Abstract
A phagocytosis assay for Streptococcus pneumoniae based on flow cytometry (FACS) with human polymorphonuclear cells and human complement was developed for the study of human vaccination antisera. Human prevaccination sera already contain high levels of C-polysaccharide (C-PS) antibodies, which are not protective in humans but which might give false positive results in a flow-cytometry-based assay. Cultures of S. pneumoniae grown to log phase on three consecutive days, followed by heat inactivation, yielded stable and highly encapsulated strains for serotypes 6A, 6B, 14, 19F, and 23F. As a result, only serotype-specific antibodies were able to facilitate phagocytosis of these strains, whereas no phagocytosis was observed with antibodies against C-PS or pneumococcal surface proteins. No, or weak, phagocytosis was observed with human prevaccination sera, whereas in general, postvaccination antisera facilitated phagocytosis. A highly significant correlation was observed between enzyme-linked immunosorbent assay titers and FACS phagocytosis titers (r = 0.98, P < 0.001) for serotype 23F pneumococci with human vaccination antisera. For all serotypes, interassay variation was below 10%. Major advantages of this assay over the classical killing assay are that (i) limited amounts of sera are required (10 μl per titration curve), (ii) 600 samples can be processed in one day by one person, and (iii) cells can be fixed and measurement of the samples can be performed up to 1 week later.
A number of pneumococcal saccharide-protein conjugate vaccines are currently under development and entering phase III trials (10, 35). In addition to other tests (enzyme-linked immunosorbent assays [ELISA], avidity-affinity tests), the efficacy of these vaccines is ultimately assessed by comparing the incidence of pneumococcal disease in the vaccinated versus nonvaccinated group. The incidence of disease caused by serotypes included in these multivalent vaccines varies, which makes it difficult to evaluate the efficacy of each component. Moreover, their composition must be adapted depending on the geographical area and probably also over time (13, 15, 25). Therefore, the introduction of this type of vaccine would be enormously facilitated by the availability of assays measuring in vitro parameters that correlate with in vivo protection.
Antibody-complement-dependent phagocytosis is the crucial defense mechanism against Streptococcus pneumoniae. The ability of serotype-specific antibodies to provide protection against infections with S. pneumoniae is beyond doubt, whereas the protective capacity of anti-pneumococcal surface protein antibodies remains to be established (4). The method most commonly used to measure levels of serotype-specific antibodies in the serum is the ELISA. This method determines the amount and isotype distribution of the antibodies present, but provides no direct information about antibody function. In addition, the correlation between antibody titer and protection depends on the pneumococcal serotype (14, 20, 34). One of the in vitro parameters that therefore provides essential information about the functioning of antibodies is their ability to promote phagocytosis as determined by phagocytosis assays based on flow cytometry (FACS) or radioactivity or classical killing assays (1–3, 8, 11, 16, 18, 21, 26, 30, 33, 37).
For human vaccination sera, conflicting data for the relation between antibody response and phagocytosis exist. Most studies have shown a weak or nonexistent relationship between these parameters (7, 17, 19, 22, 26), although a good correlation has also been reported (5, 11). These differences can in part be attributed to the differences in methodology used for measuring phagocytosis, e.g., differences in concentrations of bacteria and sera. More important, however, is the role of anti-cell-wall-polysaccharide (C-PS) antibodies. C-PS antibodies can mask the relationship between phagocytic activity and antibody concentration. Viôarsson et al. demonstrated that the correlation between ELISA titers and phagocytosis titers improved when the antisera were absorbed with C-PS before the antibody concentration was measured (37). Depending on the phagocytosis assay conditions, C-PS antibodies can facilitate phagocytosis (36a). C-PS antibodies, however, are not protective in humans, and human prevaccination sera usually contain high concentrations of these antibodies (9, 24, 27, 28, 31, 36, 37). Therefore, C-PS antibody-mediated phagocytosis should be minimized in phagocytosis assays. In principle, this can be achieved by minimizing the accessibility of C-PS by selecting highly encapsulated strains. An alternative strategy is to preabsorb the serum with C-PS.
Phagocytosis can be assessed by the classical killing assays and assays based on radioactivity or FACS. Previously, we developed a pneumococcal phagocytosis assay for mouse antisera based on FACS (1, 2). This assay gave an excellent correlation with antibody titers and protection as measured in a mouse challenge model (3). In the present study, this assay was adapted for use with human sera obtained from persons vaccinated with pneumococcal conjugate vaccines. To determine the best method for minimizing the influence of anti-C-PS antibodies, the application of highly encapsulated strains and preabsorption of antisera with C-PS was evaluated. Highly encapsulated strains (serotypes 6A, 6B, 14, 19F, and 23F) were obtained by growing pneumococcal strains to log phase on three consecutive days (28). As a result, only serotype-specific antibodies were able to promote phagocytosis of these strains. Using these strains, our FACS-based phagocytosis assay gave an excellent correlation with ELISA antibody concentrations. Our assay is now operational for the pediatric pneumococcal serotypes 6A, 6B, 14, 19F, and 23F, but can easily be set up for other serotypes when required.
MATERIALS AND METHODS
Growing of bacteria.
