Abstract
Introduction
Identification of low birthweight and small for gestational age is pivotal in clinical management and many research studies, but in low‐income countries, birthweight is often unavailable within 24 h of birth. Newborn weights measured within days after birth and knowledge of the growth patterns in the first week of life can help estimate the weight at birth retrospectively. This study aimed to generate sex‐specific prediction maps and weight reference charts for the retrospective estimation of birthweight for exclusively breastfed newborns in a low‐resource setting.
Material and methods
This was a prospective cohort study nested in a clinical trial of intermittent preventive treatment in pregnancy for malaria with either dihydroartemisinin–piperaquine with/without azithromycin or sulfadoxine–pyrimethamine in Korogwe District, north‐eastern Tanzania (Clinicaltrials.gov: NCT03208179). Newborns were weighed at birth or in the immediate hours after birth and then daily for 1 week. Reference charts, nadir, time to regain weight, and prediction maps were generated using nonlinear mixed‐effects models fitted to the longitudinal data, incorporating interindividual variation as random effects. Predictions and prediction standard deviations were computed using a linear approximation approach.
Results
Between March and December 2019, 513 live newborns with birthweights measured within 24 h of delivery were weighed daily for 1 week. Complete datasets were available from 476 exclusively breastfed newborns. There was a rapid decline in weight shortly after delivery. The average weight loss, time of nadir, and time to regain weight were 4.3% (95% confidence interval [CI] 3.8–4.9) at 27 h (95% CI 24–30) and 105 h (95% CI 91–120) in boys and 4.9% (95% CI 4.2–5.6) at 28 h (95% CI 23–33) and 114 h (95% CI 93–136) in girls, respectively. The data were used to generate prediction maps with 1‐h time intervals and 0.05 kg weight increments showing the predicted birthweights and weight‐for‐age and weight‐change‐for‐age reference charts depicting variation in weight loss from <1 to >10%.
Conclusions
The prediction maps and reference charts can be used by researchers in low‐resource settings to retrospectively estimate birthweights using weights collected up to 168 h after delivery, thereby maximizing data utilization. Clinical practitioners can also use the prediction maps to retrospectively classify newborns as low birthweight or small for gestational age.
Keywords: Newborn, infants, exclusive breastfeeding, growth, weight, weight loss
Abbreviations
- LBW
low birthweight
- SGA
small for gestational age
- LGA
large for gestational age
- IPTp
intermittent preventive treatment of malaria in pregnancy
- CI
confidence interval
- AGA
appropriate for gestational age
Key message.
In low‐income countries, newborns are often weighed for the first time several days after delivery. This jeopardizes the accuracy of birthweight. Growth curves from this study can help researchers and clinicians identify low birthweight or small for gestational age newborns retrospectively.
1. INTRODUCTION
Low birthweight (LBW) and small for gestational age (SGA) contribute considerably to neonatal morbidity and mortality, and timely diagnosis is important. 1 For the same reason, mean birthweights, LBW, and SGA are often used as outcomes measures in research studies evaluating antenatal interventions to improve newborn outcomes. 2 Newborns start to lose weight shortly after delivery, and the rate of this loss depends on the gestational age at delivery, actual birthweight, and breastfeeding patterns. 3 In low‐income countries, many deliveries occur at home or in facilities with limited resources, and newborns are often weighed for the first time several days after delivery. 4 , 5 This may jeopardize the accuracy of the LBW and SGA diagnosis and compromise the infant’s clinical care and the utility of the birthweight measurement in research studies.
To address this, one approach has been to exclude birthweights measured more than 24 or 48 h after delivery, resulting in missing data. 6 , 7 Another approach has been to adjust weights using reference charts by making flat adjustments, for example, birthweight data +2% and +4%, respectively, to correct birthweights taken 24–47 and 48–168 h after delivery. 8 , 9 Furthermore, available reference charts of newborn weights in the first days of life 10 , 11 , 12 are from developed countries, are primarily based on preterm or formula‐fed newborns, and are not applicable in exclusively breastfed term newborns in low‐resource settings.
Previous studies on early life weight changes 13 , 14 reported a wide range of weight loss, with nadirs ranging from 3.8 to 8.6% occurring from the second to fourth day postpartum. 14 A recent review pointed out that some newborns may lose ≥10%. 3 The average time to regain weight is reported as 10–14 days after delivery, 3 , 12 , 15 whereas some newborns may take up to 3 weeks. 12
The delay in birthweight measurement is especially profound in Sub‐Saharan Africa, but studies from this geographical area are sparse. Breastfed and term newborns may exhibit a different weight change pattern than preterm or formula‐fed newborns. 16 Other limitations in previous studies include the exclusion of newborns with excessive weight loss, 17 small sample sizes, 18 and lack of follow‐up after hospital discharge. 19 , 20
This study aimed to generate sex‐specific reference weight charts and prediction maps to estimate the actual birthweight using newborn weights measured up to 168 h after delivery by assessing the daily weight change among an unselected group of exclusively breastfed newborns in a low‐resource setting in Sub‐Saharan Africa.
2. MATERIAL AND METHODS
This prospective cohort study was nested in a multicountry randomized trial of intermittent preventive treatment for malaria in pregnancy (IPTp), investigating the efficacy of IPTp with dihydroartemisinin–piperaquine with/without azithromycin or sulfadoxine–pyrimethamine (IMPROVE trial; clinicaltrials.gov: NCT03208179). Pregnant women were enrolled at antenatal clinics when meeting the following inclusion criteria: HIV negative, singleton pregnancy without congenital malformation, and gestational age from 16 to 28 weeks confirmed by ultrasound, as described previously. 7
For this nested study, newborns from Korogwe District, north‐eastern Tanzania, were screened at delivery from March to December 2019. Eligible newborns with birthweight measured within 24 h of delivery and whose caregivers provided informed consent were enrolled sequentially and followed daily for the first week of life. Participants who relocated outside the study areas—jeopardizing the ability to undertake daily follow‐up visits—and newborns with major congenital malformations were excluded.
