Abstract
Background:
Device closure of multiple atrial septal defects (MASDs) is frequently done using a single centrally deployed septal or cribriform occluder, but multiple devices are needed for large defects separated more than 6 mm. There is a concern about complications while using multiple devices, especially in children.
Methods:
Patients who received multiple devices for closure of MASD were grouped according to their age and analyzed for procedural techniques, immediate and late complications. MASDs closed by a single device were not included. Balloon sizing was done when echocardiographic images were suboptimal before simultaneous device deployment through two venous accesses or sequential deployment through one access. Duration and number of antiplatelet drugs and residual flows were analyzed on follow-up.
Results:
Twenty-five patients received multiple devices. Balloon interrogation was performed in 16/18 adults but only in 2/7 children. Device size was 2–5 mm larger than echocardiographic defect size or equal to balloon waist. There were no procedural failures; 7/25 showed small postprocedural residual flows. Complications including embolization in one, arrhythmia in one, and cobra deformity in two were managed successfully. On a median follow-up of 5.5 years (1–12 years), residual flows disappeared in 4/7 and there were no major late complications.
Conclusions:
Use of multiple devices for closing MASD is feasible with good technical success. Echocardiography and balloon interrogation are the keys for success. Simultaneous deployment is often needed and sequential delivery is feasible rarely if the defects are far apart. Minor residual leaks are common but improve on follow-up. There are no significant new complications on long-term follow-up.
Keywords: Antiplatelet therapy, balloon sizing, multiple atrial septal defects, multiple atrial septal occluders, residual flows
INTRODUCTION
Transcatheter device closure of secundum atrial septal defects (ASDs) has become a safe and effective alternative for surgical closure in recent decades.[1] With the increasing experience in device closures and improved imaging techniques, varying complex defect morphologies are considered for closure in the catheterization laboratory by the interventional cardiologists.[2] Such complex defects include very large defects exceeding 35 mm, defects with deficient rims, multiple defects, and multifenestrated defects.[3,4]
Transcatheter closure of multiple defects and multifenestrated defects has been reported in the last two decades.[5,6,7,8,9,10,11,12,13] The introduction of nonself-centering Amplatzer Cribriform device (Abbott Medical, Santa Clara, CA) in 2008 has led to the closure of multifenestrated defects.[14] In case of multiple defects that are close to each other with intervening tissue smaller than 5–7 mm, they may be amenable for closure with a single self-centering Amplatzer septal occluder ASO (Abbott Medical, Santa Clara, CA).[8,9] If the satellite defects that are located more than 7 mm away from the main defect are insignificant measuring <3–4 mm, they may be ignored as the shunt through these small defects may be clinically irrelevant. If the satellite defects merit additional closure, then they require multiple devices in the atrial septum.[11,12,13]
Even though successful transcatheter closure of multiple defects using multiple devices is reported, concerns exist due to larger surface area of the multiple devices exposed in the atrium, device-to-device interactions, altered septal planes, and increased potential for thrombogenicity and residual flows.[5,6,7,8,9,10,11,12,13] Different techniques have been described till date for transcatheter closure using multiple devices.[10,11,12,13] A retrospective analysis of the techniques used for deployment of multiple occluders in the atrial septum, immediate, and long-term outcomes with special emphasis on complications will address these concerns. A comparison of procedural difficulties and complications between children and adults will indicate the appropriateness of multiple occluders in the pediatric population.
METHODS
This study is a retrospective observational analysis of all cases of multiple ASDs managed using more than one atrial septal occluder in a tertiary care institution. Adjacent defects closed with a single ASO, a large central defect with nearby satellite defects closed with a single ASO, very small insignificant satellite defects far away from the main defect that were left unclosed during transcatheter closure, and multifenestrated septum closed with a single cribriform occluder were outside the scope of the present study that analyzed the technical feasibility of implantation of multiple devices in the atrial septum and studied their follow-up data. The institutional review board permitted use of multiple devices in appropriate patients as an alternative to surgery and the ethical committee approved the analysis of data as well as anonymized reporting. Written informed consent from the patient and/or parents was obtained before implantation of multiple devices.
Preprocedural evaluation
Initial evaluation included a detailed transthoracic echocardiography. Transesophageal study was restricted to adults and patients with suboptimal transthoracic windows [Figure 1]. As thin, floppy, and hypermobile interatrial septum failed to give a good three-dimensional volume-rendered echocardiographic image of the atrial septum, we depended on two-dimensional multiplanar images in all patients despite using a three-dimensional probe. Baseline clinical parameters and additional cardiac and noncardiac illnesses were also evaluated. Indication for device closure was determined based on standard clinical guidelines to quantify the left to right shunt and presence of acceptable rims. Multiple devices were preferred over a single device if the defects were far apart with an intervening tissue of more than 6 mm. The total atrial septal length was not measured as the postrelease orientation and sandwiching of the multiple devices could never be predicted before the procedure. In case of doubt, the final decision was made on the table after balloon interrogation.
Figure 1.
