Abstract
Rice (Oryza sativa L.) is a primary food that is widely consumed throughout the world, especially in Asian countries. The two main subspecies of rice are japonica and indica which are different in physical characteristics. In general, both indica and japonica rice consist of three types of grain colors, namely white, red, and black. Furthermore, rice and rice by-products contain secondary metabolites such as phenolic compounds, flavonoids, and tocopherols that have bioactivities such as antioxidants, antimicrobial, cancer chemopreventive, antidiabetic, and hypolipidemic agents. The existence of health benefits in rice bran, especially as antioxidants, gives rice bran the opportunity to be used as a functional food. Most of the bioactive compounds in plants are found in bound form with cell wall components such as cellulose and lignin. The process of releasing bonds between bioactive components and cell wall components in rice bran can increase the antioxidant capacity. Fermentation and treatment with enzymes were able to increase the total phenolic content, total flavonoids, tocotrienols, tocopherols, and γ-oryzanol in rice bran.
Keywords: rice bran, japonica, indica, by-products, phenolic compound, bioactive compound
1. Introduction
Chronic diseases such as cancer, diabetes mellitus, stroke, and cardiovascular disease are characterized by an increase in oxidative stress status [1,2,3,4]. Oxidative stress is caused by an imbalance between the production and neutralization of free radicals in biological systems [5]. Free radicals usually come from reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive sulfur species (RSS) [6].
The free radicals produced can be neutralized in the presence of antioxidants, so it can prevent oxidative damage in the human body [5]. Many studies have reported that rice is rich in antioxidants [7,8,9,10,11,12]. In addition, rice bran is a by-product obtained after milling and also is rich in antioxidants, but previously it was used only as animal feed [13,14,15,16].
Rice (Oryza sativa L.) is widely cultivated in Asia and is important in the world because it is used as a primary food. This species consists of two main subspecies, namely japonica and indica; the difference between the two lies in morphological and agronomic characteristics, in physiological and biochemical characteristics, and in genomic structure [17]. In general, rice consists of three types of grain colors, namely white, red, and black. However, in some areas such as Thailand, there are also types of brown and purple rice [18,19,20].
In white, red, and black rice, secondary metabolites such as phenolic acids and anthocyanins are highest in rice bran compared with other parts of rice such as whole grains, embryos, and endosperm [21]. Several studies reported that secondary metabolites in rice bran have bioactivity as antioxidants, antimicrobial, cancer chemopreventive, antidiabetic, and hypolipidemic agents [22,23,24,25]. However, secondary metabolites such as phenolics exist in bound form which is insoluble and make it difficult to extract [26]. Therefore, several current studies are focusing on the release of bound phenolics so that their bioactivity is increased [27,28]. Previous research reported that rice bran can be used as functional foods such as oil, milk, flour, and bread [29,30,31].
Prior to the isolation of natural products, an extraction process is carried out to separate the bioactive components from the raw material. The extraction technique consists of solvent and non-solvent extraction. The solvents used are methanol, ethanol, acetone, chloroform, hexane, ethyl acetate, dichloromethane, and isopropanol. The solvent extraction method is more widely used but the solvent used is toxic. Therefore, there are alternative extraction methods such as ultrasound extraction (UAE) and supercritical fluid extraction (SFE) [32]. The most widely used isolation method is column chromatography using silica [33]. Isolated bioactive substances are generally identified and characterized using HPLC (High Performance Liquid Chromatography) [26], TLC (Thin Layer Chromatography [34], FTIR (Fourier-transform infrared spectroscopy), NMR (Nuclear magnetic resonance), etc. [35]
On the basis of this, this review focused on the antioxidant activity found in rice bran of rice (white, red, and black) varieties that have been extensively studied. In contrast with white rice bran, pigmented rice bran such as red and black rice contains anthocyanins which have antioxidant activity [21]. The antioxidant activity of bioactive compounds is caused by the presence of a hydroxyl group on the phenol group which is able to donate hydrogen atoms to free radicals [36]. This review will also discuss several methods that were used to increase the antioxidant capacity of rice bran.
2. Comparison of Japonica and Indica
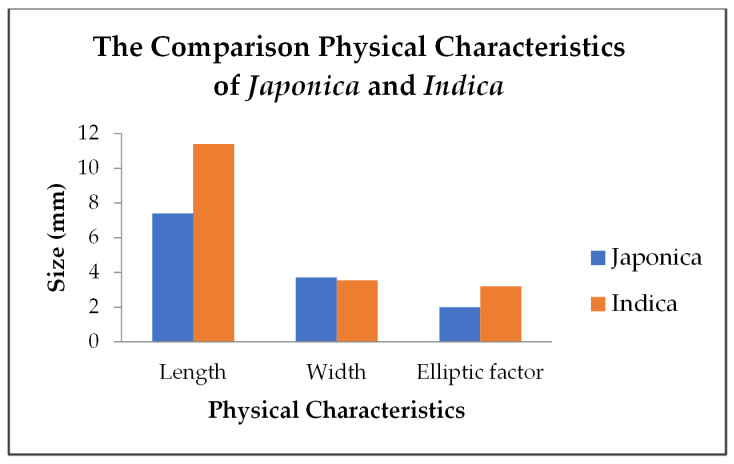
Japonica is widely consumed by the people of China, Japan, South Korea, the United States, and Egypt [37]. The grain cultivar is long with a bold shape. In general, the amylose and total carbohydrate content are lower than the indica, but the protein, lipid, and dietary fiber content are higher than the indica. Japonica have length, width, and elliptic factor of 7.40; 3.71; and 1.99 mm, respectively. [38]. Ding et al. [39] conducted a study comparing japonica and indica rice grown in the same area in China, showing that japonica rice bran and endosperm is higher in total phenolic content than indica rice. The antioxidant activity also shows higher yields in japonica rice.
Indica is widely consumed in Southeast Asian countries such as Vietnam, Thailand, Indonesia, Malaysia, Myanmar, and the Philippines [37]. The grain cultivar is extra long with a slender shape. The grains of this variety have a higher area (mm2), circumference (mm), and length (mm) than japonica shown in Figure 1 [38]. Indica have length, width, and elliptic factor of 11.34; 3.54; and 3.20 mm, respectively. In general, the amylose and total carbohydrate content are higher than in japonica, but the protein, lipid, and dietary fiber content are lower than in japonica [38].
Figure 1.
The comparison physical characteristics of japonica and indica.
3. Isolation Methods and Bioactive Compound Content in Rice Bran from Three Types of Grain Colors
3.1. White Rice Bran
Several methods have been carried out to isolate bioactive compounds in white rice bran. Peanparkdee et al. [40] used white rice bran with KDML 105 variety (indica rice) from Thailand. A total of 5 g of bran sample was dissolved in 100 mL of 65% (v/v) ethanol, isopropanol, and a mixture of isopropanol and hexane (1:1 v/v). The extracts of the three solvents were detected for the content of phenolic acids, flavonoids, and anthocyanins using high-performance liquid chromatography.
Aziz et al. [34] carried out extraction by reflux extraction using several solvents such as hexane, chloroform, ethyl acetate, dichloromethane, isopropanol, acetone, and a mixture of hexane-ethyl acetate (1:1), hexane isopropanol (1:1), and chloroform-ethyl acetate (1:1). In that study they used white rice bran from Banten Province, Indonesia. The TLC (Thin Layer Chromatography) densitometric method was used to determine the total oryzanol content. Then, column chromatography of the hexane extract was performed using silica gel 60 and gradient elution with hexane-ethyl acetate. As a result, six fractions were obtained, then the 5th fraction (F5) was subjected to centrifugal thin layer chromatography (CTLC) using silica gel GF254 and gradient elution using hexane-ethyl acetate to obtain 9 subfractions. Purification was carried out by centrifugal thin layer chromatography (CTLC) with isocratic elution using hexane-ethyl acetate (9:1) [34].
Pokkanta et al. [41] also carried out a study on KDML 105 white rice bran with another method to extract rice bran using 1:10 (w/v) methanol. Then, the mixture was centrifuged and the supernatant was taken. The methanol extract was extracted again in the same way using dichloromethane and hexane as solvents. The HPLC method was used to determine the content of tocols, γ-oryzanols, phytosterols, squalene, phylloquinone, and cholecalciferol [41]. Hansakul et al. [42] also used 80% methanol to extract rice bran. The type of white rice used was the 9311 variety [21]. In addition, there was also the extraction of rice bran by heating at 60–70 °C in distilled water 1:4 (w/v). Then, the extract was analyzed for total phenolic content by the Folin–Ciocalteu method and total flavonoid content [42].
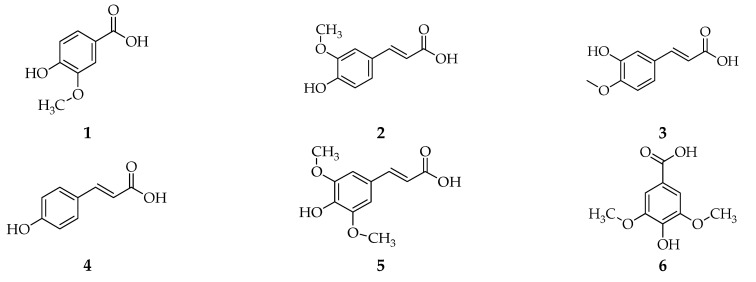
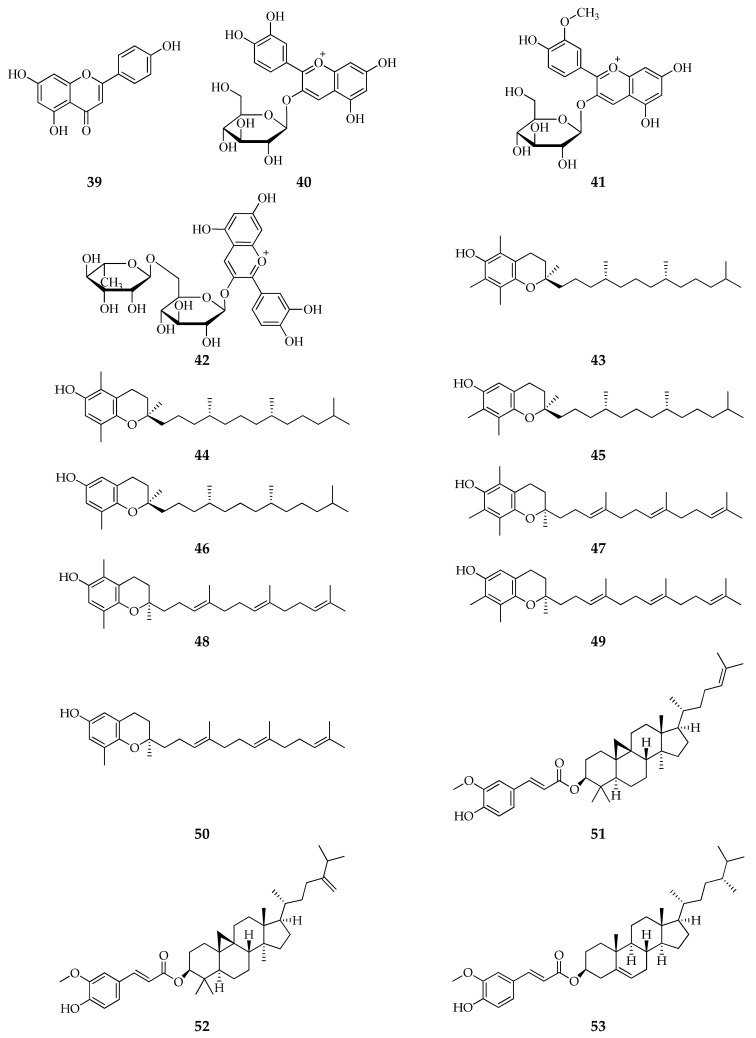
From these methods, it is known that various varieties of white rice bran contain bioactive compounds such as phenolic (vanillic acid, ferulic acid, isoferulic acid, p-coumaric acid, sinapic acid, and syringic acid), flavonoid (rutin, myricetin, and quercetin-3-glucuronide) [21,40], tocols (α-tocopherols, α-tocotrienols, γ-tocopherols, γ-tocotrienols, δ-tocopherols, and δ-tocotrienols), γ-oryzanols (cycloartenyl ferulate, 24-methylene cycloartanyl ferulate, campesteryl ferulate, and β-sitosteryl ferulate), phytosterols (stigmasterol, campesterol, and β-sitosterol), and squalene [34,41] (Figure 2).
Figure 2.
The compounds in different varieties of white rice bran use different isolation methods. (1) vanillic acid; (2) ferulic acid; (3) isoferulic acid; (4) p-coumaric acid; (5) sinapic acid; (6) syringic acid; (7) rutin; (8) myricetin; (9) quercetin-3-glucuronide [21,40]; (10) α-tocopherols; (11) γ-tocopherols; (12) δ-tocopherols; (13) α-tocotrienols; (14) γ-tocotrienols; (15) δ-tocotrienols; (16) cycloartenyl ferulate; (17) 24-methylene cycloartanyl ferulate; (18) campesteryl; (19) β-sitosteryl ferulate; (20) stigmasterol; (21) campesterol; (22) β-sitosterol; and (23) squalene [34,41].
