Abstract
Introduction
Fetal growth restriction is a major risk factor for adverse perinatal outcome. As most of the growth‐restricted fetuses are small for gestational age (SGA), an efficient antenatal screening method for SGA fetuses would have a major impact on perinatal health. The aim of this study was to compare the SGA prediction rate achieved with third‐trimester routine ultrasound estimation of fetal weight (EFW) with that obtained using ultrasound examination on indication. The secondary aim was to evaluate the clinical outcome in relation to the SGA screening method.
Material and Methods
During 1995–2009, two perinatal centers in southern Sweden offered routine ultrasound examination at 32–34 gestational weeks to 99 265 women with singleton pregnancies. Of these, 59 452 (60%) underwent the ultrasound examination. The other population, comprising 24 868 pregnancies, was cared for in another three centers that used a risk‐based method with ultrasound examinations on indication only. Of them, 5792 (23%) underwent ultrasound examination at 32–36 gestational weeks. The deviation in the EFW from the expected one was expressed as the EFW z‐score, SGA EFW being defined as the EFW z‐score less than −2. SGA prediction ability was assessed by receiver operating characteristic (ROC) curves. Crude and adjusted risk ratios were calculated for selected variables of perinatal outcome when comparing the populations.
Results
The SGA prediction ability for routine ultrasound was high, area under the ROC curve was 0.90 (95% CI 0.89–0.91). For an EFW z‐score of −1, the sensitivity was 67.3% and specificity was 90.5% among routinely screened pregnancies; corresponding numbers in the ultrasound on indication population were 34.3% and 96.6%. The screened population had a lower risk of preterm birth, birthweight z‐score less than −3, and Apgar score less than 7 at 5 min with adjusted risk ratios 0.87 (95% CI 0.82–0.92), 0.75 (95% CI 0.61–0.92), and 0.77 (95% CI 0.68–0.87), respectively. No difference in perinatal mortality was detected. There were no differences in perinatal outcome between the two subcohorts of infants born SGA.
Conclusions
Third‐trimester routine ultrasound improves the detection of SGA antenatally compared with ultrasound performed on indication, but no convincing improvement in perinatal outcome was identified.
Keywords: antenatal detection, fetal growth restriction, prenatal care, screening, small‐for‐gestational age, ultrasound
Abbreviations
- EFW
estimated fetal weight;
- FGR
fetal growth restriction;
- ROC
receiver operating characteristic;
- RR
risk ratio;
- SGA
small for gestational age
Key message.
Routine ultrasound at 32–34 gestational weeks improves detection of small for gestational age before birth compared with ultrasound examinations on indication. No convincing improvement of perinatal outcome was seen even if a higher proportion of small‐for‐gestational‐age fetuses was identified antenatally.
1. INTRODUCTION
Fetuses and infants that are small for gestational age (SGA) have a higher risk for perinatal death and adverse outcome. 1 , 2 , 3 , 4 The etiology of SGA is heterogeneous. 5 Fetuses may be constitutionally small, growth restricted by structural or chromosomal abnormalities, or suffering from placenta‐mediated growth restriction. The increased risk for adverse outcome is likely to be evident within the two latter groups.
As fetal growth restriction (FGR) is a major risk factor for adverse perinatal outcome, and most of the growth‐restricted fetuses are SGA, an efficient antenatal screening method for SGA fetuses would have a major impact on perinatal health. Strategies to estimate fetal growth vary. Serial measurements of symphysis–fundus height are routine in many countries despite the unsatisfactory detection rate. 6 Another strategy is to screen for SGA fetuses by estimated fetal weight (EFW) as part of the third‐trimester routine ultrasound examination. 7 , 8
No randomized trials have shown that screening for SGA reduces adverse perinatal outcome, 7 , 9 , 10 , 11 but there are observational studies indicating better perinatal outcome in SGA fetuses detected within screening programs in the third trimester. 12 , 13
During 1995–2009, all pregnant women living in the Malmö‐Lund area in southern Sweden were offered a routine ultrasound examination at 32–34 gestational weeks to estimate fetal growth and to detect possible fetal anomalies. During the same time period, pregnant women in nearby communities—Helsingborg, Höganäs, and Ängelholm—were instead surveilled by serial measurements of symphysis–fundus height, and in case of deviating measurements with a suspicion of SGA, or at the occurrence of clinical signs indicating an increased risk for SGA, these women were referred for an ultrasound examination on indication (risk‐based method).
As the routine ultrasound program was never fully evaluated, we aimed to assess the SGA prediction rate achieved with the third‐trimester routine ultrasound and to compare it with the corresponding rate obtained using the risk‐based method. The secondary aim was to evaluate the clinical outcome in relation to the SGA screening method, with special focus on the cohort of SGA infants.
2. MATERIAL AND METHODS
2.1. Data collection and processing
Data were collected from the perinatal quality register Perinatal Revision South, 14 containing data from all obstetric and neonatal units in the Southern Healthcare Region of Sweden. Maternal background information, such as body mass index and smoking, refers to data from women's first visit to the antenatal clinic. The gestational age was assessed on the basis of routine ultrasound in the early second trimester. Infants were defined as SGA when their birthweight z‐score was less than −2.0, that is the birthweight was more than two standard deviations below the expected weight for gestational age and gender according to the Swedish intrauterine growth curve. 15 The EFW was calculated according to the formula of Persson and Weldner, 16 which includes fetal biparietal diameter, 17 femur length, 18 and mean abdominal diameter. 19 The EFW z‐score was based on the deviation of the estimated EFW value from the expected one according to the Swedish intrauterine growth curve, one standard deviation corresponding to 11% of the mean EFW. 15 The SGA EFW was defined as the EFW z‐score less than −2.