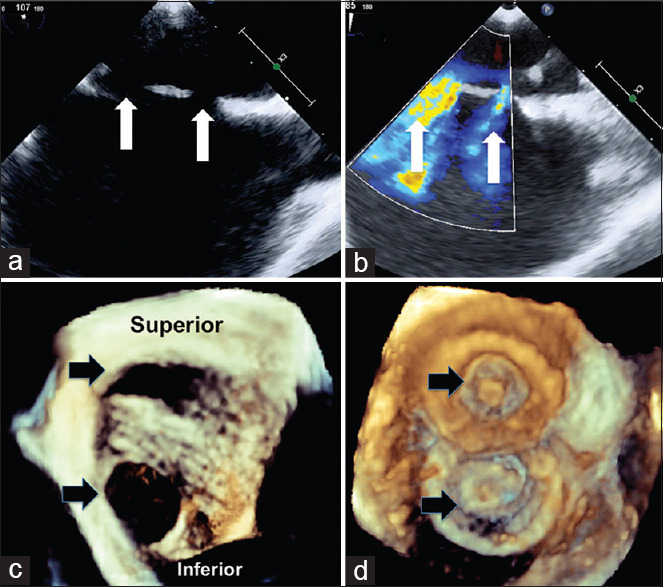
Two-dimensional transesophageal echocardiogram in vertical plane (a) shows superoinferior orientation of two defects (white arrows) with color flows (b). Three dimensional right atrial enface view in anatomical orientation (c) shows the superoinferior orientation of the two defects (black arrows) before and after (d) device closure
Procedural protocol
The procedure was done under conscious sedation in children and under local anesthesia in adults. Mild sedation was administered for a brief period of intraprocedural transesophageal echocardiography for some crucial imaging rather than throughout the entire procedure. Intubation anesthesia was restricted to apprehensive and uncooperative patients. Heparinization, a preprocedural single antiplatelet dose of aspirin and intraprocedural intravenous antibiotic prophylaxis were given according to standard guidelines.[1] Arterial access aided coronary angiography and measuring left ventricular end-diastolic pressures in adults but was avoided in children. As patients were selected only when they had clinically significant pretricuspid shunt, oximetric shunt quantification was not routinely performed. Hemodynamic assessment was confined to pressure measurements in all cardiac chambers.
Number of venous accesses
The number of venous accesses was decided based on the technique followed: simultaneous or sequential technique. If the defects were far apart from each other separated by more than 12 mm, a sequential technique was followed through a single venous access. If the intervening tissues between the defects were between 6 and 12 mm, simultaneous balloon interrogation of the defects through two groin venous access was followed by simultaneous device deployment and release. Defects <6 mm apart that were closed with a single ASO were not included in this study.
Crossing the first defect
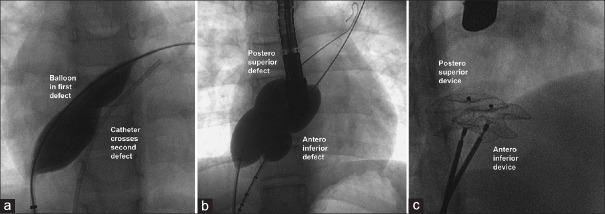
Echocardiography identified if the multiple defects had a superoinferior or anteroposterior relation between them. An angled catheter initially advanced to the superior vena cava and gradually brought down into the right atrium was used to probe the superior part of the atrial septum to enter the superior orifice, if defects were in the superoinferior relation [Figure 1]. If the two defects were in an anteroposterior relation, the angled catheter facing the anterior right atrial appendage was torqued in a clockwise direction and advanced to cross the first anterior defect in the atrial septum. After advancing a Super Stiff guidewire through this catheter, a balloon interrogation was performed using AGA Sizing balloon (Abbott, Santa Clara, CA) till a gentle waist was observed on fluoroscopy and stop-flow was observed on echocardiography. As an observation of stop-flow was challenging due to color flows through the additional defects in the atrial septum, emphasis was given for creation of a faint waist on fluoroscopy and care was taken to avoid overstretch of the defect using the sizing balloon. As balloon interrogation was primarily done for determination of the defect size, its need was decided on a case-to-case basis based on the adequacy of echocardiographic windows.
Crossing the second defect
Once the inflated balloon occluded one of the defects, another catheter from the second groin venous access was guided into the second ASD using echocardiography and fluoroscopy [Figure 2]. The inflated balloon in the first defect prevented a second entry into the same defect. Another Super Stiff guidewire and a second sizing balloon through this second access interrogated the second defect on the same principles. When both the balloons are simultaneously inflated, careful echocardiographic imaging was used to identify any additional (third) defect, measure the dimension of such an additional defect, assess its separation from the two previously identified defects, and check for the possibility of their closure with the devices in the primary and secondary orifices. After determining the sizes from the balloon waist measured on fluoroscopy and echocardiography, the balloons were withdrawn.
Figure 2.
Balloon occlusion of first defect allows cannulation of the second defect (a). This is followed by simultaneous balloon inflation of both defects (b) to identify any residual flow on echocardiography through a third defect (if any). Balloon waist guides the size of the device (c)
Selection of the device size
In children, the device size was chosen 2–4 mm more than the largest echocardiographic diameter. If balloon interrogation was done, the device size equaled the fluoroscopic waist. If there was a significant difference between the two, the larger of them is used for device selection. In adults, balloon interrogation was the main tool to decide the device size unless the defects were far apart from each other, where the largest transesophageal dimension was used to choose a device that was larger by 4–5 mm.
Simultaneous device deployment
Long sheaths of appropriate size were placed over the two Super Stiff guidewires into the left pulmonary veins. The first device was advanced (usually the smaller device) and left and right discs were deployed but not released from the delivery cable. The second device was advanced through the second long sheath and deployed sandwiching the first device [Figure 3]. After confirming the position of the devices by fluoroscopy and echocardiography, the smaller device was released earlier than the larger device. If there were concerns about the stability of the device and possibility of device displacement after release, the screw ends of both the devices were presnared before releasing to prevent their sudden displacement upon release caused by tension on the delivery cable.[15] This snare assistance was used only in devices that employed screw type of delivery cables like ASO and were not needed when angulated delivery pushers were used for Figulla septal occluder (Occlutech International, Helsingborg, Sweden). The devices were finally released from the snare after confirming their stability.
Figure 3.
