Abstract
To examine the role of the Plasmodium falciparum Exp-1 blood-stage protein in producing antibodies that cross-react with human T-cell lymphotropic virus type I (HTLV-I) proteins, we studied sera from Indonesian volunteers who seroconverted to malaria after transmigrating to an area where malaria is hyperendemic. Samples from Philippine volunteers, that were used in a prior study that examined malaria antibodies that cross-react with HTLV-I proteins, were also used. Eighty-three percent of the Indonesian transmigrants developed antibodies against the malaria Exp-1 protein by 6 months postmigration. Of these malaria seroconverters, 27% developed false-positive HTLV-I enzyme immunoassay (EIA) immunoreactivity, as indicated by indeterminate HTLV-I Western blot banding patterns. Five of the six Philippine samples tested were HTLV-I EIA false positive and Western blot indeterminate. When a recombinant Exp-1 protein was used in blocking experiments, the HTLV-I Western blot immunoreactivity of sera from both groups was either completely eliminated or greatly reduced. No effect on the Western blot immunoreactivity of truly HTLV-I-positive sera was seen. To determine if immunization with the recombinant Exp-1 protein could elicit the production of HTLV-I antibodies, six mice were inoculated with the recombinant protein. Following administration of three 50-μg doses of the protein, four of the six mice developed antibodies that cross-reacted with HTLV-I proteins on Western blot. These results indicate that the immune response against the malaria Exp-1 protein may result in HTLV-I-cross-reacting antibodies that can lead to false-positive EIA and indeterminant Western blotting results.
Plasmodium falciparum is capable of inducing antibodies that cross-react with human T-cell lymphotropic virus type I (HTLV-I) proteins to give false-positive enzyme immunoassay (EIA) results and indeterminate Western blot patterns (3, 4, 6). The specific malaria proteins responsible for this immunologic response are unknown. Recent peptide mapping studies identified a seven-amino-acid epitope, located at the carboxy-terminal end of the gag-encoded p19 protein of HTLV-I, that is recognized by P. falciparum antibodies (5). Through a computerized sequence homology search, this p19 epitope was found to be similar to a stretch of seven amino acids on P. falciparum blood-stage antigen Exp-1. The present study was conducted to clarify the role the Exp-1 antigen in the development of HTLV-I-cross-reacting antibodies. The results provide direct evidence that it is the immune response against this antigen that may produce antibodies that cross-react with several HTLV-I proteins.
MATERIALS AND METHODS
Study population.
The Indonesian samples used in this study were pre- and postmigration serum samples that had previously been obtained from 18 Indonesian volunteers who had migrated from Java, where malaria is not endemic, to the Arso region of Irian Jaya, where malaria is hyperendemic. The samples were collected as part of an earlier study examining malaria transmission rates in Indonesia. All postmigration samples were positive for P. falciparum antibodies by immunofluorescence assay. Premigration and 6-month postmigration serum samples from all volunteers were available. Three- and 12-month postmigration samples from only two and six volunteers, respectively, were available.
Previously collected serum samples from six volunteers living in the Philippines were also used. These samples were obtained as part of a prior study that examined the cross-reactivity between malaria antibodies and HTLV-I proteins (3). Two samples that were malaria and HTLV-I antibody negative and two Western blot HTLV-I-positive serum samples were used as controls. All samples were stored at −70°C until used and were obtained after informed consent had been obtained and used in accordance with approved human use protocols. The participation of the volunteers was in accordance with U.S. Navy regulations governing the use of human subjects in medical research.
Detection of HTLV-I and Exp-1 antibodies.
Samples were tested for anti-HTLV-I antibodies by EIA (Abbott Laboratories, Abbott Park, Ill.). A Western blot assay (HTLV-I Blot 2.4; Genelabs Diagnostics, Singapore, Singapore) was used to confirm EIA-positive samples. To be classified as Western blot positive, sera had to be reactive against a gag-encoded protein (p19 or p24) and two env-encoded proteins (gp46 or rgp46 and GD21). Samples not meeting these criteria but showing reactivity were considered indeterminate, and samples with no reactivity were considered negative.
