Abstract
Background:
Congenital portosystemic shunts (CPSS) are rare and present variably with hepatic encephalopathy, pulmonary arteriovenous malformations (PAVMs), and pulmonary hypertension (PH).
Objective:
The objective of the study was to see the feasibility of transcatheter closure of CPSS and their outcome.
Materials and Methods:
We analyzed the data of 24 patients of CPSS who underwent transcatheter closure from five institutions (March 2013 to April 2019). Baseline evaluation included echocardiography with bubble contrast study, ultrasound examination of the abdomen, computed tomography angiogram, and cardiac catheterization with test balloon occlusion of the CPSS. The evaluation showed cyanosis due to PAVM in 12, PH in 8, and respiratory distress in 2. Two had both cyanosis and PH. Criteria for eligibility for complete catheter closure of CPSS included demonstration of intrahepatic portal vein (PV) radicals together with a PV pressure of ≤18 mmHg on occlusion.
Results:
The median age and weight were 8 years (0.5–21) and 19.5 kg (4.2–73), respectively. Transcatheter closure was performed in 21 patients (22 procedures) using a variety of occlusive devices and stent-graft exclusion was done in one patient. Closure was not done in 3 in view of high portal venous pressures and hypoplastic PVs. During the follow-up (median: 42 months and range: 61 days–4.8 years), saturation normalized in 14 patients with PAVM. PH declined in all eight patients who underwent the procedure. Respiratory distress improved in two patients.
Conclusions:
Early and short-term follow-up results of catheter closure of CPSS appear promising. However, further, follow-up is needed to demonstrate long-term effectiveness.
Keywords: Device closure, portal vein anomaly, portosystemic shunts, Abernethy malformation, pulmonary arteriovenous malformation, pulmonary hypertension, transcatheter closure
INTRODUCTION
Portosystemic shunts are the communications between portal vein (PV) and its tributaries to one of the systemic veins.[1] The defects are either congenital or acquired secondary to liver disease. Congenital portosystemic shunts (CPSS) can also be either intrahepatic or extrahepatic. Extrahepatic CPSS are otherwise known as “Abernethy malformation” first described by John Abernethy in a child in 1793.[2] The entity is often unrecognized due to its asymptomatic nature in many cases for a long time and also due to its varied presentation. Abernethy malformations (extrahepatic CPSS) are essentially two types – end to side (Type 1) or side to side (Type 2) connection of the PV to one of the systemic veins.[3]
The manifestations of the CPSS are varied and include cyanosis due to diffuse intrapulmonary arteriovenous malformation (PAVM), pulmonary hypertension (PH), hepatic encephalopathy, and recurrent hypoglycemia. The condition is sometimes incidentally identified during routine abdominal imaging.[4] A high degree of clinical suspicion in patients with unexplained desaturation or PH is sometimes rewarding when ultrasound or computed tomography (CT) scan of the liver confirms the presence of CPSS as a potentially treatable cause.[5] There are several isolated case reports of transcatheter management of Type-2 Abernethy malformation using either occluders or stent grafts.[6,7,8] This article seeks to report the collective experience of transcatheter management of CPSS from five centers.
MATERIALS AND METHODS
The records of 24 patients from five tertiary care cardiac centers between March 2013 and April 2019 were analyzed. The baseline demographic details are given in Table 1. All patients underwent comprehensive evaluation including chest X-ray, electrocardiogram, transthoracic echocardiogram, ultrasound examination of the abdomen [Figure 1], and liver function tests. Saline bubble contrast echocardiogram was done to demonstrate PAVM. CT angiogram was performed in all to define the type and extent of communication and associated abnormalities [Figure 2]. Cardiac catheterization was planned in all cases with the intention of transcatheter closure.
Table 1.