2.2. Routine ultrasound population (population A)
From 1995 until 2009 all pregnant women in the catchment area of Malmö‐Lund were offered routine ultrasound examination with EFW at 32+0–34+6 weeks (completed gestational weeks+days), as part of the free antenatal care program. Specially trained staff of the ultrasound units performed the ultrasound examinations. Fetuses with EFW z‐score below −1.0 were followed up with a repeated ultrasound examination according to local clinical guidelines. The fetuses with EFW z‐score less than −2.0 at the first or subsequent ultrasound examination were considered SGA and submitted to an intensified clinical surveillance including cardiotocography and Doppler ultrasound examinations. The surveillance protocols developed over the years and the principles applied during years 2004–2009 have been described previously. 20 Data from all ultrasound examinations (performed as part of the routine ultrasound offer or on indication) were reported to the perinatal database.
Analyses were also made of the subgroup of women who did participate in the routine ultrasound program (scanned population; population A1).
2.3. Ultrasound on indication population (population B)
Pregnant women in the population B were not offered routine ultrasound for estimation of fetal weight. They were referred to ultrasound examination with EFW in the case of suspicion of FGR, eg deviating measurements of symphysis–fundus height or former pregnancy complicated by FGR. In the case of identified suspected SGA at ultrasound examination performed on indication, the clinical guidelines for follow up and surveillance of fetuses in population B were based on the same principles as in population A with possible minor local modifications. Data from indicated ultrasound examinations performed at 32+0–36+6 gestational weeks were evaluated to estimate SGA detection rate.
2.4. Outcome measures
Data from the scanned population (population A1) were used to define the optimal cut‐off for EFW at ultrasound examination to predict SGA at birth. Fetuses born SGA, for whom the EFW at the antenatal ultrasound examination was lower than the identified cut‐off, were classified as “detected SGA”. The perinatal outcomes considered were preterm or post‐term birth (gestational age at birth <37 weeks and ≥42 weeks, respectively), start of delivery (spontaneous, induced, or cesarean section before contractions), mode of delivery (vaginal, cesarean), Apgar score less than 7 at 5 minutes, umbilical artery pH less than 7.05, perinatal death (stillbirth, intrapartal, or early neonatal death), birthweight z‐score less than −3.0 and less than −4.0, respectively.
2.5. Exclusions
Multiple births and infants with unknown birthweight or unknown sex were excluded. Exclusions were also made if gestational age was not estimated by ultrasound in the second trimester. Women in population A who underwent ultrasound examination on other indication than screening were excluded as specified.
2.6. Statistical analyses
Differences in maternal characteristics between population A and population B were compared using chi‐squared test. Descriptive comparisons between populations regarding continuous outcome measures were performed using Mann–Whitney U tests. Perinatal outcome was analyzed using univariable and multivariable modified Poisson regression analyses. Crude and adjusted risk ratios (RR) for selected variables were calculated comparing populations A and B. Analyses were made for all infants and for infants born SGA. Adjustments were performed for maternal age (continuous variable), parity (parity 0 and parity 2+, compared with parity 1), smoking (ordinal, semi‐continuous variable: 1 = no, 2 = 1–9 cigarettes per day, 3 = more than 10 cigarettes per day) and body mass index (continuous variable). Missing values were replaced with the overall means. The SGA prediction ability for routine ultrasound examination was assessed by receiver operating characteristic (ROC) curves. Statistical analyses were performed using SPSS Statistics 26 (SPSS Inc.) and Gauss (Aptech Systems Inc.). Ninety‐five percent confidence intervals for proportions were obtained using normal approximation.
2.7. Ethical approval
The study was approved by The Swedish Ethical Review Agency on December 9, 2020 (reference no. 2020‐06088).
3. RESULTS
3.1. Populations
In population A (women offered routine ultrasound examination), 99 265 records were identified after exclusions. Of these, 59 452 (59.9%) pregnancies had undergone a screening ultrasound with measurement of EFW at 32+0–34+6 gestational weeks (population A1). Another 3408 women in population A had an ultrasound examination for another indication during 32+0–34+6 gestational weeks; hence they did not participate in the routine ultrasound screening. The characteristics of women in population A who attended or did not attend the screening program are provided in the supporting information (Table S1). In population B (ultrasound examination on indication), 24 868 singleton pregnancies were identified, among them 5792 (23.3%) had an indicated ultrasound for measurement of EFW at 32+0–36+6 gestational weeks. Overall, the population demographics of populations A and B were similar (Table 1). There was no difference in maternal age or body mass index distributions between the populations, but there were more nulliparous women, and fewer smokers in population A than in population B. A significant heterogeneity of the maternal height distribution was found, but no clear pattern was identified (Table 1).
TABLE 1.