All serum samples were screened by EIA for reactivity against a recombinant Exp-1 protein and a recombinant DR4a/b antigen as a control. The Exp-1 recombinant protein (Hoffmann-La Roche, Basel, Switzerland) was produced in vitro from recombinant plasmid pUC8-5.1 and purified as previously described (1). The Exp-1-encoding gene used to construct the recombinant plasmid was derived from P. falciparum K1 from Thailand. The Exp-1 protein encoded by this gene is also known as the 5.1 antigen (8). The control DR4a/b protein consisted of the N-terminal half of the HLA DR4a1 protein ligated to the N-terminal half of the HLA DR4b1 protein. Both the Exp-1 and DR4a/b proteins contained a C-terminal hexahistidine tail for purification.
The recombinant proteins were used to coat 96-well plates at a concentration of 2 μg/ml and reacted with serum samples diluted 1:6,250 with phosphate-buffered saline (PBS). This serum dilution was chosen after tests with serial fivefold dilutions of negative and positive control samples showed that a 1:6,250 dilution produced the lowest signal-to-noise ratio (data not shown). A sample was considered positive if the Exp-1 optical density (OD) value was at least fivefold greater than the DR4a/b OD and the mean OD obtained with the negative control sera. This stringent criterion was chosen to ensure the elimination of false-positive results due to nonspecific immunoreactivity.
Western blot blocking assays.
Experiments were conducted to see if the recombinant Exp-1 protein could block the HTLV-I Western blot immunoreactivity of the study sera. A serum sample from a Philippine volunteer, that produced a strong but indeterminate HTLV-I Western blot banding pattern, was first tested to determine the amount of Exp-1 protein needed to block HTLV-I immunoreactivity. The serum was diluted 1:500 in PBS alone and in PBS containing 102, 103, and 104 μg of recombinant Exp-1 protein per ml. The total volume of the diluted serum-protein solution was 500 μl. The diluted samples were then incubated at 4°C overnight with shaking at 100 rpm. A Western blot HTLV-I-positive serum sample was used as a control and treated identically. Following the overnight incubation, the samples were assayed by HTLV-I Western blotting as described previously (6). To accomplish this, 1.5 ml of kit blocking solution, containing Exp-1 protein at the concentrations used in the overnight incubation step, were added to each sample and subsequently assayed in accordance with the manufacturer’s instructions.
Based on the results obtained with this single sample, a serum dilution of 1:100 and a protein concentration of 102 μg/ml were used to assay the remaining samples. A lower serum dilution was used with these samples because the intensity of the Western blot indeterminate immunoreactivity was considerably less than that of the Philippine sample (Fig. 1, lane 11) used to titrate the antigen. Two samples, one Indonesian and one Philippine, that were HTLV-I EIA negative and Exp-1 EIA positive were also tested. To control for nonspecific blocking, samples were also blocked with DR4a/b recombinant protein at 50 μg/ml.
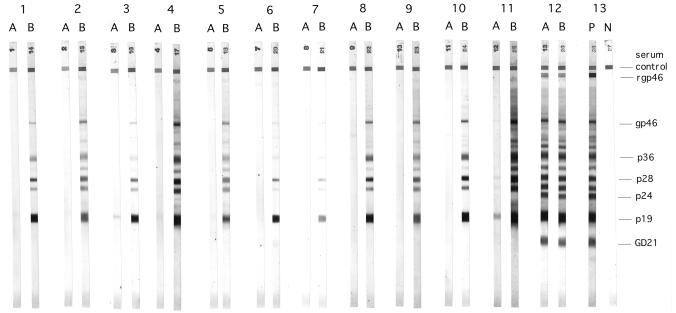
FIG. 1.
