Abstract
Introduction
Antiretroviral therapy‐naïve pregnant women living with HIV are at an increased risk for adverse pregnancy outcomes. It remains controversial whether this risk persists with antiretroviral therapy. We conducted a systematic review and meta‐analysis to evaluate whether pregnant women living with HIV and receiving antiretroviral therapy antenatally, are at an increased risk of adverse outcomes compared with HIV‐negative controls.
Material and methods
We searched MEDLINE, Embase, International Pharmaceutical Abstracts, EBM Reviews, PubMed (non‐MEDLINE records), EBSCO CINAHL Complete, Clarivate Web of Science, African Index Medicus, LILACS and Google Scholar for all observational studies comparing pregnant women living with HIV on antiretroviral therapy with HIV‐negative controls from 1 January 1994 to 10 August 2021 with no language or geographic restrictions. Perinatal outcomes included preterm birth (PTB), low birthweight, small‐for‐gestational age and preeclampsia. Using a random‐effects model we pooled raw data to generate odds ratio (OR) with 95% confidence intervals (CI) for each outcome. Sub‐analyses for high and low resource countries and time of antiretroviral therapy initiation were performed. This systematic review and meta‐analysis is registered with PROSPERO, number CRD42020182722.
Results
Of the 7900 citations identified, 27 were eligible for analysis (12 636 pregnant women living with HIV on antiretroviral therapy and 7 812 115 HIV‐negative controls). ORs (95% CI) of PTB (1.88 [1.63–2.17]), small‐for‐gestational age (1.60 [1.18–2.17]) and low birthweight (2.15 [1.58–2.92]) were significantly higher in pregnant women living with HIV than in HIV‐negative women, while the risk of preeclampsia (0.86 [0.57–1.30]) was comparable. The risk of PTB and low birthweight was higher in both high resource and low resource countries, while the risk of small‐for‐gestational age was higher only in the former. Preconceptional antiretroviral therapy was associated with a higher risk of PTB compared with antenatal initiation.
Conclusions
Pregnant women living with HIV on antiretroviral therapy have an increased risk of PTB, low birthweight and small‐for‐gestational age in high resource countries, as well as PTB and low birthweight in low income countries compared with HIV‐negative controls.
Keywords: HIV/AIDS, low birthweight, preeclampsia, preterm birth, small‐for‐gestational age
Abbreviations
- ART
antiretroviral therapy
- HIV
human immunodeficiency virus
- LBW
low birthweight
- PTB
preterm birth
- SGA
small‐for‐gestational age
- WLWH
women living with HIV
Key message.
Despite antiretroviral treatment, HIV in pregnancy remains associated with preterm birth and low birthweight, but not with preeclampsia, in both high and low resource countries. In the former it is also associated with small‐for‐gestational age.
1. INTRODUCTION
Worldwide, the human immunodeficiency virus (HIV) affects 37.9 million people, of whom 1.5 million become pregnant each year. 1 , 2 In 1994, the hallmark AIDS Clinical Trials Group (ACTG) 076 study demonstrated that antepartum and intrapartum treatment in pregnancy and postpartum treatment of the newborn with the antiretroviral agent Zidovudine successfully reduced vertical transmission of HIV by approximately two‐thirds. 3 Since then, the global standard of care for pregnant women living with HIV (WLWH) is to receive antiretroviral therapy (ART). Today, in high income settings, the risk of vertical transmission of HIV with optimal use of ART in pregnancy approaches zero. 4
A recent meta‐analysis assessing HIV infection in ART‐naive pregnant women has shown that HIV is strongly associated with increased risks of preterm birth (PTB), low birthweight (LBW), small‐for‐gestational age (SGA) and stillbirth. 5 This risk of adverse perinatal outcomes is increased further with more advanced disease. 6 , 7 It is unknown how HIV infection results in these specific outcomes, all of which are complex, multifactorial and of diverse clinical presentations. It is believed in part that a chronic state of inflammation and immune activation may disrupt normal immunological processes of pregnancy maintenance and placental function. 8 , 9
ART can decrease but not eliminate this inflammatory state. 10 , 11 , 12 If indeed the inflammatory state contributes to adverse perinatal outcomes, targeted treatment should improve outcomes. However, it is difficult to separate out antiviral activity and residual immune activation and inflammation. Observational studies in pregnant WLWH receiving treatment in both high and low income countries have reported conflicting evidence. Therefore, the association between HIV, antiviral exposure and adverse pregnancy outcomes, including PTB, 13 , 14 , 15 , 16 LBW, 17 , 18 , 19 preeclampsia, 20 , 21 and stillbirth 19 , 22 , 23 , 24 , 25 remains controversial. These studies are limited by their lack of power to detect modest effect sizes, non‐uniform definitions and differential handling of noncomparable exposure groups.
Our aim, therefore, was to conduct a systematic review and meta‐analysis to evaluate whether WLWH who receive ART antenatally, are at an increased risk of adverse pregnancy outcomes compared with HIV‐negative controls. The highest burden of HIV is in low income countries, where the incidence of adverse perinatal outcomes is high, regardless of HIV status. 25 , 26 Considering differences in resources and medical care in high vs low income countries, we aimed to evaluate these outcomes in women with HIV in both settings.
2. MATERIAL AND METHODS
This systematic review and meta‐analysis conformed to the 2009 Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines 26 and was registered in PROSPERO, #CRD42020182722 on 14 July 2020.
2.1. Inclusion and exclusion criteria
All cohort, case‐control and observational studies that compared outcomes of pregnant WLWH on ART with HIV‐negative women, from January 1994 to July 2020, were included in the analysis. We chose to begin our search in 1994 due to publication of the 076 study, which demonstrated a substantial benefit to ART in prevention of vertical HIV transmission. Case series and case reports were excluded, as were studies in which not all pregnant women were on ART. Pregnant women on any ART (mono or combination), started anytime during pregnancy were included in the analysis, whereas women taking ART only during labor were excluded. There were no language or geographic restrictions. All titles and abstracts were screened and pertinent publications were fully reviewed by two independent investigators (SS and SA) utilizing Covidence software for systematic review management. A third investigator (KEM) served as an adjudicator in cases lacking consensus. If the same cohort of pregnant women was published in more than one study, then only the larger cohort was included in the study.
2.2. Search strategy
With the assistance of a trained information specialist (MR), a comprehensive search strategy was used to screen the following sources of literature: 1) Ovid MEDLINE, 2) Ovid Embase, 3) Ovid International Pharmaceutical Abstracts, 4) Ovid EBM Reviews—Cochrane Central Register of Controlled Trials, 5) PubMed (non‐MEDLINE records), 6) EBSCO CINAHL Complete, 7) Clarivate Web of Science, 8) African Index Medicus, 9) LILACS, and 10) Google Scholar. The search strategy was designed based on the 2015 Peer Reviewed Electronic Search Strategies (PRESS) Guidelines. We included Medical Subject Headings, Emtree headings and free text terms related to HIV, pregnancy and ART (see Appendix S5 for more details). A search filter from BMJ Best Practice 27 was adapted in applicable databases to screen for cohort, case‐control and observational studies published between 1 January 1994 to 10 August 2021. No restrictions were applied to language or age. Additional references were hand‐searched from bibliographies of relevant articles. All references including duplicate records were managed using the EndNote citation management software X9.
