Abstract
Introduction
Understanding whether human papillomavirus (HPV) may establish latency in the uterine cervix is important. A better understanding of HPV natural history is useful for clinical counseling of women attending screening and to accurately inform health prevention strategies such as screening and HPV vaccination. We evaluated the extent of latent HPV infections in older women with a history of abnormal cytology.
Material and Methods
We conducted a cross‐sectional study in Aarhus, Denmark, from March 2013 through April 2015. Women were enrolled if they underwent cervical amputation or total hysterectomy because of benign disease. Prior to surgery, women completed a questionnaire and a cervical smear was collected for HPV testing and morphological assessment. For evaluation of latency (i.e., no evidence of active HPV infection, but HPV detected in the tissue), we selected women with a history of abnormal cervical cytology or histology, as these women were considered at increased risk of harboring a latent infection. Cervical tissue underwent extensive HPV testing using the SPF10‐DEIA‐LipA25 assay.
Results
Of 103 women enrolled, 26 were included in this analysis. Median age was 55 years (interquartile range [IQR] 52–65), and most women were postmenopausal and parous. The median number of sexual partners over the lifetime was six (IQR 3–10), and 85% reported no recent new sexual partner. Five women (19.2%) had evidence of active infection at the time of surgery, and 19 underwent latency evaluation. Of these, a latent infection was detected in 11 (57.9%), with HPV16 being the most prevalent type (50%). Nearly 80% (n = 14) of the 18 women with a history of previous low‐grade or high‐grade cytology with no treatment had an active or latent HPV infection, with latent infections predominating. HPV was detected in two of the six women with a history of high‐grade cytology and subsequent excisional treatment, both as latent infections.
Conclusions
HPV can be detected in cervical tissue specimens without any evidence of an active HPV infection, indicative of a latent, immunologically controlled infection. Modeling studies should consider including a latent state in their model when estimating the appropriate age to stop screening and when evaluating the impact of HPV vaccination.
Keywords: gynecological pathology, human papillomavirus, molecular biology, uterine cervix, viral latency
Abbreviations
- HPV
Human papillomavirus
- HSIL
high‐grade intraepithelial lesion
- LSIL
low‐grade intraepithelial lesion
- ASC‐H
atypical squamous cells of undetermined significance – cannot exclude HSIL
- PCR
polymerase chain reaction
- FFPE
formalin‐fixed paraffin‐embedded
- IQR
interquartile range
- H&E
hematoxylin and eosin
- CIN2+
cervical intraepithelial neoplasia grade 2+
Key message.
Among women with a previous history of abnormal cervical cytology or histology, human papillomavirus can be detected in the uterine cervix without any evidence of active infection or disease, indicative of a latent state.
1. INTRODUCTION
Human papillomavirus (HPV) is the most common sexually transmitted infection and is causally related to cancer of the anogenital region, especially the cervix. 1 Upon infection, most individuals are able to mount an effective immune response within 1–2 years, resulting in loss of HPV detectability from exfoliated cervical cell samples – a phenomenon typically defined as viral clearance. 2 , 3 However, clinical studies with long‐term follow‐up have reported an increase in HPV prevalence following immune suppressive treatment due to organ transplantation 4 and recurrent HPV detectability among sexually monogamous and sexually abstinent women. 5 , 6 Others report a dose–response relationship between decreasing CD4+ T‐cell count/increasing HIV viral load and increasing HPV incidence and prevalence, irrespective of sexual behavior. 7 , 8 These findings suggest that the natural history of cervical HPV infection is complex.
Animal studies have reported that the virus may be found in a nonproductive phase in the basal cell layer of the epithelium after lesion resolution and that these nonproductive infections can reactivate upon iatrogenic immune suppression. 9 , 10 A limited number of human studies have demonstrated evidence of focal HPV in cervical tissues that is not detectable in normal exfoliated sampling. 11 , 12 This suggests a similar process of basal cell HPV latency and reactivation in the human cervix, though the extent of latent infection in women with previous infection is unknown. Quantifying the probability that clinically resolved HPV infection establishes latency in the human uterine cervix is of great importance to accurately inform health prevention initiatives such as HPV vaccination and cervical cancer screening 13 , 14 and for counseling of women attending cervical cancer screening.
We aimed to explore the extent of latent HPV infection among older women with a history of abnormal cytology and to discuss the potential clinical implications of these findings.
