Abstract
Introduction
Cesarean scar defect (CSD) is a long‐term outcome of cesarean section (CS) and associated with numerous gynecological and obstetric problems. Previous studies indicate that infection may be a risk factor for CSD. Adjunctive azithromycin was shown to reduce the risk of postoperative infection in patients undergoing non‐elective primary cesarean delivery in labor or after the rupture of membranes compared with standard antibiotic prophylaxis. This study investigated the protective effect of adjunctive azithromycin in combination with single‐dose cephalosporin against CSD in women undergoing non‐elective cesarean delivery.
Material and methods
A randomized, double‐blind, controlled clinical trial was conducted in a University hospital in Shanghai, China. A total of 242 women who underwent their first non‐elective CS were randomly assigned to receive 1500 mg cefuroxime sodium plus 500 mg intravenous azithromycin (n = 121; experimental group) or 1500 mg cefuroxime sodium plus a placebo (n = 121; placebo group). The primary outcome was CSD prevalence, as determined by transvaginal ultrasound and saline infusion sonohysterography within 6 months of delivery. Secondary outcomes were changes in infectious indicators (eg hypersensitive C‐reactive protein and procalcitonin), postoperative morbidity, and use of postoperative antibiotics. We also examined the operative procedure, pathogenic microorganism cultures, and fetal outcomes. Outcomes were compared between groups with the chi‐squared test, Fisher's exact test, or Student's t test.
Results
Between May 2018 and May 2021, 121 women were randomized to each arm. Because the sonographic follow up was disrupted by the coronavirus disease 2019 pandemic and strict management policies, we merged the follow‐up time points (6 weeks and 6 months) into a single time period (6 weeks to 6 months); 104 and 108 women in the experimental and placebo groups, respectively, completed the first sonographic follow up. CSD was diagnosed by sonography in 34/104 (32.7%) and 50/108 (46.3%) patients in the experimental and placebo groups, respectively (relative risk 0.71, 95% confidence interval 0.50–0.99; p = 0.043). Characteristics of CSD and short‐term infection outcomes did not differ between groups.
Conclusions
A single dose of intravenous 500 mg azithromycin adjunctive to single‐dose cefuroxime prophylaxis significantly reduced the incidence of CSD in women undergoing non‐elective CS.
Keywords: azithromycin, cefuroxime sodium, cesarean delivery, cesarean scar defect, infection, randomized controlled trial
Abbreviations
- CS
cesarean section
- CSD
cesarean scar defect
- hsCRP
hypersensitive C‐reactive protein
- ROM
rupture of membranes
Key message.
One dose of 500 mg intravenous azithromycin, adjunctive to the standard single dose of cephalosporin prophylaxis, can significantly reduce the incidence of cesarean scar defect in women undergoing non‐elective cesarean sections.
1. INTRODUCTION
The rate of cesarean section (CS) delivery is high worldwide. 1 Between 2005 and 2014, the annual proportion of cesarean deliveries in the USA was 31.6%. 2 In China, the annual rate of cesarean deliveries increased between 2008 and 2014 to 34.9%. 3 Cesarean scar defect (CSD) is a long‐term outcome of CS, 4 which was formally defined by the European Niche Taskforce as an indentation of the uterine myometrium of at least 2 mm at the site of the cesarean scar, as determined by transvaginal ultrasound. 5 Due to structural changes in the anterior uterine wall and inflammatory factor aggregation or blood vessel hyperplasia in the CSD, the outflow of menstrual blood may be hindered and the endometrial cycle may be desynchronized. 6 , 7 CSD is associated with numerous gynecological and obstetric problems including abnormal uterine bleeding, postmenstrual spotting, subfertility, and even life‐threatening cesarean scar pregnancy in post‐CS women. 8 , 9 , 10 In a previous cohort study, we showed that the prevalence of CSD in Shanghai was 43.4% (95% confidence interval [CI] 39.1%–47.7%), with the number of women with CSD increasing by about 3 million each year in China. 11
The etiology of CSD has yet to be fully elucidated because conclusive data are lacking. Notably, it is unclear whether a double‐layer suture of the uterine incision is better than a single‐layer suture. 12 , 13 In particular, there is a lack of research on patient‐related factors such as infection and genetic differences, which may be underestimated. 14 Our previous cohort study found that multi‐dose administration of perioperative antibiotics had a significant protective effect against CSD compared with single‐dose administration (CSD prevalence: 31.1%, 95% CI 23.8%–38.3% vs 49.0%, 95% CI 43.8%–54.3%; adjusted odds ratio [aOR] = 0.4, 95% CI 0.3–0.7), corresponding to a 36.5% reduction in CSD rate. A subgroup analysis showed that the occurrence of CSD decreased from 47.9% (69/144) to 29.1% (30/103) after administration of multi‐dose antibiotics compared with single‐dose antibiotics for non‐elective cesarean delivery. 11
Ureaplasma urealyticum, Escherichia coli, Enterococcus, and Streptococcus are the most common agents of nosocomial infections. 15 , 16 Second‐generation cephalosporins have a more potent anti‐bacterial effect against E. coli and Enterobacteriaceae than first‐generation cephalosporins. Cefuroxime sodium was shown to have a significant advantage over cefradine in CSD prevention in our previous cohort study (aOR 0.5, 95% CI 0.3–0.8, p = 0.006), 11 which is a first‐line prophylactic antibiotic recommended by Chinese treatment guidelines for CS and is widely used in clinical practice, but azithromycin is known to exert stronger antibacterial and bacteriostatic effects against atypical pathogens such as Mycoplasma, Chlamydia, and anaerobic bacteria. 17 The C/SOAP trial demonstrated that adjunctive azithromycin reduced the risk of postoperative infection in patients undergoing non‐elective primary cesarean delivery in labor or after the rupture of membranes (ROM). 18 Based on the above findings, we speculated that adjunctive prophylactic azithromycin with standard single‐dose antibiotics for non‐elective cesarean delivery could reduce the occurrence of CSD by extending the antibacterial spectrum and enhancing antibiotic potency. To test this hypothesis, we carried out a randomized, double‐blind, controlled clinical trial to investigate the effect of adjunctive azithromycin (500 mg, intravenous) on CSD occurrence in women undergoing non‐elective cesarean delivery who were already receiving a standard single dose of cefuroxime sodium (1500 mg, intravenous).
