Abstract
Background:
Data on congenital systemic arteriovenous fistulas are largely based on individual case reports. A true systemic arteriovenous fistula needs to be differentiated from other vascular malformations like capillary or venous hemangiomas, which are far more common.
Objectives:
We sought to identify the varied symptoms, diagnostic challenges, describe interventional treatment options, and postulate an embryological basis for this uncommonly described entity.
Methods:
This is a descriptive study of a cohort of systemic arteriovenous fistulas seen in the department of pediatric cardiology at a tertiary cardiac institute from 2010 to 2020, with prospective medium-term follow-up. A total of seven cases were identified. The diagnosis was confirmed by computed tomographic imaging, magnetic resonance angiography, or conventional angiography.
Results:
All were successfully closed using duct occluders or embolization coils with no recurrence in six cases over a median duration of follow-up of 48 months (interquartile range: 16; 36–52 months). Four of the seven cases underwent follow-up imaging using echocardiography or ultrasound.
Conclusion:
The incidence of congenital systemic arteriovenous fistulas is low and accounted for 0.009% of pediatric outpatients seen over 10 years at our institute. The spectrum of clinical presentation varies from an innocuous swelling or a pulsating mass to frank heart failure. Strong clinical suspicion and advanced imaging modalities have helped identify some hitherto undescribed connections. Large malformations with multiple communications may persist or recur despite transcatheter closure.
Keywords: Arteriovenous, coil, congenital, device closure, fistula
INTRODUCTION
A systemic arteriovenous fistula (SAVF) represents a direct communication between a systemic artery or its branches to the venous system without capillary interposition.[1] While SAVF is created iatrogenically to facilitate long-term dialysis in adults with renal failure, they may also arise following traumatic penetrating injuries.[2] The exact incidence of congenital SAVF has been difficult to determine, with most descriptions confined to individual case reports. The present single-center study represents a cohort of this rare entity. Some of these unusual anatomic connections and vessel courses have not been previously described.
METHODS
This was a retrospective data analysis with prospective follow-up of SAVF over 10 years (2010–2020). All cases referred to the Department of Pediatric Cardiology at our institute, a tertiary referral center for congenital heart disease, underwent a comprehensive evaluation (including an echocardiogram) by a team of pediatric cardiologists. Only congenital SAVF which were proven on computed tomographic/magnetic resonance (CT/MR) or conventional angiography were included. Capillary or venous malformations, coronary cameral fistulas, SAVFs secondary to trauma, and those arising as iatrogenic complications of percutaneous vascular access procedures were excluded. A total of seven congenital SAVFs were diagnosed in these 10 years. All SAVFs underwent transcatheter closure and were followed up for at least 2 years for recurrence or complications. The study was approved by the local institutional ethics committee and parental consent obtained for all therapeutic procedures.
RESULTS
The clinical presentation, diagnostic approach, treatment, and follow-up data for each case are described. The youngest was a 23-day-old neonate, and the oldest boy was 12 years old. The sex distribution was equal. There was no recurrence in 6/7 cases (85%) over a median follow-up duration of 48 months (interquartile range: 16; 36–52 months) [Table 1].
Table 1.
Salient features of systemic arteriovenous fistula
| Case number | Age/sex | Mode of presentation | Artery involved | Venous drainage | Treatment strategy | Follow-up duration | Recurrence |
|---|---|---|---|---|---|---|---|
| Case 1 | 7 years/male | Incidental murmur | Descending thoracic aorta | Right atrium | 10/12 duct occluder | 48 months | No |
| Case 2 | 23 days/female | Congestive cardiac failure | Left subclavian | Portal vein | 5/6 ADO-II | 52 months | No |
| Case 3 | 5 years/male | Pulsatile mass | Right external carotid | Right external jugular | 5/4 ADO-II | 48 months | No |
| Case 4 | 12 years/female | Expansile mass | Left axillary | Left axillary | Embolization coils | 48 months | No |
| Case 5 | 40 days/female | Ascites | Common hepatic artery | Portal vein | 5/4 ADO-II | 96 months | No |
| Case 6 | 6 years/male | Scalp bleed | Right superficial temporal | Superficial venous plexus of scalp | Embolization coils | Lost to follow up after 1st month | Yes |
| Case 7 | 13 years/male | Leg swelling | Left superficial femoral artery, profunda femoris branches | Femoral vein | Embolization coils | 36 months | No |
ADO II: Amplatzer duct occluder II
Case 1 – Descending aorta to the right atrium
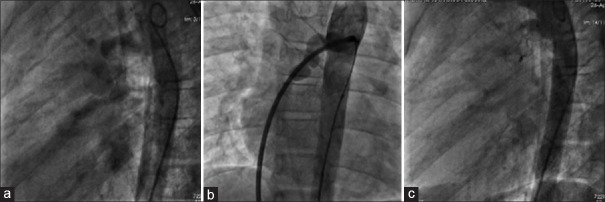
A 7-year-old boy with a history of palpitations was referred in view of a continuous murmur heard at the lower sternal border. There were no features of cardiac failure (CCF). Echocardiogram showed a mildly dilated right heart and no demonstrable intracardiac shunt. Cardiac catheterization showed normal pulmonary artery (PA) pressures with a Qp: Qs of 1.6. Descending aortogram in left lateral and anteroposterior (AP) views showed a vascular channel arising at the D4 level, coursing posteroinferior to the main and right PA and entering the posterior aspect of the right atrium (RA) just below the superior vena cava (SVC)-RA junction [Figure 1]. The fistulous channel was entered using a 5F Judkins right (JR) diagnostic catheter and 0.032” J-tipped exchange length Terumo wire. The Terumo wire was snared in the SVC to form an arteriovenous loop and exteriorized at the right femoral vein. An 8F Cook Mullins sheath was used to deploy a 10/12 mm PDA occluder (Lifetech Scientific Inc, China), with its retention skirt at the arterial end of the fistula [Videos 1–3]. There was immediate closure with the disappearance of murmur. He has been asymptomatic during the 48 months of follow-up post procedure, with normal echocardiograms.
