Abstract
Introduction
A peripartum hysterectomy is typically performed as a lifesaving procedure in obstetrics to manage severe postpartum hemorrhage. Severe hemorrhages that lead to peripartum hysterectomies are mainly caused by uterine atony and placenta accreta spectrum disorders. In this study, we aimed to estimate the incidence, risk factors, causes and management of severe postpartum hemorrhage resulting in peripartum hysterectomies, and to describe the complications of the hysterectomies.
Material and methods
Eligible women had given birth at gestational week 23+0 or later and had a postpartum hemorrhage ≥1500 mL or a blood transfusion, due to postpartum hemorrhage, at Oslo University Hospital, Norway, between 2008 and 2017. Among the eligible women, this study included those who underwent a hysterectomy within the first 42 days after delivery. The Norwegian Medical Birth Registry provided the reference group. We used Poisson regression to estimate adjusted incidence rate ratios with 95% confidence intervals to identify clinical factors associated with peripartum hysterectomy.
Results
The incidence of hysterectomies with severe postpartum hemorrhage was 0.44/1000 deliveries (42/96313). Among the women with severe postpartum hemorrhage, 1.6% ended up with a hysterectomy (42/2621). Maternal age ≥40, previous cesarean section, multiple pregnancy and placenta previa were associated with a significantly higher risk of hysterectomy. Placenta accreta spectrum disorders were the most frequent cause of hemorrhage that resulted in a hysterectomy (52%, 22/42) and contributed to most of the complications following the hysterectomy (11/15 women with complications).
Conclusions
The rate of peripartum hysterectomies at Oslo University Hospital was low, but was higher than previously reported from Norway. Risk factors included high maternal age, previous cesarean section, multiple pregnancy and placenta previa, well known risk factors for placenta accreta spectrum disorders and severe postpartum hemorrhage. Placenta accreta spectrum disorders were the largest contributor to hysterectomies and complications.
Keywords: cesarean section, maternal near miss, peripartum hysterectomy, placenta accreta spectrum disorders, placenta previa, severe postpartum hemorrhage
Abbreviations
- CI
confidence interval
- IRR
incidence rate ratio
- PAS
placenta accreta spectrum
- PPH
postpartum hemorrhage
Key message
The incidence of hysterectomies due to severe postpartum hemorrhage at Oslo University Hospital was 0.44/1000 deliveries. Placenta accreta spectrum disorders caused more than half of the cases, and were present in most women with complications after the hysterectomy.
1. INTRODUCTION
It is believed that the first successful removal of the uterus after a cesarean delivery was performed in Italy by Dr. Eduardo Porro in 1876. 1 The procedure was carefully planned in advance, due to the high risk, and the mother and child both survived. Today, acute and planned hysterectomies are performed during childbirth, mainly as a last resort, to stop or prevent massive, potentially fatal hemorrhages. In high‐income countries with low maternal mortality rates, obstetric care can be improved by identifying severe acute maternal morbidities, where survival is a “near miss” event. 2 , 3 A peripartum hysterectomy is often performed to avoid fatalities. More knowledge of the causes, management and complications of peripartum hysterectomies is needed to improve the quality of obstetric care, and thus maternal health. A peripartum hysterectomy is defined as the removal of the uterus within a specific time interval after delivering the baby, 4 , 5 , 6 , 7 and it is mainly due to postpartum hemorrhage (PPH). Worldwide, the rates of peripartum hysterectomies vary widely. 8 These rates are generally low in high‐income countries, ranging from 0.26/1000 deliveries in Denmark, to 1.07/1000 deliveries in Italy. 9 Two previous studies from Norway estimated rates similar to those observed in Denmark7, 10 In the last few decades, increasing PPH trends have been observed in several high‐income countries, including Norway. 11 , 12 , 13 Accordingly, several studies have indicated increases in the number of peripartum hysterectomies. 14 , 15 , 16 These observations have emphasized the need for updated knowledge. The present study aimed to estimate the incidence of peripartum hysterectomies after severe PPHs and explore the causes, risk factors and management. We also aimed to describe complications of the hysterectomies.