2. MATERIAL AND METHODS
2.1. Study design
This double‐blind, randomized, placebo‐controlled trial was conducted at a single hospital affiliated with the medical school of a university in Shanghai, China. The study protocol was published before the start of data analysis. 19
2.2. Participants
We trained senior residents in the wards and labor room of the hospital on the study criteria and procedure for obtaining consent from patients before their enrollment, and ensured that they were familiar with universal definitions of maternal medical comorbidities— eg (gestational) diabetes mellitus, (gestational) hypertension, and premature ROM. According to the premature ROM guidelines of the American College of Obstetricians and Gynecologists and national guidelines of China, women with ROM for more than 12 hours without Group B Streptococcus haemolyticus infection were treated with an oral antibiotic (typically 250 mg cefradine every 6 h if there was no allergy) until vaginal labor or before CS. 20 Pregnant women with singleton pregnancies who had ROM (spontaneous or iatrogenic) or were in labor were informed of the study by the senior residents in the ward or labor room. Labor was defined as regular contraction with cervical dilatation of at least 3 cm or with documented cervical change of at least 1 cm of dilatation or 50% or more effacement. Patients were eligible for the study if they underwent a primary, non‐elective CS (i.e. unscheduled CS during labor, after membrane rupture), were 18 years or older, and were at at least 37 weeks of gestation.
Patients were excluded if they had fibrinogen less than 2 g/L, platelet count less than 100 × 109/L, or hemoglobin less than 90 g/L before CS; had a known allergy to cefuroxime sodium or azithromycin; had received azithromycin within 7 days before randomization; were positive for Group B streptococci at 36 weeks of gestation (a condition requiring additional antibiotic administration after labor or ROM); were diagnosed with infection (chorioamnionitis, appendicitis, upper respiratory infection, or urinary tract infection) requiring additional antibiotic treatment; had severe maternal diseases (severe liver or renal dysfunction, pulmonary edema, cardiac structural abnormality, or a condition requiring antiarrhythmic drug use, systematic lupus erythematosus, or inadequately controlled diabetes); had a preoperative diagnosis of uterine abnormalities (eg uterine malformation, adenomyosis, or myoma); or had previously undergone CS.
The surgeons in our team checked the patients with respect to the CS indication and inclusion criteria when a non‐elective CS was decided by the ward or labor room attendant. Women who met the inclusion criteria and were willing to participate provided written, informed consent after the standard CS consent form was signed before CS.
2.3. Random allocation to intervention
After giving informed consent, participants were randomly allocated to receive 1500 mg cefuroxime sodium plus 500 mg intravenous azithromycin as the experimental (experimental) group or 1500 mg cefuroxime sodium plus placebo as the control (placebo) group within 30 min before skin incision.
2.4. Masking of intervention
Randomization codes were generated in a 1:1 ratio using SPSS v22.0 software (IBM). A list of 242 randomization codes (“A” for the azithromycin group and “B” for the placebo group) was generated where each code had a corresponding serial number. The codes were placed in a black envelope with the serial number printed on the outside. Anyone who had contact with the patient or other researchers was blinded to the list of codes.
Medicines were prepared in the hospital dispensary after randomization. The patients' envelopes were opened by an independent pharmacist, and the medications were in identical containers to ensure that they could not be identified. Envelopes were closed and locked in a non‐transparent box until follow up was completed. Participants, surgeons, operating room and ward staff, and sonographers were all blinded to the antibiotics used.
2.5. Cesarean delivery
Four experienced surgeons performed the cesarean deliveries. In the operating room, blood and vaginal secretion samples were collected from the patient and the vagina and skin of the abdomen were disinfected with povidone iodine solution. In both study arms, women underwent a CS with a standard mode transverse incision in the skin and transverse uterotomy in the lower segment of the uterus. After delivery of the fetus and placenta, the surgeon checked whether there was laceration of the incision and obtained a sample from the incision with a sterile cotton swab for pathological microorganism culture. If needed, lacerations (defined as an irregular myometrium tear longer than 5 mm along the edge of incision) were repaired with sutures. If a hemostatic procedure was required after uterotonic handling of postpartum hemorrhage, the surgeon used a B‐Lynch suture or placed a balloon in the uterine cavity (that was removed transvaginally 12 h after CS) to achieve hemostasis. We applied standard double‐layer unlocked and continuous multifilament sutures to both layers in uterine suturing; a large part of the myometrium and endometrium were included in the first layer. A continuous running suture was used for the second layer; which imbricated the first layer, including the serosal and myometrial tissues.
2.6. Outcomes
The primary outcomes were the presence of CSD 6 weeks post‐CS, CSD measurements, residual myometrium thickness, and adjacent myometrium thickness. The secondary outcomes were as follows: (a) presence of CSD 6 months post‐CS; (b) infection indicators including hypersensitive C‐reactive protein (hsCRP) and procalcitonin (measured immediately before antibiotic administration for pre‐CS and 24 h after CS for post‐CS); (c) pre‐CS vaginal secretion and intraoperative uterine cavity culture results; (d) body temperature and type and dosage of all antibiotics administered post‐CS at the hospital; and (e) postoperative morbidity, endometritis, and skin infection after CS or other infection 42 days after CS. The study was unblinded to the researchers who performed the data analysis after the first ultrasonic follow up, and they did not participate in the ongoing follow up to avoid biasing the results.
Women and newborns underwent postpartum observation and were given medical treatment at the hospital before discharge, if necessary. Other necessary antibiotics (except azithromycin) were given to patients according to medical indications (eg sign of infection). Postpartum morbidity, endometritis, skin incision infection, and other forms of infection within 42 days after delivery were recorded during follow up. Endometritis was defined as the presence of at least two of the following signs, with no other recognized cause: fever (body temperature 38°C or higher), abdominal pain, uterine tenderness, or purulent drainage from the uterus. Wound infection was defined as the presence of either superficial or deep incisional surgical‐site infection, characterized by cellulitis or erythema and induration around the incision; or purulent discharge from the incision site with or without fever and including necrotizing fasciitis. Other infections included abdominopelvic abscess, septicemia, pelvic septic thrombophlebitis, pyelonephritis, pneumonia, or meningitis.
