Abstract
Introduction
In this review and meta‐analysis we aimed to investigate whether human papilloma virus (HPV) vaccination administered after excisional treatment of cervical intraepithelial neoplasia (CIN) is associated with a reduced risk of recurrence of CIN grade 2 or worse (CIN2+).
Material and methods
We performed a systematic literature search in three online databases through June 2021. Observational studies and randomized controlled trials (RCTs) were eligible for inclusion if the prophylactic HPV vaccine was administered after excisional treatment for histologically verified CIN. Only English language literature was included. The primary outcome measure was recurrence of CIN2+ after treatment. A meta‐analysis was performed using fixed and random‐effects models, and results were reported as pooled odds ratios (OR) with 95% confidence intervals (95% CI). Quality assessment was performed using ROB2‐tool for RCTs and ROBINS‐I for observational studies. The protocol was registered in PROSPERO (CRD42021238257).
Results
A total of 1561 studies were identified, of which nine, including 19 971 women, were included. Two studies were RCTs and seven were observational studies. Using the fixed‐effect model on the two RCTs, the OR for recurrence of CIN2+ was 0.29 (95% CI 0.16–0.53). Due to considerable heterogeneity in observational studies, the random‐effects model was used to estimate pooled OR for CIN2+ recurrence in these studies. Thus, using unadjusted data from observational studies, the OR for CIN2+ recurrence was 0.35 (95% CI 0.18–0.67), whereas when using adjusted data, the OR for CIN2+ recurrence was 0.54 (95% CI 0.21–1.35). However, quality assessment revealed a serious risk of bias for the majority of the studies included.
Conclusions
HPV vaccination post‐treatment was associated with a significantly reduced risk of CIN2+ recurrence when using unadjusted estimates from observational studies and RCTs. We found no significant effect of HPV vaccination on risk of CIN2+ recurrence when using the outcome measure from observational studies with the least risk of bias. Large, well‐designed randomized placebo‐controlled trials are needed to determine whether post‐treatment HPV vaccination should be recommended to all women undergoing excisional treatment for CIN.
Keywords: cervical cancer prevention, cervical intraepithelial neoplasia, conization, excisional treatment, HPV vaccination
Abbreviations
- CI
confidence intervals
- CIN
cervical intraepithelial neoplasia
- HPV
human papilloma virus
- OR
odds ratio
- RCT
randomized controlled trial
Key message.
HPV vaccination after treatment for CIN may be associated with a reduced risk of CIN2+ recurrence but well‐designed RCTs are needed to determine whether HPV vaccination should be recommended post‐treatment.
1. INTRODUCTION
Human papillomavirus (HPV) is a necessary but not a sufficient cause of cervical cancer, and may also be causally related to anal, vulvar and vaginal cancers and their precursors. 1 Upon acquisition, most HPV infections resolve themselves within 1–2 years without causing any symptoms. 2 Women who develop a persistent infection are at significantly increased risk of precursor lesions of the cervix (hereafter referred to as cervical intraepithelial neoplasia: CIN), which may progress to cervical cancer if left undetected and untreated. 3 , 4 Whereas cervical cancer screening allows for secondary prevention through early detection and treatment of CIN, HPV vaccination allows for primary prevention.
So far, three HPV vaccines have been licensed for primary prevention of cervical cancer and its precursors: the bivalent, the quadrivalent and the nonavalent HPV vaccine. Several studies have demonstrated that HPV vaccination is associated with a significantly reduced risk of CIN 5 , 6 , 7 and recent population‐based register studies have reported an 80%–90% reduced risk of cervical cancer among HPV‐vaccinated women. 8 , 9 The most convincing evidence of a prophylactic effect was shown when individuals were vaccinated prior to sexual debut. 10 , 11
Previous studies have suggested that post‐treatment HPV vaccination may reduce the risk of recurrence in women undergoing excisional treatment for CIN, 12 , 13 whereas others have failed to demonstrate any effect. 14 Recently, four systematic reviews and meta‐analyses were published on this subject, all concluding that HPV vaccination after excisional treatment reduces the risk of recurrence. 15 , 16 , 17 , 18 However, all four reviews included studies in which women were vaccinated up to 48 months prior to surgical treatment. Thus, it is likely that a large proportion of these women were vaccinated as part of catch‐up programs and, consequently, these studies do not address the clinically important question on whether post‐treatment HPV vaccination should be offered to all women undergoing excisional treatment for CIN. Up to 8% 19 , 20 of women undergoing excisional treatment are diagnosed with recurrence within 5 years after treatment. As repeat excisional treatment may increase the risk of preterm birth and stenosis, 21 , 22 it is of great clinical relevance to explore whether HPV vaccination post‐treatment may be associated with a reduced risk of CIN.
Therefore, in this systematic review and meta‐analysis, we aimed to explore whether HPV vaccination undertaken after excisional treatment is associated with a reduced risk of CIN recurrence.
2. MATERIAL AND METHODS
2.1. Data sources
We performed a systematic literature search in PubMed and Embase through June 2021. Details of the search are included in Appendix S1. References cited in retrieved articles were also carefully evaluated. In addition, a citation search was conducted in Scopus. Two reviewers independently screened titles and abstracts. In the case of missing data or need for additional details, authors were contacted.
2.2. Main outcome measures
The primary outcome measure was recurrence rate of histologically verified CIN grade 2 or worse (CIN2+).