Figure 1.
(a) Descending aortogram in left lateral view showing the fistulous tract opacifying the right atrium and right ventricle. (b) Sheath injection in the fistula in anteroposterior projection after formation of arterio-venous loop. (c) Descending aortogram after deployment of duct occluder within the fistula
Case 2 – Subclavian artery to portal vein
A 23-day-old neonate presented with features of CCF and mild resting desaturation (SpO2-87%). Heart sounds were normal; there was mild hepatomegaly and no bruit. Echocardiography showed normal situs, atrioventricular and ventriculoatrial concordance, bidirectional shunt across a small atrial septal defect (ASD), dilated right heart, persistent neonatal pulmonary hypertension, and normal ventricular function. There were increased flows noted in the portal venous system. A vessel with continuous flow was noted to descend along the lateral border of the left ventricle (LV) and enter the diaphragm. Since the connection of two pulmonary veins to the left atrium (LA) could be demonstrated and ASD was bidirectional, infradiaphragmatic total anomalous pulmonary venous connection was ruled out [Figure 2]. Cardiac CT demonstrated a large fistulous tract 1.2 cm distal to the origin of the left subclavian artery (LSCA), from its first part, immediately after the origin of the internal mammary artery (LIMA). It descended along the left thymic border, lateral margin of the LA, close to the posterolateral wall of the LV on the pericardial surface of the heart. It pierced the left dome of the diaphragm (separate from the inferior vena cava [IVC], esophageal or descending aortic foramen), coursed inferiorly and medially through the left lobe of the liver to enter the left portal vein (PoV). The suprahepatic IVC was enlarged with normal entry of the hepatic venous confluence. The PoV was dilated (left > right), and the ductus venosus was still patent. The vertebral artery and LIMA of normal caliber arose from this proximal dilated portion of the LSCA. The LSCA distal to the fistula was of a smaller calibre. The aortic arch was left-sided with normal branching pattern [Figure 3].
Figure 2.
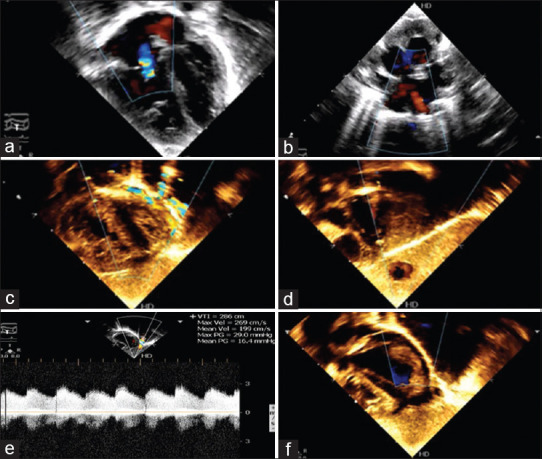
(a) Echocardiogram (4-chamber view) showing dilated right heart and mild tricuspid regurgitation. (b) Modified short axis view showing at least two pulmonary veins draining normally to the left atrium. (c) Subcostal 4-chamber view showing the fistulous tract with colour, running lateral to the left ventricle. (d) Subcostal view showing dilated portal vein with colour flows. (e) Doppler interrogation across the fistula showing restriction (gradient: 29/16 mm Hg) as it enters the diaphragm. (f) Echocardiogram (4-chamber view) post device closure of the fistula does not show any colour flow in the area lateral to the left ventricle
Figure 3.