S. pneumoniae serotypes 6A, 6B, 14, 19F, and 23F (ATCC strains; a kind gift of J. Henrichsen and U. B. Sørensen, Statens Seruminstitut, Copenhagen, Denmark) were plated on blood agar plates and grown overnight at 37°C in a 5% CO2 atmosphere. Three milliliters of Todd-Hewitt broth supplemented with 0.5% yeast extract (E. Merck Mikrobiologie, Darmstadt, Germany) was then inoculated with the strains (optical density at 660 nm [OD660] of 0.05 to 0.08). Bacteria were grown either to log phase, to stationary phase, or to log phase three times in order to obtain highly encapsulated strains. For growing bacteria three times to the log phase, 3 ml of Todd-Hewitt broth supplemented with 0.5% yeast extract and 5% heat-inactivated human pooled serum was inoculated with S. pneumoniae (OD660 of 0.05 to 0.08). Cultures were grown 4 to 5 h to the log phase (OD660 of approximately 0.5) and then stored overnight at 4°C. For the second culture, 3 ml of fresh medium was inoculated with the previous culture (OD660 of approximately 0.5), grown to the log phase, and stored overnight at 4°C. Likewise, a third culture was started from the second culture and grown to the log phase. Absence of contamination by other organisms was checked by plating the bacteria on blood agar plates. Bacteria from the third culture were washed once with phosphate-buffered saline (PBS) (2,520 × g, 15 min, 4°C) and resuspended in PBS to an OD660 of 1.0.
Inactivation of S. pneumoniae strains.
Before and after inactivation, the bacteria were resuspended in PBS to an OD660 of 1.0 (approximately 109 bacteria/ml). Three different methods were evaluated for the inactivation of the pneumococcal autolysins. The first was formaldehyde killing, in which formaldehyde (54 μl per ml of bacterial suspension) was added slowly with stirring. Bacterial suspensions were incubated for 1 h at 37°C, centrifuged, and washed twice with PBS. The second was heat killing, in which bacterial suspensions were incubated for 1 h at 60°C and washed once with PBS. The third method was killing with 70% ethanol, in which bacteria were spun down and resuspended in the same volume with 70% ethanol. After incubation for 1 h at 4°C, the bacteria were washed twice with PBS.
Fluorescence labeling of pneumococcal strains.
Bacteria were labeled with a 0.5-mg/ml solution of fluorescein isothiocyanate (FITC, Isomer I; Sigma Chemical Co., St. Louis, Mo.) in PBS for 1 h at 4°C. Bacteria were washed twice with Hanks’ balanced salt solution containing 1% bovine serum albumin (BSA-HBBS), resuspended in BSA-HBSS to an OD660 of 1.0, and stored at −20°C in 100-μl amounts (108 bacteria).
Rabbit antisera.
Adult female New Zealand White rabbits of about 3 kg were supplied by Iffa-Credo, Someren, The Netherlands, and maintained at the Central Laboratory of Experimental Animals (Utrecht University, Utrecht, The Netherlands). These rabbits were immunized subcutaneously at four sites with a total of 100 μg of a C-polysaccharide-keyhole limpet hemocyanin conjugate in Freund’s complete adjuvant (the ratio of polysaccharide to protein was 0.6; volume, 0.8 ml). A booster injection was given at day 22, and serum was obtained at day 31 (titer, 105, as determined by ELISA). Other rabbits were injected with serotype 6B, 14, 19F, or 23F formalin-killed S. pneumoniae in Freund’s complete adjuvant (total volume, 0.8 ml). Booster injections were given intraperitoneally in incomplete Freund’s adjuvant at days 14, 21, and 28 after the first immunization with 5 × 108, 1 × 109, and 5 × 109 bacteria, respectively. After 2 months’ rest, the immunization scheme was repeated. The rabbits were bled at days 0, 14, 35, 57, and 112 after the first immunization. Rabbit anti-pneumococcal protein antisera were kindly provided by Karin Overweg, Laboratory of Pediatrics, Erasmus University, Rotterdam, The Netherlands. These rabbits were injected and boosted with pneumococcal serotypes 4 and 19F hydrophobic protein fractions.
Human antisera.
Human pneumococcal S6A and S23F bivalent conjugate antisera were kindly provided by Wyeth-Lederle Vaccines and Pediatrics, Rochester, N.Y. Human antisera used for the evaluation of the reproducibility of the assay were a generous gift of G. Rijkers, Department of Immunology, Wilhelmina kinderziekenhuis, University Hospital for Children and Youth, Utrecht, The Netherlands. All sera were heated at 56°C for 30 min to inactivate complement components.
Isolation of human complement.
Human pooled serum was depleted of immunoglobulin G (IgG) by protein G affinity chromatography (Pharmacia Biotech, Roosendaal, The Netherlands) and stored at −70°C. Depleted sera did not contain detectable IgG levels (LC-Partigen IgG, Behringwerke AG, Malburg, Germany), and complement activity (as determined by a CH50 assay [23]) was conserved for at least 60% of each portion compared to undepleted source serum.
Isolation of human PMN cells.
Human polymorphonuclear (PMN) cells were isolated from the blood of healthy volunteers. A total of 30 ml of heparinized blood of a single donor was mixed with 30 ml of PBS (pH 7.4), layered on Ficoll-Histopaque gradient, and centrifuged for 20 min at 400 × g. The cell pellet, containing PMN cells and erythrocytes, was depleted of erythrocytes by two consecutive hypotonic shocks (6).
Phagocytosis assay.