2.3. Quality analysis
Quality analysis was performed by two independent investigators (SS and SA) using the study quality assessment tool provided by National Heart Lung and Blood Institute of the National Institute of Health (NIH). The tool utilized was for observational cohort studies. 28 Fourteen different research questions were addressed relating to the goal of the research, the study population, eligibility criteria, sample size justification, time frame, outcome measures, outcome assessors, follow‐up rate and statistical analysis. Studies were rated as good, fair or poor if they fulfilled 10 or more, seven to nine or fewer than seven variables, respectively.
2.4. Statistical analysis
All relevant data from individual studies for specified outcomes were extracted and entered into a spreadsheet based on the Cochrane data extraction tool. 29 Data were separately extracted from all studies for each perinatal outcome. An Odds ratio was generated for all the given outcomes. A random effects model was used to calculate the weights of the individual studies and to generate a summary estimate of odds ratios (OR) and 95% confidence intervals (CI) for the perinatal outcomes in pregnant WLWH as compared with HIV‐negative controls. This was graphically depicted using a forest plot. For each of the outcomes, a separate forest plot was created. The heterogeneity was calculated using the I2 statistic to delineate the variability among the studies which could be attributed to patient population, different ART and duration of treatment. Subgroup analysis of high and low income countries was done to study the association between HIV and perinatal outcomes in different income settings. Subgroup analysis of timing of ART inititation (preconception or antenatally) was performed to evaluate the impact of treatment initiation and duration on adverse pregnancy outcomes. Funnel plots were utilized to evaluate publication bias in all meta‐analyses with 10 or more studies and were tested for asymmetry using Egger’s test. The Egger’s meta‐regression model helped to assess the magnitude and statistical significance of the relation between observed effect sizes and the size of studies. All the analyses were done using STATASE version 16.
3. RESULTS
A total of 7900 citations were identified from our search and 6298 remained after removal of duplicates (n = 1602). We excluded 6200 studies after title and abstract screening and a further 71 studies after full text screening. The 71 studies were excluded for various reasons listed in Figure 1. Twenty‐seven studies fulfilled inclusion criteria and were included in our meta‐analysis.
FIGURE 1.
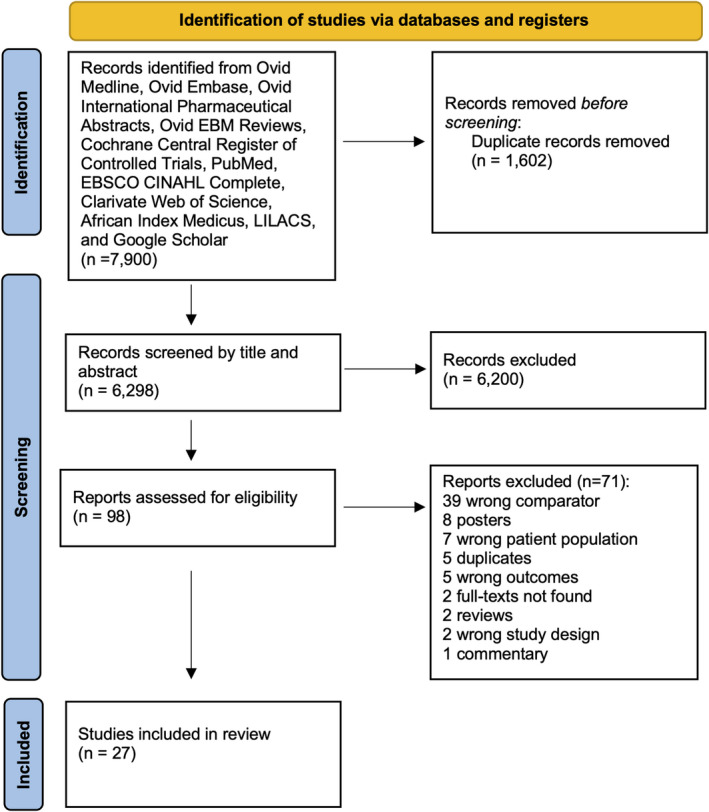
Flow chart of studies included in meta‐analysis
These studies were published between 2006 and 2021, with a wide geographic distribution. Fifteen studies originated from low income countries (China and Africa) and 12 were from high income countries (USA, France, Netherlands and Canada). Pooled together, these studies included a total of 12 636 pregnant WLWH on ART and 7 812 115 pregnant women without HIV. All the studies were cohort studies. Study characteristics are outlined in Table 1.
TABLE 1.
Characteristics of studies included in meta‐analysis
| Author, year | Country | Low/high income | Years of study | Study design | HIV+ (N) | Maternal age, years (mean(SD/ median (IQR) | ART regimen | Duration of treatment | CD4<200 cells/mm3 | GA at delivery, weeks (mean(SD/ median (IQR) | Birthweight, grams (mean(SD/ median (IQR) |
HIV– (N) |
Maternal age, years (mean(SD/ median (IQR) | GA at delivery, weeks (mean(SD/ median (IQR) | Birthweight, grams (mean(SD/ median (IQR) | Outcomes assessed | Study quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adam, 2016 | Sudan | Low | 2009–2013 | Prospective cohort | 26 | 25.9 (5.7) | cART (zidovudine/ lamivudine | From the beginning of the second trimester, or earlier if maternal condition indicated | N/A | 37.1 (3.1) | 3 (0.8) | 52 | 36.1 (5.7) | 38.2 (1.9) | 3,000 (0.4) | PTB | Fair |
| Arab, 2016 | USA | High | 2003–2012 | Retrospective cohort | 1997 | N/A | N/A | N/A | N/A | N/A | N/A | 7,777,002 | N/A | N/A | N/A | HDP, PTB | Poor |
| Azria, 2009 | France | High | 2003–2007 | Retrospective cohort | 100 | 32.4 (5) |
lopinavir/ritonavir LPV/r‐based regimen |
36 (36%) pre‐conceptional 63 (63%) antenatal |
12 (12.4%) | 37.8 (2.5) | 2,993 (629) | 200 | 32.5 (5.2) | 38.8 (2.9) | 3099 (683) | PE, SGA | Fair |
| Boer, 2006 | Netherlands | High | 1997–2003 | Retrospective matched cohort (matched for age, parity, ethnicity, multiples and time of delivery) | 143 | 29 |
93 (65%) HAART+PI 50 (35%) (HAART w/o PI |
106 (74.1%) first trimester 37 (25.9%) second or third trimester |
48 (36.3%) | 39.4 (24.1–42.4) median (range) | 3130 | 196 | 30 | 39.7 (24.8–43.0) median (range) | 3260 | PTB, LBW | Fair |
| Boyajian, 2012 | Canada | High | 2003–2010 | Retrospective matched cohort (matched for age, parity, time of delivery and multiples). | 91 | 31.0 (4.8) | HAART – 88 (97%) contained an NRTI, 18 (20%) contained nevirapine, 68 (75%) contained PI | 36 (40%) preconceptional55 (60%) antenatal | N/A | 38.3 (3.1) | 2946 (707) | 273 | 31.0 (4.7) | 38.7 (2.8) | 3260 (688) | PE, PTB, LBW, SGA | Fair |
| Carceller, 2009 | Canada | High | 1997–2005 | Retrospective cohort | 176 | N/A |
205 (99%) NRTI 176 (85%) PI 40 (19%) NNRTI |
N/A | N/A | 38 (2.2) | 3099 (586) | 206 | N/A | 39 (2.2) | 3295 (654) | PTB, SGA | Fair |
| Chen, 2012 | Botswana | Low | 2009–2011 | Retrospective cohort | 9504 | N/A |
2851 (87%) ‐ NVP/ZDV/3TC or did not have a regimen specified (and considered likely to have received NVP/ZDV/3TC) 312 (9%) LPV/r/ZDV/3TC |
2189 (27.7%) preconceptional 4726 (72.3%) antenatal |
498 (6.3%) | N/A | N/A | 22,609 | N/A | N/A | N/A | PTB, SGA | Fair |
| Dadabhai, 2019 | Malawi | Low | 2016–2017 | Prospective cohort | 614 | N/A |
Efavirenz+Lamuvidine+ Tenfovir |