Baseline characteristics of the two study populations
|
Routine ultrasound population (A) n = 99 265 |
Ultrasound on indication population (B) n = 24 868 |
p value a | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Maternal age (years) | 0.068 | ||||
| <20 | 1946 | 2.0 | 428 | 1.7 | |
| 20–24 | 13 319 | 13.4 | 3329 | 13.4 | |
| 25–29 | 30 760 | 31.0 | 7851 | 31.6 | |
| 30–34 | 34 926 | 35.2 | 8754 | 35.2 | |
| 35–39 | 15 527 | 15.6 | 3794 | 15.3 | |
| 40+ | 2787 | 2.8 | 712 | 2.9 | |
| Parity | <0.001 | ||||
| Parity 0 | 48 143 | 48.5 | 11 653 | 46.9 | |
| Parity 1 | 32 883 | 33.1 | 8680 | 34.9 | |
| Parity 2+ | 18 239 | 18.4 | 4535 | 18.2 | |
| Maternal smoking | <0.001 | ||||
| Yes | 10 506 | 10.6 | 3076 | 12.4 | |
| No | 84 175 | 84.8 | 20 870 | 83.9 | |
| Not known | 4584 | 4.6 | 922 | 3.7 | |
| Maternal body mass index (kg/m2) | 0.38 | ||||
| <18.5 | 2460 | 2.5 | 645 | 2.6 | |
| 18.5–24.9 | 56 617 | 57.0 | 14 472 | 58.2 | |
| 25–29.9 | 21 178 | 21.3 | 5412 | 21.8 | |
| 30+ | 8533 | 8.6 | 2275 | 9.1 | |
| Not known | 10 477 | 10.6 | 2064 | 8.3 | |
| Maternal height (cm) | <0.001 | ||||
| <155 | 3326 | 3.4 | 787 | 3.2 | |
| 155–164 | 33 138 | 33.4 | 7922 | 31.9 | |
| 165–174 | 48 577 | 48.9 | 12 515 | 50.3 | |
| 175+ | 10 017 | 10.1 | 2412 | 9.7 | |
| Not known | 4207 | 4.2 | 1232 | 5.0 | |
p‐values obtained by chi‐squared test.
Median gestational age at ultrasound examination was 32+5 gestational weeks in population A and 34+2 gestational weeks in population B (p < 0.001). Median gestational age at delivery did not differ between the two populations (39+6 gestational weeks). Median birthweight in population A was 3550 g (range 610–6360 g) compared with 3530 g (range 995–6110 g) in population B (p < 0.001).
3.2. Ultrasound precision
Figure 1 shows the ROC curve demonstrating the ability of routine ultrasound at 32–34 gestational weeks to predict SGA at birth as assessed in the scanned population (population A1). The overall SGA prediction ability was high, with area under the ROC curve of 0.90 (95% CI 0.89–0.91) (Figure 1 and Table S2). The highest “height over identity line” was obtained for the EFW z‐score −0.75 (sensitivity 79%, specificity 84%, false‐positive rate 16%; Table S2). In order to reduce the false‐positive rate, the EFW z‐score −1.0 was arbitrarily chosen as the optimum cut‐off level. At the EFW z‐score −1.0, the sensitivity for SGA was 67.3% (95% CI 65.0%–69.5%), specificity was 90.5% (95% CI 90.2–90.7%), and the false‐positive rate was 9.5% (95% CI 9.3%–9.8%) (Table S2).
FIGURE 1.
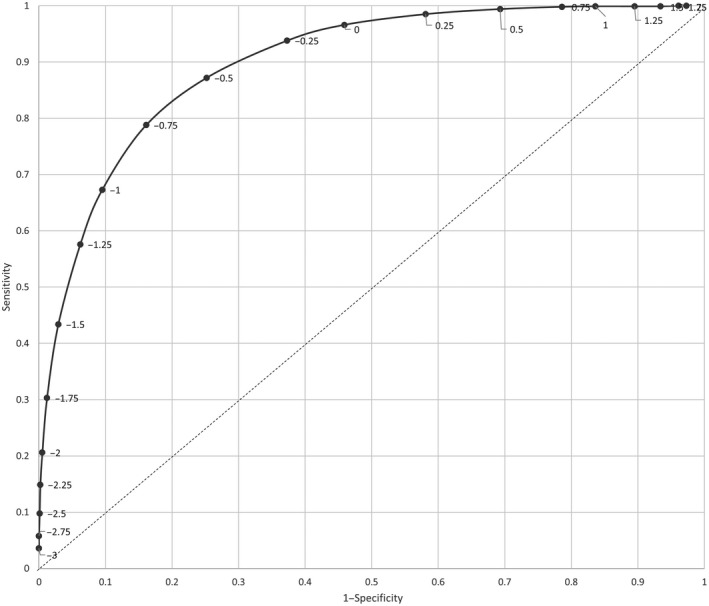
Receiver operating characteristics curve for the detection of small for gestational age by estimated fetal weight z‐score cut‐off at routine ultrasound examination performed at 32–34 gestational weeks.