3.2. Red Rice Bran
Red rice also has many types of varieties that are reported to have bioactive components. Jakwangdobyeo red rice bran from Gusan, Korea, has an antioxidant component. Rice bran was extracted twice using acetone 1:20 (w/v), methanol 1:20 (w/v), and ethanol 1:20 (w/v) with a solvent concentration range of 0 to 100%. Then, it was centrifuged at 1000 × g for 15 min and filtered. The supernatant was dissolved to 400 mL with each extract solvent. Then, the DPPH (2,2-diphenyl-1-picrylhydrazyl) test was carried out on the extract, total phenolic content was determined using the Folin–Ciocalteu method, and total flavonoid content was determined based on the Jia et al. (1999) method. The results showed the highest antioxidant activity, total phenolic content, and total flavonoid content in 40% acetone extract. This 40% acetone extract was then analyzed for phenolic acid content using HPLC [43].
Moreover, Huang and Lai [44] used three samples of red rice bran. Two of the red rice bran samples were from Taibalang and Guangfu red rice varieties were obtained from Hualien, Taiwan. Meanwhile, another sample of rice bran was obtained by importing from Thailand, of which the varieties were not mentioned. All samples of red rice bran had free phenolic and bound phenolic extracted; extraction was carried out twice using 80% ethanol. The supernatant obtained was extracted with ethyl acetate to obtain free phenolic extract while the residue was dissolved in 80% ethanol and re-extracted with ethyl acetate to obtain the bound phenolic extract. Both extracts were analyzed for phenolic acid content using HPLC. This study also analyzed the content of proanthocyanin and anthocyanin using HPLC/ESI-MS/MS. Extraction was carried out using methanol. In addition, an analysis of the content of vitamin E and γ-oryzanol was carried out using normal phase HPLC. Extraction was carried out using hexane solvent containing 0.02% butylated hydroxytoluene [44].
Pengkumsri et al. [45] also analyzed Mali red rice bran obtained from Thailand. Rice bran was extracted using 80% ethanol. Phenolic compounds were determined using reversed-phase HPLC with gradient elution. Meanwhile, to determine the anthocyanin content using 0.1 N HCl in methanol as solvent, anthocyanin content was determined by reversed-phase HPLC [45]. Shao et al. [21] also used 80% methanol to extract rice bran. The type of red rice used was the SB7 variety.
Ghasemzadeh et al. [46] carried out a study on red rice bran obtained from the Rice Research Institute of Iran (RRII). The red rice bran had previously been defatted by heating and extraction using hexane. Digital electric heating thermostat circulating water bath with double line six holes was used for extraction. The solvents used were ethanol and acetone. This research optimized various extraction conditions such as extraction time, extraction temperature, solvent-to-solid ratios (S/S), and solvent percentage to produce the highest flavonoids. Then, the content of flavonoids and phenolic acids was identified using the Agilent HPLC 1200 system coupled to an API 3200 triple quadrupole mass spectrometer.
In addition to dry bran samples, determination of the content of bioactive compounds was carried out on bran oil products. Rice bran oil was made from Mali red rice bran and was extracted using hexane (1:10). This study used different extraction methods including hexane extraction, hot press extraction, cold press extraction, and supercritical fluid extraction. The best yield of rice bran oil extraction was shown by the hexane extraction method. The content of γ-oryzanol and tocols was determined by reversed-phase HPLC [47].
Moko et al. [35] isolated stigmasterol in red rice bran obtained from Minahasa Regency, North Sulawesi, Indonesia. Samples were extracted using ethanol and fractionated using solvents (hexane, ethyl acetate, and n-butanol). Then, Thin Layer Chromatography (TLC) was performed on the hexane extract using the stationary phase of silica gel 60 Merck and eluent hexane-ethyl acetate (9:1) with a 5% stepwise elution. Stigmasterol was identified using FTIR and NMR.
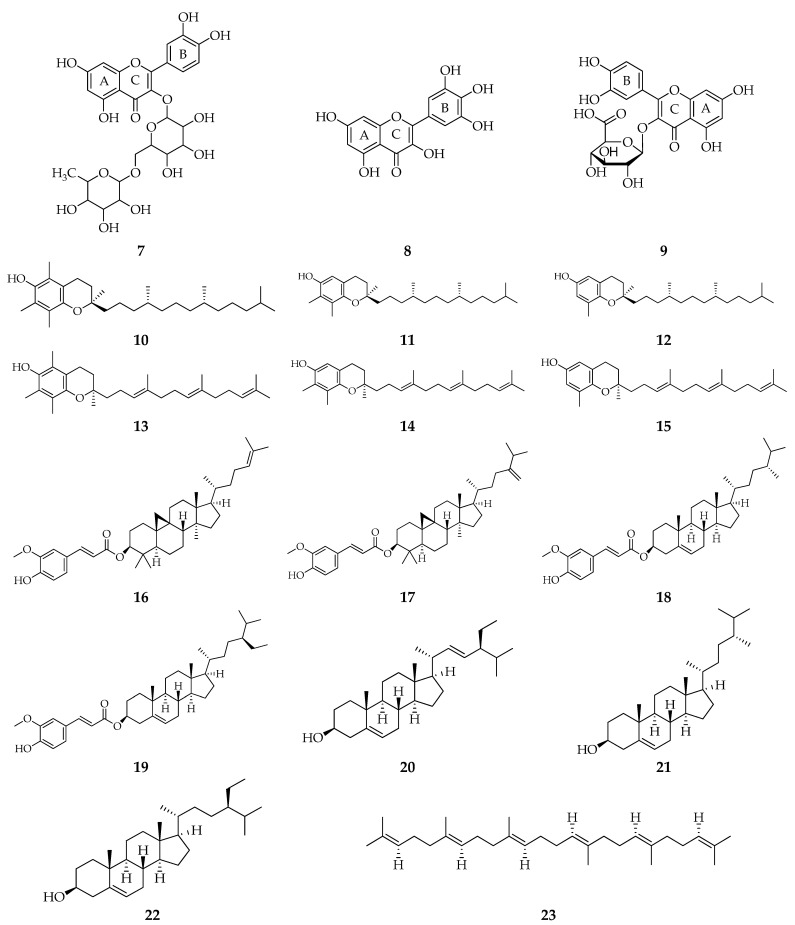
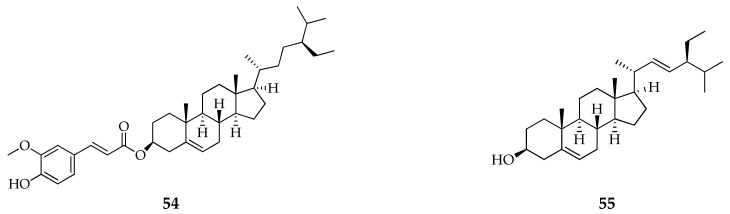
From these methods, it is known that various varieties of red rice bran contain bioactive compounds such as phenolic acid (isoferulic acid [21], ferulic acid, p-hydroxybenzoic acid, vanillic acid, protocatechuic acid, syringic acid, p-coumaric acid, sinapic acid, cinnamic acid, and gallic acid) [43,44,45,46], protocatechualdehyde [44], flavonoid (catechin, quercetin, myricetin, luteolin, and apigenin) [46], anthocyanins (cyanidin-3-glucoside, peonidin 3-glucoside, and cyanidin 3-rutinoside) [44,45], vitamin E (α-tocopherols, α-tocotrienols, β-tocopherols, β-tocotrienols, γ-tocopherols, γ-tocotrienols, δ-tocopherols, and δ-tocotrienols) [44,45,47], γ-oryzanol (cycloartenyl ferulate, 24-methylenecycloartanyl ferulate, campesteryl ferulate, and β-sitosteryl ferulate) [48], and stigmasterol [35]. In contrast to white rice bran, pigmented bran such as red rice bran contains anthocyanin compounds [28,44,45] (Figure 3).
Figure 3.
The compounds in different varieties of red rice bran use different isolation methods. (24) isoferulic acid; (25) protocatechuic acid; (26) p-hydroxybenzoic acid; (27) gallic acid; (28) vanillic acid; (29) syringic acid; (30) p-coumaric acid; (31) ferulic acid; (32) sinapic acid; (33) cinnamic acid; (34) protocatechualdehyde; (35) catechin; (36) quercetin; (37) myricetin; (38) luteolin; (39) apigenin; (40) cyanidin 3-glucoside; (41) peonidin 3-glucoside; (42) cyanidin 3-rutinoside; (43) α-tocopherols; (44) β-tocopherols; (45) γ-tocopherols; (46) δ-tocopherols; (47) α-tocotrienols; (48) β-tocotrienols; (49) γ-tocotrienols; (50) δ-tocotrienols; (51) cycloartenyl ferulate; (52) 24-methylene cycloartanyl ferulate; (53) campesteryl ferulate; (54) β-sitosteryl ferulate; and (55) stigmasterol.
3.3. Black Rice Bran
Another type of pigmented rice is black rice. After the rice milling process, black rice and bran will be produced. A study used the ultrasound-assisted extraction (UAE) method to isolate compounds in black rice bran obtained from Manipur, India. The extraction process was carried out using an ultrasonic water bath equipped with a digital timer and temperature controller. Ethanol was used as a solvent. The contents of compounds in black rice bran were analyzed using GC-MS (Gas Chromatography-Mass Spectrometry). Ultimate 3000 Liquid Chromatography Systems with a UV detector was used to identify β-carotene, tocopherols, and tocotrienols. Meanwhile, phenolic acids and anthocyanins were analyzed using HPLC [49].
Black rice bran (Kamklaing) from Thailand was macerated with 1:4 (w/v) ethanol as solvent then filtered, and the supernatant was evaporated and lyophilized. The dry extract obtained was analyzed using HPLC to determine its phenolic acid and anthocyanin content [50]. Another extraction method to determine the anthocyanin content is conventional extraction using methanol and microwave-assisted subcritical water extraction (MA-SWE). The advantage of the MA-SWE method compared with conventional extraction using methanol is the accurate temperature control [51].
Li et al. [52] used a combination of high-speed countercurrent chromatography with reversed-phase C18 column chromatography to isolate cyanidin-3-O-β-D-glucoside. This isolation method was able to produce up to 16-fold more isolates [52]. Hou et al. [53] determined the anthocyanin content of Heiyounian 96 black rice bran obtained from Japan. The bran samples were extracted using a 1:8 (w/v) acidified methanol solvent for 1 h. The methanol solvent was acidified using 1.0 M HCl in a ratio of 85:15 (v/v). Anthocyanin content was analyzed using HPLC [53]. In addition, there are also those who use 70% methanol in 1% HCl as a solvent for extraction.
Free phenolic compounds in black rice bran can be extracted using 95% methanol acidified using 1 M HCl 1:100 (w/v). The ratio of methanol and HCl was 85:15 (v/v). Meanwhile, the bound phenolic extract was extracted from the free phenolic extract using sodium hydroxide for 1 h while stirring. Lipids were extracted using hexane. The mixture was extracted five times using ethyl acetate [54].
Black rice bran samples were determined for flavonoid content using a reversed-phase HPLC with a photodiode array detector and a combined electrospray ionization tandem mass spectrometer. Rice bran samples were prepared using 0.1% formic acid in methanol as solvent, then they were sonicated for 60 min and centrifuged. The supernatant was evaporated to a volume of 2 mL [55].
Pratiwi et al. [56] reported the content of peonidin 3-glucoside and cyanidin 3-glucoside in samples of Cempo Ireng Black rice bran from DIY, Indonesia. Rice bran samples were macerated using methanol-HCl 1:10 (w/v) for 48 h. The ratio of methanol and HCl was 85:15 (v/v). Maceration was carried out 2 times and filtered. The obtained filtrate was evaporated to obtain a dry extract. The methanol extract was redissolved with methanol and fractionated. Thin layer chromatography (TLC) method with buthanol:acetic acid:water 4:1:5 (v/v) as mobile phase was used for isolation. Another method used to identify the content of peonidin 3-glucoside and cyanidine 3-glucoside is Ultra High Performance Liquid Chromatography [56].
Vardhani et al. [57] carried out the extraction of black rice bran using 96% ethanol 1:4 (w/v) to obtain a high content of γ-oryzanol [57]. In addition, the bran also contains terpenoid compounds that give the rice a good aroma character. The analysis was carried out on glutinous and non-glutinous black rice bran samples. For sampling, the SPME device with a polydimethylsiloxane (PDMS) fiber was used. The bran sample was heated at 100 °C for 30 min. The aromatic compounds were identified using the GC-MS and GC×GC-MS methods [58].
