Abstract
Introduction
Conflicting reports of increases and decreases in rates of preterm birth (PTB) and stillbirth in the general population during the coronavirus disease 2019 (COVID‐19) pandemic have surfaced. The objective of our study was to conduct a living systematic review and meta‐analyses of studies reporting pregnancy and neonatal outcomes by comparing the pandemic and pre‐pandemic periods.
Material and methods
We searched the PubMed and Embase databases and reference lists of articles published up until November 20, 2021, and included English language studies that compared outcomes between the COVID‐19 pandemic time period with pre‐pandemic time periods. Risk of bias was assessed using the Newcastle‐Ottawa scale. We conducted random‐effects meta‐analysis using the inverse variance method.
Results
Fifty‐two studies with low‐to‐moderate risk of bias, reporting on 2 372 521 pregnancies during the pandemic period and 28 518 300 pregnancies during the pre‐pandemic period, were included. There was significant reduction in unadjusted estimates of PTB (43 studies, unadjusted odds ratio [uaOR] 0.95, 95% CI 0.93–0.98), but not in adjusted estimates (five studies, adjusted OR [aOR] 0.94, 95% CI 0.74–1.19). This reduction was noted in studies from single centers/health areas (29 studies, uaOR 0.90, 95% CI 0.85–0.94) but not in regional/national studies (14 studies, uaOR 0.99, 95% CI 0.99–1.01). There was reduction in spontaneous PTB (nine studies, uaOR 0.91, 95% CI 0.88–0.94) but not in induced PTB (eight studies, uaOR 0.90, 95% CI 0.79–1.01). There was no difference in the odds of stillbirth between the pandemic and pre‐pandemic time periods (32 studies, uaOR 1.07, 95% CI 0.97–1.18 and three studies, aOR 1.18, 95% CI 0.86–1.63). There was an increase in mean birthweight during the pandemic period compared with the pre‐pandemic period (nine studies, mean difference 21 g, 95% CI 13–30 g). The odds of maternal mortality were increased (five studies, uaOR 1.15, 95% CI 1.05–1.26); however, only unadjusted estimates were available, and the result was mostly influenced by one study from Mexico. There was significant publication bias for the outcome of PTB.
Conclusions
The COVID‐19 pandemic may be associated with a reduction in PTB; however, referral bias cannot be excluded. There was no statistically significant difference in stillbirths between pandemic and pre‐pandemic periods.
Keywords: birthweight, epidemic, maternal mortality, neonatal mortality, preterm birth, severe acute respiratory syndrome coronavirus 2, stillbirth, stress
Abbreviations
- ELBW
extremely low birthweight
- LBW
low birthweight
- PTB
preterm birth
- VLBW
very low birthweight
Key message
Preterm births may have reduced during the pandemic, especially spontaneous preterm births, but there was no difference in stillbirths. The reduction in preterm birth was only noted in single‐center studies and in unadjusted estimates, raising the possibility of referral bias. Further studies from countries with high prevalence are needed and this review will be updated periodically.
Update findings
This is update #2 of this living systematic review and meta‐analyses. The search was updated to November 20, 2021. Eight new eligible studies were identified, and their data were incorporated into this new analysis. Two studies, which were included in our previous version, were excluded because of the availability of data from a larger cohort or longer duration of the pandemic, from the same region. The findings in this update are consistent with our previous version: the odds of PTB during the pandemic were significantly reduced in unadjusted estimates and in studies from single centers/single health authorities, but there was no difference in odds of PTB in studies using regional/national data. There was no difference in the odds of stillbirth between the pandemic and pre‐pandemic periods. There is a possibility of publication bias for the outcome of PTB. A post‐hoc additional subgroup analysis based on income of countries was conducted for primary outcomes. This identified a lack of studies from low‐income countries, with only one study reporting on stillbirth. The results for PTB were similar for middle‐ and high‐income countries for both single‐center/single‐health‐authority and regional/national studies. Single‐center studies from middle‐income countries and one regional study from a low‐income country showed higher odds of stillbirth; however, regional/national studies from middle‐income and high‐income countries did not show an association with stillbirth.
1. INTRODUCTION
Most pregnancies end with healthy mothers and healthy children, but a small proportion result in adverse outcomes for the mother, fetus, or neonate. Among others, such outcomes include stillbirth, preterm birth (PTB), neonatal mortality and maternal mortality—all of which can have devastating and long‐lasting effects on families. 1 , 2 , 3 Preterm birth (birth before 37 weeks of gestation) is a major determinant of neonatal mortality and morbidity 4 with long‐term adverse consequences during childhood and adulthood. 5 Medical, social, psychological, environmental, and economic factors have all been implicated in the etiopathogenesis of PTB and other adverse pregnancy outcomes.
The coronavirus disease 2019 (COVID‐19) pandemic has had an unprecedented impact on society worldwide and provided a natural experiment allowing us to study the effects of these factors on adverse pregnancy outcomes. During the early stages of the pandemic, reports emerged describing reduced PTB rates in Denmark 6 and Ireland. 7 However, these were followed by reports of increased PTB rate (births between 28 and 32 weeks of gestation) in Nepal 8 and no changes in PTB rates in the UK 9 and Sweden. 10 At the same time, increases in stillbirth rates were reported from the UK 9 and Nepal, 8 with or without changes in PTB rates, but no change in the stillbirth rate was reported from Ireland. 2
In light of these mixed reports, it is uncertain whether or not the COVID‐19 pandemic has affected pregnancy outcomes at the population level. Inconsistency among conclusions from different studies and a lack of evidence to inform the creation of evidence‐based population health guidance prompted us to undertake a comprehensive review of the influence of the COVID‐19 pandemic on pregnancy outcomes. Our objective was to systematically review and meta‐analyze studies reporting defined local, regional, or national population‐based rates for maternal, fetal, and neonatal outcomes during the pandemic period compared with the pre‐pandemic period.
2. MATERIAL AND METHODS
The review was conducted using standardized methods for systematic reviews of observational studies and reported according to the Preferred Reporting Items in Systematic Reviews and Meta‐analyses guidelines. 11 No ethical approval was obtained because all data used for these analyses were published previously. The review protocol was registered in PROSPERO (CRD42021234036). 12 This is update#2 of this living systematic review; two earlier versions included studies published until May 2021 and August 2021, respectively. 13 , 14
2.1. Data sources: search strategy and selection criteria
We searched PubMed and Embase databases, reference lists of included articles, and personal files for studies published up to November 20, 2021. The search strategy used a combination of the MeSH terms “preterm” or “stillbirth” AND “Covid19” or “SARS‐COV‐2” and included any type of study design published in the English language (Appendix S1). As this is a living systematic review, it will continue to be updated at 3‐monthly intervals for the duration of the pandemic, using the same search strategy. Studies were included if they compared pregnancy outcomes between the COVID‐19 pandemic period and pre‐pandemic time periods and reported on any of the outcomes of interest. We excluded studies that only reported outcomes of pregnant women with COVID‐19 infection. Screening of articles was conducted by two authors (PS and JY) and disagreements were resolved through discussion (JY, RD, and PS) and consensus. As we were interested in overall pregnancy outcomes, we did not restrict studies based on plurality (included singleton and multiple pregnancies).
2.2. Exposure
In most studies, the pandemic period was defined as the period beginning from the date or month of the implementation of emergency lockdown measures in relevant countries or states or cities, or when there was an emergence of cases or a surge of cases in the population studied. Some studies assessed the “post‐lockdown” period, which for the purpose of this review was included as the pandemic period, because the pandemic is ongoing. The pre‐pandemic period was defined either as the period ending immediately before lockdown measures were implemented or before the emergence of the first case or high case numbers in the population, or as a historical period, such as births in the same population in previous year(s). The lengths of these periods varied across studies.
We included studies that reported outcomes of pregnancy in the general population. The review was not designed to evaluate outcomes of pregnancies where only women affected by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection were reported.
2.3. Outcomes
The primary outcomes in this review were rates of PTB and stillbirth. Secondary outcomes included mean birthweight (continuous) and rates of low birthweight, spontaneous PTB, medically indicated PTB, and neonatal, perinatal, or maternal mortality. We contacted authors to obtain data on stillbirth and neonatal mortality when the outcomes were reported as “intrauterine fetal death” and “perinatal mortality.” The outcomes of intrauterine death and perinatal mortality, although specified in our protocol, were ultimately not included in the review (protocol deviation). Outcomes were defined as follows:
Preterm birth: Live births between 22+0 and 36+6 weeks of gestation were classified as PTB. Data on PTB at <28 weeks, <32 weeks, and <34 weeks of gestation were reported separately in some studies and were analyzed independently.
Stillbirth: Death before the complete expulsion or extraction from the parturient of a product of human conception at or after 20 weeks of gestation. 15
Birthweight: Infant weight in grams, measured as soon as possible after live birth. Birthweight <2500 g was defined as low birthweight, birthweight <1500 g was defined as very low birthweight, and birthweight <1000 g was defined as extremely low birthweight.