3.3. Detection of SGA by screening policy
Based on the total population A (irrespective of whether or not a routine ultrasound scan was performed), 46.5% (95% CI 44.7%–48.3%) of all SGA infants were detected antenatally using the cut‐off EFW z‐score of −1.0, with false‐positive rate 6.3% (95% CI 6.1%–6.4%). The corresponding SGA detection rate in population B was 34.3% (95% CI 31.1%–37.5%) with the false‐positive rate 3.4% (95% CI 3.2%–3.6%). Adjusted RR was 1.35 (95% CI 1.22–1.50) for intrauterine detection of SGA in population A compared with population B. When comparison was made between the screened population A1 and population B, the adjusted RR for SGA detection was 1.96 (95% CI 1.78–2.16) (Table 2).
TABLE 2.
Detection of small‐for‐gestational age infants
| SGA infants | RR | 95% CI | aRR | 95% CI | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Detected antenatally | Not detected antenatally | |||||||
| n | % | n | % | ||||||
| Routine ultrasound population (A) | 2951 | 1371 | 46.5 | 1580 | 53.5 | 1.35 | 1.22–1.50 | 1.35 | 1.22–1.49 |
| Scanned population (A1) | 1678 | 1129 | 67.3 | 549 | 32.7 | 1.96 | 1.78–2.16 | 1.96 | 1.78–2.17 |
| Ultrasound on indication population (B) | 860 | 295 | 34.3 | 565 | 65.7 | 1.00 | Reference | 1.00 | Reference |
SGA infant: infant with birthweight z‐score less than −2.0.
Abbreviations: aRR, adjusted risk ratio; RR, risk ratio; SGA, small‐for‐gestational age.
3.4. Pregnancy outcome by screening policy
The risk of being born preterm was lower, and the risk of being born post‐term was higher in population A (routine ultrasound) than in population B (ultrasound on indication) (Table 3). Induction of labor was less likely in population A. The risk for a birthweight z‐score less than −3.0, low Apgar score, and low umbilical artery pH, respectively, was significantly lower in population A compared with population B. There was no difference in perinatal death (Table 3).
TABLE 3.
Perinatal outcome. Comparison between routine ultrasound and ultrasound on indication; all infants
|
Routine ultrasound population (A) (n = 99 265) |
Ultrasound on indication population (B) (n = 24 868) |
RR | 95% CI | aRR | 95% CI | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| Gestational duration (weeks) | ||||||||
| Preterm delivery (<37) | 4588 | 4.6 | 1315 | 5.3 | 0.88 | 0.83–0.93 | 0.87 | 0.82–0.92 |
| Term delivery (37–42) | 89 124 | 89.8 | 22 329 | 89.8 | Reference | Reference | ||
| Post‐term delivery (≥42) | 5553 | 5.6 | 1224 | 4.9 | 1.13 | 1.06–1.20 | 1.12 | 1.06–1.19 |
| Start of delivery | ||||||||
| CS before contractions | 5169 | 5.2 | 1159 | 4.7 | 1.08 | 1.01–1.15 | 1.08 | 1.01–1.14 |
| Induction | 6280 | 6.4 | 2419 | 9.7 | 0.65 | 0.63–0.68 | 0.66 | 0.63–0.69 |
| Spontaneous start | 87 816 | 88.5 | 21 290 | 85.6 | Reference | Reference | ||
| Spontaneous start (compared with CS or induction) | 1.03 | 1.03–1.04 | 1.03 | 1.03–1.04 | ||||
| Mode of delivery a | ||||||||
| Emergency CS | 7444 | 7.9 | 1690 | 7.1 | 1.11 | 1.05–1.16 | 1.09 | 1.04–1.15 |
| Instrumental vaginal | 3742 | 4.0 | 1016 | 4.3 | 0.94 | 0.88–1.00 | 0.92 | 0.86–0.98 |
| Spontaneous vaginal | 81 112 | 86.2 | 21 003 | 88.6 | Reference | Reference | ||
| Missing information | 1798 | 0 | ||||||
| Birthweight z‐score | ||||||||
| <−3 to ≥−4 | 315 | 0.32 | 102 | 0.41 | 0.76 | 0.62–0.94 | 0.75 | 0.61–0.93 |
| <−4 | 32 | 0.03 | 12 | 0.05 | 0.67 | 0.34–1.30 | 0.64 | 0.33–1.25 |
| ≥−3 | 98 918 | 99.6 | 24 754 | 99.5 | Reference | Reference | ||
| Neonatal outcome | ||||||||
| 5‐min Apgar score <7 | 1101 | 1.1 | 353 | 1.4 | 0.78 | 0.69–0.88 | 0.77 | 0.68–0.87 |
| 5‐min Apgar score ≥7 | 98 164 | 98.9 | 24 515 | 98.6 | Reference | Reference | ||
| UA pH <7.05 b | 1390 | 1.9 | 569 | 2.8 | 0.69 | 0.63–0.76 | 0.68 | 0.62–0.75 |
| UA pH ≥7.05 b | 71 314 | 98.1 | 19 925 | 97.2 | Reference | Reference | ||
| Perinatal death | 326 | 0.33 | 84 | 0.34 | 0.97 | 0.76–1.24 | 0.97 | 0.76–1.24 |
| Alive at 1 week | 98 939 | 99.7 | 24 784 | 99.7 | Reference | Reference | ||
Abbreviations: aRR, adjusted risk ratio; CS, cesarean section; RR, risk ratio; UA, umbilical artery.
CS before contractions excluded.
Percentages and risk estimates based on known values only (population A: n = 72 704; missing n = 26 561; population B: n = 20 494, missing 4374).
