Abstract
Introduction
Rates of delivery by cesarean section have gradually risen in many parts of the world, and it is regarded as a safe surgical procedure with expanded indications. We assessed maternal complications within 6 weeks postpartum after planned cesarean section and after planned vaginal delivery among patients without medical indication for cesarean section.
Material and methods
This was a retrospective cohort study based on Swedish national registers and included 714 326 deliveries from 2008 to 2017. The study group consisted of cephalic, singleton, term pregnancies and excluded those with previous cesarean or pregnancy conditions that would qualify for cesarean section. We compared the risks of short‐term complications between planned cesarean section and planned vaginal delivery. We obtained adjusted risk ratios (ARRs) using modified Poisson regression models adjusting for maternal age, parity, body mass index, smoking, country of birth, and county.
Results
The outcomes studied were infections and thromboembolism. In the planned cesarean section group (n = 22 855), 15% had a postpartum infection compared with 10% in the planned vaginal group (n = 691 471) (ARR 1.6; 95% confidence interval [CI] 1.5–1.6), and 0.08% vs 0.05% had a postpartum pulmonary embolism (ARR 1.7; 95% CI 1.0–2.6). The obtained risk estimates corresponded to “number needed to harm” estimates of 17 and 3448, respectively. When dividing the infections into subgroups, the risk of endometritis (ARR 1.2; 95% CI 1.1–1.3), wound infection (ARR 2.7; 95% CI 2.4–3.0), urinary tract infection (ARR 1.5; 95% CI 1.3–1.7), and mastitis (ARR 2.0; 1.9–2.2) was higher after planned cesarean section.
Conclusions
Among patients without medical indication for planned cesarean section, the risks of short‐term maternal complications were higher with planned cesarean section than with planned vaginal delivery.
Keywords: cesarean section, endometritis, instrumental vaginal birth, maternal request, puerperal infection, spontaneous vaginal birth, thromboembolism
Abbreviations
- ARR
adjusted risk ratios
- BMI
body mass index
- CS
cesarean section
Key message.
The risk of infection and thrombosis is increased after planned cesarean compared with planned vaginal delivery among patients without medical indication for planned cesarean section.
1. INTRODUCTION
Rates of birth by cesarean section (CS) have gradually risen in many parts of the world. From 1990 to 2014, the global average CS rate increased three‐fold from 6.7% to 19.1%, with an average rate increase of 4.4% per year. 1 The CS rate varies considerably between different parts of the world and between counties, but the rise is a global phenomenon. 1 In Sweden, the CS rate increased from 5% in 1973 to 18% in 2017. 2 The overall rate of planned CS increased from 4% to 8% between 1991 and 2005, but no major increment was noted between 2005 and 2017. An estimated approximately 26% of the planned CS had no medical indication among primiparous patients. The corresponding percentage among multiparous patients was 33%. 2
Historically, CS was used only in life‐threatening situations. With improved healthcare, CS is regarded as a safe surgical procedure, and the indications for CS have expanded. The potential advantages of CS include decreased risk of prolapse and stress incontinence, 3 avoidance of labor pain, and convenience. However, most studies report increased risks of adverse outcomes associated with CS, such as postpartum infections and venous thromboembolism, 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 which are major causes of maternal death. 6 , 15 Long‐term effects of CS, leading to a higher risk of complications in subsequent pregnancies, include uterine rupture and invasive placenta, with a risk for subsequent need for hysterectomy. 16
Despite an emerging body of literature reporting complications after CS, 4 , 5 , 7 , 8 , 12 , 13 demand for planned CS without medical indication has increased and is termed “CS on maternal request”. A Cochrane report from 2012 described a lack of studies analyzing the risks and benefits of CS on maternal request. 17 Complications after CS vs vaginal delivery are well described in the literature. 7 , 8 , 11 , 13 However, to evaluate the risks and benefits of CS without medical indication, it is more appropriate to compare planned CS and planned vaginal delivery.
The aim of this study was to investigate the rates of short‐term complications (infections and thromboembolism within 6 weeks postpartum) with planned CS compared with those with planned vaginal delivery in a group with no formal medical indication for planned CS.