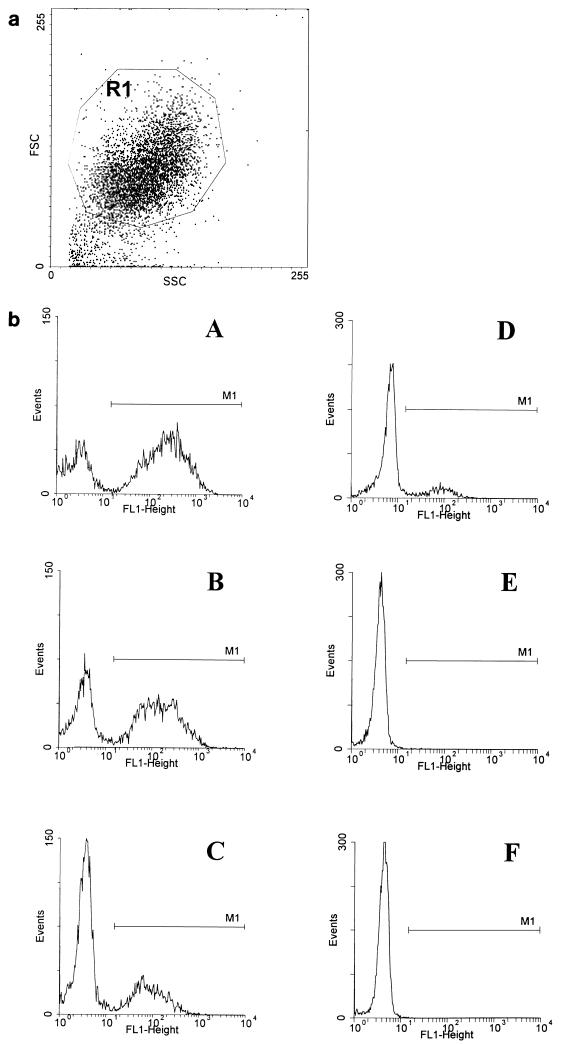
The assay was adapted from Alonso de Velasco et al. (1, 2). In short, dilutions of heat-inactivated serum and complement, which was diluted to a fixed concentration of 2% (final concentration in the assay), were made in BSA-HBSS and pipetted into round-bottomed microtiter plates (Greiner Labortechnik, Alphen aan de Rijn, The Netherlands). Samples of 2.5 × 106 pneumococci were added to each well. Opsonization was performed in a total volume of 50 μl at 37°C with shaking on a microtiter plate agitator. After 30 min of opsonization, the plates were placed on ice and 2.5 × 105 PMN cells in 50 μl of BSA-HBSS were added to each well. Phagocytosis was performed for 30 min at 37°C with shaking. An incubation time of 30 min leads to ingestion of all adherent pneumococci (29). After a wash with ice-cold BSA-HBSS, the cells were transferred to FACS tubes, fixed with paraformaldehyde (2%) in PBS, and analyzed in a flow cytometer (FACScan; Becton Dickinson, Mountain View, Calif.). The percentage of FITC-positive PMN cells was used as a measure of the phagocytic activity of serum. Percentages of FITC-positive PMN cells were corrected for the percentage of FITC-positive PMN cells of the negative control (without antiserum) (Fig. 1). Results are expressed as 25% phagocytosis titers, calculated by linear regression as the serum dilutions resulting in 25% FITC-positive PMN cells.
FIG. 1.
(a) PMN cell gating (R1) based on their specific forward (FSC) and right angle (SSC) light scatter. (b) Analysis of phagocytosis of fluorescence-labeled S. pneumoniae by PMN cells. The histograms show FITC-positive and -negative PMN cells (discriminated by marker M1) for different serum concentrations: 10% (A), 2% (B), 0.4% (C). Negative controls: PMN cells without serum (D), PMN cells without serum and complement (E), PMN cells only (F).
Inhibition of phagocytosis was performed by preincubating the sera overnight at 4°C with 100 μg of C-PS or serotype-specific polysaccharides/ml. Inhibition was calculated as follows: (phagocytosis titer without inhibitor − phagocytosis titer with inhibitor) × 100%/phagocytosis titer without inhibitor.
ELISA.
The ELISA was performed as previously described (32). Serotype-specific polysaccharide was obtained from the American Type Culture Collection (Rockville, Md.) for use as antigen. Specimens were preadsorbed with C-polysaccharide-enriched absorbent prepared by Wyeth-Lederle Vaccines and Pediatrics and quantitated against lot 89SF (Center for Biological Research and Evaluation, Food and Drug Administration, Washington, D.C.).
Statistical analysis.
The coefficient of variation was obtained after log transformation by the following formula: (standard deviation/mean) × 100. Correlations between ELISA titers and FACS phagocytosis titers were analyzed by linear regression tests. Statistical significance of correlations was assessed by Student’s t test. A P value of <0.05 was considered statistically significant.
RESULTS
Effect of pneumococcal growth phase on phagocytosis.
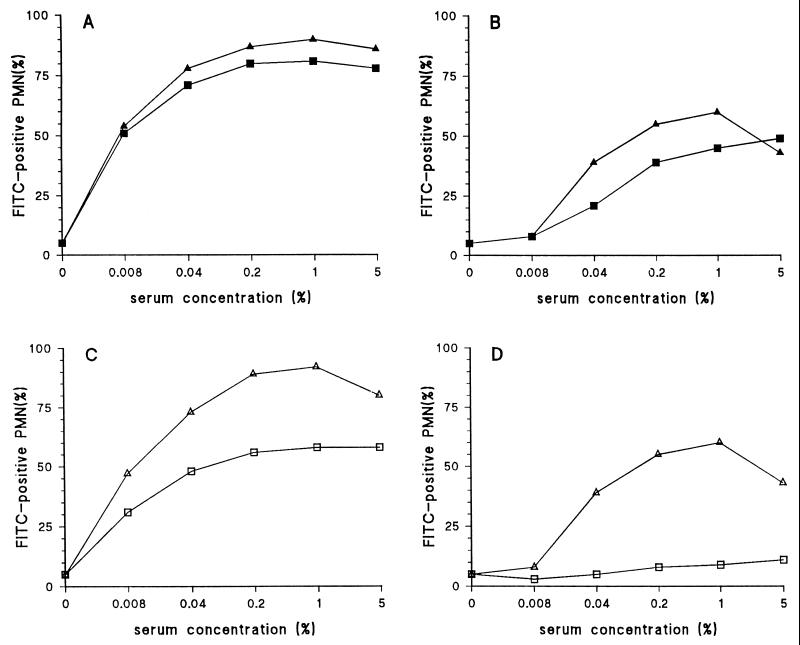
Most human antisera contain antibodies directed against C-PS of S. pneumoniae (31), which probably facilitate phagocytosis of poorly encapsulated strains. Therefore, in order to develop a serotype-specific phagocytosis assay, highly encapsulated strains are required. First, the effect of the growth phase on encapsulation was examined. To measure the effect of encapsulation, a strain grown to log phase and a strain grown to stationary phase were opsonized with either a rabbit anti-C-PS antiserum or a serotype-specific antiserum, and phagocytosis was measured. The strain grown to log phase displayed relatively low phagocytosis with the C-PS antiserum compared to the capsular polysaccharide antiserum, whereas the strain grown to stationary phase gave strong phagocytosis with the C-PS antiserum compared to the serotype-specific antiserum. Both antisera strongly promoted phagocytosis of the stationary strain, indicating that although the strain is less well encapsulated, its phagocytosis with the serotype-specific antiserum is maintained (Fig. 2A and B).