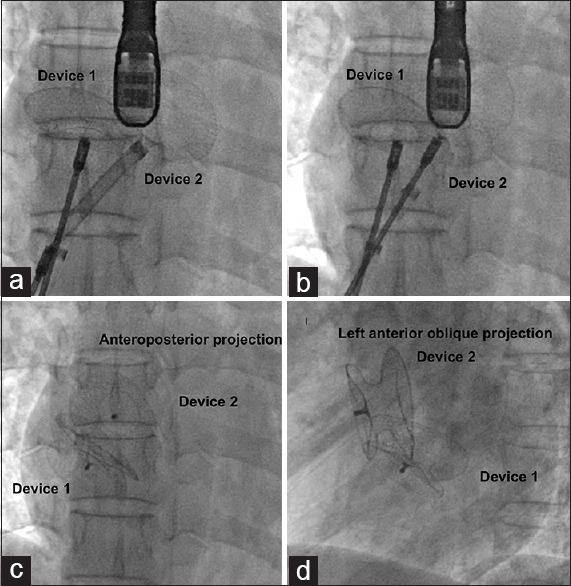
After deploying the first Figulla septal occluder (Device 1) in the posteroinferior defect, left atrial disc of the second device in the anterosuperior defect (Device 2) is brought close (a) to the first device to sandwich the former (b). However, after release, fluoroscopy in anteroposterior (c) and left anterior oblique (d) projection show a different non-sandwiched orientation due to the varying orientation of the atrial septal plane
Sequential deployment
When two defects were more than 12 mm apart from each other, only one venous access was obtained. One of the defects (smaller defect was preferred) was occluded in the usual manner. The device was deployed and released from the delivery cable, which was then withdrawn. The size of the second ASD was reassessed by echocardiography. Then, this defect was crossed with an endhole catheter, balloon interrogated if there are any concerns about its size and the same delivery sheath was advanced through this defect into the left atrium. The second occluder was deployed again in the usual manner. Final echocardiography after the release of both occluders assisted in identification of residual flows and additional defects (if any) by color flow imaging. Minor modifications were done on a case-to-case basis in both the simultaneous or sequential techniques. The use of transthoracic or transesophageal echocardiography was also decided on a case-to-case basis depending on the adequacy of images.
Device orientation
When a second larger device overlapped the initial smaller device, the appearance was akin to sandwiching of the smaller by the larger device. However, if both the devices were similar in size, they get interleaved.[5] When the devices were far apart from each other, there was no real contact with the devices. Even though these different orientations, namely “sandwich pattern” and “interleaved pattern,” were observed during deployment before release from the delivery cable, the change in the orientation of the devices in alignment to the natural lie of the atrial septum often did not maintain the original prerelease configuration [Figure 4].
Figure 4.
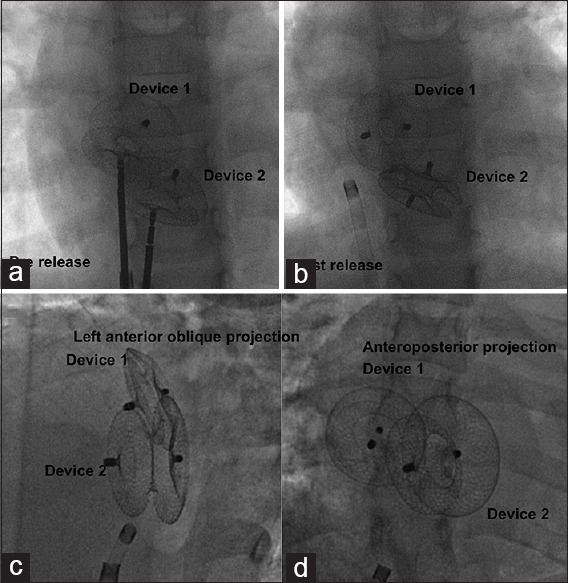
Two devices are interleaved (a) during deployment but move apart from this orientation after release (b). A set of two devices in another patient appear sandwiched in relation in left anterior oblique projection (c) but not so in anteroposterior projection (d) due to the natural curvature in the atrial septal plane
Follow-up protocol
Antiplatelet therapy was given for a total duration of 6–12 months. Dual antiplatelet therapy and longer period were advised in adults, those with larger devices, or residual shunts. Aspirin monotherapy for 6 months was considered adequate in children, smaller devices, and those with complete occlusion of the defect. Patients were followed up with clinical evaluation, electrocardiography, and transthoracic echocardiography at 1, 3, 6, and 12 months and yearly thereafter. All consenting adults underwent a transesophageal echocardiogram on one of the follow-up visits before discontinuing antiplatelets to identify residual flows [Figure 5] and thrombus on the device surface.
Figure 5.
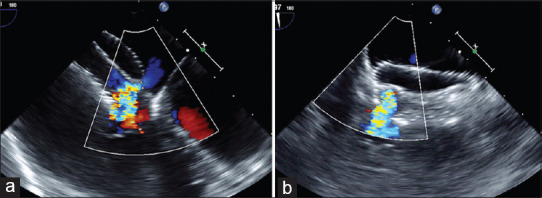
Residual flows are assessed by measurement of color width in horizontal (a) and vertical (b) planes. When immediate postprocedural residual flows are noted (b) between two devices, they often disappear on follow-up after 6–12 months possibly due to endothelialization
Data collection
Demographic details included age, weight, symptoms, presence of heart failure, and comorbidities including hypertension and atrial fibrillation. The details collected from the initial preprocedural echocardiogram included number of defects, size and associated other congenital heart defects, presence and severity of pulmonary hypertension. Procedural details included sequential versus simultaneous method of device delivery, procedural time, radiation exposure, complications, and additional procedures performed. Follow-up data collected included residual flows, late complications, and duration of antiplatelet therapy.
RESULTS
Among a total of 1256 ASD device closures done during the 12-year period from 2008 to 2019, 88 (7%) patients had multiple ASDs. Among them, 25 patients (2%) required closure using two devices and formed the study group; the rest managed with either a single self-centering ASO or a single cribriform occluder were not part of this study. Nineteen patients were found to have 2 defects, 2 patients had 3 defects, and 4 patients had more than three defects. The patients were divided into two groups based on their age to study the procedural feasibility and differences in complications: seven patients (28%) aged <16 years formed the pediatric group and older patients formed the adult group. The baseline clinical details are shown in Table 1 and procedural details are shown in Table 2. Table 3 details the follow-up differences between the pediatric and adult groups.