All participants were recalled for examination between 6 weeks and 6 months after cesarean delivery for evaluation of the presence of CSD in the scar region, CSD size and shape, residual myometrium thickness, and adjacent myometrium thickness. Procedures were conducted according to relevant guidelines. 5 Measurements were taken by two experienced sonographers blinded to the clinical information with the same standard. One sonographer examined the patient in the lithotomy position and with an empty bladder using a 3–12‐MHz transvaginal ultrasound probe (WS80A ultrasound scanner; Samsung). Saline infusion sonohysterography was performed with progression towards the inside of the endometrial cavity via the cervical os with a polyethylene insemination catheter by another sonographer to obtain a consensus diagnosis. Images and measurements were recorded by both sonographers who worked randomly.
2.7. Statistical analyses
All statistical analyses were performed with SPSS v22.0. A two‐sided p < 0.05 was considered statistically significant. Categorical measurements are presented as frequencies and percentages while continuous measurements are presented as means and standard deviations (or median and range, as appropriate). The chi‐squared or Fisher's exact test was used to analyze categorical variables and the Student's t test was used for continuous variables in the baseline and outcomes. Infective variables associated with CSD in univariate analysis (p < 0.20) were included as covariates in multivariable binary logistic regression analysis using the forward L‐R method (stepwise removal probability of 0.10). Odds ratios, aORs, and 95% CIs were calculated for outcomes and the CSD risk analysis.
2.8. Sample size calculation
Based on the assumption that the occurrence of CSD after standard cefuroxime sodium or adjunctive azithromycin prophylactics would be similar to that found in our previous data (47.9% for multi‐dose antibiotics vs 29.1% for single‐dose antibiotic administration in non‐elective CS), 11 we performed a power calculation using PASS v11.0 sample prediction software (NCSS) and determined that 220 participants (two groups of 110 women; alpha error = 0.05, beta = 0.15) were required. Assuming a 10% dropout rate, we aimed to randomize a total of 242 participants.
2.9. Ethical approval
The study was approved by the Medical Research Ethics Committee of the International Peace Maternity and Child Health Hospital (date of approval: October 10, 2017; reference number: GKLW2017‐84,). The trial was registered before the initiation of participant recruitment in the study (registration number: ChiCTR‐INR‐17013272, https://www.chictr.org.cn/). Written informed consent was obtained from all participants.
3. RESULTS
3.1. Patients
Between May 2018 and May 2021, we informed 1608 pregnant women of the study; 256 provided consent and 242 underwent randomization immediately before non‐elective CS, with 121 participants in each study arm. The indications for CS included failure to progress (n = 107, 44.2%), fetal distress (n = 86, 35.5%), failed induction (n = 18, 7.4%), and other (n = 31, 12.9%; eg breech presentation, fetal macrosomia, or severe pre‐eclampsia or intrahepatic cholestasis during pregnancy).
Because of the coronavirus disease 2019 (COVID‐19) pandemic and strict management policies from the hospital to the national level, timely ultrasonic follow up of all participants was extremely difficult during the period from January 2019 to June 2020. We therefore merged the two follow‐up time points (6 weeks and 6 months postpartum) into one period (6 weeks to 6 months postpartum) and the two ultrasound examinations into one to obtain an acceptable dropout rate (these mergers did not produce bias in the measurement of CSD; see Discussion section). Ultimately, 17 women in the experimental group and 13 in the placebo group were lost to follow up. The first postoperative ultrasound follow up was completed in 6 months by 104 and 108 participants in the experimental and placebo groups, respectively (Figure 1).
FIGURE 1.
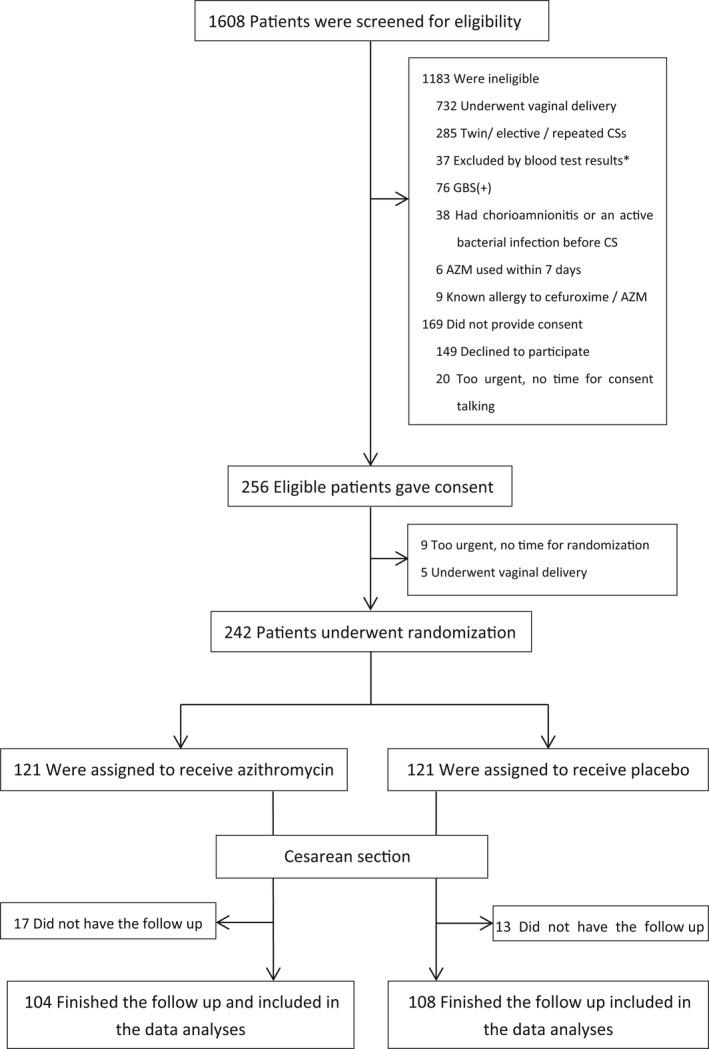
The enrollment and outcome. AZM, azithromycin; CS, cesarean section; GBS(+): Group B Streptococcus haemolyticus (positive). *Blood test exclusion criteria: fibrinogen <2 g/L, or platelet count <100 × 109/L, or hemoglobin <90 g/L.