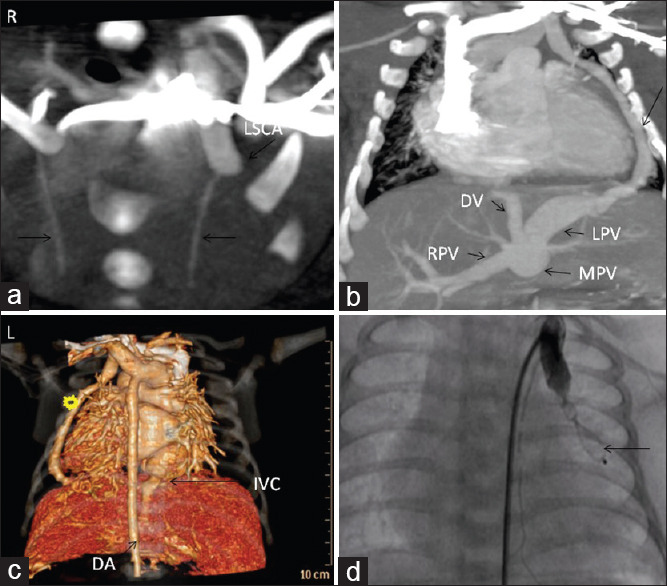
(a) Computed tomographic (axial plane) showing dilated proximal left subclavian artery (arrow) and normal calibre LIMA and RIMA. Right side has been identified as “R.” (b) Computed tomographic (coronal plane) showing the fistulous tract from the left subclavian artery, lateral to the left ventricle (arrow), piercing the diaphragm and joining the left portal vein. (c) Three-dimensional reconstruction of cardiac computed tomographic showing the separate diaphragmatic opening of the fistula and its distant relation to the inferior vena cava hiatus and descending aorta. (d) Contrast injection in the proximal left subclavian artery showing occlusion of the fistula by the ADO-II device (arrow) and LIMA arising normally from the dilated proximal left subclavian artery. MPV: Main portal vein; RPV: Right portal vein; DV: Ductus venosus.
The LSCA and the fistulous channel were entered using a 4F JR diagnostic catheter and 0.014” Whisper wire (Abbott Vascular) from the right femoral artery (RFA). The JR diagnostic catheter was subsequently exchanged for a 5F JR guide catheter, through which a 5/6 ADO-II device was deployed [Videos 4 and 5]. PoV pressures were 11 mm Hg, and PA pressure was one-third of systemic before closure. There was an immediate symptomatic improvement with spontaneous closure of ductus venosus, normal PoV size, and normal PoV flow on ultrasound abdomen at 1-month follow up. A follow-up echocardiogram and abdominal ultrasound at 52 months have not shown recurrence.
Case 3 – External carotid artery to the external jugular vein
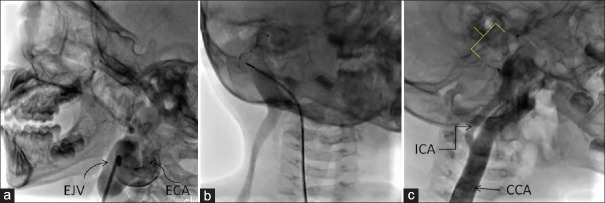
A 5-year-old boy with a swelling over the right lateral aspect of his neck since infancy, presented due to a gradual increase in its size. The swelling was diffuse (extending from the right earlobe to halfway down his neck), pulsatile, nontender, with a palpable thrill and bruit on auscultation [Video 6]. CT imaging showed a communication between the right external carotid artery (ECA) and the right external jugular vein (EJV). A RFA access was chosen, and contrast was injected into the common carotid artery (CCA) using a 4F Pigtail catheter. The right ECA was dilated, while the right internal carotid artery (ICA) was of normal caliber. The ECA was selectively cannulated using a 5F JR guide catheter and 0.032” J-tipped Terumo wire. A 5/4 ADO-II device was deployed in the ECA, the proximal disc positioned just prior to its anastomosis with the EJV. Contrast injections were given through the guide catheter to confirm device position and ensure unobstructed right ICA and proximal right ECA flows prior to device release [Figure 4 and Videos 7 and 8]. There was a visible reduction in pulsatility of the swelling and disappearance of thrill and bruit, the following day. There was no swelling or bruit at the last follow-up, 48 months after the procedure.
Figure 4.
(a) Contrast injection by hand through Judkins right diagnostic catheter in the right external carotid artery shows the tortuous fistulous communication between the external carotid artery and the right external jugular vein. Image obtained after turning the head to the left lateral position. (b) Contrast injection through the Judkins right guide catheter after deploying ADO-II in the terminal portion of external carotid artery just before its communication with the external jugular vein, shows filling of the dilated right external jugular vein and faint filling of the medial right internal jugular vein. (c) Contrast injection in the right common carotid artery after device release (device marked by the yellow box bracket) shows dilated common carotid artery and unobstructed flow to the right internal carotid artery. Image obtained after turning the head to the right lateral position
Case 4 – Axillary artery to axillary vein
A 12-year-old girl presented with swelling over the left scapula and humeral head, which had been progressively increasing over several months. The swelling was diffuse with visible pulsatile veins, tenderness on deep palpation, thrill, and an auscultable bruit. MR angiography showed a SAVF between the left axillary artery (AXA) and axillary vein (AXV). Using a right AXA approach, a 5F JR diagnostic catheter was advanced across the arch into the LSCA and left AXA. Contrast injection showed a major feeder which communicated with the AXV via numerous small anastomotic channels and another smaller branch from the AXA contributing to the SAVF. Both these feeder vessels from the left AXA were closed using Cook embolization coils [Figure 5 and Videos 9 and 10]. While there has been no recurrence of the swelling during 48 months of follow-up, additional imaging has not been performed.