Table 1.
Baseline patient characteristics between pediatric and adult groups
| Baseline characteristics | Total patients (%) | Adult group (n=18), n (%) | Pediatric group (n=7), n (%) | P |
|---|---|---|---|---|
| Age (years) | 33.92±19.63 | 44±12.44 | 8±3.87 | |
| 42 (2-66) | 47 (18-66) | 8 (2-13) | ||
| Male: female | 6 (24):19 (76) | 4 (22.2):14 (77.8) | 2 (28.6):5 (71.4) | 0.739 |
| Weight (kg) | 51.16±20.17 | 61.72±10.2 | 24±11.72 | |
| 56 (11-80) | 61.5 (40-80) | 22 (11-46) | ||
| Body surface area (m2) | 1.43±0.4 | 1.64±0.14 | 0.8±0.2 | |
| 1.6 (0.51-1.88) | 1.62 (1.33-1.88) | 0.87 (0.5-1.3) | ||
| Symptoms | ||||
| Asymptomatic | 5 | 1 (5.9) | 4 (80) | 0.01 |
| Dyspnea NYHA class II | 17 | 16 (94.1) | 1 (20) | <0.001 |
| Palpitation | 5 | 5 (29.4) | 0 | 0.168 |
| Growth delay | 1 | 0 | 1 (20) | 0.059 |
| Right heart failure | 1 | 1 (5.9) | 0 | 0.579 |
| Comorbidities | ||||
| Systemic hypertension | 2 | 2 (11.8) | 0 | 0.421 |
| Atrial fibrillation | 1 | 1 (5.9) | 0 | 0.524 |
| Pulmonary thromboembolism | 1 | 1 (5.9) | 0 | |
| Valvular PS + RPA stenosis | 1 | 1 (5.9) | 0 | |
| Pre procedural TEE | 13 (59.1) | 13 (76.5) | 0 | 0.002 |
| Number of defects | ||||
| Two | 19 (76) | 14 (77.8) | 5 (71.4) | 0.414 |
| Three | 2 (8) | 2 (11.1) | 0 | |
| Multifenestrated | 4 (16) | 2 (11.1) | 2 (28.6) | |
| Defect size-echo (mm) | ||||
| Smaller defect size | 12.52±4.27 | 13.44±4.37 | 9.6±2.41 | 0.067 |
| 11 (7-20) | 11.5 (8-20) | 9 (7-13) | ||
| Larger defect size | 18.67±5.7 | 19.88±5.81 | 14.8±3.35 | 0.057 |
| 18 (10-32) | 19.5 (11-32) | 14 (10-18) | ||
| Pulmonary hypertension | ||||
| No | 6 (24) | 3 (16.7) | 3 (42.9) | 0.390 |
| Mild | 15 (60) | 11 (61.1) | 4 (57.1) | |
| Moderate | 3 (12) | 3 (16.7) | 0 | |
| Severe | 1 (4) | 1 (5.6) | 0 |
TEE: Transesophageal echocardiography; NYHA: New York heart association; PS: Pulmonary stenosis; RPA: Right pulmonary artery; LPA: Left pulmonary artery
Table 2.
Procedural differences between pediatric and adult groups
| Total number of patients (n=25), n (%) | Adults (n=18), n (%) | Pediatric group (n=7), n (%) | P | |
|---|---|---|---|---|
| Anaesthesia | ||||
| Conscious sedation | 22 (88) | 15 (83.3) | 7 (100) | 0.250 |
| Intubation anaesthesia | 3 (12) | 3 (16.7) | 0 | |
| Access | ||||
| One venous access | 2 (8) | 1 (5.6) | 1 (14.3) | 0.470 |
| Two venous accesses | 23 (92) | 17 (94.4) | 6 (85.7) | |
| Arterial access | 19 (76) | 17 (94.4) | 2 (28.6) | 0.001 |
| Intra procedural TEE | 18 (72) | 16 (88.9) | 2 (28.6) | 0.016 |
| Balloon sizing | 18 (72) | 16 (88.8) | 2 (28.6) | 0.008 |
| ASD device size | ||||
| Smaller device (mm) | 15.0±4.4 | 16.3±4.5 | 11.7±1.7 | 0.024 |
| 13 (9-24) | 16 (10-24) | 12 (9-14) | ||
| Larger device (mm) | 22.2±6.2 | 23.7±5 | 18.6±7.7 | 0.025 |
| 21 (12-35) | 23 (14-32) | 16 (12-35) | ||
| Deployment method | ||||
| Simultaneous | 22 (88) | 17 (94.4) | 5 (71.4) | 0.187 |
| Sequential | 2 (8) | 1 (5.6) | 2 (28.6) | |
| Smaller device first deployed | 18 (72) | 16 (88.9) | 2 (28.6) | 0.003 |
| Snare used | 3 (12) | 3 (16.7) | 0 | 0.250 |
| Procedural complications | 4 (16) | 4 (22.2) | 0 | |
| Device embolization | 1 (4) | 1 (5.6) | 0.174 | |
| Arrythmia | 1 (4) | 1 (5.6) | ||
| Cobra deformation | 2 (8) | 2 (11.2) | ||
| Additional procedure | ||||
| Balloon pulmonary valvotomy | 1 (4) | 0 | 1 (14.3) | |
| Right pulmonary artery stenting | 2 (8) | 1 (5.6) | 1 (14.3) | |
| Procedure time (min) | 92.3±46.4 | 102.7±46.1 | 61±34.4 | 0.035 |
| 77.5 (30-180) | 100 (50-180) | 50 (30-120) | ||
| Fluoroscopy time (min) | 17.7±9.2 | 18.3±7.7 | 16.1±13.7 | 0.286 |
| 13 (7.2-40.3) | 16 (7.7-27.4) | 10.1 (7.2-40.3) | ||
| Dose area product (mGycm2) | 40366±23859 | 48367±17760 | 4363±950 | 0.034 |
| 45701 (3691-72381) | 50659 (16814-72381) | 4363 (3691-5035) | ||
| Residual shunt postprocedure | 7 (28) | 5 (27.8) | 2 (28.6) | 0.968 |
TEE: Transesophageal echocardiography; mGycm2: Milligray square centimeter, ASD: Atrial septal defects
Table 3.