3.2. Outcomes
The maternal and baseline obstetric characteristics of the participants in each study group are summarized in Table 1. All characteristics including pre‐CS infection index, cervix dilatation, ROM/labor duration, and CS indication were comparable between the two groups (p > 0.05). Nearly all participants were primipara except for one in each group with a previous vaginal delivery. One and two participants in the experimental and placebo groups, respectively, reached the second stage. The characteristics of the operative procedure (i.e. uterine suture [including hemostasis], operation duration, blood loss), infection indices, antibiotics administration, and fetal outcomes are presented in Table 2. There were no significant differences between groups in surgical factors, infection indicators, administration of antibiotics, or fetal outcomes (p > 0.05). Only three women underwent CSD measurement on day 42 in each group; the average follow‐up time from CS was 75.4 days and the median time was 66 days in both groups.
TABLE 1.
The characteristics of the patients at the base line
| Parameters | Units / category | Experimental (N = 121) | Placebo (N = 121) | p |
|---|---|---|---|---|
| Age | years | 30.0 ± 3.1 | 30.4 ± 3.5 | 0.221 |
| Body mass index | kg/m2 | 27.4 ± 4.0 | 27.7 ± 3.6 | 0.535 |
| ≥18.5, <25.0 | 35 | 28 | 0.528 | |
| ≥25.0, <30.0 | 61 | 62 | ||
| ≥30.0 | 25 | 31 | ||
| Pregnancy | Count | 1.5 ± 1.1 | 1.4 ± 0.7 | 0.439 |
| 1 | 84 | 89 | ||
| 2 | 23 | 25 | ||
| 3 | 11 | 5 | ||
| 4 | 2 | 1 | ||
| 5 | 0 | 1 | ||
| 9 | 1 | 0 | ||
| Diabetes mellitus | Any | 17 | 19 | 0.718 |
| Gestational only | 16 | 18 | 0.711 | |
| Hypertension | Chronic | 0 | 2 | 0.262 |
| Gestational | 4 | 5 | ||
| Pre‐eclampsia | 0 | 3 | ||
| Gestational age | weeks | 39.5 ± 1.1 | 39.5 ± 1.1 | 0.853 |
| Hemoglobin pre‐CS | g/L | 119.6 ± 11.8 | 118.6 ± 11.7 | 0.490 |
| WBC count pre‐CS | ×109/L | 8.36 ± 1.8 | 8.9 ± 2.2 | 0.119 |
| 4.5–10 | 101 | 90 | 0.083 | |
| ≥10 | 20 | 31 | ||
| NEU% pre‐CS | % | 71.8 ± 6.6 | 73.1 ± 6.5 | 0.106 |
| <60 | 5 | 3 | 0.656 | |
| ≥60, <75 | 74 | 71 | ||
| ≥75 | 42 | 47 | ||
| hsCRP pre‐CS | mg/L | 13.5 ± 13.8 | 13.5 ± 17.4 | 0.990 |
| <10 | 70 | 73 | 0.571 | |
| ≥10 | 48 | 43 | ||
| Missing data | 3 | 5 | ||
| Procalcitonin pre‐CS | μg/L | 0.08 ± 0.07 | 0.06 ± 0.04 | 0.149 |
| <0.05 | 53 | 52 | 0.755 | |
| ≥0.05 | 61 | 65 | ||
| Missing data | 7 | 4 | ||
| Body temperature pre‐CS | °C | 36.9 ± 0.3 | 36.9 ± 0.3 | 0.671 |
| <37 | 55 | 52 | 0.651 | |
| ≥37.0, <37.5 | 62 | 66 | ||
| ROM duration | hours | 15.0 ± 17.9 | 14.5 ± 17.9 | 0.845 |
| No | 4 | 6 | 0.398 | |
| <24 | 96 | 87 | ||
| ≥24 | 21 | 28 | ||
| ROM type | No | 4 | 6 | 0.060 |
| Spontaneously before labor | 77 | 87 | ||
| Spontaneously in labor | 8 | 2 | ||
| Iatrogenic ROM induction | 32 | 26 | ||
| Cervix dilatation | cm | 3.4 ± 2.4 | 3.6 ± 2.4 | 0.632 |
| <6 | 97 | 98 | 0.807 | |
| ≥6, <10 | 23 | 21 | ||
| 10 | 1 | 2 | ||
| Labor duration | hours | 5.4 ± 4.9 | 5.8 ± 4.9 | 0.505 |
| 0 | 30 | 27 | 0.953 | |
| ≥0.5, <6.0 | 38 | 38 | ||
| ≥6.0, <12.0 | 42 | 43 | ||
| ≥12.0 | 11 | 13 | ||
| CS ondication | Failure to progress | 57 | 52 | 0.524 |
| Fetal distress | 39 | 43 | ||
| Failed induction | 7 | 12 | ||
| Others | 18 | 14 | ||
Note: Data are mean ± standard deviation, or n.
Abbreviations: CS, cesarean section; hsCRP, hypersensitive C‐reactive protein; NEU%, percentage of neutrophils; ROM, rupture of membrane; WBC, white blood cells.
TABLE 2.