Spontaneous PTB: Birth of a baby between 22+0 and 36+6 weeks of gestation following spontaneous preterm labor or preterm pre‐labor rupture of membranes. 3
Medically indicated PTB: Preterm birth initiated by a healthcare provider for maternal or fetal indications. 3
Neonatal mortality: Death of a newborn due to any cause before 28 days of age.
Maternal mortality: Death of a woman either during pregnancy or childbirth from any cause related to or aggravated by pregnancy or its management, or within 42 days of end of pregnancy, irrespective of the duration and site of the pregnancy. 9
2.4. Data extraction and risk‐of‐bias assessment
Data from the eligible studies were independently extracted by two authors (JY and PS) using a predefined, standardized extraction form. Disagreements between the authors were resolved by consensus and involving a third author (RD). The information extracted included details of the publication, study setting and size, pre‐pandemic period definition, pandemic period definition, and rates of the reported outcomes in pre‐pandemic and pandemic time periods. We relied only on published information.
We anticipated that primarily observational studies would be included in this review, so we used the Newcastle‐Ottawa Scale 16 for cohort studies to assess risk of bias. This scale assesses risk of bias in domains of selection, comparability, and outcomes, and assigns a maximum score of 9. Studies with scores of 0 to 3 were considered to have high risk of bias, those with scores of 4–6 had moderate risk of bias, and those with scores of 7–9 had low risk of bias.
2.5. Statistical analyses
We planned for meta‐analyses of studies that reported similar outcomes and were methodologically homogeneous. For binary outcomes, we calculated the summary unadjusted odds ratios (uaOR), adjusted OR (aOR) when available and 95% confidence intervals (CI), whereas for birthweight we calculated the mean difference and 95% CI. Statistical heterogeneity was assessed using the Cochran's Q statistic and quantified by calculating the I 2 values. We expected clinical and methodological heterogeneity between studies so planned a priori for random effect meta‐analyses using the inverse variance method. We planned to meta‐analyze adjusted estimates from studies that reported them, understanding that studies will have adjusted for different factors based on data availability and baseline differences. We also expected that the duration of the “pre‐pandemic” period would vary across studies, so we conducted meta‐regression on the variable “duration of the pre‐pandemic period” as a covariate to explain any heterogeneity in the results. Post‐hoc subgroup analyses were conducted for the two primary outcomes after dividing studies into single‐center (or selected hospitals/centers in an area), regional (statewide or province‐wide) or national in scope. Publication bias was assessed qualitatively, using funnel plots, and quantitatively, by calculating Egger's regression intercept when more than 10 studies were included in the meta‐analyses. For the Egger test, values below 0.10 were considered indicative of publication bias. Post‐hoc subgroup analyses were conducted using income level of countries according to the World Bank classification. 17 Meta‐analyses were conducted using STATA v11.0 (StataCorp) and review manager v5.3 (Cochrane Collaboration).
3. RESULTS
3.1. General study characteristics
Of 11 875 records in the initial search, 53 articles were eligible for inclusion, of which 52 were used in the quantitative synthesis 2 , 7 , 8 , 9 , 10 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 (Figure 1). Forty full‐text reports were excluded: reasons for the exclusions are provided in Appendix S2. Three studies 6 , 9 , 10 included in the August 14, 2021 update of this review were excluded from this update because of the availability of new studies from the same area that incorporated the data in expanded data sets, or over longer study periods. 32 , 37 , 62 For one study conducted in the Netherlands by Been et al, 67 data were presented using multiple cut‐offs to define the pre‐ and post‐pandemic periods, with several different comparisons, making it difficult to select one comparison that aligned well with the other studies; we, therefore, included this study in the systematic review but not in meta‐analyses. Study characteristics are reported in Table 1: eight studies were national in scope, 11 were regional, and 33 were local, including single‐center studies; one study did not report the settings. One study included in the previous version of this review 68 was replaced by updated data from a newer study 59 that included data over longer pandemic and pre‐pandemic periods, from the same province of Ontario, Canada. To avoid double counting, Ontario data from another Canadian study 48 were excluded. Across the included studies, totals of 2 372 521 pregnancies during the pandemic period (excluding numbers from Been et al 67 ) and 28 518 300 pregnancies during the pre‐pandemic period were studied. The duration of the “pandemic period” studied varied from 4 weeks to 12 months, and the duration of the “pre‐pandemic period” varied from 2 months to 19 years across studies. The risk‐of‐bias scores for the included studies ranged from 5 to 9 (Table 2). Twenty‐six studies had moderate risk of bias and 27 studies had low risk of bias. Forty‐three studies included pregnant populations from local/regional/national data, which may have included those with COVID‐19, whereas eight studies specifically excluded women with COVID‐19 if it was known. However, it is difficult to be completely certain because testing of pregnant women was not universally applied in any of the studies. One study was from a low‐income country, 17 were from middle‐income countries, and 35 were from high‐income countries.
FIGURE 1.
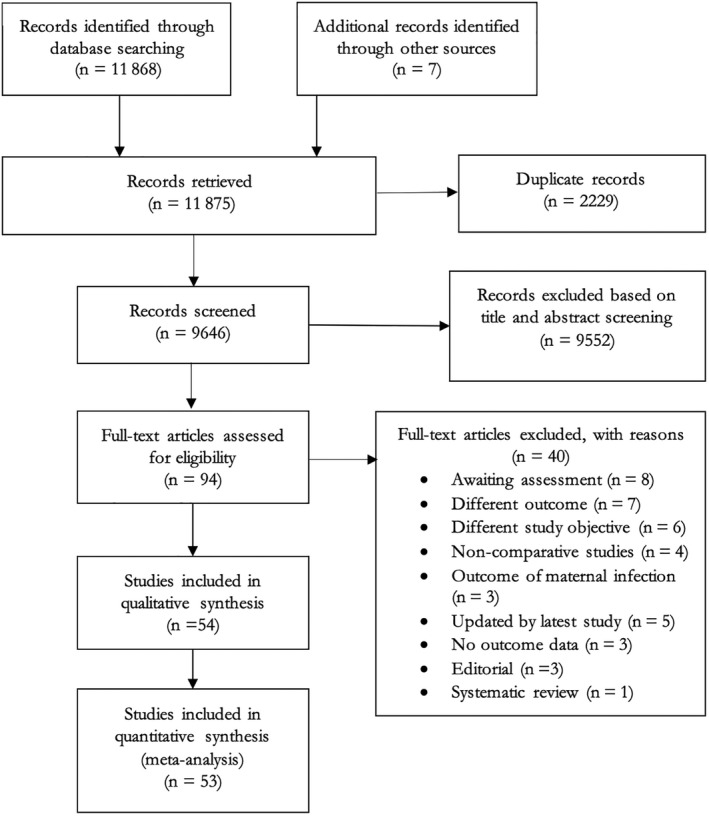
PRISMA flow diagram: article selection
TABLE 1.
