Abstract
Introduction
The association between cervical cancer screening and reduction of cervical cancer has been dealt with in much research. However, little has been published on the association between screening and cervical cancer mortality. We assessed cervical cancer deaths according to screening history, histopathology, and age among women in, under, and above screening age.
Material and methods
In this nationwide, registry‐based case–control study from Norway, we included 817 cervical cancer deaths in women diagnosed with cervical cancer in the period 1998–2009. We matched each case with 10 population‐based controls free from cervical cancer, obtained by density‐based sampling. Odds ratios (ORs) with 95% confidence intervals (CIs) for the association between screening attendance and cervical cancer mortality were estimated using conditional logistic regression models.
Results
Of all fatal cervical cancers, 35% were diagnosed among women over screening age and altogether, 83% were either in age groups not covered by the screening program or in non‐attenders of screening age. The estimated risk reduction associated with a cytology test in the preceding 3.5 years was 80% in screening age 25–69 years (OR 0.20; 95% CI 0.16–0.24) with the largest reduction in squamous cell carcinomas (84%) but also a substantial estimated risk reduction of 65% for adenocarcinomas. The associated risk reduction was strongest in women aged 45–69 years, with ORs in the range 0.09–0.18, compared with ORs 0.42–1.35 in women aged 25–39 years.
Conclusions
To reduce the mortality of cervical cancer, screening programs should focus on increasing adherence to the program, as half of all the fatal cases were in the non‐attender group. Further assessments regarding the potential preventive impact of extending screening to women over the current screening age should be considered.
Keywords: age, case–control study, cervical cancer, mortality, screening
Abbreviations
- AC
adenocarcinoma
- FIGO
International Federation of Gynecology & Obstetrics
- HPV
human papillomavirus
- NCCSP
Norwegian Cervical Cancer Screening Program
- OR
odds ratio
- SCC
squamous cell carcinoma
- Sf
self‐selection bias factor
Key message.
Screening attendance was associated with lower cancer‐specific mortality. Non‐attendance was the largest contributor, but over one‐third of the fatal cancers were diagnosed in women over screening age. Increasing coverage and screening women in their 70s may reduce cervical cancer mortality.
1. INTRODUCTION
The aim of cervical cancer screening programs is to reduce the incidence and mortality of cervical cancer by detecting and treating precursors and early‐stage disease. Organized, population‐based screening programs among Nordic countries have demonstrated a reduction in cervical cancer mortality of up to 80%. 1 To maintain a high‐quality program, the screening process and its effectiveness must be monitored, and regular audits are therefore important. 2
Since 1995, when the Norwegian Cervical Cancer Screening Program (NCCSP) was established as part of the Cancer Registry of Norway, the incidence of cervical cancer has decreased by 25%. 3 The age‐standardized mortality rate of cervical cancer in Norway was 2.4 per 100 000 women‐years in 2015, close to the Nordic average. 4 A study from Finland found that the risk of cervical cancer mortality was doubled among women who did not attend screening. 5 Several case–control studies on effectiveness of cervical screening have suggested a strong age‐dependency. 5 , 6 , 7 Consequently, there may be potential to further increase the effectiveness of cervical screening by adjusting the age range and reducing the rate of non‐attenders.
In this case–control study, we explored the screening histories of women who died from cervical cancer and compared them with controls. We investigated the impact of participation in a cytology‐based national cervical cancer screening program on cervical cancer mortality by cancer morphology and age.
2. MATERIAL AND METHODS
2.1. Data source
The cases of the study comprised 817 women who had been diagnosed with cervical cancer between January 1, 1998 and December 31, 2009 and later died from the disease, according to the Cancer Registry of Norway and the national Cause of Death Register. The databases were nearly 100% complete during the study period, 8 , 9 and no cases were excluded.
Median time from diagnosis to disease‐specific death was 503 days (range 0–5969 days). Follow‐up time for deaths included December 31, 2014.