299 (48.7%) preconceptional 315 (51.3%) antenatal |
Women with <350 cell count were excluded |
N/A | N/A | 685 | N/A | N/A | N/A | PTB, LBW, SGA | Good |
| Dara, 2018 | USA | High | 2008–2012 | Retrospective cohort (matched for birth year) | 155 | 29.2 (7.1) |
102 (80.9%) PI‐based 24 (19.2%) non‐PI based 29 (19%) unknown regimen |
N/A | N/A | 38.3 (2.5) | 2971.8 (616.3) | 775 | 28.1 (6.4) | 38.4 (2.5) | 3166.6 (644.1) | LBW | Fair |
| Elenga, 2021 | Cayenne, French Guiana | High | 2013–2015 | Retrospective cohort | 112 | 33 (27–37) | N/A | N/A | N/A | N/A | N/A | 470 | 28 (23–34) | N/A | N/A | PTB, PE | Fair |
| Gagnon, 2016 | Canada | High | 2007–2012 | Retrospective matched cohort (matched for age, parity, year of delivery) | 96 | 30.8 (5.5) |
74 (77%) PI‐based 21 (22%) ART w/o PI 1 (1%) monotherapy |
N/A | 7 (7%) | 38.2 (2.6) | 3005 (651) | 288 | 30.9 (5.6) | 38.9 (2.2) | 3334 (587) | PTB, LBW, SGA | Fair |
| Gibango, 2018 | South Africa | Low | 2012 | Prospective cohort | 206 | 28.63 (6.34) | Triple therapy or ART prophylaxis | N/A | N/A | 28.63 (6.34) | N/A | 208 | 26.34 (6.93) | 26.34 (6.93) | N/A | PTB, LBW | Good |
| Haeri, 2009 | Washington DC, North Carolina | High | 2000–2007 |
Retrospective matched cohort (matched for maternal age, race, parity, care location, insurance type, year, and mode of delivery). |
151 | 27 (6.2) |
142 (94%) NRTI 30 (20%) NNRTI 100 (74%) PI |
N/A | N/A | 37 (3.3) | 2857 (732.6) | 302 | 27 (6.1) | 38 (2.8) | 3169 (667.7) | PTB, SGA, LBW, PE | Fair |
| Huixia, 2020 | Hunan, China | High | 2014–2017 | Prospective matched (for maternal age and gestational age) | 414 | N/A |
100 mono/dual therapy 314 HAART |
138 1st trimester 159 2nd trimester 117 3rd trimester |
32 | N/A | N/A | 966 | N/A | N/A | N/A | PTB, SGA, LBW | Good |
| Ikpim, 2015 | Nigeria | Low | 2006–2010 | Retrospective matched cohort (matched for age and parity) | 181 | 28.2 (4.6) | N/A | N/A | N/A | N/A | 2920 (540) | 257 | 28.1 (4.65) | N/A | 3130 (560) | PTB, LBW | Poor |
| Iloghalu, 2019 | Nigeria, Africa | Low | 2015–2016 | Matched prospective cohort | 87 | NA | HAART | N/A | NA |
2900 (730) |
92 | NA | NA |
3000 (670) |
LBW | Good | |
| Malaba, 2017 | Cape Town, South Africa | Low | 2013–2015 | Prospective cohort | 1276 | 29 (26–34) |
1116 (87%) TDF+3TC+EFV 57 (4%) TDF+3TC+NVP 72 (6%) Other NNRTI‐based 33 (3%) PI‐based |
572 (38.3%) preconceptional 971 conceptional |
213 (14%) | N/A | 3052 (580) | 278 | 27 (23–32) | N/A | 3199 (548) | PTB, LBW, SGA | Good |
| Nyemba, 2021 | Cape Town, South Africa | Low | 2017–2018 | Prospective cohort | 431 | 31 (26–35) | N/A |
268 (62%) preconceptional 163 (38%) conceptional |
N/A | 39 (38–40) | 3100 (2750–3350) | 457 | 27 (23–32) | 39 (38–40) |
3200 (2900–3450) |
PTB, LBW, SGA | Fair |
| Olagbuji, 2010 | Nigeria | Low | 2007–2008 | Retrospective cohort | 203 | 30.1 (3.9) | Zidovudine, Lamivudine and Nevirapine | N/A | N/A | N/A | 2883 (626) | 203 | 30.4 (4.5) | N/A | 3309 (469) | LBW, HDP, PTB | Fair |
| Piske, 2021 | British Columbia, Canada | High | 1990–2012 | Retrospectve cohort (matched for age, sex, geocode) | 354 | N/A |
(1) NRTIs only (2) NRTIs +NNRTI (NNRTI); (3) NRTIs +unboosted PI (4) NRTIs +boosted PI |
95 preconceptional 255 conceptional |
N/A | N/A | N/A | 1224 | N/A | N/A | N/A | PTB | Good |
| Ramkolo, 2017 | South Africa | Low | 2012–2013 | Prospective | 2599 | N/A |
1396 (53.7%) Tenofovir disoproxil fumarate [TDF] + [3TC]/Emtracitaine [FTC] +Nevirapine [NVP]) 873 (33.6%) Zidovudine 330 (12.7%) none |
Preconception 616 (23.7%) conception 780 (30%) | N/A | N/A | N/A | 6179 | N/A | N/A | N/A | PTB, LBW, SGA | Good |
| Rempis, 2017 | Uganda, Africa | Low | 2013 | Prospective | 110 | 26 (18–42) |
97 (88.2%) Tenofovir, Lamivudine and Efavirenz 6 (5.5%) on Ziduvudine 5 (4.5%) untreated 2 (1.8%) treatment not specified. Both groups excluded from analysis |
38 (34.5%) preconceptional 59 (53.6%) antenatal 5 (4.5%) none |
N/A | 38 (30–42) | 3040 (1200–4500) | 302 | 25 (18–42) | 39 (28–42) |
3100 (500–4500) |
PTB,SGA | Good |
| Santosa, 2019 | Soweto, South Africa | Low | 2013–2016 | Prospective cohort | 229 | 32 (28, 37) | 120 (98.4%) different combinations of HAART, 2 (1.6%) Ziduvudine monotherapy |
38 (16.6%) preconceptional 71 (31%) antenatal 120 (52.4%) unknown |
9 (8.3%) | 38 (37, 39) | 2962.5 (2540, 3255) | 404 | 30 (26, 34) | 39 (37, 40) | 2995 (2652.5, 3265) | PTB, LBW, SGA | Good |
| Saums, 2019 | Georgia, USA | High | 2011–2018 | Retrospective cohort | 265 | NA | 91 (34.3%) INSTI‐based, 145 (54.7%) PI‐based, 29 (10.9%) NNRTI‐based |
117 (44.2%) preconceptional 145 (54.7%) antenatal |
34 (13%) | 38.6 (37.3–39.3) | NA | 3464 | NA | 39.1 (38.0–40.0) | NA | HDP, GH, PE, PTB | Fair |
| Sebitloane, 2017 | Durban, South Africa | Low | 2011–2014 | Matched retrospective cohort | 1159 | 28.2 (5.7) |
424 (36.6%) dual therapy during pregnancy (Zidovudine and NVP during labor). 735 (63.4%) HAART |
423 (57.6%) conceptional 312 (42.4%) antenatal |
N/A | 37.2 (3.39) | N/A | 302 | 24.4 (6.33) | 37.8 (2.96) | N/A | PTB, HDP | Fair |
| Tiam, 2019 | Lesotho, South Africa | Low | 2014–2016 | Prospective cohort | 653 | 28.7 (5.5) |
550 (84.2%) TDF/3TC/EFV Other ART regimens 89 (13.6%) |
249 (40.2%) preconceptional 370 (59.8%) antenatal |
N/A | N/A | N/A | 941 | 24.4 (5.7) | N/A | N/A | PTB, LBW | Good |
| Tukei, 2021 | Maseru, Lesotho, South Africa | Low | 2016–2017 | Prospective cohort | 562 | 28 (16–48) |
506 (90%) TDF/3TC/EFV. 56 (10%) Other ART regimens |
285 (51.6%) preconceptional 267 (48.4%) Conceptional |
N/A | N/A | N/a | 352 | 23 (14–42) | N/A | N/A | PTB, LBW | Good |
Abbreviations: ART, antiretroviral; HAART, highly active antiretroviral therapy; HDP, hypertensive disease of pregnancy (gestational hypertension and preeclampsia); LBW, low birthweight; N/A, not available; NNRTI, non‐nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PE, preeclampsia; PI, protease inhibitor; PTB, preterm delivery; SGA, small‐for‐gestational age.