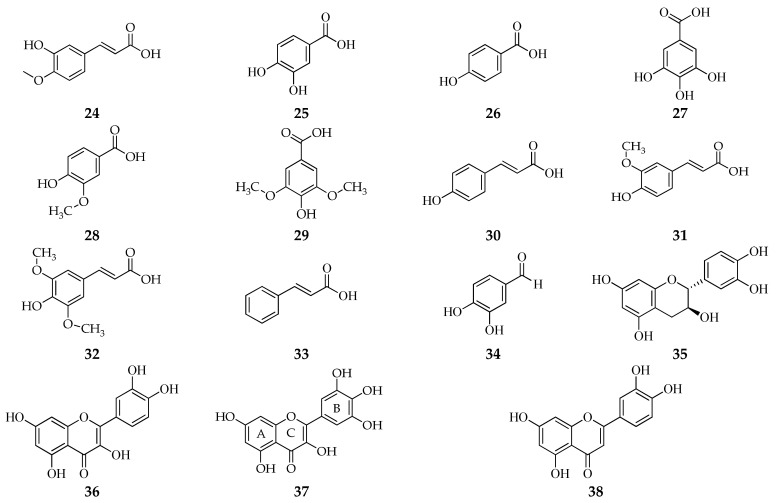
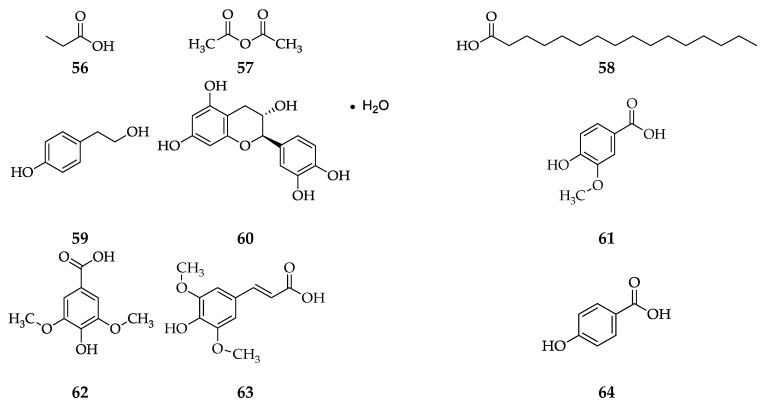
From several isolation methods and different types of black rice bran varieties, it is known that the compounds contained in black rice bran are propanoic acid, acetic anhydride, hexadecanoic acid, p-tyrosol, phenolic acid (catechin hydrate, vanillic acid, syringic acid, sinapic acid, 4-hydroxybenzoic acid, p-coumaric acid, caffeic acid, and ferulic acid), anthocyanin (cyanidin-3-rutinoside and cynanidin-3-glucoside), β-carotene, tocols (α-tocopherol and D-α-tocotrienol) [49,50,51,52,53], flavonoids (quercetin, quercetin-3-O-rutinoside, quercetin-3-O-glucoside, isorhamnetin, isorhamnetin-3-O-glucoside, and taxifolin-7-O-glucoside) [55], γ-oryzanol (Δ7-stigmastenyl ferulate, stigmasteryl ferulate, cycloartenyl ferulate, 24-methylene cycloartenol ferulate, Δ7-campestenyl ferulate, campesteryl ferulate, Δ7-sitostenyl ferulate, sitosteryl ferulate, campestanyl ferulate, and sitostanyl ferulate) [57], and terpenoids (α-pinene, sabinene, camphene, β-pinene, β-cymene, 1,4-cineol, myrcene, limonene, 1,8-cineol, trans-β-ocimene, trans-linalool oxide, γ-terpinene, cis-linalool oxide, camphor, fenchyl acetate, linalool, 10-(Acetylmethyl)-3-carene, carveol, α-copaene, α-ylangene, β-bisabolene, trans-cadina-1(6),4-diene, and 7-epi-α-selinene) [58] (See Figure 4).
Figure 4.
The compounds in different varieties of black rice bran use different isolation methods. (56) propanoic acid; (57) acetic anhydride; (58) hexadecanoic acid; (59) p-tyrosol; (60) catechin hydrate; (61) vanillic acid; (62) syringic acid; (63) sinapic acid; (64) 4-hydroxybenzoic acid; (65) p-coumaric acid; (66) caffeic acid; (67) ferulic acid; (68) cyanidin 3-glucoside; (69) cyanidin 3-rutinoside; (70) β-caroten; (71) quercetin; (72) quercetin-3-O-glucoside; (73) quercetin-3-O-rutinoside; (74) isorhamnetin; (75) isorhamnetin-3-O-glucoside; (76) taxifolin-7-O-glucoside; (77) Δ7-Stigmastenylferulate; (78) stigmasteryl ferulate; (79) cycloartenyl ferulate; (80) 24-methylene cycloartenol ferulate; (81) Δ7-Campestenyl ferulate; (82) campesteryl ferulate; (83) Δ7-Sitostenyl ferulate; (84) sitosteryl ferulate; (85) campestanyl ferulate; (86) sitostanyl ferulate; (87) α-pinene; (88) camphene; (89) sabinene; (90) β-pinene; (91) myrcene; (92) 1,4-cineol; (93) β-cymene; (94); limonene; (95) 1,8-cineol; (96) trans-β-ocimene; (97) γ-terpinene; (98) trans-linalool oxide; (99) cis-linalool oxide; (100) linalool; (101) camphor; (102) fenchyl acetate; (103) 10-(Acetylmethyl)-3-carene; (104) carveol; (105) α-copaene; (106) α-ylangene; (107) trans-cadina-1(6),4-diene; (108) β-bisabolene; and (109) 7-epi-α-selinene.
4. Extraction/Isolation Methods Using Non-Toxic Solvent
Technological advances allow the extraction process to be carried out without toxic solvents. Modern techniques allow the extraction process to be faster and more efficient because it does not use a lot of solvents. The ultrasound extraction (UAE) method is an intensification technique that has been widely used in the food and pharmaceutical industry. The principle of this extraction technique is the acoustic cavitation phenomenon which causes the release of bioactive compounds [32].
Another modern extraction technology is supercritical fluid extraction (SFE). The principle of this extraction method is using pressurized liquid as solvent. Pressure, temperature, and fluid composition affect the intermediate properties between gases and liquids of supercritical fluids. In particular, the viscosity and surface tension are similar to those of liquids. At the same time, the diffusion coefficient is similar to that of a gas, which allows for more efficient extraction by dispersing more rapidly through the solid matrix [32].
5. Rice Bran Bioactivity and Test Methods
5.1. Antimicrobial
Rice bran is known have antimicrobial activity. This antimicrobial activity is used in the food and pharmaceutical fields. A study reported that the ethanolic extract of rice bran had antimicrobial activity on emulsion-type mayonnaise. In this study, antibacterial analysis was performed using the broth microdilution method with Listeria innocua (CECT 910) and Escherichia coli (CECT 45) bacteria as strains tested [22]. Taniguchi et al. [59] carried out the synthesis of multifunctional cationic peptides identified in rice bran. The peptides were LRRHASEGGHGPHW, EKLLGKQDKGVIIRA, and SSFSKGVQRAAF. The results showed that the three peptides had antimicrobial, lipopolysaccharide-neutralizing, and angiogenic activities. The pathogens used in the antimicrobial activity test were a Gram-negative bacterium (Porphyromonas ginigivalis ATCC 33277), Gram-positive bacteria (Propionibacterium acnes JCM 6473 and Streptocossus mutans JCM5705), and a fungus (Candida albicans NBRC 1385). Antimicrobial activity assays were performed using 96-well plates [59].
Polysaccharides in rice bran are also known to have antimicrobial activity [60]. Polysaccharides extracted using hexane have better antimicrobial activity than those extracted using ethanol. The bacteria used for antimicrobial testing were Staphylococcus aureus (TISTR 517), Bacillus cereus (TISTR 687), and Escherichia coli (TISTR 1261) strains [60]. In addition to the polysaccharide content which has antimicrobial activity, the content of phenolic compounds in bran also has antimicrobial activity. Achmad et al. [61] used the bacterium Porphyromonas gingivalis to test the antimicrobial activity of rice bran using the disc diffusion method [61]. The content of γ-oryzanol in rice bran also has antimicrobial activity. The bran was extracted using isopropanol 1:25 (w/v), then the extract was dissolved using a 30% ethanol solution. This extract solution was then added to the Tryptic soy broth (TSB) medium. Antibacterial activity was carried out using the microdilution method on Gram-negative bacteria: resistant Escherichia coli, Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), and two Gram-positive bacteria: MRSA (Methicillin-resistant Staphylococcus aureus) and Staphylococcus aureus (ATCC 11632). In addition, an antifungal activity was tested using the microdilution method on Aspergillus fumigatus (ATCC 9197), Penicillium ochrochloron (ATCC 9112), Aspergillus niger (ATCC 6275), and Penicillium funiculosum (ATCC 36839) [62].
Phenolic extract from rice bran that fermented with Rhizopus oryzae was used to increase the shelf life of pizza dough. The results showed the phenolic extract that was able to increase the shelf life of pizza dough with good antifungal activity as glucosamine 17.66 µg g−1, score of molds and yeasts 1.3 × 101 CFU g−1, and invertase enzyme activity in 0.04 mg min. proteins−1 [63]. Moon et al. [64] reported that rice bran fermented using Lactiplantabillus plantarum EM had high antimicrobial activity. The antimicrobial activity test method used was spot-on-the-lawn assays. This research uses foodborne pathogenic bacteria and food spoilage fungi. The results showed high antimicrobial activity of 100–400 AU/mL [64].
Rice bran is also known to inhibit Salmonella colonization in mice. This study could be useful for protection against enteric pathogens. The results showed that rats fed a diet for one week containing 10 and 20% rice bran showed a decrease in the excretion of Salmonella feces [65]. In addition, bioprocessed (fermented) rice bran extract (BPRBE) from liquid mycelia culture of Lentinus edodes was used to inhibit Salmonella Typhimurium (SL1344). The mechanism of resistance against bacteria is by promoting upregulation of protein expression that induces bacterial destruction in autolysosomes of RAW 264.7 cells [66].
Fermented bran is known to have phenolic compounds that can inhibit fungal growth. Tests for antifungal activity were carried out in Petri dishes. The media consisted of potato dextrose agar (PDA), Penicillium verrucosum, (4 × 106 spores mL−1), and the preservatives (167 g mL−1). The results showed that the inhibition of fungi was 39.8% [67]. Rice bran fermented using Rhizopus oryzae CECT 7560 contains phenolic compounds that can inhibit fungal growth. A study reported that Fusarium, Aspergillus, and Penicillium strains were able to be inhibited by phenolic extracts from fermented rice bran. In addition, this antifungal activity was applied to bread contaminated with P. expansum. The resulting reductions were 0.6 and 0.7 log CFU g−1 [68].
5.2. Antioxidant
Research on antioxidant activity in rice bran has been widely reported. Rice bran extract using 80% methanol has the highest antioxidant activity compared with using other solvents such as 100% methanol, 80% acetone, and 100% acetone. The antioxidant activity testing methods were carried out in different systems, namely in a linoleic acid system, in a β-carotene linoleic acid system, and using sunflower oil as oxidation substrate [69].
In general, many antioxidant activity analysis methods use the DPPH radical (2,2-diphenyl-1-picrylhydrazyl) because it is stable. The principle of this method is that the DPPH color changes from purple to yellow when it receives a hydrogen atom donor from a bioactive compound. In addition, another method that is widely used for the analysis of antioxidant activity is the ABTS (2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) method. The principle of this method is to reduce the intensity the blue color of the ABTS radicals in the presence of bioactive antioxidant compounds [70].
Another study tested the antioxidant activity of rice bran using different methods such as DPPH radical scavenging ability [21,71,72,73,74,75,76,77,78,79], ABTS radical scavenging assays [45,71,79], Total antioxidant activity (TAA) [72], Nitic oxide (NO) scavenging ability [45,72], ferric reducing antioxidant power (FRAP) assay [45,74], Hydroxyl Radical Scavenging Activity [77], Superoxide Anion-Scavenging Activity [45,77], Oxygen Radical Absorbance Capacity (ORAC) assay [21], and inhibition of lipid peroxidation [45].
Several studies have shown that there is a relationship between total phenolic content and antioxidant activity. The higher the total phenolic content, the higher the antioxidant activity [45,54,80].
5.3. Cancer Chemopreventive
The process of preventing cancer using chemicals is called cancer chemoprevention [81]. Prevention, inhibition, and decrease of cancer growth can use natural products such as rice bran. Several studies have been conducted in vivo and in vitro on cancer chemopreventive activity in rice bran [82]. Colorectal cancer cells (CRC) can be inhibited with red, brown, and purple rice bran from several varieties. The results showed the influence of total phenolic content and γ-tocotrienol on the inhibition of colorectal cancer cell growth (CRC) [83]. In addition, the content of cycloartenyl ferulate also has a role in inhibiting the growth of human colorectal adenocarcinoma SW480 [84]. Peptides purified from rice bran are also known to have activity as an inhibitor of cancer growth in colon, lung, liver, and breast cancer cells [85].
Red rice bran is known to have strong activity in inhibiting cervical, stomach, and leukemia cancer cells. The test was carried out using the MTT assay. The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay is based on the conversion of MTT into formazan crystals by living cells, which determines mitochondrial activity. The content of proanthocyanidins in red rice bran has a role in inhibiting the growth of cancer cells [86]. Another study showed that purified and fractionated polysaccharides from rice bran had antitumor activity. This study used RAW 264.7 macrophages and melanoma cell line B16. The mechanism of polysaccharides in antitumor activity is by enhancing immune function [87]. The ethanol extract fraction of the black rice bran of Woja Laka variety also had chemopreventive activity on liver carcinoma HepG2 cells which was carried out using the MTT assay. Its mechanism of action is by inhibiting cell proliferation, inducing apoptosis, and causing G0/G1 phase termination in HepG2 cells [88].
In vivo studies showed that phytic acid extract from rice bran was able to suppress colon carcinogenesis in rats. The concentration of 0.2% (w/v) phytic acid extract given to rats that had been induced by azoxymethane (AOM) was able to reduce the formation of ACF [89].