Each of these cases was matched by year of birth to 10 controls drawn from Statistics Norway (population register) using density‐based sampling. Controls were therefore sampled from a risk set of all women alive of the same age, and free from a cervical cancer diagnosis at the date of the diagnosis of the case, called risk‐set sampling. Fourteen controls were excluded from analysis because of missing data on screening history. There were no further exclusion criteria for the control group. No information on hysterectomy and previous treatment of premalignant cervical lesions was available. As screening attendance during a detectable pre‐clinical phase was the main explanatory variable of interest, sampling was not performed at date of death. We linked data on each individual's screening history, collected from the NCCSP, to the death certificate from the national Cause of Death Register through an 11‐digit personal identification number that is given to all residents of Norway. All Norwegian laboratories, public and private, are obliged to report all cervical cytology regardless of result or indication, and histology to the NCCSP. 3 No distinction of indication is registered. In the study period, the national guidelines recommended cervical cytology screening for all women aged 25–69 years with screening interval every third year. The cytology was classified in accordance with the Bethesda Classification. 10 The staging of cervical cancer was according to FIGO (the International Federation of Gynecology & Obstetrics). 11
All cases were categorized into three groups according to age at diagnosis of cervical cancer: under 25 years (under screening age), 25–69 years (screening age) and over 69 years (above screening age). Age refers to age at diagnosis throughout the manuscript. Women of screening age who had a screening test registered from 6 months and up to 3.5 years before the date of diagnosis of the respective case were defined as program attenders, others were defined as non‐attenders. Tests taken during the 6 months before diagnosis do not prevent cancer, as they identify an existing cancer rather than premalignant lesions. As the data included all tests in the population, we defined the screening test exposure of women under and above screening age to estimate the association with fatal cervical cancer. However, as they were not invited to screening, we do not refer to them as attenders or non‐attenders. Cytology tests were classified as primary tests if no abnormal test results were recorded in the previous 2 years. If more than one cytology was registered, we used information on the last primary cytology.
Screening history included information on primary cervical cytology and its results. The screening history for both cases and their controls with respect to screening attendance and screening test results was obtained using the screening registry records preceding the date of diagnosis of the fatal cervical cancers. From 2005, human papillomavirus (HPV) testing was introduced in delayed triage of low‐grade cytology including atypical squamous cells of undetermined significance and low‐grade squamous intraepithelial lesion 6–12 months after the index test. We categorized cytology test results as normal, unsatisfactory, low‐grade (including persisting HPV infection), or high‐grade disease, including high‐grade squamous intraepithelial lesion, atypical squamous cells—cannot exclude high‐grade squamous intraepithelial lesion, atypical glandular cells, and adenocarcinoma in situ.
We categorized cervical cancer into Stage IA (microinvasive), Stage IB–IIA (localized), or Stage IIB+ (advanced), and the histopathological subtypes into squamous cell carcinoma (SCC), adenocarcinoma (AC), adenosquamous carcinoma, and small‐cell carcinomas representing the four most frequent histological subtypes, and “other tumor”, including other or unknown epithelial morphologies and stromal cancers.
2.2. Statistical analyses
Using conditional logistic regression, we estimated the associations of screening attendance and of result, respectively, with cervical cancer mortality. We also performed separate analyses by histopathology and age and further evaluated the impact on the main odds ratio (OR) estimates of extending the window of exposure to screening from 3 to 5 years.
We assessed the robustness of our results using correction factors for self‐selection bias (Sf) in screening attenders as outlined in Duffy et al. 12 We approximated the self‐selection factor by the risk ratio for fatal cervical cancer in two non‐overlapping age groups: invited, non‐attending women aged 65–69 years, and women aged 70–74 years, who were not invited. We divided the number of cases by the number of women of corresponding age in Norway for the elder group and by the approximate number of non‐attenders for the younger using population size according to Statistics Norway (https://www.ssb.no/en/befolkning) and screening attendance according to Cancer Registry of Norway. We estimated a self‐selection factor of 1.3.
An early Norwegian study, conducted before organized screening, identified a somewhat larger self‐selection factor of 1.6. 13 We therefore calculated corrected ORs (ORcorr) according to self‐selection factors of 1.3 and 1.6, respectively:
The participation rate (p) in the total invited population (25–69 years) has been relatively stable around 69% since the organized screening started. 4 We further calculated a corrected upper and lower confidence interval using the same formula. All analyses were performed using stata 15 (StataCorp).
2.3. Ethics approval
The study was approved by the Regional Committee for Ethics in Medical Research, West Region, Norway (no 983/ date July 1, 2013 and March 12, 2015).