2. MATERIAL AND METHODS
Eligible women had given birth at gestational week 23+0 or later and had a severe PPH at Oslo University Hospital, Norway, between 2008 and 2017. Women were included in the study when they had undergone a hysterectomy within the first 42 days after delivery, which corresponded to the puerperal period. Severe PPH was defined as a blood loss ≥1500 mL or a transfusion of red blood cells due to severe hemorrhage. Causes of PPH were classified as follows:17 Tone (atony of the uterus), Tissue (retained placenta or parts of the placenta, including placenta accreta spectrum [PAS] disorders), Trauma (birth canal trauma and uterine rupture) and Thrombin (coagulation disorders). Medical records were retrospectively scrutinized to collect information on the relevant variables. Maternal characteristics included age at delivery, parity, country of origin and previous cesarean section. Variables related to the index pregnancy included gestational age, number of fetuses (multiple pregnancies), presence of placenta previa, mode of delivery, administration of uterotonics and tranexamic acid, and interventions (eg curettage, intrauterine balloon tamponade, uterine compression sutures, embolization of pelvic arteries, and balloon occlusion of the aorta during surgery). We also recorded transfusions of blood products and characteristics of the hysterectomy. Postoperative variables included the length of hospital stay (in the intensive care unit and the postnatal ward), and complications (eg thrombotic events, sepsis, bladder injury, oophorectomy, Sheehan syndrome, severe depression and pain syndromes). The obstetrics department at Oslo University Hospital is the largest in Norway. It comprises four wards at two different locations in Oslo, performing approximately 9500 deliveries per year. The wards range from a low‐risk ward to a highly specialized ward with national referral service for specific maternal and fetal high‐risk conditions, such as maternal heart disease and severe congenital malformations. At both locations, the neonatal intensive care units treat newborns born at or after gestational week 23+0. The reference group included all mothers who delivered babies at Oslo University Hospital, based on data from the Medical Birth Registry of Norway, which stores data from compulsory reports of all pregnancies that ended after at least 12 complete weeks of gestation. We extracted aggregated data on variables relevant to the analyses, including separate analyses of each variable and analyses of all combinations of variables. Data on the country of origin and gestational age were not available in the combined data, due to privacy regulations.
2.1. Statistical analyses
Descriptive statistics are expressed as the frequency and proportion for categorical variables and the mean and range for continuous variables. The incidence of peripartum hysterectomies was calculated as the number of hysterectomies per 1000 deliveries and presented with the exact Poisson 95% confidence interval (95% CI). We performed Poisson regression analyses to investigate associations between clinical factors and the risk of hysterectomy, and we estimated incidence rate ratios (IRRs) with 95% CIs. Multivariable analyses included the following variables: age at delivery, parity, multiple pregnancies, previous cesarean section and placenta previa. To evaluate the relation between risk factors, causes and outcome, we drew a directed acyclic graph (DAG) in DAGITTY. 18 The outline for the DAG is presented as a conceptual model 19 (Figure 1). The variables placenta previa and previous cesarean section are strongly connected, but may affect the cause of PPH by various pathways, and thus are included in the multivariable analyses according to the directed acyclic graph. On the other hand, mode of delivery was not included, as the cause Tissue, more specifically PAS disorders, is associated with delivery by cesarean section. Furthermore, gestational age was not included in the multivariable analyses, as we considered this risk factor to be an intermediate factor 20 and also due to the lack of combined data on this variable in the reference group. No multicollinarity between the included variables was observed (all the variance inflation factors ≤2). In addition to Poisson regressions, we performed sensitivity analyses with negative binominal regressions to allow greater variation, when examining the effects of multiple variables on the risk of rare events. Incidence rates were compared with MEDCALC Software. 21 Associations with significance levels <0.05 were considered statistically significant. Data were analyzed with STATA/SE version 15.0.
FIGURE 1.
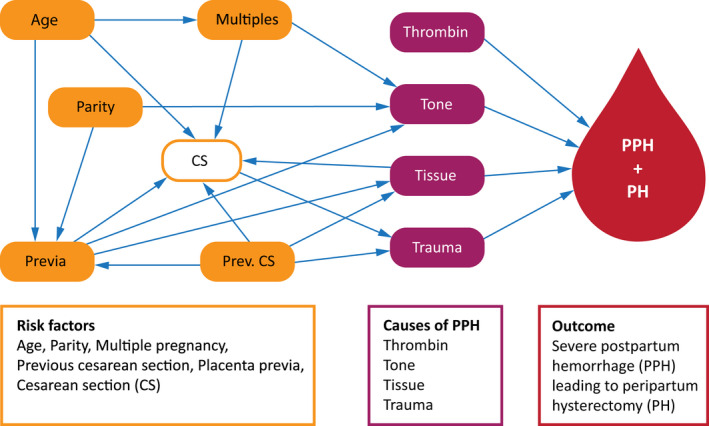
A conceptual model of peripartum hysterectomy
2.2. Ethical approval
This study was approved by the Regional Ethics Committee (REK) on January 28, 2010. The reference number is 2010/109a.