FIG. 2.
Effect of growth phase and C-PS preincubation on anti-C-PS antibody-mediated phagocytosis. A serotype 14 strain was grown to the stationary phase (panels A and C) or the log phase (panels B and D) and tested with an antiserum against C-PS and serotype 14 pneumococci. (A) Phagocytosis of the stationary-phase strain with an antiserum against C-PS (■) and serotype-specific antiserum (▴). (B) Phagocytosis of log-phase strain with an antiserum against C-PS (■) and serotype-specific antiserum (▴). (C) Preincubation of a C-polysaccharide antiserum with C-polysaccharide before opsonization of a stationary strain. C-polysaccharide antiserum was used alone (▵) or preincubated with C-PS (□). (D) Influence of C-PS preincubation and the use of the log-phase strain on C-PS antibody-mediated phagocytosis. C-polysaccharide antiserum was used alone (▵) or preincubated with C-PS (□).
C-PS preincubation.
In order to diminish the influence of C-PS antibodies on phagocytosis, anti-C-PS antisera were preincubated with C-PS. Phagocytosis assays were subsequently performed with a stationary-phase strain (Fig. 2C) and a log-phase strain (Fig. 2D). For strains grown to both phases, C-PS antibody-mediated phagocytosis was strongly reduced by this preincubation. In contrast, no effect was observed for the phagocytosis mediated by the serotype-specific antiserum (data not shown). However, in general, complete preabsorption of C-PS antibodies with C-PS was difficult to achieve. Therefore, another method was investigated for eliminating the influence of C-polysaccharide antibodies in the FACS-based assay.
Strains grown to log phase three times.
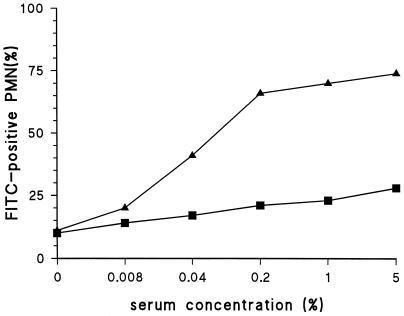
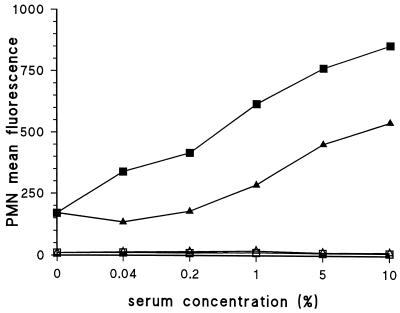
Nielsen et al. described a method for obtaining highly encapsulated strains by growing strains to log phase three times consecutively (28). Such highly encapsulated strains were tested in the phagocytosis assay with both the serotype-specific and anti-C-PS antisera. The serotype-specific antiserum strongly promoted phagocytosis, whereas almost no phagocytosis was observed with the anti-C-PS antiserum (Fig. 3). Identical results were obtained for serotypes 6A, 6B, 14, and 23F (data not shown).
FIG. 3.
Anti-C-PS antibodies do not promote phagocytosis of highly encapsulated strains. A serotype 19F strain was tested with an antiserum against C-PS (■) and 19F pneumococci (▴).
Inactivation.
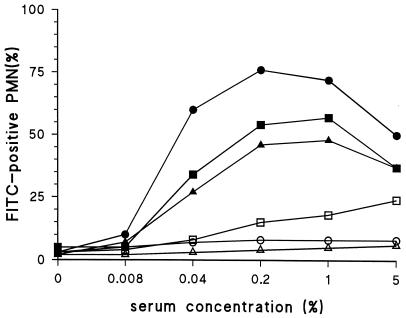
When live strains were used, some C-PS antibody-mediated phagocytosis was observed in some of the experiments. S. pneumoniae produces enzymes (autolysins) that can cause lysis of the bacterium and probably also partial release of the capsule (12). Killing the bacteria leads to inactivation of these enzymes (12). Three different methods for killing the pneumococci were evaluated: heat inactivation, killing by ethanol, and killing by formaldehyde. Heat inactivation and formaldehyde treatment both resulted in the absence of phagocytosis with anti-C-PS antibodies, whereas with ethanol treatment some residual phagocytosis was detected (Fig. 4). The strongest phagocytosis with the serotype-specific serum, however, was observed when heat inactivation was used. Therefore, subsequent experiments were performed with heat-inactivated strains grown to log phase three times. Strains treated in this way can be stored at −20°C for at least half a year without losing the properties described above (data not shown).
FIG. 4.
Comparison of the effect of three inactivation methods on phagocytosis mediated by anti-C-PS and serotype-specific antisera. Serotype 19F strains were inactivated by heat (60°C) (circles), 2% paraformaldehyde (triangles), or 70% ethanol (squares) and phagocytized by an antiserum against C-PS (open symbols) or 19F pneumococci (closed symbols).