3. RESULTS
In total, 817 cases and 8156 age‐matched controls were included in the study. Of these, 520 cases and 5189 controls were of screening age. The overall distribution of age at diagnosis for the cervical cancers leading to death had two peaks, at around 50 and 75 years, respectively (Figure 1).
FIGURE 1.
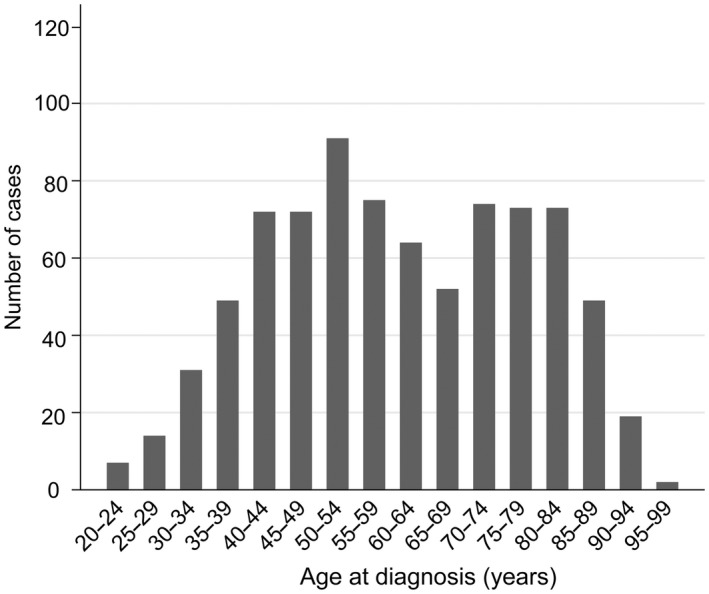
Distribution of all 817 fatal cervical cancers diagnosed in 1998–2009 by age at diagnosis
Mean age at diagnosis was 60 years (standard deviation 17) and ranged from 22 to 99 years (Table 1). Of all cervical cancers leading to death, 64% (520/817) were diagnosed in women of screening age, 1% (7/817) were under the lower screening age of 25 years, and 35% (290/817) constituted screening ages above 69 years. The majority (76%) had advanced stage at the time of diagnosis. Among attenders, 57% were diagnosed with advanced stage, whereas non‐attenders and women over screening age were more often diagnosed with advanced stage, 77% and 85%, respectively. The most frequent histological subtype was SCC (69%), followed by AC (17%), adenosquamous and small‐cell carcinomas each contributing 3%. We observed a higher proportion of AC (29%) among attenders compared with non‐attenders (16%).
TABLE 1.
Characteristics of all 817 women with cervical cancers leading to death according to age, screening attendance, stage at diagnosis and histopathology, Norway 1998–2009
| Overall | Women under screening age | Women over screening age | All women of screening age | Women of screening age not attending screening | Women of screening age attending screening | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean age in years (range) | 60 | (22–99) | 23 | (22–24) | 80 | (70–99) | 50 | (25–69) | 52 | (25–69) | 45 | (26–68) |
| n | % | n | % | n | % | n | % | n | % | n | % | |
| All cases | 817 | 100.0 | 7 | 100.0 | 290 | 100.0 | 520 | 100.0 | 382 | 100.0 | 138 | 100.0 |
| Last cytology 0.5–3.5 years | 173 | 21.2 | 5 | 71.4 | 30 | 10.3 | 138 | 26.5 | ||||
| FIGO stage | ||||||||||||
| IA | 7 | 0.7 | 1 | 14.3 | 0 | 0.0 | 6 | 1.2 | 2 | 0.5 | 4 | 2.9 |
| IB–IIA | 162 | 29.8 | 3 | 42.9 | 30 | 10.3 | 129 | 24.8 | 75 | 19.6 | 54 | 39.1 |
| IIB+ | 621 | 76.0 | 2 | 28.6 | 246 | 84.8 | 373 | 71.7 | 295 | 77.2 | 78 | 56.5 |
| Unknown | 27 | 3.3 | 1 | 14.3 | 14 | 4.8 | 12 | 2.3 | 10 | 2.6 | 2 | 1.4 |
| Morphology | ||||||||||||
| Squamous cell carcinoma | 561 | 68.7 | 3 | 42.9 | 208 | 71.7 | 350 | 67.3 | 270 | 70.7 | 80 | 58.0 |
| Adenocarcinoma | 142 | 17.4 | 2 | 28.6 | 39 | 13.5 | 101 | 19.4 | 61 | 16.0 | 40 | 29.0 |
| Adenosquamous carcinoma | 25 | 3.1 | 0 | 0.0 | 5 | 1.7 | 20 | 3.8 | 14 | 3.7 | 6 | 4.3 |
| Small‐cell carcinoma | 23 | 2.8 | 1 | 14.3 | 3 | 1.0 | 19 | 3.7 | 12 | 3.1 | 7 | 5.1 |
| Other/unknown | 66 | 8.1 | 1 | 14.3 | 35 | 12.1 | 30 | 5.8 | 25 | 6.5 | 5 | 3.6 |
In total, 83% (679/817) of women with fatal cervical cancer were either non‐attenders or in age groups outside the program (Table 1). In screening age, 73% (382/520) of cases had no cervical cytology registered 0.5–3.5 years before date of diagnosis, compared with 90% (260/290) over screening age.