2.3. Eligibility criteria
We assessed for eligibility randomized controlled trials (RCTs) and observational studies that compared recurrence of CIN2+ in women receiving any of the three prophylactic HPV vaccines after excisional treatment for CIN with the risk among women who did not receive the vaccine. Only studies that included women who had histologically verified CIN were included. Studies were excluded if the first dose of the HPV vaccine was administered more than 3 months prior to excisional treatment for CIN. Reports on new experimental and therapeutic HPV vaccines were outside the scope of this review and were therefore excluded. Only English language literature was included.
Quality assessment of the studies was evaluated using the Cochrane Risk of Bias ROB 2 tool 23 for RCTs, and the ROBINS‐I tool 24 for non‐randomized studies. The ROB 2 tool was used to assess the overall risk of bias of RCTs through evaluation of the following six domains: bias arising from the randomization process (randomization methods, allocation concealment, blinding), bias due to deviations from intended interventions, bias due to missing outcome data (high risk of bias was identified when the proportion of women lost to follow‐up exceeded 20%), bias in measurement of the outcome, and bias in selection of the reported result. The overall risk of bias was categorized as “low risk of bias,” “some concerns” or “high risk of bias.” For each of the included observational studies, the ROBINS‐I tool was used to evaluate seven domains of bias. Potential pre‐intervention biases are confounding and inclusion of participants (i.e., whether the inclusion criteria are affected by the intervention). Life‐time number of sexual partners, smoking, socioeconomic status, HPV status, cone margin status and age were evaluated as possible pre‐intervention confounders. Intervention bias may arise from misclassification of the intervention (i.e., when the HPV vaccine was administered and how many vaccine doses were administered). Finally, ROBINS‐I contains the following potential post‐intervention biases: missing data (including loss to follow‐up), deviations from the intended intervention (i.e., number of HPV vaccine doses administered), measurement of the outcome (including follow‐up time), and selection of reported results (i.e., if the reported outcome differs from the outcome planned in the protocol). The overall risk of bias was categorized as either “low risk,” “moderate risk,” “serious risk” or “critical risk” of bias.
2.4. Data collection and analysis
From each study we collected the following data: author, year of publication, country of origin, study design, age of study participants, sample size, surgical modality (eg., cold‐knife, laser or loop electrosurgical excision procedure [LEEP]), intervention (i.e., type of HPV vaccine administered and number of doses), follow‐up time, whether HPV DNA‐testing was performed, margin status of the cone biopsy, and recurrence rate of histologically confirmed CIN2+.
We conducted a meta‐analysis using the fixed‐effect model and the software REVMAN (version 5.4). 25 We also performed a meta‐analysis using the random‐effects model in case of heterogeneity. For a meta‐analysis to be conducted, at least two studies should report on our primary outcome, i.e., CIN2+. Results on RCTs and observational studies are reported separately, as are adjusted and unadjusted data from observational studies. We decided that at least two potential confounders (eg., age, number of lifetime sexual partners, margin status, smoking, socioeconomic status, HPV genotype‐status) should be adjusted for in the statistical model to allow inclusion in our meta‐analysis of adjusted risk‐estimates. We used the generic inverse‐variance as recommended by the Cochrane collaboration. 26 Egger's test was performed to assess possible publication bias, and a funnel plot was created.
The protocol was registered in PROSPERO (CRD42021238257), and the review was conducted in accordance with the PRISMA 2020 statement. 27
3. RESULTS
We identified 1561 articles in the literature search. After removal of duplicates, 1249 articles fulfilled the search criteria. Details of the inclusion process are provided in Figure 1. In total, 1203 records were excluded when screened by title and 34 records were excluded when screened by abstract, leaving 12 studies for full‐text evaluation. One article was not available in English. Four articles did not meet the inclusion criteria, as the HPV vaccine was administered before excisional treatment. Two additional studies were identified through a citation search in Scopus, and by reading reference lists, respectively, leaving nine publications with 19 971 women for inclusion in the primary analysis.
FIGURE 1.

PRISMA flowchart summarizing the inclusion of papers
3.1. General characteristics of the studies
Table 1 summarizes the characteristics of the nine included studies. Two studies were RCTs 28 , 29 and seven were observational studies, 12 , 13 , 14 , 30 , 31 , 32 , 33 one of which was a nationwide population‐based study. 14 Most studies were conducted in Europe, one was conducted in the Middle East and one in Asia. We found no studies from North America or Australia. All articles were published during 2013–2020. In the RCTs, no women in the control groups received a placebo vaccine. Thus, the women were not blinded to intervention. The largest study was a population‐based study including 17 128 women 14 and the smallest study included 178 women. 29 Most of the included studies reported only crude risk estimates. 12 , 13 , 31 , 32 The age of the women ranged from 17 to 65 years, and follow‐up time varied between 2 to 5 years. Four studies used the quadrivalent HPV‐vaccine, three studies used either the bivalent or quadrivalent HPV vaccine, one study used either the bivalent, quadrivalent or nonavalent HPV vaccine, and one study did not specify which HPV‐vaccine was used. Six studies administered three doses of an HPV‐vaccine 12 , 13 , 28 , 29 , 32 , 34 but did not necessarily exclude women who did not have all three doses. The remaining studies did not specify the number of doses given. 14 , 30 , 31 Four studies 12 , 30 , 32 , 33 performed HPV testing by cytology pre‐ and post‐treatment, whereas it was unknown whether women underwent HPV testing in the remaining studies. Only two studies 12 , 30 , 32 in which women underwent HPV testing provided sufficient information on CIN2+ by genotype.