2. MATERIAL AND METHODS
2.1. Statistical analyses
Data from three Swedish national registries (the medical birth register, 18 the Swedish national patient register 19 and the Swedish prescribed drug registry, 20 all held by the Swedish National Board of Health and Welfare) were merged using the national 12‐digit maternal identification number and date of delivery.
The medical birth register, started in 1973, contains medical information on 99% of all births in Sweden. Standardized record forms are used in all antenatal clinics, all delivery units, and for all pediatric examinations during the child's first month of life. All mothers are offered free antenatal care. Maternal data, including mode of delivery, parity, body mass index (BMI), smoking, maternal obstetric diagnosis, and procedural codes, were collected from the Medical Birth Register. Information regarding BMI is collected at the first prenatal visit, usually in gestational week 8–12.
The Swedish national patient register contains diagnosis codes for all patients admitted to hospitals in Sweden since 1987 as well as all outpatient visits since 2002. Diagnosis codes are classified according to International Classification of Diseases 10th Revision (ICD‐10) codes, since 1997. The codes used to identify the outcomes considered in the current study were postpartum endometritis (O.85, N.71), infection of obstetric surgical wound (O.860, O.861), sepsis (A.40, A.41), urinary tract infection (O.862, N.30, N.39, N.10), mastitis (O.911, O.912), deep phlebothrombosis in the puerperium (O.871), cerebral venous thrombosis (O.873, I.63), pulmonary embolism (O.882, I.74, I.82, I.26), disruption of CS delivery wound (O.900), and disruption of perineal obstetric wound (O.901).
Data on all prescribed and dispensed drugs in Sweden are available in the Swedish prescribed drug registry, from 2005. Information is available regarding the type, amount, price and dispensing date of the drug. We used the date of expedition in combination with the date of delivery to identify prescriptions of antibiotics within 2 or 6 weeks after delivery, respectively. Pharmaceutical consumption is classified according to the anatomic therapeutic chemical classification, with J01 used for antibacterials for systemic use.
To compare maternal outcomes after planned CS and after planned vaginal delivery in a low‐risk population, we excluded all people with a previous CS or any medical indication for planned CS. In Swedish clinical praxis, few medical conditions implicate a clear medical indication for planned CS. These include term breech, two or more previous CSs, placenta previa or other placental abnormalities, or severe maternal or fetal morbidity. In the absence of formal national consensus agreement, we used the medical indications for planned CS suggested in two reports published by Swedish authorities. 2 , 21 We excluded patients with diagnoses/conditions (reported as ICD‐10 codes) that implied a high risk for maternal/fetal morbidity: multiple gestation, none‐cephalic presentation, preterm birth (gestational age <37 weeks), placenta accrete, placenta abruption, placenta previa, diabetes mellitus, gestational diabetes mellitus, preeclampsia, HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets), oligohydramnios, polyhydramnios, chorioamnionitis, small for gestational age (−2 birthweight standard deviation [SD] scores according to a Swedish ultrasound‐based weight curve), large for gestational age (+2 birthweight SD scores according to a Swedish ultrasound‐based fetal weight curve 22 ), macrosomia (>4500 g), and prolonged pregnancy (gestational age ≥42 weeks). To adjust to international clinical practice, we also excluded people with one previous CS, even though one previous CS is not regarded as a medical indication according to Swedish clinical praxis. 21
We performed additional analyses to investigate whether any group, based on maternal characteristics such as age, parity, BMI etc., could benefit from planned CS or planned vaginal delivery. To achieve adequate power for those sensitivity analyses, we created a composite infection variable, including urinary tract infection, endometritis, mastitis, septicemia, wound infection, and need for prescription antibiotics.
We obtained adjusted risk ratios (ARRs) using modified Poisson regression models, adjusting for maternal age (years): <20, 20–34, 35–39, ≥40; parity: 1‐para, 2‐para, ≥3 para; BMI (kg/m2): >18.5, 18.5–24.9, 25–29.9, 30–34.9, ≥35; maternal height (cm): <155, 155–165, 165–174, ≥175; maternal country of birth (Nordic countries/non‐Nordic countries); and healthcare region: Stockholm, Uppsala/Örebro, south‐eastern region, southern region, western region, and northern region. Missing values (applies to smoking, BMI, and maternal height) were replaced by the overall mean. Findings with p‐values below 0.05 were considered statistically significant. Possible confounders with p‐value below 0.2 were included in the multivariable models.