3. RESULTS
3.1. Hysterectomy rate and associated risk factors
Between 2008 and 2017, there were 96 313 deliveries at Oslo University Hospital eligible for study inclusion (Figure 2). We identified 2621 women with severe PPH, which corresponded to 2.7% of all deliveries during the study period. Among the women with severe PPH, 49 underwent a hysterectomy. We excluded seven women: one received the hysterectomy due to malignancy, two underwent hysterectomies at more than 42 days postpartum, and four were referred from other hospitals due to suspicion of PAS disorders. Thus, 42 women, 1.6% of women with severe PPH, received a hysterectomy within 42 days postpartum. The peripartum hysterectomy rate was 0.44/1000 deliveries during the study period. The majority (38/42) were performed within the first 24 h after delivery.
FIGURE 2.
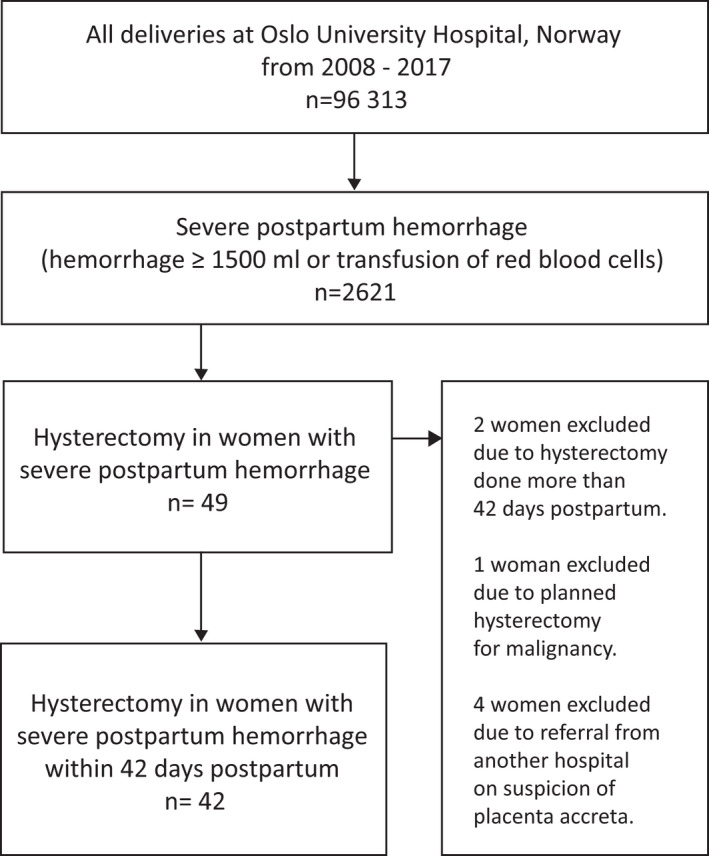
Flowchart for inclusion of women
Table 1 shows the characteristics of the study population, the hysterectomy rates and the IRRs. The majority of women who underwent a peripartum hysterectomy (n = 34/42) had required a cesarean section for delivery, but more than half of the cesarean sections were planned, for various reasons. Two of the eight women who underwent vaginal deliveries received vacuum extractions. Factors associated with increased rates of hysterectomy following severe PPH were maternal age ≥30 years, parity ≥2, delivery after gestational week 37, originating from a low‐ or middle‐income country, multiple pregnancies, previous cesarean section, delivery by cesarean section and the presence of placenta previa. However, after adjustment, results of the multivariable analyses showed that, maternal age ≥40 years, previous cesarean section, multiple pregnancies and placenta previa were positively associated with peripartum hysterectomy. The sensitivity analysis, based on negative binominal regression (data not shown), showed similar IRRs, except for the variable multiple pregnancy, where the IRR did not reach significance, although the IRR was approximately the same (2.95, 95% CI 0.78–11.2).
TABLE 1.