TABLE 1.
Characteristics of included studies
| Author, year, country | Study design | Age of participants | Number of participants | Study population | Intervention | Follow‐up | HPV genotyping | Main results | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Karimi‐Zarchi et al., 2020, Iran 28 | Randomized controlled trial | 21–45 years |
312: 154 in control group and 158 in vaccination group |
Women treated for histologically confirmed residual/recurrent CIN1 or CIN2 or CIN3 | Three doses of the quadrivalent HPV vaccine. Women were excluded if they received only one dose | 2 years | No |
Vaccine efficacy: CIN1: 54.9%. CIN2: 63.3%. CIN3: 52.3% Total for CIN1‐3: 58.7% |
Vaccine efficacy measured as difference in attack rate between vaccinated and nonvaccinated. The study did not distinguish between recurrent and residual disease |
| Pieralli et al., 2018, Italy 29 | Randomized controlled trial | Under 45 years of age |
178: 89 in control group and 89 in vaccination group |
Women treated for CIN, any grade | Three doses of the quadrivalent HPV vaccine | At least 3 years | HPV genotyping if recurrence occurred. No HPV status of primary lesion available | Recurrence of LSIL/HSIL (in cervix, vulva and vagina) was 3.4% in vaccinated group vs 13.5% in the nonvaccinated group | Persistent disease 3 months after treatment was an exclusion criterion |
| Ghelardi et al., 2018, Italy 13 | Observational study | 18–45 years |
524: 276 in control group and 248 in vaccination group |
Women treated with LEEP for histologically confirmed CIN2+ or cervical cancer stage IA1 | Three doses of the quadrivalent HPV vaccine. | Median 36 months (range 6–48 months) | HPV test and genotyping during the follow‐up program | Risk reduction of 81.2% (95% CI 34.3–95.7) in recurrence of CIN2+ |
CIN2+ independent of HPV type. Persistent histologically confirmed disease at 6‐month follow‐up visit was an exclusion criterion |
| Kang et al., 2013, Korea 12 | Observational study | 20–45 years |
737: 377 in control group and 360 in vaccination group |
Women treated with LEEP for CIN2–3 | Three doses of the quadrivalent HPV vaccine | Median 3.5 years | Before LEEP and at every visit after LEEP HPV DNA test was performed | Hazard ratio of risk of recurrence of CIN2‐3 in women not receiving the vaccine vs women receiving the vaccine: 2.84 (1335–6042) P < 0.01 | CIN2 or CIN3 at 3‐month follow‐up visit was considered as residual disease and patients were excluded |
| Sand et al., 2019, Denmark 14 | Observational study | 17–51 years |
17 128 women: 15 054 in control group and 2074 in vaccination group, of which 399 were vaccinated 0–3 months before excisional treatment and 1675 were vaccinated 0–12 months after excisional treatment |
Women treated with excisional treatment for CIN3 | HPV vaccination (not further specified) | At least 3 years | No | Adjusted hazard ratio of risk of recurrent CIN2+ in women vaccinated 0–12 months after excisional treatment: 0,88 (95% CI 0.67–1.14) | Follow‐up began 1‐year post‐treatment to ensure only women with recurrent and not residual disease were included in the analysis |
| Ortega‐Quinonero et al., 2018, Spain 30 | Observational study | 18–65 years |
242: 139 in control group and 103 in vaccination group |
Women treated with LEEP for CIN2–3 | Bivalent or quadrivalent HPV vaccination, number of doses not specified | 2 years | HPV testing and determination of genotype both before treatment and during follow‐up | Recurrence of CIN2‐3 in vaccinated group was 4.8% vs 15.8% in the nonvaccinated | Residual disease, defined as a histological diagnosis of CIN2‐3 in biopsies three months after LEEP, was an exclusion criterion |
| Petrillo et al., 2020, Italy 31 | Observational study | 32–47 years |
285: 103 in control group and 182 in vaccination group |
Women treated with LEEP due to CIN. | Bi‐ or quadrivalent HPV vaccination, number of doses not specified | 2 years | HPV testing before treatment, but not systematically at time of relapse | Odds ratio of disease recurrence: 0.4 (95% CI 0.2–0.8, P = 0,02) in vaccinated vs nonvaccinated |
Outcome was defined as a cervical lesion of any histologic type (CIN1–3). No distinction between recurrent and residual disease |
| del Pino et al., 2020, Spain 32 | Observational study | 26–64 years |
265. 112 in control group. 153 in vaccination group |
Women treated with excisional treatment for CIN | Three doses of the bi‐, quadri‐ or nonavalent HPV vaccine. Women who received less than three doses were not excluded | At least 2 years | HPV testing both at enrolment and during follow‐up | Adjusted odds ratio of persistent/recurrent HSIL: 0.2 (95% CI 0.1–0.7) in vaccinated vs nonvaccinated | No distinction between residual and recurrent disease was made. 42.3% of the women showed either HSIL or LSIL at the first post‐treatment visit after 6 months |
| Bogani et al, 2020, Italy 33 | Observational study. | 24–44 years |
300 in the propensity score‐matched comparison. 200 in control group. 100 in vaccination group |
Women treated with LEEP for CIN2 or CIN3 | Three doses of the bi‐ or quadrivalent HPV vaccine. Women who received less than three doses were not excluded | 5 years | HPV testing before and after treatment. No information on HPV type was not an exclusion criterion | Recurrence of HSIL in vaccinated group was 0% vs 4,5% (n = 9) in control group. Not statistically significant. | No information on whether women in control group had previously received the HPV vaccine. Differentiation between residual and recurrent disease in the statistical analysis |
Abbreviations: CIN, cervical intraepithelial lesion; HPV, human papillomavirus; HSIL, high‐grade squamous intraepithelial lesion; LEEP, loop electrosurgical excision procedure; LSIL, low‐grade squamous intraepithelial lesion.
