Abstract
Introduction
Hypertensive disorders of pregnancy are a leading cause of maternal and perinatal mortality and morbidity worldwide. We studied the prevalence of hypertensive disorders of pregnancy among women of migrant origin in Finland.
Material and Methods
This study used data from the nationwide Medical Birth Register. Information on the most recent singleton birth of women who delivered between 2004 and 2014 (n = 382 233) was included. Women were classified into nine regional categories based on the country of origin. Women of Finnish origin were the reference group. Generalized linear models adjusted for maternal age, socioeconomic position, smoking in pregnancy, parity, pre‐pregnancy body mass index, preexisting diabetes and delivery year were used to study the association between region/country of origin and hypertensive disorders of pregnancy.
Results
Among the study population, almost 8% were of migrant origin. The prevalence of hypertensive disorders of pregnancy varied from 1.3% (women of East Asian origin) to 4.2% (women of Sub‐Saharan African origin), compared with 4.6% in the Finnish origin reference group. Compared with women of Finnish origin, the risk for any hypertensive disorders of pregnancy after adjustment for confounders was lower for women of migrant origin, with an exception for women of Sub‐Saharan African origin. When analyzing gestational hypertension and preeclampsia outcomes separately, Sub‐Saharan African origin women had a lower risk for gestational hypertension (risk ratio [RR] 0.41, 95% confidence interval [CI] 0.30–0.56) but a higher risk for preeclampsia (RR 1.77, 95% CI 1.44–2.17) than women of Finnish origin.
Conclusions
In general, women of migrant origin in Finland had a lower risk for any hypertensive disorders of pregnancy and gestational hypertension. The risk for preeclampsia was higher among women of Sub‐Saharan African origin and may warrant special attention.
Keywords: Finland, hypertensive disorders, migrant, preeclampsia, pregnancy complication

Abbreviations
- BMI
body mass index
- CI
confidence intervals
- HDP
hypertensive disorders of pregnancy
- MBR
Medical Birth Register
- RR
risk ratio
- THL
Finnish Institute of Health and Welfare
Key message.
Women of Sub‐Saharan African origin had a lower risk for gestational hypertension but a higher risk for preeclampsia compared with women of Finnish origin in Finland.
1. INTRODUCTION
Hypertensive disorders of pregnancy (HDP) are the leading cause of maternal and perinatal mortality and morbidity worldwide, causing complications in up to 10% of all pregnancies. 1 , 2 The common HDP are chronic hypertension, gestational hypertension and preeclampsia. Preeclampsia and eclampsia account for almost 15% of all maternal deaths worldwide. 3 In addition to causing complications during pregnancy, HDP increase the risk for several post‐pregnancy complications, including post‐pregnancy hypertension, ischemic heart disease, stroke, chronic hypertension, type 2 diabetes mellitus and hypercholesterolemia. 4 , 5 , 6 The risk factors for HDP are well established. These include a higher body mass index (BMI), higher maternal age, first pregnancy, history of HDP, gestational diabetes mellitus, preexisting conditions such as type 2 diabetes, family history, genetic susceptibility, alcohol use during pregnancy, other conditions such as anemia and urinary tract infections. 7 The prevalence of HDP is expected to continue to grow in countries with increasing risk factors such as growing rates of diabetes, obesity and advanced maternal age. This is expected to also increase the rates of adverse obstetric outcomes associated with HDP, including fetal growth restriction, preterm birth and perinatal death. 4 , 6
Some studies have reported the prevalence of HDP among women of migrant origin in high‐income countries. A previous systematic review and meta‐analysis found that the prevalence of HDP was lower among women of migrant origin than among women in the general population. 8 Another systematic review reported that the risk of preeclampsia was lower among women with refugee background than women in the general population. 9 Several studies from Norway found that, in general, women of migrant origin had a lower risk for hypertensive disorder compared with women of Norwegian origin. 10 , 11 , 12 A lower prevalence of HDP was also reported among women of migrant origin of Somali, Kurdish and Russian origin than among women in the general population in Finland. 13 Therefore, in general, the existing literature shows that women of migrant origin have a lower risk of HDP compared with women in the general population. However, most of the findings that reported HDP included a broad category for the definition of migrant origin, for example, migrant origin vs the general population or refugees vs the general population. It is well acknowledged that significant differences can be observed in the health of persons of migrant origin when looking more specifically at country and region of origin. 8 , 9 , 11 , 12 , 13 Therefore, there is still a gap in the knowledge about the risk of HDP among more specifically defined migrant origin groups. In this study, we compared the risk of any HDP, and the risk of gestational hypertension and preeclampsia separately, according to the region of origin of migrant women and women of Finnish origin.