Characteristics of included studies (update #2)
| First author, country | Population level | Neonatal | Exposed cohort (pandemic period) | Non‐exposed cohort (pre‐pandemic period) | Outcomes | Statistical approach | Factors adjusted for if any |
|---|---|---|---|---|---|---|---|
|
Arnaez 18 Spain |
13 regional hospitals (Castilla‐y‐León) | Singleton | March 15–May 3, 2020 | March 15–May 3, 2015 −2019 |
PTB <37 weeks; PTB <32 weeks; PTB <28 weeks; Stillbirth; LBW; VLBW; ELBW |
Join‐point regression analysis; Multivariate binomial logistic regression models | Hospital, sex, type of delivery, and multiples |
|
Badran 19 Jordan |
Five main hospitals | Not reported |
April 2020–December 2020 |
May 2019–March 2020 | PTB <37 weeks; PTB <32 weeks; PTB <28 weeks; Stillbirth; LBW; VLB; ELBW; Neonatal mortality | Chi‐squared test; binary logistic regression; multinomial logistic regression | Mother's age, income, education, occupation, nationality, health sector, and multiplicity |
|
Been 67 Netherlands |
Nationwide | Singleton |
1 month, 2 months, 3 months and 4 months after March 9, 2020; 1 month, 2 months, 3 months and 4 months after March 15, 2020; 1 month, 2 months, 3 months and 4 months before March 23, 2020 |
1 month, 2 months, 3 months and 4 months before March 9, 2020; 1 month, 2 months, 3 months and 4 months before March 15, 2020; 1 month, 2 months, 3 months and 4 months before March 23, 2020 |
PTB <37 weeks; PTB <32 weeks | Difference‐in‐regression‐discontinuity analysis | |
|
Berghella 20 USA |
Single center (Thomas Jefferson, Philadelphia) | Singleton | March 1–July 31, 2020 | March 1–July 31, 2019 | PTB <37 weeks; PTB <34 weeks; PTB <28 weeks; Stillbirth; Spontaneous PTB; Medically indicated PTB | Chi‐squared test; multivariable logistic regression | |
|
Bian, 21 China |
Single center (Shanghai First Maternity and Infant Hospital, Shanghai) | Singleton | 2020 | 2014–2019 | PTB <37 weeks; PTB <32 weeks; Stillbirth | Chi‐squared test; Student's t test; logistic regression | Maternal age, prepregnant body mass index, education, insurance status, type of conception, parity, maternal chronic medical conditions, pregnancy complications, gender of fetus |
|
Briozzo 22 Uruguay |
Not reported | Not reported | March 15–September 30, 2020 | March 15–September 30, 2019 | PTB <37 weeks; LBW | Not reported | |
|
Caniglia 23 Botswana |
Nationwide | Singleton | April 3–July 20, 2020 | April 3–July 20, 2017–2019 | PTB <37 weeks; PTB <32 weeks; Stillbirth; Neonatal mortality | Difference‐in‐differences | |
|
Cheung 24 China a |
Regionwide (Shenzhen) | Not reported | February 1–February 29, 2020 | February 1–February 29, 2019 | PTB <37 weeks; PTB <32 weeks; PTB <28 weeks; | Not reported | |
|
Cuestas 25 Argentina |
10 birth centers (Cordoba) | Singleton | August 13–December 31, 2020 | August 13–December 31, 2019 | PTB <37 weeks; PTB <34 weeks; PTB <32 weeks; Stillbirth; Neonatal mortality; Medically indicated PTB <37 weeks; Spontaneous PTB <37 weeks; Medically indicated PTB <34 weeks; Spontaneous PTB <34 weeks; Birthweight | Chi‐squared test; Student’s t test; logistic regression | |
|
De Curtis 26 Italy |
Single center (Lazio hospital) | Singleton | March–May, 2020 | March–May, 2019 |
PTB <37 weeks; PTB <32 weeks; Stillbirth |
Z test | |
|
Dell’Utri 27 Italy |
Single center (Clinica Mangiagalli, Milan) | Not reported | February 23–June 24, 2020 | February 23–June 24, 2019 | Stillbirth | Chi‐squared test | |
|
Du 28 China |
Single center (Beijing) | Singleton | January 20–July 31, 2020 | May 20–November 30, 2019 | PTB <37 weeks; Stillbirth; LBW | Chi‐squared test; t test; Univariate and multivariate log‐binomial regression models | Age, ethnicity, occupation, education, gravidity, parity, h/o miscarriage, h/o induced abortion, BMI, GWG, f/h chronic diseases, prenatal visits |
|
Einarsdóttir 29 Iceland |
Nationwide | Singleton | 2020 | 2016–2019 |
PTB <37 weeks; PTB <32 weeks; Spontaneous PTB; Medically indicated PTB |
Generalized linear mixed models (proc glimmix) with binomial distribution and logit link |
Parity (primipara/multipara), maternal age (continuous), country of origin (Iceland, other), residential area (capital area, outside capital area), cohabitation (yes/no), employment (employed/student/homemaker/disability pension/unemployed), essential hypertension (yes/no), and pre‐existing diabetes mellitus (yes/no) |
|
Gallo 30 Australia |
Single center (Mater Mothers’ Hospital, Brisbane) | Singleton | March 30–May 1, 2020 | March 30–May 1, 2013–2019 | PTB <37 weeks; PTB <32 weeks; PTB <28 weeks |
Analysis of variance (scale); chi‐squared testing (categorical); Logistic regressions |
Maternal age, body mass index, ethnicity, parity, socioeconomic status, and history of or current asthma, diabetes mellitus, and/or hypertensive disorder |
|
Goyal 31 India |
Single center (Jodhpur) | Not reported | April 1–August 30 2020 | October 1 2019–February 29, 2020 | Maternal mortality | Chi‐squared test; Student's t test | |
|
Greenbury 32 UK |
Regionwide | Not reported | April–June, 2020 | December–February, 2012–2019 | PTB <37 weeks; PTB <32 weeks; and PTB <28 weeks | Not reported; data for national birth numbers provided by authors | |
|
Greene 33 USA |
Single center (Cedars‐Sinai, Los Angeles) | Not reported | March–April, 2020 | January–February, 2020 | PTB <37 weeks | Student's t test; Wilcoxon test; chi‐squared test; Fisher's exact test | |
|
Gu 34 China |
Single center (Zhongda Hospital, Jiangsu) | Not reported | January–February, 2020 | January–February, 2019 | PTB <37 weeks; Stillbirth; Birthweight | T test; chi‐squared test | |
|
Handley 35 USA |
2 Penn Medicine hospitals in Philadelphia | Singleton | March–June, 2020 | March–June, 2018–2019 | PTB <37 weeks; Stillbirth; Spontaneous PTB; Medically indicated PTB | Fisher’s exact | |
|
Harvey 36 USA |
Regionwide (Tennessee) | Not reported | March 22–April 30, 2020 | March 22–April 30, 2015–2019 | PTB <37 weeks; PTB <32 weeks; LBW; VLBW | Logistic regression models | Maternal age, education, race/ethnicity, diabetes, and hypertension |
|
Hedley 37 Denmark |
Nationwide | Singleton |
March 12–April 14, 2020 (Strict lockdown) February 27–September 30, 2020 |
March 12–April 14, 2015–2019 February 27–September 30, 2015–2019 |
PTB <37 weeks; PTB <32 weeks; PTB <28 weeks; Stillbirth | Fisher's exact test and proportionality test | |
|
Huseynova 38 Saudi Arabia |
Single health authority (Riyadh) | Singleton | March 1–June 30, 2020 | March 1–June 30, 2017–2019 | PTB <37 weeks; PTB <32 weeks; PTB <28 weeks | One‐sample test for binomial proportion; chi‐squared, Fisher's exact; Poisson regression model | |
|
Janevic 39 USA |
Single center | Not reported | March 28–July 31, 2020 | March 28–July 31, 2019 | PTB <37 weeks; PTB <32 weeks | Log binomial regression | |
|
Justman 40 Israel |
Single center | Not reported | March–April, 2020 | March–April, 2019 | PTB <37 weeks; PTB <32 weeks; Stillbirth; Birthweight | Chi‐squared and t test or Mann‐Whitney U test | |
|
Kassie 41 Ethiopia |
Regionwide | Not reported | March–June, 2020 | March–June, 2019 | Stillbirth; Neonatal mortality | t test | |
|
Kasuga 42 Japan |
Single center | Not reported | April 1–June 30, 2020 | April 1–June 30, 2017–2019 | PTB <37 weeks | Not reported | |
|
KC 8 Nepal |
Nine hospitals across seven provinces | Not reported | March 21–May 30, 2020 | January 1–March 20, 2020 | PTB <37 weeks; Stillbirth; LBW; Neonatal mortality | Generalized linear model with Poisson regression; Pearson's chi‐squared | Ethnicity, maternal age, and complication during admission |
|
Kirchengast 43 Austria |
Single center | Singleton | March to July, 2020 | March to July, 2005–2019 | PTB <37 weeks; PTB <32 weeks; LBW; VLBW; ELBW | t test; chi‐squared test; linear regression | |
|
Kumar 44 India |
Lady Hardinge (New Delhi) | Not reported | March to September, 2020 | March to September, 2019 | Stillbirth; LBW; ELBW; VLBW | Fisher’s exact test | |
|
Kumari 45 India |
4 regional hospitals in Western India | Not reported | March 25–June 2, 2020 | January 15–March 24, 2020 | Stillbirth; Maternal mortality | Not reported | |
|
Lemon 46 USA |
Single center | Singleton | April 1–October 27, 2020 | January 1, 2018–January 31, 2020 | PTB <37 weeks; PTB <34 weeks; PTB <28 weeks; Spontaneous PTB; Medically indicated PTB | Pearson chi‐squared or t tests | |
|
Li 47 China |
Single center | Not reported | January 23–March 24, 2020 | January 1, 2019–January 22, 2020 | PTB <37 weeks; Birthweight | Chi‐squared, t test and Fishers exact | |
|
Liu 48 Canada |
Nationwide | Singleton | March–August, 2020 | March–August, 2015–2019 | PTB <37 weeks; PTB <34 weeks; PTB <32 weeks; PTB <28 weeks; Stillbirth | Not reported | |
|
Llorca 49 Spain |
Single center | Not reported | May 26–October 22, 2020 | January 1–August 31, 2018 | PTB <37 weeks; PTB <34 weeks; LBW | Goodman–Kruskal gamma test; chi‐squared test; logistic regression | Age at delivery, educational level, and occupational status |
|
Lumbreras‐Marquez 50 Mexico |
Nationwide | Not reported | January 1–August 9, 2020 | 2011–2019 | Maternal mortality | Not reported | |
|
Main 51 USA |
Statewide | Singleton | April–July, 2020 | April–July, 2016–2019 | PTB <37 weeks; PTB <32 weeks; PTB <28 weeks | Logistic regression | |
|
Matheson 52 Australia |
3 regional hospitals | Singleton and multiple pregnancies | July–September, 2019 | July–September, 2020 | PTB <37 weeks; PTB <34 weeks; PTB <28 weeks; Stillbirth; Spontaneous PTB; Medically indicated PTB | Interrupted time‐series analysis; auto‐regressive integrated moving average (ARIMA) model | |