Newborns had their first weight measured in the hospital by study nurses or clinicians, and the birthweights and time since delivery were recorded. Field workers made seven daily home visits for the subsequent weight measurements. All weights were measured using digital weighing scales (Seca GmbH & Co. KG., precision 10 g), which were calibrated weekly using standard weights of 0.5, 1, 2, 3, and 5 kg. All measurements were performed in duplicate, and a third measurement was taken if the difference was >50 g. The average of the two closest measurements was used. The infant’s clothes, including diapers, were removed before weighing. Weight was measured just after changing a full diaper and at least 2 h since the last feed. The number of feeds since the previous visit and the type of feeding were documented (exclusive breastfeeding, mixed mainly breastfeeding, mixed mainly formula feeding, and solely formula feeding). Finally, signs and symptoms were documented. SGA was defined using a local reference chart (STOPPAM) 7 as birthweight <10th percentile, large for gestational age (LGA) as birthweight >90th percentile, and appropriate for gestational age (AGA) as 10–90th percentiles.
2.1. Statistical analyses
The data were double entered and validated using Microsoft Access. Statistical analyses were performed using Stata 16 (Stata Corp) and R (4.0.3).
Formula‐fed newborns or those who received mixed mainly formula feeding were excluded from the analysis, as were newborns for whom the time of delivery was missing, more than two of the daily follow‐up visits were missed, or combinations of weights were biologically implausible. A specific daily visit was excluded if the weight or time of measurement was missing or if information about whether the measurement took place after changing the diaper or 2 h after the last feed was missing.
Mother–newborn pair characteristics were described as proportion, mean with standard deviation, or median with interquartile range (IQR). Chi‐squared, Mann–Whitney rank sum, or Student’s t‐tests were used to compare baseline characteristics between included and excluded newborns. A two‐sided p‐value <0.05 was considered statistically significant.
About 20% of the eligible newborns had their weights measured at the time of delivery; the others were measured in the hours just after delivery (Table 1). Rather than predicting the birthweights using only those with an actual birthweight at time 0, we aimed to make full use of all data by using a well‐established model by Jenns and Bayley that described early weight development for each child. 21 Using this model, we could then predict the birthweight from a given weight measurement and its timing relative to the time of delivery. A nonlinear mixed‐effects model was fitted to the longitudinal data, incorporating interindividual variation as random effects. 22 The model assumed neonatal weight loss by initial exponential loss of weight. After reaching nadir, the growth rate tended to become constant, giving a linear weight gain (Appendix S1.1). To predict the birthweight of a newborn given subsequent measurements, the individual’s random effects were initially predicted with approximation by linearizing around the model’s random effects and estimating the conditional distribution of random effects on the estimated fixed effects and data (Appendix S1.2 formula (2)). The weights at time 0 were then predicted by evaluating the model at time 0 given the predicted random effects (the algorithm) (Appendix S1.2 formula (3)).
TABLE 1.
Timing of the first weight measurement
| Time since birth (h) | Interval (h) | Boys | Girls | Total |
|---|---|---|---|---|
| 0 | 0 | 62 | 35 | 97 |
| 1 | >0–2.8 | 136 | 157 | 293 |
| 6 | 3–8.9 | 22 | 19 | 41 |
| 12 | 9–14.9 | 10 | 13 | 23 |
| 18 | 15–20.9 | 5 | 4 | 9 |
| 24 | 21–23.8 | 6 | 7 | 13 |
| Total | 0–23.8 | 241 | 235 | 476 |
Approximate prediction variance was computed through linearization around the model’s fixed effects (Appendix S1.2).
Prediction maps were created with the predicted birthweight based on a subsequent weight measurement and the time of measurement. Predictions and prediction standard deviations were computed using a linear approximation approach (Appendix S1.2). The maps were detailed on 1‐h time intervals and 0.05 kg level for the weight measurements.
Weight‐for‐age and weight‐change‐for‐age reference charts were constructed by simulating new observations from the model over a discretized time interval given the estimated distribution of random effects and computing percentiles for each time step (Appendix S1.4).
The linearized predictions were close to those based on the nonlinear models obtained by simulations.
Further estimated quantities (i.e., time at nadir, percentage weight change at nadir, time to regained weight, and corresponding confidence intervals [CIs]), were obtained using the delta method (Appendix S1.5).
Sensitivity analysis was conducted through the exclusion of specific strata of data. The prediction model was validated with a classic train and test data splits, using 40% of newborns with the measurement at birth (time 0) as testing data (Appendix S1.6).
3. RESULTS
Overall, of 617 newborns screened, 104 (16.9%) were excluded and 513 were followed daily for 1 week, contributing 3 967 observations (Figure 1). In total, 37 newborns (7.2%) (343 observations [8.6%]) did not meet the criteria for and were excluded from the analysis (Figure 1), resulting in a cohort of 476 newborns (3 624 observations), of whom 302, 164, and 10 completed eight, seven, and six visits (including birth), respectively.
FIGURE 1.