Clinical and hemodynamic details and procedural characteristics of 24 patients with congenital portosystemic shunts
| Case number | Age (years) | Weight (kg) | Sex | Anatomical type | Defect size (mm) | Group | Saturation (%) | Baseline PAP (mmHg) (S/D/M) | Portal pressure after balloon (mmHg) | Device details | |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Pre | Post (follow-up) | ||||||||||
| 1 | 8 | 16.5 | Male | PV-IVC | 16 | III | 80 | 96 | 40/23/29 | 15 | 16.5 OSO |
| 2 | 3.5 | 12 | Male | PV-CS | 8 | II | 99 | 99 | 37/19/25 | 14 | 12 AVPII |
| 3 | 8 | 18 | Male | PV-IVC | 11 | II | 98 | 99 | 38/18/25 | 12 | 12 OSO |
| 4 | 11 | 28 | Female | PV-IVC | 15.2 | I | 80 | 96 | 28/14/18 | 14 | 18 ASO |
| 5 | 6 | 19 | Female | PV-LRV | 9 | I | 50 | 92 | NA | 16 | 14 AVPII |
| 6 | 5 | 14.6 | Male | PV-LIV | 7.5 | I | 80 | 98 | NA | 14 | 12 AVPII |
| 7 | 5 | 15 | Female | PV-IVC | 10.5 | I | 82 | 96 | 22/14/15 | 15 | 12 AMD |
| 8 | 5 | 13 | Male | PV-CS | 9.2 | I | 65 | 97 | 26/12/16 | 13 | 12 CVP |
| 9 | 1 | 6.8 | Female | PV-IVC | 8 | II | 99 | 99 | 60/22/42 | 19 | Not closed |
| 10 | 11 | 28 | Male | PV-IVC | 10 | II | 100 | 100 | 64/26/48 | 18 | 14 AMD |
| 11 | 13 | 32 | Male | PV-IVC | 11.4 | I | 78 | 92 | NA | 16 | 14 AMD |
| 12 | 0.45 | 4.2 | Male | DV-HV | 7.2 | IV | 98 | 98 | 30/18/22 | 13 | 12 AVPII |
| 13 | 13 | 54 | Male | PV-IVC | 11.5 | I | 86 | 98 | 26/10/15 | 14 | 14 AMD |
| 14 | 11 | 23 | Male | PV-IVC | 20 | I | 78 | 92 | 28/12/14 | 20 | Stent graft 22×30 and 12 AMD |
| 15 | 21 | 73 | Female | PV-LRV | 15 | II | 99 | 99 | 78/25/46 | 15 | 18 CVP |
| 16 | 3 | 12 | Female | PV-IVC | 7.5 | II | 98 | 99 | 66/29/40 | 22 | Not closed |
| 17 | 0.5 | 5.5 | Female | PV-CS | 8.5 | I | 92 | 98 | 24/12/16 | 14 | 12 CVP |
| 18 | 0.6 | 4.2 | Female | PV-LRV | 8 | IV | 97 | 97 | 36/16/22 | 12 | 12 CVP |
| 19 | 15 | 28 | Female | PV-LRV | 12.8 | I | 82 | 96 | 24/14/18 | 9 | 18 CVP |
| 20 | 10 | 24 | Male | PV-IVC | 8 | I | 94 | 90 | 26/12/14 | 12 | Not closed |
| 21 | 19 | 54 | Male | PV-IVC | 14 | III | 92 | 98 | 71/38/49 | 28 | 18 AMD (fenestrated) |
| 22 | 18 | 48 | Male | PV-IVC | 12.4 | II | 97 | 97 | 45/25/30 | 24 | 16/14 DO (fenestrated) |
| 23 | 9 | 20 | Male | PV-IVC | 14 | I | 72 | 95 | 28/12/16 | 18 | 16.5 OSO |
| 24 | 8 | 30 | Male | DV-HV | 9 | II | 97 | 98 | 44/18/26 | 22 | 14 CVP (partial closure) |
DV: Ductus venosus, HV: Hepatic vein, IVC: Inferior vena cava, LRV: Left renal vein, PV: Portal vein, CS: Coronary sinus, OSO: Occlutech septal occluder, ASO: Amplatzer septal occluder, AVPII: Amplatzer vascular plug II, AMD: Amplatzer muscular device, CVP: Cera vascular plug, PAP: Pulmonary artery pressure, LIV: Left ventricle, DO: Duct occluder
Figure 1.