Screening history differed substantially between cases and controls in screening age. Screening attendance was more than twice as high among controls as among cases (Table 2). Among the cases, 20% had a negative cytology taken within the recommended screening interval. A negative cytology was associated with 84% lower risk of cervical cancer death relative to non‐attenders. After correcting for a self‐selection factor of 1.3, this figure was reduced to 76%, and when using 1.6 as the correction factor, the associated risk reduction from a negative cytology test was still as high as 65%.
TABLE 2.
Estimated odds ratios for the association between screening attendance and cervical cancer deaths and according to the result of cytology in the previous 3.5‐year interval among women of screening age (25–69 years) in Norway 1998–2009
| Case | Control | Crude | Corr. 1.3 a | Corr. 1. 6 b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Non‐attenders | 382 | 73.5 | 1858 | 35.7 | Ref | |||||
| Attenders | 138 | 26.5 | 3331 | 64.1 | 0.20 | 0.16–0.24 | 0.30 | 0.24–0.36 | 0.44 | 0.35–0.53 |
| Negative cytology | 105 | 20.2 | 3130 | 60.2 | 0.16 | 0.13–0.20 | 0.24 | 0.20–0.30 | 0.35 | 0.28–0.44 |
| Not representative cytology | 4 | 0.8 | 51 | 1.0 | 0.38 | 0.14–1.07 | 0.57 | 0.21–1.61 | 0.83 | 0.31–2.34 |
| Low‐grade cytology | 18 | 3.5 | 110 | 2.1 | 0.76 | 0.45–1.27 | 1.14 | 0.68–1.91 | 1.66 | 0.99–2.78 |
| High‐grade cytology | 11 | 2.1 | 40 | 0.8 | 1.36 | 0.69–2.67 | 2.04 | 1.04–4.01 | 2.98 | 1.51–6.85 |
Abbreviations: CI; 95% confidence interval; OR; odds ratio.
Corr. 1.3; correction factor for self‐selection bias of 1.3.
Corr. 1.6; correction factor for self‐selection bias of 1.6.
The OR for the association of screening attendance and fatal cervical cancer using a 5‐year screening interval was 0.17 (95% CI 0.14–0.21), with an OR of 0.14 (95% CI 0.11–0.17) after a negative cytology (not tabulated). These results were similar to the estimates using a 3‐year interval (Table 2).
Women attending screening had an 84% lower risk of dying of SCC, but also a substantial risk reduction of 65% for fatal AC (Table 3). When correcting for self‐selection with a factor of 1.3, cervical screening was still associated with a substantially reduced risk of death from cervical cancer for both SCC and AC. We found, however, only weak evidence of a reduction in risk from AC with a selection correction factor of 1.6 (OR 0.77, 95% CI 0.50–1.16).
TABLE 3.