5.4. Antidiabetic
Another bioactivity that rice bran has is antidiabetic. Antidiabetic test was carried out on rice bran extracted with food grade glycerol. Antidiabetic testing was carried out using the digestive enzymes inhibition assays method and also looked at AGEs (Advanced Glycation End Products) formation and inhibition. The results showed that rice bran extract was able to inhibit the activity of α-amylase, α-glucosidase, and lipase enzymes by 34.2%, 29.9%, and 12.4%, respectively. In addition, the bran extract was able to inhibit the formation of AGEs by 52.6% at a concentration of 20 L/mL [90].
The content of momilactone A (MA) and momilactone B (MB) in rice bran of the Koshihikari variety has a role in inhibiting pancreatic α-Amylase and α-Glucosidase. α-Amylase and α-Glucosidase inhibition assays were performed using a Microplate Spectrophotometer. Momilactone A (MA) and momilactone B (MB) samples were incubated with the enzyme and substrate in each well of a microplate, then the percent inhibition was calculated. The results showed the IC50 values of a-amylase inhibition (mg/mL) using momilactone A and B were 132.56 and 129.02, respectively. Meanwhile, the IC50 values of a-glucosidase inhibition (mg/mL) using momilactone A and B were 991.95 and 612.03, respectively [91]. Another study made gluten-free composite flours from wheat (Triticum aestivum), soy (Glycine max), oat bran (Avena sativa), and rice bran (Oryza sativa). The gluten-free composite flour extract was tested for its activity in inhibiting α-amylase and α-glucosidase enzymes. Then, in vivo testing was carried out on the glycemic index and glycemic load of the formulated composite flour samples. The results showed that the inhibition of α-amylase and α-glucosidase enzymes was 66.75% and 57.40%, respectively, while the results of the glycemic load (GL) of formulated foods and control samples ranged from 24.64 to 28.5%. The glycemic index of foods in the present study varied from 40.03 to 46.41%. The activity of lowering blood glucose levels showed high results of 79.62% [92].
Egyptian rice bran was stabilized and extracted, with extract standardized to contain 2% γ-oryzanol. In addition, the rice bran extract contains γ-tocotrienol, policosanol, and γ-oryzanol. The extract was tested for insulin secretion in vitro using INS-1 cells. The results showed that rice bran extract was able to increase insulin secretion. The higher the concentration of the bran extract used, the higher the insulin secretion. The results also showed that insulin secretion was induced by the content of policosanol and γ-oryzanol. In vivo experiments GTT (Glucose Tolerance Test) in rats were then performed; the results showed that plasma insulin increased after administration of 10 mg/kg rice bran extract given orally [93]. The polyphenol content in rice bran also has therapeutic potential against type 2 diabetes mellitus [94].
Stabilized rice bran (SRB), rice bran water solubles (RBWS), and rice bran fiber concentrates (RBFC) were administered to patients with insulin-dependent and noninsulin-dependent diabetes mellitus (Type I and Type II) for 60 days. The results showed a decrease in the fasting serum glucose levels and an increase in serum insulin levels. Rice bran water solubles (RBWS) showed the best results in decreasing the fasting serum glucose levels and increasing serum insulin levels [95]. Patients with type 2 diabetes mellitus who were given a supplement of 20 g stabilized rice bran once a day for 12 weeks showed lower postprandial glucose than patients with type 2 diabetes mellitus who were given only a placebo [96]. Dietary supplementation with oil-soluble rice bran triterpenoids in healthy male adults was able to reduce blood glucose increases in subjects with higher postprandial blood glucose elevations. Self-monitoring devices were used to determine blood glucose levels. The blood samples measured were fasting blood samples and 240 min after eating [97]. Rice bran milk given to primary school teachers in the city of Makassar was able to reduce fasting blood glucose levels and body weight. Rice bran milk is given as much as 30 g once a day for 1 month [98]. Triterpene alcohol and sterol preparation (TASP) from rice bran was able to reduce blood glucose levels in mice and humans [99].
5.5. Hypolipidemic Agents
Rice bran has also been studied to have bioactivity as a hypolipidemic. The tocotrienol-rich fraction isolated from rice bran oil was able to reduce lipid levels in rats. Mice with induced hyperlipidemia from being given tocotrienol-rich fraction (TRF) supplements for three weeks were able to reduce lipid parameters such as plasma triglycerides, plasma cholesterol, LDL cholesterol, HMG-CoA reductase, TBARS, and conjugated dienes. The optimal effect of TRF administration is at a dose of 8 mg TRF/kg/day [100]. Kang et al. [101] used mice that had been fed a high-fat diet resulting in an increase in lipid parameters. The mice were then fed with the addition of rice bran and phytic acid for 7 weeks. The addition of rice bran and phytic acid to food was able to reduce triglyceride and total cholesterol levels in plasma and liver which were determined using a commercial kit (Asan Pharmaceutical, Seoul, Korea). Meanwhile, the content of triglycerides and total cholesterol in the feces increased. Rice bran and phytic acid decreased fat content by increasing fecal lipid excretion [101].
The aqueous enzymatic extract from rice bran (AEERB) has hypolipidemic activity and enhances antioxidant status. Hypolipidemic testing was carried out by in vivo on male wistar rats. Rice bran was hydrolyzed in a water bath using the enzyme trypsin at a temperature of 56 °C and pH 7,9. AEERB contains protein, γ-oryzanol, and tocol. Automatic chemistry analyzer was used to analyze metabolic parameters such as the concentrations of TC, triglyceride (TG), LDL-C, HDL-C, ApoA, ApoB, and Lp (a) in the serum lipids. Furthermore, ELISA (enzyme-linked immunosorbent assay) methods with commercial kits were used to determine the hepatic HMG-CoA reductase activity. The results showed decreased liver lipid levels, inhibition of hepatic 3-hydroxyl-3-methylglutaryl CoA reductase activity, and increased fecal excretion of total lipid and total cholesterol (TC) (p < 0.05) after administration of AEERB to male wistar rats that had been treated and fed a diet high in fat and cholesterol [102].
Feeding rice bran phenolic extracts (RBPE) for 6 weeks (200 and 400 mg/kg/d) to high-fat (HF)-diet -induced mice was able to reduce the increase in levels of TC, TG, LDL-c, and FFA and reduced the decrease in HDL-c in serum. In addition, RBPE feeding was able to increase adiponectin levels in serum which decreased after high fat (HF) diet was induced. The results of histological analysis of liver tissue using hematoxylin and eosin staining and Oil Red O staining showed a reduction in lipid droplets after RBPE feeding. In addition, RBPE is also able to inhibit SREBP1c nuclear protein translocation and promote PPARα activation. Quantification of gene expression in the liver with RT-qPCR showed that RBPE was able to inhibit the expression of the HMGR gene in which the HMGR enzyme catalyzes the reduction of HMG-CoA to mevalonic acid. In addition, RBPE also inhibited the expression of SREBP1c, SCD, and ACC activation but increased the expression of CPT1a, CYP7A1, and AMPKα proteins [103].
Justo et al. [104] used obese Zucker rats that were given rice bran enzymatic extract (RBEE) 1 and 5%. The results showed a reduction in circulating levels of TG and TC and an increase in HDL-C. RBEE was also able to reduce hypertension in obese Zucker rats [104]. The rice bran enzymatic extract (RBEE) which contains γ-oryzanol, phytosterols, and tocotrienols has hypolipidemic activity by increasing HDL-C levels in serum and increasing total cholesterol and AST. RBEE was able to inhibit HMG-CoA reductase activity and increase cholesterol excretion. In addition, RBEE was also able to reduce lipid deposition and macrophage infiltration in the aortic sinus. The expression of ICAM-1 and VCAM-1 was also able to be inhibited by RBEE. In this study, we used RBEE with a dose of 1 and 5%. According to this study, regular RBEE supplementation was able to reduce the development of plaque and hepatic steatosis [105,106]. A rice bran oil (RBO) diet in type-2 diabetic rats induced by streptozotocin/nicotinamide had lower plasma triglyceride levels than in rats fed a control diet. The content of γ-oryzanol and γ-tocotrienol in rice bran oil was able to suppress hyperlipidemia in type-2 diabetic rats [107].
Clinical studies were also conducted on overweight and obese adults who were not taking cholesterol-lowering drugs. Participants for 8 weeks were given a diet containing either pigmented rice bran (RB) or the RB with addition of plant sterols (RB + PS) snack bars. The results showed a weight loss of about 4.7 ± 2.2 kg. Total cholesterol content decreased by 36 ± 25 g/dL (for RB + PS) and 7 ± 16 g/dL (for RB). Meanwhile, LDL content decreased by 22.3 ± 25.2 g/dL (for RB + PS) and 4.4 ± 18.9 g/dL (for RB) [108]. In addition, rice bran extract containing the acylated steryl glucoside fraction was able to reduce LDL-c levels in the blood when was tested on obese men in Japan [109].
6. Phenolic Compound in Rice Bran as Antioxidant Properties
A group of small molecules having at least one phenol unit are phenolic compounds. Phenolic compounds are divided into different subgroups such as phenolic acids, flavonoids, tannins, coumarins, lignans, quinones, stilbenes, and curcuminoids based on their chemical structure [110]. Phenolic compounds are known to have very good antioxidant activity [111]. The presence of a hydroxyl group on the aromatic ring of phenolic compounds allows phenolic compounds to act as antioxidants. The hydroxyl group is able to transfer hydrogen atoms to a free radical so that it is stabilized [36]. Rice bran has the highest total phenolic content (TPC) compared with whole grain, embryo, and endosperm in white, red, and black rice [21].
Phenolic compounds in rice bran exist in bound, conjugated, and free forms [112]. Free phenolics are free soluble compounds that are localized in plant cell vacuoles where they are trapped. Meanwhile, conjugated phenolics are soluble phenolics that are covalently bonded to other molecules such as fatty acids [113]. Conjugated phenolics are soluble components that can be extracted by aqueous solutions of methanol but are considered to be bound to soluble oligosaccharides and peptides through ester bonds or ether bonds, which can be released after hydrolysis [114]. Bound phenolics are insoluble components because they are bound to cell wall components such as pectin, cellulose, arabionoxylan, and structural proteins by covalent bonds. The bound phenolic content is usually higher than the free soluble phenolic [113,115]. Studies have been conducted which showed that bound and insoluble phenolic extracts in corn, wheat, oats, and rice have higher antioxidant activity than soluble free phenolic extracts [115]. Unfortunately, insoluble bound phenolic compounds are more difficult to extract; in the extraction process, hydrolysis is required first, such as by using alkaline and enzymes [26,116,117]. Therefore, currently there are many studies that focus on increasing antioxidants in rice bran with various methods.
7. Enhancement of The Antioxidant Capacity Method in Rice Bran
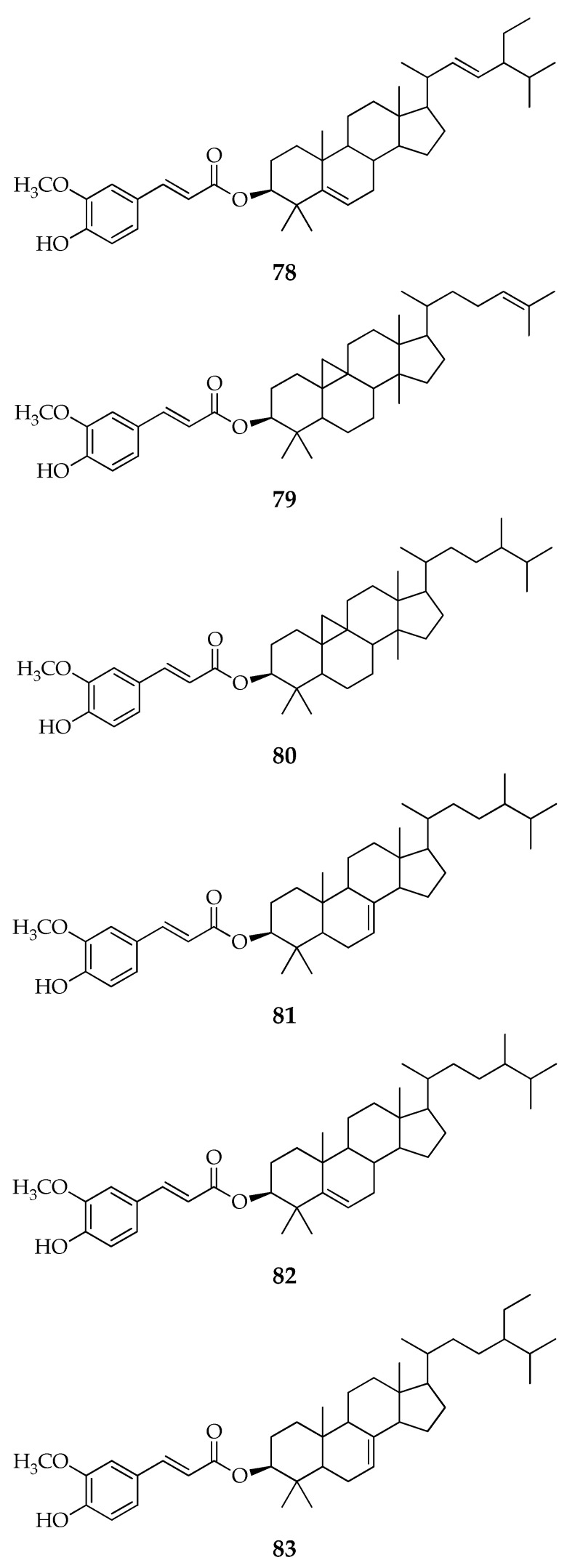
In plants, the contents of bioactive compound exist in free and bound forms. Previous studies reported that fermentation and treatment with enzymes can increase the contents of bioactive compounds in rice bran. Fermentation process and treatment with enzymes are capable of releasing bioactive compounds that bound with cell wall components, such as lignins [27,116]. The methods used to increase antioxidant capacity in rice bran from several rice varieties are summarized in Table 1.