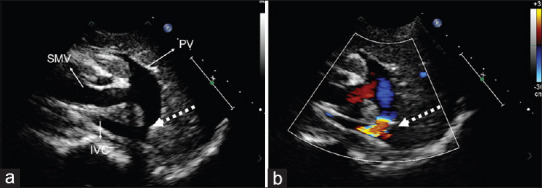
Abdominal ultrasound showing (a) Type-2 Abernethy malformation (broken arrow) (b) color flow across the defect SMV: Superior mesenteric vein, PV: portal vein, IVC: Inferior vena cava
Figure 2.
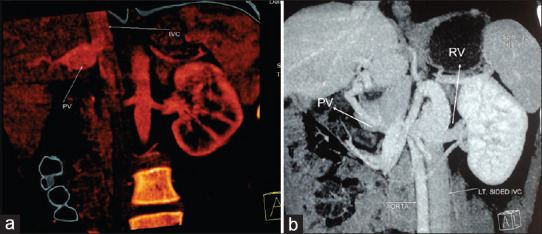
Computerized tomogram with triphasic contrast study showing (a) Portal vein draining into inferior vena cava. (b) Portal vein draining to the left renal vein in a case of left isomerism. PV: Portal vein, RV: Renal vein
Eligibility for transcatheter closure
All patients with the diagnosis of CPSS either with persistent ductus venosus (DV) or Type-2 Abernethy malformation
Normal liver and renal function
Anatomy of the defect suitable for transcatheter closure
Portal venous pressure ≤18 mmHg following test balloon occlusion of the CPSS for complete closure; with increasing experience, partial closure was considered for those with PV pressure >18 mmHg after balloon occlusion
The developed portal venous system with all major branches. Demonstrable intrahepatic PV radicles to all the lobes of the liver on CT or conventional or balloon occlusion angiography.
Cardiac catheterization
After obtaining informed consent, femoral vein and femoral artery were accessed. Internal jugular vein access was obtained in selected instances as dictated by the anatomy of the CPSS. Systemic heparinization of 100 units/kg was given after obtaining the access. Basic hemodynamic data were obtained including both right heart and left heart pressures. Portal venous pressure or hepatic venous wedge pressures were recorded at baseline and after balloon occlusion of the communication for 10 min. The balloon occlusion was done using a valvuloplasty balloon of appropriate size or a compliant sizing balloon either from the jugular or femoral vein based on anatomy of the communication. A selective angiogram in the PV [Figure 3] was performed using a separate catheter. The angiogram was useful to study the PV branches, size, and nature of the communication to systemic vein. Selective superior mesenteric artery injection followed by levophase was used to demonstrate portal anatomy in selected patients (case 12, DV communicating between PV and hepatic vein).
Figure 3.
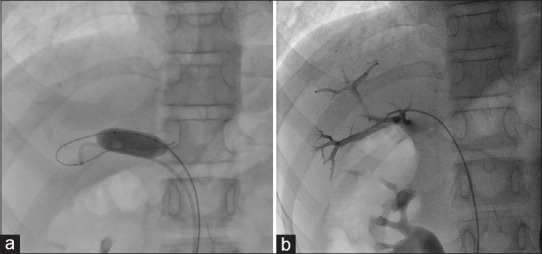
(a) Balloon occlusion of portosystemic communication to check the portal pressure. (b) Portal venous angiogram showed the branches and its radicles
Transcatheter closure
The defect and device details of all the patients underwent cardiac catheterization are given in Table 1. The type and size of the device were chosen after careful assessment by angiogram. Amplatzer muscular device or Amplatzer atrial septal occluder (Abbot/St Jude Medical MN, USA), Occlutech septal occluder (Occlutech International AB Helsingborg, Sweden) Amplatzer vascular plug II, or Cera vascular plug (Lifetech Scientific, Shenzhen, China) were used for the closure. The defect was crossed with Judkins right coronary or multipurpose catheter using an angled hydrophilic guidewire. The wire was manipulated to enter either superior mesenteric vein or splenic vein and then the catheter advanced over that. An Amplatzer extra stiff wire was exchanged and subsequently, an appropriate delivery system was positioned for the delivery of the occluder. The device position was confirmed with repeated hand injections. In one case, balloon support was used to align the muscular device in the inferior vena cava (IVC) (case no. 7). A fenestrated device [Figure 4] was used in two cases with high PV pressure (>18 mmHg). Another child with large communication (defect size-20 mm) between PV to IVC and high portal venous pressure (>18 mmHg) was excluded using a 22 mm × 30 mm Ankura stent graft (Lifetech scientific, Shenzhen, China) placed across the defect in the IVC leaving a small residual defect at the inferior end of the defect. The residual defect was closed with muscular device 1 year after the initial procedure. All the patients were observed in the intensive care and monitored for 12–24 h after the procedure.