Anti-protein antibodies.
In addition to C-PS antibodies, human antisera are expected to contain antibodies to surface proteins of S. pneumoniae. Encapsulation will probably influence the ability of these anti-protein antibodies to facilitate phagocytosis. To investigate this possibility, rabbit pre- and postvaccination sera against hydrophobic pneumococcal proteins of serotypes 4 and 19F pneumococci were used. A serotype 19F strain was grown either to stationary phase or to log phase three times and heat inactivated. The anti-protein antiserum was only able to promote phagocytosis of the stationary grown strain, whereas no phagocytosis was observed with the strain grown to log phase three times (Fig. 5).
FIG. 5.
Rabbit anti-pneumococcal protein antisera do not promote phagocytosis of heat-inactivated strains grown to log phase three times. A serotype 19F strain, grown to stationary phase (closed symbols) or to log phase three times (open symbols) was tested with rabbit prevaccination (trangles) and postvaccination (squares) sera against hydrophobic protein fractions.
Capsular polysaccharide preincubation.
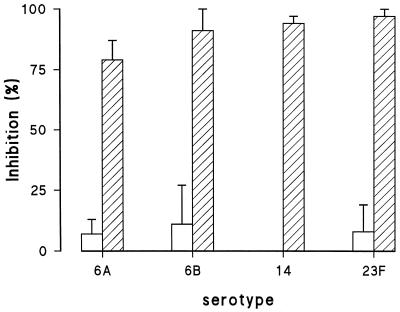
To demonstrate that only serotype-specific phagocytic antibodies are detected when heat-inactivated strains grown to log phase three times are used, sera were absorbed with serotype-specific polysaccharide or C-PS. For all serotypes, preincubation of the serotype-specific serum with serotype-specific polysaccharides resulted in a strong inhibition of phagocytosis, whereas no inhibition was observed after serum absorption with C-PS (Fig. 6).
FIG. 6.
Competitive inhibition of phagocytosis with C-polysaccharide or serotype-specific polysaccharide. Rabbit antisera were preincubated with either C-polysaccharide or serotype-specific polysaccharide, and a phagocytosis assay was performed with the highly encapsulated strains. Figure shows preincubation with C-polysaccharide (open bars) and with serotype-specific polysaccharides (hatched bars) (n = 3).
Correlation with ELISA.
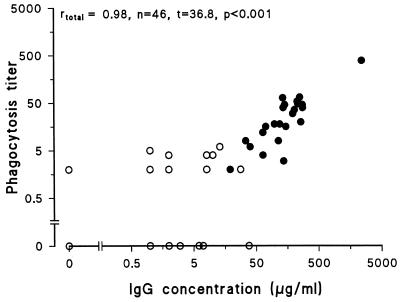
Phagocytosis of pneumococci is mediated by antibodies in cooperation with complement. To investigate the relationship between antibody concentration and phagocytosis titer, antibody concentrations and phagocytosis titers were determined in 46 human pre- and postvaccination antisera. Sera were heat inactivated, and human IgG-depleted pooled serum was used as an exogenic complement source (Fig. 7). No, or weak, phagocytosis was observed for prevaccination sera, whereas, in general, postvaccination antisera gave phagocytosis. A strong, highly significant correlation was observed between IgG antibody concentrations and phagocytosis titers (r = 0.98, P < 0.001). Without complement, similar results were obtained (r = 0.97), but no phagocytosis was observed for prevaccination antisera (data not shown).
FIG. 7.
Correlation between ELISA IgG titers and FACS-based phagocytosis titers for human prevaccination (○) and postvaccination (•) sera. ELISA titers and phagocytosis titers were determined for 48 pre- and postvaccination antisera.
Coefficient of variation.
The reproducibility of the phagocytosis assay for each serotype was determined by measuring the phagocytosis titers of a serotype-specific rabbit antiserum and a human conjugate vaccine antiserum on five different days. For all serotypes, with rabbit antisera, interassay variation was below 5%, whereas human antisera gave an interassay variation below 10% (Table 1).
TABLE 1.
Interassay coefficients of variation for rabbit and human antiseraa
| Serum type | Coefficient of variation for serotype:
|
||||
|---|---|---|---|---|---|
| 6A | 6B | 14 | 19F | 23F | |
| Rabbit | 2.6 | 3.3 | 1.3 | 1.5 | 2.6 |
| Human | 8.5 | 0.8 | 7.5 | NDb | 7.8 |
Sera were tested on five different days with different donors. For human antisera, only donors either homozygous or heterozygous for FcγRIIa-131H were used.
ND, not determined. For serotype 19F, the coefficient of variation with human antiserum could not be determined due to absence of an appropriate antiserum.
DISCUSSION
This report describes a pneumococcal phagocytosis assay which predominantly measures phagocytosis mediated by serotype-specific antibodies. Rabbit hyperimmune antisera directed against either C-PS or surface proteins failed to promote phagocytosis in this assay. Moreover, in contrast to C-PS, only preincubation of the sera with serotype-specific polysaccharide could inhibit phagocytosis.
The accessibility of C-PS on pneumococci was minimized by selecting for highly encapsulated strains. This was achieved by growing the strains three times to the log phase in the presence of 5% human pooled serum (HPS). A possible mechanism for the effect of this procedure is that C-PS antibodies present in the HPS cause agglutination and thereby inhibit growth of poorly encapsulated strains. In this way, each subsequent culture is enriched with highly encapsulated pneumococci. To ensure that every experiment was performed with highly encapsulated pneumococci, stocks of pneumococci were regularly tested for the absence of phagocytosis with the rabbit anti-C-PS antiserum. Therefore, anti-C-polysaccharide antiserum can be used as an easy quality control for strain encapsulation.