The quality analysis yielded 12 studies of overall good quality, 13 of fair quality and two of poor quality (Figure 2). Perinatal outcomes recorded included PTB in 21 studies, LBW in 14, SGA in 13 and preeclampsia in eight of the 27 studies. For the outcome of PTB, 11 studies provided information on preconceptional and antenatal initiation of ART, six studies provided outcomes of LBW and five for SGA.
FIGURE 2.
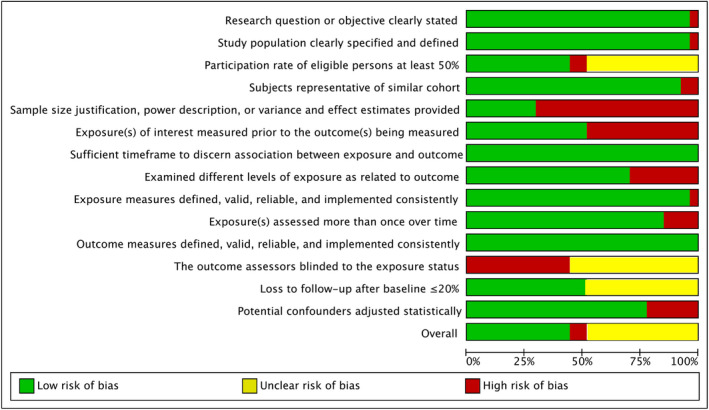
Quality analysis of the included studies. Overall quality: green ‐ good, yellow ‐ fair, red ‐ poor
3.1. Outcome definitions
PTB was defined as birth prior to 37 weeks of gestation in 17 of the 21 studies which assessed this outcome. The remaining four studies did not specify a gestational age cut‐off for prematurity. 30 , 31 , 32 , 33 LBW was defined as birthweight of <2500 g in 15 of the 17 studies that assessed this outcome. The remaining two did not provide a definition. 32 , 33 SGA was defined as a birthweight below the 10th percentile for gestational age according to standardized neonatal growth curves in 12 of 13 studies that assessed this outcome. The remaining study 34 defined SGA as birth >37 weeks’ gestation with weight of >2500 g or, for premature infants, being two standard deviations below the mean weight for gestational age. Preeclampsia was defined by the former International Society for the Study of Hypertension in Pregnancy (ISSHP) definition 35 in three of eight studies that assessed this outcome. 17 , 21 , 36 Of the five remaining, one study 37 did not differentiate between preeclampsia and gestational hypertension and the other three did not provide a specific definition. 31 , 33 , 38 , 39
The assessment of gestational age was specified in only nine of the 27 studies. 24 , 34 , 36 , 39 , 40 , 41 , 42 , 43 , 44 In eight studies it was based on the last menstrual period and corrected if needed by the first trimester ultrasound scan and in one study it was based on the ultrasound measurement obtained between 10 and 13+6 weeks. 39
3.2. Risk of adverse perinatal outcome
Maternal HIV infection was associated with a higher risk of PTB with an OR of 1.88 (95% CI 1.63–2.17, Figure 3) and a heterogeneity of 64.5%. This risk remained significantly higher in pregnant WLWH in both high (2.41, 95% CI 2.03–2.85) and low (1.61, 95% CI 1.32–1.95) income countries (Table 2, Figure S1a). Preconceptional initiation of ART was associated with a slightly higher risk of PTB compared with antenatal initiation (1.27, 95% CI 1.01–1.60, Figure S3a). Funnel plots did not show any evidence of publication bias (Figure S4a). Egger’s test suggested that smaller studies did not tend to show different results when compared with larger studies, as the CI of the intercept included the value zero with a p value of 0.099.
FIGURE 3.
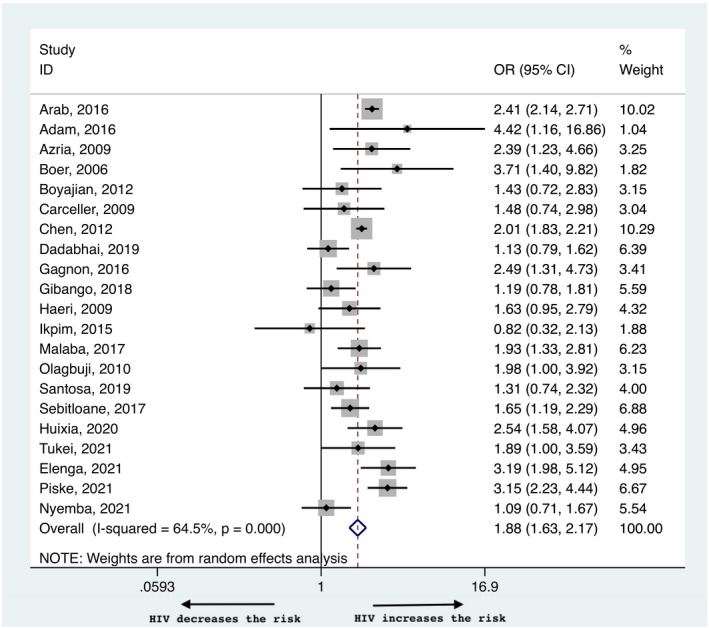
Risk of preterm birth in women living with HIV compared with negative controls
TABLE 2.
Risk of adverse pregnancy outcomes in women living with HIV compared with negative controls
| Pregnancy outcome | OR (95% CI) | OR (95% CI) in low resource countries | OR (95% CI) in high resource countries | OR (95% CI) periconceptional vs antenatal ART |
|---|---|---|---|---|
| PTB | 1.88 (1.63–2.17) | 1.61 (1.32–1.95) | 2.41 (2.03–2.85) | 1.27 (1.01–1.60) |
| LBW | 2.15 (1.58–2.92) | 1.88 (1.31–2.70) | 2.90 (1.77–4.75) | 1.04 (0.87–1.24) |
| SGA | 1.60 (1.18–2.17) | 1.49 (0.98–2.27) | 1.73 (1.22–2.47) | 1.17 (0.75–1.83) |
| Preeclampsia | 0.86 (0.57–1.30) | 0.93 (0.39–2.24) | 0.83 (0.49–1.40) | N/A |
Abbreviations: ART, artiretroviral therapy; PTB, preterm birth; SGA, small‐for‐gestational age; LBW, low birthweight.
