Abstract
Introduction
This study aimed to demonstrate the safe and effective use of the Versius surgical system (CMR Surgical, Cambridge, UK) in robot‐assisted total laparoscopic hysterectomy. This surgical robot was developed iteratively with input from surgeons to improve surgical outcomes and end‐user experience. We report data from the gynecology cohort of an early clinical trial designed in broad alignment with IDEAL‐D (Idea, Development, Exploration, Assessment, Long‐term follow‐up – Devices) stage 2b (Exploration).
Material and methods
The study is registered in the Indian clinical trials register (CTRI/2019/02/017872). Adult women requiring total hysterectomy who provided informed consent and met the eligibility criteria underwent procedures at one of three hospitals in India. Five surgeons performed robot‐assisted total laparoscopic hysterectomies using the device from March 2019 to September 2020. The primary endpoint was rate of unplanned conversion to conventional laparoscopic or open surgery. Adverse events were adjudicated by an independent clinical events committee using endoscope video recordings and clinical notes.
Results
In total, 144 women underwent surgery (median age: 44 years [range: 28–78]; median body mass index 25.8 kg/m2 [range: 14.3–47.8]). The rate of unplanned conversion to conventional laparoscopy was 2/144 (1.4%); neither conversion was device related. No surgery was converted to open. In total, 13 adverse events occurred among seven (4.9%) patients, comprising seven serious adverse events and six adverse events. One serious adverse event was deemed device‐related. Two patients were readmitted to hospital within 30 days; both made a full recovery. No patients died within 90 days of surgery.
Conclusions
The device provides a safe and effective option for total laparoscopic hysterectomy; these findings support its continued implementation in larger patient cohorts and expansion in other major minimal access indications.
Keywords: clinical trial, gynecology, minimal access surgery, robot‐assisted surgery, robotic surgical system, surgical robot
Abbreviations
- AE
adverse event
- ASA
American Society of Anesthesiologists
- BMI
body mass index
- CEC
clinical events committee
- IDEAL‐D
Idea, Development, Exploration, Assessment, Long‐term follow‐up – Devices
- MAS
minimal access surgery
- OR
operating room
- SAE
serious adverse event
Key message.
This prospective early clinical evaluation demonstrated the safe and effective performance of a next‐generation surgical device in live‐human robot‐assisted total laparoscopic hysterectomy. There were no deaths, few conversions, and low rates of intra‐ and postoperative complications.
1. INTRODUCTION
Minimal access surgery (MAS) offers numerous advantages to patients requiring gynecological procedures. 1 , 2 Substantial hospital cost savings have been projected with MAS because complication rates are lower than with open surgery. 3 However, surgeons performing MAS face extensive learning curves.
In conventional MAS, constrained instrument movements and wrist articulation, tremor transmission, and two‐dimensional visual display make the surgical steps requiring precision especially challenging. 4 , 5 , 6 Conventional MAS may also be physically detrimental to surgeons because of the inherent ergonomic challenges. 7 , 8 These factors may have limited the adoption of MAS for common gynecological procedures such as hysterectomy and sacrocolpopexy. 9 , 10 Robot‐assisted surgery offers improved dexterity, precision, and three‐dimensional visualization 11 , 12 and can extend MAS feasibility for patients with complex pathologies or higher body mass indices (BMIs). 13
Versius (CMR Surgical, Cambridge, UK), a next‐generation robotic surgical system, aims to improve patient outcomes and end‐user experience. 14 , 15 , 16 , 17 The device comprises practical‐sized mobile bedside units to provide maximum flexibility in the operating room (OR). Each wristed instrument has seven degrees of motion inside the patient and is supported by a flexible, human mimic robotic arm. This level of instrument articulation provides flexibility in port placement, which may be particularly advantageous for patients with different BMIs. The surgeon can operate in a seated or standing position, and the system features an open‐console design, negating some of the ergonomic challenges associated with conventional MAS without limiting communication between the surgeon and the surgical team. Further, the controller handgrip was based on that of a games console for optimal ergonomic design.