|
McDonnell 7 Ireland |
Single center | Not reported | January–July, 2020 | January–July, 2018–2019 | PTB <37 weeks; Stillbirth | Pearson correlation; chi‐squared, Fishers exact test | |
|
Meyer 1 53 Israel |
Single center | Singleton | March 20–June 27, 2020 | March 20–June 27, 2011–2019 | PTB <37weeks; PTB <34 weeks; PTB <32 weeks; Stillbirth; Birthweight; Neonatal mortality | Multivariate regression | |
|
Meyer 2 54 Israel |
Single center | Not reported | February–March, 2020 | February–March, 2019 | PTB <37 weeks; PTB <34 weeks; Birthweight | Chi‐squared; Fisher's exact test; Mann‐Whitney U test | |
|
Mikus 55 Croatia |
Single center (Zagreb) | Singleton | February 25–December 31, 2020 | February 25–December 31, 2019 | PTB <37 weeks; PTB <34 weeks; PTB <32 weeks; PTB <28 weeks; Stillbirth; Birthweight | Not reported | |
|
Mor 56 Israel |
Single center | Singleton | February 21–April 30, 2020 | February 21–April 30, 2017–2019 | PTB <37 weeks; PTB <34 weeks; PTB <28 weeks; Stillbirth; Birthweight | Chi‐squared test or Fisher's exact test | |
|
Philip 2 Ireland |
Regionwide | Not reported |
January–April, 2020; and March–June, 2020 |
January–April of 2001–2019; and March–June 2016–2019 |
Stillbirth; LBW; ELBW; VLBW | Poisson regression | |
|
Ranjbar 58 Iran |
Single center (Tehran) | Singleton | February 19–April 19, 2020 | February 19–April 19, 2019 | PTB <37 weeks; Stillbirth; LBW | Chi‐squared test; independent samples t test; | |
|
Rolnik 57 Australia |
Single center (Melbourne) | Singleton/ multiple pregnancies | November 1, 2019–February 29, 2020 | November 1, 2018–February 28, 2019 | PTB <37 weeks; PTB <34 weeks; PTB <28 weeks; Stillbirth; Spontaneous PTB; Medically indicated PTB; Maternal mortality, LBW; VLBW; Neonatal mortality | Independent‐samples t test; Wilcoxon rank‐sum test; chi‐squared test; Fisher's exact test; generalized linear models with Poisson n family, log link function and robust variance estimation | |
|
Shah 59 Canada |
Regionwide | All births | January 1–December 31, 2020 | July 1, 2002–December 31, 2019 | PTB <37 weeks; PTB <32 weeks; PTB <28 weeks; Stillbirth | Laney control P′ charts; the interrupted time‐series analysis; | |
|
Shakespeare 60 Zimbabwe |
Single center | Not reported | April–June, 2020 | January–March, 2020 | Stillbirth; Neonatal mortality; Maternal mortality | Not reported | |
|
Son 61 USA |
One‐third of the hospitals in USA using Epic | Not reported | March 1–December 31, 2020 | March 1–December 31, 2017–2019 | PTB <37 weeks; Stillbirth | Chi‐squared test; mixed‐effects logistic regression | |
|
Stephansson 62 Sweden |
Nationwide | Singleton | March, 2020–January, 2021 | March, 2015–January, 2020 | PTB <37 weeks; Stillbirth; Spontaneous PTB <37 weeks; Medically indicated PTB <37 weeks | Exact logistic regression; sensitivity analysis | |
|
Stowe 63 UK |
Nationwide | Not reported | April–June, 2020 | April–June, 2019 | Stillbirth | Fisher’s exact test | |
|
Sun 64 Brazil |
Single center | March 11–June 11, 2020 | March 11–June 11, 2019 | PTB <37weeks; LBW | Not reported | ||
|
Wang 65 China |
Single center | Singleton | January 24–March 31, 2020 | January 24–March 31, 2019 | PTB <37 weeks; LBW; Birthweight; | Independent sample t test; Chi‐squared test | |
|
Wood 66 USA |
4 level 3 or 4 neonatal intensive care units |
Singleton | April–July, 2020 | April–July, 2019 | PTB <37 weeks; PTB <34 weeks; PTB <32 weeks; PTB <28 weeks; Spontaneous PTB | Not reported |
Abbreviations: BMI, body mass index; GWG, gestational weight gain; ELBW, extremely low birthweight; f/h, family history of; h/o, history of; LBW, low birthweight; PTB, preterm birth, VLBW, very low birthweight.
Data only from Shenzen, China were included from this study as other data were included in Liu et al from the whole of Canada.
TABLE 2.
Risk of bias assessment using the Newcastle‐Ottawa scale (update #2)
| First author | Selection | Comparability | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non‐exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow up long enough for outcomes to occur? | Adequacy of follow up of cohorts | Total score | |
| Arnaez 18 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Badran 19 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
| Been 67 | ☆ | ☆ | ☆ | ☆ | ☆ | 5 | |||
| Berghella 20 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Bian 21 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 | |
| Briozzo 22 | ☆ | ☆ | ☆ | ☆ | ☆ | 5 | |||
| Caniglia 23 | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 7 | ||
| Cheung 24 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Cuestas 25 | ☆ | ☆ | ☆ | ☆ | ☆ | 5 | |||
| De Curtis 26 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| Dell’Utri 27 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| Du 28 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 | |
| Einarsdóttir 29 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
| Gallo 30 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Goyal 31 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| Greenbury 32 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
| Greene 33 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Gu 34 | ☆ | ☆ | ☆ | ☆ | ☆ | 5 | |||
| Handley 35 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Harvey 36 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
| Hedley 37 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
| Huseynova 38 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| Janevic 39 | ☆ | ☆ | ☆ | ☆ | ☆ | 5 | |||
| Justman 40 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| Kassie 41 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Kasuga 42 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| KC 8 | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 7 | ||
| Kirchengast 43 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Kumar 44 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| Kumari 45 | ☆ | ☆ | ☆☆ | ☆ | ☆ | 6 | |||
| Lemon 46 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Liu 48 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
| Li 47 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| Llorca 49 | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 7 | ||
| Lumbreras‐Marquez 50 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| Main 51 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Matheson 52 | ☆ | ☆ | ☆ | ☆ | ☆ | 5 | |||
| McDonnell 7 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| Meyer 1 53 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| Meyer 2 54 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| Mikus 55 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| Mor 56 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| Philip 2 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Ranjbar 58 | ☆ | ☆ | ☆ | ☆ | ☆ | 5 | |||
| Rolnik 57 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| Shakespeare 60 | ☆ | ☆ | ☆ | ☆ | ☆ | 5 | |||
| Shah 59 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Son 61 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Stephansson 62 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
| Stowe 63 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 |
| Sun 64 | ☆ | ☆ | ☆ | ☆ | ☆ | 5 | |||
| Wang 65 | ☆ | ☆ | ☆ | ☆ | ☆ | 5 | |||
| Wood 66 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
A study can be awarded a maximum of one star for each item within the Selection and Outcome categories. A maximum of two stars can be given for comparability.
3.2. Synthesis: outcomes
3.2.1. Preterm birth and its subgroups
Forty‐three studies including 986 466 women during the pandemic period and 8 716 000 women in the pre‐pandemic period reported PTB before 37 weeks of gestation; there was a reduction in the unadjusted odds of PTB during the pandemic period compared with the pre‐pandemic period (pooled uaOR 0.95, 95% CI 0.93–0.98, I 2 = 71%; Figure 2). Subgroup analyses revealed no differences in odds of PTB during the pandemic period in national or regional studies (pooled uaOR 0.99, 95% CI 0.97–1.01, I 2 = 75%); however, there was a reduction in odds of PTB in studies from single centers/single health authorities (pooled uaOR 0.90, 95% CI 0.85–0.94, I 2 = 55%, subgroup differences p = 0.0005; Figure 2). Five of the studies examining PTB reported adjusted estimates (with different factors adjusted, reported in Table 1) and pooled analyses did not show any significant differences in the odds of PTB during the pandemic, although the magnitude of the adjusted pooled estimate was similar to the unadjusted pooled estimate (pooled aOR 0.94, 95% CI 0.74–1.19; I 2 = 93%; Figure 3). The results of PTB stratified by country's income group are reported in Appendix S3 for studies from single centers/single health authorities (uaOR 0.90, 95% CI 0.85–0.94, I 2 = 0%) and Appendix S4 for regional/national studies (uaOR 0.99, 95% CI 0.97–1.01, I 2 = 75%). There was a reduction in the unadjusted odds of PTB before 34 weeks of gestation (Table 3, Appendix S5), but not before 32 weeks (Table 3, Appendix S6), or 28 weeks (Table 3, Appendix S7) of gestation. Meta‐analysis of nine studies reporting data on spontaneous PTB (Table 3, Appendix S8) revealed reductions in unadjusted odds of PTB during the pandemic period but not in eight studies of induced PTB (Table 3, Appendix S9).
FIGURE 2.