2.2. Ethical approval
Ethical approval was obtained on August 2, 2018, from the committee of ethics at Lund University (Dnr 2018/539).
3. RESULTS
The study included 714 326 births without formal medical indication for planned CS between 2008 and 2017. The selection procedure is summarized in a flowchart (Figure 1).
FIGURE 1.
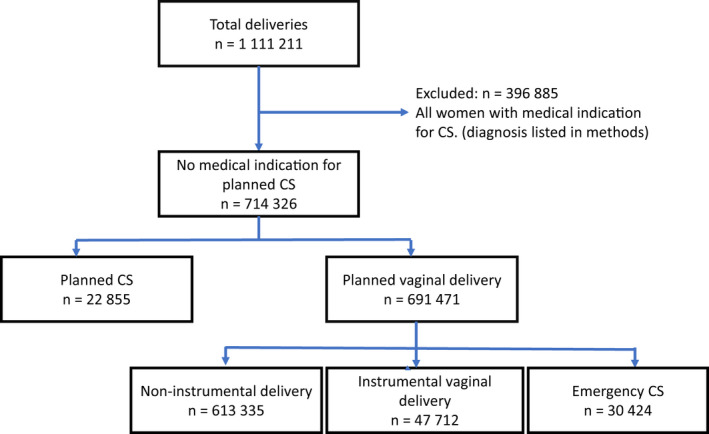
Flow diagram for selection of comparison groups.
Table 1 shows maternal characteristics in relation to planned CS vs planned vaginal delivery. Increasing maternal age, delivery of a second child (2‐para), height <155 cm, and born in a Nordic country were associated with delivery by planned CS.
TABLE 1.
Maternal characteristics among planned cesarean section and planned vaginal delivery among those without any formal medical indication for planned cesarean section
| Planned CS, N = 22 855 | Planned vaginal delivery N = 691 471 | p‐value (χ2) | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Maternal age (years) | <0.001 | ||||
| <20 | 135 | (0.6) | 10 698 | (1.5) | |
| 20–34 | 14 586 | (63.8) | 546 468 | (79.0) | |
| 35–39 | 6296 | (27.5) | 111 850 | (16.2) | |
| ≥40 | 1838 | (8.0) | 22 455 | (3.2) | |
| Parity | <0.001 | ||||
| 1 para | 7468 | (32.7) | 306 629 | (44.3) | |
| 2 para | 13 087 | (57.3) | 261 347 | (37.8) | |
| 3+ para | 2300 | (10.1) | 123 495 | (17.9) | |
| Maternal BMI (kg/m2) | <0.001 | ||||
| <18.5 | 566 | (2.5) | 17 390 | (2.5) | |
| 18.5–24.9 | 12 955 | (56.7) | 403 930 | (58.4) | |
| 25–29.9 | 5440 | (23.8) | 158 008 | (22.9) | |
| 30–34.9 | 1762 | (7.7) | 51 638 | (7.5) | |
| ≥35 | 698 | (3.1) | 19 332 | (2.8) | |
| Not known | 1434 | (6.3) | 41 173 | (6.0) | |
| Maternal smoking | <0.001 | ||||
| Non‐smoking | 21 059 | (92.1) | 630 049 | (91.1) | |
| Smoking | 1104 | (4.8) | 38 779 | (5.6) | |
| Not known | 692 | (3.0) | 22 643 | (3.3) | |
| Maternal height (cm) | <0.001 | ||||
| <155 | 1153 | (5.0) | 21 718 | (3.1) | |
| 155–164 | 8228 | (36.0) | 239 799 | (34.7) | |
| 165–174 | 10 682 | (46.7) | 339 313 | (49.1) | |
| ≥175 | 2030 | (8.9) | 65 307 | (9.4) | |
| Not known | 762 | (3.3) | 25 334 | (3.7) | |
| Maternal country of birth | |||||
| Nordic country | 19 788 | (86.6) | 587 287 | (84.9) | <0.001 |
| Outside Nordics | 3067 | (13.4) | 104 184 | (15.1) | |
| Health care region | <0.001 | ||||
| Stockholm | 8216 | (35.9) | 173 398 | (25.1) | |
| Uppsala/Örebro | 3774 | (16.5) | 131 633 | (19.0) | |
| Southeast | 1870 | (8.2) | 71 495 | (10.3) | |
| South | 3041 | (13.3) | 126 523 | (18.3) | |
| West | 4127 | (18.1) | 131 063 | (19.0) | |
| North | 1827 | (8.0) | 57 359 | (8.3) | |
Abbreviations: BMI, body mass index; CS, cesarian section.