Figure 5.
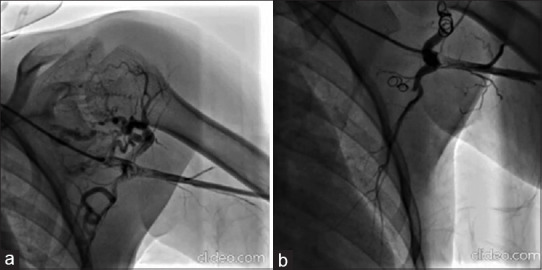
(a) Proximal left axillary artery injection using Judkins right diagnostic catheter showing multiple feeder vessels from the left axillary artery. The soft tissue swelling around the humeral head can be appreciated. (b) Closure of majority of the feeder vessels from the axillary artery after placement of two coils
Case 5 – Common hepatic artery to portal vein
A 40-day-old term infant was noted to have progressive abdominal distension and irritability since the 20th day of life. Examination showed hepato-splenomegaly with dilated veins over the abdominal wall, ascites, and no bruit on auscultation. There were no features of CCF, and liver function tests were within normal range. She was initially diagnosed to have portal hypertension based on an abdominal ultrasound, which showed dilated PoVs with flow reversal. CT abdomen with contrast, however, identified a large branch from the hepatic artery connecting to the right PoV. At therapeutic cardiac catheterization, the PoV pressure was elevated (13 mm Hg) and the feeder artery was visualized on the descending aortogram. There was a constriction at the point of entry to the PoV. A 5/4 ADO-II was deployed from the RFA using a 5F JR guide catheter to cannulate the common hepatic artery [Figure 6 and Videos 11 and 12]. The PoV was enlarged and there was no obstruction to the PoV confluence as the fistula was at the distal portion of the common PoV. Features of portal hypertension resolved completely within 10 days with no recurrence of portal hypertension over the last 8 years. This was confirmed by ultrasound abdomen during follow-up.
Figure 6.
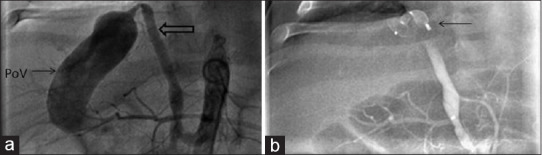
(a) Pigtail angiogram in the abdominal aorta shows the common hepatic artery (bold arrow) draining to an enlarged portal vein confluence (arrow). (b) ADO II device (arrow) deployed in the hepatic artery at its fistulous connection to the portal vein
Case 6 – Scalp arteriovenous malformation
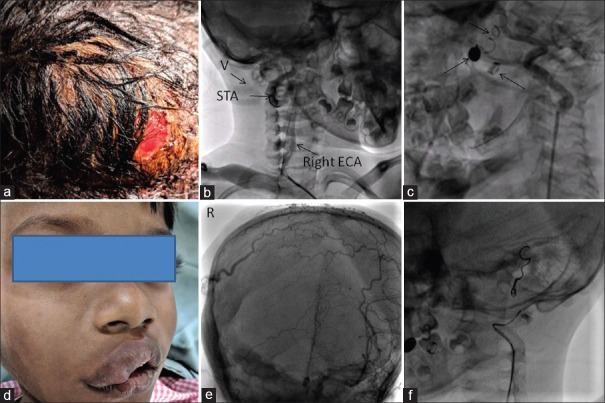
A 6-year-old boy presented with a history of swelling over the right parieto-occipital and temporal area (including swelling of the lips) with visible, pulsatile scalp veins, intermittent episodes of scalp bleeding, and associated skin erosion [Figure 7]. The lesion had been gradually increasing over the last 2 years with intermittent bleeding episodes managed by application of pressure bandage. There was a continuous bruit on local auscultation. There were no features of high output cardiac failure. A provisional diagnosis of SAVF between branches of the maxillary (MA) and superficial temporal artery (STA) and superficial veins of the scalp was made. RFA access was obtained under intravenous sedation and local anesthesia. 4F JR diagnostic and 5F JR guide catheter with 0.025” J-tipped Terumo wire were used to enter the right ECA, and digital subtraction angiography images were obtained. Multiple injections were given in the right anterior oblique, and AP views with cranial angulation to delineate a dilated right ECA, MA and its branches. There were numerous anastomoses noted between the branches of the MA and superficial venous plexus of the scalp. 8-14-0.035 and two 6-5-0.035 Nester embolization coils (Cook Medical) were deployed in the proximal portion of the right MA, right occipital, and STA [Videos 13 and 14]. Check angiogram showed a significant reduction in flows, and the bruit had disappeared. He had another episode of scalp bleeding, a week later. A repeat angiogram showed normal right ICA flow, and the coils had achieved complete occlusion of the right MA and its branches. A 4F Pigtail catheter was used for angiographic delineation of the left CCA in shallow left anterior oblique (LAO) and AP-65° cranial angulation (Towne view), which showed contra-lateral opacification of the branches of the right ST and occipital arteries from the left STA. A 5F JR guide catheter and 0.014” Whisper wire (Abbott Vascular) were advanced into the left ECA, and the wire was parked in the lingular branch. Another 0.014” floppy workhorse wire (Abbott Vascular) was advanced into the left STA, and its position was confirmed on angiogram in LAO-cranial view. A 2.7F Progreat microcatheter (Terumo Inc) was advanced over this wire to facilitate delivery of a 4-7-0.018” Nester micro-embolization coil (Cook Medical). This minimized filling of the right STA from its contra-lateral branch [Videos 15–17]. He had another episode of bleeding from the scalp, which was managed by compressive dressings and scalp sutures. He was lost to follow up a few weeks after the second procedure.