In the present review, we used HPV vaccination after treatment as the primary inclusion criterion to investigate whether women undergoing excisional treatment for CIN may or may not benefit from receiving the HPV vaccine post‐treatment. Thus, only studies in which women underwent vaccination post‐treatment were included in the analysis. However, two studies also reported risk estimates on women receiving the HPV vaccine immediately before the conization. In these studies, the vaccine was administered either 0–1 months 30 or 0–3 months 14 prior to excisional treatment.
All nine studies evaluated the effectiveness of post‐treatment HPV vaccination by reporting the risk of CIN recurrence after excisional treatment. However, there was a considerable heterogeneity between the outcome measure and statistical analyses used in the included studies. Two studies used CIN1‐3 as the primary outcome, 28 , 31 three studies used CIN2‐3, 12 , 30 , 33 and two studies reported on CIN2+. 13 , 14 One study defined the primary outcome as a subsequent diagnosis of histological HSIL or LSIL of the vagina, cervix and vulva. 29 Another study defined atypical squamous cells of undetermined significance (ASCUS) or worse or CIN1+ as the primary outcome. 32 No studies reported risk of recurrence by lesion type (glandular vs squamous). With respect to statistical analyses, two studies took person‐time at‐risk into account12, 14 whereas the remaining studies did not.
Risk of bias assessment for RCTs (Figure S1) and observational studies (Figure S2) are available, including a detailed description (Tables S1 and S2).
The risk of bias assessment revealed an overall high risk of bias for all included studies, except for one large population‐based study. 14 Neither of the two RCTs were double‐blinded, as the women were aware of the group they were allocated to. In the RCT by Karimi et al., it was unclear whether all participants underwent excisional treatment and margin status was not reported. 28 As women with positive margins have a higher risk of recurrence compared with women with negative margins, 35 the knowledge of margin status is crucial in vaccinated as well as in unvaccinated women in order to draw any firm conclusion. In addition, the study was determined to have a high risk of attrition bias, as loss‐to‐follow up was substantial; 12.6% in the vaccinated group were lost to follow‐up compared with 32.4% in the control group. Thus, in the control group the total number of women included in the analysis did not reach 150, as estimated in the power calculation. A substantial limitation in the other RCT was the noticeably low number of participants (178 women) and events of CIN2+ (zero in the vaccination group vs four in the control group). 29 Moreover, the authors did not perform a power calculation. 29 Five of the seven observational studies were assessed to have a serious risk of bias, 12 , 13 , 30 , 31 , 32 mainly due to confounding. Most studies did not adjust for known confounders such as age, margin status, smoking, socioeconomic status or HPV‐status, which is likely to overestimate the impact of the HPV vaccine on the risk of recurrence. Four studies reported adjusted risk estimates, taking into account age at excisional treatment, 14 , 32 , 33 calendar year of excisional treatment, 14 histological diagnosis in the surgical specimen, 14 , 33 prevalence of HR‐HPV before excisional treatment, 33 margin involvement, 33 body mass index 33 and educational level. 14 In one study, it was unclear which variables were included in the multivariable analysis. 30 In four studies, women decided whether they wanted to undergo HPV vaccination, 12 , 13 , 31 , 32 whereas information on how the women were allocated to intervention groups was missing in other studies. 14 , 30 , 33 One study reported that women had to pay for the vaccines themselves, 32 thus introducing healthy‐user bias; there was no information regarding payment in any of the other studies. Of importance, the absolute number of participants and events was low in the majority of the observational studies. Only one study was of significant size (17 128 women), since it was population‐based. 14 As described, the majority of studies carried a substantial risk of bias in several areas. This limits the interpretation of the true effect of HPV vaccination after excisional treatment as regards risk of recurrence.
3.2. Synthesis of the results
Both RCTs reported a reduced risk of CIN2+ recurrence in HPV‐vaccinated as compared with unvaccinated women, although the results were nonsignificant in one of these studies. The overall pooled odds ratio (OR) for recurrence of CIN2+ was 0.29 (95% CI 0.16–0.53) using the fixed‐effect model (Figure 2).
FIGURE 2.