Table 2 shows the risk of short‐term complications. The rate of planned CS was 3.2%. Among those with planned vaginal delivery, 4.3% birthed via emergency CS, 6.7% using vacuum extraction, and 0.1% using forceps. The risks for most of the evaluated outcomes were higher after planned CS than after planned vaginal delivery. For venous thrombosis and stroke, risks were higher with planned CS than with planned vaginal delivery but were not statistically significant. The risk of septicemia did not increase after planned CS. The risk of any postpartum infection was 60% higher after planned CS than after planned vaginal delivery. This was evident in each stratum: age, BMI, smoking, country of birth, and height. Hence, no group, according to the specified maternal characteristics, benefited from planned CS when evaluating the effect of planned delivery mode on maternal postpartum infections.
TABLE 2.
Risk of miscellaneous maternal postpartum complications among those without any formal medical indication for elective cesarean section: Planned cesarean section vs planned vaginal delivery
| Elective CS N = 22 855 | Vaginal trial of labor N = 691 471 | Crude RR | ARR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Per thousand | n | Per thousand | RR | 95% CI | ARR | 95% CI | |||
| Postpartum infections | ||||||||||
| Any a | 3737 | (163.5) | 70 873 | (102.5) | 1.6 | 1.5 | 1.6 | 1.6 | 1.5 | 1.6 |
| Endometritis | 388 | (17.0) | 9974 | (14.4) | 1.2 | 1.1 | 1.3 | 1.2 | 1.1 | 1.3 |
| Wound infection | 355 | (15.5) | 3534 | (5.1) | 3.0 | 2.7 | 3.4 | 2.7 | 2.4 | 3.0 |
| Urinary tract infection | 213 | (9.3) | 4209 | (6.1) | 1.5 | 1.3 | 1.8 | 1.5 | 1.3 | 1.7 |
| Mastitis | 1052 | (46.0) | 14 853 | (21.5) | 2.1 | 2.0 | 2.3 | 2.0 | 1.9 | 2.2 |
| Septicaemia | 7 | (0.3) | 149 | (0.2) | 1.4 | 0.7 | 3.0 | 1.4 | 0.7 | 3.0 |
| Antibiotics 0–14 days | 1758 | (76.9) | 39 680 | (57.4) | 1.3 | 1.3 | 1.4 | 1.3 | 1.3 | 1.4 |
| Antibiotics 0–42 days | 2937 | (128.5) | 62 631 | (90.6) | 1.4 | 1.4 | 1.5 | 1.4 | 1.4 | 1.4 |
| Other complications | ||||||||||
| Pulmonary embolism | 19 | (0.8) | 323 | (0.5) | 1.8 | 1.1 | 2.8 | 1.7 | 1.0 | 2.6 |
| Venous thrombosis | 6 | (0.3) | 133 | (0.2) | 1.4 | 0.6 | 3.1 | 1.3 | 1.3 | 1.4 |
| Cerebral venous thrombosis | 4 | (0.2) | 51 | (0.1) | 2.4 | 0.9 | 6.6 | 1.4 | 1.4 | 1.4 |
Note: ARRs were computed adjusting for maternal age, parity, smoking, body mass index, country of birth, and county.
Abbreviations: ARR, adjusted RR; CI, confidence interval; CS, cesarean section; RR, risk ratio.
Endometritis, wound infection, urinary tract infection, mastitis, septicaemia, prescribed and dispensed antibiotics within 6 weeks.
Table 3 shows the maternal characteristics and final mode of delivery among those with planned vaginal delivery (n = 691 471). Increasing age, delivery of the first child (1‐para), increasing BMI, and height <155 cm were associated with delivery by emergency CS.
TABLE 3.