The device was developed in broad alignment with the IDEAL‐D (Idea, Development, Exploration, Assessment, Long‐term follow‐up – Devices) recommendations throughout its evolution. 15 , 16 , 17 , 18 , 19 , 20 First, its design was based on testing by and feedback from end users to better meet their needs, 16 and usability was demonstrated early in the development process. 15 Several procedures were then successfully performed in cadaver and live porcine studies, 14 , 17 , 18 and a purpose‐designed training program was validated. 21 A small number of first‐in‐human minor surgeries were then safely completed. 22
This prospective, early clinical study broadly aligns with stage 2b (Exploration) and aimed to provide a full device safety and performance analysis from a larger cohort of more than 120 women requiring total laparoscopic hysterectomy.
2. MATERIAL AND METHODS
2.1. Surgeons
The participating surgeons were accredited, practicing, high‐volume consultant gynecology surgeons with extensive experience in MAS. Surgeons had no prior experience using other robotic systems and had limited or no experience using this robotic device in humans prior to the clinical trial (two or fewer cases per surgeon). In accordance with the training protocol, all participating surgeons completed approximately 10 h of online training and a minimum 6 h of simulator training and passed the validated 3.5‐day training program immediately prior to the start of the study. 21 Surgeons completed procedures at two study sites in Maharashtra, India: the Deenanath Mangeshkar Hospital and Research Center (March 12, 2019, to January 24, 2020) and the HCG Manavata Cancer Center (October 30, 2019, to January 20, 2020). Following a regulatory request for “final finished device” data, an additional 25 cases were performed as part of a bridging study completed at both centers and at an additional third center (because of the COVID‐19 pandemic), the Healing Hands Clinic, Pune, Maharashtra, India, between August 24, 2020, and September 04, 2020.
2.2. Patients
Eligible women requiring total hysterectomy, aged ≥18 years, and able to provide informed consent were prospectively enrolled in the study. Exclusion criteria included pregnancy and any of the following conditions: uncontrolled hypertension and/or diabetes mellitus (blood glucose concentration >200 mg/dl), known presence of regional and/or distant metastases, medical instability prior to surgery, and any obvious contraindications to abdomino‐pelvic surgery. Patients were excluded if their physical status was American Society of Anesthesiologists (ASA) class III or higher. 23 In June 2020, this exclusion criterion was changed to ASA class IV or higher to extend eligibility once several procedures had been safely performed. Eligible participants were identified from study hospital surgical lists and approached directly by their surgeon or clinical team. After being provided with relevant study information, patients provided written informed consent to participate in the study, and audio‐visual consent was recorded for patients who enrolled at the Deenanath Mangeshkar Hospital and Research Center.
2.3. Study design
Patients completed pre‐operative screening, then underwent total hysterectomy on Day 1 (the surgical procedure steps are provided in Table S1). Perioperative care was uniform across all patients unless adverse events (AEs) occurred. Following surgery, patients were discharged from hospital when deemed safe by the operating surgeon and postoperative care team and were followed‐up on postoperative Day 30 (±2 days) and Day 90 (±7 days) via telephone or clinic visit (Figure S1). Patients in the bridging study were followed up to at least Day 30 (±2 days).
2.4. Device set‐up and OR layout
The device consisted of a surgeon console, instrument bedside units (two or three instrument bedside units can be used according to procedure type and surgeon preference; three were used in this study), and one visualization bedside unit (Figure 1A+B). Energy instruments, including a Monopolar Hook and Bipolar Maryland Grasper, were used during the procedures. The most frequently used port positioning and OR layouts are illustrated in Figure 1C+D. The camera port was positioned up to 2 cm above the umbilicus on the midline, with a 5 mm robotic port on the right and left mid‐clavicular line, at the level of the umbilicus. A 5–10 mm assistant port was positioned below the umbilicus at the midline. For women with high BMI (≥30 kg/m2), the camera port was positioned below the umbilicus.
FIGURE 1.