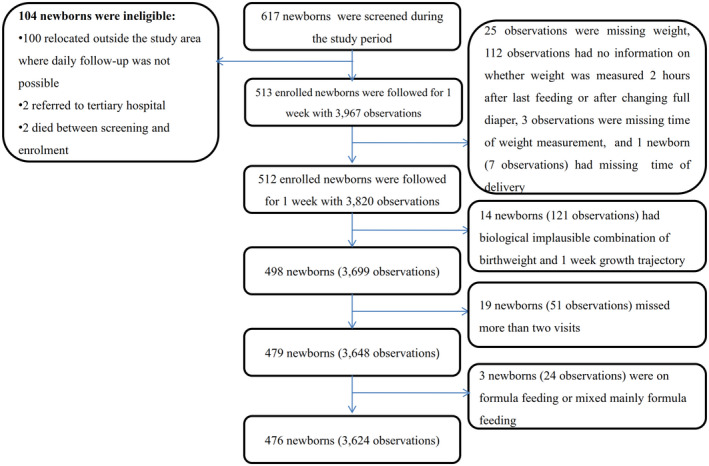
Flow chart of newborns enrolled and weighed daily for the first week of life
Among the 476 newborns, the time of birthweight measurement ranged from 0 to 23.8 h after delivery, with a median of 5 min (IQR 1–68), and 390 (82%) measured within 2.9 h after delivery (Table 1). The mean birthweight was 3.04 (standard deviation 0.44) kg; 16.5% (77/470) were SGA and 8.7% (41/470) LGA (Table 2). The median gestational age at delivery was 279 (IQR 271–297) days, and 6.7% (32/476) were preterm (Table 2). There were no differences in maternal characteristics and pregnancy outcomes between the 476 included and 141 excluded mother–newborn pairs, except for maternal malaria prevalence at enrolment, which was twofold higher among excluded mothers (p < 0.01), and maternal mean hemoglobin level, which was 0.4 g/dl higher among included women (p = 0.01) (Table S1).
TABLE 2.
Descriptions of mother–newborn pairs (n = 476)
| Maternal characteristics | N | n (%)/median (IQR) |
|---|---|---|
| Age (years) | 472 | 26.8 (6.7) a |
| Education level | ||
| None | 472 | 25 (5.3) |
| Primary school | 472 | 314 (66.5) |
| Secondary school | 472 | 119 (25.2) |
| Higher | 472 | 14 (3.0) |
| Source of income | ||
| None | 472 | 88 (14.6) |
| Subsistence farming | 472 | 252 (53.4) |
| Selling items | 472 | 94 (19.9) |
| Wages | 472 | 35 (7.4) |
| Unknown | 472 | 3 (0.6) |
| Paucigravide | 476 | 224 (46.9) |
| Trial arm | ||
| IPTp‐SP | 476 | 170 (35.7) |
| IPTp‐DP | 476 | 306 (64.3) |
| Height (cm) | 474 | 155.4 (151.6–165.8) |
| Weight (kg) | 475 | 57.9 (51.5–83.1) |
| Mid‐upper arm circumference <23 cm | 474 | 29 (6.1) |
| Body mass index (kg/m2) | ||
| <18.5 | 474 | 17 (3.6) |
| 18.5 to <25 | 474 | 264 (55.7) |
| 25 to <30 | 474 | 136 (28.7) |
| ≥30 | 474 | 57 (12.0) |
| Positive malaria rapid diagnostic test at enrolment | 474 | 50 (10.5) |
| Hemoglobin level at enrolment (g/dl) | 474 | 11.3 (1.5) a |
| Delivered by cesarean section | 476 | 46 (9.7) |
| Newborn characteristics | ||
| Male newborn | 476 | 241 (50.6) |
| Gestational age at delivery (days) | 476 | 279 (271–297) |
| Preterm (<37 weeks) | 476 | 32 (6.7) |
| Birthweight (kg) | 476 | 3.04 (0.44) a |
| Time of birthweight measurement since birth (h) | 476 | 0.08 (0.02–1.14) |
| Small for gestational age a | 470 | 77 (16.4) |
| Large for gestational age a | 470 | 41 (8.7) |
| Ever sick b | 476 | 128 (26.9) |
| Type of feeding | ||
| Exclusive breastfeeding | 476 | 471 (98.9) |
| Mixed mainly breastfeeding c | 476 | 5 (1.1) |
| Mixed mainly formula feeding | 476 | 0 (0.0) |
| Solely formula feeding | 476 | 0 (0.0) |
Note: Body mass index: underweight (<18.5), normal (18.5 to <25), overweight (25 to <30), or obesity (≥30).
Abbreviations: IPTp‐SP/‐DP: intermittent preventive treatment with sulfadoxine–pyrimethamine or dihydroartemisinin–piperaquine with/without azithromycin at a ratio of 1:2, respectively; IQR: interquartile range
Mean (standard deviation).
Six newborns had gestational age at delivery beyond the limits of the reference chart.
History of fever or temperature ≥37.5 °C, vomiting, diarrhea or a condition entailing admission to the hospital.
Received one supplementary feeding in a single isolated visit.
3.1. Weight development during the first week of life
The statistical model indicated a decline in weight during the first couple of days of life (Table 3 and Table S2). The weight loss was rapid and reached a nadir of 4.7% (95% CI 4.2–5.1) at 28 h (95% CI 25–31) after delivery (Table 4). The percentage of weight loss was slightly larger among girls than among boys. The nadir was 4.3% (95% CI 3.8–4.9) at 27 h (95% CI 24–30) for boys and 4.9% (95% CI 4.2–5.6) at 28 h (95% CI 23–33) for girls (Table 4). The time to regain birthweight was also shorter for boys than for girls (105 h [95% CI 90–120] vs. 114 h [95% CI 93–136], respectively).
TABLE 3.