2. MATERIAL AND METHODS
The data for this study were based on information from the Finnish national Medical Birth Register (MBR) and Statistics Finland. MBR is responsible for collecting all the data related to women’s sociodemographic information, pregnancy, monitoring during pregnancy, delivery and its complications and information on the newborns. 14 The information on the country of origin was obtained from Statistics Finland. Information from these two registers was linked using the unique personal identification code. Permission to use the data was obtained from the respective registries from the Finnish Institute of Health and Welfare (THL) and Statistics Finland. We analyzed and stored the pseudonymized data at THL, following THL’s data safety regulations.
This study included information on each woman’s most recent pregnancy in Finland between January 2004 and December 2014 (n = 389 758). After excluding women with multiple births (n = 7525), the final study sample was comprised of 382 233 women. In Finland, gestational hypertension is diagnosed if the systolic blood pressure of ≥140 mmHg or the diastolic blood pressure of ≥90 mmHg is identified after 20 weeks’ gestation and before the delivery, or if it increases by more than 30/15 mmHg from the baseline level during pregnancy. Preeclampsia is diagnosed when the blood pressure exceeds 140/90 mmHg after 20 weeks’ gestation and presence of proteinuria. 15 Pregnant women are regularly screened for their blood pressure levels at the maternity clinic. When necessary, blood pressure is measured more often and women with preeclampsia and eclampsia are referred for closer monitoring at the delivery hospital. 15
Migrant origin groups were categorized based on the United Nations’ geographic classification of countries. 16 Country of origin was defined as the country of birth of the pregnant woman's parents. If both parents were born abroad, the country of birth of the woman's biological mother was the primary country of origin. If at least one of the parents was born in Finland, the country of origin was Finland. Women were classified into nine regional categories: Finland; Western Europe/North America/Oceania (later referred to as Western high‐income countries); Eastern Europe; Russia and the former Union of Soviet Socialist Republics (USSR); South Asia; East Asia; Sub‐Saharan Africa; Middle East/North Africa; and Latin America/Caribbean. Women with an unknown country of origin (n = 231, 0.06%) were excluded from the final analyses.
The composite outcome variable for any hypertensive disorders was based on specific hypertensive disorders, namely, chronic hypertension, gestational hypertension, preeclampsia, eclampsia and unspecified maternal hypertension. The diagnosis of these specific hypertensive disorders was extracted from the MBR based on the International Statistical Classification of Diseases and Related Health Problems ICD‐10 codes. We included ICD‐10 codes O10, O10.0–O10.9, O11 (chronic hypertension), O13 (gestational hypertension), O14, O14.0, O14.1 and O14.9 (mild, moderate, severe and unspecified preeclampsia), O15, O15.1, O15.2 and O15.9 (eclampsia in pregnancy, labor and unspecified) and O16 (unspecified/transient hypertension of pregnancy). In addition to the composite HDP variable, we presented the risk for gestational hypertension and preeclampsia by migrant origin separately. It was not possible to analyze the risk for chronic hypertension, eclampsia and transient/unspecified maternal hypertension separately by region of origin due to a low number of cases for these specific conditions.
Mother’s age at delivery was categorized into four categories: <25, 25–29, 30–34 and ≥35 years. Socioeconomic position was categorized into five categories: upper‐level employees (administrative, managerial, professional and related occupations), lower‐level employees (administrative and clerical occupations), manual workers, other (including pensioners/homemakers/students), and unknown. Smoking in pregnancy was categorized as yes/no. Parity (number of previous births) was categorized as 0, 1 and ≥2. Pre‐pregnancy BMI was categorized as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) and obesity (≥30 kg/m2). The delivery year was categorized as 2004–2007, 2008–2011 and 2012–2014.
Descriptive data were reported as numbers of observations and prevalence (%). Generalized linear models with the log‐link function were used to obtain relative risk (risk ratio [RR]) estimates with 95% confidence intervals (CI) for the composite HDP variable, gestational hypertension and preeclampsia. women of Finnish origin were the reference group. Model I was an unadjusted model and Model II was adjusted for age, socioeconomic position, smoking in pregnancy, parity, pre‐pregnancy BMI, preexisting diabetes (Type 1 or Type 2) and delivery year. Age, BMI, parity and delivery year were used as continuous variables in the adjusted model. All analyses were performed in Statistical Package for the Social Sciences (SPSS, version 23; SPSS, Inc.).
2.1. Ethical approval
This is original research based on data from the Medical Birth Register of Finland. The permission to use the data was obtained from the respective registries from the Finnish Institute of Health and Welfare (THL) and Statistics Finland. No separate ethical permission was required for this study.
