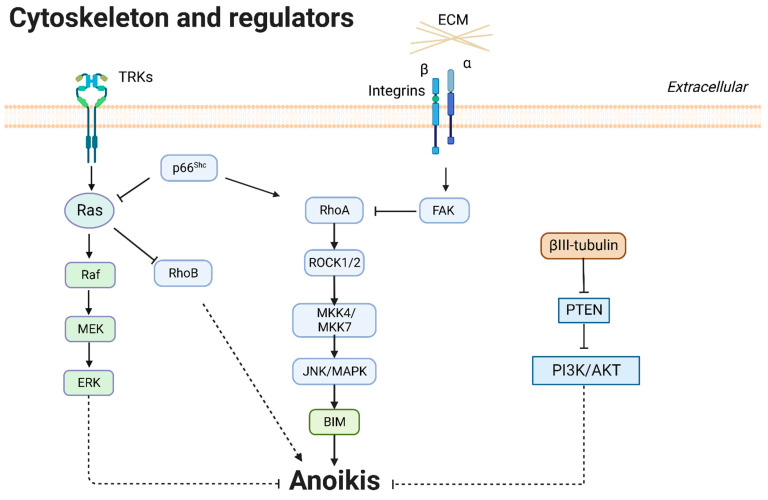
The Rho family of GTPases are small signaling G proteins from the Ras superfamily. RhoA regulates cell morphology change, cell–matrix adhesion, and cytoskeletal reorganization [83,84]. Cell adhesion molecules regulate RhoA; activation of FAK counteracted RhoA inducing anoikis [10], and p66Shc promoted RhoA, causing anoikis [56]. RhoB can inhibit invasion and proliferation and induce anoikis, whereas growth signals Ras, ERK, and PI3K/AKT counteract the effects of RhoB [65,73]. In summary, RhoA and RhoB promote anoikis and antagonize Ras. ROCK kinases are critical downstream effectors of Rho GTPases [85]. Haun et al. showed that RhoA/ROCK activated MKK4/MKK7 and JNK to promote BIM phosphorylation and caspase-3 processing, activating the anoikis signaling pathway [10]. A summary of cytoskeleton and regulators is presented in Figure 4.
Figure 4.
Cytoskeleton and regulators in anoikis.
3.4. Cell Detachment and Directional Migration
3.4.1. CAV1
CAV1 is a vital component of plasma membrane caveolae; it undergoes extracellular changes and regulates caveola-dependent signaling and endocytosis [86]. CAV1 is a cellular membrane raft structure that responds to external environmental stimuli such as ROS, shear stress, and mechanical stress. In addition, CAV1 can regulate cell polarization and directional migration [87]. CAV1 functions as a membrane adaptor to kinase Fyn in integrin signaling, critical for anchorage-dependent cell growth [88]. Several studies have proven that CAV1 overexpression enhanced anchorage-independent growth in H460 cells in vitro [89,90]. Myeloid leukemia-1 (MCL-1) is an anti-apoptotic protein required to escape anoikis in several tumors, including breast cancer, osteosarcoma, and melanoma [91,92]. CAV1 interacted with MCL-1 to prevent degradation and resist anoikis [93].
Tumor cells suffer fluid shear stress through the venous and lymphatic systems, which can acquire anoikis resistance. CAV1 can help cells overcome fluid shear stress and anoikis [94,95]. Free radicals stimulate CAV1 to a large extent during cell detachment. Oxidative stress products, including nitric oxide (NO) and hydrogen peroxide (H2O2), inhibited CAV1 degradation to resist anoikis [90,96]. NO-mediated S-nitrosylation of CAV1 is a protein modification that stabilizes CAV1 protein and protects it from ubiquitination [89]. As feedback, anoikis resistance enhanced CAV1 expression [97,98]. H2O2 inhibited the formation of the CAV1–ubiquitin complex through the consumption of catalase and N-acetylcysteine to cause anoikis resistance in H460 cells [96,99,100,101].
The relationships between miRNAs and CAV1 participate in anoikis. CAV1 mRNA is directly inhibited by miR-1827; downregulated CAV1 induces anoikis in A549 cells [102]. CAV1 and lipid raft-dependent endocytosis promote the transfer of miR-222-3p. Exosomes drive miR-222-3p targeting the promoter of suppressor of cytokine signaling 3 (SOCS3) to enhance gemcitabine resistance, migration, and anti-anoikis effects in vivo and in vitro [103].
3.4.2. EMT
EMT is characterized by epithelial cells undergoing remarkable morphologic changes to the mesenchymal phenotype, leading to increased motility and invasion in cancer [104]. Loss of the epithelial marker E-cadherin makes cells lose attachment and antagonizes the response to anoikis after cell detachment [105,106]. Some features of EMT, including loss of the epithelial cell–cell junction, reorganization of the actin cytoskeleton, and gain of directional migration capability, are critical for anoikis resistance [107,108]. Upregulation of N-cadherin, vimentin, twist, snail, and ZEB1, feature proteins of EMT, inhibits anoikis [63,68,109]. In summary, EMT is a process of adaptation to anchorage-independent growth, which is a factor against anoikis.
Some critical proteins (i.e., integrin, claudin-1, p66Shc, RhoA, CAV1, contactin 1, and TrkB) are involved in EMT in the context of anoikis, as previously mentioned [63] [27,33,68]. RhoA downstream signaling can affect actin remodeling and prevent the formation of contractile stress fibers during EMT [109]. Several pathways (i.e., the PI3K/AKT, Notch, and Wnt pathways) are related to EMT. Contactin 1 promotes EMT and anoikis resistance by enhancing the PI3K/AKT pathway [106]. Notch-1 enhanced the expression of vimentin and snail to suppress anoikis [110]. Fas apoptotic inhibitory molecule 2 (FAIM2) induced EMT and anoikis resistance through the Wnt/β-catenin pathway in NSCLC bone metastasis [111]. NO exposure upregulated EMT and enhanced anoikis resistance, as shown by anoikis assays with poly-HEMA-coated plates [90].
3.5. Apoptotic Signal Transduction
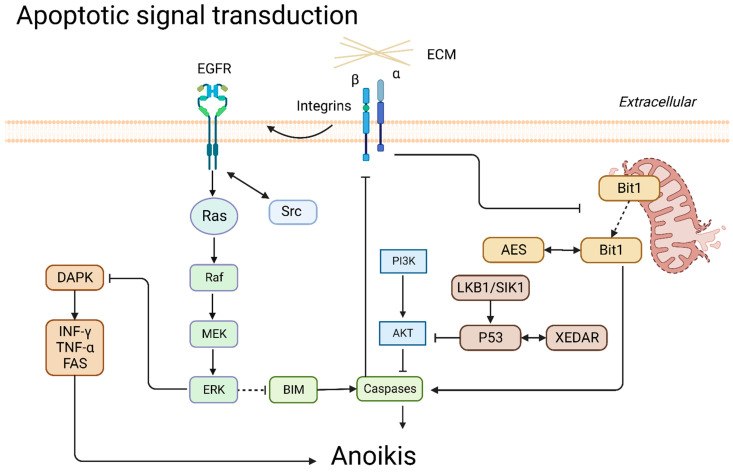
3.5.1. Death-Associated Protein Kinase (DAPK)
DAPK is a Ser/Thr protein kinase that mediates apoptosis signaling with upregulation of interferon-γ, tumor necrosis factor-α, and Fas to initiate apoptosis and anoikis [112,113]. CCN2 assisted DAPK in overcoming the inhibition by MAPK/ERK signaling [52].
3.5.2. p53
As a principal apoptosis regulator of tumors, p53 plays a pivotal role in anoikis-mediated cell death. p53-dependent anoikis has been demonstrated in several cell lines in lung cancer [44,114]. p53 upregulated cleavage caspase-3 to inhibit integrin α6β4, AKT signaling, and tumor metastasis [115]. XEDAR is an effector downstream of p53. p53 binds to intron 1 of the XEDAR gene and promotes transcription [44]. The expression of p53 and liver kinase B1 (LKB1) is positive feedback; however, loss of p53 or p14 cooperating with mutant Kras results in the inactivation of LKB1 [116,117,118]. Regulation of the p53 pathway is essential for tumor treatment, and some drugs interfere with the p53 pathway, as detailed below.
3.5.3. BIM
The pro-apoptotic protein BIM, a BH3-only protein, is a critical executor of apoptosis in anoikis. BIM disrupts the outer mitochondrial membrane to induce apoptosis, a response to anchorage-independent growth [119]. 14-3-3ζ knockdown in A549 cells was accompanied by upregulation of the pro-apoptotic protein BIM rather than Bad and susceptibility to anoikis. BIM inhibits BCL-2, Bcl-xL, and MCL-1, leading to Bax activation and anoikis [120]. In addition, when RhoA/ROCK senses cell detachment, signals are transmitted to BIM to initiate the apoptosis process [10].
3.5.4. Bit1
BCL-2 inhibitor of transcription 1 (Bit1) is named for the ability to reduce BCL-2 promoter activity. After integrin- or ECM-mediated cell attachment, Bit1 is released from the mitochondria to the cytoplasm and forms a complex with the transcriptional regulator amino-terminal enhancer split (AES) protein to initiate cell death against anchorage-independent growth [121,122]. In addition, the Bit1/AES complex upregulated E-cadherin to inhibit EMT and enhanced anoikis in lung cancer cells in vitro [121,123]. Transducin-like enhancer of split 1 (TLE1) sequestrates AES into the nucleus to reduce the formation of the Bit1/AES complex. Bit1 induced cytoplasmic translocation and degradation of TLE1 during anoikis [122,124,125]. A summary is presented in Figure 5.
Figure 5.
Apoptotic signal transduction in anoikis.
3.6. Other Regulators
3.6.1. LKB1
Several studies demonstrated that LKB1 accumulation promotes anoikis in tumors [126]. LKB1 decreases anchorage-independent growth of A549 and HeLa cells, suggesting that LKB1 is a tumor suppressor and anti-metastasis factor [127]. Salt-inducible kinase 1 (SIK1) is an LKB1-dependent kinase that inversely correlates with poor prognosis and distal metastases in breast cancer. The SIK1/LKB1 complex promotes p53-dependent anoikis and suppresses metastasis in lung cancer [114]. Meanwhile, loss of LKB1 is connected to poor prognosis in lung cancer. In LKB1-deficient lung cancer, pleomorphic adenoma gene 1 (PLAG1) mediates the upregulation of a glutaminolysis enzyme, glutamate dehydrogenase 1 (GDH1), and calcium/calmodulin-dependent protein kinase kinase 2 bound to AMP-activated protein kinase (AMPK). PLAG1/GDH1-mediated activation of AMPK confers anti-anoikis effects by inhibiting the mechanistic target of rapamycin (mTOR) [128,129,130].
3.6.2. Other Regulators
There are several studies on the negative regulation of anoikis. Phosphorylated Pyk2, MET amplification, glycolysis regulator (TIGAR), and BUB1B are anoikis suppressors; overexpression of these factors attenuated anoikis in lung cancer [131,132,133,134]. PARP1 binds near the promoter region of CLDN7 and S100A4 to upregulate expression at the transcriptional level, which induces DNA repair and attenuates anoikis [135]. Activating transcription factor 4 binds to the heme oxygenase 1 promoter and coordinates with nuclear factor erythroid 2-related factor 2 to induce cytoprotective autophagy, ROS suppression, and anoikis resistance [136]. Deletion of NAD(P)H/quinone oxidoreductase 1 (NQO1) increases ROS formation and anoikis sensitization in NSCLC. NQO1 potentiates glycometabolism, enhancing cell proliferation and anoikis resistance [137,138]. ΔNp63α, the major isoform of p63, protects tumor cells from oxidative stress, DNA damage, anoikis, and ferroptosis through transcriptional upregulation of glutathione metabolism genes GCLC, GSS, IDH2, and GPX2 [139]. Interleukin enhancer-binding factor 2 (ILF2) directly binds to the PTEN upstream regulatory region, promoting anchorage independence in NSCLC [140]. Activated transcription factor Spi-B (SPIB) enhances SNAP47 transcription and mitigates anoikis in lung cancer [141]. Keratin 14 contributes to anoikis repression via upregulation of gastrokine 1 in lung cancer [142]. Loss of CoA desaturase (SCD1) induces cellular damage and anoikis in circulating tumor cells (CTCs) [143]. Macrophage-stimulating protein (MSP) binds to recepteur d’origine nantais (RON) to promote liver metastases by affecting the organ microenvironment in SCLC [144].
As for promoting anoikis factors, Krüppel-like factor 12 (KLF12) promotes cell cycle transition through the S phase to induce anoikis [145]. Trim62 E3 ligase is an anoikis protective factor; the loss of Trim62 E3 ligase promotes EMT, tumorigenesis, and anti-anoikis effects in lung cancer [146]. Src homology 2-b3 (SH2B3) is reduced in lung cancer tissues and cells. SH2B3 interacts with Janus kinase 2 (JAK2) and Src homology region 2-containing protein tyrosine phosphatase 2 to inhibit JAK2/STAT3 and PI3K/AKT signaling pathway-mediated anoikis in lung cancer [147].