Comparisons between the population A1 and population B gave results similar to those for populations A and B. The results are presented in Tables S3 and S4.
During the study period there was a gradual decrease of deliveries with spontaneous start from 90.6% to 84.1% and an increase of deliveries by elective and emergency CS from 8.3% to 14.4% over time. The proportion of infants with Apgar score less than 7 at 5 minutes was stable during the study period.
3.5. Pregnancy outcome among children born SGA
Among infants born SGA, no significant difference in the gestational duration was detected between populations A and B (Table 4). Spontaneous start of delivery was more common and induction of labor was less frequent in population A than in population B. The birthweight distribution and risk of perinatal death among children born SGA did not differ between populations A and B (Table 4).
TABLE 4.
Perinatal outcome. Comparison between routine ultrasound and ultrasound on indication; infants born SGA
|
Routine ultrasound population (A) (n = 2951) |
Ultrasound on indication population (B) (n = 860) |
RR | 95% CI | aRR | 95% CI | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| Gestational duration (weeks) | ||||||||
| Preterm delivery (<37) | 460 | 15.6 | 149 | 17.3 | 0.92 | 0.78–1.08 | 0.90 | 0.76–1.06 |
| Term delivery (37–42) | 2309 | 78.2 | 674 | 78.4 | Reference | Reference | ||
| Post term delivery (≥42) | 182 | 6.2 | 37 | 4.3 | 1.40 | 1.00–1.98 | 1.40 | 0.99–1.96 |
| Start of delivery | ||||||||
| CS before contractions | 167 | 5.7 | 32 | 3.7 | 1.41 | 0.97–2.04 | 1.37 | 0.95–1.98 |
| Induction | 475 | 16.1 | 188 | 21.9 | 0.75 | 0.65–0.87 | 0.75 | 0.65–0.88 |
| Spontaneous start | 2309 | 78.2 | 640 | 74.4 | Reference | Reference | ||
| Spontaneous start (compared with CS or induction) | 1.05 | 1.01–1.10 | 1.05 | 1.01–1.10 | ||||
| Mode of delivery a | ||||||||
| Emergency CS | 710 | 25.5 | 195 | 23.6 | 1.06 | 0.93–1.21 | 1.04 | 0.91–1.19 |
| Instrumental vaginal | 114 | 4.1 | 33 | 4.0 | 1.28 | 0.85–1.92 | 1.20 | 0.80–1.80 |
| Spontaneous vaginal | 1898 | 68.2 | 600 | 72.5 | Reference | Reference | ||
| Missing information | 62 | 0 | ||||||
| Birthweight z‐score | ||||||||
| 3 to ≥−4 | 315 | 10.7 | 102 | 11.9 | 0.89 | 0.73–1.08 | 0.88 | 0.72–1.07 |
| <−4 | 32 | 1.1 | 12 | 1.4 | 0.77 | 0.40–1.48 | 0.72 | 0.37–1.39 |
| ≥−3 | 2604 | 88.2 | 746 | 86.7 | Reference | Reference | ||
| Neonatal outcome | ||||||||
| 5‐min Apgar score <7 | 124 | 4.2 | 39 | 4.5 | 0.93 | 0.65–1.32 | 0.86 | 0.60–1.23 |
| 5‐min Apgar score ≥7 | 2827 | 95.8 | 821 | 95.5 | Reference | Reference | ||
| UA pH <7.05 b | 48 | 2.4 | 26 | 4.0 | 0.60 | 0.38–0.96 | 0.58 | 0.36–0.95 |
| UA pH ≥7.05 b | 1956 | 97.6 | 625 | 96.0 | Reference | Reference | ||
| Perinatal death | 73 | 2.5 | 15 | 1.7 | 1.42 | 0.82–2.46 | 1.39 | 0.80–2.40 |
| Alive at 1 week | 2878 | 97.5 | 845 | 98.3 | Reference | Reference | ||
Abbreviations: aRR, adjusted risk ratio; CS, cesarean section; RR, risk ratio; SGA, small for gestational age; UA, umbilical artery.
CS before contractions excluded.
Percentages and risk estimates based on known values only (population A: n = 2004; missing n = 947; population B: n = 651, missing 209).
4. DISCUSSION
Routine ultrasound assessment of EFW in the third trimester improved the prediction of SGA at birth compared with the method using ultrasound on indication. For screened pregnancies, the area under the ROC curve (0.90) indicated a high ability of routine ultrasound to detect SGA fetuses at 32–34 gestational weeks. At a cut‐off EFW z‐score of −1, the sensitivity was 67.3%, and the false‐positive rate was 9.5%. In the two populations—one offering routine ultrasound screening, the other with ultrasound on indication—the sensitivities for SGA detection were 46.5% and 34.3%, respectively. In the routine ultrasound population there were fewer obstetric interventions compared with the on‐indication population and the risk of being born preterm, having birthweight z‐score less than −3, and Apgar‐score less than 7 at 5 minutes was reduced. Despite the high detection rate, there was no indication of improved perinatal outcome in the routine ultrasound population when comparing the two subcohorts of infants born SGA.