2. MATERIAL AND METHODS
We conducted a cross‐sectional study at the Department of Obstetrics and Gynecology, Aarhus University Hospital, Denmark, from March 1, 2013, through April 1, 2015. Women were eligible for enrollment if they were scheduled to have their cervix removed, (i.e., cervical amputation or as part of total hysterectomy) unrelated to epithelial abnormality of the cervix or cancer, were aged ≥50 years, were able to speak and understand Danish, and had no records of histologically verified cervical dysplasia within 5 years of surgery.
Information on basic demographics and medical and sexual behavior history was collected at enrollment via a self‐administered questionnaire. Dates and results of all previous cervical cytology and histology samples were retrieved from the Danish Pathology Databank, which was established in 1998 and collects information on all cyto‐ and histopathological specimens at the individual level. 15
Prior to surgery, an exfoliated cell sample was collected from enrolled women. Samples underwent morphological assessment using Focal Point™ (BD) and categorized according to the Bethesda classification system. 16 Samples were tested for HPV using the US FDA‐approved clinical assay cobas® (Roche Diagnostics), which allows for simultaneous detection of HPV 16 and 18 and pooled detection of 12 other high‐risk HPV types. Cervical samples were processed at the Department of Pathology, Randers, Denmark.
Immediately after surgery, the cervix was separated from the uterine corpus, sliced open in the anterior wall, and fixated to a Styrofoam plate covered with a sterile surgical glove, as illustrated elsewhere. 11 The specimen was fixed in formalin for a maximum of 24 h. At 1 day post‐surgery, the cervix was cut in 2–3 mm sections using sterile utensils to avoid cross‐contamination and embedded in paraffin. From each formalin‐fixed paraffin‐embedded (FFPE) tissue block, a hematoxylin and eosin (H&E) stained slide was evaluated by an experienced gynecological pathologist (EM) at the Department of Pathology, Aarhus University Hospital.
Women were eligible for the present analysis if they had a record of previous abnormal cytology or histology in the Danish Pathology Databank but no record of histologically verified cervical dysplasia within 5 years of surgery. Low‐grade disease was defined as a history of atypical squamous cells of undetermined significance, low‐grade intraepithelial lesion, or cervical intraepithelial neoplasia grade 1 (CIN1), whereas high‐grade disease was defined as a history of atypical squamous cells of undetermined significance – cannot exclude high‐grade intraepithelial lesion (ASC‐H), high‐grade intraepithelial lesion, or CIN grades 2 or 3. We focused on women with a previous history of cervical dysplasia as we hypothesized that they were more likely to have an active or latent infection detected, as previously demonstrated. 11 Women were defined as having an active infection if liquid‐based cytology tested positive for HPV or they had abnormal cytology or histology at the time of surgery. Women without evidence of an active infection were eligible for evaluation of HPV latency. If HPV was detected in tissue samples but without evidence of an active infection, women were defined as having a latent infection.
For 17 of the 19 women selected for latent HPV testing with available tissue blocks, each tissue block was sectioned into six sets; each set consisted of one section for H&E staining, three sections for HPV testing, five sections for additional analyses, and one section for H&E staining. Tissue sections for HPV testing underwent macro dissection to allow targeted collection of epithelial cells for analysis. 17 Sections from three sets were pooled into one tube, resulting in two HPV tests being performed on each block. DNA extraction was performed using the QIAsymphony DSP DNA mini kit (Qiagen) following the manufacturer's instructions at the Department of Pathology, Aarhus University Hospital, Denmark. The remaining two patients underwent more extensive testing, as described previously. 11
HPV testing of tissue specimens was carried out using the SPF10 polymerase chain reaction (PCR) followed by DEIA/LiPA25, version 1 at DDL Diagnostic Laboratory (Rijswijk, the Netherlands). The SPF10 PCR‐DEIA‐LiPA25, version 1 assay is highly sensitive and allows qualitative identification of the following HPV genotypes: 6, 11, 16, 18, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68/73, 70, and 74. Additionally, cervical cytology specimens from women for whom tissue testing indicated HPV‐positive results were retested using the SPF10 PCR‐DEIA‐LiPA25, version 1 system to confirm cobas® negative results.