Table 1.
Molecular factors and mechanisms related to anoikis in lung cancer.
| Factors | Anoikis | Model(s) | Mechanism | Ref |
|---|---|---|---|---|
| Fibronectin | ↓ | A549, H460, and H1975 | Fibronectin upregulated cell desmosomal interactions. | [19] |
| Laminin 5 | ↓ | A549, PC-14, LC-2/ad, RERF-LC-KJ, NCI-H322, and NCI-H358 | Laminin 5/integrin/FAK signaling pathway activated the expression of EGFR. | [20] |
| Collagen XVII | ↓ | A549 | S727 phosphorylation of STAT3 activated collagen XVII to maintain the stability of laminin 5. | [21] |
| Collagen IV | ↓ | Lung-metastasizing M-27 cells | Collagen IV activated integrin α2/FAK and increased reactivity to IGF-I. | [22] |
| CRABP2 | ↓ | L1, C9F6, and H1650 | CRABP2 coupled with HuR promoted integrin β1/FAK/ERK signaling. | [25] |
| Integrin β3 | ↓ | HCC827, H1975, A549, H292, and H1299 | The activation of the TGFβ1/integrin β3 axis overcame acquired resistance to EGFR-TKIs and anoikis. | [27] |
| CEA | ↓ | L6 and LR-73 | CEA inactivated caspase-9 and caspase-8 and enhanced PI3K/AKT pathway. | [28,30,31] |
| Claudin-1, Serglycin, and CD44 | ↓ | H1299, H322, H358, H23, H928, H460, and A549 | Serglycin interacting with CD44 enhanced claudin-1 expression to promote EMT and anoikis resistance. | [33] |
| Claudin-18 | ↑ | A549, RERF-LC-AI, IA-LM, WA-hT, PC-3, and RERF-LC–MS | Claudin-18 inactivated PDK1 and phospho-AKT levels. | [34] |
| Occludin and TTF-1 | ↑ | H441, A549, H1299 | TTF-1 transactivated occludin to promote anoikis. | [35] |
| CDCP1 | ↓ | A549, PC14, H322, H520, and H157 | Tyr734-phosphorylated CDCP1 regulated PKCδ and inhibited autophagy. | [36,38,39] |
| FAK | ↓ | A549 | Phosphorylated tyrosine sites (Tyr397, Tyr861, Tyr925) in FAK bound to intracellular proteins of Src and stimulated PI3K/AKT pathway, MAPK/ERK pathway, and MAPK/p38 pathway. | [42] |
| Olfactomedin III | ↓ | Poorly differentiated human squamous carcinoma, named DLKP cell line | Olfactomedin III upregulated phospho-FAK and phospho-Paxillin and kept procaspase-3 from activation. | [43] |
| Src | ↓ | SK-LU-1, H522, H1437, A549, H460, and H1792 | EGFR, PDGFR, and ZIC2 increased the phosphorylation of Src to induce anoikis resistance. | [48,50,52] |
| p130Cas | ↓ | A549 and H1792 | Src contributed to the phosphorylation of p130Cas in the tumor cells. | [49] |
| ZIC2 | ↓ | HFL1, Calu-3, H1975, H1395, H520, A549, H1299, H226, SK-MES-1, and BEAS-2B | ZIC2 promoted tumorigenesis and anoikis resistance of NSCLC by Src/FAK signaling. | [50] |
| FAM188B | ↓ | A549, H1299, H1975 | FAM188B potentiated the stabilization of EGFR expression. | [52] |
| p66Shc | ↑ | LLC, H69, and H209 | p66Shc potentiated autophagy through cleavage of caspase 7 and PARP. | [57,58] |
| Aiolos | ↓ | A549, H1155 | Aiolos disrupted the enhancer of p66Shc at the transcription level. | [61] |
| PRDM1 | ↑ | HUVEC, A549, Beas-2B, H209, H69, H1155 | PRDM1 was silenced by aiolos. | [62] |
| EGFR | ↓ | A549, H1703, Calu-6, H460, H358, HCC2279, BEAS-2B | EGFR mediated phosphorylation of c-Src and ERK to attenuate anoikis. | [51,52,66] |
| NOX4 | ↓ | A549, H1703, Calu-6, H460, H358, HCC2279, BEAS-2B | NOX4 increased the activation of Src and EGFR, attenuating anoikis | [51] |
| TrkB | ↓ | Primary cell cultures derived from malignant pleural effusions, L1 sarcoma cells from a primary, spontaneous lung tumor in Balb/c | TrkB inhibited EMT. The expression levels of TrkB and E-cadherin were opposite in lung adenocarcinoma samples. | [68,69,70] |
| Ras | ↓ | A549, PC14, H322, H520, and H157 | Ras/ERK signaling activated CDCP1 to induce anoikis resistance. Ras/ERK pathway was regulated through CAV1. | [38,88] |
| βIII-tubulin | ↓ | H460 and A549 | High levels of βIII-tubulin enhanced phospho-AKT activity via PTEN suppression. | [76]. |
| RhoA | ↑ | BEAS-2B, LLC, H69, and H209 | RhoA counteracted FAK and was activated by p66Shc, causing anoikis. | [10,56] |
| RhoB | ↑ | NIH-3T3, A549 | RhoB was suppressed by Ras/PI3K/AKT. Suppression of RhoB resulted in anoikis resistance. | [65,73] |
| ROCK | ↑ | BEAS-2B | RhoA/ROCK activated MKK4/MKK7/JNK/BIM to promote apoptosis. | [10] |
| NO | ↓ | H460, H23, H292 | NO exposure enhanced EMT and upregulated CAV1, allowing escape from anoikis and promoting migration. | [89,90] |
| MCL-1 | ↓ | NIH3T3, H460 | Mcl-1 degradation activated anoikis. CAV1 interacted with MCL-1 to prevent MCL-1 from degradation via ubiquitination. | [92,93] |
| H2O2 | ↓ | H460, G361 | H2O2 inhibited the formation of the CAV1–ubiquitin complex. | [96,100] |
| miR-222-3p | ↓ | A549-GR | miR-222-3p directly targeted the promoter of SOCS3 to enhance gemcitabine resistance and anti-anoikis. | [103] |
| CAV1 | ↓ | H460 | CAV1, as a membrane adaptor to kinase Fyn, activated integrin signaling. CAV1 was negatively regulated by oxidative stress. | [88,90,96] |
| ZEB1 | ↓ | HBECs, HepG2, H358, H1155, H1299, and A549 | ZEB1 was a negative regulator of p66Shc. | [63] |
| Notch-1 | ↓ | PC-9 | Notch-1 enhanced EMT markers, including vimentin and snail. | [110] |
| FAIM2 | ↓ | HARA, HARA-B4, H1395, A549, and NIH3T3 | FAIM2 enhanced Wnt/β-catenin signaling pathway to facilitate EMT and anoikis resistance. | [111] |
| DAPK | ↑ | H460, A427, A549, and CL1-0 | CCN2 promoted DAPK kinase activity and then activated the p53 pathway. | [52] |
| p53 and XEDAR | ↑ | A549, H1299, and HeLa | p53 enhanced cleavage caspase 3 and AKT activity and promoted XEDAR expression to inhibit integrin/FAK signaling. | [44,115] |
| CRR9 | ↓ | A549, H838 | CRR9 interacted with PI3K to activate oncogene Ras. | [74] |
| Contactin 1 | ↓ | H446, H526 | Contactin 1 activated the AKT pathway to promote EMT phenotype. | [77] |
| MMP-7 | ↓ | BALB/c 3T3 | MMP-7 degraded IGFBP-3 and activated the AKT pathway, resulting in anoikis. | [79] |
| IL13Rα2 | ↓ | HBE-135, HBE-154, PC9, H1975, A549, HTB-57, H2170, H1299, H358, H3255, H1838, and HCC827 | IL13Rα2 activated PI3K/AKT and TAZ, resulting in migration, invasion, and anoikis resistance. | [80] |
| lncRNA VAL | ↓ | A549, HCC827, H1650, H596, H1975, H1299, H292, H2009, and H2030 | lncRNA VAL bound to vimentin and inhibited vimentin degradation. | [81] |
| Farnesylated AKT1 | ↓ | MCF10A and A549 | Farnesylated AKT1 suppressed anoikis and caused resistance to DNA-reactive agents, but not altered cell cycle (M-phase) specific chemotherapeutics. | [78] |
| BIM | ↑ | NIH3T3, A549, BEAS-2B | BIM induced endangerment of mitochondria and apoptosis. | [92,119] |
| 14-3-3ζ | ↓ | A549 | Deficiency of 14-3-3ζ upregulated BAD and BIM and decreased MCL-1 to increase BAX, cleaved caspase 7, and cleaved caspase 3 toward tumor cell anoikis. | [120] |
| Bit1 | ↑ | A549 | Bit1/AES complex initiated cell death complex and inhibited EMT through upregulation of E-cadherin. | [121,122] |
| TLE1 | ↓ | A549 | TLE1 interfered with AES formation Bit1/AES complex. | [122,124] |
| NQO1 | ↓ | A549, H292 | NQO1 decreased ROS formation and anoikis sensitization. | [137,138] |
| ΔNp63α | ↓ | HCC95 | ΔNp63α protected cells from oxidative stress, DNA damage, anoikis, and ferroptosis through upregulation of glutathione metabolism. | [139] |
| ILF2 | ↓ | HUVEC-C, HBEC-5i, BEAS-2B, A549, H460, H1155, and H1299 | ILF2 directly inhibited PTEN via binding to its upstream regulatory region. | [140] |
| SPIB | ↓ | A549, H1703, H1975, H446, H520, H226, SK-MES-1, H460, H1299 | Activated SPIB directly enhanced SNAP47 transcription in lung cancer cells and increased anoikis resistance. | [141] |
| Keratin 14 | ↓ | KrasG12D/Trp53L/L cell lines from de novo KP tumors | Gastrokine 1 cooperated with keratin 14, inhibited anoikis, and promoted cancer metastasis. | [142] |
| SCD1 | ↓ | H460 | Loss of SCD1 induced cellular damage and anoikis. | [143] |
| MSP | ↓ | SBC-5, H1048 | MSP phosphorylated RON to promote liver metastases in lung cancer. | [144] |
| KLF12 | ↑ | A549, H23, H460 | KLF12 promotes cell cycle transition through the S phase to induce anoikis. | [145] |
| TRIM62 | ↑ | Trim62+/− and Trim62−/− C57BL/6J mice | The loss of TRIM62 synergizes with K-Ras mutation, promoting EMT, tumorigenesis, and metastasis in lung cancer. | [146] |
| SH2B3 | ↑ | A549, NCI-H358, NCI-H1650, NCI-H460, NCI-H1688, Calu-1; A549 and NCI-H1688 tumor-bearing nude mouse |
SH2B3 inhibits JAK2/STAT3 and PI3K/AKT signaling pathways to induce anoikis in lung cancer. | [147] |
↓: downregulation of anoikis; ↑: upregulation of anoikis.
4. Therapy for Anoikis in Lung Cancer
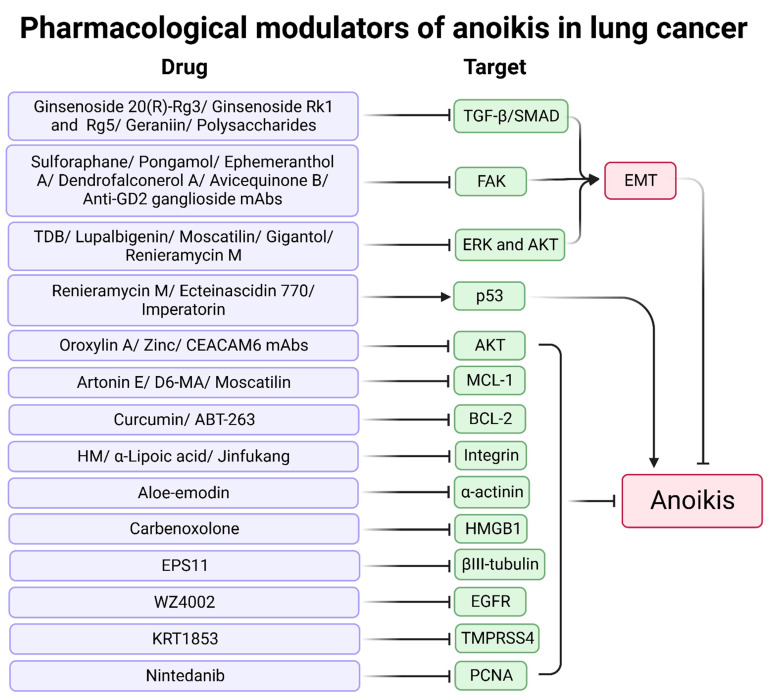
Investigations in lung cancer models demonstrate the potent anti-tumor activity of treatments such as monoclonal antibodies (mAbs), natural products, chemosynthetic drugs, and multi-component drugs against lung cancer metastasis via activation of anoikis (Table 2 and Figure 6).
Figure 6.