In the early 1980s, our research group performed two prospective studies on detection of SGA fetuses with routine ultrasound at 33 gestational weeks. The sensitivity levels, 77% both for fetometry and for EFW, 21 , 22 were similar to that found in the screened population of the present study. Interestingly, the selected z‐score cut‐off levels were also similar in the study by Laurin and Persson and in the present study, 22 −1.1 and −1.0, respectively. In concordance with the current study, a randomized controlled trial in a non‐selected Norwegian population, screening ultrasound improved the detection of SGA from 46% to 80%. 7 An English prospective cohort study reported a tripled SGA detection rate with universal ultrasonography compared with selective ultrasonography. 10
Despite the high SGA detection rates reported above, the 13 trials reviewed in the Cochrane Library could not show that routine ultrasound screening for SGA in late pregnancy would reduce perinatal mortality or adverse perinatal outcomes in general. 9 There are a few observational studies indicating a better outcome with ultrasound SGA detection. Lindqvist and Molin found that antenatal detection of SGA fetuses in a large collection of background material (n = 26 968) lowered four‐fold the risk of severe adverse perinatal outcome. 12 However, that study did not consider two screening strategies, because it compared the outcome among fetuses who were appropriate for gestational age at the time of ultrasound examination and subsequently SGA at birth with those who had SGA EFW at the examination, were submitted to intensified surveillance, and had an SGA birthweight. Hence, the group of infants born SGA and undetected antenatally was more likely to suffer from true growth restriction with a worse outcome. In a cohort study from England, FGR was identified as the major risk factor for stillbirth, and in the subgroup of growth‐restricted infants the risk was significantly higher when not detected antenatally. 23 In that study, the median gestational age was 10 days shorter in infants with antenatally detected FGR compared with the non‐detected group, indicating a more active obstetrical management. A French study reported a weak protective effect of antenatally detected FGR on the risk of stillbirth. 24 In another study the same research group found no benefit from antenatal suspicion of SGA. They reported on a large number of false‐positive diagnoses with higher rates of CS, resuscitation, and admission to neonatal units compared with infants with normal birthweight and no suspicion of SGA. 25
It has been suggested that late routine ultrasound screening might have clinically important spin‐off effects, such as improved detection of structural fetal abnormalities or identification of fetal malpresentations otherwise not detected. Unfortunately, no evidence supporting these speculations has been found. 9 In our study, we did not have the possibility to evaluate this.
None of the published studies included a follow up of infants up to childhood. Our research group systematically followed a cohort of late‐onset FGR infants and controls up to the age of 25 years. We found that the growth‐restricted individuals had suboptimal neurocognitive development at the ages of 7 and 18 years. 26 , 27 , 28 , 29 Their cardiovascular system also showed impaired morphology and function. 30 , 31 These findings might suggest that a more active management of FGR fetuses in the perinatal period could diminish the negative effects of intrauterine hypoxia superposed on the process of growth restriction. Such active management necessitates strict clinical protocols enabling identification of true FGR and optimal timing of delivery. Similarly important is to avoid unnecessary interventions in false‐positive cases. In such clinical protocols, fetoplacental and uteroplacental Doppler velocimetry will have a given place. 20 , 32
The choice of screening models must include cost–benefit considerations and balancing of the positive and false‐positive rates when selecting the proper SGA risk group to be submitted to repeated fetometry and intensified surveillance. For this purpose, an addition of maternal risk factors and Doppler ultrasound parameters has been suggested. 13 Individualized growth assessment and differentiation into a number of types of growth restriction have also been suggested to distinguish infants born SGA with elevated risks. 33 The POP‐study reported an association between prediction of SGA and neonatal morbidity if growth velocity of fetal abdominal circumference was added. 10 This was contradicted by more recent studies. 34 Strategies including additional routine examinations or postponing the screening examination close to term improved the SGA detection slightly, 8 , 21 but did not improve the perinatal outcome, possibly because the majority of severe late‐onset FGR cases have already been delivered for clinical reasons—detected or undetected. Screening for SGA with a combination of ultrasonography and biochemical markers has been suggested and is being evaluated. 35 , 36 There are ongoing randomized trials investigating intervention based on Doppler measures when there is a suspicion of FGR. These studies will hopefully provide knowledge on how to identify and manage surveillance and timing of delivery when growth restriction is suspected.
Strengths of the current study are the large size of a non‐selected population and evaluation of the total populations, as the coverage is a matter of concern for all screening programs. This guarantees the generalizability of our results for populations with similar antenatal care systems. Within population A, we compared the characteristics of pregnant women who attended the ultrasound screening (population A1) and those who did not. The absolute differences were small although statistically significant because of the large numbers involved. Hence, we have no explanation for the low pick‐up rate in screening population A. The information on neonatal outcome was retrieved from a population‐based, high‐quality clinical register, keeping the lack of information and dropout rate to a minimum. Limitations of the study are its retrospective design and possible changes in the clinical management at participating centers during the time period covered. Another drawback is the lack of detailed information on the clinical surveillance protocols used on detected SGA fetuses. There were also some differences between the two populations but adjustments were made for this in the statistical analysis.
5. CONCLUSION
Routine ultrasound at 32+0–34+6 gestational weeks improves detection of SGA before birth compared with ultrasound examinations on indication. However, this study, in concordance with others, did not show convincing improvement of perinatal outcome even if a higher proportion of SGA fetuses was identified before birth. This emphasizes the need for effective and evidence‐based protocols for the management of pregnancies complicated by suspected growth restriction.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
All authors participated in the concept, design, interpretation of data, drafting and revising of the manuscript. AB and KK performed the analyses.