HTLV-I Western blot blocking results obtained with Indonesian transmigrant and Philippine samples. Lanes: 1, 2, 4, and 5, samples from the four Indonesian transmigrant HTLV-I EIA seroconverters in Table 1; 3, sample from a volunteer who was HTLV-I negative but Exp-1 positive by EIA; 6 to 11, Philippine samples; 12, HTLV-I-positive control; 13, kit positive (P) and negative (N) controls. (A) Samples preabsorbed and blocked with recombinant Exp-1 protein. (B) Samples preabsorbed and blocked with HLA DR control recombinant protein. Note that GD21 is a recombinant env-encoded glycoprotein that migrates at a faster rate than the other HTLV-I proteins.
Statistical analysis.
To look for a correlation between HTLV-I and Exp-1 antigen EIA positivity, the sample OD values obtained with these proteins and the DR4a/b protein were grouped according to the times when they were collected and subjected to correlation analysis by the software StatView, version 4.5 for Macintosh (Abacus Concepts, Berkeley, Calif.). The P values for each comparison were calculated by using Fisher’s r to z test.
Mouse immunization with recombinant Exp-1.
Four- to 6-week-old BALB/cByJ mice were immunized with the recombinant Exp-1 protein to elicit HTLV-I-cross-reactive antibodies. Each of six mice was given three 50-μg subcutaneous injections at 2-week intervals. The first injection was prepared in complete Freund’s adjuvant, and the subsequent injections were prepared in incomplete Freund’s adjuvant. Serum samples were collected 2 weeks after the final immunization and tested for immunoreactivity against HTLV-I proteins by Western blotting. To adapt this assay for use with mouse serum, the anti-human immunoglobulin G-horseradish peroxidase conjugate was replaced with an anti-mouse immunoglobulin G-horseradish peroxidase conjugate. Serum from a mouse immunized with a recombinant dengue virus antigen in complete Freund’s adjuvant was used as a negative control. This animal was shown to have high titers of dengue virus antibodies, as demonstrated by enzyme-linked immunosorbent assay and plaque reduction neutralization assay (data not shown).
RESULTS
Detection of HTLV-I and Exp-1 antibodies.
All samples from the Indonesian volunteers were HTLV-I negative by EIA premigration. At 6 months postmigration, 3 of the 18 volunteers had seroconverted to HTLV-I, as shown by EIA (Table 1). One volunteer seroconverted 12 months postmigration. Confirmatory Western blot analysis of the EIA-positive samples showed indeterminate banding patterns, as shown in Fig. 1. Five of the six Philippine samples tested were HTLV-I positive by EIA, and all five showed indeterminate Western blot banding patterns (Table 1). One of the Philippine samples HTLV-I positive by EIA showed relatively weak Western blot immunoreactivity that was barely visible at the serum dilutions used in the blocking assay (Fig. 1, lane 7B).
TABLE 1.
Serology results obtained with the Exp-1 recombinant and HTLV-I proteins
| Sample group | EIAa
|
HTLV Western blot confirmationb
|
||
|---|---|---|---|---|
| Exp-1 | HTLV-I | Pos | Ind | |
| Indonesian | 15/18 | 4/18 | 0/4 | 4/4 |
| Philippine | 4/6c | 5/6 | 0/5 | 5/5 |
| HTLV-I positive | 0/2 | 2/2 | 2/2 | 0/2 |
Results are expressed as the number of volunteers positive/number tested. The Indonesian data represent those individuals who seroconverted postmigration.
Results are expressed as the number of volunteers falling into the indicated category/number tested. Pos, positive; Ind, indeterminate.
One additional sample showed Exp-1 immunoreactivity, but the level of reactivity was not strong enough to be considered positive.
Serum samples from all Indonesian volunteers were negative for immunoreactivity to the Exp-1 protein prior to migration. Fifteen of the 18 Indonesian volunteers seroconverted to the P. falciparum Exp-1 antigen by 6 months postmigration, including the four who were also shown to have seroconverted to HTLV-I by EIA. No immunoreactivity was seen against the DR4a/b recombinant protein with any of the samples (data not shown).