To avoid cross‐contamination during sectioning, utensils were carefully cleaned with 1% sodium dodecyl sulphate and 99% ethanol between each block. Controls were incorporated as follows: (a) Sectioning included cutting an empty paraffin block between each patient, (b) PCR included two negative and one positive control, and (c) DEIA included a negative, a borderline (to establish the cut‐off for positivity), and a positive control. All controls were tested using the DEIA.
The frequency and proportions of HPV detection in exfoliated and tissue samples are presented, stratified by screening and treatment history.
2.1. ETHICAL APPROVAL
The study was approved by the Central Denmark Region Ethics Committee (1–10–72‐432‐12) and the Danish Data Protection Agency (1–16–02‐211‐12) on March 7, 2013. All women signed an informed consent form before enrollment.
3. RESULTS
A total of 103 women were enrolled and completed all study procedures, 26 of whom were included for analysis. According to the most severe prior abnormality, 11 (42.3%) women had documented low‐grade disease, nine (34.6%) had prior high‐grade disease without treatment, and six (23.1%) had prior high‐grade disease with subsequent excisional treatment (Figure 1). Women included were predominately postmenopausal (median age 55 years; interquartile range [IQR] 52–65), and the majority of women were parous and had previously used oral contraceptives (Table 1). The median age of sexual debut was 17 years (IQR 15–18), and the median number of lifetime sexual partners was six (IQR 3–10). Only four women (15.4%) reported having had a new sexual partner in their current decade. We found no meaningful difference in basic characteristics between these 26 women and all women enrolled, except for differences in previous history of cervical dysplasia and the proportion of women reporting more than five sexual partners in a lifetime.
FIGURE 1.
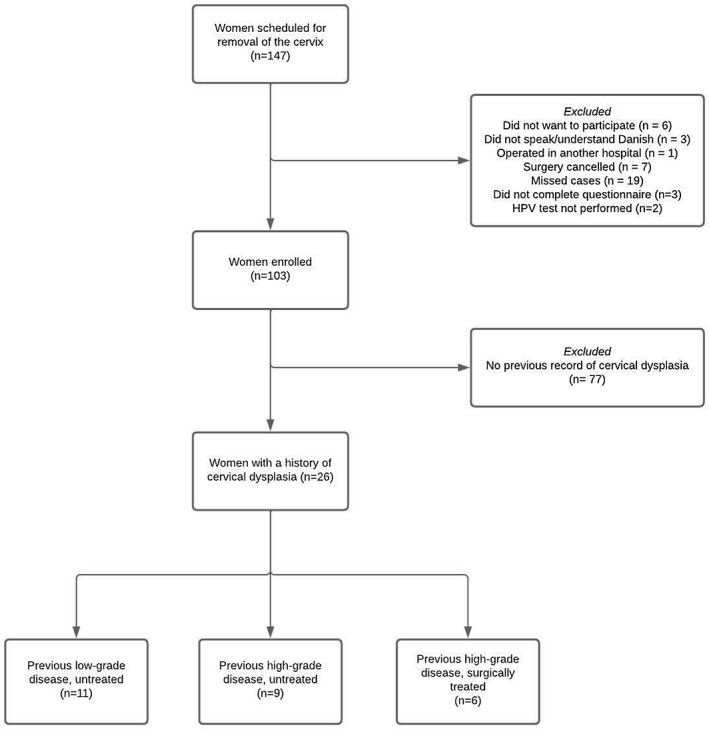
Flowchart of women included in the present analysis. HPV, human papillomavirus
TABLE 1.