Estimated odds ratios of the association of screening attendance with cervical cancer deaths according to histopathology in women of screening age (25–69 years), n = 5709 (including 520 cases and 5189 controls)
| Crude | Corr. 1.3 a | Corr. 1.6 b | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Squamous carcinoma | ||||||
| Not screened | 1 (reference) | 1 (reference) | 1 (reference) | |||
| Screened | 0.16 | 0.12–0.21 | 0.24 | 0.18–0.32 | 0.35 | 0.26–0.46 |
| Adenocarcinoma | ||||||
| Not screened | 1 (reference) | 1 (reference) | 1 (reference) | |||
| Screened | 0.35 | 0.23–0.53 | 0.53 | 0.35–0.80 | 0.77 | 0.50–1.16 |
| Adenosquamous carcinoma | ||||||
| Not screened | 1 (reference) | 1 (reference) | 1 (reference) | |||
| Screened | 0.27 | 0.10–0.72 | 0.41 | 0.15–1.08 | 0.59 | 0.22–1.58 |
| Small‐cell carcinoma | ||||||
| Not screened | 1 (reference) | 1 (reference) | 1 (reference) | |||
| Screened | 0.34 | 0.13–0.92 | 0.51 | 0.20–1.38 | 0.77 | 0.28–2.02 |
| Other | ||||||
| Not screened | 1 (reference) | 1 (reference) | 1 (reference) | |||
| Screened | 0.12 | 0.05–0.33 | 0.18 | 0.08–0.50 | 0.26 | 0.11–0.72 |
Abbreviations: CI; 95% confidence interval; OR; odds ratio.
Corr. 1.3; correction factor for self‐selection bias of 1.3.
Corr. 1.6; correction factor for self‐selection bias of 1.6.
The OR for fatal cervical cancer according to cervical cytology as a function of age is plotted in Figure 2. We found no indices of a reduction in risk of cervical cancer death after cervical cytology in the last 0.5–3.5 years before diagnosis in women under the age of 30 years. From ages 30 to 75, having a cytology was associated with substantially lower risk of cervical cancer death. The association was strongest in women aged 45–69 years, with ORs in the range 0.09 to 0.18, compared with ORs from 0.42 to 1.35 in women aged 25–39 years. Also, above the screening age of 69 years, we found a tendency of lower risk after cervical cytology in the last 3.5 years.
FIGURE 2.
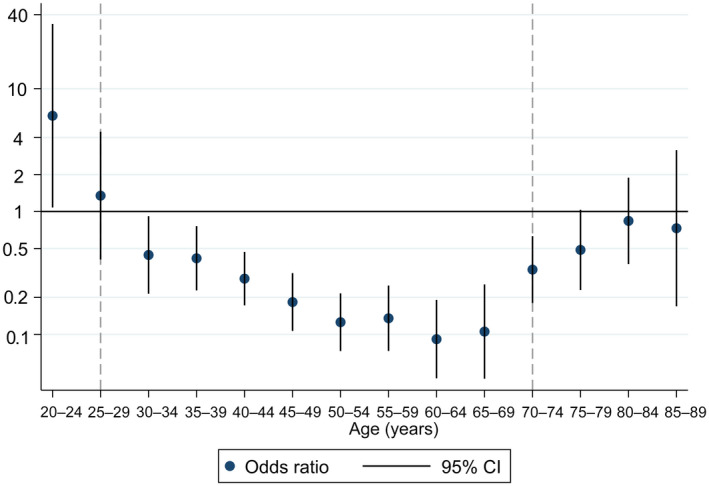
Odds ratio for the association of any cervical cytology registered in the preceding 0.5–3.5 years with risk of fatal cervical cancer according to age at diagnosis
4. DISCUSSION
In this national series of women dying from cervical cancer, 83% were either non‐attenders or in age groups not covered by the screening program. Among women of screening age, attenders had an overall estimated risk reduction of 80%. The association was strongest for SCC, but was also substantial for AC. The risk reduction associated with screening attendance was age dependent with no reduction apparent in women under the age of 30. There was a tendency to a lower mortality in women aged 75 years and older if a cytology was performed even though the evidence was weak.
The few previous studies regarding fatal cervical cancer have also showed lower risk among screening attenders. 5 , 14 , 15 , 16 A UK study estimated a 92% risk reduction comparing regular screening every 5.5 years with no screening, 16 whereas in Finland, attending 5‐year screening was associated with a 66% reduction in cervical cancer mortality in women over 40 years. 5 Similarly, a Canadian study found an age‐dependent protection from mortality of cervical cancer by 40%–72% in women over the age of 30 years when screening was performed 3–36 months before cervical cancer diagnosis, but no association under the age of 30. 14 This age‐dependent risk reduction was demonstrated in our data with an increasing protective association in women aged 30–69 years and may represent a cumulative effect of multiple screening. Although we presented ORs according to the recommended screening interval of 3 years when using cytology as the primary test, results were similar using a 5‐year interval.