3. RESULTS
Women of Finnish origin constituted almost 92% (n = 350 548) of the study population and women of migrant origin 8% (n = 31 454). Table 1 presents the background characteristics of the study population. Among women of migrant origin, those originating from Russia/former USSR were the largest group (n = 11 994) and those originating from Latin America/Caribbean were the smallest (n = 739) group. The proportion of women in the upper‐level employee category was lower for all other migrant groups except for women in the Western Europe group and Latin America/Caribbean compared with women of Finnish origin. Finnish, Eastern European and Russian/former USSR‐origin women were more likely to smoke during pregnancy (16%–17%) than were women originating from the other groups (1%–9%). Women of Sub‐Saharan African and Middle Eastern/North African origin had the highest proportion with two or more previous births than any other group. Approximately half of women of Sub‐Saharan African (52%) and Middle Eastern/North African (49%) origin were overweight or obese compared with 36% of women of Finnish origin.
TABLE 1.
Background characteristics by region of origin during the most recent delivery, all singleton births, 2004–2014 (number and crude percentage)
| Variables |
Finland (n = 350 548) |
Western high‐income countries a (n = 2290) |
Eastern Europe (n = 2566) |
Russia/former USSR (n = 11 994) |
South Asia (n = 1904) |
East Asia (n = 4948) |
Sub‐Saharan Africa (n = 3548) |
Middle East/North Africa (n = 3465) |
Latin America, Caribbean (n = 739) |
|---|---|---|---|---|---|---|---|---|---|
| n (%) | |||||||||
| Age at delivery (years) | |||||||||
| <25 | 44 671 (12.7) | 161 (7.0) | 492 (19.2) | 1924 (16.0) | 355 (18.6) | 538 (10.9) | 743 (20.9) | 682 (19.7) | 73 (9.9) |
| 25–29 | 93 177 (26.6) | 445 (19.4) | 799 (31.1) | 3666 (30.6) | 728 (38.2) | 1335 (27.0) | 978 (27.6) | 1009 (29.1) | 167 (22.6) |
| 30–34 | 122 549 (35.0) | 870 (38.0) | 771 (30.0) | 3614 (30.1) | 583 (30.6) | 1704 (34.5) | 1010 (28.5) | 929 (26.8) | 256 (34.6) |
| >35 | 90 151 (25.7) | 814 (35.5) | 504 (19.6) | 2790 (23.3) | 238 (12.5) | 1371 (27.7) | 817 (23.0) | 845 (24.4) | 243 (32.9) |
| Socioeconomic position | |||||||||
| Upper level employees | 72 235 (20.6) | 772 (33.7) | 298 (11.6) | 1253 (10.4) | 313 (16.4) | 716 (14.5) | 139 (3.9) | 156 (4.5) | 177 (24.0) |
| Lower level employees | 136 900 (39.1) | 556 (24.3) | 439 (17.1) | 2515 (21.0) | 244 (12.8) | 777 (15.7) | 438 (12.3) | 291 (8.4) | 159 (21.5) |
| Manual workers | 77 953 (22.2) | 436 (19.0) | 885 (34.5) | 4193 (35.0) | 448 (23.5) | 1813 (36.6) | 683 (19.3) | 889 (25.7) | 160 (21.7) |
| Others b | 43 168 (12.3) | 269 (11.7) | 559 (21.8) | 2392 (19.9) | 579 (30.4) | 1072 (21.7) | 1137 (32.0) | 1146 (33.1) | 163 (22.1) |
| Unknown | 20 292 (5.8) | 257 (11.2) | 385 (15.0) | 1641 (13.7) | 320 (16.8) | 570 (11.5) | 1151 (32.4) | 983 (28.4) | 80 (10.8) |
| Smoking in pregnancy | 56 030 (16.0) | 200 (8.7) | 438 (17.1) | 1992 (16.6) | 19 (1.0) | 209 (4.2) | 72 (2.0) | 226 (6.5) | 43 (5.8) |
| Previous birth | |||||||||
| None | 104 720 (29.9) | 887 (38.8) | 833 (32.5) | 4331 (36.1) | 814 (42.8) | 2004 (40.5) | 879 (24.8) | 955 (27.6) | 329 (44.5) |
| One | 142 413 (40.6) | 868 (38.0) | 999 (38.9) | 4992 (41.6) | 670 (35.3) | 1885 (38.1) | 912 (25.8) | 1187 (34.3) | 275 (37.2) |
| Two or more | 103 251 (29.5) | 530 (23.2) | 734 (28.6) | 2665 (22.2) | 416 (21.9) | 1057 (21.4) | 1755 (49.5) | 1320 (38.1) | 135 (18.3) |
| Pre‐pregnancy body mass index c | |||||||||