Basic characteristics of all women enrolled and women with a previous history of cervical dysplasia
| Variable |
All women enrolled (n = 103) |
Women with previous cervical dysplasia (n = 26) |
|---|---|---|
| Age at enrolment | 56 (52–67) | 55 (52–65) |
| Age at sexual debut | 17 (16–19) | 17 (15–18) |
| Number of sexual partners in a lifetime | 5 (3–11) | 6 (3–10) |
| Parity | 2 (2–3) | 2 (2–3) |
| Surgery | ||
| Total hysterectomy | 88 (85.4) | 22 (84.6) |
| Cervical amputation | 15 (14.6) | 4 (15.4) |
| Currently married | ||
| Yes | 62 (60.2) | 13 (50.0) |
| No | 40 (38.8) | 12 (46.2) |
| Missing | 1 (1.0) | 1 (3.9) |
| Children | ||
| Yes | 94 (91.3) | 25 (96.2) |
| No | 8 (7.8) | 1 (3.9) |
| Missing | 1 (1.0) | 0 (0) |
| Education (college degree or higher) | ||
| Yes | 56 (54.4) | 14 (53.9) |
| No | 45 (43.7) | 11 (42.3) |
| Missing | 2 (1.9) | 1 (3.9) |
| Smoking (current or previous) | ||
| Yes | 50 (48.5) | 14 (53.9) |
| No | 51 (49.5) | 11 (42.3) |
| Missing | 2 (1.9) | 1 (3.9) |
| Medication (any) | ||
| Yes | 69 (67.0) | 14 (53.9) |
| No | 33 (32.0) | 11 (42.3) |
| Missing | 1 (1.0) | 1 (3.9) |
| Postmenopausal | ||
| Yes | 67 (65.1) | 17 (65.4) |
| No | 36 (35.0) | 9 (34.6) |
| Missing | 0 (0) | 0 (0) |
| HPV vaccination | ||
| Yes | 4 (3.9) | 2 (7.7) |
| No | 97 (94.2) | 23 (88.5) |
| Missing | 2 (1.9) | 1 (3.9) |
| Previous use of oral contraceptives | ||
| Yes | 72 (69.9) | 16 (62.5) |
| No | 30 (29.1) | 9 (34.6) |
| Missing | 1 (1.0) | 1 (3.9) |
| Previous or current hormonal replacement therapy | ||
| Yes | 26 (25.2) | 3 (11.5) |
| No | 72 (69.9) | 20 (76.9) |
| Missing | 5 (4.9) | 3 (11.5) |
| Age at sexual debut | ||
| <17 years | 35 (34.0) | 11 (42.3) |
| ≥17 years | 68 (66.0) | 15 (57.7) |
| Missing | 0 (0) | 0 (0) |
| Number of sexual partners in a lifetime | ||
| <5 | 48 (46.6) | 3 (18.8) |
| ≥5 | 54 (52.4) | 13 (81.3) |
| Missing | 1 (1.0) | 0 (0) |
| History of genital warts (self‐reported) | ||
| Yes | 13 (12.6) | 4 (15.4) |
| No | 89 (86.4) | 22 (84.6) |
| Missing | 1 (1.0) | 0 (0) |
| History of abnormal cervical cytology or histology a | ||
| Low grade | 10 (9.7) | 11 (42.3) |
| High grade | 15 (14.6) | 15 (57.7) |
| Normal | 78 (75.7) | 0 (0) |
| Previous excisional treatment of the cervix | ||
| Yes | 6 (5.8) | 6 (23.1) |
| No | 97 (94.2) | 15 (57.7) |
| Missing | 0 (0) | 0 (0) |
Note: Data are presented as median (interquartile range) or n (%).
Abbreviations: HPV, human papillomavirus; HSIL, high‐grade intraepithelial lesion.
Record of abnormal cervical cytology or histology in the Danish National Pathology Databank. Low grade refers to a history of atypical squamous cells of undetermined significance, low‐grade squamous intraepithelial lesion, or cervical intraepithelial neoplasia grade 1. High grade refers to high‐grade squamous intraepithelial lesion, atypical squamous cells – cannot exclude high‐grade squamous intraepithelial lesion, adenocarcinoma in situ, or cervical intraepithelial neoplasia grades 2 and 3.
Of the 26 women included, five (19.2%) had evidence of active infection at the time of surgery (Figure 2). Two women were positive for non‐HPV16/18 HPV types, one had atypical squamous cells of undetermined significance – cannot exclude high‐grade intraepithelial lesion (cytology), one had high‐grade intraepithelial lesion (cytology), and one had low‐grade intraepithelial lesion (cytology) in combination with non‐HPV16/18 HPV types. In women with prior low‐grade disease or high‐grade disease without treatment, three (27.3%) and two (22.2%), respectively, had evidence of active infection. No women with previously treated high‐grade cytology had active HPV infection detected. Of women with active infection, one reported having had a new sexual partner, two reported no new partner, and two did not reply. All women had more than five sexual partners over their lifetime.
FIGURE 2.