Characteristics of the operation procedure, infection indices, and fetal outcome
| Parameters | Units category | Experimental (N = 104) | Placebo (N = 108) | p |
|---|---|---|---|---|
| Uterine incision lancination | No | 100 | 107 | 0.161 |
| Yes | 4 | 1 | ||
| Uterine suture | Double layer only | 98 | 107 | 0.136 |
| Double layer + B‐lynch | 5 | 1 | ||
| Double layer + balloon | 1 | 0 | ||
| Operation duration | min | 35.3 ± 7.3 | 35.1 ± 7.3 | 0.859 |
| <30 | 12 | 15 | 0.796 | |
| ≥30, <40 | 63 | 67 | ||
| ≥40, <60 | 28 | 24 | ||
| ≥60 | 1 | 2 | ||
| Blood loss | mL | 228.4 ± 63.8 | 231.1 ± 193.9 | 0.893 |
| Hemoglobin post‐CS | g/L | 113.0 ± 12.7 | 112.8 ± 12.2 | 0.945 |
| <113 | 50 | 52 | 0.992 | |
| ≥113 | 54 | 56 | ||
| WBC count post‐CS | ×109/L | 12.8 ± 2.8 | 12.9 ± 2.7 | 0.731 |
| <10 | 17 | 14 | 0.739 | |
| ≥10, <15 | 64 | 67 | ||
| ≥15 | 23 | 27 | ||
| NEU% post‐CS | % | 80.9 ± 4.2 | 81.5 ± 4.1 | 0.274 |
| <75 | 6 | 6 | 0.461 | |
| ≥75, <90 | 81 | 77 | ||
| ≥90 | 17 | 25 | ||
| hsCRP post‐CS | mg/L | 48.0 ± 29.9 | 50.0 ± 31.7 | 0.651 |
| <50 | 64 | 63 | 0.619 | |
| ≥50 | 37 | 42 | ||
| 3 | 3 | |||
| CRP ratio | (Post/pre‐CS) | 5.8 ± 4.9 | 6.1 ± 5.2 | 0.616 |
| 7 | 10 | |||
| Procalcitonin post‐CS | μg/L | 0.14 ± 0.17 | 0.11 ± 0.08 | 0.069 |
| <0.05 | 20 | 18 | 0.482 | |
| ≥0.05 | 75 | 87 | ||
| 9 | 3 | |||
| Procalcitonin ratio | (Post/pre‐CS) | 2.2 ± 2.2 | 2.0 ± 1.3 | 0.391 |
| 13 | 7 | |||
| Vaginal secretion culture pre‐CS | Negative | 56 | 57 | 0.725 |
| Mycoplasma | 40 | 46 | ||
| Others | 7 | 5 | ||
| Uterine cavity culture in‐CS | Negative | 87 | 94 | 0.426 |
| Mycoplasma | 8 | 9 | ||
| Escherichia coli etc. | 8 | 4 | ||
| Body temperature post‐CS | 36.83 ± 0.25 | 36.80 ± 0.29 | 0.370 | |
| <37°C | 77 | 80 | ||
| ≥37°C, <37.5°C | 27 | 27 | ||
| ≥37.5°C | 0 | 1 | ||
| Antibiotics doses before randomization | Doses | 1.8 ± 2.6 | 1.9 ± 2.7 | 0.783 |
| Antibiotics doses after CS | Doses | 1.0 ± 2.0 | 1.4 ± 2.9 | 0.222 |
| Antibiotics used after CS % | Cases/No.* % | 24 | 31.5 | 0.228 |
| In‐hospital | Days | 4.09 ± 0.46 | 4.16 ± 0.63 | 0.353 |
| APGAR score 1 min | 10 | 100 | 98 | 0.491 |
| 9 | 1 | 1 | ||
| 8 | 2 | 4 | ||
| 7 | 1 | 4 | ||
| 4 | 0 | 1 | ||
| APGAR score 5 min | 10 | 104 | 105 | 0.231 |
| 9 | 0 | 2 | ||
| 7 | 0 | 1 | ||
| Fetal body weight | g | 3423.9 ± 414.8 | 3437.5 ± 429.2 | 0.816 |
| Fetal macrosomia | 95 | 101 | 0.549 | |
| 9 | 7 | |||
| Days from CS to follow up | Days | 75.4 ± 32.7 | 75.4 ± 32.0 | 0.994 |
| ≥42, <66 | 50 | 55 | 0.680 | |
| ≥66 | 54 | 53 |
Note: Data are mean ± standard deviation, or n.
Abbreviations: CS, cesarean Section; hsCRP, hypersensitive C‐reactive protein; NEU%, percentage neutrophils; WBC, white blood cell.
To determine if the follow‐up time differentially affected the two groups, we divided the data using a cut‐off value of 66 days. There were 50 women in the experimental group and 55 women in the placebo group with a follow up shorter than 66 days, and 54 and 53 women, respectively, with a follow‐up time of 66 days or longer (Table 2). Given the small sample size of each subgroup, there was no significant difference between experimental and placebo groups in CSD occurrence or CSD characteristics (width, length, or depth; residual myometrium thickness; and adjacent myometrium thickness) in either subgroup stratified by follow‐up time. We therefore pooled the data and found that CSD was diagnosed in 34/104 patients (32.7%) in the experimental group and 50/108 (46.3%) in the placebo group, as determined by transvaginal ultrasound and sonohysterography (OR 0.71, 95% CI 0.50–0.90, p = 0.043) using univariate analysis (Table 3). We observed a variety of CSD shapes including triangular (63.1%), oval (16.7%), circular (19.0%), and square (1.2%). There were no differences in CSD characteristics or short‐term (within 42 days) infection outcomes between groups. One patient in each group suffered an abdominal skin wound infection. There were no puerperal infections in the experimental group, whereas two patients in the placebo group had puerperal infection (one case of pyelonephritis and one case of septicemia).
TABLE 3.