| Underweight | 10 393 (3.1) | 112 (5.3) | 146 (6.0) | 857 (7.5) | 106 (5.9) | 673 (14.4) | 143 (4.3) | 106 (3.3) | 30 (4.4) |
| Normal weight | 203 463 (60.7) | 1420 (66.6) | 1557 (63.9) | 7725 (67.8) | 1043 (58.1) | 3406 (72.9) | 1431 (43.2) | 1561 (48.1) | 489 (71.4) |
| Overweight | 76 324 (22.8) | 383 (18.0) | 531 (21.8) | 1952 (17.1) | 496 (27.6) | 499 (10.7) | 1059 (31.9) | 1086 (33.5) | 122 (17.8) |
| Obese | 44 826 (13.4) | 218 (10.2) | 201 (8.3) | 860 (7.5) | 150 (8.4) | 94 (2.0) | 682 (20.6) | 489 (15.1) | 44 (6.4) |
| Year of delivery | |||||||||
| 2004–2007 | 95 233 (27.2) | 574 (25.1) | 458 (17.8) | 2595 (21.6) | 287 (15.1) | 1001 (20.2) | 446 (12.5) | 631 (18.2) | 160 (21.7) |
| 2008–2011 | 117 851 (33.6) | 776 (33.9) | 833 (32.5) | 4061 (33.9) | 590 (31.0) | 1744 (35.3) | 989 (27.9) | 1152 (33.2) | 274 (37.1) |
| 2012–2014 | 137 464 (39.2) | 940 (41.0) | 1275 (49.7) | 5338 (44.5) | 1027 (53.9) | 2203 (44.5) | 2113 (59.6) | 1682 (48.5) | 305 (41.3) |
Missing values for all other variables were <1% in each category.
Western Europe, North America & Oceania.
‘Others’ includes students, housewives, unemployed and pensioners.
Missing values for pre‐pregnancy body mass index in each category from the left to the right were 4.4%, 6.8%, 5.1%, 5.0%, 5.7%, 5.5%, 6.5%, 6.4% and 7.3% respectively.
The prevalence of any HDP varied from 1.3% (n = 62) among women of East Asian origin to 4.6% (n = 16 040) among the women of Finnish origin (Table 2). The prevalence of gestational hypertension was the lowest among women of East Asian origin (n = 17, 0.3%) and the highest among the women of Finnish origin (n = 8091, 2.3%). The prevalence of preeclampsia was the lowest among women of East Asian origin (n = 4, 0.9%) and the highest among women of Sub‐Saharan African origin (n = 105, 3.0%). The proportion of eclampsia was quite low (<0.2%) among all the study groups.
TABLE 2.
Prevalence of hypertensive disorders during the most recent pregnancy by region of origin, all singleton births, 2004–2014
| Regions |
Any hypertensive disorders n (%) |
Gestational hypertension n (%) |
Preeclampsia n (%) |
|---|---|---|---|
| Finland | 16 040 (4.6) | 8091 (2.3) | 5699 (1.6) |
| Western high‐income countriesa | 62 (2.7) | 18 (0.8) | 36 (1.6) |
| Eastern Europe | 58 (2.3) | 24 (0.9) | 28 (1.1) |
| Russia/former USSR | 274 (2.3) | 122 (1.0) | 123 (1.0) |
| South Asia | 51 (2.7) | 20 (1.1) | 28 (1.5) |
| East Asia | 62 (1.3) | 17 (0.3) | 44 (0.9) |
| Sub Saharan Africa | 149 (4.2) | 38 (1.1) | 105 (3.0) |
| Middle East/North Africa | 65 (1.9) | 20 (0.6) | 43 (1.2) |
| Latin America/ Caribbean | 16 (2.2) | 6 (0.8) | 9 (1.2) |
Table 3 presents the unadjusted and adjusted RR and 95% CI for the risk of any HDP in each migrant origin group compared with the Finnish origin group. Except for women of Sub‐Saharan African origin, women o f migrant origin had a lower risk for any HDP than the women of Finnish origin in the unadjusted and adjusted models. The women of Sub‐Saharan African origin did not differ from women of Finnish origin in the risk for any HDP when these were examined jointly. However, following adjustment for confounders, the separate analyses for gestational hypertension and preeclampsia showed that women originating from Sub‐Saharan Africa had a lower risk for gestational hypertension (RR 0.41, 95% CI 0.30–0.56) but a higher risk for preeclampsia (RR 1.77, 95% CI 1.44–2.17) compared with women of Finnish origin (Figures 1 and 2). The risk for gestational hypertension was lower also among all other women of migrant origin compared with women of Finnish origin (Figure 1).
TABLE 3.