Four of the six Philippine samples tested were positive for Exp-1 by EIA. One additional sample showed Exp-1 immunoreactivity, but the level of reactivity was not strong enough to be considered positive. This sample, however, was positive for HTLV-I by EIA. The two Western blot HTLV-I-positive control samples did not react with either the Exp-1 protein or the DR4a/b protein.
When the raw OD values for the samples HTLV-I positive by EIA, grouped according to the times when they were collected, were compared, a statistically significant correlation between the OD values for the Exp-1 and HTLV-I proteins was seen (Table 2). The correlation coefficient for this comparison was 0.755 (P < 0.02). There was no correlation between the OD values obtained with the HTLV-I and DR4a/b proteins (data not shown).
TABLE 2.
OD values for the four transmigrants who were shown to have seroconverted to HTLV-I by EIA
| Sample no. | Premigration
|
6 months postmigration
|
||
|---|---|---|---|---|
| HTLV-I | Exp-1 | HTLV-I | Exp-1 | |
| 1 | 0.193 | 0.350 | 1.110 | 1.390 |
| 2 | 0.150 | 0.143 | 1.761 | 1.460 |
| 3 | 0.154 | 0.190 | 0.801 | 1.630 |
| 4a | 0.159 | 0.104 | 0.264 | 1.402 |
Seroconverted to HTLV-I 12 months postmigration with OD values of 1.940 for HTLV-I and 1.596 for Exp-1.
Western blot blocking assays.
Figure 1 shows the blocking results obtained with five Indonesian (four 6-month, one 12-month) postmigration samples and six Philippine samples by using the Exp-1 protein and the negative control DR4a/b protein. Two of the samples used in the blocking assay, one Indonesian (lane 3) and one Philippine (lane 6), although HTLV-I negative by EIA, did show indeterminate Western blot patterns with the HTLV-I Blot 2.4 kit. The Exp-1 protein blocked or greatly reduced the indeterminate immunoreactivity of all of the Philippine and Indonesian sera tested, whereas the DR4a/b protein had no effect. The immunoreactivity of the truly HTLV-I-positive sample was not affected.
Mouse immunization with recombinant Exp-1.
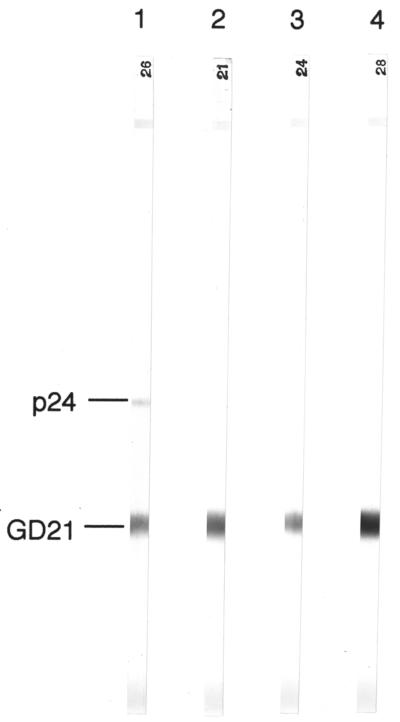
Mice were given three immunizations with the recombinant Exp-1 protein to see if HTLV-I-cross-reactive antibodies could be produced. Two weeks after the final immunization, all animals developed Exp-1 antibody titers of approximately 1:20,000, as measured by EIA (data not shown). When assayed by HTLV-I Western blotting, immunoreactivity against the recombinant GD21 env-encoded protein was seen with four of the six samples (Fig. 2). One of these samples also showed weak immunoreactivity against the p24 antigen. The mouse serum sample that contained high titers of dengue virus antibodies showed no Western blot immunoreactivity (data not shown).
FIG. 2.
HTLV-I Western blots of sera from mice immunized with recombinant Exp-1 proteins (stripes 1 to 4). Sera from two Exp-1-immunized mice showed no Western blot immunoreactivity, as did sera from a dengue virus-immune mouse (data not shown).