When live, highly encapsulated bacterial stocks were used, particularly those of strain 23F, appearance of C-polysaccharide antibody-mediated phagocytosis was observed after freezing and thawing, probably due to the release and activation of pneumococcal enzymes (autolysins). Comparing methods for inactivating these enzymes, such as heat treatment, ethanol fixation, and paraformaldehyde fixation, indicated that heat inactivation for 1 h at 60°C gave the best results (Fig. 4). Strains treated this way gave no phagocytosis with the anti-C-polysaccharide antiserum and the strongest phagocytosis with the serotype-specific antiserum. A potential disadvantage of the use of heat treatment is the potential denaturation of protein epitopes on the pneumococcus. Therefore, when the purpose is to evaluate the opsonic capacity of anti-pneumococcal protein antibodies, live and heat-inactivated strains should initially be compared.
Interassay variation of the FACS-based assay was excellent and in the same range as that recently reported for the classical killing assay in which HL-60 cells were used as a source of phagocytes (33). With rabbit antisera, the interassay variation was below 5% for all serotypes, whereas with human antisera, an interassay variation of around 10% was observed (Table 1). Part of this difference is probably due to Fcγ polymorphisms on the PMN cells used. For testing of the human antisera, care was taken to use donors who were either heterozygous or homozygous for FcγRIIa-131H. These donors, however, possessed different FcγRIIIb receptors, which affects the phagocytosis mediated by IgG1 and IgG3 antibodies (NA1/NA2). Rabbits produce only a single type of IgG, and as far as we know, the interaction with human FcγRIIa and FcγRIIIb is not dependent on the polymorphisms of these receptors. The influence of FcγRIIa and FcγRIIIb polymorphisms on the performance of the phagocytosis assay is currently under investigation in our laboratory.
To evaluate the performance of the FACS-based assay with human antisera, the phagocytic capacities of pre- and postvaccination sera obtained from adults vaccinated with various conjugate vaccines or Pneumovax were determined. Independent of antibody concentrations, no phagocytosis was observed for prevaccination antisera without addition of complement, suggesting that these prevaccination sera probably contained phagocytic IgM antibodies. Comparison of the FACS-based phagocytosis titers and the IgG antibody concentrations demonstrated a strong and highly significant correlation between these two parameters. In this respect, the FACS-based phagocytosis assay adapted for use of human antisera displayed characteristics identical to those of our previously described assay for mouse antisera, in which we used the J774 mouse cell line (2, 3). This assay not only correlated strongly with the serotype-specific IgG and IgM antibody levels but was (in combination with the IgG antibody levels) also the best predictor of survival of vaccinated mice upon challenge with a lethal dose of pneumococci (3).
The classical killing assay is considered to be the “gold standard” for the assessment of the phagocytic capacity of antisera. That assay, however, is cumbersome to perform, only a limited number of antisera (at most 30) can be processed per assay, and the test has to be evaluated the next day (33). With the FACS-based assay, 600 samples (150 antisera/4 serum dilutions) can be processed in 1 day by one person. Moreover, the cells can be fixed and the flow cytometry can be performed up to 1 week later. In addition, in the FACS-based assay, the results are measured by a machine and analyzed by computer, whereas with the classical killing assay, the colonies are scored by eye, leading more easily to human errors. Moreover, the use of antibiotics by patients or vaccinated people affects the outcome of the killing assay. The FACS-based assay uses inactivated strains and is therefore not affected by the presence of antibiotics.
Upon comparison of our FACS-based assay with the killing assays described in the literature, at first sight, our FACS-based assay seems to be less sensitive (33). There are, however, essential differences between both assays. To avoid bacterial outgrowth, effector/target ratios used in classical killing assays vary from 100:1 to 500:1, and baby rabbit serum is utilized as the complement source. The FACS-based assay uses an effector/target ratio of 1:10 and human complement. At the moment, it is difficult to establish what type of assay correlates best with in vivo protection in humans or animals, but effector/target cell ratios during pneumococcal infections of 100:1 to 500:1 seem to be less likely compared to an effector/target ratio of 1:10. In addition, the FACS-based assay for mouse antisera is an excellent predictor of the survival of vaccinated mice after challenge with pneumococci (3). The question raised above, however, can probably be answered only at the end of the current phase III pneumococcal conjugate trials. Large numbers of antisera have to be evaluated for their phagocytic capacity by both assays, and the correlation with protection has to be established. In particular, sera obtained from toddlers who are infected with S. pneumoniae despite having being vaccinated will be important to study. At the moment, we are in the process of setting up interlaboratory studies on the correlation between FACS phagocytosis titers and classical killing titers.
In conclusion, the FACS-based assay is an easy-to-perform method for measuring the phagocytic capacity of large numbers of pneumococcal vaccine antisera. It has low interassay variation and displays a good correlation with postvaccination IgG concentrations.
ACKNOWLEDGMENTS
We thank G. Rijkers, Department of Immunology, Wilhelmina kinderziekenhuis, University Hospital for Children and Youth, Utrecht, and K. Overweg, Laboratory of Pediatrics, Erasmus University, Rotterdam, for providing antisera; J. van der Winkel and N. Westerdaal, Department of Immunology, Utrecht University Hospital, Utrecht, The Netherlands, for providing the facilities and help for typing the donors for their FcγRIIa and FcγRIIIb allotypes; J. Henrichsen and U. B. Sørensen, Statens Seruminstitut, Copenhagen, Denmark, for their advice on how to obtain highly encapsulated pneumococcal strains; and C. Kraaijeveld for helpful advice and careful reading of the manuscript.
This research was funded in part by the World Health Organization, Global Program for Vaccines and Immunization, Vaccine Research & Development (V23/181/92).