Pharmacological modulators of anoikis in lung cancer.
4.1. Natural Products
Numerous studies suggest that drugs enhancing anoikis are derived from natural products, of which small molecule compounds are the most common, including natural herbs such as Thai medicine, Chinese medicine, and marine natural products. The primary targets of natural products for anoikis include inhibition of MCL-1, AKT, FAK, and EMT.
For MCL-1 and anoikis-related pathways, renieramycin M and ecteinascidin 770, isolated from Thai tunicate Ecteinascidia thurstoni, exhibit promising anticancer activity via a p53-dependent pathway and inhibition of MCL-1 and BCL-2 [148,149]. Imperatorin from Angelica dahurica root was first reported by Pithi et al.; it enhances protein expression of p53 and Bax and reduces MCL-1, which promotes anoikis and cell death sensitization in several lung cancer cell lines [150]. Artonin E is extracted from the bark of Artocarpus gomezianus; it enhances the anoikis of lung cancer cells in a dose-dependent manner by downregulating MCL-1 [151].
Inhibition of AKT is an effective anoikis promoter. Several natural products inhibit lung cancer metastasis through the PI3K/AKT pathway. Oroxylin A, a flavonoid isolated from Scutellaria root, induces anoikis via the inhibition of the c-Src/AKT pathway and then increases the loss of mitochondrial membrane potential [152]. 4,5,4′-Trihydroxy-3,3′-dimethoxybibenzyl (TDB), isolated from D. ellipsophyllum, inhibits the anchorage-independent growth of lung cancer cells. TDB inhibits EMT through the inactivation of phospho-ERK and phospho-AKT [153]. Lupalbigenin, isolated from Derris scandens, inhibits phospho-AKT, phospho-ERK, and BCL-2 for sensitivity to anoikis in lung cancer [154].
FAK is associated with cell adhesion, and inhibition of FAK contributes to anoikis. Sulforaphane is an isothiocyanate found in cruciferous vegetables that promotes anoikis and cell death. Sulforaphane reduces FAK, AKT, and β-catenin and upregulates p21 to induce anoikis in lung cancer cells with wild-type p53 [155]. Pongamol is a chalcone derived from the T. purpurea stem extract that mitigates EMT and permits anoikis through downregulation of the FAK and AKT/mTOR signaling pathways [156]. EPS11 is a purified polysaccharide from a crude extract of marine bacterial polysaccharide; it inhibits the expression of βIII-tubulin at the transcription level, triggering suppression of the downstream effectors phospho-PKB and phospho-AKT in vitro and in vivo [157].
EMT promotes the progression and metastasis of lung cancer, and some natural products target anoikis by inhibiting EMT. Ginsenoside Rg3 promotes anoikis and EMT inhibition via the TGF-β/SMAD pathway in A549 [158]. Ginsenosides Rk1 and Rg5 suppress EMT through downregulation of MMP2/9 activity, inhibitory actions of Smad2/3, and the NF-kB/ERK pathway in A549 cells [159]. Jorunnamycin A is a bis-tetrahydroisoquinoline quinone isolated from the blue sponge Xestospongia sp.; it reduces EMT and anchorage-independent survival by regulating apoptosis-related proteins and EMT markers. p53 and E-cadherin are upregulated after treatment with Jorunnamycin A [160].
The active compounds from Dendrobii caulis inhibit lung cancer through anoikis. Moscatilin, gigantol, and ephemeranthol A exhibit strong anti-EMT and anti-migration activity involving suppression of ERK, AKT, and CAV1 [161,162,163]. Dendrofalconerol A, extracted from Dendrobium falconeri (Orchidaceae), sensitizes anoikis-induced cell death involving inhibitory effects of phospho-FAK and Rho-GTP in H460 cells [164]. Four bibenzyls isolated from the stem of Dendrobium pulchellum (chrysotobibenzyl, chrysotoxine, crepidatin, and moscatilin) facilitate anoikis and inhibit metastasis in lung cancer cells [165]. Phenolic compounds from Dendrobium ellipsophyllum (4,4′-dihydroxy-3,5-dimethoxybibenzyl, 4,5,4′-trihydroxy-3,3′-dimethoxybibenzyl, chrysoeriol, and luteolin) have cytotoxic and anoikis-related activities [166].
There are also natural products used for anti-tumor effects that stimulate anoikis. Aloe-emodin induces anoikis by interfering with the downregulation of α-actinin and the MAPK pathway (i.e., JNK, ERK1, and p38) and upregulation of PKCδ [167,168]. Curcumin increases BCL-2 ubiquitination and degradation, sensitizing cells to detachment-induced anoikis [169]. Geraniin, extracted from Phyllanthus amarus, exhibits anti-anoikis activity via TGF-β suppression [170].
Crude extracts of plants and Chinese medicine formulas target anoikis in lung cancer. A polysaccharide extract obtained from rough extraction of persimmon leaves inhibited EMT and anoikis resistance through the canonical TGF-β/SMAD pathway and inhibited the MAPK pathway [171]. Oat avenanthramides inhibited EGFR, suppressing lung cancer cell growth and migration [172]. Jinfukang is a traditional Chinese medicine prescription for lung cancer treatment; it inhibits the integrin/Src pathway, suppressing ECM–receptor interaction and focal adhesion-related genes [173]. Modified Bu-Fei decoction inhibits hypoxia-inducible factor 1α (HIF-1α) signaling and angiogenin-like protein 4 (ANGPTL4) to inhibit A549 cells anoikis and lung metastasis of LLC-bearing mice [174].
4.2. Synthetic Products
Chemosynthetic products have more precise targets and lower active concentrations for treating malignant tumors; these include the inhibitors of some targets such as BCL-2 and EGFR. BCL-2 inhibitor ABT-263 acts as adjunctive therapy to promote cell death-related proteins and anoikis [175]. WZ4002, a third-generation EGFR inhibitor, causes anoikis and inhibits lung cancer metastasis [176]. TMPRSS4 serine protease inhibitor KRT1853 promotes anoikis of lung cancer cells by inhibiting the JNK/MAPK, PI3K/AKT, and NF-κB pathways [177]. Nintedanib is available for idiopathic pulmonary fibrosis treatment. High-dose nintedanib (5 uM) promotes anoikis and apoptosis via downregulation of PCNA in NSCLC [178].
Derivatives of natural products were studied in lung cancer treatment, and modified natural products had anti-tumor activity. Renieramycin M exhibits anoikis activity by inhibiting phospho-ERK, phospho-AKT, BCL-2, and MCL-1 [179]. Avicequinone B (chemically synthesized from lawsone) inhibits MCL-1 and BCL-2 through the integrin/FAK/Src axis to promote anoikis [180]. Carbenoxolone (chemically derived from glycyrrhizin) enhances anoikis by inhibiting high mobility group box 1 (HMGB1) [181]. α-l-Rhamnose monosaccharide derivative (D6-MA), a novel synthetic derivative of digitoxin, has anticancer activity in lung cancer cell lines; it attenuated MCL-1 expression via glycogen synthase kinase-3β-mediated ubiquitin proteasomal degradation [182].
Chemical synthesis products and chemical element supplements also show good anti-anoikis activity. N,N-Bis(5-ethyl-2-hydroxy benzyl) methylamine (HM) enhances anoikis by targeting the integrin β3/FAK/AKT axis [183]. α-Lipoic acid decreases integrin β1 and β3 to induce anoikis [184]. Zinc sensitizes to anoikis by inhibiting AKT and CAV1 [185].
Macromolecules related to biological agents treat anoikis in lung cancer. Several mAbs inhibit lung cancer metastasis. Anti-ganglioside (GD2) mAbs reduce FAK phosphorylation levels and promote p38/MAPK for SCLC treatment [186]. LN-332 mAbs reduce anoikis resistance and migration, associated with tumor cell–matrix interaction [187]. CEACAM6 mAbs decrease phospho-AKT to promote anoikis in lung cancer. CEACAM6 mAbs with paclitaxel treatment markedly decreased tumor growth by 40% to 80% compared to CEACAM6 mAbs alone and were more effective in CEACAM6-targeting albumin-based nanoparticles [188,189].
Table 2.
Pharmacological modulators of anoikis in lung cancer.
| Modulators | Tested Model(s) | Mechanism | Ref |
|---|---|---|---|
| Renieramycin M | H460 | p53 activation | [148] |
| Ecteinascidin 770 | H23, H460 | p53 activation | [149] |
| Imperatorin | H23, H292, and A549 | p53 activation and MCL-1 downregulation | [150] |
| Artonin E | H460, A549, and H292 | MCL-1 downregulation | [151] |
| Oroxylin A | A549 | Inhibitory effect of c-Src/AKT pathway | [152] |
| TDB | H292 | EMT inhibition via inactivation of phospho-ERK and phospho-AKT | [153] |
| Lupalbigenin | H460 | Phospho-AKT, phospho-ERK, and BCL-2 inhibition | [154] |
| Sulforaphane | A549 and CL1-5 | Reduction of FAK, AKT, and β-catenin and upregulation of p21 | [155] |
| Pongamol | H460 | Inhibition of EMT through FAK and AKT/mTOR signaling pathways | [156] |
| EPS11 | H460, A549, and H1299 A549 xenograft with BALB/c-nu mice |
Inhibitory effect on βIII-tubulin | [157] |
| Ginsenoside 20(R)-Rg3 | A549 | EMT inhibition through the TGF-β/SMAD pathway | [158] |
| Ginsenosides Rk1 and Rg5 | A549 | Inhibition of TGF-β1-induced EMT | [159] |
| Jorunnamycin A | H460, H292, and H23 | Suppression of EMT and apoptosis-related protein | [160] |
| Moscatilin | H460 | EMT inhibition involves repression of ERK, AKT, MCL-1, and CAV1 | [161] |
| Gigantol | H460 | EMT inhibition involves suppression of ERK, AKT, and CAV1 | [162] |
| Ephemeranthol A | H460 | EMT inhibition via FAK/AKT pathway | [163] |
| Dendrofalconerol A | H460 | Suppression of phospho-FAK and Rho-GTP | [164] |
| Chrysotobibenzyl, chrysotoxine, crepidatin, and moscatilin | H23 | Anti-anoikis activities | [165] |
| 4,4′-Dihydroxy-3,5-dimethoxybibenzyl, 4,5,4′-trihydroxy-3,3′-dimethoxybibenzyl, chrysoeriol, and luteolin | H292 | Anti-anoikis activities | [166] |
| Aloe-emodin | H460 | Downregulation of α-actinin and MAPK pathway (JNK, ERK1, p38) and upregulation of PKCδ | [167,168] |
| Curcumin | H460 | Upregulation of the degradation of BCL-2 | [169] |
| Geraniin | A549 | EMT inhibition via TGF-β1 | [170] |
| Polysaccharide | A549 | Inhibition of TGF-β1-induced EMT | [171] |
| Oat avenanthramides | A549 | Suppression of EGFR | [172] |
| Jinfukang | H1975 | Suppression of integrin/Src pathway | [173] |
| Modified Bu-Fei decoction | A549 and LLC-bearing mice | Inhibition of ANGPTL4 expression through suppressing HIF-1α signaling | [174] |
| ABT-263 | LC-KJ, HCC827, H1650, and H1975 | A BCL-2 inhibitor enhanced Src inhibitors | [175] |
| WZ4002 | HCC827 and H1975 | A third-generation EGFR inhibitor | [176] |
| KRT1853 | H322 | TMPRSS4 serine protease inhibitors, repression of JNK/MAPK, PI3K/AKT, and NF-κB pathways | [177] |
| Nintedanib | A549, H1299, and H460 | Downregulation of PCNA | [178] |
| Renieramycin M | H460 | Downregulation of phospho-ERK, phospho-AKT, BCL-2, and MCL-1 | [179] |
| Avicequinone B | H460, H292, and H23 | Diminution of integrin/FAK/Src, MCL-1, and BCL-2 | [180] |
| Carbenoxolone | C57BL/6J mice injected with LLC | Inhibition of HMGB1 | [181] |
| D6-MA | H460 | MCL-1 downregulation | [182] |
| HM | H292 | Inhibition of integrin β3 | [183] |
| α-Lipoic acid | H460 | Integrin β1 and β3 inhibition | [184] |
| Zinc | H460 | Downregulation of AKT and CAV1 | [185] |
| Anti-GD2 ganglioside mAbs | NCI-417, ACC-LC-171, and ACC-LC-96 in vitro | FAK reduction and p38 activation | [186] |
| Anti-LN-332 mAbs | KLN-205 tumors in DBA/2 mice | Blocking cell–matrix interaction | [187] |
| CEACAM6 mAbs | Balb/C xenotransplanted A549 | Inhibitory effects on phospho-AKT and upregulation of paclitaxel chemosensitivity | [188,189] |
5. Discussion
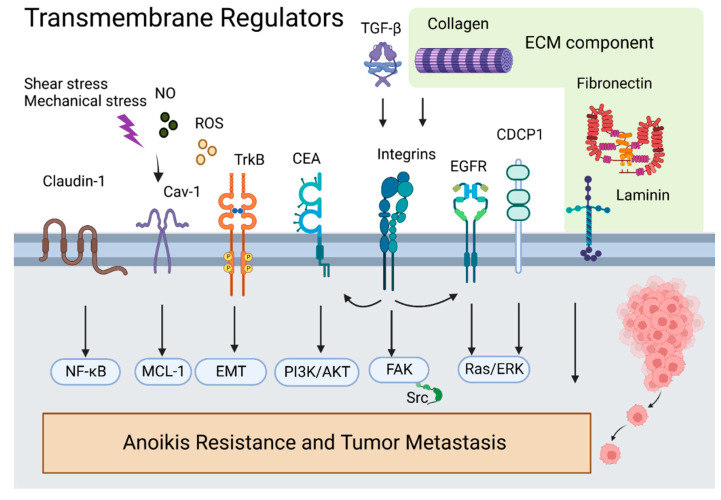
Anoikis is a critical biological process that antagonizes lung cancer metastasis. Tumor metastasis begins when cells detach from their native environment and adapt to new sites through blood vessels, lymphatics, or body cavities. Anoikis prevents tumor cells from detachment and re-adhesion to new matrices in incorrect locations or the new organism. During this process, the ECM, including collagen IV; laminin 5; fibronectin; and cell membrane proteins including integrins, CEA, EGFR, CDCP1, and CAV1, acts as a sensor and is the first site to receive cell detachment signals. CEA and EGFR engage in crosstalk with integrins and promote metastasis. Integrins can trigger FAK/Src, an essential pathway for inhibiting anoikis. CAV1 is a membrane adapter to kinase Fyn in integrin signaling; it interacts with MCL-1 to inhibit anoikis (Figure 7).