Mean percentage weight change per newborn per hour and the percentiles stratified by sex
| Day | Hours | Boys | Girls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean change (50p) | SD | 2.5p | 10p | 90p | 97.5p | Mean change (50p) | SD | 2.5p | 10p | 90p | 97.5p | ||
| 0 | 0 | 100.0 | ‐ | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | ‐ | 100.0 | 100.0 | 100.0 | 100.0 |
| 6 | 97.7 | 1.5 | 93.6 | 95.4 | 99.0 | 99.4 | 97.6 | 2.0 | 92.1 | 94.7 | 99.1 | 99.6 | |
| 12 | 96.4 | 2.4 | 89.9 | 92.8 | 98.5 | 99.2 | 96.4 | 3.0 | 88.2 | 91.8 | 98.8 | 99.5 | |
| 18 | 95.8 | 3.0 | 87.6 | 91.2 | 98.4 | 99.2 | 95.7 | 3.7 | 85.6 | 90.1 | 98.7 | 99.7 | |
| 1 | 24 | 95.5 | 3.5 | 86.2 | 90.3 | 98.5 | 99.5 | 95.4 | 4.2 | 83.9 | 88.9 | 98.8 | 100.0 |
| 30 | 95.5 | 3.8 | 85.3 | 89.8 | 98.7 | 99.9 | 95.3 | 4.6 | 82.6 | 88.2 | 99.1 | 100.4 | |
| 36 | 95.6 | 4.1 | 84.7 | 89.5 | 99.1 | 100.3 | 95.3 | 5.0 | 81.7 | 87.6 | 99.4 | 100.8 | |
| 42 | 95.8 | 4.3 | 84.4 | 89.4 | 99.5 | 100.9 | 95.5 | 5.3 | 81.0 | 87.3 | 99.8 | 101.3 | |
| 2 | 48 | 96.1 | 4.4 | 84.2 | 89.5 | 99.9 | 101.4 | 95.7 | 5.6 | 80.5 | 87.2 | 100.2 | 101.9 |
| 54 | 96.4 | 4.6 | 84.2 | 89.6 | 100.4 | 102.0 | 96.0 | 5.8 | 80.1 | 87.1 | 100.7 | 102.5 | |
| 60 | 96.8 | 4.7 | 84.3 | 89.8 | 100.9 | 102.7 | 96.3 | 6.0 | 79.8 | 87.1 | 101.2 | 103.1 | |
| 66 | 97.2 | 4.8 | 84.5 | 90.1 | 101.5 | 103.3 | 96.7 | 6.2 | 79.7 | 87.2 | 101.7 | 103.7 | |
| 3 | 72 | 97.5 | 4.9 | 84.7 | 90.4 | 102.0 | 104.0 | 97.0 | 6.3 | 79.6 | 87.4 | 102.2 | 104.4 |
| 78 | 97.9 | 5.0 | 85.0 | 90.8 | 102.6 | 104.7 | 97.4 | 6.5 | 79.6 | 87.5 | 102.8 | 105.1 | |
| 84 | 98.3 | 5.1 | 85.3 | 91.1 | 103.1 | 105.4 | 97.8 | 6.6 | 79.6 | 87.7 | 103.3 | 105.8 | |
| 90 | 98.7 | 5.2 | 85.6 | 91.5 | 103.7 | 106.1 | 98.1 | 6.8 | 79.7 | 88.0 | 103.9 | 106.5 | |
| 4 | 96 | 99.2 | 5.3 | 85.9 | 91.8 | 104.3 | 106.8 | 98.5 | 6.9 | 79.8 | 88.3 | 104.5 | 107.2 |
| 102 | 99.6 | 5.4 | 86.3 | 92.2 | 104.9 | 107.6 | 98.9 | 7.0 | 79.9 | 88.6 | 105.0 | 107.9 | |
| 108 | 100.0 | 5.5 | 86.6 | 92.5 | 105.5 | 108.3 | 99.4 | 7.1 | 80.1 | 88.9 | 105.6 | 108.6 | |
| 114 | 100.4 | 5.6 | 87.0 | 92.9 | 106.2 | 109.1 | 99.8 | 7.3 | 80.3 | 89.2 | 106.2 | 109.4 | |
| 5 | 120 | 100.8 | 5.7 | 87.3 | 93.2 | 106.8 | 109.9 | 100.2 | 7.4 | 80.5 | 89.5 | 106.8 | 110.1 |
| 126 | 101.3 | 5.8 | 87.7 | 93.5 | 107.4 | 110.6 | 100.6 | 7.5 | 80.8 | 89.9 | 107.5 | 110.9 | |
| 132 | 101.7 | 5.9 | 88.1 | 93.9 | 108.1 | 111.4 | 101.0 | 7.6 | 81.1 | 90.2 | 108.1 | 111.6 | |
| 138 | 102.2 | 6.0 | 88.4 | 94.2 | 108.7 | 112.2 | 101.4 | 7.7 | 81.3 | 90.6 | 108.7 | 112.4 | |
| 6 | 144 | 102.6 | 6.1 | 88.7 | 94.5 | 109.4 | 113.0 | 101.9 | 7.8 | 81.7 | 90.9 | 109.3 | 113.2 |
| 150 | 103.0 | 6.2 | 89.1 | 94.9 | 110.0 | 113.8 | 102.3 | 7.9 | 81.9 | 91.2 | 110.0 | 113.9 | |
| 156 | 103.5 | 6.3 | 89.4 | 95.2 | 110.7 | 114.6 | 102.7 | 8.1 | 82.2 | 91.6 | 110.6 | 114.7 | |
| 162 | 103.9 | 6.5 | 89.7 | 95.5 | 111.3 | 115.4 | 103.1 | 8.2 | 82.5 | 92.0 | 111.2 | 115.5 | |
| 7 | 168 | 104.3 | 6.6 | 90.0 | 95.8 | 112.0 | 116.2 | 103.6 | 8.3 | 82.8 | 92.3 | 111.9 | 116.3 |
Note: Weight change <100% indicate weight loss. This table represents the statistical model, not the actual measurements. Bold formatting indicates the approximate nadir time and weight regained time.
Abbreviations: SD, standard deviation; p, percentiles.