Forest plot for odds of preterm birth <37 weeks’ gestation in pandemic vs pre‐pandemic periods. CI, confidence interval; IV, inverse variance[Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
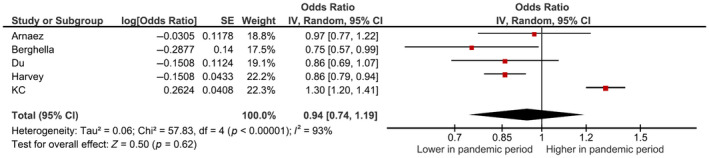
Forest plot for adjusted odds of PTB <37 weeks’ gestation in pandemic vs. pre‐pandemic periods. CI, confidence interval; IV, inverse variance
TABLE 3.
Results of studies reporting other outcomes (update #2)
| Outcome | Number of studies | Pandemic period (n/N) | Pre‐pandemic period (n/N) | OR (95% CI) | I 2 (%) |
|---|---|---|---|---|---|
| PTB <34 weeks (all) | 12 | 2230/102 839 | 9569/448 001 | 0.84 (0.74–0.95) | 66 |
| PTB <34 weeks (regional/national) | 2 | 1554/76810 | 7749/379 934 | 0.86 (0.72–1.03) | 79 |
| PTB <34 weeks (single center/single health authority) | 10 | 676/26 029 | 1820/68 027 | 0.82 (0.68–0.98) | 66 |
| PTB <32 weeks (all) | 21 |
7772/626 445 |
94612/7 548 313 | 0.92 (0.81–1.04) | 94 |
| PTB <32 weeks (regional/national) | 9 | 6757/550 959 | 90 948/7 278 288 | 0.93 (0.78–1.11) | 97 |
| PTB <32 weeks (single center/ single health authority) | 12 | 1015/75 486 | 3664/270 025 | 0.91 (0.78–1.06) | 67 |
| PTB <28 weeks (all) | 18 | 2472/569 336 | 32 077/7 274 415 | 0.92 (0.84–1.01) | 55 |
| PTB <28 weeks (regional/national) | 7 | 2220/530 309 | 31 570/7 211 858 | 0.94 (0.86–1.02) | 61 |
| PTB <28 weeks (single center/ single health authority) | 11 | 251/39 027 | 507/62 557 | 0.89 (0.68–1.16) | 55 |
| Spontaneous PTB | 9 | 4207/123 778 | 19 009/535 635 | 0.91 (0.88–0.94) | 0 |
| Induced PTB | 8 | 2562/119 066 | 9168/53 0991 | 0.90 (0.79–1.01) | 75 |
| Low birthweight (all) | 14 | 4197/52 964 | 10 103/14 0701 | 0.91 (0.83–1.00) | 70 |
| Low birthweight (regional/national) | 3 | 1547/19 690 | 5721/70 660 | 0.91 (0.85–0.97) | 14 |
| Low birthweight (single center/ single health authority) | 11 | 2650/33 274 | 4382/70 041 | 0.89 (0.77–1.03) | 72 |
| Very low birthweight | 6 | 373/31 087 | 1566/129 947 | 0.98 (0.75–1.27) | 64 |
| Extremely low birthweight | 6 | 170/26 149 | 611/91 541 | 0.65 (0.38–1.11) | 81 |
| Neonatal mortality | 10 | 1855/88 131 | 2153/279 601 | 1.17 (0.81–1.70) | 94 |
| Birthweight, grams | 9 | 25310 a | 61 599 a | 21 (13–29) b | 0 |
Abbreviations: PTB, preterm birth.
Birthweight is shown as total numbers.
Value shown is mean difference (95% CI) in grams.
Most of the studies presented data for the entire pregnant population, but some explicitly excluded individuals with a known confirmed diagnosis of COVID‐19. When such studies were included in meta‐analyses, we identified no difference in the odds of PTB or stillbirth (for PTB: regional/national data from two studies had a pooled uaOR 1.05 [95% CI 0.87–1.26], whereas six single‐center studies had pooled uaOR of 0.89 [95% CI 0.79–1.01]; for stillbirth: regional/national data from two studies had a pooled uaOR of 1.14 [95% CI 0.58–2.22], while four single‐center studies had uaOR of 1.97 [95% CI 0.85–4.55]).
3.2.2. Stillbirth
Thirty‐two studies of 797 416 women during the pandemic period and 4 440 616 women in the pre‐pandemic period assessed stillbirth. There was no difference in the odds of stillbirth between the pandemic and pre‐pandemic periods (pooled uaOR 1.07, 95% CI 0.97–1.18, I 2 = 75%, Figure 4). Subgroup analyses also revealed no difference in stillbirth during the pandemic period vs the pre‐pandemic period in single‐center studies and regional/national studies (Figure 4). The results of stillbirth stratified by country's income group are reported in Appendix S10 for studies from single centers/single health authorities (which was higher for middle‐income countries [uaOR 1.14, 95% CI 1.01–1.28] but not for high‐income countries [uaOR 1.17, 95% CI 0.82–1.68]) and Appendix S11 for regional/national studies (which was high for low‐income countries [uaOR 1.72, 95% CI 1.50–1.97] based on one study, but not for middle‐income and high‐income countries). Meta‐analysis of adjusted estimates from three studies revealed no difference in stillbirth between pandemic and pre‐pandemic periods (aOR 1.18, 95% CI 0.86–1.63; I 2 = 72%; Appendix S12).
FIGURE 4.

Forest plot for odds of stillbirth in pandemic vs pre‐pandemic periods. CI, confidence interval; IV, inverse variance
3.2.3. Birthweight
Nine studies of 25 310 women during the pandemic period and 61 599 women in the pre‐pandemic period reported birthweight. There was a small increase in mean birthweight during the pandemic compared with the pre‐pandemic period (pooled mean difference 21 g, 95% CI 13–30 g, I 2 = 0%; Table 3, Appendix S13). There was no difference in the odds of low birthweight (Table 3, Appendix S14), very low birthweight (Table 3, Appendix S15), or extremely low birthweight (Table 3, Appendix S16).
3.2.4. Neonatal mortality
Ten studies of 88 131 neonates during the pandemic period did not show any difference in neonatal mortality between the pandemic and pre‐pandemic periods (pooled uaOR 1.17, 95% CI 0.81–1.70, I 2 = 94%; Table 3, Appendix S17), however, the heterogeneity of results across studies was very high. One national study from nine hospitals in Nepal 8 reported a higher neonatal mortality rate during the pandemic period, which may reflect significant local impact on access to care during the lockdown period.
3.2.5. Maternal mortality
Four studies reported on maternal mortality. Three reported no significant difference in maternal mortality; however, one study from Mexico 50 reported a significant increase in maternal mortality during the pandemic (Figure 5). The study from Mexico contributed to 98.7% of the weight in this meta‐analysis and it also reported that a significant portion of excess mortality was due to respiratory infections including COVID‐19.
FIGURE 5.
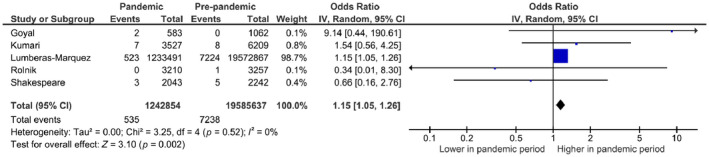
Forest plot for odds of maternal mortality in pandemic vs pre‐pandemic periods. CI, confidence interval; IV, inverse variance
In meta‐regression analyses, duration of the pre‐pandemic study period did not emerge as a significant covariate for any outcome (p > 0.05 for all outcomes). We found evidence of publication bias for PTB (Egger's p = 0.001; Appendix S18) but not for stillbirth (Appendix S19), with fewer studies reporting higher rates of PTB during the pandemic period.
4. DISCUSSION
In this updated systematic review and meta‐analysis, we identified a 6% reduction in the unadjusted odds of PTB during the pandemic compared with pre‐pandemic, for both spontaneous PTB and medically indicated PTB. However, in subgroup analyses, a significant reduction in PTB was only observed in single‐center studies, not in regional or national studies. Although there was no statistically significant difference in the pooled adjusted odds ratio for PTB the magnitude of the pooled estimate was the same as the pooled unadjusted estimate. Subgroup analyses identified reduced odds of preterm birth earlier than 34 weeks of gestation especially in studies from single centers/single health authorities. We identified no difference in any other fetal/neonatal outcomes, including stillbirths and neonatal mortality, and only a marginal increase of 21 g in mean birthweight during the pandemic period compared with the pre‐pandemic period. The increased incidence of maternal mortality noted in our meta‐analysis was mostly driven by one study from Mexico 50 that included deaths due to COVID‐19, which was the leading cause of maternal mortality during the pandemic period.
This review was designed to evaluate the impact of the COVID‐19 pandemic period on pregnancy and neonatal outcomes and not to evaluate studies that report only on maternal SARS‐CoV‐2 infection itself, which has been discussed in other reviews. 69 , 70 , 71 We specifically excluded studies that only reported outcomes of pregnant individuals with SARS‐CoV‐2 infection/COVID‐19 illness. We identified conflicting evidence from the included studies based on whether they were single‐center or regional/national studies. In addition to potential referral bias, other potential explanations for this finding include variation in sample sizes, outcome definitions, lengths of the pandemic and pre‐pandemic periods, differences in timing and enforcement of lockdown orders, failure of some studies to account for natural variation in pregnancy outcomes over time, and dissimilarities among COVID‐19 mitigation strategies. 8 , 10 , 23 , 35 , 54 Moreover, the study populations were heterogeneous; for example, baseline PTB rates ranged from 4.4% to 21.0% during pre‐pandemic period across the included studies; however, the change in PTB rate between periods was not baseline rate dependent.