Table 1.
Enhancement of The Antioxidant Capacity Method in Rice Bran.
| Variety (Source) | Method | Effect | Reference |
|---|---|---|---|
| Japonica type variety unknown (Seoul, Korea) | treated with different carbohydrases (Viscozyme, Termamyl, Celluclast, AMG, Ultraflo, and Pentopan) | ethanol extract (%), reducing sugar (mg/g), and total phenolic content (mg GAE/g) increased | [118] |
| Jyothi variety red rice paddy (Karnataka, India) | treated with Bacillus Endoxylanase (EXB) and Trichoderma Endoxylanase (EXF) | total flavonoid; individual soluble phenolic components cycloartenyl ferulate; β-sitosteryl ferulate; and δ, γ, α-tocotrienols and tocopherols increased | [48] |
| Unknown (Yogyakarta, Indonesia) | fermented with Lactobacillus plantarum and Lactobacillus lactic | total phenolic content (mg GAE/g dw) increased | [119] |
| Khao Bahn Nah and Thai jasmine (Prachin Buri province, Thailand) | fermented with Lactobacillus casei and Lactobacillus plantarum | polysaccharide (mg/mL) decreased and total phenolic content (mg/mL) increased | [120] |
| BR-IRGA 417 (Brazil) | fermented with Rizhopus oryzae | total phenolic content (mg/g) increased | [121] |
| Unknown (Stuttgart, Ark., U.S.A.) | fermented with Bacillus subtilis subspecies subtilis NRRL NRS-744 | total phenolic content (mg FAE/g) increased | [122] |
| PB-1121 (Sirsa, India) | solid-state fermentation (SSF) with Aspergillus oryzae MTCC 3107 | total phenolic content (g GAE/g dwb) and condensed tannin content (mg CE/g dwb) increased | [123] |
| IR 64 and Jyothi (Karnataka, India) | treated with Cellulase from Aspergillus niger (1.4 U/mg solid) from Sigma-Aldrich, USA and Xylanase D-762-p (400 U/g solid) from Biocatalyst | γ-oryzanol; soluble, bound, and total polyphenols; flavonoid and tannin content increased | [124] |
| RD6 (Chiang Mai Rice Research Center, Thailand) | Three different methods were used for comparison, namely enzymatically stabilized rice bran (ESRB) using (trypsin, chymotrypsin, papain, bromelain, or Flavorzyme) enzymes; raw rice bran (RRB) without any treatment; and thermally stabilized rice bran (TSRB) which is raw rice bran heated at 100 °C in open steam for 15 min | free phenolics, γ-oryzanol, tocopherols, and tocotrienols content in ESRB is higher compared with TSRB and RRB | [125] |
| Jyothi paddy (Karnataka, India) | Rice bran was treated with a combination of endo-1,4-beta-xylanase (EXYL) and Fiberzyme (Fzyme) enzymes. Three combinations of the two enzymes were performed, namely 1.5 BGU + 3 EXU (CXC1), 3 BGU + 2 EXU (CXC2), and 4.5 BGU + 1 EXU (CXC3) enzymes. | The content of ferulic acid in soluble phenolics, p-coumaric acid in bound phenolics, and γ-oryzanol fractions increased the most in the combination CXC2. In addition, ferric reducing power, DPPH• scavenging capacity, nitric oxide scavenging, and inhibition of human LDL oxidation were increased for all three types of enzyme combination. Superoxide anion and hydroxyl radical scavenging activities increased for the combination of CXC1 and CXC2 enzymes. | [126] |
| Khao Dok Mali 105 (Suphan Buri Province, Thailand) | Six different pretreatment methods were carried out in rice bran to extract rice bran oil, such as microwave heating (60–110 °C for 3 min), hot air heating (70–180 °C for 10 min), roasting (60 and 80 °C for 3 min), parboiling (75 °C for 60 min), autoclave heating (121 °C for 15 min), and hydrolysis with α-amylase 1375 units/mL under the optimum condition for enzyme activity (180 rpm, at 50 °C for 120 min) | Pretreatment with roasting at 60 °C produced the highest γ-oryzanol content which was 46.9 mg/mL of rice bran oil. | [127] |
| Unknown (Kuala Lumpur, Malaysia) | fermented with Rhizopus oligosporus (strain F0020) and Monascus purpureus (strain F0061) | The phenolic acid content in methanol extracts of fermented rice bran such as ferulic, sinapic, vanillic, caffeic, syringic, and 4-hydroxybenzoic acids increased | [128] |
| KDML 105 (Northeastern Thailand) | Three different types of pretreatment were carried out on rice bran compared with raw bran, namely hot-air (120°C for 30 min), far-infrared radiation (FIR), and hydrolysis with cellulase | There was an increase in DPPH radical scavenging activities, ferric reducing antioxidant power (FRAP), total phenolic content (TPC), several phenolic acids, α-tocopherols, γ-tocopherols, and δ-tocopherols; the highest was in rice bran treated with FIR-treated | [129] |
| Axios-Long A type (Unknown) | infrared radiation heating | There was an increase in phenolic content and antioxidant activity in the bound extract of rice bran which was directly proportional to the increase in IR power. | [130] |
| Unknown (Rio Grande do Sul, Brazil) | fermented rice bran by Saccharomyces cerevisiae for gluten-free cookies formulation with different fermented rice bran composition, namely 7.08%, 14.16%, and 28.33% | Cookie formulations with a fermented rice bran composition of 7.08% showed the highest phenolic compound content compared with the others and was higher than cookies without fermented rice bran. | [131] |
8. Conclusions
Bioactive compounds in white, red, and black rice bran can be isolated by column chromatography using silica gel. The solvents used for the extraction process are methanol, ethanol, acetone, chloroform, hexane, ethyl acetate, dichloromethane, and isopropanol. However, these solvents are toxic so that in the future alternative extraction methods such as ultrasound extraction (UAE) and supercritical fluid extraction (SFE) can be used. Several methods of identification and characterization of bioactive compounds in rice bran used HPLC (High Performance Liquid Chromatography), TLC (Thin Layer Chromatography), FTIR (Fourier-transform infrared spectroscopy), and NMR (Nuclear magnetic resonance). The process of releasing bonds between the components of bioactive compounds and cell wall components affects the amount of bioactive compounds extracted so that the antioxidant activity of rice bran increases. Fermentation method with Lactobacillus plantarum, Lactobacillus lactic, Lactobacillus casei, Rizhopus oryzae, Bacillus subtilis, Aspergillus oryzae, Rhizopus oligosporus (strain F0020), Monascus purpureus (strain F0061), and Saccharomyces cerevisiae have been used on rice bran. Treatment using enzymes carbohydrases, cellulase, xylanase, trypsin, chymotrypsin, papain, bromelain, or flavorzyme, fiberzyme, and endo-1,4-beta-xylanase has been carried out. In the future, methods such as infrared radiation heating, which is easier and can be applied on an industrial scale, can be developed to increase the antioxidant capacity of rice bran.
9. Patents
This section is not mandatory but may be added if there are patents resulting from the work reported in this manuscript.
Acknowledgments
The authors are grateful to Academic Leadership Grant (ALG) and to Universitas Padjadjaran for all research facilities.
Author Contributions
Conceptualization, R.A. and T.S.; methodology, T.S., D.K. and S.I.; validation, T.S. and D.K.; formal analysis, T.S.; resources, R.A., T.S. and D.K.; data curation, R.A. and S.I.; writing—original draft preparation, R.A.; writing—review and editing, R.A., T.S. and D.K.; visualization, R.A.; supervision, T.S. and D.K.; project administration, T.S.; funding acquisition, D.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The study did not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Academic Leadership Grant (ALG) Dikdik Kurnia, Indonesia (2203/UN6.3.1/PT.00/2022; May 20 May 2022).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arfin S., Jha N.K., Jha S.K., Kesari K.K., Ruokolainen J., Roychoudhury S., Rathi B., Kumar D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants. 2021;10:642. doi: 10.3390/antiox10050642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ighodaro O.M. Molecular Pathways Associated with Oxidative Stress in Diabetes Mellitus. Biomed. Pharmacother. 2018;108:656–662. doi: 10.1016/j.biopha.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 3.Chehaibi K., Trabelsi I., Mahdouani K., Slimane M.N. Correlation of Oxidative Stress Parameters and Inflammatory Markers in Ischemic Stroke Patients. J. Stroke Cerebrovasc. Dis. 2016;25:2585–2593. doi: 10.1016/j.jstrokecerebrovasdis.2016.06.042. [DOI] [PubMed] [Google Scholar]
- 4.Shaik F.B., Nagajothi G., Swarnalatha K., Kumar C.S., Rajendra W., Maddu N. Correlation between Smokeless Tobacco (Gutkha) and Biomarkers of Oxidative Stress in Plasma with Cardiovascular Effects. Heliyon. 2021;7:e05487. doi: 10.1016/j.heliyon.2020.e05487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francenia Santos-Sánchez N., Salas-Coronado R., Villanueva-Cañongo C., Hernández-Carlos B. Antioxidant Compounds and Their Antioxidant Mechanism. In: Shalaby E., editor. Antioxidants. IntechOpen; London, UK: 2019. [Google Scholar]
- 6.Lü J.-M., Lin P.H., Yao Q., Chen C. Chemical and Molecular Mechanisms of Antioxidants: Experimental Approaches and Model Systems. J. Cell. Mol. Med. 2010;14:840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bordiga M., Gomez-Alonso S., Locatelli M., Travaglia F., Coïsson J.D., Hermosin-Gutierrez I., Arlorio M. Phenolics Characterization and Antioxidant Activity of Six Different Pigmented Oryza Sativa L. Cultivars Grown in Piedmont (Italy) Food Res. Int. 2014;65:282–290. doi: 10.1016/j.foodres.2014.03.007. [DOI] [Google Scholar]
- 8.Dutta A.K., Gope P.S., Banik S., Makhnoon S., Siddiquee M.A., Kabir Y. Antioxidant Properties of Ten High Yielding Rice Varieties of Bangladesh. Asian Pac. J. Trop. Biomed. 2012;2:S99–S103. doi: 10.1016/S2221-1691(12)60137-3. [DOI] [Google Scholar]
- 9.Apea-Bah F.B., Li X., Beta T. Phenolic Composition and Antioxidant Properties of Cooked Rice Dyed with Sorghum-Leaf Bio-Colorants. Foods. 2021;10:2058. doi: 10.3390/foods10092058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du S., Huang X., Cai Y., Hao Y., Qiu S., Liu L., Cui M., Luo L. Differential Antioxidant Compounds and Activities in Seedlings of Two Rice Cultivars Under Chilling Treatment. Front. Plant Sci. 2021;12:631421. doi: 10.3389/fpls.2021.631421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao J., Zhu H., Zhang Z., Yang S., Li H. Identification of Anthocyanins in Black Rice (Oryza Sativa L.) by UPLC/Q-TOF-MS and Their in Vitro and in Vivo Antioxidant Activities. J. Cereal Sci. 2015;64:92–99. doi: 10.1016/j.jcs.2015.05.003. [DOI] [Google Scholar]
- 12.Palungwachira P., Tancharoen S., Phruksaniyom C., Klungsaeng S., Srichan R., Kikuchi K., Nararatwanchai T. Antioxidant and Anti-Inflammatory Properties of Anthocyanins Extracted from Oryza Sativa L. in Primary Dermal Fibroblasts. Oxidative Med. Cell. Longev. 2019;2019:2089817. doi: 10.1155/2019/2089817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moongngarm A., Daomukda N., Khumpika S. Chemical Compositions, Phytochemicals, and Antioxidant Capacity of Rice Bran, Rice Bran Layer, and Rice Germ. APCBEE Procedia. 2012;2:73–79. doi: 10.1016/j.apcbee.2012.06.014. [DOI] [Google Scholar]