Follow up data comparison between pediatric and adult groups
| Follow up parameters | Total (n=25), n (%) | Adults (n=18), n (%) | Pediatric group (n=7), n (%) | P |
|---|---|---|---|---|
| Follow up duration (months) | 55.7±37.5 | 60.3±29.1 | 43.9±54.7 | |
| 64 (7-147) | 67 (11-120) | 15 (7-147) | ||
| Residual shunt at last follow up | 3 (15) | 2 (11.1) | 1 (14.3) | 0.826 |
| Antiplatelet/anticoagulant use | ||||
| Aspirin monotherapy | 4 (16) | 0 | 4 (57.1) | 0.001 |
| Aspirin + clopidogrel | 19 (76) | 17 (94.4) | 2 (28.6) | |
| Aspirin + warfarin | 2 (8) | 1 (5.6) | 1 (14.3) | |
| Aspirin duration (months) | 9.5 (6-18) | 11 (6-18) | 6 (6-12) | 0.385 |
| Clopidogrel duration (months) | 6 (2-12) | 6 (3-12) | 2 | 0.111 |
| Late complication | ||||
| Device thrombosis | 1 (4) | 1 (5.6) | 0 | |
| Deep vein thrombosis | 1 (4) | 0 | 1 (14.3) | |
| Bleeding manifestations | 2 (8) | 1 (5.6) | 1 (14.3) | |
| Late embolization | 0 | 0 | 0 | |
| Erosion | 0 | 0 | 0 | |
| Late arrhythmias | 0 | 0 | 0 | |
| Thrombo embolic events | 0 | 0 | 0 | |
| Headaches | 0 | 0 | 0 |
Baseline differences between children and adults
While most of the adults (94.1%) were symptomatic, 80% of children had no symptoms [Table 1]. Adults had comorbidities including systemic hypertension, paroxysmal atrial fibrillation, and thromboembolic pulmonary artery occlusions. One child had features of Noonan syndrome, pulmonary valve stenosis, and right pulmonary artery stenosis. Preprocedural transesophageal echocardiography for assessing suitability for device closure was never required in children as transthoracic images were adequate. The defect size and severity of pulmonary hypertension did not differ between the two groups.
Procedural differences between children and adults
The procedure was done under conscious sedation in all 7 children and 15 (83.3%) adults. Intubation anesthesia was used in 3 adults [Table 2]. Balloon interrogation of the ASD was done in 16 adults (89%). However, it was only sparingly used in children (28%) as either echocardiographic images were adequate for decision making or due to concerns of additional hardwares and procedural time. Simultaneous deployment was used in 22 patients and sequential deployment in three patients. In a child with multifenestrated ASD, after deployment of a cribriform occluder, an additional ASO was deployed to cover an additional defect. Intraprocedural transesophageal echocardiographic guidance was used in 16 adults (89%) and 2 children (28%). Prerelease snaring of the device that needed a larger vascular access was used in three adult cases during deployment of device to identify impending embolization but not used in children.[15] Snare assistance was successful in all the cases. Concurrent additional procedures included balloon pulmonary valvotomy in one and stenting of the right pulmonary artery in two patients. An adult with pulmonary thromboembolic complete left pulmonary artery occlusion and stenosis of right upper and lower lobar branch arteries underwent separate stenting of the branches resulting in the normalization of pulmonary artery pressures that allowed closure of both the ASDs [Figure 6].
Figure 6.
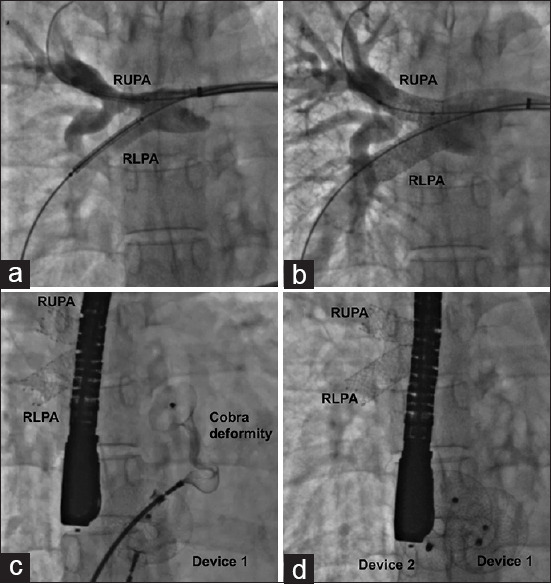
Right pulmonary artery angiogram (a) shows stenosis of right upper (RUPA) and lower (RLPA) lobar branch stenosis due to chronic thromboembolism. Stent angioplasty normalizes the flows (b) and allows device closure of multiple atrial septal defects, complicated by transient cobra deformity of one device (c) that recovers memory with gentle manipulations (d)
Procedural complications
Out of the total 25 patients, we found a residual leak immediately after the procedure in seven patients (28%) that included 5 adults and 2 children. Minor procedural complications such as device embolization, arrhythmia, and cobra deformation were observed in 4 adults, but none were noted in children. An adult patient with two defects measuring 16 and 19 mm on a transesophageal echocardiogram had balloon waist measurements of 20 and 22 mm. There was embolization of both the devices (22 mm and 20 mm) into the left ventricle immediately after release from the delivery cable. One of the devices was moved into the left atrium with a pigtail catheter and snared from the venous end, while the other was snared from the arterial end. A reassessment with balloon interrogation recognized an under-sizing and the defects were closed successfully with 22 mm and 28 mm devices successfully with snare assistance. Multiple runs of atrial arrythmia were noted in a patient and settled after amiodarone infusion. Transient cobra deformation of the left atrial disc of a 28 mm ASO and a 20 mm Cera ASD occluder (Lifetech Scientific Inc, Shenzhen, PRC) got corrected with minimal manipulations.