Supporting information
TABLE S1. Characteristics of subpopulations of women who attended screening ultrasound and those who did not within Population A
TABLE S2. Ability of routine ultrasound at 32–34 gestational weeks to predict SGA at birth in the scanned population (population A1). Based on the ROC curve with AUC 0.9 (95% CI 0.89–0.91)
TABLE S3. Perinatal outcome. Comparison between scanned pregnancies (population A1) and ultrasound on indication (population B); all infants
TABLE S4. Perinatal outcome. Comparison between scanned pregnancies (population A1) and ultrasound on indication (population B); infants born SGA
Bonnevier A, Maršál K, Källén K. Detection and clinical outcome of small‐for‐gestational‐age fetuses in the third trimester—A comparison between routine ultrasound examination and examination on indication. Acta Obstet Gynecol Scand.2022;101:102–110. doi: 10.1111/aogs.14278
Funding information
Region Skane, Department of Research and Development financially supported this study.
REFERENCES
- 1. Kramer MS, Olivier M, McLean FH, Willis DM, Usher RH. Impact of intrauterine growth retardation and body proportionality on fetal and neonatal outcome. Pediatrics. 1990;86:707‐713. [PubMed] [Google Scholar]
- 2. Mc Intire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999;340:1234‐1238. [DOI] [PubMed] [Google Scholar]
- 3. Flenady V, Koopmans L, Middleton P, et al. Major risk factors for stillbirth in high‐income countries: a systematic review and meta‐analysis. Lancet. 2011;377:1331‐1340. [DOI] [PubMed] [Google Scholar]
- 4. Moraitis AA, Wood AM, Fleming M, Smith GCS. Birth weight percentile and the risk of term perinatal death. Obstet Gynecol. 2014;124:274‐283. [DOI] [PubMed] [Google Scholar]
- 5. Deter RL, Lee W, Sangi‐Haghpeykar H, Kingdom J, Romero R. Third trimester growth restriction patterns: individualized assessment using a fetal growth pathology score. J Matern Fetal Neonatal Med. 2018;16:2155‐2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kayem G, Grange G, Bréart G, Goffinet F. Comparison of fundal height measurement and sonographically measured fetal abdominal circumference in the prediction of high and low birth weight at term birth weight at term. Ultrasound Obstet Gynecol. 2009;34:566‐571. [DOI] [PubMed] [Google Scholar]
- 7. Skråstad RB, Eik‐Nes SH, Sviggum O, et al. A randomized controlled trial of third‐trimester routine ultrasound in a non‐selected population. Acta Obstet Gynecol Scand. 2013;92:1353‐1360. [DOI] [PubMed] [Google Scholar]
- 8. Roma E, Arnau A, Berdala R, Bergos C, Montesinos J, Figueras F. Ultrasound screening for fetal growth restriction at 36 vs 32 weeks’ gestation: a randomized trial (ROUTE). Ultrasound Obstet Gynecol. 2015;46:391‐397. [DOI] [PubMed] [Google Scholar]
- 9. Bricker L, Medley N, Pratt JJ. Routine ultrasound in late pregnancy (after 24 weeks’ gestation). Cochrane Database Syst Rev. 2015;2015(6):CD001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sovio U, White IR, Dacey A, Pasupathy D, Smith GCS. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study. Lancet. 2015;386:2089‐2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henrichs J, Verfaille V, Jellema P, et al. Effectiveness of routine third trimester ultrasonography to reduce adverse perinatal outcomes in low risk pregnancy (the IRIS study): nationwide, pragmatic, multicentre, stepped wedge cluster randomised trial. BMJ. 2019;367:l5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lindqvist PG, Molin J. Does antenatal identification of small‐for‐gestational age fetuses significantly improve their outcome? Ultrasound Obstet Gynecol. 2005;25:258‐264. [DOI] [PubMed] [Google Scholar]
- 13. Akolekar R, Panaitescu AM, Ciobanu A, Syngelaki A, Nicolaides KH. Two‐stage approach for prediction of SGA neonate and adverse perinatal outcome by routine ultrasound examination at 35–37 weeks gestation. Ultrasound Obstet Gynecol. 2019;54:484‐491. [DOI] [PubMed] [Google Scholar]
- 14. Molin J. A regional perinatal database in southern Sweden—a basis for quality assurance in obstetrics and neonatology. Acta Obstet Gynecol Scand Suppl. 1997;164:37‐39. [PubMed] [Google Scholar]
- 15. Maršál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85:843‐848. [DOI] [PubMed] [Google Scholar]
- 16. Persson P‐H, Weldner B‐M. Intra‐uterine weight curves obtained by ultrasound. Acta Obstet Gynecol Scand. 1986;65:169‐173. [DOI] [PubMed] [Google Scholar]
- 17. Shepard M, Filly RA. A standard plane for biparietal diameter measurement. Ultrasound Med. 1982;1(4):145‐150. [DOI] [PubMed] [Google Scholar]
- 18. O’Brian GD, Queenan JT, Campell S. Assessement of gestational age in the second trimester by real time ultrasound measurement of the femur length. Am J Obstet Gynecol. 1981;139:540‐545. [DOI] [PubMed] [Google Scholar]