Figure 4.

Custom-made fenestration of muscular ventricular septal occluder
Liver function tests were monitored routinely for all patients. Predischarge echocardiogram and ultrasound of the abdomen were done for all patients. Oral aspirin 3 mg/kg was started for all patients and continued for 6 months.
The follow-up protocol included clinical assessment, echocardiogram to assess PH by right ventricular systolic pressure, and saline bubble contrast study to demonstrate regression of PAVM. Follow-up was recommended at 1, 3, 6, 12 months, and yearly thereafter.
RESULTS
The study population distribution and their course are given in the flowchart [Figure 5 and Table 1]. There were 24 (male: 15) patients with CPSS. The evaluation showed cyanosis due to PAVM in 12, PH in 8, and respiratory distress in 2 (case no. 12 and 18) probably due to hyperammonemia (serum ammonia 159 and 132 μmol/lt.). Two patients had both cyanosis and PH (case no. 1, 21). Twenty-two patients were diagnosed as CPSS Type-2 Abernethy malformation and two patients (case no. 12 and 24) with DV communicating between PV and hepatic veins [Figure 6]. Angiographic anatomy in Type-2 Abernethy (n = 22) showed communication between PV and IVC in 14 cases [Figure 7], between PV and right atrium [Figure 8] in 3 cases, between PV and left renal vein [Figure 9] in 4 cases, and in 1 patient, the connection was to iliac vein. Hypoplastic portal venous system with poor ramification was seen in 1 patient (case no. 20).
Figure 5.
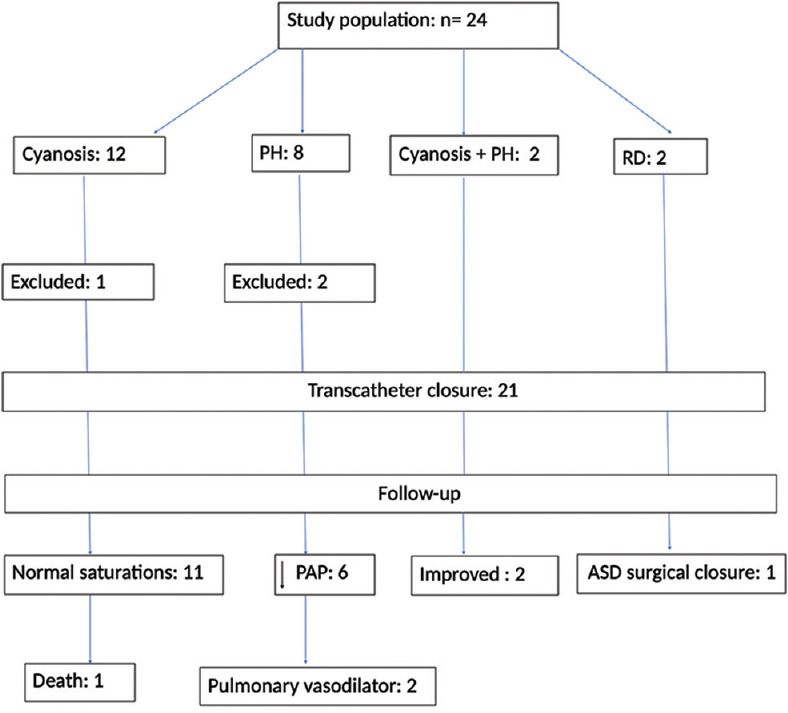
Flow chart: showing the distribution of the study population and their course. PH: Pulmonary hypertension, RD: Respiratory distress, PAP: Pulmonary artery pressure, ASD: Atrial septal defect
Figure 6.