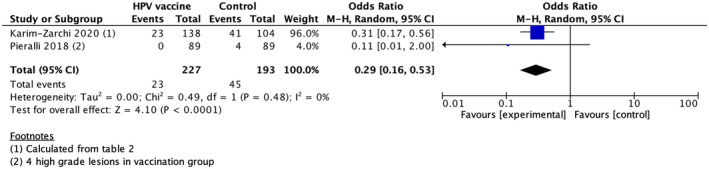
Forest plot of randomized controlled trials using the fixed‐effect model. The figure summarizes odds ratios (OR) of recurrence of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) comparing women receiving the HPV vaccine after excisional treatment with women who did not receive the vaccine
Crude risk estimates varied across observational studies. For example, one nationwide population‐based cohort study including more than 17 000 individuals found no reduced risk of CIN2+ recurrence in women undergoing HPV vaccination post‐treatment compared with women who did not undergo vaccination, whereas five smaller studies reported a significant risk reduction. Four studies 14 , 30 , 32 , 33 reported adjusted risk‐estimates in addition to unadjusted estimates. One study was excluded from the meta‐analysis of adjusted data because it only included age in the multivariate analysis. 32 Due to heterogeneity, we applied the random‐effects model on crude estimates in our main analysis of observational studies and found an overall pooled OR for CIN2+ recurrence of 0.35 (95% CI 0.18–0.67) (Figure 3A). When applying the random‐effects model on adjusted risk‐estimates, OR for CIN2+ recurrence was no longer statistically significant (pooled OR of 0.54, 95% CI 0.21–1.35) (Figure 3B). For transparency, we also conducted a meta‐analysis using the fixed‐effect model and found a pooled OR for recurrence of CIN2+ of 0.69 (95% CI 0.56–0.86) when using crude estimates (Figure 4A). When using the fixed‐effect model on adjusted risk‐estimates, the pooled OR for CIN2+ recurrence remained low but was no longer statistically significant (OR 0.82, 95% CI 0.63–1.06) (Figure 4B). Restricting to studies that reported the use of the quadrivalent HPV vaccine only, did not change the main conclusions (data not shown).
FIGURE 3.
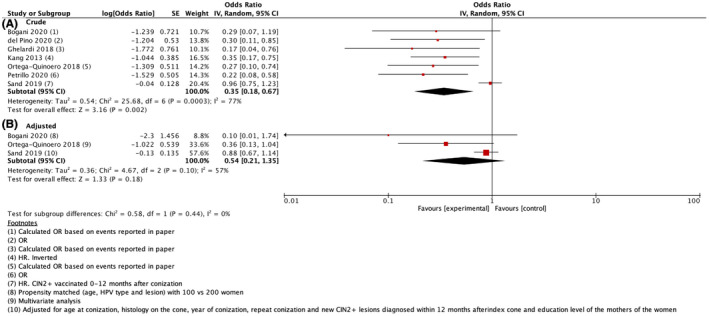
Forest plot of observational studies using the random‐effects model on crude estimates (A) and adjusted estimates (B). The figure summarizes odds ratios (OR) of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) comparing women receiving the HPV vaccine after excisional treatment with women who did not receive the vaccine
FIGURE 4.
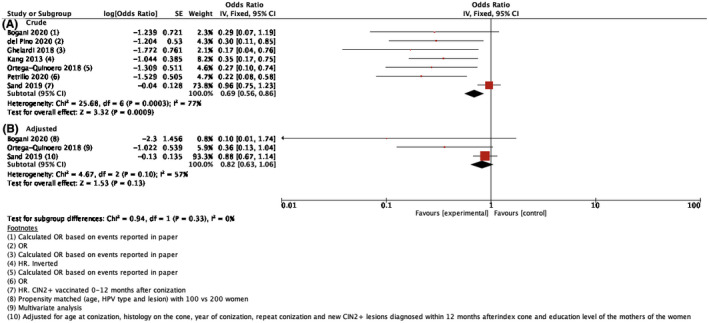
Forest plot of observational studies using the fixed‐effect model on crude estimates (A) and adjusted estimates (B). The figure summarizes odds ratios (OR) of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) comparing women receiving the HPV vaccine after excisional treatment with women who did not receive the vaccine
We created a funnel plot to assess publication bias and found a high risk of publication bias, as indicated by the asymmetry of the funnel plot (Figure S3).
4. DISCUSSION
This paper summarizes the existing literature regarding the effect of post‐treatment HPV vaccination on risk of recurrence after excisional treatment for CIN. Similar to previous systematic reviews, our findings suggest that HPV vaccination post‐surgery is associated with a reduced risk of recurrence of CIN when using crude risk estimates from observational studies and data from two RCTs. However, most included studies were assessed to have a serious risk of bias in addition to a high risk of publication bias and data heterogeneity. None of the two RCTs were sufficiently powered to address the research question. Importantly, HPV vaccination was not associated with a reduced risk of recurrence when using adjusted risk‐estimates. These findings suggest a need for well‐designed randomized controlled trials to assess whether post‐treatment HPV vaccination should be recommended to women undergoing excisional treatment for CIN.
Previous systematic reviews and meta‐analyses on this subject have all suggested a significantly reduced risk of recurrence among women receiving the HPV vaccine in relation to excisional treatment of CIN. 15 , 16 , 17 , 18 However, unlike our study, they also included studies in which individuals received the HPV vaccine up to 48 months prior to excisional treatment for CIN. This implies that these women were likely vaccinated as part of a prophylactic HPV vaccination program. Pooling data from studies with women who underwent prophylactic HPV vaccination with women who underwent post‐treatment HPV vaccination is likely to lead to an overestimation of the impact of post‐treatment HPV vaccination on risk of CIN2+ recurrence. Moreover, previous systematic reviews mainly used crude risk estimates in their meta‐analyses, whereas we performed a meta‐analysis on crude and adjusted risk‐estimates. These differences may to some extent explain the observed differences between our study and previous studies.