Antithrombotic prophylaxis
All adults received dual antiplatelet therapy at discharge except one patient who received warfarin in addition to antiplatelets in view of chronic pulmonary thromboembolism. Among the pediatric population, 4 children received aspirin monotherapy and 2 children with relatively large devices received dual antiplatelet therapy. One child with Noonan syndrome who underwent concurrent balloon pulmonary valvotomy and right pulmonary artery stenting and multiple hemodynamic pressure recordings developed postprocedural femoral vein thrombosis possibly related to prolonged procedure warranting warfarin in addition to aspirin. Despite oral anticoagulation, the venous occlusion on vascular Doppler persisted without causing any symptoms on late follow-up.
Follow-up
The median duration of follow-up was 5.5 years ranging from 1 to 12 years [Table 3]. We encountered late complications in four patients-one adult presented with asymptomatic device thrombosis on the left atrial surface detected on routine transesophageal echocardiography that resolved later, one child with persistent femoral vein occlusion already described and two patients on oral anticoagulation developing cutaneous ecchymosis. Of the 7 patients with a residual shunt at discharge, only 3 continued to have one at the last follow-up. There was a resolution of residual leak at 1 month in one case, 3 months in two cases, and 11 months of follow-up in one patient. One patient with a residual leak between the devices measuring 9 mm immediately after procedure had a decrease in the size of leak on follow-up. There were no late embolizations, erosions, arrhythmia, embolic events, or headaches.
DISCUSSION
Increasing experience accumulated over the past 2 decades of safe use of ASO for closure of secundum ASD has encouraged operators to include patients with multiple atrial defects after careful evaluation of the number of defects, their size, the length and sturdiness of the intervening tissue between the defects and age of the patient.[16,17] The most common strategy uses a single ASO through the largest and most central of the defects if the surrounding satellite defects are small and close to the main orifice.[8,9,10] In some instances, this is facilitated by a balloon enlargement of the central orifice to accommodate a larger device.[18] While nonself-centering cribriform occluders are applicable in relatively smaller orifices of multifenestrated defects, larger multiple defects need multiple devices.[11,12,13,14] A concern exists about delayed endothelialization and persistent residual flows when multiple devices are used.
Feasibility of closure with multiple devices
Our study adds to the existing evidence by showing successful implantation of more than one ASO for multiple defects.[11,12,13] Lack of procedural complications in children in our cohort shows their applicability in children weighing more than 10 kg, as shown previously.[5] The key tool of preprocedural evaluation for the feasibility of closure was echocardiography that was transthoracic in all children and transesophageal in almost all adults when transthoracic images were suboptimal. Balloon interrogation was an essential component of the decision-making process and was done in almost all adults; however, it was avoided in most children to reduce additional catheter exchanges and procedural time. Excellent multiplanar echocardiographic visualization may remove the need for balloon sizing.
Choice of technique decides the vascular access
In our study, simultaneous deployment of two devices through separate venous accesses was done in most patients. Bilateral femoral venous puncture might not be a major issue in adults. Simultaneous deployment provided control over the devices throughout the procedure. Sequential deployment through a single groin venous access was followed only when the defects were far apart leading to limited interaction between the devices during deployment. While simultaneous deployment through two venous accesses was a more common strategy, sequential technique avoided multiple venous punctures, especially in children.[5]
Are complications common with multiple devices?
Device embolization in one patient accounted for 4% of our patients. Similar embolizations were reported in 3%–4% of patients in previous studies using multiple devices.[11,12] This was higher compared to an incidence of 1.1% following single device use in a large meta-analysis.[19] This might be attributed to difficulties in determining the exact size of defects, improper assessment of sturdiness of the tissue between the defects, and abnormal interaction between the two devices after their release. We used a prophylactic prerelease snaring in three of our patients successfully when we anticipated a possible embolization.[15] Cardiac erosion due to devices is another concern following multiple devices. One study reported an erosion among 33 patients when they significantly oversized the device with a device: defect ratio of 1.5–1.8.[12] With our conservative device oversizing of 2–5 mm more than the defect diameter measured on echocardiography, we did not encounter any erosion on a reasonably long follow-up ranging 1–12 years.
Are residual leaks more common?
Studies comparing single versus multiple devices have also found higher residual leaks when using multiple devices.[20] Incidence of immediate postprocedural residual leak of 28% in our study is comparable to 17%–40% incidence observed in other studies.[10,11,12,13,16] While 19 patients in our cohort had two defects, two had three defects and four patients had more than three defects. We closed them using two devices with the smaller additional defects trapped between the left and right atrial discs protruding from the waist. These led to immediate postprocedural residual flows that improved with time possibly due to tissue ingrowth. Even when a larger device was chosen in another study of 34 patients with a device: defect ratio of 1.5–1.9, 32.4% of patients showed immediate residual leaks.[16,20] We chose a device around 4–5 mm larger than the defect size in adults and 2–4 mm larger than the defect size in children and observed similar rates of residual leaks.[10,16] While some of the previous reports of use of multiple devices described relatively smaller defects, the defects were relatively larger in our cohort.[10,16]
Do residual leaks resolve on follow-up?