- 19. Eik‐Nes SH, Grøttum P, Persson P‐H, Maršál K. Prediction of fetal growth deviation by ultrasound biometry I. Methodology. Acta Obstet Gynecol Scand. 1982;61:53‐58. [DOI] [PubMed] [Google Scholar]
- 20. Maršál K. Obstetric management of intrauterine growth restriction. Best Pract Res Clin Obstet Gynaecol. 2009;23:857‐870. [DOI] [PubMed] [Google Scholar]
- 21. Eik‐Nes SH, Persson P‐H, Gröttum P, Maršál K. Prediction of fetal growth deviation by ultrasonic biometry. II Clinical application. Acta Obstet Gynecol Scand. 1983;62:117‐123. [DOI] [PubMed] [Google Scholar]
- 22. Laurin J, Persson P‐H. Ultrasound screening for detection of intra‐uterine growth retardation. Acta Obstet Gynecol Scand. 1987;66:493‐500. [DOI] [PubMed] [Google Scholar]
- 23. Gardosi J, Madurasinghe V, Williams M, Malik A, Francis A. Maternal and fetal risk factors for stillbirth: population based study. BMJ. 2013;346:f108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ego A, Monier I, Skaare K, Zeitlin J. Antenatal detection of fetal growth restriction and stillbirth risk: a population‐based case–control study. Ultrasound Obstet Gynecol. 2020;55:613‐620. [DOI] [PubMed] [Google Scholar]
- 25. Monier I, Blondel B, Ego A, Kaminiski M, Goffinet F, Zeitlin J. Poor effectiveness of antenatal detection of fetal growth restriction and consequences for obstetric management and neonatal outcomes: a French national study. Br J Obstet Gynecol. 2015;122:518‐527. [DOI] [PubMed] [Google Scholar]
- 26. Ley D, Laurin J, Bjerre I, Maršál K. Abnormal fetal aortic velocity waveform and minor neurological dysfunction at 7 years of age. Ultrasound Obstet Gynecol. 1996;8:152‐159. [DOI] [PubMed] [Google Scholar]
- 27. Ley D, Tideman E, Laurin J, Bjerre I, Maršál K. Abnormal fetal aortic velocity waveform and intellectual function at 7 years of age. Ultrasound Obstet Gynecol. 1996;8:160‐165. [DOI] [PubMed] [Google Scholar]
- 28. Tideman E, Maršál K, Ley D. Cognitive function in young adults following intrauterine growth restriction with abnormal fetal aortic blood flow. Ultrasound Obstet Gynecol. 2007;29:614‐618. [DOI] [PubMed] [Google Scholar]
- 29. Ley D, Maršál K, Dahlgren J, Hellström A. Abnormal retinal optic nerve morphology in young adults after intrauterine growth restriction. Pediatres. 2004;56:139‐143. [DOI] [PubMed] [Google Scholar]
- 30. Hellström A, Dahlgren J, Maršál K, Ley D. Abnormal retinal vascular morphology in young adults following intrauterine growth restriction. Pediatrics. 2004;113:e77‐e80. [DOI] [PubMed] [Google Scholar]
- 31. Brodszki J, Länne T, Maršál K, Ley D. Vascular growth in late adolescence after intrauterine growth restriction. Circulation. 2005;111:2623‐2628. [DOI] [PubMed] [Google Scholar]
- 32. Rizzo G, Mappa I, Bitsadze V, et al. Role of Doppler ultrasound at time of diagnosis of late‐onset fetal growth restriction in predicting adverse perinatal outcome: prospective cohort study. Ultrasound Obstet Gynecol. 2020;55:793‐798. [DOI] [PubMed] [Google Scholar]
- 33. Deter RL, Lee W, Yeo L, et al. Individualized growth assessment: conceptual framework and practical implementation for the evaluation of fetal growth and neonatal growth outcome. Am J Obstet Gynecol. 2018;218:S656‐S678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ciobanu A, Formuso C, Syngelaki A, Akolekar R, Nicolaides KH. Prediction of small‐for‐gestational age neonates at 35–37 weeks’ gestation: contribution of maternal factors and growth velocity between 20 and 36 weeks. Ultrasound Obstet Gynecol. 2019;53:488‐495. [DOI] [PubMed] [Google Scholar]
- 35. Gaccioli F, Sovio U, Cook E, Hund M, Charnock‐Jones DS, Smith GCS. Screening for fetal growth restriction using ultrasound and the sFLT1/PlGF ratio in nulliparous women: a prospective cohort study. Lancet Child Adolesc Health. 2018;8:569‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sovio U, Goulding N, McBride N, et al. A maternal serum metabolite ratio predicts fetal growth restriction at term. Nat Med. 2020;26:348‐353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1. Characteristics of subpopulations of women who attended screening ultrasound and those who did not within Population A
TABLE S2. Ability of routine ultrasound at 32–34 gestational weeks to predict SGA at birth in the scanned population (population A1). Based on the ROC curve with AUC 0.9 (95% CI 0.89–0.91)
TABLE S3. Perinatal outcome. Comparison between scanned pregnancies (population A1) and ultrasound on indication (population B); all infants
TABLE S4. Perinatal outcome. Comparison between scanned pregnancies (population A1) and ultrasound on indication (population B); infants born SGA