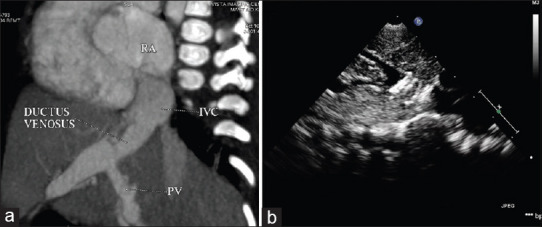
(a) Computed tomography angiogram showing ductus venosus. (b) Ultrasound abdomen showing vascular plug occluding the intrahepatic shunt of the same patient. RA: right atrium, IVC: inferior vena cava, PV: portal vein
Figure 7.
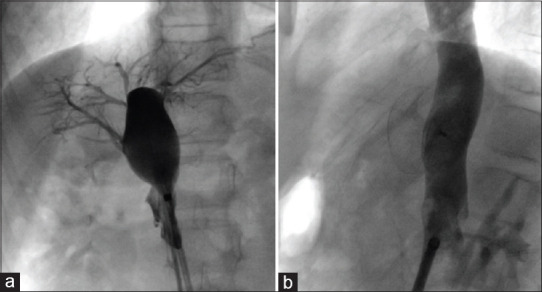
(a) Selective angiogram of portal vein through the portosystemic shunt demonstrating all branches of portal veins. (b) Atrial septal occluder (Occlutech International AB Helsingborg, Sweden) successfully deployed across the defect in the same patient
Figure 8.
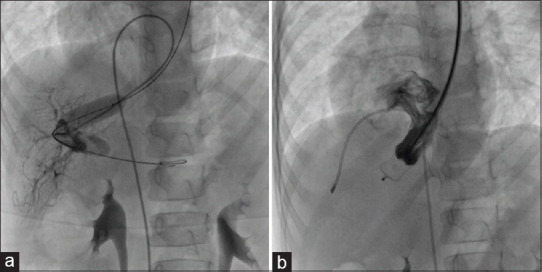
(a) Balloon occlusion from the left jugular vein and angiogram from femoral vein demonstrating portal venous branches. (b) The communicating channel was completely occluded using vascular plug
Figure 9.
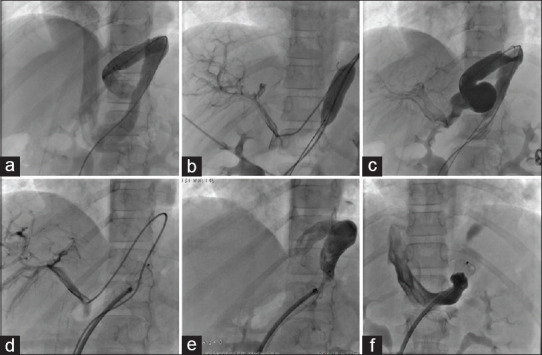
Various steps of closure of congenital portosystemic shunt draining to the left renal vein. (a-c) Angiographic demonstration of tortuous venous channel and balloon showing well ramified portal venous system. (d-f) Deployment of Cera vascular plug
Three patients were found to have associated cardiac defects – fossa ovalis atrial septal defect (case no. 12), ventricular septal defect (case no. 1), and DV patent ductus arteriosus (case no. 2). They underwent surgical closure (case no. 1 and 12) or catheter closure (case no. 2) of the defects on another occasion. One patient (case no. 15) had isolated left isomerism (interruption of IVC). The postocclusion portal venous pressure was ≤18 mmHg in 18 patients and >18 mmHg in 6 patients.