Ultrasonic follow‐up and CSD characteristics in 6 months
| Parameters | Units category | Experimental | Placebo | p | OR | 95% CI |
|---|---|---|---|---|---|---|
| Follow up ≥42,< 60 days | 50 | 55 | ||||
| Uterine length | 48.4 ± 6.4 | 47.6 ± 5.5 | 0.480 | |||
| Uterine width | 51.1 ± 6.6 | 50.9 ± 6.4 | 0.824 | |||
| Uterine thickness | 39.6 ± 6.4 | 39.7 ± 5.0 | 0.937 | |||
| CSD | Yes | 18 (36.0) | 26 (47.3) | 0.242 | 0.76 | 0.48–1.21 |
| No | 32 (64.0) | 29 (52.7) | ||||
| CSD width | mm | 10.6 ± 4.7 | 10.0 ± 5.9 | 0.740 | ||
| CSD length | mm | 7.4 ± 3.7 | 7.0 ± 4.1 | 0.750 | ||
| CSD depth | mm | 6.2 ± 3.3 | 6.4 ± 3.3 | 0.832 | ||
| RMT | mm | 6.3 ± 2.4 | 5.3 ± 3.1 | 0.266 | ||
| AMT | mm | 12.0 ± 2.7 | 11.4 ± 4.2 | 0.640 | ||
| Follow up ≥66 days | 54 | 53 | ||||
| Uterine length | 44.4 ± 4.8 | 45.7 ± 4.6 | 0.182 | |||
| Uterine width | 46.1 ± 5.5 | 47.6 ± 4.8 | 0.137 | |||
| Uterine thickness | 35.9 ± 6.0 | 38.8 ± 4.5 | 0.008 | |||
| CSD | Yes | 16 (29.6) | 24 (45.3) | 0.094 | 0.65 | 0.39–1.09 |
| No | 38 (70.4) | 29 (54.7) | ||||
| CSD width | mm | 9.8 ± 4.7 | 9.7 ± 4.3 | 0.984 | ||
| CSD length | mm | 6.9 ± 3.9 | 6.7 ± 3.4 | 0.893 | ||
| CSD depth | mm | 5.3 ± 2.7 | 7.0 ± 3.0 | 0.072 | ||
| RMT | mm | 4.6 ± 2.5 | 4.7 ± 3.2 | 0.885 | ||
| AMT | mm | 9.7 ± 2.5 | 10.9 ± 3.5 | 0.239 | ||
| Pooled data | 104 | 108 | ||||
| Uterine length | 46.4 ± 6.0 | 46.7 ± 5.1 | 0.688 | |||
| Uterine width | 48.5 ± 6.5 | 49.3 ± 5.9 | 0.382 | |||
| Uterine thickness | 37.7 ± 6.4 | 39.3 ± 4.8 | 0.053 | |||
| CSD | Yes | 34 (32.7) | 50 (46.3) | 0.043 | 0.71 | 0.50–0.99 |
| No | 70 (67.3) | 58 (53.7) | ||||
| CSD width | mm | 10.18 ± 4.63 | 9.87 ± 5.15 | 0.284 | ||
| CSD length | mm | 7.14 ± 3.71 | 6.86 ± 3.74 | 0.734 | ||
| CSD depth | mm | 10.18 ± 4.63 | 9.87 ± 5.15 | 0.777 | ||
| RMT | mm | 5.51 ± 2.59 | 5.05 ± 3.16 | 0.479 | ||
| AMT | mm | 10.88 ± 2.83 | 11.17 ± 3.84 | 0.706 |
Note: Data are mean ± standard deviation, n, or n (%).
Abbreviations: AMT, adjacent myometrium thicknesses; CSD, cesarean scar defect; RMT, residual myometrium thickness; SIS, saline infusion sonohysterography.
The associations between CSD and the infective factors (white blood cells, neutrophil ratio, hsCRP and procalcitonin pre‐ and post‐CS, cervix dilatation, pre‐CS cervix secretion culture, in‐CS uterine cavity culture) were all found to be non‐significant in our univariate analysis, except for ROM duration (p = 0.001). However, we observed a non‐significant tendency towards a higher risk of CSD in patients with larger cervix dilatation, higher infectious index (procalcitonin/hsCRP) values, and a positive pathogenic microorganism culture (0.065 < p < 0.160; Table S1). Multifactor binary logistic regression was applied to further investigate the association between infective factors/prophylactic antibiotics and CSD. We found that prolonged (≥24 h) duration of ROM was a significant risk factor (aOR 1.5, 95% CI 1.2–1.9, p = 0.001) for CSD, further subclassification analysis of ROM duration and CSD risk also showed a similar detailed result (Table S2), while single‐dose standard cefuroxime was a significant risk factor for CSD relative to adjunctive azithromycin to standard cefuroxime (aOR 2.1, 95% CI 1.1–3.8, p = 0.023) (Table 4).
TABLE 4.
Infective factors and the CSD risk
| Parameters | Category | Counts | CSD prevalence (95% CI) | aOR (95% CI) | p |
|---|---|---|---|---|---|
| Antibiotics prophylaxis group | Experimental | 104 | 32.7 (23.5–41.9) | Reference | 0.023 |
| Placebo | 108 | 46.3 (36.7–55.9) | 2.1 (1.1–3.8) | ||
| ROM duration (h) | <24 | 165 | 34.6 (27.2–41.9) | Reference | 0.001 |
| ≥24 | 40 | 62.5 (46.8–78.2) | 1.5 (1.2–1.9) | ||
| Cervix dilatation (cm) | <6 | 174 | 37.4 (30.1–44.6) | Reference | 0.161 |
| ≥6 | 38 | 50.0 (33.3–66.6) | NA | ||
| hsCRP pre‐CS (mg/L) | <10 | 125 | 35.2 (26.7–43.7) | Reference | 0.273 |
| ≥10 | 82 | 45.1 (34.1–56.1) | NA | ||
| hsCRP post‐CS (mg/L) | < 50 | 127 | 35.4 (27.0–43.9) | Reference | 0.304 |
| ≥50 | 79 | 45.6 (34.3–56.8) | NA | ||
| Procalcitonin pre‐CS (μg/L) | < 0.05 | 92 | 34.8 (24.9–44.7) | Reference | 0.121 |
| ≥0.05 | 113 | 44.3 (35.0–53.6) | NA | ||
| Procalcitonin post‐CS (μg/L) | <0.05 | 38 | 29.0 (13.8–44.1) | Reference | 0.228 |
| ≥0.05 | 162 | 42.0 (34.3–49.7) | NA | ||
| Cervix secretion culture pre‐CS | Negative | 113 | 35.4 (26.5–44.4) | Reference | 0.465 |
| Positive | 98 | 44.9 (34.9–54.9) | NA | ||
| Uterine cavity culture in‐CS | Negative | 183 | 37.2 (30.1–44.2) | reference | 0.321 |
| Positive | 29 | 55.2 (35.9–74.4) | NA |
Note: N/A denotes not applicable. By univariate analysis of the factor associated with antibiotics prophylaxis, factors with p <0.20 were included in the logistic regression model. The adjusted odds ratios (aOR) were adjusted for cervix dilatation, pre‐/post‐CS hsCRP/ptocalcitonin, cervix secretion culture pre‐CS and uterine cavity culture in‐CS.