Maternal characteristics and mode of delivery among women with planned vaginal delivery among women without any formal medical indication for elective cesarean section
| Trial of labor, total | Vaginal‐noninstr | Emergency CS | VE | Forceps | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | n | (%) | n | (%) | n | (%) | n | (%) | |
| Total births | 691 471 | 612 072 | (88.5) | 30 623 | (4.4) | 47 946 | (6.9) | 830 | (0.1) |
| Maternal age (years) | |||||||||
| <20 | 10 698 | 9662 | (90.3) | 316 | (3.0) | 706 | (6.6) | 14 | (0.1) |
| 20–34 | 546 468 | 484 838 | (88.7) | 22 379 | (4.1) | 38 569 | (7.1) | 682 | (0.1) |
| 35–39 | 111 850 | 98 621 | (88.2) | 6000 | (5.4) | 7122 | (6.4) | 107 | (0.1) |
| ≥40 | 22 455 | 18 951 | (84.4) | 1928 | (8.6) | 1549 | (6.9) | 27 | (0.1) |
| Parity | |||||||||
| 1 para | 306 629 | 245 318 | (80.0) | 22 147 | (7.2) | 38 494 | (12.6) | 670 | (0.2) |
| 2 para | 261 347 | 246 890 | (94.5) | 6622 | (2.5) | 7704 | (2.9) | 131 | (0.1) |
| ≥3 para | 123 495 | 119 864 | (97.1) | 1854 | (1.5) | 1748 | (1.4) | 29 | (0.0) |
| Maternal BMI (kg/m2) | |||||||||
| <18.5 | 17 390 | 15 501 | (89.1) | 474 | (2.7) | 1388 | (8.0) | 27 | (0.2) |
| 18.5–24.9 | 403 930 | 359 030 | (88.9) | 15 043 | (3.7) | 29 334 | (7.3) | 523 | (0.1) |
| 25–29.9 | 158 008 | 139 097 | (88.0) | 8307 | (5.3) | 10 452 | (6.6) | 152 | (0.1) |
| 30–34.9 | 51 638 | 45 273 | (87.7) | 3372 | (6.5) | 2946 | (5.7) | 47 | (0.1) |
| ≥35 | 19 332 | 16 705 | (86.4) | 1558 | (8.1) | 1051 | (5.4) | 18 | (0.1) |
| Not known | 41 173 | 36 466 | (88.6) | 1869 | (4.5) | 2775 | (6.7) | 63 | (0.2) |
| Maternal smoking | |||||||||
| Non‐smoking | 630 049 | 557 088 | (88.4) | 27 919 | (4.4) | 44 291 | (7.0) | 751 | (0.1) |
| Smoking | 38 779 | 34 825 | (89.8) | 1664 | (4.3) | 2240 | (5.8) | 50 | (0.1) |
| Not known | 22 643 | 20 159 | (89.0) | 1040 | (4.6) | 1415 | (6.2) | 29 | (0.1) |
| Maternal height (cm) | |||||||||
| <155 | 21 718 | 17 646 | (81.3) | 2135 | (9.8) | 1908 | (8.8) | 29 | (0.1) |
| 155–164 | 239 799 | 207 789 | (86.7) | 13 600 | (5.7) | 18 095 | (7.5) | 315 | (0.1) |
| 165–174 | 339 313 | 304 420 | (89.7) | 12 079 | (3.6) | 22 450 | (6.6) | 364 | (0.1) |
| ≥175 | 65 307 | 59 699 | (91.4) | 1649 | (2.5) | 3873 | (5.9) | 86 | (0.1) |
| Not known | 25 334 | 22 518 | (88.9) | 1160 | (4.6) | 1620 | (6.4) | 36 | (0.1) |
| Maternal country of birth | |||||||||
| Nordic country | 587 287 | 519 932 | (88.5) | 25 627 | (4.4) | 41 017 | (7.0) | 711 | (0.1) |
| Outside Nordics | 104 184 | 92 140 | (88.4) | 4996 | (4.8) | 6929 | (6.7) | 119 | (0.1) |
| Health care region | |||||||||
| Stockholm | 173 398 | 149 718 | (86.3) | 9222 | (5.3) | 14 292 | (8.2) | 166 | (0.1) |
| Uppsala/Örebro | 131 633 | 115 971 | (88.1) | 6100 | (4.6) | 9529 | (7.2) | 33 | (0.0) |
| Southeast | 71 495 | 64 582 | (90.3) | 2269 | (3.2) | 4602 | (6.4) | 42 | (0.1) |
| South | 126 523 | 112 732 | (89.1) | 5082 | (4.0) | 8325 | (6.6) | 384 | (0.3) |
| West | 131 063 | 118 257 | (90.2) | 5684 | (4.3) | 7009 | (5.3) | 113 | (0.1) |
| North | 57 359 | 50 812 | (88.6) | 2266 | (4.0) | 4189 | (7.3) | 92 | (0.2) |
Abbreviations: CS, cesarean section; noninstr, noninstrumental; VE, vacuum extraction.