There was a gradual resolution of leak on follow-up and residual leak was persistent in only 12% of cases; none of them were hemodynamically significant. Residual leak often resolves on follow-up due to progressive endothelialization and right atrial remodeling which reduces the size of the atrium and the residual defects.[5,11,12] Residual leak may also result from the presence of multiple fenestrations that are not closed by the discs of the devices or the orientation of devices in different planes. The orientation may improve over time leading to the resolution of leak. Small additional defects hidden between the protruding left and right atrial device edges may close with tissue growth over time as noted in few of our patients.
Need for dual and longer antiplatelet therapy?
In our study, all adults were treated with additional clopidogrel for a duration varying from 3 to 12 months. In the pediatric group, half were on aspirin monotherapy and the rest received additional clopidogrel for 2 months. Few prior studies of multiple devices provided data on antiplatelet therapy which was usually given for 6 months, similar to those with single devices.[5,8] The concern of delay in endothelialization led to indefinite aspirin therapy in one study.[3] Despite dual antiplatelet therapy, one of our adult patients with residual leak developed an asymptomatic small left atrial surface thrombosis detected on routine transesophageal echocardiogram after 5 months following the procedure. The recommendation regarding the dose and duration of antiplatelet therapy after device closure continues to be a point of debate with one randomized trial showing a lower incidence of new-onset migraine in those who received dual antiplatelet therapy, though there was no mention about device thrombosis.[21] Variable platelet reactivity has been the basis for number of drugs and duration of therapy.[22,23] Higher level of aspirin and clopidogrel resistance in different populations may justify population-specific guidelines for longer duration of therapy instead of an uniform universal approach.[24] This issue is contentious and needs more research as few recent studies do not observe differences in antiplatelet resistance among different population.[25,26]
Evolution of our experience
Over the last 12 years, there was a progressive evolution in our methodology: (i) inclusion of children in the study followed 2–3 years after initial adult experience; (ii) a prolonged transesophageal imaging under intubation anesthesia was replaced by a brief prerelease study under a conscious sedation; (iii) a brief prerelease intraprocedural transesophageal imaging was used in the initial two relatively older children with a brief increase of sedation; but in the last five children, we relied on transthoracic images; (iv) the adoption of sequential device deployment through a single access in defects that were far apart from each other was relatively recent compared to our earlier experience of two venous access for a simultaneous deployment; and (v) recent adoption of new techniques like snare assisted release to identify a relatively unstable device position.
Limitations
The limitations inherent to any retrospective study applied to this study also. Even though a prospective study with clear methodology defining imaging and standard operating procedures is the ideal way of research, but our increasing experience with more challenging defects permitted the inclusion of more and more patients, and this forced us to do a retrospective analysis. Cranially angulated left anterior oblique projections for assessment of the postrelease device profile in relation to the fluoroscopic orientation of the atrial septum and inter-device relationship were not available for all cases. The number of cases was less for adequate comparison between the pediatric and adult cases. The long period of follow-up was the key strength of the study. While similar studies were reported in the past using multiple devices, our group had relatively larger defect sizes; the extent of oversizing was less than those studies and the study period was more recent indicating an evolved learning curve with the use of multiple devices.
CONCLUSIONS
Transcatheter closure of multiple defects using multiple devices is feasible with good technical success similar to closure of single defects. Adequate preprocedural echocardiographic evaluation and balloon interrogation are the keys for a successful procedure. Simultaneous deployment using two groin accesses is a commonly followed strategy especially if the devices are separated by 6–12 mm. Sequential deliveries using a single groin access, though less cumbersome, is feasible rarely if the defects are far apart from each other. Residual leak remains higher but without any major clinical consequences. Long-term follow-up does not show any significant new complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K Amplatzer Investigators. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: Results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002;39:1836–44. doi: 10.1016/s0735-1097(02)01862-4. [DOI] [PubMed] [Google Scholar]
- 2.Podnar T, Martanovic P, Gavora P, Masura J. Morphological variations of secundum-type atrial septal defects: Feasibility for percutaneous closure using Amplatzer septal occluders. Catheter Cardiovasc Interv. 2001;53:386–91. doi: 10.1002/ccd.1187. [DOI] [PubMed] [Google Scholar]
- 3.Suárez De Lezo J, Medina A, Pan M, Romero M, Segura J, Pavlovic D, et al. Transcatheter occlusion of complex atrial septal defects. Catheter Cardiovasc Interv. 2000;51:33–41. doi: 10.1002/1522-726x(200009)51:1<33::aid-ccd9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Sivakumar K, Viswambaran B, Bhattacharjya S. Feasibility, safety and midterm follow-up of patients after nonsurgical closure of atrial septal defects using very large 40-46 mm nitinol septal occluders. Catheter Cardiovasc Interv. 2019;93:466–73. doi: 10.1002/ccd.27957. [DOI] [PubMed] [Google Scholar]