Figure 7.
Overview of transmembrane regulators in anoikis-associated lung cancer metastasis.
p66shc is a focal adhesion regulatory protein that initiates apoptosis signals for anoikis. Apoptosis-related proteins (i.e., BIM, P53, DAPK, and caspases) are critical targets driving anoikis. Inhibitors of apoptosis proteins MCL-1, Bit, and BCL-2 suppress anoikis. CEA, TrkB, FAK/Src, and βIII-tubulin inhibit anoikis through upregulation of the PI3K/AKT pathway. Next, growth factors negatively regulate anoikis, and TGF-β activates integrins to trigger intracellular apoptosis resistance. EFGR and TrkB activate ERK/MAPK or Ras/Raf/ERK signaling pathways to counteract anoikis. Furthermore, cytoskeleton regulator RhoA and the downstream effector ROCK antagonize Ras and FAK signaling and promote apoptotic signaling (Figure 8).
Figure 8.
Overview of primary regulators in anoikis-associated lung cancer metastasis.
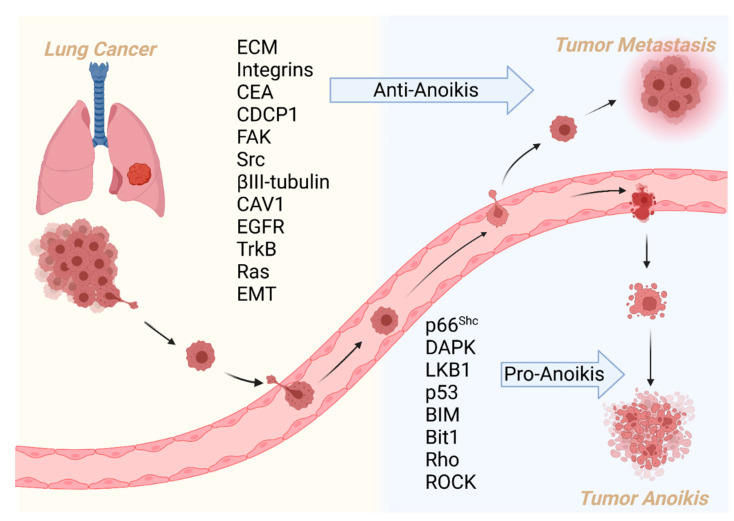
Because of evidence of anoikis reducing metastasis, preclinical investigations in lung cancer focus on targeting anoikis using novel small molecule compounds (natural and synthetic products), mAbs, and repurposed FDA-approved compounds. Therapeutic targets include increased p53 and inhibition of TGF-β/SMAD, FAK, AKT, and MCL-1. Despite several preclinical studies on anoikis-related inhibitors, a gap urgently needs to be filled, including an analysis of compounds that combat lung cancer metastases in vivo.
6. Conclusions
Anoikis plays an important role in lung cancer metastasis and is associated with tumor progression and therapy failure. The composition of the extracellular matrix, cell adhesion-related membrane proteins, cytoskeletal regulators, and epithelial–mesenchymal transition are involved in the process of anoikis, and the initiation of apoptosis signals is a critical step in anoikis. Several natural and synthetic products, including artonin E, imperatorin, oroxylin A, lupalbigenin, sulforaphane, renieramycin M, avicequinone B, and carbenoxolone, exhibit pro-anoikis potential. This review provides an overview of the major regulators and mechanisms of anoikis in lung cancer and discusses the therapeutic potential of targeting anoikis in the treatment of lung cancer.
Acknowledgments
Figures in the article were drawn using BioRender.com (accessed on 29 September 2022).
Author Contributions
Conceptualization, J.W., X.S., E.L.-H.L. and Q.W. (Qibiao Wu); writing—original draft preparation, J.W., Z.L. and L.Y.; writing—review and editing, J.W., L.L., R.Z., C.X., Z.Z., Q.Z., B.A., Q.W. (Qiao Wang) and B.C.; visualization, Z.L.; supervision, J.W. and Q.W. (Qibiao Wu). All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Natural Science Foundation of Jilin Province (grant number YDZJ202101ZYTS035); the Education Department of Jilin Province (grant number JJKH20210983KJ); the Science and Technology Development Fund, Macau SAR (grant number 0168/2019/A3, 0099/2018/A3, 0098/2021/A2, and 130/2017/A3); and the Science and Technology Planning Project of Guangdong Province (grant number 2020B1212030008).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Fehlmann T., Kahraman M., Ludwig N., Backes C., Galata V., Keller V., Geffers L., Mercaldo N., Hornung D., Weis T., et al. Evaluating the Use of Circulating MicroRNA Profiles for Lung Cancer Detection in Symptomatic Patients. JAMA Oncol. 2020;6:714–723. doi: 10.1001/jamaoncol.2020.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohn M., Shin S., Yoo J.-Y., Goh Y., Lee I.H., Bae Y.S. Ahnak promotes tumor metastasis through transforming growth factor-β-mediated epithelial-mesenchymal transition. Sci. Rep. 2018;8:14379. doi: 10.1038/s41598-018-32796-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talbot L.J., Bhattacharya S.D., Kuo P.C. Epithelial-mesenchymal transition, the tumor microenvironment, and metastatic behavior of epithelial malignancies. Int. J. Biochem. Mol. Biol. 2012;3:117–136. [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang B., Zhang T., Liu F., Sun Z., Shi H., Hua D., Yang C. The co-stimulatory molecule B7-H3 promotes the epithelial-mesenchymal transition in colorectal cancer. Oncotarget. 2016;7:31755–31771. doi: 10.18632/oncotarget.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamińska K., Szczylik C., Bielecka Z.F., Bartnik E., Porta C., Lian F., Czarnecka A.M. The role of the cell-cell interactions in cancer progression. J. Cell. Mol. Med. 2015;19:283–296. doi: 10.1111/jcmm.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frisch S.M., Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kockx M.M., Herman A.G. Apoptosis in atherosclerosis: Beneficial or detrimental? Cardiovasc. Res. 2000;45:736–746. doi: 10.1016/S0008-6363(99)00235-7. [DOI] [PubMed] [Google Scholar]
- 9.Dobler D., Ahmed N., Song L., Eboigbodin K.E., Thornalley P.J. Increased Dicarbonyl Metabolism in Endothelial Cells in Hyperglycemia Induces Anoikis and Impairs Angiogenesis by RGD and GFOGER Motif Modification. Diabetes. 2006;55:1961–1969. doi: 10.2337/db05-1634. [DOI] [PubMed] [Google Scholar]
- 10.Haun F., Neumann S., Peintner L., Wieland K., Habicht J., Schwan C., Østevold K., Koczorowska M.M., Biniossek M., Kist M., et al. Identification of a novel anoikis signalling pathway using the fungal virulence factor gliotoxin. Nat. Commun. 2018;9:3524. doi: 10.1038/s41467-018-05850-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson C.D., Anyiwe K., Schimmer A.D. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272:177–185. doi: 10.1016/j.canlet.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Chaffer C.L., San Juan B.P., Lim E., Weinberg R.A. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35:645–654. doi: 10.1007/s10555-016-9648-7. [DOI] [PubMed] [Google Scholar]
- 13.Zhao K., Wang Z., Hackert T., Pitzer C., Zöller M. Tspan8 and Tspan8/CD151 knockout mice unravel the contribution of tumor and host exosomes to tumor progression. J. Exp. Clin. Cancer Res. 2018;37:312. doi: 10.1186/s13046-018-0961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baethge C., Goldbeck-Wood S., Mertens S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019;4:5. doi: 10.1186/s41073-019-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theocharis A.D., Skandalis S.S., Gialeli C., Karamanos N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Taddei M.L., Giannoni E., Fiaschi T., Chiarugi P. Anoikis: An emerging hallmark in health and diseases. J. Pathol. 2012;226:380–393. doi: 10.1002/path.3000. [DOI] [PubMed] [Google Scholar]
- 17.Pickup M.W., Mouw J.K., Weaver V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enkhbat M., Zhong B., Chang R., Geng J., Lu L.-S., Chen Y.-J., Wang P.-Y. Harnessing Focal Adhesions to Accelerate p53 Accumulation and Anoikis of A549 Cells Using Colloidal Self-Assembled Patterns (cSAPs) ACS Appl. Bio Mater. 2022;5:322–333. doi: 10.1021/acsabm.1c01109. [DOI] [PubMed] [Google Scholar]
- 19.Han H.-J., Sung J.Y., Kim S.-H., Yun U.-J., Kim H., Jang E.-J., Yoo H.-E., Hong E.K., Goh S.-H., Moon A., et al. Fibronectin regulates anoikis resistance via cell aggregate formation. Cancer Lett. 2021;508:59–72. doi: 10.1016/j.canlet.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Kodama K., Ishii G., Miyamoto S., Goya M., Zhang S.-C., Sangai T., Yoshikawa T., Hasebe T., Hitomi Y., Izumi K., et al. Laminin 5 expression protects against anoikis at aerogenous spread and lepidic growth of human lung adenocarcinoma. Int. J. Cancer. 2005;116:876–884. doi: 10.1002/ijc.21136. [DOI] [PubMed] [Google Scholar]
- 21.Liu C.-C., Lin S.-P., Hsu H.-S., Yang S.-H., Lin C.-H., Yang M.-H., Hung M.-C., Hung S.-C. Suspension survival mediated by PP2A-STAT3-Col XVII determines tumour initiation and metastasis in cancer stem cells. Nat. Commun. 2016;7:11798. doi: 10.1038/ncomms11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnier J.V., Wang N., Michel R.P., Hassanain M., Li S., Lu Y., Metrakos P., Antecka E., Burnier M.N., Ponton A., et al. Type IV collagen-initiated signals provide survival and growth cues required for liver metastasis. Oncogene. 2011;30:3766–3783. doi: 10.1038/onc.2011.89. [DOI] [PubMed] [Google Scholar]
- 23.Jang I., Beningo K. Integrins, CAFs and Mechanical Forces in the Progression of Cancer. Cancers. 2019;11:721. doi: 10.3390/cancers11050721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamidi H., Ivaska J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer. 2018;18:533–548. doi: 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu J.-I., Lin Y.-P., Tseng C.-W., Chen H.-J., Wang L.-H. Crabp2 Promotes Metastasis of Lung Cancer Cells via HuR and Integrin β1/FAK/ERK Signaling. Sci. Rep. 2019;9:845. doi: 10.1038/s41598-018-37443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winograd-Katz S.E., Fässler R., Geiger B., Legate K.R. The integrin adhesome: From genes and proteins to human disease. Nat. Rev. Mol. Cell Biol. 2014;15:273–288. doi: 10.1038/nrm3769. [DOI] [PubMed] [Google Scholar]
- 27.Wang C., Wang T., Lv D., Li L., Yue J., Chen H.-Z., Xu L. Acquired Resistance to EGFR TKIs Mediated by TGFβ1/Integrin β3 Signaling in EGFR-Mutant Lung Cancer. Mol. Cancer Ther. 2019;18:2357–2367. doi: 10.1158/1535-7163.MCT-19-0181. [DOI] [PubMed] [Google Scholar]
- 28.Ordonez C., Zhai A.B., Camacho-Leal P., Demarte L., Fan M.M., Stanners C.P. GPI-anchored CEA family glycoproteins CEA and CEACAM6 mediate their biological effects through enhanced integrin α5β1-fibronectin interaction. J. Cell. Physiol. 2007;210:757–765. doi: 10.1002/jcp.20887. [DOI] [PubMed] [Google Scholar]
- 29.Stanners C.P. Cell Adhesion and Communication Mediated by the CEA Family. CRC Press; London, UK: 1998. [DOI] [Google Scholar]
- 30.Camacho-Leal P., Zhai A.B., Stanners C.P. A co-clustering model involving α5β1 integrin for the biological effects of GPI-anchored human carcinoembryonic antigen (CEA) J. Cell. Physiol. 2007;211:791–802. doi: 10.1002/jcp.20989. [DOI] [PubMed] [Google Scholar]
- 31.Camacholeal P., Stanners C.P. The human carcinoembryonic antigen (CEA) GPI anchor mediates anoikis inhibition by inactivation of the intrinsic death pathway. Oncogene. 2008;27:1545–1553. doi: 10.1038/sj.onc.1210789. [DOI] [PubMed] [Google Scholar]