Thus, the lack of adjustment for well‐known confounders, differences in how the women were allocated to intervention groups, and the presentation of unadjusted numbers in studies included in the present meta‐analysis may result in an overestimation of the effect of HPV vaccination administered post‐treatment. Additionally, the studies in which women were allocated by self‐payment are severely biased by behavioral differences between women accepting vaccines and women who do not; the “healthy user effect”. 36 This may result in an overestimation of the effect of HPV vaccination post‐treatment, unless accounted for in the analyses. For transparency, we decided to conduct a meta‐analysis using both the fixed‐effect and random‐effects model. The fact that the pooled OR of CIN2+ recurrence was lower in the random‐effects model than in the fixed‐effect model likely occurred because the large population‐based study 14 (which did not find a significant effect of HPV vaccination) was more influential in the fixed‐effect than in the random‐effects model. Results from large, randomized, double‐blinded, placebo‐controlled studies are needed to overcome all these potential biases with the aim to estimate the true effectiveness of HPV vaccination post‐surgery. Currently, one RCT is ongoing; the NOVEL trial. 37 Results from this study are awaited. Until then, the current literature does not allow a firm conclusion regarding the effect of post‐treatment HPV vaccination for reducing risk of CIN recurrence.
Previous studies have reported that women with free margins in the conization specimen have a decreased risk of recurrence compared with women with margin involvement. 35 , 38 In line with this finding, one of the included studies concluded that margin involvement was a significant and independent risk factor for recurrence. 12 Also, two of the included studies in this review reported that risk of recurrence was lower among vaccinated individuals who had free margins than vaccinated individuals who did not. 30 , 33 This emphasizes the importance of adjusting for margin status when evaluating the effect of HPV vaccination after excisional treatment. Only five studies included in this review reported on margin status. 12 , 13 , 31 , 32 , 33 Unfortunately, we were unable to perform a meta‐analysis by margin status due to missing information and heterogeneity. However, new evidence suggests that a negative test of cure (i.e., cytology and HPV testing 6 months post‐conization) provides greater reassurance against recurrence than free margins do. 19 , 35 In all studies, women had received the first vaccine dose during the 6‐month time period following treatment. This prevents us from adjusting for test of cure result and conducting a meta‐analysis using this outcome.
As only two studies provided sufficient information on CIN2+ recurrence by HPV type, we were unable to conduct a meta‐analysis stratified by HPV genotype (ie HPV 16/18 vs other HPV types). Two of the previously published meta‐analyses conducted a meta‐analysis by genotype and both found that HPV vaccination was associated with lower odds for CIN2+ in women who tested positive for HPV 16/18 (pooled OR 0.35–0.37). 17 , 18 However, as previously described, these meta‐analyses also included studies in which women received the vaccine up to 48 months prior to excisional treatment, which likely affected the results. More studies are therefore needed to determine whether effectiveness of post‐treatment vaccination differs by HPV genotype.
None of the included studies had a follow‐up time >5 years. Previous studies show that women who have undergone excisional treatment for CIN have an increased risk of cervical cancer and other HPV‐related cancers compared with the general population, 39 a risk that increases with age and over time. 39 , 40 The underlying reasons for these findings are unclear but may be due to a higher susceptibility to HPV infections or shared risk factors such as a high number of sexual partners and smoking. Moreover, genetic variation in susceptibility for cervical cancer development in HPV‐infected individuals has been suggested. 41 It remains unclear whether HPV vaccination after excisional treatment may potentially reduce the long‐term risk of cervical cancer and other HPV‐related diseases.
The mechanism in secondary prevention of CIN remains unclear. Several reasons why a prophylactic HPV vaccine may prevent recurrence of CIN can be hypothesized. Previous studies highlight the possible effect of cross‐immunization for the primary prevention of HPV‐related disease, but the effect in secondary prevention is unclear. 42 , 43 Although the HPV vaccine can protect against infection with HPV strains which an individual has not been exposed to previously, it is unclear whether it may also prevent re‐activation of a potential latent HPV infection, 44 lateral spread or auto‐inoculation from other infection sites, including the anus. 45
A strength of this systematic review and meta‐analysis was the inclusion of studies in which HPV vaccination was undertaken after excisional treatment only. Secondly, we performed a thorough systematic literature search in more than one online database. Thirdly, a detailed risk of bias assessment was performed for all studies to critically address potential bias. Due to substantial heterogeneity, a random‐effects model was applied in our meta‐analysis, thereby allowing for a fair weighting of large studies. Of note, in the random‐effects model, the largest study including more than 17 000 individuals was less influential than in the fixed‐effect model despite being assessed as having the best quality of all observational studies. Finally, a great strength is the fact that we performed a meta‐analysis on adjusted data.
However, several limitations should be addressed. First, we observed a substantial heterogeneity between studies with regard to outcome, inclusion and exclusion criteria, and confounders. Additionally, we were unable to conduct a meta‐analysis by margin status, vaccine type, number of vaccine doses administered or HPV genotype due to limited or missing information on these variables. Secondly, a considerable indirectness of evidence has to be emphasized due to the lack of adjusting for confounders in the studies included. Thirdly, a small number of studies were included and only two were RCTs; these two studies had different inclusion criteria, thus questioning the pooling of the odds ratios. Thus, our results may be subject to an overestimation of the effect of HPV vaccination post‐treatment.
5. CONCLUSION
Based on a meta‐analysis of two RCTs and seven observational studies, HPV vaccination post‐treatment was associated with a significantly reduced risk of CIN2+ recurrence. However, applying the recommended tools for bias assessment revealed a serious risk of bias in all included studies except for one observational study. 14 When using the outcome measure with the least risk of bias and performing a meta‐analysis on adjusted risk estimates from observational studies, HPV vaccination post‐treatment remained associated with a reduced odds for CIN2+ recurrence; however, this finding was no longer statistically significant. Large well‐designed randomized placebo‐controlled trials with sufficient power and follow‐up time are needed to adequately address whether HPV vaccination should be recommended to women after excisional treatment for CIN.