Chmielewska et al. 72 reported results from a systematic review and meta‐analysis including studies evaluating studies assessing population‐level impact during the pandemic period published up to January 8, 2021. They reported no difference in the PTB rate (15 studies, uaOR 0.94, 95% CI 0.87–1.02) and an increase in stillbirth (12 studies, uaOR 1.28, 95% CI 1.07–1.54) and maternal mortality. With availability of data from 29 more studies on PTB and 20 more studies for stillbirth, the results have remarkably changed, although this could also partly relate to minor differences in study inclusion criteria and data extraction. The larger number of participants included in pooled analyses in our review has improved the precision of pooled estimates, thus increasing confidence in the findings particularly for less common secondary outcomes; however, this is the main reason for conducting this as a living systematic review—so that the information can be updated regularly.
The effects of lockdowns and mitigation strategies had contrasting effects in high‐income vs low‐ and middle‐income countries. 72 This was assessed in post‐hoc subgroup analyses in this review update. Reports from low‐resource settings described increased fear and stress among pregnant individuals, reluctance to access in‐hospital care during a pandemic, financial or employment issues, childcare or home schooling challenges, maternity staff shortages, reduced access to in‐hospital care, and perceived or actual reductions in available obstetric services, resulting in a significant reduction in institutional births. 8 , 9 , 23 , 44 , 45 Some reports noted a reduction in PTB and attributed this to a number of social and health behaviors associated with the pandemic, 2 , 7 including decreased physical and mental stress because of a better work‐life balance, 6 , 20 , 53 better support systems and financial assistance, 20 , 42 improved nutrition, better hygiene, 8 , 12 reduced physical activity, 6 , 20 , 42 , 47 reduced exposure to infection, 8 , 20 , 53 , 73 lower incidence of smoking and drug use due to reduced access and being indoors, 20 lower pollution exposure and levels in environment, 20 , 74 and fewer medical interventions secondary to reduced antenatal surveillance. 7 , 20 , 53 , 67 There continues to be a lack of studies from low‐income countries, with only one study reporting on stillbirth at the time of this review update. The results for PTB were similar for middle‐ and high‐income countries for both single‐center/single health authority and regional/national studies. Single‐center studies from middle‐income countries and one regional study from a low‐income country showed higher odds of stillbirth; however, pooled results from regional/national studies from middle‐income and high‐income countries did not identify an association with odds of stillbirth. The differences in PTB findings between single‐center/adjacent hospitals studies and national/regional studies could reflect a change in referral patterns due to reduced access or the fact that pregnant individuals opted to give birth in hospitals with lower prevalence of COVID‐19 or in non‐COVID‐designated hospitals. 40 Future studies should explore these differences.
Although we did not observe an overall change in the odds of stillbirth during the pandemic period, several individual studies, mostly single center in scope, reported increased odds of stillbirth compared with pre‐pandemic time periods. The increase in stillbirth reported by these studies was attributed to reduced antenatal surveillance, a reluctance to access in‐hospital care due to increased stress and anxiety, 9 , 26 , 44 , 47 , 56 or missed appointments due to rapid changes in maternity services during the pandemic. 73 These reasons may also explain an increase in maternal mortality identified in Mexico; 50 however, according to the authors, the data from the government website were preliminary in scope and may change as more data become available. This could be a signal to be vigilant in attending the mother‐fetus dyad during difficult public health emergency situations.
We did not find any significant differences between the pandemic and pre‐pandemic periods for other outcomes, except for a marginal difference in birthweight. As these data came from only nine studies, further studies are needed to clarify this association, as a difference of 21 g is unlikely to be of clinical significance. Other factors that could be responsible for the differences between study findings include variations in the etiology of adverse pregnancy outcomes in different countries, 2 , 23 initiatives by local governments to provide support to those at risk for higher stress, 7 and changes to national legislation on pregnancy termination during the study period potentially influencing the incidences of stillbirth and PTB. 2 , 7
A key strength of our review was the inclusion of large populations from 29 countries, mainly arising from national or state/provincial data. Most included studies came from registries or similar types of data sets. In addition, we only included studies that reported on temporal changes in outcomes in the overall population, and not data specifically from women affected by COVID‐19. However, our study also has limitations. There may be other relevant studies that are not yet published (and so are not included) as the pandemic is still ongoing and many countries are facing additional waves of infections and associated public health restrictions. There was clinical and methodological heterogeneity across studies regarding pandemic and pre‐pandemic period definitions, population bases (single center/adjacent hospitals vs regional/national), and choices of statistical methodologies. To overcome these limitations, we planned a priori to include pre‐pandemic duration in meta‐regression analyses, and we conducted post‐hoc subgroup analyses on type of studies. We were able to explain statistical heterogeneity to an extent for both of our primary outcomes. Some studies included the entire population of pregnant women, comprising those who did and did not have COVID‐19 infection in their sample. When studies that categorically excluded women with a COVID‐19 infection were included in our review, we identified no difference in PTB or stillbirth. Finally, there were insufficient studies to assess some of the pre‐specified outcomes, including maternal mortality.
The COVID‐19 pandemic has affected many countries with very high case numbers, such as India, Brazil, and Italy, but large, population‐based estimates of pregnancy outcomes from these countries were lacking in this review. National registries from these and other countries would be ideally suited to investigate the impact of the pandemic on perinatal health at a population level. A harmonization of methodological approaches would also facilitate the assessment of the effects of the pandemic period on fetal, neonatal, and maternal outcomes, as high methodological heterogeneity makes direct comparisons challenging. One important point to consider going forward will be that the rates of these outcomes fluctuate with natural variation over time. We hope to capture these fluctuations through further 3‐monthly updates of this living systematic review. Future investigations should use approaches that can elucidate whether any fluctuation observed in a particular setting during the pandemic period is outside the range of expected natural variation.
5. CONCLUSION
In pooled analyses, we observed reductions in the unadjusted odds of PTB between the pandemic and pre‐pandemic periods in both induced and spontaneous PTB. However, this finding was driven by single‐center studies. There was no difference in analyses of adjusted estimates of PTB. Although we did not observe meaningful differences in other outcomes, including odds of stillbirth, the data were more limited and precluded a robust assessment. Higher maternal mortality reported from Mexico indicates that further studies from low‐ and middle‐income regions highly affected by COVID‐19 are needed where drastic changes in the healthcare access, healthcare availability, and personal, social, and environmental factors contributed disproportionately to adverse pregnancy outcomes. As the findings have changed between the review published recently and the current review, there is a need for such living systematic review, which can be updated regularly.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
All authors are responsible for the reported research; all participated in the study concept and design or data analysis and interpretation and in the drafting or revising of the manuscript; and all have approved the manuscript as submitted.
Supporting information
Appendix S1. Search strategies
Appendix S2. Excluded studies and reasons for exclusion
Appendix S3. Forest plot for odds of preterm birth in single‐center/single‐health‐authority studies by income level of country
Appendix S4. Forest plot for odds of preterm birth in regional/national studies by income level of country
Appendix S5. Forest plot for odds of preterm birth before 34 weeks of gestation by single‐center/single‐health‐authority vs regional/national studies
Appendix S6. Forest plot for odds of preterm birth before 32 weeks of gestation by single‐center/single‐health‐authority vs regional/national studies
Appendix S7. Forest plot for odds of preterm birth before 28 weeks of gestation by single‐center/single‐health‐authority vs regional/national studies
Appendix S8. Forest plot for odds of spontaneous preterm birth
Appendix S9. Forest plot for odds of induced preterm birth
Appendix S10. Forest plot for odds of stillbirth in single centre/single health authority by income level of country
Appendix S11. Forest plot for odds of stillbirth in regional/national studies by income level of country
Appendix S12. Forest plot of adjusted odds of stillbirth
Appendix S13. Forest plot for mean difference in birthweight
Appendix S14. Forest plot for odds of low birth‐weight by single‐center/single‐health‐authority vs. regional/national studies
Appendix S15. Forest plot for odds of very low birth‐weight
Appendix S16. Forest plot for odds of extremely low birth‐weight
Appendix S17. Forest plot for odds of neonatal mortality
Appendix S18. Funnel plot for preterm birth before 37 weeks of gestation
Appendix S19. Funnel plot for stillbirth
ACKNOWLEDGMENTS
We thank Heather McDonald Kinkaid, for editorial support in preparing previous versions of this manuscript. Dr Kinkaid was a scientific writer employed with the Maternal‐infant Care Research Centre (MiCare) at Mount Sinai Hospital in Toronto, Ontario, Canada, and received a salary for her work. MiCare is supported by Sinai Health and the participating hospitals, and in turn provides organizational support for the Canadian Preterm Birth Network. We also thank Drs Berghella, Greenbury, Modi, and Olaphsson for providing clarification and/or data from their studies.