Abbreviations: aOR, adjusted odds ratio; CS, cesarean section; hsCRP, hypersensitive C‐reactive protein; NEU, neutrophils; ROM, rupture of membrane; WBC, white blood cell.
4. DISCUSSION
Our pooled results showed that prophylaxis with adjunctive azithromycin can help to prevent CSD. The pooled result showed that adjunctive azithromycin prophylaxis resulted in a 29.4% reduction in CSD compared with the standard single‐dose cefuroxime sodium prophylaxis, whereas no significant differences were observed in short‐term conditions such as endometritis (1.92% vs 3.7%, p = 0.434), wound infection (0.96% vs 0.93%, p = 0.973), and puerperal infection (0.00% vs 1.85%, p = 0.165). Hence, adjunctive azithromycin prophylaxis had a slight protective effect but this was not significant, possibly because the morbidity of these complications is lower than that of CSD and because the sample size was small (the C/SOAP trial examined postoperative infection in a sample that was about 10 times larger).
A key strength of our study is that we found that azithromycin reduced the occurrence of CSD; the specific reduction in absolute risk was determined to be 13.6% (95% CI 0.4%–26.8%, p = 0.022). This finding can guide the development of preventive strategies for CSD. Additionally, this study uncovered the relation between prolonged ROM duration (≥24 h) and CSD. Our results can help to identify high‐risk patients so that appropriate interventions can be implemented.
A limitation of our study is that the inclusion criteria were limited to singleton term pregnancies and cases of first non‐elective CS among ethnic Han women at a single center in Shanghai, China. The protective effect of adjunctive azithromycin for CSD in other contexts requires validation. Furthermore, our ultrasound follow up was not completed within the planned time point (6 weeks) because of the COVID‐19 outbreak and strict management policies. Therefore, the mean follow‐up duration was 75 ± 32 days and the median follow up was 66 days.
Wound healing in cesarean incisions of the uterus and the natural process of CSD development are not fully understood, and the uterine scar and CSD may change over time. One prospective observational study reported the persistence of CSDs from 6 weeks to 6 months or longer 21 ; and another prospective study found that some women with or without a CSD may present the opposite from 6 weeks to 6 months. 22 There was no significant difference in CSD incidence between women who were followed up before vs after the median number of days, nor between the number of women in the experimental and placebo groups before vs after the median number of days. We therefore concluded that merging the follow‐up time points (6 weeks and 6 months) into one period (6 weeks to 6 months) did not introduce bias into the primary outcome (Table 3).
Because of the COVID‐19 pandemic and the associated management policies, the dropout rate was higher than expected (14.0% and 10.7% in the experimental and placebo groups, respectively); this may have reduced the significance or statistical power in the data analyses. Given the limited sample size, we failed to detect a relation between CSD and the results of pathogen cultures, which are the reference standard for infection testing. Pathogen cultures were conducted at the hospital laboratory using traditional methods, which are limited to specific and common forms of bacteria, fungi, Mycoplasma, and Chlamydia. Additionally, the abundance and metabolic characteristics of the detected pathogens could not be determined and false negatives were inevitable during testing. Hence, the causal relation between infection and CSD development requires further investigation.
Most guidelines recommend the administration of a single dose of first‐generation cephalosporin 60 min before CS. 23 , 24 Our standard antibiotic is cefuroxime sodium, which is administered as appropriate. Guidelines related to the management of post‐CS infections and prophylactic antibiotics mainly focus on obvious short‐term outcomes such as endometritis and wound infections.
The addition of azithromycin to standard antibiotic prophylaxis regimens before non‐elective CS was reported to reduce short‐term infections including endometritis, wound infection, and serious maternal adverse events. 18 A previous study of cost‐effectiveness showed that adjunctive azithromycin prophylaxis is a cost‐saving strategy that can be used for both unscheduled and scheduled cesarean deliveries. 25 The prevention of CSD is very important; a 1% reduction in the incidence of CSD means that hundreds of thousands of women would be spared from CSD worldwide. There is a lack of standardized preventive strategies because the etiology of CSD remains unclear. Infection has been considered as a risk factor for CSD, but the specific association between them has not been determined. Some researchers have proposed that infection can lead to CSD through adhesions caused by inflammation. 14 Our previous investigation of risk factors was the first to show that perioperative infection should be considered as a predictor of CSD and that multi‐dose antibiotics had a protective effect against its occurrence. 11 However, we did not observe a significant relationship between direct indicators of infection (such as procalcitonin or pathogens) and CSD. This study indicates that infection is a key risk factor in CSD, and that adjunctive azithromycin may be helpful in the prevention of this condition in non‐elective primary CS.
5. CONCLUSION
Our analyses showed that a single dose of 500 mg intravenous azithromycin in addition to the standard single dose of cephalosporin significantly reduced the prevalence of CSD in women undergoing non‐elective CS.
ACKNOWLEDGEMENTS
The authors thank Prof. Ben W. J. Mol for his guidence and help in the trial protocol. The authors wish to acknowledge the valuable help provided by the staff of the IPMCH who engeged in the study but not listed in the authors.
AUTHOR CONTRIBUTIONS
HP, DH, SC, and YC designed the study. WC and JZ co‐ordinated the trial. SC, YS, and MZ collected the data. DH, YC, MZ, YS, and SC managed the trial in the hospital and all commented on the draft paper. HP and DH performed the statistical analyses. HP and DH drafted the paper. All authors interpreted the data, critically revised the article and approved the final version.
FUNDING INFORMATION
Ding Huang was supported by funding from the Shanghai Shenkang Hospital Development Center Clinical Science and Technology Innovation Project (no. SHDC12017X05) and Hongjie Pan was supported by the medical youth funds from Shanghai Municipal Health Commission (no. 20174Y0074).
CONFLICT OF INTEREST
None.
Supporting information
Table S1.
Table S2.
Huang D, Chen S, Cai Y, et al. Adjunctive azithromycin prophylaxis protects women from uterine cesarean scar defect: A randomized controlled trial. Acta Obstet Gynecol Scand. 2022;101:889‐900. doi: 10.1111/aogs.14387
Ding Huang and Sha Chen contributed equally to this research.