- 14.Aguilar-Garcia C., Gavino G., Baragaño-Mosqueda M., Hevia P., Gavino V.C. Correlation of Tocopherol, Tocotrienol, γ-Oryzanol and Total Polyphenol Content in Rice Bran with Different Antioxidant Capacity Assays. Food Chem. 2007;102:1228–1232. doi: 10.1016/j.foodchem.2006.07.012. [DOI] [Google Scholar]
- 15.Martillanes S., Ayuso-Yuste M.C., Gil M.V., Manzano-Durán R., Delgado-Adámez J. Bioavailability, Composition and Functional Characterization of Extracts from Oryza Sativa L. Bran. Food Res. Int. 2018;111:299–305. doi: 10.1016/j.foodres.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 16.Ghasemzadeh A., Karbalaii M.T., Jaafar H.Z.E., Rahmat A. Phytochemical Constituents, Antioxidant Activity, and Antiproliferative Properties of Black, Red, and Brown Rice Bran. Chem. Cent. J. 2018;12:17. doi: 10.1186/s13065-018-0382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y., Zhu K., Xia H., Chen L., Chen K. Comparative Proteomic Analysis of Indica and Japonica Rice Varieties. Genet. Mol. Biol. 2014;37:652–661. doi: 10.1590/S1415-47572014005000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang Y., Ahmed S., Xu Y., Beta T., Zhu Z., Shao Y., Bao J. Bound Phenolic Compounds and Antioxidant Properties of Whole Grain and Bran of White, Red and Black Rice. Food Chem. 2018;240:212–221. doi: 10.1016/j.foodchem.2017.07.095. [DOI] [PubMed] [Google Scholar]
- 19.Oppong D., Panpipat W., Chaijan M. Chemical, Physical, and Functional Properties of Thai Indigenous Brown Rice Flours. PLoS ONE. 2021;16:e0255694. doi: 10.1371/journal.pone.0255694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fongfon S., Pusadee T., Prom-u-thai C., Rerkasem B., Jamjod S. Diversity of Purple Rice (Oryza Sativa L.) Landraces in Northern Thailand. Agronomy. 2021;11:2029. doi: 10.3390/agronomy11102029. [DOI] [Google Scholar]
- 21.Shao Y., Xu F., Sun X., Bao J., Beta T. Identification and Quantification of Phenolic Acids and Anthocyanins as Antioxidants in Bran, Embryo and Endosperm of White, Red and Black Rice Kernels (Oryza Sativa L.) J. Cereal Sci. 2014;59:211–218. doi: 10.1016/j.jcs.2014.01.004. [DOI] [Google Scholar]
- 22.Martillanes S., Rocha-Pimienta J., Gil M.V., Ayuso-Yuste M.C., Delgado-Adámez J. Antioxidant and Antimicrobial Evaluation of Rice Bran (Oryza Sativa L.) Extracts in a Mayonnaise-Type Emulsion. Food Chem. 2020;308:125633. doi: 10.1016/j.foodchem.2019.125633. [DOI] [PubMed] [Google Scholar]
- 23.Verschoyle R.D., Greaves P., Cai H., Edwards R.E., Steward W.P., Gescher A.J. Evaluation of the Cancer Chemopreventive Efficacy of Rice Bran in Genetic Mouse Models of Breast, Prostate and Intestinal Carcinogenesis. Br. J. Cancer. 2007;96:248–254. doi: 10.1038/sj.bjc.6603539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boue S.M., Daigle K.W., Chen M.-H., Cao H., Heiman M.L. Antidiabetic Potential of Purple and Red Rice (Oryza Sativa L.) Bran Extracts. J. Agric. Food Chem. 2016;64:5345–5353. doi: 10.1021/acs.jafc.6b01909. [DOI] [PubMed] [Google Scholar]
- 25.Chandrashekar P., Kumar P.K.P., Ramesh H.P., Lokesh B.R., Krishna A.G.G. Hypolipidemic Effect of Oryzanol Concentrate and Low Temperature Extracted Crude Rice Bran Oil in Experimental Male Wistar Rats. J. Food Sci. Technol. 2014;51:1278–1285. doi: 10.1007/s13197-012-0628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W., Guo J., Zhang J., Peng J., Liu T., Xin Z. Isolation, Identification and Antioxidant Activity of Bound Phenolic Compounds Present in Rice Bran. Food Chem. 2015;171:40–49. doi: 10.1016/j.foodchem.2014.08.095. [DOI] [PubMed] [Google Scholar]
- 27.Shin H.-Y., Kim S.-M., Lee J.H., Lim S.-T. Solid-State Fermentation of Black Rice Bran with Aspergillus Awamori and Aspergillus Oryzae: Effects on Phenolic Acid Composition and Antioxidant Activity of Bran Extracts. Food Chem. 2019;272:235–241. doi: 10.1016/j.foodchem.2018.07.174. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y., Ma Y., Dong L., Jia X., Liu L., Huang F., Chi J., Xiao J., Zhang M., Zhang R. Extrusion and Fungal Fermentation Change the Profile and Antioxidant Activity of Free and Bound Phenolics in Rice Bran Together with the Phenolic Bioaccessibility. LWT. 2019;115:108461. doi: 10.1016/j.lwt.2019.108461. [DOI] [Google Scholar]
- 29.Jan-on G., Sangartit W., Pakdeechote P., Kukongviriyapan V., Sattayasai J., Senaphan K., Kukongviriyapan U. Virgin Rice Bran Oil Alleviates Hypertension through the Upregulation of ENOS and Reduction of Oxidative Stress and Inflammation in L-NAME–Induced Hypertensive Rats. Nutrition. 2020;69:110575. doi: 10.1016/j.nut.2019.110575. [DOI] [PubMed] [Google Scholar]
- 30.Pacheco de Delahaye E., Jiménez P., Pérez E. Effect of Enrichment with High Content Dietary Fiber Stabilized Rice Bran Flour on Chemical and Functional Properties of Storage Frozen Pizzas. J. Food Eng. 2005;68:1–7. doi: 10.1016/j.jfoodeng.2004.05.048. [DOI] [Google Scholar]
- 31.Irakli M., Mygdalia A., Chatzopoulou P., Katsantonis D. Impact of the Combination of Sourdough Fermentation and Hop Extract Addition on Baking Properties, Antioxidant Capacity and Phenolics Bioaccessibility of Rice Bran-Enhanced Bread. Food Chem. 2019;285:231–239. doi: 10.1016/j.foodchem.2019.01.145. [DOI] [PubMed] [Google Scholar]
- 32.Chaves J.O., de Souza M.C., da Silva L.C., Lachos-Perez D., Torres-Mayanga P.C., Machado A.P.d.F., Forster-Carneiro T., Vázquez-Espinosa M., González-de-Peredo A.V., Barbero G.F., et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020;8:507887. doi: 10.3389/fchem.2020.507887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q.-W., Lin L.-G., Ye W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chinese Med. 2018;13:1–26. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aziz S., Elfahmi Y., Soemardji A.A., Sukrasno S. Anti-Hypercholesterolemic Agent from Indonesian Rice Bran. Int. J. Res. Pharm. Sci. 2019;10:2733–2738. doi: 10.26452/ijrps.v10i4.1538. [DOI] [Google Scholar]
- 35.Moko E.M., Rahardiyan D. Structure of Stigmasterols in Bran of Red Rice from Minahasa Regency, North Sulawesi, Indonesia. Fuller. J. Chem. 2020;5:16. doi: 10.37033/fjc.v5i1.145. [DOI] [Google Scholar]
- 36.Rungratanawanich W., Memo M., Uberti D. Redox Homeostasis and Natural Dietary Compounds: Focusing on Antioxidants of Rice (Oryza Sativa L.) Nutrients. 2018;10:1605. doi: 10.3390/nu10111605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koizumi T., Furuhashi G. Global Rice Market Projections Distinguishing Japonica and Indica Rice under Climate Change. JARQ. 2020;54:63–91. doi: 10.6090/jarq.54.63. [DOI] [Google Scholar]
- 38.Morales-Martínez L.E., Bello-Pérez L.A., Sánchez-Rivera M.M., Ventura-Zapata E., Jiménez-Aparicio A.R. Morphometric, Physicochemical, Thermal, and Rheological Properties of Rice (Oryza Sativa L.) Cultivars Indica & Japonica. Food Nutr. Sci. 2014;05:271–279. doi: 10.4236/fns.2014.53034. [DOI] [Google Scholar]
- 39.Ding C., Liu Q., Li P., Pei Y., Tao T., Wang Y., Yan W., Yang G., Shao X. Distribution and Quantitative Analysis of Phenolic Compounds in Fractions of Japonica and Indica Rice. Food Chem. 2019;274:384–391. doi: 10.1016/j.foodchem.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Peanparkdee M., Patrawart J., Iwamoto S. Effect of Extraction Conditions on Phenolic Content, Anthocyanin Content and Antioxidant Activity of Bran Extracts from Thai Rice Cultivars. J. Cereal Sci. 2019;86:86–91. doi: 10.1016/j.jcs.2019.01.011. [DOI] [Google Scholar]
- 41.Pokkanta P., Sookwong P., Tanang M., Setchaiyan S., Boontakham P., Mahatheeranont S. Simultaneous Determination of Tocols, γ-Oryzanols, Phytosterols, Squalene, Cholecalciferol and Phylloquinone in Rice Bran and Vegetable Oil Samples. Food Chem. 2019;271:630–638. doi: 10.1016/j.foodchem.2018.07.225. [DOI] [PubMed] [Google Scholar]
- 42.Hansakul P., Srisawat U., Kaendee N., Panunto W. Determination of Phenolic Compounds, Flavonoids, and Antioxidant Activities in Crude Water Extracts of Thai Red and White Rice Cultivars. Planta Med. 2010;76:s-0030-1264431. doi: 10.1055/s-0030-1264431. [DOI] [PubMed] [Google Scholar]
- 43.Jun H.-I., Song G.-S., Yang E.-I., Youn Y., Kim Y.-S. Antioxidant Activities and Phenolic Compounds of Pigmented Rice Bran Extracts. J. Food Sci. 2012;77:C759–C764. doi: 10.1111/j.1750-3841.2012.02763.x. [DOI] [PubMed] [Google Scholar]
- 44.Huang Y.-P., Lai H.-M. Bioactive Compounds and Antioxidative Activity of Colored Rice Bran. J. Food Drug Anal. 2016;24:564–574. doi: 10.1016/j.jfda.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pengkumsri N., Chaiyasut C., Saenjum C., Sirilun S., Peerajan S., Suwannalert P., Sirisattha S., Sivamaruthi B.S. Physicochemical and Antioxidative Properties of Black, Brown and Red Rice Varieties of Northern Thailand. Food Sci. Technol. 2015;35:331–338. doi: 10.1590/1678-457X.6573. [DOI] [Google Scholar]
- 46.Ghasemzadeh A., Baghdadi A., Jaafar Z.E.H., Swamy M., Megat Wahab P. Optimization of Flavonoid Extraction from Red and Brown Rice Bran and Evaluation of the Antioxidant Properties. Molecules. 2018;23:1863. doi: 10.3390/molecules23081863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pengkumsri N., Chaiyasut C., Sivamaruthi B.S., Saenjum C., Sirilun S., Peerajan S., Suwannalert P., Sirisattha S., Chaiyasut K., Kesika P. The Influence of Extraction Methods on Composition and Antioxidant Properties of Rice Bran Oil. Food Sci. Technol. 2015;35:493–501. doi: 10.1590/1678-457X.6730. [DOI] [Google Scholar]
- 48.Sapna I., Jayadeep A. Role of Endoxylanase and Its Concentrations in Enhancing the Nutraceutical Components and Bioactivities of Red Rice Bran. LWT. 2021;147:111675. doi: 10.1016/j.lwt.2021.111675. [DOI] [Google Scholar]
- 49.Das A.B., Goud V.V., Das C. Extraction of Phenolic Compounds and Anthocyanin from Black and Purple Rice Bran (Oryza Sativa L.) Using Ultrasound: A Comparative Analysis and Phytochemical Profiling. Ind. Crops Prod. 2017;95:332–341. doi: 10.1016/j.indcrop.2016.10.041. [DOI] [Google Scholar]
- 50.Phetpornpaisan P., Tippayawat P., Jay M., Sutthanut K. A Local Thai Cultivar Glutinous Black Rice Bran: A Source of Functional Compounds in Immunomodulation, Cell Viability and Collagen Synthesis, and Matrix Metalloproteinase-2 and -9 Inhibition. J. Funct. Foods. 2014;7:650–661. doi: 10.1016/j.jff.2013.12.020. [DOI] [Google Scholar]
- 51.Moirangthem K., Ramakrishna P., Amer M.H., Tucker G.A. Bioactivity and Anthocyanin Content of Microwave-Assisted Subcritical Water Extracts of Manipur Black Rice (Chakhao) Bran and Straw. Future Foods. 2021;3:100030. doi: 10.1016/j.fufo.2021.100030. [DOI] [Google Scholar]