A major strength of our study is that the nationwide organized screening program registers all tests, both organized and opportunistic. This provides a complete overview regarding exposure to screening, covering the entire population. The non‐attender group is not diluted with opportunistically screened women in the screening period, except for immigrants who may choose to be screened in their home countries. 17 We should acknowledge that some residual protection is likely from earlier screening among women who were not screened in the past 3.5 years, but were screened before the observation time (as shown by the similar results when considering 3.5 years and 5.5 years).
The choice to attend screening may reflect a lifestyle involving unknown confounders leading to self‐selection bias. 18 The magnitude of this selection bias is unknown. A Danish study found that all‐cause mortality in non‐attenders was 1.5–2 times higher than in attenders, indicating that this is a high‐risk group. 19 A Finnish study compared non‐attenders and not‐invited women at the same age and found a 29% higher risk for incident cervical cancer in those choosing not to respond to an invitation. 7 We estimated the selection bias to be of similar magnitude in our study sample, using non‐overlapping age groups. The non‐invited group was older than the non‐attending group with all the implications that higher age might have on health and cause‐specific mortality. Less aggressive treatment and hence a lower proportion of cured women in the eldest, non‐invited group might lead to an underestimated self‐selection factor. On the other hand, the correction factor regarding self‐selection bias may be overestimated because 41% of women aged 70–74 years had a cervical cytology registered during the last 3.5 years, according to the NCCSP. 3 Using the relative risk of 1.6 to correct for self‐selection bias, screening attendance was still associated with a substantially lower risk of cervical cancer death. 13
Nonetheless, screening coverage and distribution of risk factors in the study group will vary, hence corrections factors are not immediately transferable. The confidence intervals of the corrected intervals should be taken even more cautiously, as we were not able to include the uncertainty of the self‐selection factors in their estimation. To fully explain the observed association between screening attendance and lower risk of death from cervical cancer (i.e. to calculate a corrected OR of 1 using the formula presented in the Material and methods section), one would need the relative risk for death from cervical cancer in non‐attenders compared with non‐invited (i.e. the correction factor) to be as high as 2.23, which is not plausible.
The effect of screening on mortality will likely be overestimated without correcting for self‐selection among women in screening age, but as these women probably represent healthier women, the opposite might be true for women outside screening age. Women who are not invited to the screening program may seek medical examination for several reasons, including symptoms or perceived high risk of cancer, or as follow up after treatment of premalignant lesions earlier in life. Hence, those who have a cytology might constitute a high‐risk group. 20
Causes of death could be misclassified despite quality assurance and almost complete registration, as data recorded may be based on limited information regarding actual and underlying diseases. 9 We can assume this misclassification to be non‐differential because it does not depend on screening exposure or vice versa, it would therefore lead to an underestimation of the observed association. The majority of cervical cancer deaths occur within 5 years of diagnosis, corresponding to the shortest follow‐up time in our study. However, we may have excluded cases with the longest survival, because they may have died after the end of follow up.
No national registry with individual data on hysterectomies was available for this period. The control group might therefore include some hysterectomized women, not at risk of developing cervical cancer. Lifetime risk of hysterectomy is approximately 16% in Norway, 21 hence an underestimation of the impact of attending screening may result.