Risk ratio (RR) and 95% confidence interval (CI) for having any hypertensive disorder during the most recent pregnancy by region of origin
| Regions |
Model I b RR (95% CI) |
p value |
Model II c RR (95% CI) |
p value |
|---|---|---|---|---|
| Finland | Reference | Reference | ||
| Western high‐income countries a | 0.58 (0.45–0.75) | <0.001 | 0.60 (0.47–0.78) | <0.001 |
| Eastern Europe | 0.48 (0.37–0.63) | <0.001 | 0.54 (0.42–0.70) | <0.001 |
| Russia/former USSR | 0.49 (0.43–0.55) | <0.001 | 0.56 (0.50–0.64) | <0.001 |
| South Asia | 0.57 (0.43–0.76) | <0.001 | 0.63 (0.48–0.83) | 0.001 |
| East Asia | 0.27 (0.21–0.34) | <0.001 | 0.33 (0.26–0.43) | <0.001 |
| Sub Saharan Africa | 0.91 (0.78–1.08) | 0.286 | 0.84 (0.71–1.00) | 0.051 |
| Middle East/North Africa | 0.40 (0.31–0.51) | <0.001 | 0.39 (0.30–0.50) | <0.001 |
| Latin America/ Caribbean | 0.46 (0.28–0.76) | 0.002 | 0.52 (0.32–0.85) | 0.010 |
Statistically significant values are highlighted with bold text.
Western Europe, North America & Oceania.
Unadjusted generalized linear model.
Generalized linear model adjusted for age, socioeconomic position, smoking during pregnancy, pre‐pregnancy body mass index, parity, preexisting diabetes and year of delivery.
FIGURE 1.
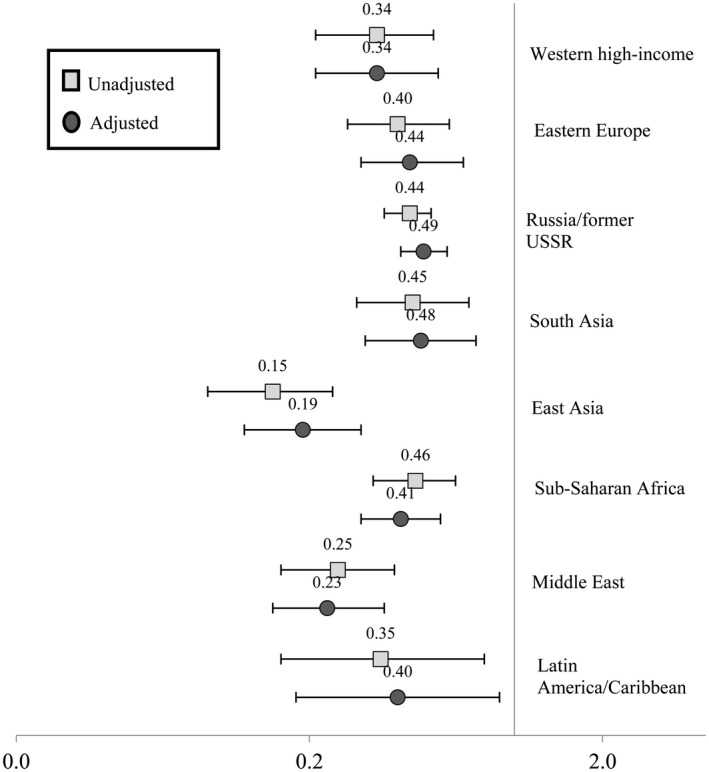
Risk ratio (RR) and 95% confidence interval (CI) for having gestational hypertension during the most recent pregnancy by region of origin (unadjusted generalized linear model and generalized linear model adjusted for age, socioeconomic position, smoking during pregnancy, pre‐pregnancy body mass index, parity, preexisting diabetes and year of delivery)
FIGURE 2.
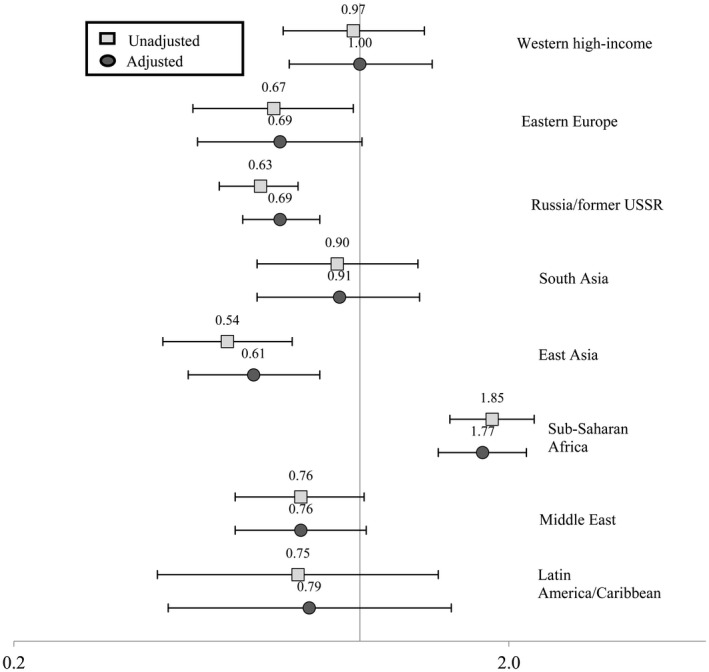
Risk ratio (RR) and 95% confidence interval (CI) for having preeclampsia during the most recent pregnancy by region of origin (unadjusted generalized linear model and generalized linear model adjusted for age, socioeconomic position, smoking during pregnancy, pre‐pregnancy body mass index, parity, preexisting diabetes and year of delivery)
The risk of preeclampsia was lower for women of Russia/former USSR origin (RR 0.69, 95% CI 0.58–0.83) and East Asian origin (RR 0.61, 95% CI 0.45–0.83) whereas the other groups did not differ from women of Finnish origin except for women of Sub‐Saharan African origin (Figure 2).