Tables S1 to S3 (Supporting Information) correspond to Tables 1, 2, 3 but show results from the unselected study group, which included those with and without medical indications or other antenatal conditions for CS (n = 1 111 211). As in the selected group, planned CS was associated with increasing age and height <155 cm. An opposite association was found regarding parity, as giving birth to the first child (1‐para) was associated with planned CS in the unselected group. No association was found regarding Nordic/non‐Nordic country. Regarding BMI, an association was found in the unselected group but showed no correlation in the selected group.
Both groups had risks of short‐term complications but not to the same magnitude as in the unselected group.
4. DISCUSSION
Our study included over 700 000 deliveries among patients without formal medical indications for planned CS and showed a higher risk of short‐term complications after planned CS than after planned vaginal delivery. This low‐risk group had overall encouraging pregnancy outcomes. In trial of labor, almost 90% had a vaginal noninstrumental delivery, and only 4.4% underwent emergency CS. Among those with nonindicated planned CS, over 15% had a postpartum infection, compared with 10% in the planned vaginal group (ARR 1.6), and 0.08% had a postpartum pulmonary embolism (ARR 1.7). The risk estimates correspond to “number needed to harm” estimates of 17 and 3448, respectively. We did not identify any group, based on maternal characteristics (such as age, height, BMI, smoking, and country of birth), that benefited from planned CS rather than planned vaginal delivery in terms of the risk of postpartum infections.
Several studies have compared maternal complications after CS and vaginal birth, 4 , 7 although the complications after planned and emergency CS differ considerably. Other studies have analyzed emergency CS and planned CS separately but have used those with vaginal deliveries as the control group. 5 , 8 Studies that compare complications only after planned CS and noninstrumental deliveries systematically underestimate the complications after vaginal birth since they do not consider complications in the planned vaginal group occurring after emergency CS, forceps, or vacuum extractions. To estimate the true impact of nonindicated planned CS on maternal morbidity, it is important to keep an intention‐to‐treat perspective, making the study of outcomes with planned CS and planned vaginal deliveries appropriate.
No randomized trials have compared maternal complications after planned CS and planned vaginal birth. 17 In cohort studies, Liu et al. (2007), 12 Dahlgren et al. (2009), 23 and Larsson et al. (2011) 24 included patients with planned CS indicated by term breech presentation as a surrogacy for low‐risk CS, and in the comparison group they selected a group who attempted a vaginal birth with fetuses in cephalic presentation. The selection procedures differed between the quoted studies, but the intention was to include healthy patients with no known pregnancy complications. In the current study, we used strict selection criteria for both the planned CS and the planned vaginal group to create two comparable low‐risk groups. We excluded those with breech deliveries, multiple births, diabetes, gestational diabetes, ablatio placentae, placenta accrete, and pre‐eclampsia.