- 32.Kim E.Y., Cha Y.J., Jeong S., Chang Y.S. Overexpression of CEACAM6 activates Src-FAK signaling and inhibits anoikis, through homophilic interactions in lung adenocarcinomas. Transl. Oncol. 2022;20:101402. doi: 10.1016/j.tranon.2022.101402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo J.-Y., Hsu H.-S., Tyan S.-W., Li F.-Y., Shew J.-Y., Lee W.-H., Chen J.-Y. Serglycin in tumor microenvironment promotes non-small cell lung cancer aggressiveness in a CD44-dependent manner. Oncogene. 2017;36:2457–2471. doi: 10.1038/onc.2016.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimobaba S., Taga S., Akizuki R., Hichino A., Endo S., Matsunaga T., Watanabe R., Yamaguchi M., Yamazaki Y., Sugatani J., et al. Claudin-18 inhibits cell proliferation and motility mediated by inhibition of phosphorylation of PDK1 and Akt in human lung adenocarcinoma A549 cells. Biochim. Biophys. Acta. 2016;1863:1170–1178. doi: 10.1016/j.bbamcr.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Runkle E.A., Rice S.J., Qi J., Masser D., Antonetti D.A., Winslow M.M., Mu D. Occludin Is a Direct Target of Thyroid Transcription Factor-1 (TTF-1/NKX2–1) J. Biol. Chem. 2012;287:28790–28801. doi: 10.1074/jbc.M112.367987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uekita T., Jia L., Narisawa-Saito M., Yokota J., Kiyono T., Sakai R. CUB Domain-Containing Protein 1 Is a Novel Regulator of Anoikis Resistance in Lung Adenocarcinoma. Mol. Cell. Biol. 2007;27:7649–7660. doi: 10.1128/MCB.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyazawa Y., Uekita T., Hiraoka N., Fujii S., Kosuge T., Kanai Y., Nojima Y., Sakai R. CUB Domain–Containing Protein 1, a Prognostic Factor for Human Pancreatic Cancers, Promotes Cell Migration and Extracellular Matrix Degradation. Cancer Res. 2010;70:5136–5146. doi: 10.1158/0008-5472.CAN-10-0220. [DOI] [PubMed] [Google Scholar]
- 38.Uekita T., Fujii S., Miyazawa Y., Iwakawa R., Narisawa-Saito M., Nakashima K., Tsuta K., Tsuda H., Kiyono T., Yokota J., et al. Oncogenic Ras/ERK Signaling Activates CDCP1 to Promote Tumor Invasion and Metastasis. Mol. Cancer Res. 2014;12:1449–1459. doi: 10.1158/1541-7786.MCR-13-0587. [DOI] [PubMed] [Google Scholar]
- 39.Uekita T., Fujii S., Miyazawa Y., Hashiguchi A., Abe H., Sakamoto M., Sakai R. Suppression of autophagy by CUB domain-containing protein 1 signaling is essential for anchorage-independent survival of lung cancer cells. Cancer Sci. 2013;104:865–870. doi: 10.1111/cas.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaller M.D. FAK and paxillin: Regulators of N-cadherin adhesion and inhibitors of cell migration? J. Cell Biol. 2004;166:157–159. doi: 10.1083/jcb.200406151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alanko J., Ivaska J. Endosomes: Emerging Platforms for Integrin-Mediated FAK Signalling. Trends Cell Biol. 2016;26:391–398. doi: 10.1016/j.tcb.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Liu G., Meng X., Jin Y., Bai J., Zhao Y., Cui X., Chen F., Fu S. Inhibitory role of focal adhesion kinase on anoikis in the lung cancer cell A549. Cell Biol. Int. 2008;32:663–670. doi: 10.1016/j.cellbi.2008.01.292. [DOI] [PubMed] [Google Scholar]
- 43.Keenan J., Joyce H., Aherne S., O’Dea S., Doolan P., Lynch V., Clynes M. Olfactomedin III expression contributes to anoikis-resistance in clonal variants of a human lung squamous carcinoma cell line. Exp. Cell Res. 2012;318:593–602. doi: 10.1016/j.yexcr.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 44.Tanikawa C., Furukawa Y., Yoshida N., Arakawa H., Nakamura Y., Matsuda K. XEDAR as a putative colorectal tumor suppressor that mediates p53-regulated anoikis pathway. Oncogene. 2009;28:3081–3092. doi: 10.1038/onc.2009.154. [DOI] [PubMed] [Google Scholar]
- 45.Thomas S.M., Brugge J.S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 46.Schlessinger J. New Roles for Src Kinases in Control of Cell Survival and Angiogenesis. Cell. 2000;100:293–296. doi: 10.1016/S0092-8674(00)80664-9. [DOI] [PubMed] [Google Scholar]
- 47.Sakuma Y., Takeuchi T., Nakamura Y., Yoshihara M., Matsukuma S., Nakayama H., Ohgane N., Yokose T., Kameda Y., Tsuchiya E., et al. Lung adenocarcinoma cells floating in lymphatic vessels resist anoikis by expressing phosphorylated Src. J. Pathol. 2009;220:574–585. doi: 10.1002/path.2676. [DOI] [PubMed] [Google Scholar]
- 48.Wei L., Yang Y., Zhang X., Yu Q. Altered regulation of Src upon cell detachment protects human lung adenocarcinoma cells from anoikis. Oncogene. 2004;23:9052–9061. doi: 10.1038/sj.onc.1208091. [DOI] [PubMed] [Google Scholar]
- 49.Wei L., Yang Y., Zhang X., Yu Q. Anchorage-independent phosphorylation of p130Cas protects lung adenocarcinoma cells from anoikis. J. Cell. Biochem. 2002;87:439–449. doi: 10.1002/jcb.10322. [DOI] [PubMed] [Google Scholar]
- 50.Liu A., Xie H., Li R., Ren L., Yang B., Dai L., Lu W., Liu B., Ren D., Zhang X., et al. Silencing ZIC2 abrogates tumorigenesis and anoikis resistance of non-small cell lung cancer cells by inhibiting Src/FAK signaling. Mol. Ther. Oncolytics. 2021;22:195–208. doi: 10.1016/j.omto.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim H., Sung J.Y., Park E.-K., Kho S., Koo K.H., Park S.-Y., Goh S.-H., Jeon Y.K., Oh S., Park B.-K., et al. Regulation of anoikis resistance by NADPH oxidase 4 and epidermal growth factor receptor. Br. J. Cancer. 2017;116:370–381. doi: 10.1038/bjc.2016.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jang E.-J., Sung J.Y., Yoo H.-E., Jang H., Shim J., Oh E.-S., Goh S.-H., Kim Y.-N. FAM188B Downregulation Sensitizes Lung Cancer Cells to Anoikis via EGFR Downregulation and Inhibits Tumor Metastasis In Vivo. Cancers. 2021;13:247. doi: 10.3390/cancers13020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Debnath J. p66(Shc) and Ras: Controlling anoikis from the inside-out. Oncogene. 2010;29:5556–5558. doi: 10.1038/onc.2010.347. [DOI] [PubMed] [Google Scholar]
- 54.Frisch S.M., Schaller M.D. The Wind God Promotes Lung Cancer. Cancer Cell. 2014;25:551–552. doi: 10.1016/j.ccr.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 55.Ma Z., Myers D.P., Wu R.F., Nwariaku F.E., Terada L.S. p66Shc mediates anoikis through RhoA. J. Cell Biol. 2007;179:23–31. doi: 10.1083/jcb.200706097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma Z., Liu Z., Wu R.-F., Terada L.S. p66Shc restrains Ras hyperactivation and suppresses metastatic behavior. Oncogene. 2010;29:5559–5567. doi: 10.1038/onc.2010.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng Z., Yang J., Zhao D., Gao D., Yan X., Yao Z., Liu Z., Ma Z. Downregulated adaptor protein p66Shcmitigates autophagy process by low nutrient and enhances apoptotic resistance in human lung adenocarcinoma A549 cells. FEBS J. 2013;280:4522–4530. doi: 10.1111/febs.12416. [DOI] [PubMed] [Google Scholar]
- 58.Cai Z., Zhao D., Sun Y., Gao D., Li X., Yang J., Ma Z. Detachment-Based Equilibrium of Anoikic Cell Death and Autophagic Cell Survival Through Adaptor Protein p66Shc. Anat. Rec. 2018;299:325–333. doi: 10.1002/ar.23299. [DOI] [PubMed] [Google Scholar]
- 59.Du W., Jiang Y., Zheng Z., Zhang Z., Chen N., Ma Z., Yao Z., Terada L., Liu Z. Feedback loop between p66Shc and Nrf2 promotes lung cancer progression. Cancer Lett. 2013;337:58–65. doi: 10.1016/j.canlet.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 60.Billot K., Soeur J., Chereau F., Arrouss I., Merle-Beral H., Huang M.-E., Mazier D., Baud V., Rebollo A. Deregulation of Aiolos expression in chronic lymphocytic leukemia is associated with epigenetic modifications. Blood. 2011;117:1917–1927. doi: 10.1182/blood-2010-09-307140. [DOI] [PubMed] [Google Scholar]
- 61.Li X., Xu Z., Du W., Zhang Z., Wei Y., Wang H., Zhu Z., Qin L., Wang L., Niu Q., et al. Aiolos Promotes Anchorage Independence by Silencing p66Shc Transcription in Cancer Cells. Cancer Cell. 2014;25:575–589. doi: 10.1016/j.ccr.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu Z., Wang H., Wei Y., Meng F., Liu Z., Zhang Z. Downregulation of PRDM1 promotes cellular invasion and lung cancer metastasis. Tumor Biol. 2017;39:1010428317695929. doi: 10.1177/1010428317695929. [DOI] [PubMed] [Google Scholar]
- 63.Li X., Gao D., Wang H., Yang J., Yan X., Liu Z., Ma Z. Negative feedback loop between p66Shc and ZEB1 regulates fibrotic EMT response in lung cancer cells. Cell Death Dis. 2015;6:e1708. doi: 10.1038/cddis.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reginato M.J., Mills K.R., Paulus J.K., Lynch D.K., Sgroi D.C., Debnath J., Muthuswamy S.K., Brugge J.S. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat. Cell Biol. 2003;5:733–740. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- 65.Jiang K., Sun J., Cheng J., Djeu J.Y., Wei S., Sebti S. Akt Mediates Ras Downregulation of RhoB, a Suppressor of Transformation, Invasion, and Metastasis. Mol. Cell. Biol. 2004;24:5565–5576. doi: 10.1128/MCB.24.12.5565-5576.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang C.-C., Yang M.-H., Lin B.-R., Chen S.-T., Pan S.-H., Hsiao M., Lai T.-C., Lin S.-K., Jeng Y.-M., Chu C.-Y., et al. CCN2 inhibits lung cancer metastasis through promoting DAPK-dependent anoikis and inducing EGFR degradation. Cell Death Differ. 2013;20:443–455. doi: 10.1038/cdd.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miłoszewska J., Przybyszewska M., Gos M., Swoboda P., Trembacz H. TrkB expression level correlates with metastatic properties of L1 mouse sarcoma cells cultured in non-adhesive conditions. Cell Prolif. 2013;46:146–152. doi: 10.1111/cpr.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smit M.A., Geiger T.R., Song J.-Y., Gitelman I., Peeper D.S. A Twist-Snail Axis Critical for TrkB-Induced Epithelial-Mesenchymal Transition-Like Transformation, Anoikis Resistance, and Metastasis. Mol. Cell. Biol. 2009;29:3722–3737. doi: 10.1128/MCB.01164-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ricci A., De Vitis C., Noto A., Fattore L., Mariotta S., Cherubini E., Roscilli G., Liguori G., Scognamiglio G., Rocco G., et al. TrkB is responsible for EMT transition in malignant pleural effusions derived cultures from adenocarcinoma of the lung. Cell Cycle. 2013;12:1696–1703. doi: 10.4161/cc.24759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geiger T.R., Song J.-Y., Rosado A., Peeper D.S. Functional Characterization of Human Cancer-Derived TRKB Mutations. PLoS ONE. 2011;6:e16871. doi: 10.1371/journal.pone.0016871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Voisin L., Julien C., Duhamel S., Gopalbhai K., Claveau I., Saba-El-Leil M.K., Rodrigue-Gervais I.G., Gaboury L., Lamarre D., Basik M., et al. Activation of MEK1 or MEK2 isoform is sufficient to fully transform intestinal epithelial cells and induce the formation of metastatic tumors. BMC Cancer. 2008;8:337. doi: 10.1186/1471-2407-8-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pearson G., Robinson F., Beers Gibson T., Xu B.E., Karandikar M., Berman K., Cobb M.H. Mitogen-Activated Protein (MAP) Kinase Pathways: Regulation and Physiological Functions. Endocr. Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 73.Jiang K., Delarue F.L., Sebti S.M. EGFR, ErbB2 and Ras but not Src suppress RhoB expression while ectopic expression of RhoB antagonizes oncogene-mediated transformation. Oncogene. 2004;23:1136–1145. doi: 10.1038/sj.onc.1207236. [DOI] [PubMed] [Google Scholar]