TABLE 4.
Nadir and time to regained weight
| Group | N | Nadir (%) | 95% CI | Nadir (h) | 95% CI | Regain ~100% (h) | 95% CI |
|---|---|---|---|---|---|---|---|
| All | 476 | 95.3 | 94.9–95.8 | 27.8 | 24.7–30.8 | 111.5 | 98.2–124.8 |
| Male | 241 | 95.7 | 95.1–96.2 | 27.3 | 24.1–30.4 | 105.1 | 90.6–119.6 |
| Female | 235 | 95.1 | 94.4–95.8 | 27.9 | 22.6–33.2 | 114.1 | 92.5–135.7 |
| Term a | 444 | 95.2 | 94.7–95.6 | 26.3 | 23.6–28.9 | 110.0 | 98.0–122.0 |
| AGA a | 352 | 95.0 | 94.5–95.5 | 26.0 | 22.7–29.3 | 112.3 | 98.7–126.0 |
| Healthy b | 348 | 95.5 | 95.1–96.2 | 26.4 | 22.5–30.2 | 109.1 | 90.9–127.4 |
Abbreviations: AGA, appropriate for gestational age; CI, confidence interval.
Excluding preterm.
Excluding small for gestational and large for gestational age.
Excluding ever sick (history of fever or temperature ≥37.5 °C, vomiting, diarrhea, or a condition requiring admission to the hospital).
The models’ estimated response curves for each newborn in the dataset revealed that only 0.9% of boys and 2.4% of girls had gained weight 24 h after their birth, whereas 9.5% of boys and 12% of girls had gained weight 48 h after birth.
Sex‐specific weight‐for‐age and weight‐change‐for‐age reference charts showed considerable variation in weight decline, with some newborns having a percentage weight loss >10% and some <1% (Figures 2 and 3). For example, at 30 h after delivery, the 10th and 90th percentiles for weight loss was 10.2 and 1.3%, respectively, for boys and 11.8 and 0.9% for girls (Table 3 and Figure 3).
FIGURE 2.
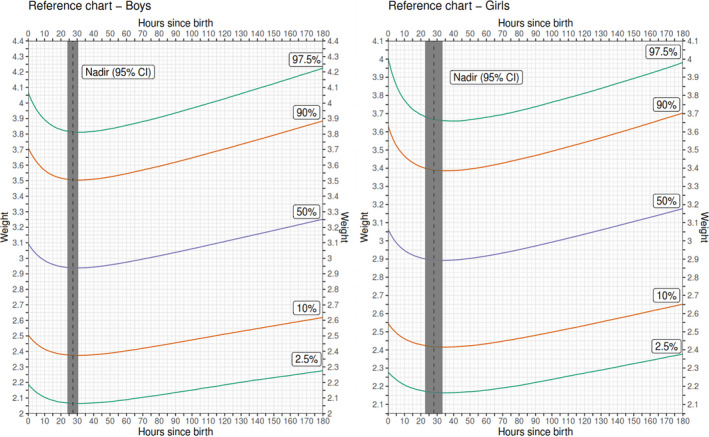
Sex‐specific weight‐for‐age reference charts
FIGURE 3.
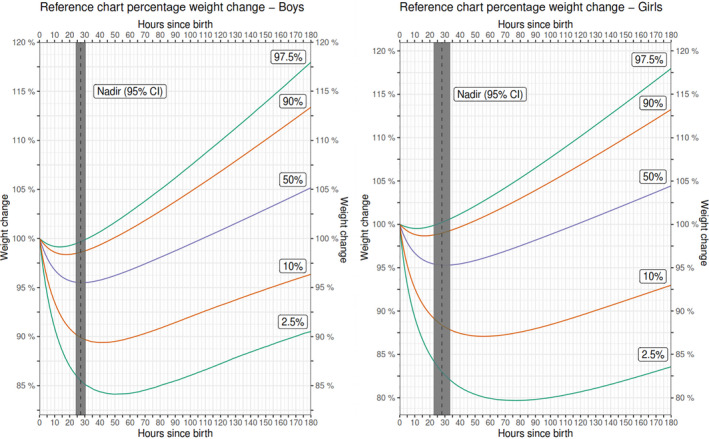
Sex‐specific percentage weight‐change‐for‐age reference charts
The number of newborns and timing of weight measurements in 6 h intervals is shown in Table S3, and the mean weights in 8 h intervals from birth until 180 h after delivery is shown in Tables S4 and S5.
Sensitivity analyses with subgroups of only AGA, only term, or only healthy newborns did not display significant differences in the magnitude and time of nadir and weight regain time (Table 4).
3.2. Prediction of birthweight
We developed a prediction formula for birthweight given the weight measured subsequently and the time since delivery (Appendix S1.2 formula (3)).
We constructed reference charts and predictions maps using the Jenss and Bayley model‐based approach (Appendix S1.2 formula (1–3)). The prediction maps (Figures S1 and S2) based on the weights with 0.05 kg weight increments and the time of measurement (rounded to hours) give an estimated birthweight. The prediction model was validated by splitting the data of those with birthweights measured at the time of delivery into a test and validation dataset and then comparing the predictions with the actual birthweights in the test dataset (Appendix S1.6). A linear correlation between the predicted and measured birthweights was observed, as also reflected in the Bland–Altman plot (Appendix S1.6), indicating the prediction model worked well.
4. DISCUSSION
We generated sex‐specific weight‐for‐age and weight‐change‐for‐age reference charts, an algorithm, and sex‐specific prediction maps that can be used to retrospectively estimate birthweight from weights collected up to 168 h after delivery. These tools provide useful information for clinical practitioners and researchers in similar resource‐poor settings.