Figure 7.
(a) Scalp arteriovenous fistula showing scalp erosion. (b) Contrast injection through Judkins right diagnostic catheter in the right external carotid artery showing a dilated external carotid artery and superficial temporal artery and immediate opacification of venous tributaries (v) of the external jugular vein. (c) Left external carotid artery shoot showing a mildly dilated left external carotid artery and coils in situ, suggestive of contra-lateral filling of the scalp arteries. Image obtained after turning the head to the right. (d) The huge scalp arteriovenous fistula has caused swelling of the right face and lips. (e) Left external carotid artery injection (Towne view) showing contralateral filling of the superficial scalp plexus. Right side has been marked “R.” (f) Contrast injection via end-hole catheter placed deep within the left external carotid artery showing opacification of the left occipital artery and coil in the left posterior auricular artery
Case 7 – Left superficial femoral artery and profunda femoris artery to femoral vein
A 13-year-old boy presented with painless swelling of the left thigh, which had been increasing in size over the last few years. The enlarged left thigh was pulsatile, nontender, warm, and without an auscultable bruit. Contralateral femoral arterial access was obtained and a 5F LIMA catheter was used to cross over to the left iliac and femoral arteries. Multiple feeder vessels from the left superficial femoral and lateral circumflex branches of the profunda femoris artery were occluded using three 0.035” embolization coils (Cook Inc., Bloomington, IN, USA) and three 0.018” Nester embolization coils (Cook Inc., Bloomington, IN, USA) [Videos 18 and 19]. The femoral vein was enlarged and tortuous distally due to multiple fistulous communications. Arteriovenous malformation over SAVF is a better terminology to describe the lesion. There was a reduction in limb dimension a month after the procedure.
DISCUSSION
The study describes seven cases of SAVF with discrete anatomic features, presenting at different age groups with variable presentation. When classified by location, congenital SAVF is most commonly intra-abdominal, followed by craniocervical, and rarely intra-thoracic.[3] Others have classified them as aortocaval, dural, carotid-cavernous, coronary, and hepatic in location.[4] Due to their rarity, there are no accurate data on the incidence or prevalence of congenital SAVF. We recorded seven cases out of 71,204 new pediatric cardiology outpatient visits to our institute over a period of 10 years. This constitutes an in-hospital incidence of 0.009%. They also accounted for 0.074% (7/9349) of all pediatric cardiac hospital admissions over 10 years. Coronary cameral fistulas (with a reported prevalence on CT as high as 0.9%),[5] being a separate entity, have not been included in our series of SAVF, although those draining into the vena cava or RA could still qualify as congenital SAVF. The vein of Galen (voG) malformation, an important cause of neonatal heart failure with an incidence as high as 1:25,000, is usually managed by neuro-interventional radiologists.[6] This referral bias might have resulted in none being seen at a cardiac center in over 10 years.
The diagnosis of a congenital SAVF is not easy and requires a high degree of suspicion. Those with features of CCF with no apparent cause may be suspected on echocardiography or ultrasound abdomen but usually require a CT/MR imaging to delineate the exact communication. These modalities also help differentiate SAVF from capillary or cavernous hemangiomas, which are much more common and could have manifestations of high output failure. Several children with a diffuse swelling, with or without features of heart failure and unexplained hepatomegaly had been referred to us with a suspicion of SAVF. Pulsatility of the lesion and bruit favor a diagnosis of SAVF over capillary or cavernous hemangioma. During the initial few years, four children had been subjected to cardiac catheterization with an intention to treat, but angiographic findings did not favor the diagnosis of SAVF, with only a leash of capillaries and veins visualized in the lesion. Elwatidy et al. first reported a fistulous communication between the descending aorta (DA) and RA, which was surgically ligated.[7] The fistula between the LSCA and the ligamentum arteriosum terminated close to the SVC-RA junction. Ho et al. reported successful closure of a DA-RA fistula in a neonate with cardiovascular collapse, using a vascular plug.[8] Our first case is similar to the one described by these authors and was closed with a duct occluder. While there are several reports of fistulae between the ascending aorta, brachiocephalic trunk, and right SCA to the RA,[9,10] the embryological basis of a communication between the DA and RA has not been postulated. Rana et al. have documented a distance of 1.5 mm between the left CCA and the 7th intersegmental artery (future subclavian artery), and this dorsal aortic distance stays constant between Carnegie stage 13 and the developed heart.[11] This part of the dorsal aorta, which becomes a part of the aortic arch, is very close to the sinus venosus (which gets incorporated into the RA).