- 74.James M.A., Vikis H.G., Tate E., Rymaszewski A.L., You M. CRR9/CLPTM1L Regulates Cell Survival Signaling and Is Required for Ras Transformation and Lung Tumorigenesis. Cancer Res. 2014;74:1116–1127. doi: 10.1158/0008-5472.CAN-13-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei L., Yang Y., Yu Q. Tyrosine kinase-dependent, phosphatidylinositol 3′-kinase, and mitogen-activated protein ki-nase-independent signaling pathways prevent lung adenocarcinoma cells from anoikis. Cancer Res. 2001;61:2439–2444. [PubMed] [Google Scholar]
- 76.McCarroll J.A., Gan P.P., Erlich R.B., Liu M., Dwarte T., Sagnella S.S., Akerfeldt M.C., Yang L., Parker A.L., Chang M.H., et al. TUBB3/βIII-Tubulin Acts through the PTEN/AKT Signaling Axis to Promote Tumorigenesis and Anoikis Resistance in Non–Small Cell Lung Cancer. Cancer Res. 2015;75:415–425. doi: 10.1158/0008-5472.CAN-14-2740. [DOI] [PubMed] [Google Scholar]
- 77.Xu S., Lam S.-K., Cheng P.N.-M., Ho J.C.-M. Contactin 1 modulates pegylated arginase resistance in small cell lung cancer through induction of epithelial-mesenchymal transition. Sci. Rep. 2019;9:12030. doi: 10.1038/s41598-019-48476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmidt M., Hövelmann S., Beckers T.L. A novel form of constitutively active farnesylated Akt1 prevents mammary epithelial cells from anoikis and suppresses chemotherapy-induced apoptosis. Br. J. Cancer. 2002;87:924–932. doi: 10.1038/sj.bjc.6600566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miyamoto S., Yano K., Sugimoto S., Ishii G., Hasebe T., Endoh Y., Kodama K., Goya M., Chiba T., Ochiai A. Matrix Metalloproteinase-7 Facilitates Insulin-Like Growth Factor Bioavailability through Its Proteinase Activity on Insulin-Like Growth Factor Binding Protein 3. Cancer Res. 2004;64:665–671. doi: 10.1158/0008-5472.CAN-03-1916. [DOI] [PubMed] [Google Scholar]
- 80.Xie M., Wu X.-J., Zhang J.-J., He C.-S. IL-13 receptor α2 is a negative prognostic factor in human lung cancer and stimulates lung cancer growth in mice. Oncotarget. 2015;6:32902–32913. doi: 10.18632/oncotarget.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tian H., Lian R., Li Y., Liu C., Liang S., Li W., Tao T., Wu X., Ye Y., Yang X., et al. AKT-induced lncRNA VAL promotes EMT-independent metastasis through diminishing Trim16-dependent Vimentin degradation. Nat. Commun. 2020;11:5127. doi: 10.1038/s41467-020-18929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jordan M.A., Wilson L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 83.Heasman S.J., Ridley A.J. Mammalian Rho GTPases: New insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 84.Ren X.D., Kiosses W.B., Schwartz M.A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Riento K., Ridley A.J. ROCKs: Multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 86.Sargiacomo M., Scherer P.E., Tang Z., Kübler E., Song K.S., Sanders M.C., Lisanti M.P. Oligomeric structure of caveolin: Implications for caveolae membrane organization. Proc. Natl. Acad. Sci. USA. 1995;92:9407–9411. doi: 10.1073/pnas.92.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grande-García A., Echarri A., de Rooij J., Alderson N.B., Waterman-Storer C.M., Valdivielso J.M., del Pozo M.A. Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J. Cell Biol. 2007;177:683–694. doi: 10.1083/jcb.200701006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wary K.K., Mariotti A., Zurzolo C., Giancotti F.G. A Requirement for Caveolin-1 and Associated Kinase Fyn in Integrin Signaling and Anchorage-Dependent Cell Growth. Cell. 1998;94:625–634. doi: 10.1016/S0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- 89.Chanvorachote P., Nimmannit U., Lu Y., Talbott S., Jiang B.-H., Rojanasakul Y. Nitric Oxide Regulates Lung Carcinoma Cell Anoikis through Inhibition of Ubiquitin-Proteasomal Degradation of Caveolin-1. J. Biol. Chem. 2009;284:28476–28484. doi: 10.1074/jbc.M109.050864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chanvorachote P., Pongrakhananon V., Chunhacha P. Prolonged Nitric Oxide Exposure Enhances Anoikis Resistance and Migration through Epithelial-Mesenchymal Transition and Caveolin-1 Upregulation. BioMed Res. Int. 2014;2014:941359. doi: 10.1155/2014/941359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boisvert-Adamo K., Longmate W., Abel E.V., Aplin A.E. Mcl-1 Is Required for Melanoma Cell Resistance to Anoikis. Mol. Cancer Res. 2009;7:549–556. doi: 10.1158/1541-7786.MCR-08-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Woods N.T., Yamaguchi H., Lee F.Y., Bhalla K.N., Wang H.-G. Anoikis, Initiated by Mcl-1 Degradation and Bim Induction, Is Deregulated during Oncogenesis. Cancer Res. 2007;67:10744–10752. doi: 10.1158/0008-5472.CAN-07-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chunhacha P., Pongrakhananon V., Rojanasakul Y., Chanvorachote P. Caveolin-1 regulates Mcl-1 stability and anoikis in lung carcinoma cells. Am. J. Physiol. Physiol. 2012;302:C1284–C1292. doi: 10.1152/ajpcell.00318.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang H., Guan L., Li S., Jiang Y., Xiong N., Li L., Wu C., Zeng H., Liu Y. Mechanosensitive caveolin-1 activation-induced PI3K/Akt/mTOR signaling pathway promotes breast cancer motility, invadopodia formation and metastasis in vivo. Oncotarget. 2016;7:16227–16247. doi: 10.18632/oncotarget.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li S., Chen Y., Zhang Y., Jiang X., Jiang Y., Qin X., Yang H., Wu C., Liu Y. Shear stress promotes anoikis resistance of cancer cells via caveolin-1-dependent extrinsic and intrinsic apoptotic pathways. J. Cell. Physiol. 2019;234:3730–3743. doi: 10.1002/jcp.27149. [DOI] [PubMed] [Google Scholar]
- 96.Rungtabnapa P., Nimmannit U., Halim H., Rojanasakul Y., Chanvorachote P. Hydrogen peroxide inhibits non-small cell lung cancer cell anoikis through the inhibition of caveolin-1 degradation. Am. J. Physiol. Physiol. 2011;300:C235–C245. doi: 10.1152/ajpcell.00249.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Halim H., Luanpitpong S., Chanvorachote P. Acquisition of anoikis resistance up-regulates caveolin-1 expression in human non-small cell lung cancer cells. Anticancer Res. 2012;32:1649–1658. [PubMed] [Google Scholar]
- 98.Chanvorachote P., Pongrakhananon V., Halim H. Caveolin-1 regulates metastatic behaviors of anoikis resistant lung cancer cells. Mol. Cell. Biochem. 2015;399:291–302. doi: 10.1007/s11010-014-2255-4. [DOI] [PubMed] [Google Scholar]
- 99.Songserm T., Pongrakhananon V., Chanvorachote P. Sub-toxic cisplatin mediates anoikis resistance through hydrogen peroxide-induced caveolin-1 up-regulation in non-small cell lung cancer cells. Anticancer Res. 2012;32:1659–1669. [PubMed] [Google Scholar]
- 100.Halim H., Chanvorachote P. Long-term hydrogen peroxide exposure potentiates anoikis resistance and anchorage-independent growth in lung carcinoma cells. Cell Biol. Int. 2012;36:1055–1066. doi: 10.1042/CBI20120111. [DOI] [PubMed] [Google Scholar]
- 101.Lloyd P.G., Hardin C.D. Caveolae in cancer: Two sides of the same coin? Focus on “Hydrogen peroxide inhibits non-small cell lung cancer cell anoikis through the inhibition of caveolin-1 degradation”. Am. J. Physiol. Physiol. 2011;300:C232–C234. doi: 10.1152/ajpcell.00483.2010. [DOI] [PubMed] [Google Scholar]
- 102.Guo X., Wang Z., Sun Q., Sun C., Hua H., Huang Q. The inhibitory effect of microRNA-1827 on anoikis resistance in lung adenocarcinoma A549 cells via targeting caveolin-1. Acta Biochim. Biophys. Sin. 2020;52:1148–1155. doi: 10.1093/abbs/gmaa102. [DOI] [PubMed] [Google Scholar]
- 103.Wei F., Ma C., Zhou T., Dong X., Luo Q., Geng L., Ding L., Zhang Y., Zhang L., Li N., et al. Exosomes derived from gemcitabine-resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Mol. Cancer. 2017;16:132. doi: 10.1186/s12943-017-0694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thiery J.P., Acloque H., Huang R.Y.J., Nieto M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 105.Powan P., Luanpitpong S., He X., Rojanasakul Y., Chanvorachote P. Detachment-induced E-cadherin expression promotes 3D tumor spheroid formation but inhibits tumor formation and metastasis of lung cancer cells. Am. J. Physiol. Physiol. 2017;313:C556–C566. doi: 10.1152/ajpcell.00096.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Frisch S.M., Schaller M., Cieply B. Mechanisms that link the oncogenic epithelial–mesenchymal transition to suppression of anoikis. J. Cell Sci. 2013;126:21–29. doi: 10.1242/jcs.120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Denlinger C.E., Ikonomidis J.S., Reed C.E., Spinale F.G. Epithelial to mesenchymal transition: The doorway to metastasis in human lung cancers. J. Thorac. Cardiovasc. Surg. 2010;140:505–513. doi: 10.1016/j.jtcvs.2010.02.061. [DOI] [PubMed] [Google Scholar]
- 108.Adeshakin F.O., Adeshakin A.O., Afolabi L.O., Yan D., Zhang G., Wan X. Mechanisms for Modulating Anoikis Resistance in Cancer and the Relevance of Metabolic Reprogramming. Front. Oncol. 2021;11:626577. doi: 10.3389/fonc.2021.626577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salvi A., Thanabalu T. WIP promotes in-vitro invasion ability, anchorage independent growth and EMT progression of A549 lung adenocarcinoma cells by regulating RhoA levels. Biochem. Biophys. Res. Commun. 2017;482:1353–1359. doi: 10.1016/j.bbrc.2016.12.040. [DOI] [PubMed] [Google Scholar]
- 110.Xie M., Zhang L., He C.-S., Xu F., Liu J.-L., Hu Z.-H., Zhao L.-P., Tian Y. Activation of Notch-1 enhances epithelial-mesenchymal transition in gefitinib-acquired resistant lung cancer cells. J. Cell. Biochem. 2012;113:1501–1513. doi: 10.1002/jcb.24019. [DOI] [PubMed] [Google Scholar]
- 111.She K., Yang W., Li M., Xiong W., Zhou M. FAIM2 Promotes Non-Small Cell Lung Cancer Cell Growth and Bone Metastasis by Activating the Wnt/β-Catenin Pathway. Front. Oncol. 2021;11:690142. doi: 10.3389/fonc.2021.690142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bialik S., Kimchi A. The Death-Associated Protein Kinases: Structure, Function, and Beyond. Annu. Rev. Biochem. 2006;75:189–210. doi: 10.1146/annurev.biochem.75.103004.142615. [DOI] [PubMed] [Google Scholar]