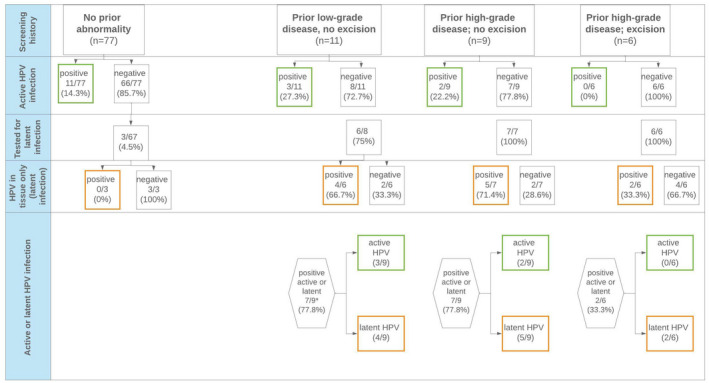
Active and latent human papillomavirus (HPV) infections among women aged ≥50 years with a previous history of cervical abnormality. Active infection is defined as women with HPV‐positive results on liquid‐based cytology and/or who had atypical squamous cells of undetermined significance or worse detected on cytology and/or who had koilocytosis or cervical intraepithelial neoplasia grade 1 or worse detected on histology at the time of surgery. Latent infection is defined as no evidence of active HPV infection but HPV detected in the cervical tissue
A total of 21 women were eligible for latency testing. Tissue blocks could not be retrieved in two women, leaving 19 women for evaluation. HPV was detected in the cervical tissue of 11 women (57.9%), indicative of latency. For 17 of the 19 women, a total of 161 tissue blocks were available for testing, resulting in nearly 20 HPV analyses for each woman. Of 305 HPV analyses completed, 16 (5.2%) were positive for HPV. The remaining two women were previously tested using more extensive sampling as previously described. 11 Of 428 HPV analyses completed, 15 (3.5%) were positive for HPV.
Half of the HPV‐positive PCR tests were positive for HPV16, and HPV6 was detected in two (12.5%). HPV18, HPV33, HPV56, and HPV66 were each detected in one PCR test (5%). An additional two PCR tests were positive on the DEIA, but the specific genotype was not identified. The number of positive HPV test results per woman ranged from one to four. HPV16, HPV18, and HPV66 was detected in separate blocks for one woman. No single tissue section contained multiple HPV types. One of the controls, derived from a clean paraffin block, tested positive for HPV 16, but the same genotype was not detected in the adjacent tissue specimen (HPV33).
Among women with samples undergoing latency testing, the prevalence of latent infection was highest in those with prior low‐grade cytology (4/6; 66.7%) and women with prior high‐grade cytology and no excisional treatment (5/7; 71.4%) (Figure 2). Women with prior high‐grade cytology and subsequent excisional treatment were less likely to have HPV detected in tissue (2/6; 33.3%). Among the women with evidence of latent HPV infection, two (18.2%) reported having a new sexual partner and four (36.4%) reported having five or more sexual partners over their lifetime (data not shown).
We retested liquid‐based cytology samples from women with HPV‐positive tissue samples using the SPF10‐PCR‐DEIA‐LiPA25 HPV assay. One cobas‐negative liquid‐based cytology sample tested positive by DEIA, but we were unable to determine the genotype. Results from the remaining 10 women were confirmed as HPV negative.
4. DISCUSSION
In our study, we found that 19.2% (n = 5) of older women with a previous history of cervical dysplasia had evidence of active HPV infection at the time of surgery, and latent HPV was detected in 57.9% (n = 11) of women undergoing evaluation of latency. Although the sample size was very small, women with a history of excisional treatment were less likely to have HPV detected (active and latent) than were women without this treatment.
Measurement of an individual's HPV status in both clinical and epidemiological studies is typically based on detection of HPV DNA from an exfoliated cell sample. This sample is insufficient for determination of the presence of immunologically controlled (latent) HPV infection, as there is no viral replication and therefore no shedding of viral particles. 9 , 10 Although immunologically controlled infections (i.e., HPV‐DNA‐negative cervical cytology samples) pose low immediate risk of CIN2+, 18 loss of immune control may lead to viral reactivation and subsequent development of cervical precancer, as has been observed following iatrogenic immune suppression of transplant patients and following HIV infection. 4 , 7 , 8 Evidence for HPV DNA in full‐thickness cervical tissue that is not detected from exfoliated samples has been previously described. 11 In this report, we sought to expand this anecdotal evidence by estimating the proportion of women with previous cervical dysplasia harboring undetectable, controlled (latent) HPV infections.