There was a higher risk of LBW babies born to WLWH with an OR of 2.15 (95% CI 1.58–2.92, Figure 4), and a heterogeneity of 77%. The risk was higher among WLWH, independent of the income setting, with a risk of 2.9 (95% CI 1.77–4.75) in high and 1.88 (95% CI 1.31–2.70) in low income countries (Table 2, Figure S1b). The OR of SGA was higher in WLWH than in HIV‐negative controls (1.60, 95% CI 1.18–2.17, Figure 5) with a heterogeneity of 91.4%. In subgroup analysis for high and low income countries, the OR remained higher in WLWH in the former (1.73, 95% CI 1.22–2.47) but was comparable in the latter (1.49, 95% CI 0.98–2.27), Figure S2a). The risk of SGA and LBW was not influenced by preconceptional vs antenatal initiation of ART; 1.17 (95% CI 0.75–1.83) for SGA and 1.04 (95% CI 0.87–1.24) for LBW (Table 2, Figure S3b,c). There was mild asymmetry in the funnel plots for LBW and SGA indicating publication bias (Figure S4b and S4c, respectively).
FIGURE 4.
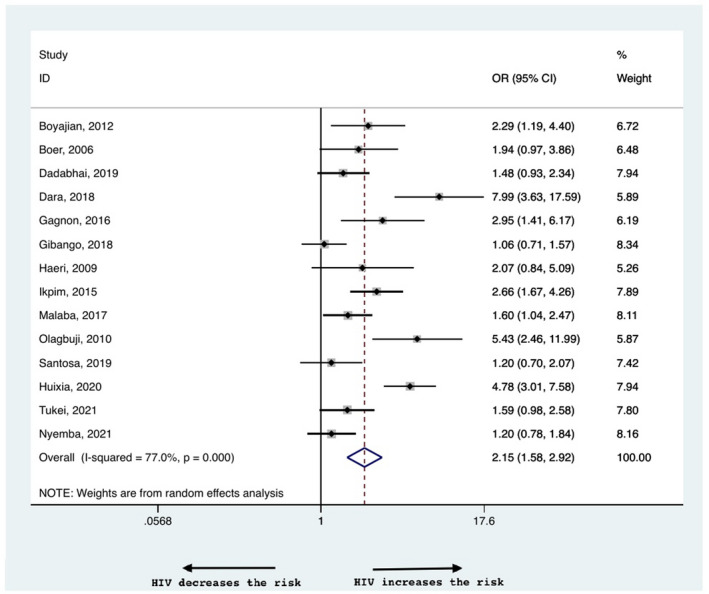
Risk of low birthweight in women living with HIV compared with negative controls
FIGURE 5.
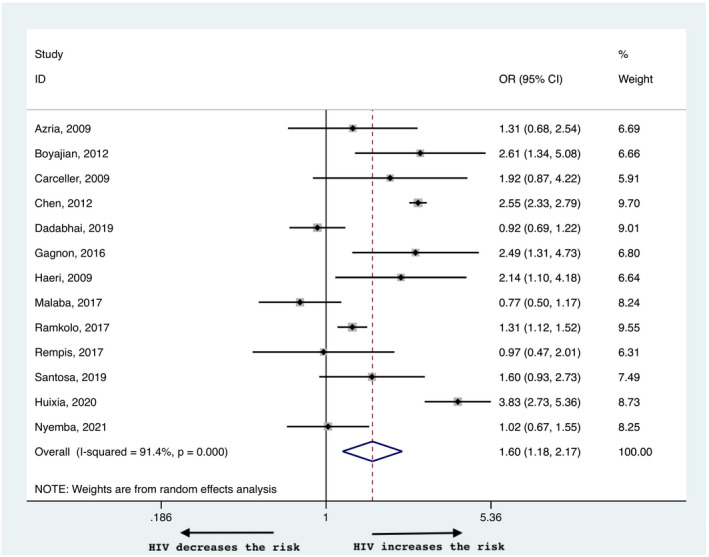
Risk of small‐for‐gestational age in women living with HIV compared with negative controls
The incidence of preeclampsia did not differ between WLWH and HIV‐negative women (0.86, 95% CI 0.57–1.30, Figure 6). The heterogeneity was 71.9%. Subgroup analysis for high and low income countries yielded similar results, with no difference between the groups (0.83, 95% CI 0.49–1.40 and 0.93, 95% CI 0.39–2.24 in high and low income countries, respectively; Table 2, Figure S2c).
FIGURE 6.
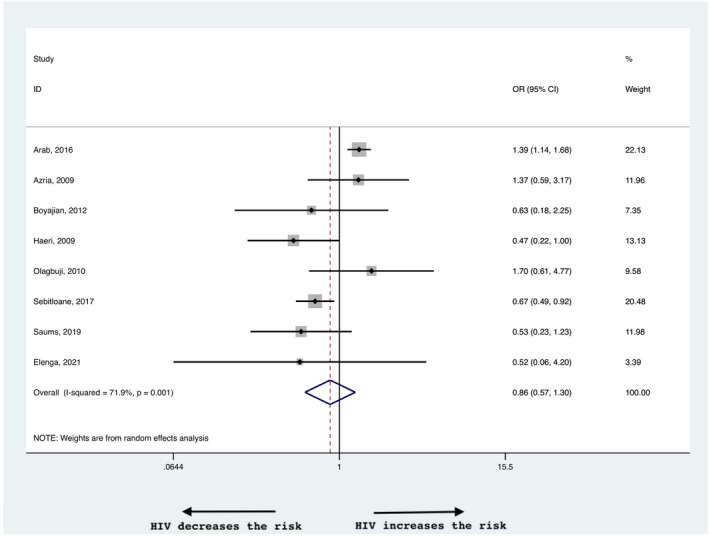
Risk of preeclampsia in women living with HIV compared with negative controls
4. DISCUSSION
This systematic review and meta‐analysis found that the risks of PTB, LBW and SGA were higher in WLWH on ART compared with HIV‐negative controls. The risk of preeclampsia did not differ. In sub‐group analyses of high and low income countries, the increased risk of PTB and LBW persisted in both settings, whereas the risk of SGA remained higher only in high income countries. Compared with antenatal initiation of ART, preconceptional initiation was associated with a higher risk of PTB, but did not affect the risk of SGA and LBW.
Out meta‐analysis is noteworthy for its extensive scientific literature search, incorporating 10 search engines, including the gray literature, thereby overcoming potential publication bias. It included all study designs with no language or geographic restriction. Our search terms did not define outcomes, thereby increasing the power of our analyses. A random effects model was used to overcome substantial heterogeneity between studies. We included only studies in which all women were on ART prior to labor or studies in which the results were provided separately for the subset of medicated WLWH. Sub‐analysis was done for high and low income countries to account for the different quality of medical care provided and the different baseline characteristics of the populations studied. Therefore, we believe our results are relevant for countries with a high as well as a low burden of HIV infection.