4. DISCUSSION
In this study, the risk for any HDP was lower for all women of migrant origin with an exception for women originating from Sub‐Saharan Africa compared with women of Finnish origin. When analyzing gestational hypertension and preeclampsia separately, all other migrant origin groups had a lower risk for gestational hypertension, but women of Sub‐Saharan African origin had a higher risk for preeclampsia than women of Finnish origin.
A recent study based on the Norwegian Birth Register data reported that the prevalence of preeclampsia was 3.5% among Norwegian women and 2.5% among all women of migrant origin. 14 Furthermore, compared with women of Norwegian origin, the adjusted odds ratio for preeclampsia was lower among migrants with all types of reasons for migration: labor immigrants, family immigrants, immigrant students and refugees. The study reported no results by region of origin and was also unable to adjust for the pre‐pregnancy BMI, which is an important confounder. 10 Another similar study from Norway found that women of migrant origin had lower risks of HDP compared with women of Norwegian origin. 11 This study reported the outcomes by region of origin based on mother’s country of birth, and the risk of HDP was lower for all women of migrant origin. 11 Although the reason for migration may be indicative of some pre‐migration conditions in the country of origin, region of origin is more indicative of the pre‐migration environment and genetic factors that are likely to contribute to a variety of health consequences in the long‐term. Lower risk of preeclampsia among migrant women of refugee background is also supported by a recent systematic review on pregnancy complications among refugee women. 9
Consist with our findings, another Norwegian study found that the adjusted odds for preeclampsia were higher among women of Somali origin compared with women of Norwegian origin. 12 Women of Somali origin constituted a large population group within the Sub‐Saharan African group in our study. Among all women of migrant origin, there were lower odds for preeclampsia but, when analyzed separately by country of birth, women of Somali origin had higher odds for preeclampsia. 12 Another study found that the prevalence of any HDP was lower among women of Somali origin compared with women of Swedish origin (4.3% vs 5.1%), although other adverse pregnancy‐related outcomes were more prevalent among women of Somali origin. 17 In Finland, the majority of persons of Somali origin have a refugee background. 18
In previous studies, the lower prevalence of any HDP among women of migrant origin has been attributed to the healthy migrant effect, according to which people of migrant origin have more favorable health outcomes compared with the native population in the host countries. 19 , 20 According to this hypothesis, many women who migrate to another country are more likely to be healthier and younger than women who do not migrate. So, when they migrate, they carry health benefits compared with the average person in their country of origin. However, the healthy migrant effect may not explain the lower risk of HDP among women of migrant origin because some of these migrant origin groups also have an increased risk of several other adverse pregnancy outcomes such as gestational diabetes mellitus, emergency cesarean delivery, pre‐term birth and low birthweight. 21 , 22 It is likely that a variety of genetic and lifestyle factors play a significant role for HDP. A previous study examining the metabolic syndrome among migrants in Finland found that while many metabolic risk factors were present, average blood pressure levels and the prevalence of hypertension was lower among women of Somali and Kurdish origin compared with women in the general Finnish population. 23 Furthermore, alcohol consumption was lower, and less smoking was observed among migrant women of Russian, Somali and Kurdish origin than the women in the general Finnish population. 24 Therefore, in general, genetic and lifestyle‐related factors may be possible explanations of lower prevalence of any HDP, gestational hypertension or preeclampsia among women of migrant origin in Finland.
A recent review on migrants of humanitarian background (refugees, asylum seekers and undocumented migrants) and their experience of maternity care in the Nordic countries concludes that migrant women of humanitarian background face obstacles in maternity care due to diminished negotiation power, sense of insecurity, and experienced care‐related discrimination. 25 However, this might not be a major problem in Finland where the services of maternity clinics are free of charge for all pregnant women permanently living in Finland, including migrants. The average number of antenatal check‐up visits for all women in our study was 16. The women of Sub‐Saharan origin had the lowest mean total number of antenatal visits (13.8) whereas the women of Finnish origin had the highest (16.1). Further, 0.3% (n = 988) of all women had not had an antenatal check‐up at a maternity clinic. When analyzed separately by country of origin, the women originating from East Asia (0.5%, n = 25) and Sub‐Saharan Africa (0.5%, n = 16) constituted the largest group who did not visit maternity clinics for check‐up (data not shown). So, it is less likely that migrant women had a lack of access to maternity care in Finland. However, undocumented migrants and short‐term visitors may need to pay for the maternity services in Finland.