CONFLICT OF INTEREST
PJ and AH have received a speaker's fee from Astra Zeneca, outside the submitted work. AH has received reagents at reduced cost from Roche, Denmark, outside the submitted work. DOE and JBS declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
DOE performed the literature search, screened titles and abstracts, assessed studies for inclusion, performed risk of bias assessment, and drafted the manuscript. AH performed the literature search, screened titles and abstracts, assessed studies for inclusion, contributed in discussions, and critically read and commented on the manuscript. PJ read the articles assessed for eligibility, contributed in discussions, and critically read and commented on the manuscript. JBS read the articles assessed for eligibility, performed risk of bias assessment and conducted the meta‐analyses, contributed in discussions, and critically read the manuscript.
Supporting information
Appendix S1
Figure S1.
Figure S2.
Figure S3.
Table S1.
Table S2.
Eriksen DO, Jensen PT, Schroll JB, Hammer A. Human papillomavirus vaccination in women undergoing excisional treatment for cervical intraepithelial neoplasia and subsequent risk of recurrence: A systematic review and meta‐analysis. Acta Obstet Gynecol Scand. 2022;101:597–607. doi: 10.1111/aogs.14359
REFERENCES
- 1. Curado MP, Shin HR, Ferlay J, Heanue M, Boyle P, Storm H. Cancer incidence in five continents. IARC Sci Publ. 2008;9(160):1‐837. [Google Scholar]
- 2. Frazer IH. Interaction of human papillomaviruses with the host immune system: a well evolved relationship. Virology. 2009;384:410‐414. [DOI] [PubMed] [Google Scholar]
- 3. Walboomers JMM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12‐19. [DOI] [PubMed] [Google Scholar]
- 4. Bosch FX, Lorincz A, Muñoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baldur‐Felskov B, Dehlendorff C, Junge J, Munk C, Kjaer SK. Incidence of cervical lesions in Danish women before and after implementation of a national HPV vaccination program. Cancer Causes Control. 2014;25:915‐922. [DOI] [PubMed] [Google Scholar]
- 6. Baldur‐Felskov B, Dehlendorff C, Munk C, Kjaer SK. Early impact of human papillomavirus vaccination on cervical neoplasia‐‐Nationwide follow‐up of young Danish women. J Natl Cancer Inst. 2014;106:djt460. [DOI] [PubMed] [Google Scholar]
- 7. Drolet M, Bénard É, Pérez N, et al. Population‐level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta‐analysis. Lancet. 2019;394:497‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340‐1348. [DOI] [PubMed] [Google Scholar]
- 9. Kjaer SK, Dehlendorff C, Belmonte F, Baandrup L. Real‐world effectiveness of human papillomavirus vaccination against cervical cancer. J Natl Cancer Inst. 2021;113:1329‐1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: updating the natural history of HPV and anogenital cancer. Vaccine. 2006;24(Suppl 3):42‐51. [DOI] [PubMed] [Google Scholar]
- 11. Arbyn M, Xu L, Simoens C, Martin‐Hirsch PPL. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Systc Rev. 2018;5:CD009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kang WD, Choi HS, Kim SM. Is vaccination with quadrivalent HPV vaccine after loop electrosurgical excision procedure effective in preventing recurrence in patients with high‐grade cervical intraepithelial neoplasia (CIN2‐3)? Gynecol Oncol. 2013;130:264‐268. [DOI] [PubMed] [Google Scholar]
- 13. Ghelardi A, Parazzini F, Martella F, et al. SPERANZA project: HPV vaccination after treatment for CIN2+. Gynecol Oncol. 2018;151:229‐234. [DOI] [PubMed] [Google Scholar]
- 14. Sand FL, Kjaer SK, Frederiksen K, Dehlendorff C. Risk of cervical intraepithelial neoplasia grade 2 or worse after conization in relation to HPV vaccination status. Int J Cancer. 2020;147:641‐647. [DOI] [PubMed] [Google Scholar]
- 15. Bartels HC, Postle J, Rogers AC, Brennan D. Prophylactic human papillomavirus vaccination to prevent recurrence of cervical intraepithelial neoplasia: a meta‐analysis. Int J Gynecol Cancer. 2020;30:777‐782. [DOI] [PubMed] [Google Scholar]
- 16. Lichter K, Krause D, Xu J, et al. Adjuvant human papillomavirus vaccine to reduce recurrent cervical dysplasia in unvaccinated women: a systematic review and meta‐analysis. Obstet Gynecol. 2020;135:1070‐1083. [DOI] [PubMed] [Google Scholar]
- 17. Jentschke M, Kampers J, Becker J, Sibbertsen P, Hillemanns P. Prophylactic HPV vaccination after conization: a systematic review and meta‐analysis. Vaccine. 2020;38:6402‐6409. [DOI] [PubMed] [Google Scholar]
- 18. Di Donato V, Caruso G, Petrillo M, et al. Adjuvant hpv vaccination to prevent recurrent cervical dysplasia after surgical treatment: A meta‐analysis. Vaccines (Basel). 2021;9:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Verguts J, Bronselaer B, Donders G, et al. Prediction of recurrence after treatment for high‐grade cervical intraepithelial neoplasia: the role of human papillomavirus testing and age at conisation. BJOG. 2006;113:1303‐1307. [DOI] [PubMed] [Google Scholar]
- 20. Bogani G, DI Donato V, Sopracordevole F, et al. Recurrence rate after loop electrosurgical excision procedure (LEEP) and laser conization: A 5‐year follow‐up study. Gynecol Oncol. 2020;159:636‐641. [DOI] [PubMed] [Google Scholar]
- 21. Nøhr B, Tabor A, Frederiksen K, Krüger Kjær S. Loop electrosurgical excision of the cervix and the subsequent risk of preterm delivery. Acta Obstet Gynecol Scand. 2007;86:596‐603. [DOI] [PubMed] [Google Scholar]
- 22. Kyrgiou M, Athanasiou A, Paraskevaidi M, et al. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: systematic review and meta‐analysis. BMJ. 2016;354:i3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sterne JACSJ, Page MJ, Elbers RG, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 24. Sterne JACHM, Reeves BC, Savović J, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomized studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. (RevMan) RM. 5.4 ed.: The Cochrane Collaboration, 2020.