- 5.Bramlet MT, Hoyer MH. Single pediatric center experience with multiple device implantation for complex secundum atrial septal defects. Catheter Cardiovasc Interv. 2008;72:531–7. doi: 10.1002/ccd.21668. [DOI] [PubMed] [Google Scholar]
- 6.Farhaj Z, Hongxin L, Wenbin G, Zhang WL, Liang F, Zhang HZ, et al. Device closure of diverse layout of multi-hole secundum atrial septal defect: Different techniques and long-term follow-up. J Cardiothorac Surg. 2019;14:130. doi: 10.1186/s13019-019-0952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roman KS, Jones A, Keeton BR, Salmon AP. Different techniques for closure of multiple interatrial communications with the Amplatzer septal occluder. J Interv Cardiol. 2002;15:393–7. doi: 10.1111/j.1540-8183.2002.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 8.Zanchetta M, Rigatelli G, Pedon L, Zennaro M, Carrozza A, Onorato E. Catheter closure of perforated secundum atrial septal defect under intracardiac echocardiographic guidance using a single amplatzer device: Feasibility of a new method. J Invasive Cardiol. 2005;17:262–5. [PubMed] [Google Scholar]
- 9.Szkutnik M, Masura J, Bialkowski J, Gavora P, Banaszak P, Kusa J, et al. Transcatheter closure of double atrial septal defects with a single Amplatzer device. Catheter Cardiovasc Interv. 2004;61:237–41. doi: 10.1002/ccd.10753. [DOI] [PubMed] [Google Scholar]
- 10.Masseli J, Bertog S, Stanczak L, Blankenbach K, Majunke N, Reiffenstein I, et al. Transcatheter closure of multiple interatrial communications. Catheter Cardiovasc Interv. 2013;81:825–36. doi: 10.1002/ccd.24329. [DOI] [PubMed] [Google Scholar]
- 11.Cao Q, Radtke W, Berger F, Zhu W, Hijazi ZM. Transcatheter closure of multiple atrial septal defects. Initial results and value of two- and three-dimensional transoesophageal echocardiography. Eur Heart J. 2000;21:941–7. doi: 10.1053/euhj.1999.1909. [DOI] [PubMed] [Google Scholar]
- 12.Awad SM, Garay FF, Cao QL, Hijazi ZM. Multiple Amplatzer septal occluder devices for multiple atrial communications: Immediate and long-term follow-up results. Catheter Cardiovasc Interv. 2007;70:265–73. doi: 10.1002/ccd.21145. [DOI] [PubMed] [Google Scholar]
- 13.Mahadevan VS, Gomperts N, Haberer K, Silversides C, Benson LN, McLaughlin PR, et al. Transcatheter closure of atrial septal defects with multiple devices in adults: Procedural and clinical outcomes. Int J Cardiol. 2009;133:359–63. doi: 10.1016/j.ijcard.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Numan M, El Sisi A, Tofeig M, Gendi S, Tohami T, El-Said HG. Cribriform amplatzer device closure of fenestrated atrial septal defects: Feasibility and technical aspects. Pediatr Cardiol. 2008;29:530–5. doi: 10.1007/s00246-007-9079-x. [DOI] [PubMed] [Google Scholar]
- 15.Pavithran S, Sivakumar K. A novel snare assistance safeguards against early embolization of devices and facilitates quick retrieval of malpositioned devices in atrial septal defects with deficient margins. Ann Pediatr Cardiol. 2015;8:189–95. doi: 10.4103/0974-2069.164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Xu Z, Jiang S, Zhao S, Zhang G, Jin J, et al. Simultaneous transcatheter closure of multiple atrial septal defects using dual Amplatzer septal occluder devices. Am J Med Sci. 2016;352:245–51. doi: 10.1016/j.amjms.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Pedra CA, Fontes-Pedra SR, Esteves CA, Assef J, Fontes VF, Hijazi ZM. Multiple atrial septal defects and patent ductus arteriosus: Successful outcome using two Amplatzer septal occluders and Gianturco coils. Cathet Cardiovasc Diagn. 1998;45:257–9. doi: 10.1002/(sici)1097-0304(199811)45:3<257::aid-ccd8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 18.Carano N, Hagler DJ, Agnetti A, Squarcia U. Device closure of fenestrated atrial septal defects: Use of a single Amplatz atrial septal occluder after balloon atrial septostomy to create a single defect. Catheter Cardiovasc Interv. 2001;52:203–7. doi: 10.1002/1522-726x(200102)52:2<203::aid-ccd1048>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 19.Abaci A, Unlu S, Alsancak Y, Kaya U, Sezenoz B. Short and long term complications of device closure of atrial septal defect and patent foramen ovale: Meta-analysis of 28,142 patients from 203 studies. Catheter Cardiovasc Interv. 2013;82:1123–38. doi: 10.1002/ccd.24875. [DOI] [PubMed] [Google Scholar]
- 20.Hu Z, Zhang Y, Zhang T, Cheng G, Xie X, He X, et al. Comparison of the effectiveness and safety of single versus dual occluders for the closure of multiple atrial septal defects. J Invasive Cardiol. 2015;27:E90–7. [PubMed] [Google Scholar]
- 21.Wintzer-Wehekind J, Horlick E, Ibrahim R, Cheema AN, Labinaz M, Nadeem N, et al. Effect of clopidogrel and aspirin vs aspirin alone on migraine headaches after transcatheter atrial septal defect closure: One-year results of the CANOA randomized clinical trial. JAMA Cardiol. 2021;6:209–13. doi: 10.1001/jamacardio.2020.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olasinska-Wisniewska A, Grygier M. Antithrombotic/Antiplatelet treatment in transcatheter structural cardiac interventions-PFO/ASD/LAA occluder and interatrial shunt devices. Front Cardiovasc Med. 2019;6:75. doi: 10.3389/fcvm.2019.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polzin A, Dannenberg L, Sophia Popp V, Kelm M, Zeus T. Antiplatelet effects of clopidogrel and aspirin after interventional patent foramen ovale/atrium septum defect closure. Platelets. 2016;27:317–21. doi: 10.3109/09537104.2015.1096335. [DOI] [PubMed] [Google Scholar]
- 24.Sadiq PA, Puri A, Dixit M, Ghatak A, Dwivedi SK, Narain VS, et al. Profile and prevalence of aspirin resistance in Indian patients with coronary artery disease. Indian Heart J. 2005;57:658–61. [PubMed] [Google Scholar]
- 25.Pareed SA, Vijayaraghavan G, Kartha CC, Manoj MT. Antiplatelet drug resistance in Indians. Ann Clin Cardiol. 2020;2:36–41. [Google Scholar]
- 26.Patel S, Arya V, Saraf A, Bhargava M, Agrawal CS. Aspirin and clopidogrel resistance in Indian patients with ischemic stroke and its associations with gene polymorphisms: A pilot study. Ann Indian Acad Neurol. 2019;22:147–52. doi: 10.4103/aian.AIAN_4_18. [DOI] [PMC free article] [PubMed] [Google Scholar]