Because our prior work showed that the tissue distribution of controlled latent infection is likely limited and extremely focal, 11 for efficiency we focused this evaluation of latent HPV infection on women with a national registry‐confirmed history of cytological or histological abnormality. Our results suggest that over two‐thirds of women with a history of any abnormality, particularly those who went untreated, harbored HPV infections, the majority of which were only detectable as latent infection and likely reflected controlled infection. Previous studies have shown that women without detectable HPV in screening are adequately controlling infections and are at a low risk of cervical precancer and cancer at least for the next 5 years. 18 However, given that immunologic control of HPV (or latency) is a reversible state, in contrast to immune clearance, our data may suggest that guidelines recommending the age and conditions under which women may be safely exited from cervical screening be reconsidered. Current guidelines from the United States 19 , 20 recommend against screening in women aged >65 years if (a) their last three consecutive cytology tests in the past 10 years were negative or their last two consecutive HPV tests were negative and (b) they have not been diagnosed with CIN2+ in the past 25 years. Given the results presented here, a majority of women without histologically confirmed disease but a history of positive cervical cytology or histology may be exiting screening with latent HPV. Immunosenescence of aging, as well as increasing comorbidities and medication use in older women, might be expected to result in reversal of immune control (i.e., HPV reactivation) in at least a proportion of these women after exiting routine screening. These findings may suggest a need to also include previous low‐grade disease as a screening exit criterion. However, this may prove to be difficult as not all countries have a national pathology register, so some clinicians must rely on the woman's ability to recall previous screening test results.
Given the transitioning to HPV‐based screening, our understanding of age‐specific HPV natural history and cervical cancer risk is evolving. New HPV detection can occur at all ages, but an increasing proportion of newly detected HPV in women aged >40 years represents recurrent detection of a previously acquired infection, not a newly acquired infection 5 , 6 , 21 , 22 . We emphasize that, although incident detection of HPV is often lower at older ages in well‐screened populations, the proportion of newly HPV‐positive women ultimately diagnosed with high‐grade lesions or cancer is similar across all ages. 23 In fact, recent studies performing diagnostic excision in older women with persistent HPV infection in the absence of cytological abnormalities found a significant prevalence of underlying CIN2+, 24 suggesting that the risk of precancer in older women may be underestimated. Gilham et al 25 highlighted that 40% of cancers in the UK diagnosed after the age of 65 years occurred in women who had regular screening and were appropriately exited from the screening program. Clinical HPV tests set viral load detection thresholds to optimize the sensitivity and specificity for CIN2+ detection, which was largely driven by precancers diagnosed in younger women. These data, as well as the possible differences in viral replication capacity in atrophied epithelium, suggest that a more sensitive HPV test may be needed to safely exit women from screening.
The persistent risk of precancer and cancer across the lifespan described is particularly concerning given evidence that older women are screened less frequently, 26 may be more likely to actively decide not to screen, 26 and are more likely to perceive a lower risk of cervical cancer because of their current sexual activity. 6 , 26
Finally, because routine HPV testing has only relatively recently been initiated on a population level, we rely heavily on simulation models to set policy decisions for older women, such as those regarding age to exit screening and cost effectiveness of vaccination. 13 , 19 , 27 To our knowledge, only one model specifically included a latent‐reactivated infection transition state, 14 and this demonstrated substantially lower vaccine efficacy in previously exposed women compared with models that did not include latency. Time series study designs such as those proposed for younger women 28 are needed across diverse age groups to accurately inform model assumptions about the source and determinants of fluctuating HPV detection. 5
A major limitation of the present study was the low sample size, which precludes a formal statistical analysis. Thus, results should be interpreted with caution. As we tested a selected group of women considered at increased risk of testing positive, we cannot infer that the observed prevalence reflects the true burden of HPV latency in a general population. To obtain an accurate prevalence of true latency, an intensive sampling is required, 11 and we only tested a small fraction of the uterine cervix compared with previous work. 11 Thus, we cannot rule out that the prevalence observed in the current study would have been higher had we analyzed the entire cervix in all women. The use of FFPE tissue may have resulted in a lower detection rate as the FFPE process is known to affect PCR performance. 29 However, for this study, a lower detection rate is not likely as the SPF10‐PCR amplifies a fragment of only 65 base pairs and has been shown to have equal HPV detection rates when comparing smears and FFPE tissue taken from the same women. 30 Pooling of tissue from three sets into one tube may have diluted a latent infection below detectable levels. Yet, our findings clearly demonstrate that HPV can be detected in the cervical epithelium with no evidence of an active infection, suggesting a latent state. Strengths include the use of a very sensitive PCR‐based HPV assay and use of sterile utensils throughout the entire process, limiting the risk of contamination. Unfortunately, one of the controls tested positive for HPV16, which may suggest cross‐contamination during sectioning or macro dissection. However, the same genotype was not found in the adjacent tissue slide.