REFERENCES
- 1. Betrán AP, Ye J, Moller AB, Zhang J, Gülmezoglu AM, Torloni MR. The increasing trend in Caesarean section rates: global, regional and national estimates: 1990‐2014. PLoS One. 2016;11:e0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hehir MP, Ananth CV, Siddiq Z, Flood K, Friedman AM, D'Alton ME. Cesarean delivery in the United States 2005 through 2014: a population‐based analysis using the Robson 10‐Group Classification System. Am J Obstet Gynecol. 2018;219:105.e1‐105.e11. [DOI] [PubMed] [Google Scholar]
- 3. Li HT, Luo S, Trasande L, et al. Geographic variations and temporal trends in Cesarean delivery rates in China, 2008‐2014. JAMA. 2017;317:69‐76. [DOI] [PubMed] [Google Scholar]
- 4. van der Voet LF, Bij de Vaate AM, Veersema S, Brölmann HA, Huirne JA. Long‐term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121:236‐244. [DOI] [PubMed] [Google Scholar]
- 5. Jordans IPM, de Leeuw RA, Stegwee SI, et al. Sonographic examination of uterine niche in non‐pregnant women: a modified Delphi procedure. Ultrasound Obstet Gynecol. 2019;53:107‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Voet LLF, Limperg T, Veersema S, et al. Niches after cesarean section in a population seeking hysteroscopic sterilization. Eur J Obstet Gynecol Reprod Biol. 2017;214:104‐108. [DOI] [PubMed] [Google Scholar]
- 7. Karpathiou G, Chauleur C, Dridi M, et al. Histologic findings of uterine niches. Am J Clin Pathol. 2020;154:645‐655. [DOI] [PubMed] [Google Scholar]
- 8. Tulandi T, Cohen A. Emerging manifestations of cesarean scar defect in reproductive‐aged women. J Minim Invasive Gynecol. 2016;23:893‐902. [DOI] [PubMed] [Google Scholar]
- 9. Vissers J, Hehenkamp W, Lambalk CB, Huirne JA. Post‐Caesarean section niche‐related impaired fertility: hypothetical mechanisms. Hum Reprod. 2020;35:1484‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morlando M, Buca D, Timor‐Tritsch I, et al. Reproductive outcome after cesarean scar pregnancy: a systematic review and meta‐analysis. Acta Obstet Gynecol Scand. 2020;99:1278‐1289. [DOI] [PubMed] [Google Scholar]
- 11. Pan H, Zeng M, Xu T, et al. The prevalence and risk predictors of cesarean scar defect at 6 weeks postpartum in Shanghai, China: a prospective cohort study. Acta Obstet Gynecol Scand. 2019;98:413‐422. [DOI] [PubMed] [Google Scholar]
- 12. Di Spiezio Sardo A, Saccone G, McCurdy R, Bujold E, Bifulco G, Berghella V. Risk of Cesarean scar defect following single‐ vs double‐layer uterine closure: systematic review and meta‐analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2017;50:578‐583. [DOI] [PubMed] [Google Scholar]
- 13. Yılmaz Baran Ş, Kalaycı H, Doğan Durdağ G, et al. Single‐ or double‐layer uterine closure techniques following cesarean: a randomized trial. Acta Obstet Gynecol Scand. 2021;100:531‐537. [DOI] [PubMed] [Google Scholar]
- 14. Vervoort AJ, Uittenbogaard LB, Hehenkamp WJ, Brölmann HA, Mol BW, Huirne JA. Why do niches develop in Caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum Reprod. 2015;30:2695‐2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lamey JR, Eschenbach DA, Mitchell SH, Blumhagen JM, Foy HM, Kenny GE. Isolation of mycoplasmas and bacteria from the blood of postpartum women. Am J Obstet Gynecol. 1982;143:104‐112. [DOI] [PubMed] [Google Scholar]
- 16. D'Angelo LJ, Sokol RJ. Determinants of postpartum morbidity in laboring monitored patients: a reassessment of the bacteriology of the amniotic fluid during labor. Am J Obstet Gynecol. 1980;136:575‐578. [DOI] [PubMed] [Google Scholar]
- 17. Mackeen AD, Packard RE, Ota E, Berghella V, Baxter JK. Timing of intravenous prophylactic antibiotics for preventing postpartum infectious morbidity in women undergoing cesarean delivery. Cochrane Database Syst Rev. 2014;5:CD009516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231‐1241.27682034 [Google Scholar]
- 19. Cai Y, Pan H, Zhang J, et al. Efficacy of adjunctive azithromycin versus single‐dose cephalosporin prophylaxis for caesarean scar defect: study protocol for a randomised controlled trial. BMJ Open. 2020;10:e032379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Committee on Practice Bulletins‐Obstetrics . ACOG Practice Bulletin No. 188: prelabor rupture of membranes. Obstet Gynecol. 2018;131:e1‐e14. [DOI] [PubMed] [Google Scholar]
- 21. Dosedla E, Calda P. Can the final sonographic assessment of the cesarean section scar be predicted 6 weeks after the operation? Taiwan J Obstet Gynecol. 2016;55:718‐720. [DOI] [PubMed] [Google Scholar]
- 22. Bamberg C, Hinkson L, Dudenhausen JW, Bujak V, Kalache KD, Henrich W. Longitudinal transvaginal ultrasound evaluation of cesarean scar niche incidence and depth in the first two years after single‐ or double‐layer uterotomy closure: a randomized controlled trial. Acta Obstet Gynecol Scand. 2017;96:1484‐1489. [DOI] [PubMed] [Google Scholar]
- 23. van Schalkwyk J, Van Eyk N. No. 247‐antibiotic prophylaxis in obstetric procedures. J Obstet Gynaecol Can. 2017;39:e293‐e299. [DOI] [PubMed] [Google Scholar]
- 24. Committee on Practice Bulletins‐Obstetrics . ACOG Practice Bulletin No. 199: use of prophylactic antibiotics in labor and delivery. Obstet Gynecol. 2018;132:e103‐e119. [DOI] [PubMed] [Google Scholar]
- 25. Harper LM, Kilgore M, Szychowski JM, Andrews WW, Tita ATN. Economic evaluation of adjunctive azithromycin prophylaxis for Cesarean delivery. Obstet Gynecol. 2017;130:328‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.