Our meta‐analysis has several important limitations. First, as with all meta‐analyses, the results are dependent on the methodologic quality of the studies included. Although the majority of the studies were of fair to good quality, two 31 , 32 were of low quality. The majority of the studies included originated from North American and Africa, and there was little to no representation of Asia and Latin America. As such, our results may not be generalizable to all WLWH. South Africa was uniformly classified as a low resource country, although different parts within this country differ substantially in their quality of care. All studies included were observational, with no randomized controlled trials on the subject. Observational studies are prone to confounding and bias because the comparison groups may be different in characteristics that are associated with the outcomes studied. 45 These include maternal age, ethnicity, previous obstetric history, previous preterm births, multiple preganncies, poor prenatal care, stage of maternal illness, CD4 counts, viral loads, smoking and recreational drug use. Second, while some studies adjusted for potential confounding variables, others did not. When adjustments were made they varied between the studies and therefore a pooled analysis of adjusted estimates could only be performed for timing of ART inititation. Thus, residual confounding cannot be excluded. For example, we could not assess the effect of maternal viral loads and CD4 cell counts due to limited reporting and lack of adjustment for these confounders in most of the studies included. 17 , 21 , 30 , 31 , 32 , 34 , 37 , 43 , 44 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 Still, for some of the outcomes studied, such as PTB, the risk was significantly elevated in the majority of the studies with narrow CIs. Third, some of the measured outcomes, such as preeclampsia, 17 , 21 , 31 , 33 , 36 , 37 , 39 were reported by only a few studies (seven). Nonetheless, among the studies that assessed this outcome, one (Arab et al. 31 ), was substantially larger than the other studies in this systematic review. Fourth, the definition of the outcomes assessed was non‐uniform across all studies and not all studies provided a definition. Additionally, included studies did not differentiate between spontaneous and iatrogenic preterm birth. Fifth, WLWH differed in their ART regimen and it is difficult to assume that medicated WLWH were adequately treated, since we do not have any information on compliance to medication and only partial information on treatment success (CD4 counts and viral loads). Lastly, one may argue that a better comparator to help delineate the association between HIV, ART and adverse pregnancy outcomes is non‐ART treated pregnant WLWH. 5 Given the important reduction in perinatal transmission, for over 25 years, providing ART to pregnant WLWH is standard care. Thus, studies involving non‐ART‐treated pregnant WLWH are in the earlier years of the epidemic and originate primarily from low income countries, making it difficult to compare their outcomes directly with ART‐treated women of today.
Our results are supported in part by a previous meta‐analysis by Brocklehurst that found higher rates of PTB in both high and low income patients. 55 The lack of difference in SGA rates in low income countries may be due to inaccurate determination of SGA given the lack of accurate pregnancy timing in these countries. It should be noted that Brocklehurst et al.’s metanalysis 55 only included studies published prior to 1997, 3 years after antiretroviral treatment in pregnancy was found to decrease vertical transmission rates. 3 Thus there was no differentiation between ART‐treated and non–ART‐treated WLWH. Concordant with our findings, Wedi et al. 5 in their recent meta‐analysis also found a similar increase in the risk of PTB in WLWH compared with healthy controls in sub‐Saharan Africa but, unlike our study, focused on a population naïve to treatment. This may explain their higher risk of additional adverse outcomes in this population, such as SGA.
Our results suggest that despite perinatal antiviral treatment, pregnant WLWH remain at an increased risk for significant adverse perinatal outcomes. The risk of PTB increased with preconceptional initiation of ART. This finding is in accordance with previous evidence, which found an increased risk of PTB with earlier ART initiation; 56 , 57 however, it may be due to a selection bias with advancing gestational age at initiation of ART. 58 Another possible explanation for poorer outcomes with preconceptional initiation of treatment is that these women had more severe advanced disease and severe inflammation than women who initiated treatment in pregnancy. With the present data we are unable to establish the relative contributions of ART compared with that of the HIV infection and the inflammatory state to these outcomes. It is speculated that both HIV and ART have a direct impact on placental dysfunction, 59 , 60 , 61 with the effect differing by type of ART. 62 , 63 This association is supported by the finding of an increased risk of SGA in WLWH. Our finding of no change in preeclampsia risk may point to non‐placental contributing factors, such as socioeconomic status and maternal health, which were not adequately controlled for in most studies.
Clearly, the benefit of perinatal ART to maternal health and prevention of perinatal HIV transmission far outweighs the risks for pregnant WLWH. 4 The question as to whether the drugs, the disease or the patient demographics contribute to the increased risk in adverse pregnancy outcomes, is important to establish, since drug classes are modifiable. In order to do so, strict control for classes of suppressive ART, CD4 levels, population and obstetric characteristics are required. In the present study, we could not perform a pooled analysis of adjusted estimates controlling for competing risk factors for PTB (i.e. socioeconomic status, history of PTB and indication for preterm delivery) and SGA (i.e. chronic hypertension, early onset preeclampsia, history of SGA, smoking and drug use), as not all studies controlled for the same confounding variables. Nonetheless, for PTB and LBW the risk remained high in both low and high income countries. This might indicate that this risk is higher in WLWH irrespective of the availability and the quality of prenatal care. Acknowledging this, increased risk is key in risk reduction. Future studies should evaluate the role of cervical length surveillance as well as serial fetal growth on perinatal outcomes in pregnant WLWH treated with ART. Progesterone supplementation may also be beneficial, 64 , 65 although the evidence for this intervention is lacking.
5. CONCLUSION
WLWH on ART remain at an increased risk for adverse pregnancy outcomes including PTB and LBW independent of resurce setting and for SGA in high income countries. We did not find them to be at a greater risk for preeclampsia compared with HIV‐negative controls. These associations may, in part, be due to bias including uncontrolled or residual confounding. Pre‐conceptional counseling should review this increased risk of adverse outcomes. Further study is indicated to assess pregnancy outcomes in women on modern suppressive ART with more robust control for potential confounding variables in effort to determine if there is a general residual risk or a medication related risk associated with specific classes of antiviral therapy.
CONFLICT OF INTERESTS
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
AUTHOR CONTRIBUTIONS
SS: protocol writing, scientific literature search, search results screening, eligibility assessment, methodological quality assessment, data extraction, data interpretation and manuscript writing. SA: protocol writing, scientific literature search, search results screening, eligibility assessment, methodological quality assessment, data extraction, meta‐analysis, sensitivity analyses, and subgroup analyses and data interpretation, editing. MR: the scientific literature search. KEM: protocol writing, screening, study coordination and editing. SW, LS and MY: idea conception, content expertise and editing. All authors read and approved the final version of the manuscript.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Supplementary Material
Shinar S, Agrawal S, Ryu M, et al. Perinatal outcomes in women living with HIV‐1 and receiving antiretroviral therapy—a systematic review and meta‐analysis. Acta Obstet Gynecol Scand. 2022;101:168–182. doi: 10.1111/aogs.14282
Shiri Shinar and Swati Agrawal contributed equally.
Funding information
This study was funded by the Canadian Foundation for AIDS research (CANFAR) (#26‐021).
REFERENCES
- 1. https://www.who.int/news‐room/fact‐sheets/detail/hiv‐aids. 2019.