Peripartum hysterectomy rates and incidence rate ratios according to associated factors
| Hysterectomy n (%) | Deliveries a n (%) | Hysterectomy rate per 1000 deliveries (95% CI) | Crude IRR (95% CI) | Adjusted IRR (95% CI) b | |
|---|---|---|---|---|---|
| All | 42 | 96 313 | 0.44 (0.31–0.59) | ||
| Age at delivery, years | 96 312 | ||||
| <30 | 2 (4.8) | 29 315 (30.4) | 0.07 (0.01–0.25) | 1.00 (ref) | 1.00 (ref) |
| 30–39 | 26 (61.9) | 61 762 (64.1) | 0.42 (0.27–0.62) | 6.16 (1.46–26.0) | 3.66 (0.80–16.6) |
| ≥40 | 14 (33.3) | 5235 (5.5) | 2.67 (1.46–4.49) | 39.2 (8.91–172.2) | 12.3 (2.67–56.7) |
| Gestational age, weeks | 95 907 | ||||
| ≥37 | 26 (61.9) | 89 365 (93.2) | 0.29 (0.19–0.43) | 1.00 (ref) | |
| <37 | 16 (38.1) | 6542 (6.8) | 2.45 (1.4–3.97) | 8.41 (4.51–15.7) | |
|
Parity |
96 313 | ||||
| Para 0 | 9 (21.4) | 47 682 (49.5) | 0.19 (0.09–0.36) | 1.00 (ref) | 1.00 (ref) |
| Para 1 | 14 (33.3) | 33 549 (34.8) | 0.45 (0.25–0.74) | 2.21 (0.96–5.11) | 0.91 (0.35–2.37) |
| Para ≥2 | 19 (45.3) | 15 082 (15.7) | 1.26 (0.76–1.97) | 6.67 (3.02–14.8) | 2.24 (0.88–5.69) |
|
Country of origin |
96 313 | ||||
| Norway | 17 (40.5) | 64 033 (66.5) | 0.27 (0.15–0.43) | 1.00 (ref) | |
| High income countries | 3 (7.1) | 8491 (8.8) | 0.35 (0.07–1.03) | 1.33 (0.39–4.54) | |
| LMIC | 21 (50.0) | 22 580 (23.5) | 0.93 (0.58–1.42) | 3.50 (1.85–6.64) | |
| Unknown | 1 (2.4) | 1209 (1.2) | 0.83 (0.02–4.61) | 3.12 (0.41–23.41) | |
|
Multiple pregnancy |
|||||
| No | 39 (92.9) | 94 162 (97.8) | 0.41 (0.29–0.57) | 1.00 (ref) | 1.00 (ref) |
| Yes | 3 (7.1) | 2151 (2.2) | 1.39 (0.29–4.08) | 3.37 (1.04–10.9) | 3.68 (1.12–12.1) |
|
Previous CS |
96 313 | ||||
| No | 20 (47.6) | 86 779 (90.1) | 0.23 (0.14–0.36) | 1.00 (ref) | 1.00 (ref) |
| Yes | 22 (52.4) | 9534 (9.9) | 2.31 (1.45–3.49) | 9.10 (4.97–16.7) | 4.69 (2.27–9.66) |
|
Mode of delivery |
96 313 | ||||
| Vaginal delivery | 8 (19.0) | 77 038 (80.0) | 0.1 (0.04–0.2) | 1.00 (ref) | |
| CS | 34 (81.0) | 19 275 (20.0) | 1.76 (1.22–2.46) | 17.0 (7.87–36.7) | |
|
Placenta previa |
96 313 | ||||
| No | 28 (66.7) | 95 942 (99.6) | 0.29 (0.19–0.42) | 1.00 (ref) | 1.00 (ref) |
| Yes | 14 (33.3) | 371 (0.4) | 37.7 (20.6–63.3) | 129.3 (68.1–245.6) | 71.6 (36.7–139.7) |
Statistically significant results (P < 0.05) are presented in bold.
CI, confidence interval; IRR, incidence rate ratio; LMIC, low‐ and middle‐income countries; CS, cesarean section.
Number of deliveries at Oslo University Hospital provided by+ the Medical Birth Registry of Norway.
Adjusted for age at delivery, parity, previous cesarean section, multiple pregnancy and placenta previa.
3.2. Trends in incidence rates
Two previous studies reported the incidences of peripartum hysterectomies in Norway. The estimated incidences were 0.16/1000 deliveries in 1981–19967 and 0.29/1000 deliveries in 2009–2012. 10 In the present study, the incidence was higher, at 0.44/1000 deliveries in 2008–2017 (P = 0.0015 and P = 0.09, respectively), although the difference was not significant in the most recent study, where the study period overlapped with ours.
3.3. Causes of PPH
The causes of severe PPHs that led to hysterectomies are presented in Table 2. PAS disorders were diagnosed clinically in 22 women (52.4%) and all were histologically confirmed. In six women (6/22) the condition was suspected before delivery; all of these occurred in the last 3 years of the study period (2015–2017). All six women with suspected PAS disorders had a previous cesarean section and placenta previa. Among the 14 women with placenta previa, 11 had confirmed PAS disorders. Uterine atony was identified as a cause of PPH in 19 women (45%) and it was the sole cause in 13 women (31%). Trauma was diagnosed as a cause in five women; of these, four received a hysterectomy due to uterine ruptures. Two of these four women had no previous uterine surgery, but they had both experienced induced labor and large lateral ruptures. Three women were diagnosed with disseminated intravascular coagulation, and in one woman, this condition was the sole cause of PPH. Multiple pregnancy occurred in three women, all pregnant with twins, delivered by cesarean section around week 37, and atony was the cause of severe PPH. In eight women, overlapping causes were reported; mainly atony combined with another condition.