Twenty-two transcatheter procedures were done in 21 cases. Amplatzer muscular device was used in six, Cera vascular plug in six, Amplatzer vascular plug II in four, septal occluder in four patients. Duct occluder was used in one patient (case no. 22). Dual procedure was done in one patient (case no. 14) using a stent graft placed in IVC to partially exclude communication in a child with large defect and high portal pressure. This patient underwent 12-mm muscular device closure 1 year later after a repeat diagnostic catheterization. Partial closure of DV was done in one case with hypoplastic portal branches (case no. 24).
Three patients did not undergo closure of the CPSS; two of them (case no. 9 and 16) had high portal venous pressure (>18 mmHg) and one (case no. 20) had hypoplastic portal radicles.
There was no procedure-related mortality. One patient had transient lower limb venous congestion where larger size sheath was used to deploy stent graft. There was no rise in liver enzymes after the procedure in any patient.
The median follow-up of 42 months (range: 61 days–4.8 years) is available. Cyanosis and PAVM (saline bubble contrast) disappeared in all 14 cases within 6–12 weeks after the procedure. In the PH group, the pulmonary pressure by tricuspid regurgitation velocity showed improvement in all eight patients and only two were on pulmonary vasodilator therapy. Respiratory distress subsided in two patients with raised ammonia levels after the procedure. One patient died (case no. 8) 3 months after the procedure due to a brain abscess.
DISCUSSION
CPSS are rare with varied presentation and often may remain unnoticed. Although Abernethy[2] described this entity a long ago, the pathophysiology and manifestations were not understood for many years. CPSS may be asymptomatic or present with (i) cyanosis, (ii) pulmonary arterial hypertension, (iii) hepatic encephalopathy, and (iv) recurrent hypoglycemia.[4]
Unexplained cyanosis and PH are common presentations in our study population. Respiratory distress, a manifestation of hyperammonemia, was seen in two of our young patients. Several postulations for varied presentations of CPSS have been described; we would like to summarize them based on our experience of 24 patients who underwent transcatheter procedures into four groups:
Cyanotic group: Systemic desaturation results from the development of arteriovenous shunt at the capillary level and diffusion-perfusion defects from unfiltered molecules from the gut that bypass the liver either partially or completely due to CPSS to reach the pulmonary circulation.[9,10,11] All patients with systemic desaturation became asymptomatic with normal saturation in 6–12 weeks after transcatheter closure of CPSS
PH group: There are several postulated mechanisms that are thought to result in PH in patients with CPSS.[11,12,13] There were eight patients presented with PH in our study but only six were candidates for transcatheter closure. Patients with PH who underwent transcatheter closure were weaned off from the medication during follow-up in five patients. One adult patient was still on medications but symptomatically became better after closure[7]
Mixed group: The presence of both cyanosis or demonstrable PAVM and PH was seen in two of our patients (case no. 1 and 21). Both the patients were weaned off from the pulmonary vasodilator therapy after the procedure
Hyperammonemia group: Intrahepatic portosystemic shunt including DV may present with hyperammonemia.[14,15] In our series, two patients (both infants) presented with respiratory distress and showed increased serum ammonia levels and improved dramatically after closure of the defect with ammonia levels normalizing within 72 h.
The treatment modalities are dependent on the type and size of the CPSS. Closure of Type-I Abernethy malformations is contraindicated because of absent portal communication and may require hepatic transplantation when they become symptomatic.[16] Type-2 Abernethy can undergo either surgical or transcatheter closure after thorough angiographic and hemodynamic assessment after balloon occlusion. Surgical closure carries morbidity and appears to be high risk.[17,18]
Several case reports of transcatheter closure using Amplatzer muscular or septal occluder showed immediate good results but there is inadequate information about case selection and technical aspects including short-term follow-up.[19,20] Our study addresses some of the issues and provides a road map for the transcatheter closure in CPSS.
Diagnostic cardiac catheterization is needed to assess the feasibility of transcatheter closure. Test balloon occlusion is important to assess PV pressure and its branches. Early in our series, three of patients were not considered eligible for the transcatheter closure in view of high portal pressures and paucity of the PV radicles. If the portal venous pressure ≤18 mmHg, it is perhaps safe to close. A fenestrated device may be a cautious choice if portal venous pressure is >18 mmHg. The presence of high portal pressure together with poorly developed portal branches is perhaps a contraindication for complete closure and may be candidates for a staged approach to allow portal radicles to develop over a period of time after partial closure.[19]
The choice of device is purely based on the anatomy and size of the defect. A detailed anatomical delineation by CT angiogram before the procedure may be useful to plan the transcatheter closure.