5. CONCLUSION
HPV can be detected in the cervix without any evidence of an active infection, indicative of viral latency. A better understanding of risk factors associated with loss of immunologic control and subsequent redetection is needed and may enable the creation of targeted intervention strategies. Moreover, modeling studies should carefully consider including a latent state when estimating the effectiveness of HPV vaccination and the appropriate age at which to exit screening.
CONFLICT OF INTEREST
AH has received a grant from Sanofi Pasteur, Denmark, for the current study. The funders had no role in the design of the study, in the interpretation of results, or the decision to publish. AH has received a speaker’s fee from Astra Zeneca and reagents from Roche, Denmark, outside the submitted work. MK is employed by and is a <1% equity holder of the Viroclinics group of companies, Rotterdam, the Netherlands and has no conflicts of interest. JB, TS, EM, HS, MR, KF, JD, RA, WQ, and PG have no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors: conceptualization, methodology, supervision, review and editing. AH, KF, MR: data curation. AH, KF, MR, MdK, WQ, JD, TS, EM, HS, RHA, PG: formal analysis. AH, JB, PG, WQ: Funding acquisition. AH, JB, TS, HS, EM: Project administration. AH and PG: writing draft. The final manuscript was approved by all authors.
ACKNOWLEDGMENTS
The authors thank Ulla Kehlet for assistance with enrolment of participants.
Hammer A, Blaakaer J, de Koning MN, et al. Evidence of latent HPV infection in older Danish women with a previous history of cervical dysplasia. Acta Obstet Gynecol Scand. 2022;101:608‐615. doi: 10.1111/aogs.14362
Funding informationThis study was funded by research grants from the Danish Cancer Society, Einar Willumsen's Foundation, Dagmar Marshall's foundation, Aase and Einar Danielsen's foundation, and Sanofi Pasteur, Denmark.
REFERENCES
- 1. Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12‐19. [DOI] [PubMed] [Google Scholar]
- 2. Moscicki AB, Schiffman M, Burchell A, et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine. 2012;30(Suppl 5):F24‐F33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890‐907. [DOI] [PubMed] [Google Scholar]
- 4. Hinten F, Hilbrands LB, Meeuwis KAP, et al. Reactivation of latent HPV infections after renal transplantation. Am J Transplant. 2017;17:1563‐1573. [DOI] [PubMed] [Google Scholar]
- 5. Liu SH, Cummings DA, Zenilman JM, et al. Characterizing the temporal dynamics of human papillomavirus DNA detectability using short‐interval sampling. Cancer Epidemiol Biomark Prev. 2014;23:200‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paul P, Hammer A, Rositch AF, et al. Rates of new human papillomavirus detection and loss of detection in middle‐aged women by recent and past sexual behavior. J Infect Dis. 2021;223:1423‐1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus‐positive women. J Natl Cancer Inst. 2005;97:577‐586. [DOI] [PubMed] [Google Scholar]
- 8. Heard I, Tassie JM, Schmitz V, Mandelbrot L, Kazatchkine MD, Orth G. Increased risk of cervical disease among human immunodeficiency virus‐infected women with severe immunosuppression and high human papillomavirus load(1). Obstet Gynecol. 2000;96:403‐409. [DOI] [PubMed] [Google Scholar]
- 9. Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology. 2011;414:153‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maglennon GA, McIntosh PB, Doorbar J. Immunosuppression facilitates the reactivation of latent papillomavirus infections. J Virol. 2014;88:710‐716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hammer A, de Koning MN, Blaakaer J, et al. Whole tissue cervical mapping of HPV infection: molecular evidence for focal latent HPV infection in humans. Papillomavirus Res. 2019;7:82‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leonard SM, Pereira M, Roberts S, et al. Evidence of disrupted high‐risk human papillomavirus DNA in morphologically normal cervices of older women. Sci Rep. 2016;6:20847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malagon T, Kulasingam S, Mayrand MH, et al. Age at last screening and remaining lifetime risk of cervical cancer in older, unvaccinated, HPV‐negative women: a modelling study. Lancet Oncol. 2018;19:1569‐1578. [DOI] [PubMed] [Google Scholar]