TABLE 2.
Causes of severe postpartum hemorrhage leading to hysterectomy, n = 42
| Cause a | n (%) |
|---|---|
| Tone | |
|
Uterine atony Uterine atony alone (no other coexisting causes) |
19 (45.2) 13 (31.0) |
| Tissue | |
Placenta accrete spectrum disorder
|
22 (52.4) 2 (4.8) 13 (31.0) 7 (16.7) |
| Trauma | |
| Uterine rupture | 4 (9.5) |
| Surgical trauma | 1 (2.4) |
| Thrombin | |
| Disseminated intravascular coagulation | 3 (7.1) |
Overlapping causes of postpartum hemorrhage were identified in seven women (16.7%).
3.4. Management and complications
The majority of severe PPH cases were treated with a multimodal management regimen, before resorting to a hysterectomy (Table 3). However, five women (12%) did not receive either uterotonics or surgical management. Nine women, including seven with PAS disorders, received resuscitative endovascular balloon occlusions of the aorta, and all nine were performed in the last 5 years of the study (2013–2017).
TABLE 3.
Management of severe postpartum hemorrhage in women with peripartum hysterectomy according to cause
| All causes n = 42 | Atony aloneas cause n = 13 | PAS as cause n = 22 | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Medications | |||
| Prophylactic oxytocin | 36 (85.7) | 13 (100) | 18 (81.8) |
| Oxytocin infusion | 28 (66.7) | 12 (92.3) | 11 (50.0) |
| Prostaglandins | 15 (35.7) | 10 (76.9) | 2 (9.1) |
| Ergometrin | 7 (16.7) | 2 (15.4) | 3 (13.6) |
| Tranexamic acid | 21 (50.0) | 8 (61.5) | 9 (40.9) |
| Surgical procedures | |||
| Curettage | 7 (16.7) | 3 (23.1) | 3 (13.6) |
| Intrauterine balloon | 12 (28.6) | 6 (46.2) | 5 (22.7) |
| Uterine suture | 6 (14.3) | 1 (7.7) | 3 (13.6) |
| Ligation of uterine arteries | 5 (11.9) | 3 (23.1) | 1 (4.5) |
| Embolization of pelvic arteries | 9 (21.4) | 5 (38.5) | 2 (9.1) |
| REBOA | 9 (21.4) | 1 (7.7) | 7 (31.8) |
| Transfusion of blood products | |||
| Red blood cells ≥8 units | 26 (61.9) | 9 (69.2) | 11 (50.0) |
| Red blood cells ≥12 units | 16 (38.1) | 4 (30.8) | 8 (36.4)) |
| Plasma ≥8 units a | 15 (35.7) | 4 (30.8) | 7 (31.8) |
PAS, placenta accrete spectrum disorders; REBOA, resuscitative endovascular balloon occlusion of aorta.
One missing.
All women received packed red blood cells (packed in a saline‐adenine‐glucose‐mannitol solution; mean volume administered 10.3 units, range 1–38 units), and 26 women received more than eight units. Additional fresh frozen plasma was administered to 36 women (range 2–23 units). Thrombocyte concentrate was administered to 25 women (range 1–10 units). The mean length of hospital stay was 9.0 days (range 3–40 days). Six women stayed in the intensive care unit for 2 or more days.
Severe complications were identified in 15 women (Table 4). Five women had more than one severe complication, and one woman experienced a thrombotic event, a bladder injury and a unilateral oophorectomy. The majority of women with severe complications (11/15) had a PAS disorder. One of the women with a twin pregnancy ended up with an unintended oophorectomy, but no other complications were registered in the three multiple pregnancies. No one died due to PPH during the study period.
TABLE 4.
Severe complications in women with a peripartum hysterectomy due to severe postpartum hemorrhage, n = 42
| Severe complication | n (%) |
|---|---|
| Women with complications | 15 (35.7) a |
| Thrombotic event | 4 (9.5) |
| Bladder injury | 4 (9.5) |
| Unintended unilateral oophorectomy | 4 (9.5) |
| Sepsis | 2 (4.8) |
| Pain syndrome | 2 (4.8) |
| Severe depression | 2 (4.8) |
| Sheehan syndrome | 2 (4.8) |
| Resuscitation | 1 (2.4) |
| Rupture of fascia | 1 (2.4) |
Five women had more than one complication.