- 2. Luo C, Hirnschall G, Rodrigues J, et al. Translating technical support into country action: the role of the interagency task team on the prevention and treatment of HIV infection in pregnant women, mothers, and children in the global plan era. J Acquir Immune Defic Syndr. 2017;75:S7‐S16. [DOI] [PubMed] [Google Scholar]
- 3. Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal‐infant transmission of human immunodeficiency virus type 1 with Zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173‐1180. [DOI] [PubMed] [Google Scholar]
- 4. ACOG Committee Opinion No. 751: Labor and Delivery Management of Women With Human Immunodeficiency Virus Infection. Obstet Gynecol. 2018;132:e131‐e137. [DOI] [PubMed] [Google Scholar]
- 5. Wedi CO, Kirtley S, Hopewell S, Corrigan R, Kennedy SH, Hemelaar J. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta‐analysis. Lancet HIV. 2016;3:e33–e48. [DOI] [PubMed] [Google Scholar]
- 6. Coley JL, Msamanga GI, Fawzi MC, et al. The association between maternal HIV‐1 infection and pregnancy outcomes in Dar es Salaam, Tanzania. BJOG. 2001;108:1125‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Datta P, Embree JE, Kreiss JK, et al. Mother‐to‐child transmission of human immunodeficiency virus type 1: report from the Nairobi Study. J Infect Dis. 1994;170:1134‐1140. [DOI] [PubMed] [Google Scholar]
- 8. Kolte L, Gaardbo JC, Karlsson I, et al. Dysregulation of CD4+CD25+CD127lowFOXP3+ regulatory T cells in HIV‐infected pregnant women. Blood. 2011;117:1861‐1868. [DOI] [PubMed] [Google Scholar]
- 9. Mikyas Y, Aziz N, Harawa N, et al. Immunologic activation during pregnancy: serial measurement of lymphocyte phenotype and serum activation molecules in HIV‐infected and uninfected women. J Reprod Immunol. 1997;33:157‐170. [DOI] [PubMed] [Google Scholar]
- 10. Bianconi V, Schiaroli E, Pirro M, et al. Effects of antiretroviral therapy on proprotein convertase subtilisin/kexin 9: focus on lipids, inflammation and immunovirological parameters. HIV Medicine. 2020;21:512‐522. [DOI] [PubMed] [Google Scholar]
- 11. Delagreverie HM, Bauduin C, De Castro N, et al. Impact of Raltegravir or Efavirenz on cell‐associated human immunodeficiency virus‐1 (HIV‐1) deoxyribonucleic acid and systemic inflammation in HIV‐1/tuberculosis coinfected adults initiating antiretroviral therapy. Open Forum Infect Dis. 2020;7:ofz549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li JZ, Segal FP, Bosch RJ, et al. Antiretroviral therapy reduces T‐cell activation and immune exhaustion markers in human immunodeficiency virus controllers. Clin Infect Dis. 2020;70:1636‐1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel K, Shapiro D, Brogly S, et al. Prenatal protease inhibitor use and risk of preterm birth among HIV‐infected women initiating antiretroviral drugs during pregnancy. J Infect Dis. 2010;201:1035‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grosch‐Woerner I, Puch K, Maier RF, et al. Increased rate of prematurity associated with antenatal antiretroviral therapy in a German/Austrian cohort of HIV‐1‐infected women. HIV Med. 2008;9:6‐13. [DOI] [PubMed] [Google Scholar]
- 15. Powis KM, Smeaton L, Ogwu A, et al. Effects of in utero antiretroviral exposure on longitudinal growth of HIV‐exposed uninfected infants in Botswana. J Acquir Immune Defic Syndr. 2011;56:131‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schulte J, Dominguez K, Sukalac T, Bohannon B, Fowler MG. Declines in low birth weight and preterm birth among infants who were born to HIV‐infected women during an era of increased use of maternal antiretroviral drugs: pediatric Spectrum of HIV Disease, 1989–2004. Pediatrics. 2007;119:e900‐e906. [DOI] [PubMed] [Google Scholar]
- 17. Haeri S, Shauer M, Dale M, et al. Obstetric and newborn infant outcomes in human immunodeficiency virus‐infected women who receive highly active antiretroviral therapy. Am J Obstet Gynecol. 2009;201:315.e1‐5. [DOI] [PubMed] [Google Scholar]
- 18. Lopez M, Figueras F, Hernandez S, et al. Association of HIV infection with spontaneous and iatrogenic preterm delivery: effect of HAART. AIDS. 2012;26:37‐43. [DOI] [PubMed] [Google Scholar]
- 19. Thorne C, Patel D, Newell ML. Increased risk of adverse pregnancy outcomes in HIV‐infected women treated with highly active antiretroviral therapy in Europe. AIDS. 2004;18:2337‐2339. [DOI] [PubMed] [Google Scholar]
- 20. Suy A, Martínez E, Coll O, et al. Increased risk of pre‐eclampsia and fetal death in HIV‐infected pregnant women receiving highly active antiretroviral therapy. AIDS. 2006;20:59‐66. [DOI] [PubMed] [Google Scholar]
- 21. Boyajian T, Shah PS, Murphy KE. Risk of preeclampsia in HIV‐positive pregnant women receiving HAART: a matched cohort study. J Obstet Gynaecol Can. 2012;34:136‐141. [DOI] [PubMed] [Google Scholar]
- 22. Cotter AM, Garcia AG, Duthely ML, Luke B, O’Sullivan MJ. Is antiretroviral therapy during pregnancy associated with an increased risk of preterm delivery, low birth weight, or stillbirth? J Infect Dis. 2006;193:1195‐1201. [DOI] [PubMed] [Google Scholar]
- 23. Marazzi MC, Palombi L, Nielsen‐Saines K, et al. Extended antenatal use of triple antiretroviral therapy for prevention of mother‐to‐child transmission of HIV‐1 correlates with favorable pregnancy outcomes. AIDS. 2011;25:1611‐1618. [DOI] [PubMed] [Google Scholar]
- 24. Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV‐infected women in Botswana. J Infect Diseases. 2012;206:1695‐1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tuomala RE, Watts DH, Li D, et al. Improved obstetric outcomes and few maternal toxicities are associated with antiretroviral therapy, including highly active antiretroviral therapy during pregnancy. J Acquir Immune Defic Syndr. 2005;38:449‐473. [DOI] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. 2021. https://bestpractice.bmj.com/info/toolkit/learn-ebm/study-design-search-filters/.
- 28. 2021. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 29. 2014. https://dplp.cochrane.org/data-extraction-forms.