DISCUSSION
Prior studies showed that anti-P. falciparum antibodies can cross-react with HTLV-I proteins by EIA and Western blotting. These cross-reactive antibodies recognize a seven-amino-acid epitope on the HTLV-I p19 protein. The sequence of this epitope is similar to a stretch of seven amino acids located on the P. falciparum Exp-1 protein, suggesting that this protein has a role in generating HTLV-I-cross-reacting antibodies. The function of this protein, as it relates to the P. falciparum life cycle, is not completely understood. However, it is known that this protein is exported from the malaria parasite to the parasitophorous vacuole membrane and to membranous compartments of the erythrocyte (2). This protein is also expressed on the infected-hepatocyte surface (7).
Eighty-three percent of the Indonesian transmigrants we studied clearly demonstrated seroconversion to the P. falciparum Exp-1 blood-stage antigen postmigration to an area where malaria is endemic. Of those who seroconverted, 27% simultaneously seroconverted to HTLV-I, as shown by EIA. These seroconversions were false positives, as indicated by indeterminate Western blot banding patterns obtained upon confirmatory testing. The simultaneous development of a false-positive HTLV-I EIA result and a statistically significant correlation between HTLV-I and Exp-1 OD values provides suggestive evidence that this malaria protein is responsible for the production of the HTLV-I antibodies.
The ability of Exp-1 protein to block indeterminate Western blot HTLV-I immunoreactivity of the Indonesian sera and not the immunoreactivity of Western blot HTLV-I-positive sera provides conclusive evidence that this protein elicits antibodies that cross-react with HTLV-I antibodies. This cross-reactive immune response is not seen in all Exp-1 antibody-positive individuals. Although the reason for this is not clear, this observation does suggest that other factors are involved, such as HLA type and number of malaria re-exposures. However, the data generated in our study are not sufficient to make any conclusions regarding the influence of these factors on the generation of HTLV-I cross-reactive antibodies. Thus, risk factors related to the development of these antibodies cannot be established. The successful blocking of indeterminate Western blot HTLV-I immunoreactivity in sera from Philippine volunteers does indicate that this phenomenon is not confined to Indonesia.
It is unlikely that the only Exp-1 epitope responsible for these antibodies is the seven-amino-acid sequence similar to the previously identified HTLV-I p19 malaria antibody-cross-reactive epitope. This view is supported by the fact that although the other Western blot HTLV-I-reactive proteins have amino acid sequences different from that of the p19 protein, immunoreactivity to these proteins was also blocked or reduced by the recombinant Exp-1 antigen.
The recombinant Exp-1 antigen had no effect on the p19 Western blot immunoreactivity of truly HTLV-I-positive sera. This result was not unexpected. We speculate that during HTLV-I infection, antibodies are generated against p19 epitopes different from the malaria antibody-cross-reactive p19 epitopes. Therefore, adsorption with the Exp-1 malaria protein would have little to no effect on the Western blot p19 immunoreactivity of truly HTLV-I-positive sera.
Two of the samples (one Philippine and one Indonesian), used in the blocking assay as EIA HTLV-I-negative; Exp-1-positive controls, did show indeterminate immunoreactivity to HTLV-I by Western blotting. This Western blot immunoreactivity was also completely eliminated by blocking with the recombinant Exp-1 protein (Fig. 1, lanes 3 and 6). This indicates that the cross-reactive immune response of these volunteers was not strong enough to produce a positive reaction in the HTLV-I EIA.