The findings of the present study are in line with the findings of our previous study that was based on the same data. 22 In that study, we found that women of Sub‐Saharan African origin had higher risks for emergency cesarean, pre‐term birth, low birthweight, lower Apgar score and neonatal intensive unit care for newborns compared with women of Finnish origin. Preeclampsia is one of the main causes leading to these adverse outcomes. To meet the specific needs of women of Sub‐Saharan African origin, it should be assured that high‐quality interpreter services are always offered when needed.
It was important to study the risk for gestational hypertension and preeclampsia separately, as this revealed significant differences in the risk of these outcomes among women of Sub‐Saharan origin that would not have been observed had HDP only been analyzed jointly. A similar analysis should be conducted for eclampsia due to its serious and life‐threatening nature, even though this requires larger datasets. Therefore, future research on the topic is recommended to update the results and to check whether the risk profile for HDP among women of migrant origin changes.
This study contributed to the limited literature on HDP among women of migrant origin in Finland and identified women originating from Sub‐Saharan Africa as a high‐risk migrant origin group for preeclampsia. The outcome measures (any HDP, gestational hypertension and preeclampsia) were based on standard ICD‐10 codes extracted from the MBR. Furthermore, MBR is based on nationwide register‐based information with a high degree of completeness 26 and limited risk of selection bias. The migrant groups were categorized based on the United Nation’s geographic classification based on the country of origin. The sample size was quite large. These results are likely to be generalizable to countries outside of Finland with similar healthcare systems.
Our data were based on the ICD‐10 codes registered in the Finnish MBR from 2004 to 2014. During this period, there have been slight changes in the definition of some HDP. We had no information on several important migration indicators, such as migration status, length of stay and language skills, which might have contributed to the differences between the groups. Although we have adjusted for some known confounders, we could not adjust for diet and physical activity, which are well‐known risk factors for HDP. Our variable on the socioeconomic position was based on employment and occupation, which was unknown for almost one‐third of women from Sub‐Saharan Africa and the Middle East/North Africa. Better indicators of socioeconomic position, such as the highest educational attainment and family income, should be used in future studies whenever possible. It was not possible to compare the prevalence of early and late‐onset HDP between the groups due to low numbers of cases in some of the groups.
5. CONCLUSION
This study provided evidence that, in general, women of migrant origin had a lower risk for any HDP as well as gestational hypertension compared with women of Finnish origin. However, women of Sub‐Saharan African origin had a higher risk of preeclampsia than women of Finnish origin. Therefore, women from Sub‐Saharan African origin may need more attention in maternity clinics.
AUTHOR CONTRIBUTIONS
KB, TK, PK and MG conceived and designed the study, KB analyzed and prepared the manuscript, PK, NS, M and TIK revised, reviewed, provided critical feedbacks and approved the manuscript for final submission.
Bastola K, Koponen P, Skogberg N, Gissler M, Kinnunen TI. Hypertensive disorders of pregnancy among women of migrant origin in Finland: A population‐based study. Acta Obstet Gynecol Scand. 2022;101:127–134. doi: 10.1111/aogs.14291
REFERENCES
- 1. American College of Obstetricians and Gynecologists . Task force on hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013;122:1122‐1131. [DOI] [PubMed] [Google Scholar]
- 2. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170:1‐7. [DOI] [PubMed] [Google Scholar]
- 3. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323‐e333. [DOI] [PubMed] [Google Scholar]
- 4. Bellamy L, Casas J‐P, Hingorani AD, Williams DJ. Pre‐eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta‐analysis. BMJ. 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stuart JJ, Tanz LJ, Missmer SA, et al. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study. Ann Intern Med. 2018;169:224‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benschop L, Duvekot JJ, Roeters van Lennep JE. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart. 2019;105:1273‐1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. 2017;40:213‐220. [DOI] [PubMed] [Google Scholar]
- 8. Mogos MF, Salinas‐Miranda AA, Salemi JL, Medina IM, Salihu HM. Pregnancy‐related hypertensive disorders and immigrant status: a systematic review and meta‐analysis of epidemiological studies. J Immigr Minor Health. 2017;19:1488‐1497. [DOI] [PubMed] [Google Scholar]
- 9. Harakow HI, Hvidman L, Wejse C, Eiset AH. Pregnancy complications among refugee women: a systematic review. Acta Obstet Gynecol Scand. 2021;100:649‐657. [DOI] [PubMed] [Google Scholar]
- 10. Nilsen RM, Vik ES, Rasmussen SA, et al. Preeclampsia by maternal reasons for immigration: a population‐based study. BMC Pregnancy Childbirth. 2018;18:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sole KB, Staff AC, Laine K. The association of maternal country of birth and education with hypertensive disorders of pregnancy: a population‐based study of 960 516 deliveries in Norway. Acta Obstet Gynecol Scand. 2018;97:1237‐1247. [DOI] [PubMed] [Google Scholar]
- 12. Naimy Z, Grytten J, Monkerud L, Eskild A. The prevalence of pre‐eclampsia in migrant relative to native Norwegian women: a population‐based study. BJOG. 2015;122:859‐865. [DOI] [PubMed] [Google Scholar]
- 13. Bastola K, Koponen P, Härkänen T, Luoto R, Gissler M, Kinnunen TI. Pregnancy complications in women of Russian, Somali, and Kurdish origin and women in the general population in Finland. Womens Health (Lond). 2020;16. doi: 10.1177/1745506520910911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Medical Birth Register. National Institute of Health Welfare . 2021. https://www.thl.fi/en/web/thlfi‐en/statistics/information‐on‐statistics/register‐descriptions/newborns Accessed May 25, 2021.