- 113.Kwon T., Youn H., Son B., Kim D., Seong K.M., Park S., Kim W., Youn B. DANGER is involved in high glucose-induced radioresistance through inhibiting DAPK-mediated anoikis in non-small cell lung cancer. Oncotarget. 2016;7:7193–7206. doi: 10.18632/oncotarget.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cheng H., Liu P., Wang Z.C., Zou L., Santiago S., Garbitt V., Gjoerup O.V., Iglehart J.D., Miron A., Richardson A.L., et al. SIK1 Couples LKB1 to p53-Dependent Anoikis and Suppresses Metastasis. Sci. Signal. 2009;2:ra35. doi: 10.1126/scisignal.2000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bachelder R.E., Ribick M.J., Marchetti A., Falcioni R., Soddu S., Davis K.R., Mercurio A.M. P53 Inhibits α6β4 Integrin Survival Signaling by Promoting the Caspase 3–Dependent Cleavage of Akt/PKB. J. Cell Biol. 1999;147:1063–1072. doi: 10.1083/jcb.147.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marcus A.I., Zhou W. LKB1 Regulated Pathways in Lung Cancer Invasion and Metastasis. J. Thorac. Oncol. 2010;5:1883–1886. doi: 10.1097/JTO.0b013e3181fbc28a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ji H., Ramsey M.R., Hayes D.N., Fan C., McNamara K., Kozlowski P., Torrice C., Wu M.C., Shimamura T., Perera S.A., et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 118.Platt R.J., Chen S., Zhou Y., Yim M.J., Swiech L., Kempton H.R., Dahlman J.E., Parnas O., Eisenhaure T.M., Jovanovic M., et al. CRISPR-Cas9 Knockin Mice for Genome Editing and Cancer Modeling. Cell. 2014;159:440–455. doi: 10.1016/j.cell.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Akiyama T., Dass C.R., Choong P.F. Bim-targeted cancer therapy: A link between drug action and underlying molecular changes. Mol. Cancer Ther. 2009;8:3173–3180. doi: 10.1158/1535-7163.mct-09-0685. [DOI] [PubMed] [Google Scholar]
- 120.Li Z., Zhao J., Du Y., Park H.R., Sun S.-Y., Bernal-Mizrachi L., Aitken A., Khuri F.R., Fu H. Down-regulation of 14-3-3ζ suppresses anchorage-independent growth of lung cancer cells through anoikis activation. Proc. Natl. Acad. Sci. USA. 2008;105:162–167. doi: 10.1073/pnas.0710905105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yao X., Pham T., Temple B., Gray S., Cannon C., Chen R., Abdel-Mageed A.B., Biliran H. The Anoikis Effector Bit1 Inhibits EMT through Attenuation of TLE1-Mediated Repression of E-Cadherin in Lung Cancer Cells. PLoS ONE. 2016;11:e0163228. doi: 10.1371/journal.pone.0163228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yao X., Gray S., Pham T., Delgardo M., Nguyen A., Do S., Ireland S.K., Chen R., Abdel-Mageed A.B., Biliran H. Downregulation of Bit1 expression promotes growth, anoikis resistance, and transformation of immortalized human bronchial epithelial cells via Erk activation-dependent suppression of E-cadherin. Biochem. Biophys. Res. Commun. 2018;495:1240–1248. doi: 10.1016/j.bbrc.2017.11.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yao X., Jennings S., Ireland S.K., Pham T., Temple B., Davis M., Chen R., Davenport I., Biliran H. The Anoikis Effector Bit1 Displays Tumor Suppressive Function in Lung Cancer Cells. PLoS ONE. 2014;9:e101564. doi: 10.1371/journal.pone.0101564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yao X., Pham T., Temple B., Gray S., Cannon C., Hardy C., Fletcher K., Ireland S.K., Hossain A., Chen R., et al. TLE1 inhibits anoikis and promotes tumorigenicity in human lung cancer cells through ZEB1-mediated E-cadherin repression. Oncotarget. 2017;8:72235–72249. doi: 10.18632/oncotarget.19703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brunquell C., Biliran H., Jennings S., Ireland S.K., Chen R., Ruoslahti E. TLE1 Is an Anoikis Regulator and Is Downregulated by Bit1 in Breast Cancer Cells. Mol. Cancer Res. 2012;10:1482–1495. doi: 10.1158/1541-7786.MCR-12-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shaw R.J. Tumor Suppression by LKB1: SIK-ness Prevents Metastasis. Sci. Signal. 2009;2:pe55. doi: 10.1126/scisignal.286pe55. [DOI] [PubMed] [Google Scholar]
- 127.Mack H.I., Munger K. The LKB1 tumor suppressor differentially affects anchorage independent growth of HPV positive cervical cancer cell lines. Virology. 2013;446:9–16. doi: 10.1016/j.virol.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jin L., Chun J., Pan C., Kumar A., Zhang G., Ha Y., Li D., Alesi G.N., Kang Y., Zhou L., et al. The PLAG1-GDH1 Axis Promotes Anoikis Resistance and Tumor Metastasis through CamKK2-AMPK Signaling in LKB1-Deficient Lung Cancer. Mol. Cell. 2018;69:87–99.e7. doi: 10.1016/j.molcel.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Glutaminolysis Drives Lung Cancer Metastasis via the PLAG1–GDH1 Axis. Cancer Discov. 2018;8:135. doi: 10.1158/2159-8290.CD-RW2018-004. [DOI] [PubMed] [Google Scholar]
- 130.Coloff J.L. Glutamate Dehydrogenase to the Rescue. Mol. Cell. 2018;69:1–2. doi: 10.1016/j.molcel.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 131.Kuang B.-H., Zhang M.-Q., Xu L.-H., Hu L.-J., Wang H.-B., Zhao W.-F., Du Y., Zhang X. Proline-rich tyrosine kinase 2 and its phosphorylated form pY881 are novel prognostic markers for non-small-cell lung cancer progression and patients’ overall survival. Br. J. Cancer. 2013;109:1252–1263. doi: 10.1038/bjc.2013.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baldacci S., Kherrouche Z., Cockenpot V., Stoven L., Copin M.C., Werkmeister E., Marchand N., Kyheng M., Tulasne D., Cortot A.B. MET amplification increases the metastatic spread of EGFR-mutated NSCLC. Lung Cancer. 2018;125:57–67. doi: 10.1016/j.lungcan.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 133.Tykhomyrov A.A., Nedzvetsky V.S., Aĝca C.A., Guzyk M.M., Korsa V.V., Grinenko T.V. Plasminogen/plasmin affects expression of glycolysis regulator TIGAR and induces autophagy in lung adenocarcinoma A549 cells. Exp. Oncol. 2020;42:270–276. doi: 10.32471/exp-oncology.2312-8852.vol-42-no-4.15253. [DOI] [PubMed] [Google Scholar]
- 134.Chen H., Lee J., Kljavin N.M., Haley B., Daemen A., Johnson L., Liang Y. Requirement for BUB1B/BUBR1 in tumor progression of lung adenocarcinoma. Genes Cancer. 2015;6:106–118. doi: 10.18632/genesandcancer.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Choi E.-B., Yang A.-Y., Kim S.C., Lee J., Choi J.K., Choi C., Kim M.-Y. PARP1 enhances lung adenocarcinoma metastasis by novel mechanisms independent of DNA repair. Oncogene. 2016;35:4569–4579. doi: 10.1038/onc.2016.3. [DOI] [PubMed] [Google Scholar]
- 136.Dey S., Sayers C.M., Verginadis I.I., Lehman S.L., Cheng Y., Cerniglia G.J., Tuttle S.W., Feldman M.D., Zhang P.J., Fuchs S.Y., et al. ATF4-dependent induction of heme oxygenase 1 prevents anoikis and promotes metastasis. J. Clin. Investig. 2015;125:2592–2608. doi: 10.1172/JCI78031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Madajewski B., Boatman M.A., Chakrabarti G., Boothman D.A., Bey E.A. Depleting Tumor-NQO1 Potentiates Anoikis and Inhibits Growth of NSCLC. Mol. Cancer Res. 2016;14:14–25. doi: 10.1158/1541-7786.MCR-15-0207-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cheng X., Liu F., Liu H., Wang G., Hao H. Enhanced glycometabolism as a mechanism of NQO1 potentiated growth of NSCLC revealed by metabolomic profiling. Biochem. Biophys. Res. Commun. 2018;496:31–36. doi: 10.1016/j.bbrc.2017.12.160. [DOI] [PubMed] [Google Scholar]
- 139.Wang G.X., Tu H.-C., Dong Y., Skanderup A.J., Wang Y., Takeda S., Ganesan Y.T., Han S., Liu H., Hsieh J.J., et al. ΔNp63 Inhibits Oxidative Stress-Induced Cell Death, Including Ferroptosis, and Cooperates with the BCL-2 Family to Promote Clonogenic Survival. Cell Rep. 2017;21:2926–2939. doi: 10.1016/j.celrep.2017.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li N., Liu T., Li H., Zhang L., Chu L., Meng Q., Qiao Q., Han W., Zhang J., Guo M., et al. ILF2 promotes anchorage independence through direct regulation of PTEN. Oncol. Lett. 2019;18:1689–1696. doi: 10.3892/ol.2019.10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang H., Wang G., Zhou R., Li X., Sun Y., Li Y., Du W., Yan X., Yang J., Chang X., et al. SPIB promotes anoikis resistance via elevated autolysosomal process in lung cancer cells. FEBS J. 2020;287:4696–4709. doi: 10.1111/febs.15272. [DOI] [PubMed] [Google Scholar]
- 142.Yao S., Huang H.-Y., Han X., Ye Y., Qin Z., Zhao G., Li F., Hu G., Hu L., Ji H. Keratin 14-high subpopulation mediates lung cancer metastasis potentially through Gkn1 upregulation. Oncogene. 2019;38:6354–6369. doi: 10.1038/s41388-019-0889-0. [DOI] [PubMed] [Google Scholar]
- 143.Noto A., Raffa S., De Vitis C., Roscilli G., Malpicci D., Coluccia P., Di Napoli A., Ricci A., Giovagnoli M.R., Aurisicchio L., et al. Stearoyl-CoA desaturase-1 is a key factor for lung cancer-initiating cells. Cell Death Dis. 2013;4:e947. doi: 10.1038/cddis.2013.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sato S., Hanibuchi M., Kuramoto T., Yamamori N., Goto H., Ogawa H., Mitsuhashi A., Van T.T., Kakiuchi S., Akiyama S.-I., et al. Macrophage stimulating protein promotes liver metastases of small cell lung cancer cells by affecting the organ microenvironment. Clin. Exp. Metastasis. 2013;30:333–344. doi: 10.1007/s10585-012-9540-y. [DOI] [PubMed] [Google Scholar]
- 145.Godin-Heymann N., Brabetz S., Murillo M.M., Saponaro M., Santos C.R., Lobley A., East P., Chakravarty P., Matthews N., Kelly G., et al. Tumour-suppression function of KLF12 through regulation of anoikis. Oncogene. 2016;35:3324–3334. doi: 10.1038/onc.2015.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Quintás-Cardama A., Post S.M., Solis L.M., Xiong S., Yang P., Chen N., Wistuba I.I., Killary A.M., Lozano G. Loss of the novel tumour suppressor and polarity gene Trim62 (Dear1) synergizes with oncogenic Ras in invasive lung cancer. J. Pathol. 2014;234:108–119. doi: 10.1002/path.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang L.N., Zhang Z.T., Wang L., Wei H.X., Zhang T., Zhang L.M., Lin H., Zhang H., Wang S.Q. TGF-β1/SH2B3 axis regulates anoikis resistance and EMT of lung cancer cells by modulating JAK2/STAT3 and SHP2/Grb2 signaling pathways. Cell Death Dis. 2022;13:472. doi: 10.1038/s41419-022-04890-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Halim H., Chunhacha P., Suwanborirux K., Chanvorachote P. Anticancer and antimetastatic activities of Renieramycin M, a marine tetrahydroisoquinoline alkaloid, in human non-small cell lung cancer cells. Anticancer Res. 2011;31:193–201. [PubMed] [Google Scholar]
- 149.Powan P., Saito N., Suwanborirux K., Chanvorachote P. Ecteinascidin 770, a tetrahydroisoquinoline alkaloid, sensitizes human lung cancer cells to anoikis. Anticancer Res. 2013;33:505–512. [PubMed] [Google Scholar]
- 150.Choochuay K., Chunhacha P., Pongrakhananon V., Luechapudiporn R., Chanvorachote P. Imperatorin sensitizes anoikis and inhibits anchorage-independent growth of lung cancer cells. J. Nat. Med. 2013;67:599–606. doi: 10.1007/s11418-012-0719-y. [DOI] [PubMed] [Google Scholar]
- 151.Wongpankam E., Chunhacha P., Pongrakhananon V., Sritularak B., Chanvorachote P. Artonin E mediates MCL1 down-regulation and sensitizes lung cancer cells to anoikis. Anticancer Res. 2012;32:5343–5351. [PubMed] [Google Scholar]
- 152.Wei L., Dai Q., Zhou Y., Zou M., Li Z., Lu N., Guo Q. Oroxylin A sensitizes non-small cell lung cancer cells to anoikis via glucose-deprivation-like mechanisms: C-Src and hexokinase II. Biochim. Biophys. Acta. 2013;1830:3835–3845. doi: 10.1016/j.bbagen.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 153.Chaotham C., Pongrakhananon V., Sritularak B., Chanvorachote P. A Bibenzyl from Dendrobium ellipsophyllum inhibits epithelial-to-mesenchymal transition and sensitizes lung cancer cells to anoikis. Anticancer Res. 2014;34:1931–1938. [PubMed] [Google Scholar]