- 26. Reeves BC DJ, Higgins JPT, Shea B, Tugwell P, Wells GA. Chapter 24: including non‐randomized studies on intervention effects., http://www.training.cochrane.org/handbook (2021, accessed July 25 2021).
- 27. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karimi‐Zarchi M, Allahqoli L, Nehmati A, Kashi AM, Taghipour‐Zahir S, Alkatout I. Can the prophylactic quadrivalent HPV vaccine be used as a therapeutic agent in women with CIN?. A randomized trial. BMC Public Health. 2020;20:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pieralli A, Bianchi C, Auzzi N, et al. Indication of prophylactic vaccines as a tool for secondary prevention in HPV‐linked disease. Arch Gynecol Obstet. 2018;298:1205‐1210. [DOI] [PubMed] [Google Scholar]
- 30. Ortega‐Quiñonero P, Remezal‐Solano M, Carazo‐Díaz MC, et al. Impact of the human papillomavirus vaccination on patients who underwent conization for high‐grade cervical intraepithelial neoplasia. Eur J Gynaecoll Oncol. 2019;40:402‐407. [Google Scholar]
- 31. Petrillo M, Dessole M, Tinacci E, et al. Efficacy of HPV vaccination in women receiving LEEP for cervical dysplasia: A single institution's experience. Vaccines (Basel). 2020;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Del Pino M, Martí C, Torras I, et al. HPV vaccination as adjuvant to conization in women with cervical intraepithelial neoplasia: A study under real‐life conditions. Vaccines (Basel). 2020;8:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bogani G, Raspagliesi F, Sopracordevole F, et al. Assessing the Long‐Term Role of Vaccination against HPV after Loop Electrosurgical Excision Procedure (LEEP): A Propensity‐Score Matched Comparison. Vaccines (Basel). 2020;8:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barra F, Leone Roberti Maggiore U, Bogani G, et al. New prophylactics human papilloma virus (HPV) vaccines against cervical cancer. J Obstet Gynaecol. 2019;39:1‐10. [DOI] [PubMed] [Google Scholar]
- 35. Arbyn M, Redman CWE, Verdoodt F, et al. Incomplete excision of cervical precancer as a predictor of treatment failure: a systematic review and meta‐analysis. Lancet Oncol. 2017;18:1665‐1679. [DOI] [PubMed] [Google Scholar]
- 36. Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med. 2011;26:546‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. ClinicalTrials.gov. Nonavalent Prophylactic HPV Vaccine (GARDASIL9) After Local Conservative The NOVEL Trial (NOVEL), https://clinicaltrials.gov/ct2/show/NCT03979014?term=Kyrgiou&type=Intr&cond=HPV+vaccine&draw=2&rank=1 (2019, accessed 15.09.2021.
- 38. Chen JY, Wang ZL, Wang ZY, Yang XS. The risk factors of residual lesions and recurrence of the high‐grade cervical intraepithelial lesions (HSIL) patients with positive‐margin after conization. Medicine (Baltimore). 2018;97:e12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kalliala I, Athanasiou A, Veroniki AA, et al. Incidence and mortality from cervical cancer and other malignancies after treatment of cervical intraepithelial neoplasia: a systematic review and meta‐analysis of the literature. Ann Oncol. 2020;31:213‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Strander B, Hällgren J, Sparén P. Effect of ageing on cervical or vaginal cancer in Swedish women previously treated for cervical intraepithelial neoplasia grade 3: population based cohort study of long term incidence and mortality. BMJ. 2014;348:f7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bowden SJ, Bodinier B, Kalliala I, et al. Genetic variation in cervical preinvasive and invasive disease: a genome‐wide association study. Lancet Oncol. 2021;22:548‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ault KA. Human papillomavirus vaccines and the potential for cross‐protection between related HPV types. Gynecolc Oncol. 2007;107:S31‐S33. [DOI] [PubMed] [Google Scholar]
- 43. Jenkins D. A review of cross‐protection against oncogenic HPV by an HPV‐16/18 AS04‐adjuvanted cervical cancer vaccine: importance of virological and clinical endpoints and implications for mass vaccination in cervical cancer prevention. Gynecol Oncol. 2008;110:S18‐S25. [DOI] [PubMed] [Google Scholar]
- 44. Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: Role of viral latency. Viruses. 2017;9:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nyitray AG, Chang M, Villa LL, et al. The natural history of genital human papillomavirus among HIV‐negative men having sex with men and men having sex with women. J Infect Dis. 2015;212:202‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Figure S1.
Figure S2.
Figure S3.
Table S1.
Table S2.