Yang J, D’Souza R, Kharrat A, Fell DB, Snelgrove JW, Shah PS. COVID‐19 pandemic and population‐level pregnancy and neonatal outcomes in general population: A living systematic review and meta‐analysis (Update#2: November 20, 2021). Acta Obstet Gynecol Scand. 2022;101:273–292. doi: 10.1111/aogs.14318
This version updates the previous Living Systematic Review (https://doi.org/10.1111/aogs.14277).
Funding information
Although no specific funding was received for this study, the Canadian Preterm Birth Network is funded by a grant from the Canadian Institutes of Health Research (CIHR) (PBN 150642).
REFERENCES
- 1. Ohlsson A, Shah PS. Effects of the September 11, 2001 disaster on pregnancy outcomes: a systematic review. Acta Obstet Gynecol Scand. 2011;90:6‐18. [DOI] [PubMed] [Google Scholar]
- 2. Philip RK, Purtill H, Reidy E, et al. Unprecedented reduction in births of very low birthweight (VLBW) and extremely low birthweight (ELBW) infants during the COVID‐19 lockdown in Ireland: a ‘natural experiment’ allowing analysis of data from the prior two decades. BMJ Glob Health. 2020;5:e003075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stout MJ, Busam R, Macones GA, Tuuli MG. Spontaneous and indicated preterm birth subtypes: interobserver agreement and accuracy of classification. Am J Obstet Gynecol MFM. 2014;211:530.e531‐530.e5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu LI, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post‐2015 priorities: an updated systematic analysis. Lancet. 2015;385:430‐440. [DOI] [PubMed] [Google Scholar]
- 5. Crump C. An overview of adult health outcomes after preterm birth. Early Hum Dev. 2020;150:105187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hedermann G, Hedley PL, Bækvad‐Hansen M, et al. Danish premature birth rates during the COVID‐19 lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106:93‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McDonnell S, McNamee E, Lindow SW, O'Connell MP. The impact of the Covid‐19 pandemic on maternity services: a review of maternal and neonatal outcomes before, during and after the pandemic. Eur J Obstet Gynecol Reprod Biol. 2020;255:172‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kc A, Gurung R, Kinney MV, et al. Effect of the COVID‐19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: a prospective observational study. Lancet Glob Health. 2020;8:e1273‐e1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O'Brien P, Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID‐19 pandemic. JAMA. 2020;324:705‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pasternak B, Neovius M, Söderling J, et al. Preterm birth and stillbirth during the COVID‐19 pandemic in Sweden: a nationwide cohort study. Ann Intern Med. 2021;174:873‐875. doi: 10.7326/M20-6367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta‐Analyses: the PRISMA statement. PLoS Medicine. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang JSP. COVID‐19 pandemic and population level pregnancy and neonatal outcomes: a systematic review. Accessed December 14, 2021. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021234036 [DOI] [PMC free article] [PubMed]
- 13. Yang J, D’Souza R, Kharrat A, et al. COVID‐19 pandemic and population‐level pregnancy and neonatal outcomes: a living systematic review and meta‐analysis. Acta Obstet Gynecol Scand. 2021;100:1756‐1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang J, D’Souza R, Kharrat A, et al. Coronavirus disease 2019 pandemic and pregnancy and neonatal outcomes in general population: a living systematic review and meta‐analysis (updated Aug 14, 2021). Acta Obstet Gynecol Scand. 2022;101:7‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Statistics Canada. Deaths 2004: Vital Statistics‐Stillbirth Database. Accessed December 14, 2021. https://www150.statcan.gc.ca/n1/pub/84f0211x/2004000/4068009‐eng.htm
- 16. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Accessed December 14, 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 17. The World Bank . World Bank Country and Lending Groups. Accessed December 14, 2021. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519
- 18. Arnaez J, Ochoa‐Sangrador C, Caserío S, et al. Lack of changes in preterm delivery and stillbirths during COVID‐19 lockdown in a European region. Eur J Pediatr. 2021;180:1997‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Badran EF, Darwish RM, Khader Y, et al. Adverse pregnancy outcomes during the COVID‐19 lockdown. A descriptive study. BMC Pregnancy Childbirth. 2021;21:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berghella V, Boelig R, Roman A, Burd J, Anderson K. Decreased incidence of preterm birth during coronavirus disease 2019 pandemic. Am J Obstet Gynecol MFM. 2020;2:100258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bian Z, Qu X, Ying H, Liu X. Are COVID‐19 mitigation measures reducing preterm birth rate in China? BMJ Glob Health. 2021;6:e006359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Briozzo L, Tomasso G, Viroga S, Nozar F, Bianchi A. Impact of mitigation measures against the COVID 19 pandemic on the perinatal results of the reference maternity hospital in Uruguay. J Matern Fetal Neonatal Med. 2021;1–3. [DOI] [PubMed] [Google Scholar]
- 23. Caniglia EC, Magosi LE, Zash R, et al. Modest reduction in adverse birth outcomes following the COVID‐19 lockdown. Am J Obstet Gynecol. 2021;224:615.e1‐615.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheung PY, Alshaikh B, Yang C. COVID‐19 pandemic: Different associative relationships of city lockdown with preterm births in three cities—An ecological study. Front Pediatr. 2021;9:644771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cuestas E, Gómez‐Flores ME, Charras MD, et al. Association between COVID‐19 mandatory lockdown and decreased incidence of preterm births and neonatal mortality. J Perinatol. 2021;41:2566‐2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Curtis M, Villani L, Polo A. Increase of stillbirth and decrease of late preterm infants during the COVID‐19 pandemic lockdown. Arch Dis Child Fetal Neonatal Ed. 2020;106:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dell’Utri C, Manzoni E, Cipriani S, et al. Effects of SARS Cov‐2 epidemic on the obstetrical and gynecological emergency service accesses. What happened and what shall we expect now? Eur J Obstet Gynecol Reprod Biol. 2020;254:64‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Du M, Yang J, Han N, Liu M, Liu J. Association between the COVID‐19 pandemic and the risk for adverse pregnancy outcomes: a cohort study. BMJ Open. 2021;11:e047900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Einarsdóttir K, Swift EM, Zoega H. Changes in obstetric interventions and preterm birth during COVID‐19: a nationwide study from Iceland. Acta Obstet Gynecol Scand. 2021;100:1924‐1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gallo LA, Gallo TF, Borg DJ, Moritz KM, Clifton VL, Kumar S. A decline in planned, but not spontaneous, preterm birth rates in a large Australian tertiary maternity centre during COVID‐19 mitigation measures. Aust N Z J Obstet Gynaecol. 2021. doi: 10.1111/ajo.13406. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goyal M, Singh P, Singh K, Shekhar S, Agrawal N, Misra S. The effect of the COVID‐19 pandemic on maternal health due to delay in seeking health care: experience from a tertiary center. Int J Gynaecol Obstet. 2021;152:231‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Greenbury SF, Longford N, Ougham K, et al. Changes in neonatal admissions, care processes and outcomes in England and Wales during the COVID‐19 pandemic: a whole population cohort study. BMJ Open. 2021;11:e054410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Greene NH, Kilpatrick SJ, Wong MS, Ozimek JA, Naqvi M. Impact of labor and delivery unit policy modifications on maternal and neonatal outcomes during the coronavirus disease 2019 pandemic. Am J Obstet Gynecol MFM. 2020;2:100234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gu XX, Chen K, Yu H, Liang GY, Chen H, Shen Y. How to prevent in‐hospital COVID‐19 infection and reassure women about the safety of pregnancy: Experience from an obstetric center in China. J Int Med Res. 2020;48:300060520939337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Handley SC, Mullin AM, Elovitz MA, et al. Changes in preterm birth phenotypes and stillbirth at 2 philadelphia hospitals during the SARS‐CoV‐2 pandemic, march‐june 2020. JAMA. 2020;325:87‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harvey EM, McNeer E, McDonald MF, et al. Association of preterm birth rate with COVID‐19 statewide stay‐at‐home orders in Tennessee. JAMA Pediatr. 2021;175:635‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hedley PL, Hedermann G, Hagen CM, et al. Preterm birth, stillbirth and early neonatal mortality during the Danish COVID‐19 lockdown. Eur J Pediatr. 2021;1‐10. 10.1007/s00431-021-04297-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huseynova R, Bin Mahmoud L, Abdelrahim A, et al. Prevalence of preterm birth rate during COVID‐19 lockdown in a tertiary care hospital, Riyadh . Cureus. 2021;13:e13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Janevic T, Glazer KB, Vieira L, et al. Racial/ethnic disparities in very preterm birth and preterm birth before and during the COVID‐19 pandemic. JAMA Netw Open. 2021;4:e211816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Justman N, Shahak G, Gutzeit O, et al. Lockdown with a price: the impact of the COVID‐19 pandemic on prenatal care and perinatal outcomes in a tertiary care center. Isr Med Assoc J. 2020;22:533‐537. [PubMed] [Google Scholar]