We observed rapid weight loss, with a mean nadir of 4.7% occurring just over 1 day after delivery. This is an important finding, as some studies include birthweight measured within 24 or 48 h of delivery that are not adjusted retrospectively for the physiological decline in newborn’s weight. 6 , 7 The use of these delayed birthweights may underestimate mean birthweight and overestimate LBW or SGA. It could also affect a study’s ability to detect differences in mean birthweight or z‐scores for birthweight by gestational age if the timing of measurements is not equally divided between study arms, because the percentage increase in mean birthweight resulting from interventions such as insecticide‐treated nets or IPTp with sulfadoxine–pyrimethamine (+2.5 to 3%) or iron supplementation (1%) is modest. 23 , 24
Our study indicates that a much lower cut‐off than the commonly used 24 h may be more appropriate in these settings when relying on unadjusted birthweights. However, using a 6‐ or 12‐h cut‐off would exclude valuable data, jeopardizing study power. It may also introduce a potential bias as women giving birth at home or in facilities without access to functioning weighing scales may differ from those delivering in better‐equipped facilities. Our reference charts and algorithm address this challenge and enable researchers to estimate birthweight using weights measured up to 168 h after delivery using 1‐h increments instead of using a flat adjustment by day or group of days. 8 , 9 The algorithm contains rather complex statistical mathematics and may not be easily applied in clinical settings. However, the prediction maps with birthweight already estimated serve as an easy tool for clinical practitioners to identify vulnerable SGA newborns, even when delivery occurred several days previously. As an example of using the prediction map (Supporting Information Figure S1), if a boy weighs 2.8 kg (as indicated in the y‐axis) at 36 h after birth as shown in the x‐axis, then his actual birthweight is predicted as 2.94 kg, as indicated in the cell.
Our study revealed a smaller nadir (4.7%) occurring at an earlier time than the previously reported average weight loss of 7–8% and nadir time of 48–72 h after delivery among breastfed newborns in well‐resourced settings. 12 , 17 , 25 Some of the previous studies only included newborns admitted to the hospital, potentially causing selection bias. 19 , 20 Illness may prolong a hospital stay, and inadequate feeding among sick infants 26 may result in more weight loss. Conversely, healthy newborns are discharged early before reaching the nadir, unlike those who are ill. This may account for the lower percentage of weight loss (<4%) reported in other studies that did not follow newborns after discharge compared with ours. 10 , 17 , 27 Ethnic diversity may also partially explain the differences, as previous studies were conducted outside Africa. 3 , 13 , 14
The type of feeding is pivotal for weight trajectories but is not always documented systematically. 3 Formula‐fed newborns tend to lose less weight than breastfed newborns. Breastfed newborns depend on smaller amounts of colostrum during the first 2 days and are thus expected to continue losing weight until day 3, when stage II of lactogenesis usually begins. Previous studies with weight loss <4% did not include full reports on feeding type but included a large proportion of formula‐fed newborns. 3 , 10 , 13 , 14 , 28 However, we observed a nadir as early as day 2, despite our study population being exclusively breastfed. One possible explanation is that our study used a robust methodology, thereby minimizing potential bias compared with previous studies. Also, women in this study received IPTp, which might have improved the health of newborns, and the daily newborn monitoring might have led mothers to optimize breastfeeding, resulting in less weight loss.
Weight trajectories are also affected by maternal factors, including obesity, older age, and cesarean section delivery. 15 , 17 , 20 , 29 Cesarean section, depending on the indication, may delay the establishment of sufficient breastfeeding, resulting in longer weight decline. 17 , 20 , 30 This may partially explain the low percentage of weight loss in our study: our enrolled mothers were younger, less likely to be obese, and <10% required a cesarean section, a lower rate than in other studies. 17 , 19 , 20 , 30 Sex and newborn size also affect weight trajectories. 17 , 19 , 20 However, the effect of newborn size could not be confirmed, as excluding SGA, LGA, and preterm newborns did not significantly alter the nadir. As the sample size among these subgroups was small, it was not possible to perform analyses on only SGA, LGA, or preterm newborns.
The reference chart from this study provides an additional tool for the clinical care of newborns. Using these reference charts, clinical practitioners may be able to assess the newborn’s growth pattern and may consider using the percentage‐weight‐change percentiles instead of a 10% fixed cut‐off 31 for excess weight loss; for example, the percentage weight change below the 10th percentile may be considered excessive and warrant close monitoring of the newborn and/or supportive breastfeeding. From our reference chart, weight loss below the 10th percentiles of the nadir is 10% for boys and 13% for girls. The predictive ability of percentage‐weight‐change percentiles and neonatal morbidity needs to be investigated in future studies.