PV to IVC defects are morphologically window type and hence can be effectively closed using a septal occluder or muscular device based on the length and space for the retention disc in the PV chamber. Predeployment angiogram using additional catheter is useful to see the impingement of the device disc in the portal system. Muscular device or septal occluder is probably a good choice as it has a double retention disc with a central waist. The retention disc on the portal venous side gets accommodated easily in the capacious vessel without causing any obstruction to the portal venous system [Figure 5]. The disc on the IVC side sometimes may be difficult to align and configure to circular-shaped tubular IVC as we experienced in one of our cases that needed balloon-assisted technique for the proper position
CPSS communicating to the right atrium coronary sinus and persistent DV can be closed from the jugular approach (n = 5). The communicating channels are generally straight and can be closed with vascular plugs
In left isomerism perhaps due to lack of laterality in a right-sided PV, the anatomy and course of the venous channel are highly variable and these cases may be best suited for catheter closure using vascular plugs
A large defect directly communicating to IVC may be best managed by stent-graft exclusion
Fenestrated device for partial closure may be considered in cases with borderline high portal pressures or hypoplasia of the portal system.
Limitations
This is a small series and only a short-term follow-up study. The rarity and heterogeneity of the individual lesions required a multicenter data collection to enable sufficient representation of individual subtypes. The patients with PH have only been followed noninvasively and not undergone a repeat catheterization to document pulmonary arterial pressures.
CONCLUSIONS
The clinical manifestations of CPSS are varied and include cyanosis from PAVM, pulmonary arterial hypertension, and features of hyperammonemia. A high degree of clinical suspicion in the abovementioned circumstances followed by meticulous evaluation by ultrasound examination and CT angiogram can enable the diagnosis of CPSS. Transcatheter closure appears to be safe in carefully selected cases using conventional occlusive devices. Immediate and short-term follow-up results are encouraging. Long-term follow-up is needed to demonstrate overall effectiveness.
Ethical approval
This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individuals participants included in the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Blanc T, Guerin F, Franchi-Abella S, Jacquemin E, Pariente D, Soubrane O, et al. Congenital portosystemic shunts in children: A new anatomical classification correlated with surgical strategy. Ann Surg. 2014;260:188–98. doi: 10.1097/SLA.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 2.Abernethy J. Account of two instances of uncommon formation in the viscera of the human body: From the Philosophical Transactions of the Royal Society of London. Med Facts Obs. 1797;7:100–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CP, Yoo SJ, Babyn PS. Congenital extrahepatic portosystemic shunts. Pediatr Radiol. 2003;33:614–20. doi: 10.1007/s00247-003-1002-x. [DOI] [PubMed] [Google Scholar]
- 4.Papamichail M, Pizanias M, Heaton N. Congenital portosystemic venous shunt. Eur J Pediatr. 2018;177:285–94. doi: 10.1007/s00431-017-3058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alonso-Gamarra E, Parrón M, Pérez A, Prieto C, Hierro L, López-Santamaría M. Clinical and radiologic manifestations of congenital extrahepatic portosystemic shunts: A comprehensive review. Radiographics. 2011;31:707–22. doi: 10.1148/rg.313105070. [DOI] [PubMed] [Google Scholar]