A US study suggested a potential benefit of including women aged 65–79 years in screening programs. 15 A Canadian simulation study suggested that increasing upper screening age from 69 to 75 might also reduce the risk of cervical cancer among unvaccinated women. 22 The authors also estimated a very low lifetime risk of developing cervical cancer if women tested negative on HPV test at the age of 55. In our data, there may be a cohort effect as elderly women have not been offered screening to the same extent as younger women. The peak in incidence of cervical cancer among elderly women in Nordic countries may therefore represent residuals from unscreened and under‐screened birth cohorts. 23 Other possible explanations may be new transmission of high‐risk HPV in women in their 40s and 50s leading to disease 15 to 20 years later. Biological explanations, including activation of latent HPV infections as the immune system declines with increasing age, may also contribute. 24
We noticed a substantially reduced risk of cervical cancer mortality according to cytology‐based screening attendance for AC. This result was surprising, as screening has proven less effective in preventing AC incidence. 7 , 25 , 26 , 27 At the same time, a UK study concluded that screening led to early diagnosis and down‐staging of AC, which may explain the stronger association of protection from screening on mortality compared with incidence. 28 We found evidence of a reduced risk even when correcting for self‐selection using our estimate of 1.3. When using the larger correction factor of 1.6, the evidence was weaker, in line with data from Finland. 5 Small numbers reduce the statistical power to identify moderate associations in both studies. Since 2015, HPV‐based screening has been gradually introduced in Norway because it provides greater protection against cervical cancer than cytology‐based screening. 29 Hence, a larger reduction in fatal cervical cancers could be expected from screening in the future.
5. CONCLUSION
The majority of cervical cancer deaths occurred among women who had not attended screening, and women over screening age contributed substantially to cervical cancer deaths. In the women of screening age, the effect of screening was age dependent with the lowest effect in the youngest women and a higher and similar protective effect in the mid and upper part of the screening age range. Screening attendance reduced mortality from non‐squamous as well as squamous carcinomas. In addition to efforts to increase coverage in the currently recommended screening, the potential benefit of expanding the screening program to cover women in their 70s should be evaluated further. For this purpose, exploring these older women's full screening history including treatment of premalignant cervical lesions, would be valuable.
AUTHOR CONTRIBUTIONS
SL conceptualized the research objective and collected data. GÅV and IB performed the statistical analyses and visualization. IB and GÅV drafted the manuscript. SL and PRR provided statistical support for the analyses. SL, GÅV, PRR, and IB discussed the results and critically reviewed the manuscript.
FUNDING INFORMATION
The study was supported by the Liaison Committee between the Central Norway Regional Health Authority (RHA) and the Norwegian University of Science and Technology (NTNU). GÅV was funded by the Norwegian Research Council (grant number 250335).
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENTS
The authors thank Gry Baadstrand Skare and Soheil Mashayekhi for their contributions to the data management required for this study at the Cancer Registry of Norway and Nancy Lea Eik‐Nes for providing valuable linguistic advice.
Baasland I, Vie GÅ, Romundstad PR, Lönnberg S. Cervical cancer mortality in Norway according to screening attendance and age. Acta Obstet Gynecol Scand. 2022;101:952‐959. doi: 10.1111/aogs.14402
REFERENCES
- 1. Laara E, Day NE, Hakama M. Trends in mortality from cervical cancer in the Nordic countries: association with organised screening programmes. Lancet. 1987;1:1247‐1249. [DOI] [PubMed] [Google Scholar]
- 2. Arbyn M, Anttila A, Jordan J, et al. European guidelines for quality assurance in cervical cancer screening. second edition‐‐summary document. Ann Oncol. 2010;21:448‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cancer Registry of Norway . Annual report 2016 of The Norwegian Cervical Cancer Screening Programme. Institute of Populationbased Cancer Research; 2018. [Google Scholar]
- 4. NORDCAN . 2018. Accessed January 2, 2019. http://www‐dep.iarc.fr/NORDCAN.htm
- 5. Lonnberg S, Nieminen P, Luostarinen T, Anttila A. Mortality audit of the Finnish cervical cancer screening program. Int J Cancer. 2013;132:2134‐2140. [DOI] [PubMed] [Google Scholar]