Our second case, a fistula from the LSCA to PoV has not been reported earlier. Görlach et al. have reported a similar case of a fistula from the right SCA to PoV, which was managed surgically.[12] There have been three earlier reports of a communication between the LIMA and PoV.[13,14,15] While this neonate presented with CCF and had normal PoV pressures, a similar fistula from the hepatic artery to PoV (Case 5) had features of elevated portal pressure, hepatomegaly, and ascites. The long, tortuous channel along with its compression at the diaphragm could have prevented elevation of PoV pressure while still allowing for enough steal from the aortic branches to cause high output cardiac failure. This fistula was unique, not only for being hitherto undescribed but also for its anatomical course and diaphragmatic entry via a separate orifice, bypassing all three anatomic hiatuses in the diaphragm. During the initial stages of embryonic development (Carnegie stages 9–11), the lung bud and liver primordium are separated by a distance of <500 μm. This ventral side of the embryonic foregut is surrounded by visceral mesoderm encompassing a huge venous vascular plexus. The same region is fed by the 7th segmental artery, which eventually differentiates as the LSCA.[16] An erroneous connection at such an early stage might have resulted in this rare SAVF. While this postulation is attractive, it does not explain the rarity of such communications.
Fistulous connections between the ECA and EJV have been more often described compared to other SAVF. Pulsatile tinnitus has been described as one of the distinguishing features of carotid fistulas, although this was not a symptom in our patient. Undiagnosed carotid fistulas may cause headache, sleep, and psychomotor disorders.[17] Earlier papers have reported on the use of coils to successfully embolize such fistulas.[18,19] However, there is a risk of coil embolization to the PA if the fistulous communication is relatively large. Regina et al. have described a hybrid approach wherein the EJV was transiently clamped to prevent coil migration.[20] Vascular plugs or the ADO-II device employed in our case are simpler to use and might reduce procedural risks. It is important to note that the ICA and internal jugular vein may appear smaller compared to the involved external branches.
While there are reports of SAVF between the SCA and SCV (more often seen on the right side),[21] a literature search did not yield any published report of a congenital fistula from the AXA to AXV. Fistulas involving the upper extremities have been known to recur.[22] An early description of extremity SAVF by Horton in 1932 described surgical ligation of arterial feeders, which had a lower success rate as congenital malformations usually had multiple feeder vessels with difficulty in isolation of every feeder during surgery.[23] Transcatheter embolization is relatively easier and might have helped prevent a recurrence, as in our cases.
Large arterioportal flows are a cause of pre-sinusoidal portal hypertension and may even cause hepatofugal flow with alterations in portal flow dynamics.[24] A bruit may not be auscultable in moderate-sized fistulas or in the presence of gross ascites, as in our case. Most congenital arterioportal fistulas have been described as being diffuse,[25] unlike the finding in our case, where a single branch from the common hepatic artery entered the right PoV. Our patient was probably one of the smallest infants to be treated using an ADO-II device. The common hepatic artery, a branch of the coeliac trunk is very close to the developing PoV. The position of the celiac artery changes from the (thoracic) T5 to (lumbar) L1 vertebral level as the dorsal aortic growth is much faster than the growth of the vertebral bodies between (cervical) C7 and T5 levels. We postulate this could have led to the development of such a communication.
SAVF of the scalp involves the superficial and deep temporal, posterior auricular, occipital or ophthalmic arteries with one or more communications with the superficial or deep venous plexus of the scalp and constitute roughly 8% of all SAVF.[26] Most large scalp AVF have been treated using a combination of endovascular treatment and surgery; larger fistulas with multiple feeders often require extensive scalp reconstruction after ligation and excision of the feeder vessels. Our child had a similar presentation with scalp bleeding despite the placement of coils from the ipisilateral MA and bilateral STA. He was also in an advanced grade of SAVF (Schobinger Grade III).[27] Unfortunately, he was lost to follow-up, and further surgical procedures could not be completed. Nagasaka et al. have presented their series of seven adult patients and discussed treatment options in detail.[28] More recently, a series of five children with scalp SAVF was reported by Gupta and Kayal.[29] The temporal and maxillary branches are pharyngeal arch arteries and drain to the superior cardinal vein (future jugular vein). We postulate that defective programming or signaling in the establishment of arterial and venous identity could have resulted in a complete anastomosing network in this region.