- 6.Guneyli S, Cinar C, Bozkaya H, Parildar M, Oran I, Akin Y. Successful transcatheter closure of a congenital high-flow portosystemic venous shunt with the Amplatzer vascular plug II. Perspect Vasc Surg Endovasc Ther. 2012;24:202–5. doi: 10.1177/1531003513496850. [DOI] [PubMed] [Google Scholar]
- 7.Venkateshwaran S, Krishnamoorthy KM, Sivasankaran S. Percutaneous device closure of Abernethy malformation – A treatable cause of hepatopulmonary syndrome. Catheter Cardiovasc Interv. 2014;83:968–70. doi: 10.1002/ccd.25275. [DOI] [PubMed] [Google Scholar]
- 8.Kraus C, Sheynzon V, Hanna R, Weintraub J. Single stage endovascular treatment of a type 2 Abernethy malformation: Successful nonsurgical outcome in a case report. Case Rep Radiol. 2015;2015:491867. doi: 10.1155/2015/491867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edell ES, Cortese DA, Krowka MJ, Rehder K. Severe hypoxemia and liver disease. Am Rev Respir Dis. 1989;140:1631–5. doi: 10.1164/ajrccm/140.6.1631. [DOI] [PubMed] [Google Scholar]
- 10.Krowka MJ, Porayko MK, Plevak DJ, Pappas SC, Steers JL, Krom RA, et al. Hepatopulmonary syndrome with progressive hypoxemia as an indication for liver transplantation: Case reports and literature review. Mayo Clin Proc. 1997;72:44–53. doi: 10.4065/72.1.44. [DOI] [PubMed] [Google Scholar]
- 11.Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and hepatopulmonary syndrome. Lancet. 2004;363:1461–8. doi: 10.1016/S0140-6736(04)16107-2. [DOI] [PubMed] [Google Scholar]
- 12.Budhiraja R, Hassoun PM. Portopulmonary hypertension: A tale of two circulations. Chest. 2003;123:562–76. doi: 10.1378/chest.123.2.562. [DOI] [PubMed] [Google Scholar]
- 13.Edwards BS, Weir EK, Edwards WD, Ludwig J, Dykoski RK, Edwards JE. Coexistent pulmonary and portal hypertension: Morphologic and clinical features. J Am Coll Cardiol. 1987;10:1233–8. doi: 10.1016/s0735-1097(87)80123-7. [DOI] [PubMed] [Google Scholar]
- 14.Ferrero GB, Porta F, Biamino E, Mussa A, Garelli E, Chiappe F, et al. Remittent hyperammonemia in congenital portosystemic shunt. Eur J Pediatr. 2010;169:369–72. doi: 10.1007/s00431-009-1031-z. [DOI] [PubMed] [Google Scholar]
- 15.Kitagawa S, Gleason WA, Jr, Northrup H, Middlebrook MR, Ueberschar E. Symptomatic hyperammonemia caused by a congenital portosystemic shunt. J Pediatr. 1992;121:917–9. doi: 10.1016/s0022-3476(05)80341-5. [DOI] [PubMed] [Google Scholar]
- 16.Benedict M, Rodriguez-Davalos M, Emre S, Walther Z, Morotti R. Congenital extrahepatic portosystemic shunt (Abernethy malformation type Ib) with associated hepatocellular carcinoma: Case report and literature review. Pediatr Dev Pathol. 2017;20:354–62. doi: 10.1177/1093526616686458. [DOI] [PubMed] [Google Scholar]
- 17.Witjes CD, Ijzermans JN, Vonk Noordegraaf A, Tran TK. Management strategy after diagnosis of Abernethy malformation: A case report. J Med Case Rep. 2012;6:167. doi: 10.1186/1752-1947-6-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saad WE. Portosystemic shunt syndrome and endovascular management of hepatic encephalopathy. Semin Intervent Radiol. 2014;31:262–5. doi: 10.1055/s-0034-1382795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruckheimer E, Dagan T, Atar E, Schwartz M, Kachko L, Superina R, et al. Staged transcatheter treatment of portal hypoplasia and congenital portosystemic shunts in children. Cardiovasc Intervent Radiol. 2013;36:1580–5. doi: 10.1007/s00270-013-0581-7. [DOI] [PubMed] [Google Scholar]
- 20.Passalacqua M, Lie KT, Yarmohammadi H. Congenital extrahepatic portosystemic shunt (Abernethy malformation) treated endovascularly with vascular plug shunt closure. Pediatr Surg Int. 2012;28:79–83. doi: 10.1007/s00383-011-2944-y. [DOI] [PubMed] [Google Scholar]