4. DISCUSSION
Studies of rare but severe complications of childbirth are particularly relevant in high‐income countries, where maternal mortality rates are low. This retrospective, hospital‐based study has made an important contribution to existing knowledge about peripartum hysterectomies. We found a higher incidence of peripartum hysterectomy than reported from Norway previously, and the rate of hysterectomy in women delivered by cesarean section was similar to rates in countries with higher frequencies of cesarean deliveries. Another important finding in our study was that more than 50% of the hysterectomies performed after a severe PPH occurred in women with PAS disorders, and these women experienced the majority of complications.
In this study, 1.6% of women with severe PPHs required a hysterectomy. This rate was somewhat lower than the 2.7% rate observed among women with PPHs studied in the WOMAN trial, conducted in Europe and the Americas. 4 We found that the overall rate of peripartum hysterectomies was 0.44/1000 deliveries, close to the rate of 0.52/1000 deliveries reported in a recent study that included nine European countries. 9 However, compared with previous studies from Norway, 7 , 10 we observed a higher incidence of peripartum hysterectomies. This finding may indicate a true increase in recent years or may reflect different study populations and problems with generalizability.
The rates of peripartum hysterectomy associated with both concurrent and prior cesarean sections was surprisingly similar to findings from studies conducted in countries with higher frequencies of cesarean sections. 16 , 22 , 23 For example, in Italy the proportion of women with a previous cesarean section in the reference group (16.8%) was higher than the proportion observed in our reference group (9.9%). However, our finding that a previous cesarean section was a risk factor for hysterectomy was consistent with findings from a study conducted in Italy: indeed, our hysterectomy rate among women with previous cesarean sections was comparable to that observed in Italy (2.31 and 2.98 hysterectomies/1000 deliveries, respectively). 23 This is an important clinical finding because it supports the notion that a previous cesarean section is an independent risk factor for hysterectomy even in a country with a low cesarean section rate. Thus, these findings underlined the importance of maintaining a low rate of cesarean sections.
We found an increased risk with placenta previa and being pregnant with multiples, both well known risk factors in severe PPH and peripartum hysterectomy. 6 , 16 However, when considering the increased risk for hysterectomy with maternal age ≥40 years and parity ≥2, the risk might also be influenced by the surgeon, as the clinical threshold for performing a hysterectomy in an older woman with two or more children may be lower than in primiparas.
For the last two decades, PAS disorders have emerged as the main cause of peripartum hysterectomies, particularly in high‐income countries. 5 , 9 , 10 , 22 , 23 Despite the fact that PAS disorders remain uncommon in Norway, 24 they were the main cause of hysterectomies in our study. In our study of severe PPH, confirmed PAS disorders were the cause of severe PPH in 2.9% (76/2545). Due to the rarity of the PAS diagnosis, a hysterectomy was probably the preferred management, compared with conservative strategies for sparing the uterus. Thus, this preference contributed to the high proportion of PAS disorders among women who required peripartum hysterectomies. In 27% of women with PAS disorders, the condition was suspected antenatally, and all were reported in the last 3 years of the study. This finding could be due to the increased focus on PAS disorders in recent years. During the study, Norway had no national guideline on the management of invasive placentas. The present national guideline, published in 2020, 25 recommended that pregnant women with previous cesarean sections and anterior placenta previas should be examined with PAS disorders specifically in mind. Hopefully, this guideline will increase the antenatal diagnosis of PAS disorders.
Overall, severe complications occurred in around 35% of the women in our study. This rate was similar to the 32.2% of women with major complications reported in a previous Nordic study. 10 Similar to studies by Knight et al.5 and Kallianidis et al., 26 we found a low number of thrombotic events. All four women that experienced a thrombotic event had a histologically verified placenta increta, surgery that lasted over 90 min, and a resuscitative endovascular balloon occlusion of the aorta. Almost every woman in the study had long‐lasting surgery; thus, we speculated that a PAS disorder or an aortic balloon occlusion probably contributed to the thrombosis. A study conducted in South America 27 described three cases of arterial thrombosis after an aortic balloon occlusion, and a recent study by Matsuo et al. demonstrated that the risk of arterial thrombosis increased after an intra‐arterial balloon occlusion during a cesarean hysterectomy. 28
Furthermore, bladder injuries exclusively occurred in women with PAS disorders. Knight et al.5 showed that women with PAS disorders had a 3.41 higher risk of bladder damage after a hysterectomy, compared with women that required peripartum hysterectomies due to other causes. Jakobsson et al.10 found fewer women with bladder damage (3.3%) than we observed in the present study (9.5%), but their cohort was smaller and included a smaller proportion of women with PAS disorders.
This study had some limitations. First, the results may lack generalizability. The study mainly included women from Oslo, the largest city in Norway, and its surrounding area; thus, the cohort may not reflect the entire country. Oslo University Hospital is a tertiary referral hospital for the entire country, and women may have been referred here due to prematurity, maternal heart disease or fetal malformations; thus, women with existing morbidities were included in our material. Secondly, although Oslo University Hospital had the largest labor ward in Norway, and the study time‐frame was quite long, the number of women with severe PPH and peripartum hysterectomies was low. Thus, the confidence intervals were broad, which made the results assumptive, rather than conclusive.