- 52.Li B., Du W., Qian D., Du Q. Combination of High-Speed Countercurrent Chromatography and Reversed Phase C18 Chromatography for Large-Scale Isolation of Cyanidin-3-O-β-d-Glucoside from Black Rice Bran Extract. Ind. Crops Prod. 2012;37:88–92. doi: 10.1016/j.indcrop.2011.12.009. [DOI] [Google Scholar]
- 53.Hou F., Zhang R., Zhang M., Su D., Wei Z., Deng Y., Zhang Y., Chi J., Tang X. Hepatoprotective and Antioxidant Activity of Anthocyanins in Black Rice Bran on Carbon Tetrachloride-Induced Liver Injury in Mice. J. Funct. Foods. 2013;5:1705–1713. doi: 10.1016/j.jff.2013.07.015. [DOI] [Google Scholar]
- 54.Zhang M.W., Zhang R.F., Zhang F.X., Liu R.H. Phenolic Profiles and Antioxidant Activity of Black Rice Bran of Different Commercially Available Varieties. J. Agric. Food Chem. 2010;58:7580–7587. doi: 10.1021/jf1007665. [DOI] [PubMed] [Google Scholar]
- 55.Sriseadka T., Wongpornchai S., Rayanakorn M. Quantification of Flavonoids in Black Rice by Liquid Chromatography-Negative Electrospray Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2012;60:11723–11732. doi: 10.1021/jf303204s. [DOI] [PubMed] [Google Scholar]
- 56.Pratiwi R., Tunjung W.A.S., Rumiyati R., Amalia A.R. Black Rice Bran Extracts and Fractions Containing Cyanidin 3-Glucoside and Peonidin 3-Glucoside Induce Apoptosis in Human Cervical Cancer Cells. Indones. J. Biotechnol. 2016;20:69. doi: 10.22146/ijbiotech.15271. [DOI] [Google Scholar]
- 57.Vardhani A., Jufri M., Purwaningsih E. Potency of γ-Oryzanol Rich Black Rice Bran (Oryza Sativa L. Indica) Extract for Tyrosinase Inhibition. Int. J. Pharm. Pharm. Sci. 2020;12:90–93. doi: 10.22159/ijpps.2020v12i5.37197. [DOI] [Google Scholar]
- 58.Chumpolsri W., Wijit N., Boontakham P., Nimmanpipug P., Sookwong P., Luangkamin S., Wongpornchai S. Variation of Terpenoid Flavor Odorants in Bran of Some Black and White Rice Varieties Analyzed by GC×GC-MS. J. Food Nutr. Res. 2015;3:114–120. doi: 10.12691/jfnr-3-2-7. [DOI] [Google Scholar]
- 59.Taniguchi M., Kameda M., Namae T., Ochiai A., Saitoh E., Tanaka T. Identification and Characterization of Multifunctional Cationic Peptides Derived from Peptic Hydrolysates of Rice Bran Protein. J. Funct. Foods. 2017;34:287–296. doi: 10.1016/j.jff.2017.04.046. [DOI] [Google Scholar]
- 60.Surin S., Phimolsiripol Y. Antioxidant and Antimicrobial Properties of Polysaccharides from Rice Brans. Chiang Mai J. Sci. 2018;45:1372–1382. [Google Scholar]
- 61.Achmad H., Singgih M.F., Ramdhani A.F., Ramadhany Y.F. Inhibitory Power Test of White Rice Bran Extract (Oryza Sativa L.) with the Solution of Ethanol and Aquades on Porphyromonas Gingivalis (In Vitro) Bacteria. Syst. Rev. Pharm. 2020;11:6. [Google Scholar]
- 62.Castanho A., Lageiro M., Calhelha R.C., Ferreira I.C.F.R., Sokovic M., Cunha L.M., Brites C. Exploiting the Bioactive Properties of γ-Oryzanol from Bran of Different Exotic Rice Varieties. Food Funct. 2019;10:2382–2389. doi: 10.1039/C8FO02596G. [DOI] [PubMed] [Google Scholar]
- 63.Christ-Ribeiro A., Bretanha C.C., Luz G.G., de Souza M.M., Badiale-Furlong E. <b>Antifungal Compounds Extracted from Rice Bran Fermentation Applied to Bakery Product Conservation. Acta Sci. Technol. 2017;39:263. doi: 10.4025/actascitechnol.v39i3.29099. [DOI] [Google Scholar]
- 64.Moon S.-H., Chang H.-C. Rice Bran Fermentation Using Lactiplantibacillus Plantarum EM as a Starter and the Potential of the Fermented Rice Bran as a Functional Food. Foods. 2021;10:978. doi: 10.3390/foods10050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar A., Henderson A., Forster G.M., Goodyear A.W., Weir T.L., Leach J.E., Dow S.W., Ryan E.P. Dietary Rice Bran Promotes Resistance to Salmonella Enterica Serovar Typhimurium Colonization in Mice. BMC Microbiol. 2012;12:71. doi: 10.1186/1471-2180-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim S.P., Lee S.J., Nam S.H., Friedman M. The Composition of a Bioprocessed Shiitake (Lentinus Edodes) Mushroom Mycelia and Rice Bran Formulation and Its Antimicrobial Effects against Salmonella Enterica Subsp. Enterica Serovar Typhimurium Strain SL1344 in Macrophage Cells and in Mice. BMC Complement. Altern. Med. 2018;18:322. doi: 10.1186/s12906-018-2365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Christ-Ribeiro A., Graça C.S., Kupski L., Badiale-Furlong E., de Souza-Soares L.A. Cytotoxicity, Antifungal and Anti Mycotoxins Effects of Phenolic Compounds from Fermented Rice Bran and Spirulina sp. Process Biochem. 2019;80:190–196. doi: 10.1016/j.procbio.2019.02.007. [DOI] [Google Scholar]
- 68.Denardi-Souza T., Luz C., Mañes J., Badiale-Furlong E., Meca G. Antifungal Effect of Phenolic Extract of Fermented Rice Bran with Rhizopus Oryzae and Its Potential Use in Loaf Bread Shelf Life Extension: Antifungal Effect of Phenolic Extract of Fermented Rice Bran. J. Sci. Food Agric. 2018;98:5011–5018. doi: 10.1002/jsfa.9035. [DOI] [PubMed] [Google Scholar]
- 69.Shahid Chatha S.A., Anwar F., Manzoor M., Rehman Bajwa J. Evaluation of the Antioxidant Activity of Rice Bran Extracts Using Different Antioxidant Assays. Grasas Aceites. 2006;57:328–335. doi: 10.3989/gya.2006.v57.i3.56. [DOI] [Google Scholar]
- 70.Sunitha D. A review on antioxidant methods. Asian J. Pharm. Clin. Res. 2016;9:14–32. doi: 10.22159/ajpcr.2016.v9s2.13092. [DOI] [Google Scholar]
- 71.Zaky A.A., Chen Z., Qin M., Wang M., Jia Y. Assessment of Antioxidant Activity, Amino Acids, Phenolic Acids and Functional Attributes in Defatted Rice Bran and Rice Bran Protein Concentrate. Prog. Nutr. 2021;22:e2020069. doi: 10.23751/pn.v22i4.8971. [DOI] [Google Scholar]
- 72.Rao A.S., Reddy S.G., Babu P.P., Reddy A.R. The Antioxidant and Antiproliferative Activities of Methanolic Extracts from Njavara Rice Bran. BMC Complement. Altern. Med. 2010;10:4. doi: 10.1186/1472-6882-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mohd Daud N.S., Abang Zaidel D.N., Kok Song L., Muhamad I.I., Mohd Jusoh Y.M. Antioxidant Properties of Rice Bran Oil from Different Varieties Extracted by Solvent Extraction Methods. J. Teknol. 2016;78 doi: 10.11113/jt.v78.9239. [DOI] [Google Scholar]
- 74.Sukrasno S., Tuty S., Fidrianny I. Antioxidant Evaluation and Phytochemical Content of Various Rice Bran Extracts of Three Varieties Rice from Semarang-Central Java, Indonesia. Asian J. Pharm. Clin. Res. 2017;10:377. doi: 10.22159/ajpcr.2017.v10i6.16565. [DOI] [Google Scholar]
- 75.Khammanit R., Lomarat P., Anantachoke N., Sato V.H., Ungsurungsie M., Mangmool S. Inhibition of Oxidative Stress through the Induction of Antioxidant Enzymes of Pigmented Rice Bran in HEK-293 Cells. Nat. Prod. Commun. 2017;12:1934578X1701200. doi: 10.1177/1934578X1701200727. [DOI] [Google Scholar]
- 76.Hull J., Kannan A., Hettiarachchy N.S. Antioxidant and Antihypertensive Activities of Rice Bran Peptides. Discov. Stud. J. Dale Bump. Coll. Agric. Food Life Sci. 2011;12:7. [Google Scholar]
- 77.Hefnawy H.T.M., El-Shourbagy G.A. Chemical Analysis and Antioxidant Activity of Polysaccharide Extracted from Rice Bran. World J. Dairy Food Sci. 2014;10:95–104. [Google Scholar]
- 78.Rhee Y.-H., Rhee C.H., Chung P.-S., Ahn J.-C. Anti-Oxidant and Anti-Inflammatory Effects of Rice Bran and Green Tea Fermentation Mixture on Lipopolysaccharideinduced RAW 264.7 Macrophages. Trop. J. Pharm. Res. 2018;16:2943. doi: 10.4314/tjpr.v16i12.19. [DOI] [Google Scholar]
- 79.Ruen-ngam D., Thawai C., Sukonthamut S., Nokkoul R., Tadtong S. Evaluation of Nutrient Content and Antioxidant, Neuritogenic, and Neuroprotective Activities of Upland Rice Bran Oil. ScienceAsia. 2018;44:257. doi: 10.2306/scienceasia1513-1874.2018.44.257. [DOI] [Google Scholar]
- 80.Zhang H., Kai G., Xia Y., Wang G., Ai L. Antioxidant and in Vitro Digestion Property of Black Rice (Oryza Sativa L.): A Comparison Study between Whole Grain and Rice Bran. Int. J. Food Eng. 2020;16 doi: 10.1515/ijfe-2019-0260. [DOI] [Google Scholar]
- 81.Ritchie K.J., Sarker S.D. Annual Reports in Medicinal Chemistry. Volume 55. Elsevier; Amsterdam, The Netherlands: 2020. Cancer Chemopreventive Natural Products; pp. 273–295. [Google Scholar]
- 82.Henderson A.J., Ollila C.A., Kumar A., Borresen E.C., Raina K., Agarwal R., Ryan E.P. Chemopreventive Properties of Dietary Rice Bran: Current Status and Future Prospects. Adv. Nutr. 2012;3:643–653. doi: 10.3945/an.112.002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Forster G.M., Raina K., Kumar A., Kumar S., Agarwal R., Chen M.-H., Bauer J.E., McClung A.M., Ryan E.P. Rice Varietal Differences in Bioactive Bran Components for Inhibition of Colorectal Cancer Cell Growth. Food Chem. 2013;141:1545–1552. doi: 10.1016/j.foodchem.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kong C.K.L., Lam W.S., Chiu L.C.M., Ooi V.E.C., Sun S.S.M., Wong Y.-S. A Rice Bran Polyphenol, Cycloartenyl Ferulate, Elicits Apoptosis in Human Colorectal Adenocarcinoma SW480 and Sensitizes Metastatic SW620 Cells to TRAIL-Induced Apoptosis. Biochem. Pharmacol. 2009;77:1487–1496. doi: 10.1016/j.bcp.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 85.Kannan A., Hettiarachchy N.S., Lay J.O., Liyanage R. Human Cancer Cell Proliferation Inhibition by a Pentapeptide Isolated and Characterized from Rice Bran. Peptides. 2010;31:1629–1634. doi: 10.1016/j.peptides.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 86.Chen M.-H., Choi S.H., Kozukue N., Kim H.-J., Friedman M. Growth-Inhibitory Effects of Pigmented Rice Bran Extracts and Three Red Bran Fractions against Human Cancer Cells: Relationships with Composition and Antioxidative Activities. J. Agric. Food Chem. 2012;60:9151–9161. doi: 10.1021/jf3025453. [DOI] [PubMed] [Google Scholar]
- 87.Wang L., Li Y., Zhu L., Yin R., Wang R., Luo X., Li Y., Li Y., Chen Z. Antitumor Activities and Immunomodulatory of Rice Bran Polysaccharides and Its Sulfates in Vitro. Int. J. Biol. Macromol. 2016;88:424–432. doi: 10.1016/j.ijbiomac.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 88.Rukmana R.M., Soesilo N.P., Rumiyati R., Pratiwi R. Chemopreventive Activities of ‘Woja Laka’ Black Rice Bran Fractions on Liver Carcinoma HepG2 Cells. Biomed. Pharmacol. J. 2017;10:1677–1684. doi: 10.13005/bpj/1279. [DOI] [Google Scholar]
- 89.Norazalina S., Norhaizan M.E., Hairuszah I., Norashareena M.S. Anticarcinogenic Efficacy of Phytic Acid Extracted from Rice Bran on Azoxymethane-Induced Colon Carcinogenesis in Rats. Exp. Toxicol. Pathol. 2010;62:259–268. doi: 10.1016/j.etp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 90.Aalim H., Belwal T., Jiang L., Huang H., Meng X., Luo Z. Extraction Optimization, Antidiabetic and Antiglycation Potentials of Aqueous Glycerol Extract from Rice (Oryza Sativa L.) Bran. LWT. 2019;103:147–154. doi: 10.1016/j.lwt.2019.01.006. [DOI] [Google Scholar]