- 154.Ausawasamrit A., Itthiwarapornkul N., Chaotham C., Sukrong S., Chanvorachote P. Lupalbigenin from Derris scandens Sensitizes Detachment-induced Cell Death in Human Lung Cancer Cells. Anticancer Res. 2015;35:2827–2834. [PubMed] [Google Scholar]
- 155.Tsai J.-Y., Tsai S.-H., Wu C.-C. The chemopreventive isothiocyanate sulforaphane reduces anoikis resistance and anchorage-independent growth in non-small cell human lung cancer cells. Toxicol. Appl. Pharmacol. 2019;362:116–124. doi: 10.1016/j.taap.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 156.Putri H.E., Sritularak B., Chanvorachote P. Pongamol Inhibits Epithelial to Mesenchymal Transition Through Suppression of FAK/Akt-mTOR Signaling. Anticancer Res. 2021;41:6147–6154. doi: 10.21873/anticanres.15434. [DOI] [PubMed] [Google Scholar]
- 157.Cao R., Jin W., Shan Y., Wang J., Liu G., Kuang S., Sun C. Marine Bacterial Polysaccharide EPS11 Inhibits Cancer Cell Growth via Blocking Cell Adhesion and Stimulating Anoikis. Mar. Drugs. 2018;16:85. doi: 10.3390/md16030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim Y.-J., Choi W.-I., Jeon B.-N., Choi K.-C., Kim K., Kim T.-J., Ham J., Jang H.J., Kang K.S., Ko H. Stereospecific effects of ginsenoside 20-Rg3 inhibits TGF-β1-induced epithelial–mesenchymal transition and suppresses lung cancer migration, invasion and anoikis resistance. Toxicology. 2014;322:23–33. doi: 10.1016/j.tox.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 159.Kim H., Choi P., Kim T., Kim Y., Song B.G., Park Y.-T., Choi S.-J., Yoon C.H., Lim W.-C., Ko H., et al. Ginsenosides Rk1 and Rg5 inhibit transforming growth factor-β1-induced epithelial-mesenchymal transition and suppress migration, invasion, anoikis resistance, and development of stem-like features in lung cancer. J. Ginseng Res. 2021;45:134–148. doi: 10.1016/j.jgr.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ecoy G.A.U., Chamni S., Suwanborirux K., Chanvorachote P., Chaotham C. Jorunnamycin A from Xestospongia sp. Suppresses Epithelial to Mesenchymal Transition and Sensitizes Anoikis in Human Lung Cancer Cells. J. Nat. Prod. 2019;82:1861–1873. doi: 10.1021/acs.jnatprod.9b00102. [DOI] [PubMed] [Google Scholar]
- 161.Busaranon K., Plaimee P., Sritularak B., Chanvorachote P. Moscatilin inhibits epithelial-to-mesenchymal transition and sensitizes anoikis in human lung cancer H460 cells. J. Nat. Med. 2016;70:18–27. doi: 10.1007/s11418-015-0931-7. [DOI] [PubMed] [Google Scholar]
- 162.Unahabhokha T., Chanvorachote P., Pongrakhananon V. The attenuation of epithelial to mesenchymal transition and induction of anoikis by gigantol in human lung cancer H460 cells. Tumor Biol. 2016;37:8633–8641. doi: 10.1007/s13277-015-4717-z. [DOI] [PubMed] [Google Scholar]
- 163.Nonpanya N., Prakhongcheep O., Petsri K., Jitjaicham C., Tungsukruthai S., Sritularak B., Chanvorachote P. Ephemeranthol A Suppresses Epithelial to Mesenchymal Transition and FAK-Akt Signaling in Lung Cancer Cells. Anticancer Res. 2020;40:4989–4999. doi: 10.21873/anticanres.14502. [DOI] [PubMed] [Google Scholar]
- 164.Pengpaeng P., Sritularak B., Chanvorachote P. Dendrofalconerol A sensitizes anoikis and inhibits migration in lung cancer cells. J. Nat. Med. 2015;69:178–190. doi: 10.1007/s11418-014-0876-2. [DOI] [PubMed] [Google Scholar]
- 165.Chanvorachote P., Kowitdamrong A., Ruanghirun T., Sritularak B., Mungmee C., Likhitwitayawuid K. Anti-metastatic Activities of Bibenzyls from Dendrobium pulchellum. Nat. Prod. Commun. 2013;8:115–118. doi: 10.1177/1934578X1300800127. [DOI] [PubMed] [Google Scholar]
- 166.Tanagornmeatar K., Chaotham C., Sritularak B., Likhitwitayawuid K., Chanvorachote P. Cytotoxic and anti-metastatic activities of phenolic compounds from Dendrobium ellipsophyllum. Anticancer Res. 2014;34:6573–6579. [PubMed] [Google Scholar]
- 167.Lee H.-Z., Yang W.-H., Hour M.-J., Wu C.-Y., Peng W.-H., Bao B.-Y., Han P.-H., Bau D.-T. Photodynamic activity of aloe-emodin induces resensitization of lung cancer cells to anoikis. Eur. J. Pharmacol. 2010;648:50–58. doi: 10.1016/j.ejphar.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 168.Chang W.-T., You B.-J., Yang W.-H., Wu C.-Y., Bau D.-T., Lee H.-Z. Protein kinase C delta-mediated cytoskeleton remodeling is involved in aloe-emodin-induced photokilling of human lung cancer cells. Anticancer Res. 2012;32:3707–3713. [PubMed] [Google Scholar]
- 169.Pongrakhananon V., Nimmannit U., Luanpitpong S., Rojanasakul Y., Chanvorachote P. Curcumin sensitizes non-small cell lung cancer cell anoikis through reactive oxygen species-mediated Bcl-2 downregulation. Apoptosis. 2010;15:574–585. doi: 10.1007/s10495-010-0461-4. [DOI] [PubMed] [Google Scholar]
- 170.Ko H. Geraniin inhibits TGF-β1-induced epithelial–mesenchymal transition and suppresses A549 lung cancer migration, invasion and anoikis resistance. Bioorganic Med. Chem. Lett. 2015;25:3529–3534. doi: 10.1016/j.bmcl.2015.06.093. [DOI] [PubMed] [Google Scholar]
- 171.Lim W.-C., Choi J.W., Song N.-E., Cho C.-W., Rhee Y.K., Hong H.-D. Polysaccharide isolated from persimmon leaves (Diospyros kaki Thunb.) suppresses TGF-β1-induced epithelial-to-mesenchymal transition in A549 cells. Int. J. Biol. Macromol. 2020;164:3835–3845. doi: 10.1016/j.ijbiomac.2020.08.155. [DOI] [PubMed] [Google Scholar]
- 172.Trabalzini L., Ercoli J., Trezza A., Schiavo I., Macrì G., Moglia A., Spiga O., Finetti F. Pharmacological and In Silico Analysis of Oat Avenanthramides as EGFR Inhibitors: Effects on EGF-Induced Lung Cancer Cell Growth and Migration. Int. J. Mol. Sci. 2022;23:8534. doi: 10.3390/ijms23158534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Que Z.-J., Yang Y., Liu H.-T., Shang-Guan W.-J., Yu P., Zhu L.-H., Li H.-G., Liu H.-M., Tian J.-H. Jinfukang regulates integrin/Src pathway and anoikis mediating circulating lung cancer cells migration. J. Ethnopharmacol. 2021;267:113473. doi: 10.1016/j.jep.2020.113473. [DOI] [PubMed] [Google Scholar]
- 174.Hao H., Guo Z., Li Z., Li J., Jiang S., Fu J., Jiao Y., Deng X., Han S., Li P. Modified Bu-Fei decoction inhibits lung metastasis via suppressing angiopoietin-like 4. Phytomedicine. 2022;106:154409. doi: 10.1016/j.phymed.2022.154409. [DOI] [PubMed] [Google Scholar]
- 175.Sakuma Y., Tsunezumi J., Nakamura Y., Yoshihara M., Matsukuma S., Koizume S., Miyagi Y. ABT-263, a Bcl-2 inhibitor, enhances the susceptibility of lung adenocarcinoma cells treated with Src inhibitors to anoikis. Oncol. Rep. 2011;25:661–667. doi: 10.3892/or.2010.1123. [DOI] [PubMed] [Google Scholar]
- 176.Sakuma Y., Yamazaki Y., Nakamura Y., Yoshihara M., Matsukuma S., Nakayama H., Yokose T., Kameda Y., Koizume S., Miyagi Y. WZ4002, a third-generation EGFR inhibitor, can overcome anoikis resistance in EGFR-mutant lung adenocarcinomas more efficiently than Src inhibitors. Lab. Investig. 2012;92:371–383. doi: 10.1038/labinvest.2011.187. [DOI] [PubMed] [Google Scholar]
- 177.Kim S., Ko D., Lee Y., Jang S., Lee Y., Lee I.Y., Kim S. Anti-cancer activity of the novel 2-hydroxydiarylamide derivatives IMD-0354 and KRT1853 through suppression of cancer cell invasion, proliferation, and survival mediated by TMPRSS4. Sci. Rep. 2019;9:10003. doi: 10.1038/s41598-019-46447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shochet G.E., Israeli-Shani L., Koslow M., Shitrit D. Nintedanib (BIBF 1120) blocks the tumor promoting signals of lung fibroblast soluble microenvironment. Lung Cancer. 2016;96:7–14. doi: 10.1016/j.lungcan.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 179.Sirimangkalakitti N., Chamni S., Suwanborirux K., Chanvorachote P. Renieramycin M Sensitizes Anoikis-resistant H460 Lung Cancer Cells to Anoikis. Anticancer Res. 2016;36:1665–1671. [PubMed] [Google Scholar]
- 180.Prateep A., Sumkhemthong S., Karnsomwan W., De-Eknamkul W., Chamni S., Chanvorachote P., Chaotham C. Avicequinone B sensitizes anoikis in human lung cancer cells. J. Biomed. Sci. 2018;25:32. doi: 10.1186/s12929-018-0435-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Karsch-Bluman A., Avraham S., Assayag M., Schwob O., Benny O. Encapsulated Carbenoxolone Reduces Lung Metastases. Cancers. 2019;11:1383. doi: 10.3390/cancers11091383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pongrakhananon V., Stueckle T.A., Wang H.-Y.L., O’Doherty G.A., Dinu C.Z., Chanvorachote P., Rojanasakul Y. Monosaccharide digitoxin derivative sensitize human non-small cell lung cancer cells to anoikis through Mcl-1 proteasomal degradation. Biochem. Pharmacol. 2014;88:23–35. doi: 10.1016/j.bcp.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sriratanasak N., Nonpanya N., Wattanathana W., Chanvorachote P. Benzoxazine Dimer Analogue Targets Integrin β3 in Lung Cancer Cells and Suppresses Anoikis Resistance and Migration. Anticancer Res. 2020;40:2583–2589. doi: 10.21873/anticanres.14229. [DOI] [PubMed] [Google Scholar]
- 184.Puchsaka P., Chaotham C., Chanvorachote P. α-Lipoic acid sensitizes lung cancer cells to chemotherapeutic agents and anoikis via integrin β1/β3 downregulation. Int. J. Oncol. 2016;49:1445–1456. doi: 10.3892/ijo.2016.3624. [DOI] [PubMed] [Google Scholar]
- 185.Pramchu-Em C., Meksawan K., Chanvorachote P. Zinc Sensitizes Lung Cancer Cells to Anoikis through Down-Regulation of Akt and Caveolin-1. Nutr. Cancer. 2016;68:312–319. doi: 10.1080/01635581.2016.1142582. [DOI] [PubMed] [Google Scholar]
- 186.Aixinjueluo W., Furukawa K., Zhang Q., Hamamura K., Tokuda N., Yoshida S., Ueda R., Furukawa K. Mechanisms for the Apoptosis of Small Cell Lung Cancer Cells Induced by Anti-GD2 Monoclonal Antibodies. J. Biol. Chem. 2005;280:29828–29836. doi: 10.1074/jbc.M414041200. [DOI] [PubMed] [Google Scholar]
- 187.Salo S., Boutaud A., Hansen A.J., He L., Sun Y., Morales S., Venturini A., Martin P., Nokelainen P., Betsholtz C., et al. Antibodies blocking adhesion and matrix binding domains of laminin-332 inhibit tumor growth and metastasis in vivo. Int. J. Cancer. 2009;125:1814–1825. doi: 10.1002/ijc.24532. [DOI] [PubMed] [Google Scholar]
- 188.Hong K.P., Shin M.H., Yoon S., Ji G.Y., Moon Y.R., Lee O.-J., Choi S.-Y., Lee Y.-M., Koo J.H., Lee H.-C., et al. Therapeutic effect of anti CEACAM6 monoclonal antibody against lung adenocarcinoma by enhancing anoikis sensitivity. Biomaterials. 2015;67:32–41. doi: 10.1016/j.biomaterials.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 189.Lee H., Jang Y., Park S., Jang H., Park E.-J., Kim H.J., Kim H. Development and evaluation of a CEACAM6-targeting theranostic nanomedicine for photoacoustic-based diagnosis and chemotherapy of metastatic cancer. Theranostics. 2018;8:4247–4261. doi: 10.7150/thno.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]