- 6. Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case‐control study of prospectively recorded data. BMJ. 2009;339:b2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lonnberg S, Anttila A, Luostarinen T, Nieminen P. Age‐specific effectiveness of the Finnish cervical cancer screening programme. Cancer Epidemiol Biomarkers Prev. 2012;21:1354‐1361. [DOI] [PubMed] [Google Scholar]
- 8. Bilet EF, Langseth H, Thoresen SO, Bray F. Completeness of invasive cervical cancer at the Cancer Registry of Norway. Acta Oncol. 2009;48:1070‐1073. [DOI] [PubMed] [Google Scholar]
- 9. Pedersen AG, Ellingsen CL. Data quality in the causes of death registry. Tidsskr Nor Legeforen. 2015;8:768‐770. [DOI] [PubMed] [Google Scholar]
- 10. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda system: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114‐2119. [DOI] [PubMed] [Google Scholar]
- 11. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103‐104. [DOI] [PubMed] [Google Scholar]
- 12. Duffy SW, Cuzick J, Tabar L, et al. Correcting for non‐compliance bias in case–control studies to evaluate cancer screening programmes. J Royal Stat Soc C. 2002;51:235‐243. [Google Scholar]
- 13. Magnus K, Langmark F, Andersen A. Mass screening for cervical cancer in Ostfold county of Norway 1959‐77. Int J Cancer. 1987;39:311‐316. [DOI] [PubMed] [Google Scholar]
- 14. Vicus D, Sutradhar R, Lu Y, Elit L, Kupets R, Paszat L. The association between cervical cancer screening and mortality from cervical cancer: a population based case‐control study. Gynecol Oncol. 2014;133:167‐171. [DOI] [PubMed] [Google Scholar]
- 15. Rustagi AS, Kamineni A, Weinmann S, Reed SD, Newcomb P, Weiss NS. Cervical screening and cervical cancer death among older women: a population‐based, case‐control study. Am J Epidemiol. 2014;179:1107‐1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Landy R, Pesola F, Castanon A, Sasieni P. Impact of cervical screening on cervical cancer mortality: estimation using stage‐specific results from a nested case‐control study. Br J Cancer. 2016;115:1140‐1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lamkaddem M, Spreeuwenberg PM, Deville WL, Foets MM, Groenewegen PP. Importance of quality aspects of GP care among ethnic minorities: role of cultural attitudes, language and healthcare system of reference. Scand J Public Health. 2012;40:25‐34. [DOI] [PubMed] [Google Scholar]
- 18. Croswell JM, Ransohoff DF, Kramer BS. Principles of cancer screening: lessons from history and study design issues. Sem Oncol. 2010;37:202‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dugue PA, Lynge E, Rebolj M. Mortality of non‐participants in cervical screening: register‐based cohort study. Int J Cancer. 2014;134:2674‐2682. [DOI] [PubMed] [Google Scholar]
- 20. Kalliala I, Anttila A, Pukkala E, Nieminen P. Risk of cervical and other cancers after treatment of cervical intraepithelial neoplasia: retrospective cohort study. BMJ. 2005;331:1183‐1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moen MH. Different practice in hysterectomy. Tidsskrift for den Norske laegeforening: tidsskrift for praktisk medicin, ny raekke. 2004;124:767. [PubMed] [Google Scholar]
- 22. Malagon T, Kulasingam S, Mayrand MH, et al. Age at last screening and remaining lifetime risk of cervical cancer in older, unvaccinated, HPV‐negative women: a modelling study. Lancet Oncol. 2018;19:1569‐1578. [DOI] [PubMed] [Google Scholar]
- 23. Lynge E, Lonnberg S, Tornberg S. Cervical cancer incidence in elderly women‐biology or screening history? Eur J Cancer. 2017;74:82‐88. [DOI] [PubMed] [Google Scholar]
- 24. Gravitt PE. The known unknowns of HPV natural history. J Clin Invest. 2011;121:4593‐4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lonnberg S, Hansen BT, Haldorsen T, Campbell S, Schee K, Nygard M. Cervical cancer prevented by screening: long‐term incidence trends by morphology in Norway. Int J Cancer. 2015;137:1758‐1764. [DOI] [PubMed] [Google Scholar]
- 26. Andrae B, Kemetli L, Sparen P, et al. Screening‐preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J Nat Cancer Inst. 2008;100:622‐629. [DOI] [PubMed] [Google Scholar]
- 27. Sasieni P, Castanon A, Cuzick J. Screening and adenocarcinoma of the cervix. Int J Cancer. 2009;125:525‐529. [DOI] [PubMed] [Google Scholar]
- 28. Castanon A, Landy R, Sasieni PD. Is cervical screening preventing adenocarcinoma and adenosquamous carcinoma of the cervix? Int J Cancer. 2016;139:1040‐1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ronco G, Dillner J, Elfstrom KM, et al. Efficacy of HPV‐based screening for prevention of invasive cervical cancer: follow‐up of four European randomised controlled trials. Lancet. 2014;383:524‐532. [DOI] [PubMed] [Google Scholar]