- 91.Quan N.V., Xuan T.D., Tran H.-D., Ahmad A., Khanh T.D., Dat T.D. Contribution of Momilactones A and B to Diabetes Inhibitory Potential of Rice Bran: Evidence from in Vitro Assays. Saudi Pharm. J. 2019;27:643–649. doi: 10.1016/j.jsps.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ijarotimi O.S., Fakayejo D.A., Oluwajuyitan T.D. Nutritional Characteristics, Glycaemic Index and Blood Glucose Lowering Property of Gluten-Free Composite Flour from Wheat (Triticum Aestivum), Soybean (Glycine Max), Oat-Bran (Avena Sativa) and Rice-Bran (Oryza Sativa) Appl. Food Res. 2021;1:100022. doi: 10.1016/j.afres.2021.100022. [DOI] [Google Scholar]
- 93.Kaup R.M., Khayyal M.T., Verspohl E.J. Antidiabetic Effects of a Standardized Egyptian Rice Bran Extract: Rice Bran and Diabetes. Phytother. Res. 2013;27:264–271. doi: 10.1002/ptr.4705. [DOI] [PubMed] [Google Scholar]
- 94.Saji N., Francis N., Schwarz L.J., Blanchard C.L., Santhakumar A.B. Rice Bran Derived Bioactive Compounds Modulate Risk Factors of Cardiovascular Disease and Type 2 Diabetes Mellitus: An Updated Review. Nutrients. 2019;11:2736. doi: 10.3390/nu11112736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qureshi A.A., Sami S.A., Khan F.A. Effects of Stabilized Rice Bran, Its Soluble and Fiber Fractions on Blood Glucose Levels and Serum Lipid Parameters in Humans with Diabetes Mellitus Types I and II. J. Nutr. Biochem. 2002;13:175–187. doi: 10.1016/S0955-2863(01)00211-X. [DOI] [PubMed] [Google Scholar]
- 96.Cheng H.-H., Huang H.-Y., Chen Y.-Y., Huang C.-L., Chang C.-J., Chen H.-L., Lai M.-H. Ameliorative Effects of Stabilized Rice Bran on Type 2 Diabetes Patients. Ann. Nutr. Metab. 2010;56:45–51. doi: 10.1159/000265850. [DOI] [PubMed] [Google Scholar]
- 97.Misawa K., Jokura H., Shimotoyodome A. Rice Bran Triterpenoids Improve Postprandial Hyperglycemia in Healthy Male Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Food Nutr. Res. 2018;62 doi: 10.29219/fnr.v62.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sirajuddin S., Masni S.A. Abdul the Effect of Giving Rice Bran Milk on Blood Glucose Levels and Body Weight in Hyperglycemic Primary School Teachers in Makassar City. Int. J. Pharm. Res. 2021;13:2973–2978. doi: 10.31838/ijpr/2021.13.01.438. [DOI] [Google Scholar]
- 99.Okahara F., Suzuki J., Hashizume K., Osaki N., Shimotoyodome A. Triterpene Alcohols and Sterols from Rice Bran Reduce Postprandial Hyperglycemia in Rodents and Humans. Mol. Nutr. Food Res. 2016;60:1521–1531. doi: 10.1002/mnfr.201500897. [DOI] [PubMed] [Google Scholar]
- 100.Minhajuddin M., Beg Z.H., Iqbal J. Hypolipidemic and Antioxidant Properties of Tocotrienol Rich Fraction Isolated from Rice Bran Oil in Experimentally Induced Hyperlipidemic Rats. Food Chem. Toxicol. 2005;43:747–753. doi: 10.1016/j.fct.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 101.Kang M.Y., Kim S.M., Rico C.W., Lee S.-C. Hypolipidemic and Antioxidative Effects of Rice Bran and Phytic Acid in High Fat-Fed Mice. Food Sci. Biotechnol. 2012;21:123–128. doi: 10.1007/s10068-012-0015-3. [DOI] [Google Scholar]
- 102.Wang Y.-X., Li Y., Sun A.-M., Wang F.-J., Yu G.-P. Hypolipidemic and Antioxidative Effects of Aqueous Enzymatic Extract from Rice Bran in Rats Fed a High-Fat and -Cholesterol Diet. Nutrients. 2014;6:3696–3710. doi: 10.3390/nu6093696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang R., Ma Q., Tong X., Liu L., Dong L., Huang F., Deng Y., Jia X., Chi J., Zhang M. Rice Bran Phenolic Extract Supplementation Ameliorates Impaired Lipid Metabolism in High-Fat-Diet Fed Mice through AMPK Activation in Liver. J. Funct. Foods. 2020;73:104131. doi: 10.1016/j.jff.2020.104131. [DOI] [Google Scholar]
- 104.Justo M.L., Rodriguez–Rodriguez R., Claro C.M., Alvarez de Sotomayor M., Parrado J., Herrera M.D. Water-Soluble Rice Bran Enzymatic Extract Attenuates Dyslipidemia, Hypertension and Insulin Resistance in Obese Zucker Rats. Eur. J. Nutr. 2013;52:789–797. doi: 10.1007/s00394-012-0385-6. [DOI] [PubMed] [Google Scholar]
- 105.Justo M.L., Claro C., Zeyda M., Stulnig T.M., Herrera M.D., Rodríguez-Rodríguez R. Rice Bran Prevents High-Fat Diet-Induced Inflammation and Macrophage Content in Adipose Tissue. Eur. J. Nutr. 2016;55:2011–2019. doi: 10.1007/s00394-015-1015-x. [DOI] [PubMed] [Google Scholar]
- 106.Perez-Ternero C., Claro C., Parrado J., Herrera M.D., Alvarez de Sotomayor M. Rice Bran Enzymatic Extract Reduces Atherosclerotic Plaque Development and Steatosis in High-Fat Fed ApoE−/− Mice. Nutrition. 2017;37:22–29. doi: 10.1016/j.nut.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 107.Chen C.-W., Cheng H.-H. A Rice Bran Oil Diet Increases LDL-Receptor and HMG-CoA Reductase mRNA Expressions and Insulin Sensitivity in Rats with Streptozotocin/Nicotinamide-Induced Type 2 Diabetes. J. Nutr. 2018;136:1472–1476. doi: 10.1093/jn/136.6.1472. [DOI] [PubMed] [Google Scholar]
- 108.Hongu N., Kitts D.D., Zawistowski J., Dossett C.M., Kopeć A., Pope B.T., Buchowski M.S. Pigmented Rice Bran and Plant Sterol Combination Reduces Serum Lipids in Overweight and Obese Adults. J. Am. Coll. Nutr. 2014;33:231–238. doi: 10.1080/07315724.2013.869772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ito Y., Nakashima Y., Matsuoka S. Rice Bran Extract Containing Acylated Steryl Glucoside Fraction Decreases Elevated Blood LDL Cholesterol Level in Obese Japanese Men. J. Med. Investig. 2015;62:80–84. doi: 10.2152/jmi.62.80. [DOI] [PubMed] [Google Scholar]
- 110.Gan R.-Y., Chan C.-L., Yang Q.-Q., Li H.-B., Zhang D., Ge Y.-Y., Gunaratne A., Ge J., Corke H. Sprouted Grains. Elsevier; Amsterdam, The Netherlands: 2019. Bioactive Compounds and Beneficial Functions of Sprouted Grains; pp. 191–246. [Google Scholar]
- 111.Arab F., Alemzadeh I., Maghsoudi V. Determination of Antioxidant Component and Activity of Rice Bran Extract. Sci. Iran. 2011;18:1402–1406. doi: 10.1016/j.scient.2011.09.014. [DOI] [Google Scholar]
- 112.Begum A., Goswami A., Chowdhury P. A Comparative Study on Free and Bound Phenolic Acid Content and Their Antioxidant Activity in Bran of Rice (Oryza Sativa L.) Cultivars of Eastern Himalayan Range. Int. J. Food Sci. Technol. 2015;50:2529–2536. doi: 10.1111/ijfs.12920. [DOI] [Google Scholar]
- 113.Shahidi F., Yeo J. Insoluble-Bound Phenolics in Food. Molecules. 2016;21:1216. doi: 10.3390/molecules21091216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gao Y., Ma S., Wang M., Feng X.-Y. Characterization of Free, Conjugated, and Bound Phenolic Acids in Seven Commonly Consumed Vegetables. Molecules. 2017;22:1878. doi: 10.3390/molecules22111878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Adom K.K., Liu R.H. Antioxidant Activity of Grains. J. Agric. Food Chem. 2002;50:6182–6187. doi: 10.1021/jf0205099. [DOI] [PubMed] [Google Scholar]
- 116.Chen D., Shi J., Hu X., Du S. Alpha-Amylase Treatment Increases Extractable Phenolics and Antioxidant Capacity of Oat (Avena Nuda L.) Flour. J. Cereal Sci. 2015;65:60–66. doi: 10.1016/j.jcs.2015.06.019. [DOI] [Google Scholar]
- 117.Ribeiro B.D., Barreto D.W., Coelho M.A.Z. Enzyme-Enhanced Extraction of Phenolic Compounds and Proteins from Flaxseed Meal. ISRN Biotechnol. 2013;2013:521067. doi: 10.5402/2013/521067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim S.-M., Lim S.-T. Enhanced Antioxidant Activity of Rice Bran Extract by Carbohydrase Treatment. J. Cereal Sci. 2016;68:116–121. doi: 10.1016/j.jcs.2016.01.006. [DOI] [Google Scholar]
- 119.Nisa K., Rosyida V.T., Nurhayati S., Indrianingsih A.W., Darsih C., Apriyana W. Total Phenolic Contents and Antioxidant Activity of Rice Bran Fermented with Lactic Acid Bacteria. IOP Conf. Ser. Earth Environ. Sci. 2019;251:012020. doi: 10.1088/1755-1315/251/1/012020. [DOI] [Google Scholar]
- 120.Sawangwan T., Porncharoennop C., Nimraksa H. Antioxidant Compounds from Rice Bran Fermentation by Lactic Acid Bacteria. AIMS Agric. Food. 2021;6:578–587. doi: 10.3934/agrfood.2021034. [DOI] [Google Scholar]
- 121.Schmidt C.G., Gonçalves L.M., Prietto L., Hackbart H.S., Furlong E.B. Antioxidant Activity and Enzyme Inhibition of Phenolic Acids from Fermented Rice Bran with Fungus Rizhopus Oryzae. Food Chem. 2014;146:371–377. doi: 10.1016/j.foodchem.2013.09.101. [DOI] [PubMed] [Google Scholar]
- 122.Webber D.M., Hettiarachchy N.S., Li R., Horax R., Theivendran S. Phenolic Profile and Antioxidant Activity of Extracts Prepared from Fermented Heat-Stabilized Defatted Rice Bran: Phenolic Extracts from Fermented Rice Bran. J. Food Sci. 2014;79:H2383–H2391. doi: 10.1111/1750-3841.12658. [DOI] [PubMed] [Google Scholar]
- 123.Punia S., Sandhu K.S., Grasso S., Purewal S.S., Kaur M., Siroha A.K., Kumar K., Kumar V., Kumar M. Aspergillus Oryzae Fermented Rice Bran: A Byproduct with Enhanced Bioactive Compounds and Antioxidant Potential. Foods. 2020;10:70. doi: 10.3390/foods10010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Prabhu A.A., Jayadeep A. Enzymatic Processing of Pigmented and Non Pigmented Rice Bran on Changes in Oryzanol, Polyphenols and Antioxidant Activity. J. Food Sci. Technol. 2015;52:6538–6546. doi: 10.1007/s13197-015-1761-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Laokuldilok T., Rattanathanan Y. Protease Treatment for the Stabilization of Rice Bran: Effects on Lipase Activity, Antioxidants, and Lipid Stability. Cereal Chem. J. 2014;91:560–565. doi: 10.1094/CCHEM-02-14-0022-R. [DOI] [Google Scholar]
- 126.Sapna I., Jayadeep A. Cellulolytic and Xylanolytic Enzyme Combinations in the Hydrolysis of Red Rice Bran: A Disparity in the Release of Nutraceuticals and Its Correlation with Bioactivities. LWT. 2022;154:112856. doi: 10.1016/j.lwt.2021.112856. [DOI] [Google Scholar]
- 127.Ruen-Ngam D., Thawai C., Sukonthamut S. Pretreatment to Increase Yield and Antioxidant Activity of γ-Oryzanol in Rice Bran Oil. ScienceAsia. 2016;42:75. doi: 10.2306/scienceasia1513-1874.2016.42.075. [DOI] [Google Scholar]
- 128.Abd Razak D.L., Abd Rashid N.Y., Jamaluddin A., Sharifudin S.A., Long K. Enhancement of Phenolic Acid Content and Antioxidant Activity of Rice Bran Fermented with Rhizopus Oligosporus and Monascus Purpureus. Biocatal. Agric. Biotechnol. 2015;4:33–38. doi: 10.1016/j.bcab.2014.11.003. [DOI] [Google Scholar]
- 129.Wanyo P., Meeso N., Siriamornpun S. Effects of Different Treatments on the Antioxidant Properties and Phenolic Compounds of Rice Bran and Rice Husk. Food Chem. 2014;157:457–463. doi: 10.1016/j.foodchem.2014.02.061. [DOI] [PubMed] [Google Scholar]
- 130.Irakli M., Kleisiaris F., Mygdalia A., Katsantonis D. Stabilization of Rice Bran and Its Effect on Bioactive Compounds Content, Antioxidant Activity and Storage Stability during Infrared Radiation Heating. J. Cereal Sci. 2018;80:135–142. doi: 10.1016/j.jcs.2018.02.005. [DOI] [Google Scholar]
- 131.Christ-Ribeiro A., Chiattoni L.M., Mafaldo C.R.F., Badiale-Furlong E., Souza-Soares L.A. de Fermented Rice-Bran by Saccharomyces Cerevisiae: Nutritious Ingredient in the Formulation of Gluten-Free Cookies. Food Biosci. 2021;40:100859. doi: 10.1016/j.fbio.2020.100859. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study did not report any data.