REFERENCES
- 1.Alonso de Velasco E, Verheul A F M, van Steijn A M P, Dekker H A T, Feldman R G, Fernández I M, Kamerling J P, Vliegenthart J F, Verhoef J, Snippe H. Epitope specificity of rabbit immunoglobulin G (IgG) elicited by pneumococcal type 23F synthetic oligosaccharide- and native polysaccharide-protein conjugate vaccines: comparison with human anti-polysaccharide 23F IgG. Infect Immun. 1994;62:799–808. doi: 10.1128/iai.62.3.799-808.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso De Velasco E, Dekker H A, Antal P, Jalink K P, van Strijp J A, Verheul A F M, Verhoef J, Snippe H. Adjuvant Quil A improves protection in mice and enhances opsonic capacity of antisera induced by pneumococcal polysaccharide conjugate vaccines. Vaccine. 1994;12:1419–1422. doi: 10.1016/0264-410x(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 3.Alonso De Velasco E, Dekker B A, Verheul A F M, Feldman R G, Verhoef J, Snippe H. Anti-polysaccharide immunoglobulin isotype levels and opsonic activity of antisera: relationships with protection against Streptococcus pneumoniae infection in mice. J Infect Dis. 1995;172:562–565. doi: 10.1093/infdis/172.2.562. [DOI] [PubMed] [Google Scholar]
- 4.Alonso De Velasco E, Verheul A F M, Verhoef J, Snippe H. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol Rev. 1995;59:591–603. doi: 10.1128/mr.59.4.591-603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardardottir E, Jonsson S, Jonsdottir I, Sigfusson A, Valdimarsson H. IgG subclass response and opsonization of Streptococcus pneumoniae after vaccination of healthy adults. J Infect Dis. 1990;162:482–488. doi: 10.1093/infdis/162.2.482. [DOI] [PubMed] [Google Scholar]
- 6.Böyum X. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Investig Suppl. 1986;97:77–89. [PubMed] [Google Scholar]
- 7.Braconier J H, Pedersen F K, Odeberg H, Rosen C. Opsonic and antibody responses to pneumococcal polysaccharide types 6A, 19F and 23F after vaccination of immunocompromised patients. Scand J Infect Dis. 1984;16:161–167. doi: 10.3109/00365548409087136. [DOI] [PubMed] [Google Scholar]
- 8.Briles D E, Forman C, Horowitz J C, Volanakis J E, Benjamin W H, Jr, McDaniel L S, Eldridge J, Brooks J. Antipneumococcal effects of C-reactive protein and monoclonal antibodies to pneumococcal cell wall and capsular antigens. Infect Immun. 1989;57:1457–1464. doi: 10.1128/iai.57.5.1457-1464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown E J, Joiner K A, Cole R M, Berger M. Localization of complement component 3 on Streptococcus pneumoniae: anti-capsular antibody causes complement deposition on the pneumococcal capsule. Infect Immun. 1983;39:403–409. doi: 10.1128/iai.39.1.403-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan C Y, Molrine D C, George S, Tarbell N J, Mauch P, Diller L, Shamberger R C, Phillips N R, Goorin A, Ambrosino D M. Pneumococcal conjugate vaccine primes for antibody responses to polysaccharide pneumococcal vaccine after treatment of Hodgkin’s disease. J Infect Dis. 1996;173:256–258. doi: 10.1093/infdis/173.1.256. [DOI] [PubMed] [Google Scholar]
- 11.Chudwin D S, Artrip S G, Korenblit A, Schiffman G, Rao S. Correlation of serum opsonins with in vitro phagocytosis of Streptococcus pneumoniae. Infect Immun. 1985;50:213–217. doi: 10.1128/iai.50.1.213-217.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coonrod J D, Varble R, Yoneda K. Mechanism of killing of pneumococci by lysozyme. J Infect Dis. 1991;164:527–532. doi: 10.1093/infdis/164.3.527. [DOI] [PubMed] [Google Scholar]
- 13.Dixon J M, Lipinski A E. Pneumococcal serotypes causing bacteremia and meningitis: relevance to composition of pneumococcal vaccine. Can Med Assoc J. 1981;125:263–267. [PMC free article] [PubMed] [Google Scholar]
- 14.Fine D P, Kirk J L, Schiffman G, Schweinle J E, Guckian J C. Analysis of humoral and phagocytic defenses against Streptococcus pneumoniae serotypes 1 and 3. J Lab Clin Med. 1988;112:487–497. [PubMed] [Google Scholar]
- 15.Finland M, Barnes M W. Changes in occurrence of capsular serotypes of Streptococcus pneumoniae at Boston City Hospital during selected years between 1935 and 1974. J Clin Microbiol. 1977;5:154–166. doi: 10.1128/jcm.5.2.154-166.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner S E, Anderson D C, Webb B J, Stitzel A E, Edwards M S, Spitzer R E, Baker C J. Evaluation of Streptococcus pneumoniae type XIV opsonins by phagocytosis-associated chemiluminescence and a bactericidal assay. Infect Immun. 1982;35:800–808. doi: 10.1128/iai.35.3.800-808.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giebink G S, Foker J E, Kim Y, Schiffman G. Serum antibody and opsonic responses to vaccination with pneumococcal capsular polysaccharide in normal and splenectomized children. J Infect Dis. 1980;141:404–412. doi: 10.1093/infdis/141.3.404. [DOI] [PubMed] [Google Scholar]
- 18.Giebink G S, Koskela M, Vella P P, Harris M, Le C T. Pneumococcal capsular polysaccharide-meningococcal outer membrane protein complex conjugate vaccines: immunogenicity and efficacy in experimental pneumococcal otitis media. J Infect Dis. 1993;167:347–355. doi: 10.1093/infdis/167.2.347. [DOI] [PubMed] [Google Scholar]