Larsson et al. 24 included only 541 people and did not have sufficient power to detect any association between planned delivery mode and risk of maternal complications. The power of the study by Dahlgren et al. 23 was also low, but they detected a five‐fold risk for wound infection after planned CS compared with after planned vaginal delivery. Our results confirm many of the associations reported by Liu et al., 12 albeit often with lower point estimates of the relative risks. The present study showed an increased risk for mastitis after planned CS compared with after planned vaginal delivery (ARR 2.0), which to our knowledge has not been previously reported. The risk was surprisingly high given that, in Sweden, the newborn is encouraged to breastfeed while still in the operation theater. The higher prevalence of mastitis after planned CS compared with after planned vaginal birth could perhaps, at least partly, be explained by higher registration rates due to longer length of hospitalization in the former group. However, it is unlikely that hospitalization duration is a major source of bias since most cases of mastitis occur after at least 10 days post partum 25 and very few hospital stays are longer than 4 days after planned CS. Another explanation for the increased risk for mastitis in the planned CS group could be the comparatively short time for the hormonal system to adjust for the postpartum period, which could lead to breast‐feeding problems, which in turn could increase the risk for mastitis. A third explanation could be that those who request planned CS are less likely than others to initiate any breast feeding. 26
The current study used a large study population based on high‐quality register data and with a clear intention‐to‐treat perspective. The group of nonindicated CS was created by excluding patients with diagnoses regarded as possible indications for CS. Even though our ambition was to include low‐risk pregnancies only, we cannot exclude the possibility that those choosing planned CS had unrecorded pregnancy complications or other subtle conditions conferring smaller chances of vaginal delivery than in the planned vaginal group. Another topic for discussion is whether pregnancies with macrosomic fetuses should be included in the low‐risk population. It could be argued that fetal size is not estimated before birth and that excluding those pregnancies would result in an advantage for the planned vaginal group. Including them would instead bias the results in the opposite direction. We excluded macrosomic pregnancies as we believe that most obstetric units recognize the importance of identifying high‐risk groups prior to delivery. The scope of the current study was limited to comparing the risk for short‐term maternal complications by planned delivery mode. We did not evaluate complications that could only occur after vaginal delivery such as perineal lacerations and shoulder dystocia. Furthermore, we did not consider maternal long‐term complications, perinatal outcomes such as complications due to fetal asphyxia, neonatal breathing difficulties, or long‐term consequences among children. Another important topic that was not addressed was possible psychological effects. Thus, the results from the current study only bring one piece of information to the complex topic of the advantages and disadvantages of planned CS.
5. CONCLUSION
Patients who request CS without medical indication should be informed that planned CS confers an increased risk of maternal short‐term complications compared with planned vaginal delivery, with elevated risks for mastitis, endometritis, cystitis, and/or need of prescription antibiotics. Long‐term complications, psychological effects, or complications occurring exclusively after vaginal birth (such as perineal ruptures) were not evaluated.
AUTHOR CONTRIBUTIONS
KD, AS, and KK designed and carried out the study and wrote the paper. KK performed the statistical analysis; all authors were present and analyzed the data. All authors approved the final version of the manuscript.
FUNDING INFORMATION
Gorthon Foundation.
CONFLICT OF INTEREST
None.
Supporting information
Table S1
Table S2
Table S3
ACKNOWLEDGMENT
Open access funding enabled and organized by ProjektDEAL.
Dahlquist K, Stuart A, Källén K. Planned cesarean section vs planned vaginal delivery among women without formal medical indication for planned cesarean section: A retrospective cohort study of maternal short‐term complications. Acta Obstet Gynecol Scand. 2022;101:1026‐1032. doi: 10.1111/aogs.14408
REFERENCES