- 14. van Schalkwyk C, Moodley J, Welte A, Johnson LF. Estimated impact of human papillomavirus vaccines on infection burden: the effect of structural assumptions. Vaccine. 2019;37:5460‐5465. [DOI] [PubMed] [Google Scholar]
- 15. Erichsen R, Lash TL, Hamilton‐Dutoit SJ, Bjerregaard B, Vyberg M, Pedersen L. Existing data sources for clinical epidemiology: the Danish National Pathology Registry and data Bank. Clin Epidemiol. 2010;2:51‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nayer RWG. The Bethesda System for Reporting Cervical Cytology, Definitions, Criteria, and Explanatory Notes. 3rd ed. Springer; 2015. [Google Scholar]
- 17. Lade‐Keller J, Romer KM, Guldberg P, et al. Evaluation of BRAF mutation testing methodologies in formalin‐fixed, paraffin‐embedded cutaneous melanomas. J Mol Diagn. 2013;15:70‐80. [DOI] [PubMed] [Google Scholar]
- 18. Hammer A, Demarco M, Campos N, et al. A study of the risks of CIN3+ detection after multiple rounds of HPV testing: results of the 15‐year cervical cancer screening experience at Kaiser Permanente northern California. Int J Cancer. 2020;147:1612‐1620. [DOI] [PubMed] [Google Scholar]
- 19. Force USPST, Curry SJ, Krist AH, et al. Screening for cervical cancer: US preventive services task Force recommendation statement. JAMA. 2018;320:674‐686. [DOI] [PubMed] [Google Scholar]
- 20. Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk‐based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rositch AF, Burke AE, Viscidi RP, Silver MI, Chang K, Gravitt PE. Contributions of recent and past sexual partnerships on incident human papillomavirus detection: acquisition and reactivation in older women. Cancer Res. 2012;72:6183‐6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fu TC, Carter JJ, Hughes JP, et al. Re‐detection vs. new acquisition of high‐risk human papillomavirus in mid‐adult women. Int J Cancer. 2016;139:2201‐2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gage JC, Katki HA, Schiffman M, et al. Age‐stratified 5‐year risks of cervical precancer among women with enrollment and newly detected HPV infection. Int J Cancer. 2015;136:1665‐1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. St‐Martin G, Viborg PH, Andersen ABT, et al. Histological outcomes in HPV‐screened elderly women in Denmark. PLoS One. 2021;16:e0246902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gilham C, Crosbie EJ, Peto J. Cervical cancer screening in older women. BMJ. 2021;372:n280. [DOI] [PubMed] [Google Scholar]
- 26. Marlow LAV, Ryan M, Waller J. Increasing the perceived relevance of cervical screening in older women who do not plan to attend screening. Sex Transm Infect. 2020;96:20‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Massad LS. New guidelines on cervical cancer screening: more than just the end of annual pap testing. J Low Genit Tract Dis. 2012;16:172‐174. [DOI] [PubMed] [Google Scholar]
- 28. Murall CL, Rahmoun M, Selinger C, et al. Natural history, dynamics, and ecology of human papillomaviruses in genital infections of young women: protocol of the PAPCLEAR cohort study. BMJ Open. 2019;9:e025129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greer CE, Lund JK, Manos MM. PCR amplification from paraffin‐embedded tissues: recommendations on fixatives for long‐term storage and prospective studies. PCR Methods Appl. 1991;1:46‐50. [DOI] [PubMed] [Google Scholar]
- 30. Quint WG, Scholte G, van Doorn LJ, et al. Comparative analysis of human papillomavirus infections in cervical scrapes and biopsy specimens by general SPF(10) PCR and HPV genotyping. J Pathol. 2001;194:51‐58. [DOI] [PubMed] [Google Scholar]