- 19.Giebink G S, Verhoef J, Peterson P K, Quie P G. Opsonic requirements for phagocytosis of Streptococcus pneumoniae types VI, XVIII, XXIII, and XXV. Infect Immun. 1977;18:291–297. doi: 10.1128/iai.18.2.291-297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamill R J, Musher D M, Groover J E, Zavell P J, Watson D A. IgG antibody reactive with five serotypes of Streptococcus pneumoniae in commercial intravenous immunoglobulin preparations. J Infect Dis. 1992;166:38–42. doi: 10.1093/infdis/166.1.38. [DOI] [PubMed] [Google Scholar]
- 21.Hof D G, Repine J E, Giebink G S, Hoidal J R. Production of opsonins that facilitate phagocytosis of Streptococcus pneumoniae by human alveolar macrophages or neutrophils after vaccination with pneumococcal polysaccharide. Am Rev Respir Dis. 1981;124:193–195. doi: 10.1164/arrd.1981.124.2.193. [DOI] [PubMed] [Google Scholar]
- 22.Johansen K S, Pedersen F K. Antibody response and opsonization after pneumococcal vaccination in splenectomized children and healthy persons. Acta Pathol Microbiol Immunol Scand Sect C. 1982;90:265–270. doi: 10.1111/j.1699-0463.1982.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 23.Klerx J P A M, Beukelman C J, Dijk H V, Willers J. Microassay for colorimetric estimation of complement activity in guinea pig, human and mouse serum. J Immunol Methods. 1983;63:215. doi: 10.1016/0022-1759(83)90425-8. [DOI] [PubMed] [Google Scholar]
- 24.Koskela M. Serum antibodies to pneumococcal C polysaccharide in children: response to acute pneumococcal otitis media or to vaccination. Pediatr Infect Dis J. 1987;6:519–526. doi: 10.1097/00006454-198706000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Moulin A, Fleurette J, Ekong G. Serotypie des pneumocoques isoles dans les produits pathologiques au cours de deux periodes, 1972–1977 et 1978. Pathol Biol. 1979;27:567–570. [PubMed] [Google Scholar]
- 26.Musher D M, Chapman A J, Goree A, Jonsson S, Briles D, Baughn R E. Natural and vaccine-related immunity to Streptococcus pneumoniae. J Infect Dis. 1986;154:245–256. doi: 10.1093/infdis/154.2.245. [DOI] [PubMed] [Google Scholar]
- 27.Musher D M, Watson D A, Baughn R E. Does naturally acquired IgG antibody to cell wall polysaccharide protect human subjects against pneumococcal infection? J Infect Dis. 1990;161:736–740. doi: 10.1093/infdis/161.4.736. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen S V, Sorensen U B, Henrichsen J. Antibodies against pneumococcal C-polysaccharide are not protective. Microb Pathog. 1993;14:299–305. doi: 10.1006/mpat.1993.1029. [DOI] [PubMed] [Google Scholar]
- 29.Obaro S K, Henderson D C, Monteil M A. Defective antibody-mediated opsonisation of S. pneumoniae in high risk patients detected by flow cytometry. Immunol Lett. 1996;49:83–89. doi: 10.1016/0165-2478(95)02487-5. [DOI] [PubMed] [Google Scholar]
- 30.Paoletti L C, Kasper D L, Michon F, DiFabio J, Jennings H J, Tosteson T D, Wessels M R. Effects of chain length on the immunogenicity in rabbits of group B Streptococcus type III oligosaccharide-tetanus toxoid conjugates. J Clin Invest. 1992;89:203–209. doi: 10.1172/JCI115564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedersen F K, Henrichsen J, Sorensen U B, Nielsen J L. Anti-C-carbohydrate antibodies after pneumococcal vaccination. Acta Pathol Microbiol Immunol Scand Sect C. 1982;90:353–355. doi: 10.1111/j.1699-0463.1982.tb01462.x. [DOI] [PubMed] [Google Scholar]
- 32.Quataert S A, Kirch C S, Quackenbush Wiedl L J, Phipps D C, Strohmeyer S, Cimino C O, Skuse J, Madore D V. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin Diagn Lab Immunol. 1995;2:590–597. doi: 10.1128/cdli.2.5.590-597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero-Steiner S, Libutti D, Pais L B, Dykes J, Anderson P, Whitin J C, Keyserling H L, Carlone G M. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997;4:415–422. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiffman G, Douglas R M, Bonner M J, Robbins M, Austrian R. A radioimmunoassay for immunologic phenomena in pneumococcal disease and for the antibody response to pneumococcal vaccines. I. Method for the radioimmunoassay of anticapsular antibodies and comparison with other techniques. J Immunol Methods. 1980;33:133–144. doi: 10.1016/s0022-1759(80)80004-4. [DOI] [PubMed] [Google Scholar]
- 35.Shelly M A, Jacoby H, Riley G J, Graves B T, Pichichero M, Treanor J J. Comparison of pneumococcal polysaccharide and CRM197-conjugated pneumococcal oligosaccharide vaccines in young and elderly adults. Infect Immun. 1997;65:242–247. doi: 10.1128/iai.65.1.242-247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sjogren A, Lindholm B, Holme T. Availability of reaction with antibodies of the pneumococcal C-polysaccharide on the surface of capsulated pneumococci. Acta Pathol Microbiol Immunol Scand Sect B. 1987;95:371–378. doi: 10.1111/j.1699-0463.1987.tb03141.x. [DOI] [PubMed] [Google Scholar]
- 36a.Verheul, A. F. M., et al. Unpublished results.
- 37.Viôarsson G, Jonsdottir I, Jonsson S, Valdimarsson H. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J Infect Dis. 1994;170:592–599. doi: 10.1093/infdis/170.3.592. [DOI] [PubMed] [Google Scholar]