We also observed a mean time to regain weight of approximately 5 days, which is earlier than in previous studies. 12 , 13 , 15 The mothers in this study may have optimized breastfeeding knowing that newborns were monitored daily, thereby ensuring early regain. Previous studies included only admitted newborns, who are likely to have delayed weight gain especially if they were ill or born by cesarean section. 12 , 17 It is also possible that the time to regain weight may have occurred earlier in previous studies but not been identified because of time gaps in follow‐up. 12 , 28
To our knowledge, this is the first study in a low‐resource African setting with a robust analysis of longitudinal weight data converted into clinically and scientifically applicable tools. Our study has several advantages over previous studies. In addition to the prospective design with a large sample of exclusively breastfed newborns, weight being measured daily, and documentation of the type and number of feedings, we also took all measures to reduce error by weighing newborns in duplicate and using the average and ensuring newborns were weighed after a diaper change and at least 2 h after breastfeeding. Furthermore, gestational age estimated using transabdominal ultrasound ensured accurate classification of newborns as term, preterm, SGA, AGA, or LGA. Previous studies indicated that weight change patterns may vary according to newborn size and gestational age at delivery. 16 , 18
The study has several limitations. First, not all newborns were measured at time 0, but 82% of newborns were measured within 3 h of birth, and our use of the Jenss and Bayley model allowed us to include all data. The most optimal time for birthweight measurement would be at time 0, but this was not possible in our real‐life setting, and designing a study of this type by enforcing structural assumptions might have led to bias compared with real‐life data. Therefore, to get unbiased estimates of growth development, we instead worked with a real‐life sample so were forced to use a model to infer weight development over time. The model prediction performance was satisfactory in the training/testing set up, so we do not think it jeopardized the robustness of the results. Second, the follow‐up was only 1 week, whereas some newborns may not surpass their birthweight until 3 weeks after delivery. 12 Third, the sample size was too small to allow the development of growth curves specific for preterm or SGA or LGA newborns. Fourth, there could be a potential bias if breastfeeding was unconsciously optimized by the mothers because their newborns were included in a weight trajectory study. Finally, there were differences in exposure to malaria and anemia for analyzed and excluded mothers. However, it is unlikely that this could have affected the results significantly, as other baseline characteristics and pregnancy outcomes were similar between the two groups. In addition, the socio‐demographic characteristics of the analyzed cohort are comparable to those reported in community surveys from the study area. 32
5. CONCLUSION
This study provides useful information on weight trajectories among breastfed newborns in a low‐resource African setting. The reference chart, algorithm, and prediction maps can help research studies to maximize the use of birthweight data by adjusting birthweights measured more than 6 h or several days after delivery. The prediction maps can help clinical practitioners accurately classify newborns as LBW or SGA retrospectively in a timely manner. Furthermore, replacing the fixed 10% cut‐off for excessive weight loss with the percentage weight change below the 10th percentile may be considered, allowing a more dynamic evaluation of the newborn over time, but its predictive ability in neonatal morbidity needs further investigation.
CONFLICT OF INTEREST
None.
ETHICAL APPROVAL
All procedures involving human participants were in compliance with the principles of the Declaration of Helsinki (1996) and its later amendments, and in accordance with the Tanzanian National Health Research Ethics Committee`s ethical standards and regulatory requirements. Enrolled participants provided written informed consent. The study procedures were performed in accordance with the Principles of God Clinical Practice. All the preparations and the equipments used were officially certified for the clinical use.
AUTHOR CONTRIBUTIONS
GM, SG, DTRM, JPAL, MA, FOTK, and CS conceived and designed the study. DTRM, JPAL, MM, MA, FOTK, and CS obtained the funding for the study. GM, FK, OA, SG, DTRM, JPAL, and CS conducted and supervised the study. GM, FMA, and TS performed the statistical analysis. GM drafted the manuscript. GM, FMA, and TS had full access to all of the data and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors reviewed the manuscript for important intellectual content. All authors approved the final version for publication.
Supporting information
Figure S1
Figure S2
Tables S1
Table S2
Table S3
Table S4
Table S5
Appendix S1
ACKNOWLEDGMENTS
The authors are grateful to Claud Tesha, Humphrey Mathew, Emmanuel Kessy, Doris Bakari, Walter Maranga, Regina Malugu, Jacqueline Kichungo, and David Ngulizi for the field work; Christian Msokame, Eva Rimoy, and Celina Mzava for data entry; and Mohamed Mapondela for managing the database.
Mtove GP, Abdul O, Kullberg F, et al. Weight change during the first week of life and a new method for retrospective prediction of birthweight among exclusively breastfed newborns. Acta Obstet Gynecol Scand. 2022;101:293–302. doi: 10.1111/aogs.14323
Funding informationThis study was supported by the Joint Global Health Trials Scheme of the UK Medical Research Council (MRC/ Wellcome Trust of Great Britain (WT) and the UK Department of International Development (DFID) and by the European and Developing Countries Clinical Trials Partnership (EDCTP) which is funded by the European Union (EU), European Participating States and third parties including Product Development Partners (PDPs), Private Sector Industry, and International Development Partners. Grant number TRIA‐2015‐1076 IMPROVE.
REFERENCES