- 30. Adam GK, Ahmed MA, Ali AA. Human immune deficiency virus (HIV) infection during pregnancy at Gadarif hospital, Eastern Sudan. J Obstet Gynaecol. 2016;36:962‐963. [DOI] [PubMed] [Google Scholar]
- 31. Arab K, Spence AR, Czuzoj‐Shulman N, Abenhaim HA. Pregnancy outcomes in HIV‐positive women: a retrospective cohort study. Arch Gynecol Obstet. 2017;295:599‐606. [DOI] [PubMed] [Google Scholar]
- 32. Ikpim EM, Edet UA, Bassey AU, Asuquo OA, Inyang EE. HIV infection in pregnancy: maternal and perinatal outcomes in a tertiary care hospital in Calabar, Nigeria. Trop Doct. 2016;46:78‐86. [DOI] [PubMed] [Google Scholar]
- 33. Olagbuji BN, Ezeanochie MC, Ande AB, Oboro VO. Obstetric and perinatal outcome in HIV positive women receiving HAART in urban Nigeria. Arch Gynecol Obstet. 2010;281:991‐994. [DOI] [PubMed] [Google Scholar]
- 34. Carceller A, Ferreira E, Alloul S, Lapointe N. Lack of effect on prematurity, birth weight, and infant growth from exposure to protease inhibitors in utero and after birth. Pharmacotherapy. 2009;29:1289‐1296. [DOI] [PubMed] [Google Scholar]
- 35. Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Preg. 2001;20(1):ix–xiv [DOI] [PubMed] [Google Scholar]
- 36. Azria E, Moutafoff C, Schmitz T, et al. Pregnancy outcomes in women with HIV type‐1 receiving a lopinavir/ritonavir‐containing regimen. Antivir Ther. 2009;14:423‐432. [PubMed] [Google Scholar]
- 37. Sebitloane HM, Moodley J. Maternal and obstetric complications among HIV‐infected women treated with highly active antiretroviral treatment at a Regional Hospital in Durban, South Africa. Niger J Clin Pract. 2017;20:1360‐1367. [DOI] [PubMed] [Google Scholar]
- 38. Saums MK, King CC, Adams JC, et al. Combination antiretroviral therapy and hypertensive disorders of pregnancy. Obstet Gynecol. 2019;134:1205‐1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Elenga N, Djossou FÉL, Nacher M. Association between maternal human immunodeficiency virus infection and preterm birth: a matched case‐control study from a pregnancy outcome registry. Medicine (Baltimore). 2021;100:e22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boer K, Nellen JF, Patel D, et al. The AmRo study: pregnancy outcome in HIV‐1‐infected women under effective highly active antiretroviral therapy and a policy of vaginal delivery. BJOG. 2007;114:148‐155. [DOI] [PubMed] [Google Scholar]
- 41. Tiam A, Kassaye SG, Machekano R, et al. Comparison of 6‐week PMTCT outcomes for HIV‐exposed and HIV‐unexposed infants in the era of lifelong ART: results from an observational prospective cohort study. PLoS One. 2019;14:e0226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ramokolo V, Goga AE, Lombard C, Doherty T, Jackson DJ, Engebretsen IM. In utero ART exposure and birth and early growth outcomes among HIV‐exposed uninfected infants attending immunization services: results from National PMTCT Surveillance, South Africa. Open Forum Infect Dis. 2017;4:ofx187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Piske M, Qiu AQ, Maan EJ, et al. Preterm birth and antiretroviral exposure in infants HIV‐exposed uninfected. Pediatr Infect Dis J. 2021;40:245‐250. [DOI] [PubMed] [Google Scholar]
- 44. Tukei VJ, Hoffman HJ, Greenberg L, et al. Adverse pregnancy outcomes among HIV‐positive women in the era of universal antiretroviral therapy remain elevated compared with HIV‐negative women. Pediatr Infect Dis J. 2021;40:821‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jepsen P, Johnsen SP, Gillman MW, Sørensen HT. Interpretation of observational studies. Heart. 2004;90:956‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dara JS, Hanna DB, Anastos K, Wright R, Herold BC. Low birth weight in human immunodeficiency virus‐exposed uninfected infants in Bronx, New York. J Pediatric Infect Dis Soc. 2018;7:e24‐e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Malaba TR, Phillips T, Le Roux S, et al. Antiretroviral therapy use during pregnancy and adverse birth outcomes in South African women. Int J Epidemiol. 2017;46:1678‐1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gagnon LH, MacGillivray J, Urquia ML, Caprara D, Murphy KE, Yudin MH. Antiretroviral therapy during pregnancy and risk of preterm birth. Eur J Obstet Gynecol Reprod Biol. 2016;201:51‐55. [DOI] [PubMed] [Google Scholar]
- 49. Gibango NN, Mda S, Ntuli TS. Factors associated with delivering premature and/or low birth weight infants among pregnant HIV‐positive women on antiretroviral treatment at Dr George Mukhari Hospital, South Africa. S Afr J Infect Dis. 2018;33:42‐45. [Google Scholar]
- 50. Santosa WB, Staines‐Urias E, Tshivuila‐Matala COO, Norris SA, Hemelaar J. Perinatal outcomes associated with maternal HIV and antiretroviral therapy in pregnancies with accurate gestational age in South Africa. AIDS. 2019;33:1623‐1633. [DOI] [PubMed] [Google Scholar]
- 51. Iloghalu EI, Dim CC, Ugwu EO, Onwuka CI, Ozumba BC. Effect of maternal HIV infection on treatment with HAART on neonatal birth weight and other anthropometry: a Cohort Study of HIV sero‐positive women in Enugu, South‐East Nigeria. J Clin Diag Res. 2019;13:QC01–QC04. [Google Scholar]
- 52. Dadabhai S, Gadama L, Chamanga R, et al. Pregnancy outcomes in the era of universal antiretroviral treatment in Sub‐Saharan Africa (POISE Study). J Acquir Immune Defic Syndr. 2019;80:7‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li H, Liu J, Tan D, et al. Maternal HIV infection and risk of adverse pregnancy outcomes in Hunan province, China: a prospective cohort study. Medicine (Baltimore). 2020;99:e19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nyemba DC, Kalk E, Madlala HP, et al. Lower birth weight‐for‐age and length‐for‐age z‐scores in infants with in‐utero HIV and ART exposure: a prospective study in Cape Town, South Africa. BMC Pregnancy Childbirth. 2021;21:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta‐analysis. BJOG. 1998;105:836‐848. [DOI] [PubMed] [Google Scholar]
- 56. Uthman OA, Nachega JB, Anderson J, et al. Timing of initiation of antiretroviral therapy and adverse pregnancy outcomes: a systematic review and meta‐analysis. Lancet HIV. 2017;4:e21‐e30. [DOI] [PubMed] [Google Scholar]
- 57. Combination antiretroviral therapy and duration of pregnancy. AIDS. 2000;14:2913‐2920. [DOI] [PubMed] [Google Scholar]
- 58. Stoner MCD, Cole SR, Price J, Winston J, Stringer JSA. Timing of initiation of antiretroviral therapy and risk of preterm birth in studies of HIV‐infected pregnant women: the role of selection bias. Epidemiology. 2018;29:224‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wt A, Kwiek JJ. Role of the placenta in adverse perinatal outcomes among HIV‐1 seropositive women. J Nippon Med Sch. 2013;80:90‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mohammadi H, Papp E, Cahill L, et al. HIV antiretroviral exposure in pregnancy induces detrimental placenta vascular changes that are rescued by progesterone supplementation. Sci Rep. 2018;8:6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kala S, Dunk C, Acosta S, Serghides L. Periconceptional exposure to lopinavir, but not darunavir, impairs decidualization: a potential mechanism leading to poor birth outcomes in HIV‐positive pregnancies. Hum Reprod. 2020;35:1781‐1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Papp E, Balogun K, Banko N, et al. Low prolactin and high 20‐α‐hydroxysteroid dehydrogenase levels contribute to lower progesterone levels in HIV‐infected pregnant women exposed to protease inhibitor‐based combination antiretroviral therapy. J Infect Dis. 2016;213:1532‐1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McDonald CR, Conroy AL, Gamble JL, et al. Estradiol levels are altered in human immunodeficiency virus‐infected pregnant women randomized to Efavirenz‐ versus Lopinavir/Ritonavir‐based antiretroviral therapy. Clin Infect Dis. 2018;66:428‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Price JT, Phiri WM, Freeman BL, et al. Vaginal progesterone to prevent preterm delivery among HIV‐infected pregnant women in Zambia: a feasibility study. PLoS One. 2020;15:e0224874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Siou K, Walmsley SL, Murphy KE, et al. Progesterone supplementation for HIV‐positive pregnant women on protease inhibitor‐based antiretroviral regimens (the ProSPAR study): a study protocol for a pilot randomized controlled trial. Pilot Feasibility Stud. 2016;2:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Supplementary Material