The ability of the Exp-1 protein to elicit antibodies that cross-react with HTLV-I proteins was demonstrated by immunizing mice with the recombinant protein. Immunoreactivity against the HTLV-I GD21 recombinant env-encoded protein developed in 67% of the mice, and one mouse also showed immunoreactivity against p24. The immunoreactivity elicited in mice against predominantly a single HTLV-I protein (GD21) is contrary to the broad HTLV-I immunoreactivity seen with the human sera. Further, immunoreactivity against GD21 was not seen with any of the human samples used in this study, although prior studies with human samples did show definite but infrequent immunoreactivity against this glycoprotein (6). The differences in the number and type of HTLV-I proteins recognized by murine compared to human sera could, perhaps, be explained by the obvious differences between murine and human immune systems. The longer duration of P. falciparum antigen exposure in humans compared to the mice we immunized could also explain why the human sera reacted to more HTLV-I proteins. Volunteers who transmigrated to an area where malaria is endemic were presumably exposed repeatedly to P. falciparum over a 6-month period, unlike the mice, which were immunized over a shorter period of time. Because data supporting any of these hypotheses are lacking, we cannot say definitely why murine anti-Exp-1 antibodies react predominately with the HTLV-I GD21 protein.
We have shown that the unique phenomenon of P. falciparum generating an immune response against HTLV-I proteins is due to an antibody response directed against epitopes located on the Exp-1 blood-stage protein. Although this immune response may have a profound impact on screening assays for HTLV-I, the impact of these antibodies on the biology of the virus remains to be determined.
ACKNOWLEDGMENT
This work was supported by the U.S. Naval Medical Research and Development Command for Work Units 61152N MR00001 001 2108 and 63105A 3M263105 DH29 AA1.
REFERENCES
- 1.Caspers P, Etlinger H, Matile H, Pink J R, Stuber D, Takacs B. A Plasmodium falciparum malaria vaccine candidate which contains epitopes from the circumsporozoite protein and a blood stage antigen, 5.1. Mol Biochem Parasitol. 1991;47:143–150. doi: 10.1016/0166-6851(91)90173-4. [DOI] [PubMed] [Google Scholar]
- 2.Gunther K, Tummler M, Arnold H H, Ridley R, Goman M, Scaife J G, Lingelbach K. An exported protein of Plasmodium falciparum is synthesized as an integral membrane protein. Mol Biochem Parasitol. 1991;46:149–157. doi: 10.1016/0166-6851(91)90208-n. [DOI] [PubMed] [Google Scholar]
- 3.Hayes C G, Burans J P, Oberst R B. Antibodies to human T lymphotropic virus type I in a population from the Philippines: evidence for cross-reactivity with Plasmodium falciparum. J Infect Dis. 1991;163:257–262. doi: 10.1093/infdis/163.2.257. [DOI] [PubMed] [Google Scholar]
- 4.Lal R B, Rudolph D, Alpers M P, Sulzer A J, Ya-Ping S, Lal A A. Immunologic cross-reactivity between structural proteins of human T-cell lymphotropic virus type I and the blood stage of Plasmodium falciparum. Clin Diagn Lab Immunol. 1994;1:5–10. doi: 10.1128/cdli.1.1.5-10.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter K R, Anthony R L, Solihin A, Hayes C G. Mapping of a human T-lymphotropic virus type I Gag protein epitope that cross-reacts with anti-Plasmodium falciparum antibodies. J Med Virol. 1995;45:469–474. doi: 10.1002/jmv.1890450419. [DOI] [PubMed] [Google Scholar]
- 6.Porter K R, Liang L, Long G W, Bangs M J, Anthony R L, Anderson E M, Hayes C G. Evidence for anti-Plasmodium falciparum antibodies that cross-react with HTLV-I proteins in a population in Irian Jaya, Indonesia. Clin Diagn Lab Immunol. 1994;1:11–15. doi: 10.1128/cdli.1.1.11-15.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez G L, Rogers W O, Mellouk S, Hoffman S L. Plasmodium falciparum exported protein-1, a blood stage antigen, is expressed in liver stage parasites. Exp Parasitol. 1994;79:59–62. doi: 10.1006/expr.1994.1060. [DOI] [PubMed] [Google Scholar]
- 8.Simmons D, Woollet G, Bergin-Cartwright M, Kay D, Scaife J. A malaria protein exported into a new compartment within the host erythrocyte. EMBO J. 1987;6:485–491. doi: 10.1002/j.1460-2075.1987.tb04779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]