- 1. Betrán AP, Ye J, Moller AB, Zhang J, Gülmezoglu AM, Torloni MR. The increasing trend in caesarean section rates: global, regional and national estimates: 1990‐2014. PLoS One. 2016;11:e0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Källén K. Kejsarsnitt i Sverige 2008–2017. [Cesarean section in Sweden 2008–2017]. 2019. Assessed January 17, 2022. Available at kejsarsnitt i Sverige 2008–2017 (socialstyrelsen.se) or https://www.socialstyrelsen.se/globalassets/sharepoint‐dokument/artikelkatalog/ovrigt/2019‐12‐6529.pdf
- 3. Leijonhufvud A, Lundholm C, Cnattingius S, Granath F, Andolf E, Altman D. Risks of stress urinary incontinence and pelvic organ prolapse surgery in relation to mode of childbirth. Am J Obstet Gynecol. 2011;204(70):e1‐e7. [DOI] [PubMed] [Google Scholar]
- 4. Blondon M, Casini A, Hoppe KK, Boehlen F, Righini M, Smith NL. Risks of venous thromboembolism after cesarean sections: a meta‐analysis. Chest. 2016;150:572‐596. [DOI] [PubMed] [Google Scholar]
- 5. Burrows LJ, Meyn LA, Weber AM. Maternal morbidity associated with vaginal versus cesarean delivery. Obstet Gynecol. 2004;103:907‐912. [DOI] [PubMed] [Google Scholar]
- 6. Clark SL, Belfort MA, Dildy GA, Herbst MA, Meyers JA, Hankins GD. Maternal death in the 21st century: causes, prevention, and relationship to cesarean delivery. Am J Obstet Gynecol 2008;199:36.e1, 36.e5. discussion 91–2. e7‐11. [DOI] [PubMed] [Google Scholar]
- 7. Deneux‐Tharaux C, Carmona E, Bouvier‐Colle MH, Bréart G. Postpartum maternal mortality and cesarean delivery. Obstet Gynecol. 2006;108:541‐548. [DOI] [PubMed] [Google Scholar]
- 8. Hall MH, Bewley S. Maternal mortality and mode of delivery. Lancet. 1999;354:776. [DOI] [PubMed] [Google Scholar]
- 9. Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ III. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30‐year population‐based study. Ann Intern Med. 2005;143:697‐706. [DOI] [PubMed] [Google Scholar]
- 10. Holm C, Langhoff‐Roos J, Petersen KB, Norgaard A, Diness BR. Severe postpartum haemorrhage and mode of delivery: a retrospective cohort study. BJOG. 2012;119:596‐604. [DOI] [PubMed] [Google Scholar]
- 11. Lindqvist P, Dahlbäck B, Marŝál K. Thrombotic risk during pregnancy: a population study. Obstet Gynecol. 1999;94:595‐599. [DOI] [PubMed] [Google Scholar]
- 12. Liu S, Liston RM, Joseph KS, Heaman M, Sauve R, Kramer MS. Maternal mortality and severe morbidity associated with low‐risk planned cesarean delivery versus planned vaginal delivery at term. CMAJ. 2007;176:455‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smaill FM, Gyte GM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev. 2014;2014(10):CD007482. doi: 10.1002/14651858.CD007482.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Virkus RA, Løkkegaard E, Lidegaard Ø, et al. Risk factors for venous thromboembolism in 1.3 million pregnancies: a nationwide prospective cohort. PLoS One. 2014;9:e96495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323‐e333. [DOI] [PubMed] [Google Scholar]
- 16. Keag OE, Norman JE, Stock SJ. Long‐term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: systematic review and meta‐analysis. PLoS Med. 2018;15(1):e1002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lavender T, Hofmeyr GJ, Neilson JP, Kingdon C, Gyte GM. Caesarean section for non‐medical reasons at term. Cochrane Database Syst Rev. 2012;(3):CD004660. doi: 10.1002/14651858.CD004660.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karin Källén BK. The Swedish Medical Birth Register ‐ a summary of content and quality. Socialstyrelsen. 2003. https://www.socialstyrelsen.se/globalassets/sharepoint‐dokument/artikelkatalog/ovrigt/2003‐112‐3_20031123.pdf [Google Scholar]
- 19. 2011–11–22 Vr. Patient Registry . Swedish National Board of Health 2017.
- 20. Swedish registry of prescribed and dispensed drugs National Board of Health, Sweden; 2010. 2010.
- 21. Andolf I. Indikationer för kejsarsnitt på moderns önskan. [Indications for cesarean section at the mother's request.] https://www.sfog.se/media/336234/nationella‐indikationer‐kejsarsnitt‐moderns‐onskan.pdf. 2011.
- 22. Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85:843‐848. [DOI] [PubMed] [Google Scholar]
- 23. Dahlgren LS, von Dadelszen P, Christilaw J, et al. Caesarean section on maternal request: risks and benefits in healthy nulliparous women and their infants. J Obstet Gynaecol Can. 2009;31:808‐817. [DOI] [PubMed] [Google Scholar]
- 24. Larsson C, Saltvedt S, Wiklund I, Andolf E. Planned vaginal delivery versus planned caesarean section: short‐term medical outcome analyzed according to intended mode of delivery. J Obstet Gynaecol Can. 2011;33:796‐802. [DOI] [PubMed] [Google Scholar]
- 25. World Health O . Mastitis: causes and management. World Health Organization; 2000. [Google Scholar]
- 26. Prior E, Santhakumaran S, Gale C, Philipps LH, Modi N, Hyde MJ. Breastfeeding after cesarean delivery: a systematic review and meta‐analysis of world literature. Am J Clin Nutr. 2012;95:1113‐1135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3