- 1. Marchant T, Willey B, Katz J, et al. Neonatal mortality risk associated with preterm birth in East Africa, adjusted by weight for gestational age: individual participant level meta‐analysis. PLoS Med. 2012;9:e1001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Unger HW, Ome‐Kaius M, Wangnapi RA, et al. Sulphadoxine‐pyrimethamine plus azithromycin for the prevention of low birthweight in Papua New Guinea: a randomized controlled trial. BMC Med. 2015;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DiTomasso D, Cloud M. Systematic review of expected weight changes after birth for full‐term, breastfed newborns. J Obstet Gynecol Neonatal Nurs. 2019;48:593‐603. [DOI] [PubMed] [Google Scholar]
- 4. Rijken M, Rijken J, Papageorghiou AT, et al. Malaria in pregnancy: the difficulties in measuring birthweight. BJOG. 2011;118:671‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang J, Merialdi M, Platt LD, Kramer MS. Defining normal and abnormal fetal growth: promises and challenges. Am J Obstet Gynecol. 2010;202:522‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luntamo M, Kulmala T, Cheung YB, Maleta K, Ashorn P. The effect of antenatal monthly sulphadoxine‐pyrimethamine, alone or with azithromycin, on foetal and neonatal growth faltering in Malawi: a randomized controlled trial. Trop Med Int Health. 2013;18:386‐397. [DOI] [PubMed] [Google Scholar]
- 7. Schmiegelow C, Scheike T, Oesterholt M, et al. Development of a fetal weight chart using serial trans‐abdominal ultrasound in an East African population: a longitudinal observational study. PLoS One. 2012;7:e44773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D'Alessandro U, Langerock P, Bennett S, Francis N, Cham K, Greenwood BM. The impact of a national impregnated bed net programme on the outcome of pregnancy in primigravidae in The Gambia. Trans R Soc Trop Med Hyg. 1996;90:487‐492. [DOI] [PubMed] [Google Scholar]
- 9. Greenwood BM, Greenwood AM, Snow RW, Byass P, Bennett S, Hatib‐N'Jie AB. The effects of malaria chemoprophylaxis given by traditional birth attendants on the course and outcome of pregnancy. Trans R Soc Trop Med Hyg. 1989;83:589‐594. [DOI] [PubMed] [Google Scholar]
- 10. Crossland DS, Richmond S, Hudson M, Smith K, Abu‐Harb M. Weight change in the term baby in the first 2 weeks of life. Acta Paediatr. 2008;97:425‐429. [DOI] [PubMed] [Google Scholar]
- 11. Fenton TR. A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr. 2003;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paul IM, Schaefer EW, Miller JR, et al. Weight change nomograms for the first month after birth. Pediatrics. 2016;138:e20162625. [DOI] [PubMed] [Google Scholar]
- 13. Noel‐Weiss J, Courant G, Woodend AK. Physiological weight loss in the breastfed neonate: a systematic review. Open Med. 2008;2:e99‐e110. [PMC free article] [PubMed] [Google Scholar]
- 14. Thulier D. Weighing the facts: a systematic review of expected patterns of weight loss in full‐term, breastfed infants. J Hum Lact. 2016;32:28‐34. [DOI] [PubMed] [Google Scholar]
- 15. DiTomasso D, Paiva AL. Neonatal weight matters: an examination of weight changes in full‐term breastfeeding newborns during the first 2 weeks of life. J Hum Lact. 2018;34:86‐92. [DOI] [PubMed] [Google Scholar]
- 16. Goyal NK, Attanasio LB, Kozhimannil KB. Hospital care and early breastfeeding outcomes among late preterm, early‐term, and term infants. Birth. 2014;41:330‐338. [DOI] [PubMed] [Google Scholar]
- 17. Flaherman VJ, Schaefer EW, Kuzniewicz MW, Li SX, Walsh EM, Paul IM. Early weight loss nomograms for exclusively breastfed newborns. Pediatrics. 2015;135:e16‐e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hossain MA, Islam MN, Shahidullah M, Akhter H. Pattern of change of weight following birth in the early neonatal period. Mymensingh Med J. 2006;15:30‐32. [DOI] [PubMed] [Google Scholar]
- 19. Gallardo López M, Gallardo Cadenasso E, Gallardo CL. Weight decrease in full‐term newborns in the first 48 hours post natal. Rev Chil Pediatr. 2018;89:325‐331. [DOI] [PubMed] [Google Scholar]
- 20. Mezzacappa MA, Ferreira BG. Excessive weight loss in exclusively breastfed full‐term newborns in a Baby‐Friendly Hospital. Rev Paul Pediatr. 2016;34:281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jenss RM, Bayley N. A mathematical method for studying the growth of a child. Human Biology. 1937;9:556. [Google Scholar]
- 22. Vonesh EF, Chinchilli VM. Linear and nonlinear models for the analysis of repeated measurements. In: Dekker M, ed. Statistics, textbooks and monographs. 154th ed. CRC Press; 1997:560. [Google Scholar]
- 23. Gamble C, Ekwaru PJ, Garner P, ter Kuile FO. Insecticide‐treated nets for the prevention of malaria in pregnancy: a systematic review of randomized controlled trials. PLoS Med. 2007;4:e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Radeva‐Petrova D, Kayentao K, ter Kuile FO, Sinclair D, Garner P. Drugs for preventing malaria in pregnant women in endemic areas: any drug regimen versus placebo or no treatment. Cochrane Database Syst Rev. 2014;2014:CD000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thulier D. Challenging expected patterns of weight loss in full‐term breastfeeding neonates born by cesarean. J Obstet Gynecol Neonatal Nurs. 2017;46:18‐28. [DOI] [PubMed] [Google Scholar]
- 26. Fonseca MJ, Severo M, Barros H, Santos AC. Determinants of weight changes during the first 96 hours of life in full‐term newborns. Birth. 2014;41:160‐168. [DOI] [PubMed] [Google Scholar]
- 27. Mulder PJ, Johnson TS, Baker LC. Excessive weight loss in breastfed infants during the postpartum hospitalization. J Obstet Gynecol Neonatal Nurs. 2010;39:15‐26. [DOI] [PubMed] [Google Scholar]
- 28. Macdonald PD, Ross SR, Grant L, Young D. Neonatal weight loss in breast and formula fed infants. Arch Dis Child Fetal Neonatal Ed. 2003;88:F472‐F476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Preusting I, Brumley J, Odibo L, Spatz DL, Louis JM. Obesity as a predictor of delayed lactogenesis II. J Hum Lact. 2017;33:684‐691. [DOI] [PubMed] [Google Scholar]
- 30. Davanzo R, Cannioto Z, Ronfani L, Monasta L, Demarini S. Breastfeeding and neonatal weight loss in healthy term infants. J Hum Lact. 2013;29:45‐53. [DOI] [PubMed] [Google Scholar]
- 31. Bertini G, Breschi R, Dani C. Physiological weight loss chart helps to identify high‐risk infants who need breastfeeding support. Acta Paediatr. 2015;104:1024‐1027. [DOI] [PubMed] [Google Scholar]
- 32. Kamugisha ML, Mmbando BP, Francis F, Ishengoma DS, Challe DP, Lemnge MM. Establishing and implementing Demographic Surveillance System as a tool for monitoring health interventions in Korogwe District, northastern Tanzania. Tanzan J Health Res. 2011;13:57‐67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Tables S1
Table S2
Table S3
Table S4
Table S5
Appendix S1