The angioarchitecture of SAVF has been classified by Yakes and Baumgartner.[30] Eradicating the nidus of the lesion is crucial to prevent recurrence. Occlusion of the major feeding artery is usually the first step and suffices in those cases with a single large feeder artery draining into a single vein. This approach was sufficient in cases 1, 2, 3, and 5 in our series. Those with multiple feeding arteries or draining veins/venules need a staged approach to initially decrease flow from the major feeder and subsequent embolization using micro-coils or liquid agents by distal entry of all accessible feeder arteries, direct ultrasound-guided puncture of the nidus or blocking the venous effluence and complimentary surgical resection.[31] This combined approach has been recommended for most scalp malformations but could not be pursued in our case. Antegrade occlusion of multiple feeder arteries was employed in cases 4 and 7 in our series. This seems to have prevented progression of the lesions, but additional imaging and long-term follow-up are needed to state that the SAVF has completely regressed.
Different liquid embolic agents used to treat SAVF include ethanol, ethylene vinyl alcohol copolymer (Onyx), and glue.[31] Ethanol destroys vascular endothelium and has high success rates as it causes nidal destruction. Pulmonary hypertension is a serious complication that might occur during ethanol therapy, and doses should be limited to 1 ml/kg body weight. The latter two agents do not cause nidal destruction and have higher recurrence rates following initial occlusion.
Although we have presented only a small number of subjects, data on this orphan disease is confined to individual case reports. This limitation may be addressed if more publications on the subject become available in the future, permitting a meta-analysis. Several of these cases reported earlier were managed surgically as interventional options were limited. Barring a large case series of voG malformations from Lasjaunias,[32] there have been no large data published on congenital SAVF.
CONCLUSIONS
Congenital SAVF is more easily diagnosed in the current era of advanced imaging. They usually present in infancy and childhood. Routine cath lab hardware can be used to successfully manage most SAVF with a minimal risk of recurrence or long-term complications. It is important to differentiate SAVF from the more common low-flow vascular malformations, as treatment options vary considerably. While we have offered some embryological hypothesis to explain SAVF in various locations, further research in this area is needed to provide a better understanding of the pathophysiology of these communications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on: www.annalspc.com
REFERENCES
- 1.Steiner JE, Drolet BA. Classification of vascular anomalies: An update. Semin Intervent Radio. 2017;34:225–32. doi: 10.1055/s-0037-1604295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reinhoff WF., Jr Congenital arteriovenous fistula: An embryologic study with report of case. JAMA. 1924;83:743–50. [Google Scholar]
- 3.Jabari S, Hartmann A, Cesnjevar R. A rare case of congenital thoracic arteriovenous fistula between the brachiocephalic truncus and the superior vena cava resulting in heart failure. J Pediatr Surg Case Rep. 2016;15:50–2. [Google Scholar]
- 4.Jayroe H, Foley K. StatPearls. Treasure Island (FL): Stat Pearls Publishing; 2021. Arteriovenous fistula. PMID 32644639 Bookshelf ID: NBK559213. Last updated on 2020 Aug 10. [PubMed] [Google Scholar]
- 5.Lim JJ, Jung JI, Lee BY, Lee HG. Prevalence and types of coronary artery fistulas detected with coronary CT angiography. AJR Am J Roentgenol. 2014;203:W237–43. doi: 10.2214/AJR.13.11613. [DOI] [PubMed] [Google Scholar]
- 6.Sood S, Sharma S, Verma R, Bansal V. Vein of galen aneurysmal malformation: Antenatal diagnosis by ultrasound and MRI. Online J Health Allied Scs. 2015;14:16. [Google Scholar]
- 7.Elwatidy AF, Galal AN, Rhydderch D, Ashmeg AK. Aorto-right atrial fistula. Ann Thorac Surg. 2003;76:929–31. doi: 10.1016/s0003-4975(03)00448-x. [DOI] [PubMed] [Google Scholar]
- 8.Ho AB, Magee AG, Hayes N. Descending aorta to right atrial fistula. Catheter Cardiovasc Interv. 2017;90:1158–60. doi: 10.1002/ccd.27129. [DOI] [PubMed] [Google Scholar]
- 9.Soler P, Mehta AV, Garcia OL, Kaiser G, Tamer D. Congenital systemic arteriovenous fistula between the descending aorta, azygos vein, and superior vena cava. Chest. 1981;80:647–9. doi: 10.1378/chest.80.5.647. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez FR, Monaco MP, Hartmann AF, Jr, McKnight RC. Congenital arteriovenous malformations between brachiocephalic arteries and systemic veins. Chest. 1987;92:897–9. doi: 10.1378/chest.92.5.897. [DOI] [PubMed] [Google Scholar]