- 15. Raskaudenaikainen verenpaineen nousu, Duodecium terveyskirjasto [Increase in blood pressure during pregnancy]. Duodecium Health Library (in Finnish). 2021. https://www.terveyskirjasto.fi/dlk00167 Accessed May 25, 2021.
- 16. UN Classifications of World’s Regions . 2021. https://unstats.un.org/unsd/methodology/m49 Accessed May 25, 2021.
- 17. Råssjö EB, Byrskog U, Samir R, Klingberg‐Allvin M. Somali women’s use of maternity health services and the outcome of their pregnancies: a descriptive study comparing Somali immigrants with native‐born Swedish women. Sex Reprod Healthc. 2013;4:99‐106. [DOI] [PubMed] [Google Scholar]
- 18. Castaneda AE, Rask S, Koponen P, Mölsa M, Koskinen S, Keskimäki I. Maahanmuuttajien terveys ja hyvinvointi. Tutkimus venäläis‐, somalialais‐ ja kurdi‐taustaisista Suomessa [Migrant health and wellbeing. A study on persons of Russian, Somali and Kurdish origin in Finland]. Report 61/2012. Helsinki: National Institute for Health and Welfare (THL); 2012.
- 19. Hauck FR. The healthy immigrant effect: improved reproductive health outcomes among African refugee women compared with US‐born women. J Women’s Health. 2019;28:739‐740. [DOI] [PubMed] [Google Scholar]
- 20. Weitoft GR, Gullberg A, Hjern A, Rosén M. Mortality statistics in immigrant research: method for adjusting underestimation of mortality. Int J Epidemiol. 1999;28:756‐763. [DOI] [PubMed] [Google Scholar]
- 21. Bastola K, Koponen P, Skogberg N, Gissler M, Kinnunen TI. Gestational diabetes among women of migrant origin in Finland—a population‐based study. Eur J Public Health. 2021;31:784‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bastola K, Koponen P, Gissler M, Kinnunen TI. Differences in caesarean delivery and neonatal outcomes among women of migrant origin in Finland: a population‐based study. Paediatr Perinat Epidemiol. 2020;34:12‐20. [DOI] [PubMed] [Google Scholar]
- 23. Skogberg N, Laatikainen T, Koskinen S, et al. Cardiovascular risk factors among Russian, Somali and Kurdish migrants in comparison with the general Finnish population. Eur J Public Health. 2016;26:667‐673. [DOI] [PubMed] [Google Scholar]
- 24. Skogberg N, Laatikainen T, Jula A, Härkänen T, Vartiainen E, Koponen P. Contribution of sociodemographic and lifestyle‐related factors to the differences in metabolic syndrome among Russian, Somali and Kurdish migrants compared with Finns. Int J Cardiol. 2017;232:63‐69. [DOI] [PubMed] [Google Scholar]
- 25. Leppälä S, Lamminpää R, Gissler M, Vehviläinen‐Julkunen K. Humanitarian migrant women’s experiences of maternity care in Nordic countries: a systematic integrative review of qualitative research. Midwifery. 2020;80:102572. [DOI] [PubMed] [Google Scholar]
- 26. Gissler M, Teperi J, Hemminki E, Meriläinen J. Data quality after restructuring a national medical registry. Scand J Soc Med. 1995;23:75‐80. [DOI] [PubMed] [Google Scholar]