- 41. Kassie A, Wale A, Yismaw W. Impact of coronavirus diseases‐2019 (COVID‐19) on utilization and outcome of reproductive, maternal, and newborn health services at governmental health facilities in South West Ethiopia, 2020: comparative cross‐sectional study. Int J Womens Health. 2021;13:479‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kasuga Y, Tanaka M, Ochiai D. Preterm delivery and hypertensive disorder of pregnancy were reduced during the COVID‐19 pandemic: a single hospital‐based study. J Obstet Gynaecol Res. 2020; doi: 10.1111/jog.14518. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kirchengast S, Hartmann B. Pregnancy outcome during the first COVID 19 lockdown in Vienna, Austria. Int J Environ Res Public Health. 2021;18:3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kumar M, Puri M, Yadav R, et al. Stillbirths and the COVID‐19 pandemic: looking beyond SARS‐CoV‐2 infection. Int J Gynaecol Obstet. 2020;153:76‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kumari V, Mehta K, Choudhary R. COVID‐19 outbreak and decreased hospitalisation of pregnant women in labour. Lancet Glob Health. 2020;8:e1116‐e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lemon L, Edwards RP, Simhan HN. What is driving the decreased incidence of preterm birth during the coronavirus disease 2019 pandemic? Am J Obstet Gynecol MFM. 2021;3:100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li M, Yin H, Jin Z, et al. Impact of Wuhan lockdown on the indications of cesarean delivery and newborn weights during the epidemic period of COVID‐19. PLoS One. 2020;15:e0237420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu S, Dzakpasu S, Nelson C, et al. Pregnancy outcomes during the COVID‐19 pandemic in Canada, March to August 2020. J Obstet Gynaecol Can. 2021;43:1406‐1415. [DOI] [PubMed] [Google Scholar]
- 49. Llorca J, Lechosa‐Muñiz C, Frank de Zulueta P, et al. Results of pregnancy control before and during the COVID‐19 pandemic: a comparison of two cohorts. Int J Environ Res Public Health. 2021;18:8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lumbreras‐Marquez MI, Campos‐Zamora M, Seifert SM, et al. Excess maternal deaths associated with coronavirus disease 2019 (COVID‐19) in Mexico. Obstet Gynecol. 2020;136:1114‐1116. [DOI] [PubMed] [Google Scholar]
- 51. Main EK, Chang S‐C, Carpenter AM, et al. Singleton preterm birth rates for racial and ethnic groups during the coronavirus disease 2019 pandemic in California. Am J Obstet Gynecol. 2021;224:239‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Matheson A, McGannon CJ, Malhotra A, et al. Prematurity rates during the coronavirus disease 2019 (COVID‐19) pandemic lockdown in Melbourne, Australia. Obstet Gynecol. 2021;137:405‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Meyer R, Bart Y, Tsur A, et al. A marked decrease in preterm deliveries during the coronavirus disease 2019 pandemic. Am J Obstet Gynecol. 2021;224:234‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Meyer R, Levin G, Hendin N, Katorza E. Impact of the COVID‐19 outbreak on routine obstetrical management. Isr Med Assoc J. 2020;22:483‐488. [PubMed] [Google Scholar]
- 55. Mikuš M, Sokol Karadjole V, Kalafatić D, Orešković S, Šarčević A. Increase of stillbirths and unplanned out‐of‐hospital births during coronavirus disease 2019 lockdown and the Zagreb earthquake. Acta Obstet Gynecol Scand. 2021;100:2119‐2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mor M, Kugler N, Jauniaux E, et al. Impact of the COVID‐19 pandemic on excess perinatal mortality and morbidity in Israel. Am J Perinatol. 2020;38:398‐403. [DOI] [PubMed] [Google Scholar]
- 57. Rolnik DL, Matheson A, Liu Y, et al. Impact of COVID‐19 pandemic restrictions on pregnancy duration and outcome in Melbourne, Australia. Ultrasound Obstet Gynecol. 2021;58:677‐687. [DOI] [PubMed] [Google Scholar]
- 58. Ranjbar F, Allahqoli L, Ahmadi S, et al. Changes in pregnancy outcomes during the COVID‐19 lockdown in Iran. BMC Pregnancy Childbirth. 2021;21:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shah PS, Ye XY, Yang J, Campitelli MA. Preterm birth and stillbirth rates during the COVID‐19 pandemic: a population‐based cohort study. CMAJ. 2021;193:E1164‐E1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shakespeare Clare DH, Moyo S, Ngwenya S. Resilience and vulnerability of maternity services in Zimbabwe: a comparative analysis of the effect of Covid‐19 and lockdown control measures on maternal and perinatal outcomes at Mpilo Central Hospital. Accessed December 14, 2021. https://www.researchsquare.com/article/rs‐52159/v1 [DOI] [PMC free article] [PubMed]
- 61. Son M, Gallagher K, Lo JY, et al. Coronavirus disease 2019 (COVID‐19) pandemic and pregnancy outcomes in a U.S. Population. Obstet Gynecol. 2021;138:542‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stephansson O, Pasternak B, Ahlberg M, et al. SARS‐CoV‐2 and pregnancy outcomes under universal and non‐universal testing in Sweden: register‐based nationwide cohort study. BJOG. 2022;129:282‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stowe J, Smith H, Thurland K, Ramsay ME, Andrews N, Ladhani SN. Stillbirths during the COVID‐19 pandemic in England, April‐June 2020. JAMA. 2021;325:86‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sun SY, Guazzelli CAF, Morais LR, et al. Effect of delayed obstetric labor care during the COVID‐19 pandemic on perinatal outcomes. Int J Gynaecol Obstet. 2020;151:287‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang J, Wang Y, He M‐Y, et al. Maternal and infant outcomes during the COVID‐19 pandemic: a retrospective study in Guangzhou, China. Reprod Biol Endocrinol. 2021;19:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wood R, Sinnott C, Goldfarb I, Clapp M, McElrath T, Little S. Preterm birth during the coronavirus disease 2019 (COVID‐19) pandemic in a large hospital system in the United States. Obstet Gynecol. 2021;137:403‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Been JV, Burgos Ochoa L, Bertens LCM, Schoenmakers S, Steegers EAP, Reiss IKM. Impact of COVID‐19 mitigation measures on the incidence of preterm birth: a national quasi‐experimental study. Lancet Public Health. 2020;5:e604‐e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Simpson AN, Snelgrove JW, Sutradhar R, Everett K, Liu N, Baxter NN. Perinatal outcomes during the COVID‐19 pandemic in Ontario, Canada. JAMA Netw Open. 2021;4:e2110104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta‐analysis. BMJ. 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Juan J, Gil MM, Rong Z, Zhang Y, Yang H, Poon LC. Effect of coronavirus disease 2019 (COVID‐19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56:15‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Smith V, Seo D, Warty R, et al. Maternal and neonatal outcomes associated with COVID‐19 infection: a systematic review. PLoS One. 2020;15:e0234187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chmielewska B, Barratt I, Townsend R, et al. Effects of the COVID‐19 pandemic on maternal and perinatal outcomes: a systematic review and meta‐analysis. Lancet Glob Health. 2021;9:e759‐e772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Coxon K, Turienzo CF, Kweekel L, et al. The impact of the coronavirus (COVID‐19) pandemic on maternity care in Europe. Midwifery. 2020;88:102779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bauwens M, Compernolle S, Stavrakou T, et al. Impact of coronavirus outbreak on NO(2) pollution assessed using TROPOMI and OMI observations. Geophys Res Lett. 2020;47:e2020GL087978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Search strategies
Appendix S2. Excluded studies and reasons for exclusion
Appendix S3. Forest plot for odds of preterm birth in single‐center/single‐health‐authority studies by income level of country
Appendix S4. Forest plot for odds of preterm birth in regional/national studies by income level of country
Appendix S5. Forest plot for odds of preterm birth before 34 weeks of gestation by single‐center/single‐health‐authority vs regional/national studies
Appendix S6. Forest plot for odds of preterm birth before 32 weeks of gestation by single‐center/single‐health‐authority vs regional/national studies
Appendix S7. Forest plot for odds of preterm birth before 28 weeks of gestation by single‐center/single‐health‐authority vs regional/national studies
Appendix S8. Forest plot for odds of spontaneous preterm birth
Appendix S9. Forest plot for odds of induced preterm birth
Appendix S10. Forest plot for odds of stillbirth in single centre/single health authority by income level of country
Appendix S11. Forest plot for odds of stillbirth in regional/national studies by income level of country
Appendix S12. Forest plot of adjusted odds of stillbirth
Appendix S13. Forest plot for mean difference in birthweight
Appendix S14. Forest plot for odds of low birth‐weight by single‐center/single‐health‐authority vs. regional/national studies
Appendix S15. Forest plot for odds of very low birth‐weight
Appendix S16. Forest plot for odds of extremely low birth‐weight
Appendix S17. Forest plot for odds of neonatal mortality
Appendix S18. Funnel plot for preterm birth before 37 weeks of gestation
Appendix S19. Funnel plot for stillbirth