The main strength of the study was the detailed information about clinical, maternal and fetal characteristics, which was collected directly from hospital records. Peripartum hysterectomies are not routinely recorded in the Medical Birth Registry of Norway and thus had to be studied by collecting retrospective data from hospital records. However, this provided an opportunity to explore every case thoroughly. In addition, the combined aggregated population data in the Medical Birth Registry of Norway provided a unique opportunity to perform multivariate analyses, which suggested causal associations, although the number of cases was small. The sensitivity analysis showed the robustness of the significant findings, independent of the choice of regression model, although the variable multiple pregnancy was no longer a significant risk factor. This illustrates the problem with samples containing low number of cases, as only three women had a multiple pregnancy (3/42). Moreover, this retrospective study did not require consent from the participating women; thus all identified cases could be included (ie no selection bias).
5. CONCLUSION
The present study showed that the rate of peripartum hysterectomies after a severe PPH at Oslo University Hospital was 0.44/1000 deliveries in the years 2008–2017. This rate was higher than previous rates reported in Norway. Risk factors for peripartum hysterectomy due to severe PPH included previous cesarean section, multiple pregnancies and placenta previa. PAS disorders were the main cause of severe PPHs that led to hysterectomies, and they were associated with the majority of complications after surgery.
CONFLICT OF INTEREST
Lill T. Nyfløt has received payment for lectures on postpartum hemorrhage from CSL Behring. None of the other authors has any conflict of interest.
AUTHOR CONTRIBUTIONS
SP contributed to protocol/project development, data collection, data analysis, and manuscript writing and editing. LTN contributed to protocol/project development, data collection, and manuscript writing and editing. RSF contributed to data analysis and manuscript writing and editing. SV contributed to protocol/project development and manuscript writing and editing.
ACKNOWLEDGMENTS
Pernille Frese for assisting with design of tables and figures.
Pettersen S, Falk RS, Vangen S, Nyfløt LT. Peripartum hysterectomy due to severe postpartum hemorrhage: A hospital‐based study. Acta Obstet Gynecol Scand. 2022;101:819–826. doi: 10.1111/aogs.14358
Funding informationThe study was funded by South‐Eastern Norway Regional Health Authority; project number 2018024.
REFERENCES
- 1. Godson C. Porro’s Operation. Br Med J. 1884;1:142‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Say L, Souza JP, Pattinson RC. Maternal near miss‐‐towards a standard tool for monitoring quality of maternal health care. Best Pract Res Clin Obstet Gynaecol 2009;23:287–96, Maternal near miss – towards a standard tool for monitoring quality of maternal health care. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization . Trends in maternal mortality: 1990 to 2015 Geneva, Switzerland: World Health Organization; 2015 [Available from: http://www.who.int/reproductivehealth/publications/monitoring/maternal‐mortality‐2015/en/.
- 4. Huque S, Roberts I, Fawole B, Chaudhri R, Arulkumaran S, Shakur‐Still H. Risk factors for peripartum hysterectomy among women with postpartum haemorrhage: analysis of data from the WOMAN trial. BMC Pregnancy Childbirth. 2018;18:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knight M. Peripartum hysterectomy in the UK: management and outcomes of the associated haemorrhage. BJOG. 2007;114:1380‐1387. [DOI] [PubMed] [Google Scholar]
- 6. Vandenberghe G, Guisset M, Janssens I, et al. A nationwide population‐based cohort study of peripartum hysterectomy and arterial embolisation in Belgium: results from the Belgian Obstetric Surveillance System. BMJ Open. 2017;7:e016208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Engelsen IB, Albrechtsen S, Iversen OE. Peripartum hysterectomy‐incidence and maternal morbidity. Acta Obstet Gynecol Scand. 2001;80:409‐412. [PubMed] [Google Scholar]
- 8. van den Akker T, Brobbel C, Dekkers OM, Bloemenkamp KW. Prevalence, Indications, Risk Indicators, and Outcomes of Emergency Peripartum Hysterectomy Worldwide: A Systematic Review and Meta‐analysis. Obstet Gynecol. 2016;128:1281‐1294. [DOI] [PubMed] [Google Scholar]