- 11.Rana MS, Sizarov A, Christoffels VM, Moorman AF. Development of the human aortic arch system captured in an interactive three-dimensional reference model. Am J Med Genet A. 2014;164A:1372–83. doi: 10.1002/ajmg.a.35881. [DOI] [PubMed] [Google Scholar]
- 12.Görlach G, Hagel KH, Mulch J, Scheld HH, Hehrlein FW. Congenital arteriovenous fistula between the subclavian artery and the portal vein. J Cardiovasc Surg (Torino) 1984;25:378–80. [PubMed] [Google Scholar]
- 13.Glass IH, Rowe RD, Duckworth JW. Congenital arterio-venous fistula between the left internal mammary artery and ductus venosus: Unusual cause of congestive heart failure in the newborn infant. Pediatrics. 1960;26:604–10. [PubMed] [Google Scholar]
- 14.Stanford W, Fixler DE, Armstrong RG, Lindberg EF, Johnson HH. Congenital arteriovenous fistula between the left internal mammary artery and the ductus venosus. A case report. J Thorac Cardiovasc Surg. 1970;60:248–52. [PubMed] [Google Scholar]
- 15.Cobby MJ, Culling W, Jordan SC, Hartnell GG. Balloon embolization of a congenital arterio-venous fistula between the internal mammary artery and a portal vein radicle. Br J Radiol. 1989;62:371–3. doi: 10.1259/0007-1285-62-736-371. [DOI] [PubMed] [Google Scholar]
- 16.Congdon ED. Transformation of the aortic-arch system during the development of the human embryo. Contrib Embryol. 1922;14:47–110. [Google Scholar]
- 17.Chi MH, Wang NK, Lu YY. Treatment of a rare congenital external carotid arteriovenous fistula with transcatheter coil embolization. Acta Cardiol Sing. 2010;26:272–5. [Google Scholar]
- 18.Halbach VV, Higashida RT, Hieshima GB, Hardin CW. Arteriovenous fistula of the internal maxillary artery: Treatment with transarterial embolization. Radiology. 1988;168:443–5. doi: 10.1148/radiology.168.2.3393664. [DOI] [PubMed] [Google Scholar]
- 19.Berenstein A, Scott J, Choi IS, Persky M. Percutaneous embolization of arteriovenous fistulas of the external carotid artery. AJNR Am J Neuroradiol. 1986;7:937–42. [PMC free article] [PubMed] [Google Scholar]
- 20.Regina G, Impedovo G, Angiletta D, Marotta V, Lillo A, Pestrichella F, et al. A new strategy for treatment of a congenital arteriovenous fistula of the neck. Case report. Eur J Vasc Endovasc Surg. 2006;32:107–9. doi: 10.1016/j.ejvs.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 21.Dogan R, Yilmaz M, Ozkutlu S, Elsharshari H. Congenital subclavian artery to subclavian vein fistula presenting with congestive heart failure in an infant. Pediatr Cardiol. 2000;21:269–70. doi: 10.1007/s002460010056. [DOI] [PubMed] [Google Scholar]
- 22.Shoab SS, Scurr JH. Arteriovenous malformations of the upper limb. In: Chang JB, editor. Textbook of Angiology. New York: Springer; 2000. [Google Scholar]
- 23.Horton BT. Hemihypertrophy of extremities associated with congenital arteriovenous fistula. JAMA. 1932;98:373–9. [Google Scholar]
- 24.Vauthey JN, Tomczak RJ, Helmberger T, Gertsch P, Forsmark C, Caridi J, et al. The arterioportal fistula syndrome: Clinicopathologic features, diagnosis, and therapy. Gastroenterology. 1997;113:1390–401. doi: 10.1053/gast.1997.v113.pm9322535. [DOI] [PubMed] [Google Scholar]
- 25.Guzman EA, McCahill LE, Rogers FB. Arterioportal fistulas: Introduction of a novel classification with therapeutic implications. J Gastrointest Surg. 2006;10:543–50. doi: 10.1016/j.gassur.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Valdeci WP, Gilmone RL, Rosa RM, Rafael C. Giant scalp arteriovenous malformation. Rev Assoc Med Bras. 2016;9:828–30. doi: 10.1590/1806-9282.62.09.828. [DOI] [PubMed] [Google Scholar]
- 27.Finn MC, Glowacki J, Mulliken JB. Congenital vascular lesions: Clinical application of a new classification. J Pediatr Surg. 1983;18:894–900. doi: 10.1016/s0022-3468(83)80043-8. [DOI] [PubMed] [Google Scholar]
- 28.Nagasaka S, Fukushima T, Goto K, Ohjimi H, Iwabuchi S, Maehara F. Treatment of scalp arteriovenous malformation. Neurosurgery. 1996;38:671–7. [PubMed] [Google Scholar]
- 29.Gupta R, Kayal A. Scalp arteriovenous malformations in young. J Pediatr Neurosci. 2014;9:263–6. doi: 10.4103/1817-1745.147587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yakes W, Baumgartner I. Interventional treatment of arterio-venous malformations. Gefasschirurgie. 2014;19:325–30. [Google Scholar]
- 31.Gilbert P, Dubois J, Giroux MF, Soulez G. New treatment approaches to arteriovenous malformations. Semin Intervent Radiol. 2017;34:258–71. doi: 10.1055/s-0037-1604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lasjaunias P, Alvarez H, Rodesch G, Garcia-Monaco R, Ter Brugge K, Burrows P, et al. Aneurysmal malformation of the vein of Galen, follow up of 120 children treated between 1984 and 1994. Interv Neuroradiol. 1996;2:15–26. doi: 10.1177/159101999600200102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.