- 9. Kallianidis AF, Maraschini A, Danis J, et al. Epidemiological analysis of peripartum hysterectomy across 9 European countries. Acta Obstet Gynecol Scand. 2020;99:1364‐1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jakobsson M, Tapper AM, Colmorn LB, et al. Emergency peripartum hysterectomy: results from the prospective Nordic Obstetric Surveillance Study (NOSS). Acta Obstet Gynecol Scand. 2015;94:745‐754. [DOI] [PubMed] [Google Scholar]
- 11. Knight M, Callaghan WM, Berg C, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth. 2009;9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Stralen G, von Schmidt Auf Altenstadt JF, Bloemenkamp KW, van Roosmalen J, Hukkelhoven CW. Increasing incidence of postpartum hemorrhage: the Dutch piece of the puzzle. Acta Obstet Gynecol Scand. 2016;95:1104‐1110. [DOI] [PubMed] [Google Scholar]
- 13. Rossen J, Okland I, Nilsen OB, Eggebo TM. Is there an increase of postpartum hemorrhage, and is severe hemorrhage associated with more frequent use of obstetric interventions? Acta Obstet Gynecol Scand. 2010;89:1248‐1255. [DOI] [PubMed] [Google Scholar]
- 14. Bodelon C, Bernabe‐Ortiz A, Schiff MA, Reed SD. Factors associated with peripartum hysterectomy. Obstet Gynecol. 2009;114:115‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sakse A, Weber T, Nickelsen C, Secher NJ. Peripartum hysterectomy in Denmark 1995‐2004. Acta Obstet Gynecol Scand. 2007;86:1472‐1475. [DOI] [PubMed] [Google Scholar]
- 16. Campbell SM, Corcoran P, Manning E, Greene RA. Peripartum hysterectomy incidence, risk factors and clinical characteristics in Ireland. Eur J Obstet Gynecol Reprod Biol. 2016;207:56‐61. [DOI] [PubMed] [Google Scholar]
- 17. Anderson JM, Etches D. Prevention and management of postpartum hemorrhage. Am Fam Physician. 2007;75:875‐882. [PubMed] [Google Scholar]
- 18. dagitty.net. Dagitty [28.02.2022]. Available from: http://www.dagitty.net/dags.html. Accessed February 10.2022
- 19. Victora CG, Huttly SR, Fuchs SC, Olinto MT. The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. Int J Epidemiol. 1997;26:224‐227. [DOI] [PubMed] [Google Scholar]
- 20. Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. Am J Obstet Gynecol. 2017;217:167‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ldt MS. Comparioson of two rates [Version 20.015]. Available from: https://www.medcalc.org/calc/rate_comparison. Accessed February 10, 2022
- 22. de la Cruz CZ, Thompson EL, O’Rourke K, Nembhard WN. Cesarean section and the risk of emergency peripartum hysterectomy in high‐income countries: a systematic review. Arch Gynecol Obstet. 2015;292:1201‐1215. [DOI] [PubMed] [Google Scholar]
- 23. Maraschini A, Lega I, D’Aloja P, Buoncristiano M, Dell’Oro S, Donati S. Women undergoing peripartum hysterectomy due to obstetric hemorrhage: A prospective population‐based study. Acta Obstet Gynecol Scand. 2020;99:274‐282. [DOI] [PubMed] [Google Scholar]
- 24. Colmorn LB, Petersen KB, Jakobsson M, et al. The Nordic Obstetric Surveillance Study: a study of complete uterine rupture, abnormally invasive placenta, peripartum hysterectomy, and severe blood loss at delivery. Acta Obstet Gynecol Scand. 2015;94:734‐744. [DOI] [PubMed] [Google Scholar]
- 25. NGF. Invasiv placenta, placenta previa og vasa previa: Dnlf; 2020 [Available from: https://www.legeforeningen.no/foreningsledd/fagmed/norsk‐gynekologisk‐forening/veiledere/veileder‐i‐fodselshjelp/invasiv‐placenta‐placenta‐previa‐og‐vasa‐previa/. Accessed October 22, 2021
- 26. Kallianidis AF, Maraschini A, Danis J, et al. Management of major obstetric hemorrhage prior to peripartum hysterectomy and outcomes across nine European countries. Acta Obstet Gynecol Scand. 2021;100:1345‐1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nieto‐Calvache AJ, Hidalgo‐Cardona A, Lopez‐Girón MC, et al. Arterial thrombosis after REBOA use in placenta accreta spectrum: a case series. J Matern Fetal Neonatal Med. 2020. Nov;18:1‐4. doi: 10.1080/14767058.2020.1846178 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28. Matsuo K, Matsuzaki S, Vestal NL, et al. Utilizations and outcomes of intra‐arterial balloon occlusion at cesarean hysterectomy for placenta accreta spectrum. Acta Obstet Gynecol Scand. 2021;100:2234‐2243. [DOI] [PubMed] [Google Scholar]