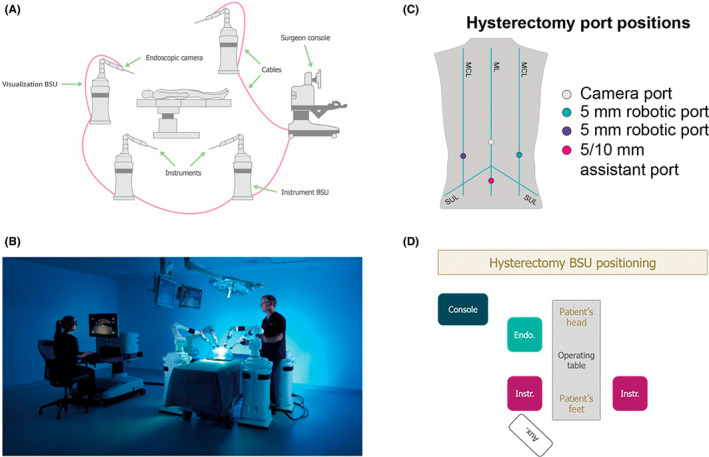
Overview of the device, port positioning, and operating room layout. Schematic representation of the setup of the device (A) and real‐world image of the device setup (B); adapted from Haig et al. 15 Common port positioning (C) with corresponding BSU positions (D); adapted from Kelkar et al. 22 The assistant port was for nonrobotic laparoscopic instruments. Umbilicus is where the ML crosses the SUL. Aux: auxiliary monitor; BSU: bedside unit; Console: surgeon console; Endo: endoscope; Instr: instrument; MCL: mid‐clavicular line; ML: midline; SUL: supine‐umbilical line
2.5. Study procedures and evaluations
The primary endpoint was the rate of unplanned conversion of robot‐assisted procedures to conventional laparoscopic or open surgery. Secondary endpoints included operative time (from first incision to skin closure), estimated intra‐operative blood loss, intra‐operative complications, return to the OR within 24 h, length of hospital stay, hospital readmission within 30 days, postoperative complications through 30 and 90 days, and mortality rate at 90 days. Uterine weight was measured postoperatively at a histopathology laboratory. As a standard protocol, length of stay was pre‐emptively extended to 4 days for some patients living a long distance from the study site because of transportation limitations if readmission was required.
2.6. Adverse events
All AEs were adjudicated by an independent clinical events committee (CEC), comprising four members who convened across a series of meetings between October 23 and November 17, 2020 (CEC member details are listed in the Acknowledgements). All postoperative AEs were graded according to the Clavien–Dindo classification, first by the operating surgeon and then by the CEC. 24 Serious AEs (SAEs) included all medical occurrences that were life‐threatening or led to death, required hospital admission, prolonged hospitalization, or resulted in persistent disability or permanent damage. Any other AE the CEC deemed “medically significant” was also classified as an SAE.
Prior to the adjudication meetings, each CEC member was provided with detailed information on each AE/SAE, as recorded by the operating surgeon. For SAEs classified by the surgeon, additional information, including ethics committee notifications, follow‐up reports, and summary operative and recovery notes, were provided. The CEC then systematically reviewed each AE regarding expectedness (expected/unexpected), seriousness (AE/SAE), and relatedness to the device (not related/possibly related/probably related/related) until consensus. Expectedness was determined based on whether the complication is typically listed on the patient consent form for hysterectomy and in view of the comorbidities present for each patient. If required, the committee had access to endoscope video recordings for all surgeries to aid their review. CEC members “upgraded” the event classification in uncertain cases.
2.7. Statistical analyses
A sample size of at least 120 women was determined sufficient to estimate conversion rates with satisfactory accuracy, with confidence intervals of an appropriate size; alpha was set at 0.05, and the conversion rate was assumed to be 1.8% based upon a literature search. The inclusion of patients in the bridging study further increased the sample size. Unless otherwise stated, continuous data summaries were used to present the number of observations, the median, and range. Data were collected in SAS format, and all analyses were performed using SAS version 9.4.
2.8. Ethical approval
This study was reviewed and approved by the Institutional Ethics Committee of the Deenanath Mangeshkar Hospital & Research Center, Erandwane, Pune, Maharashtra, India, on February 23, 2019 (Reference number ECR/15/Inst/Maha/2013/RR‐19), and the Manavata Clinical Research Institute Ethics Committee of the HCG Manavata Cancer Center, Mumbai Naka, Nashik, Maharashtra, India, on October 11, 2019 (Reference number ECR/500/Inst/MH/2013/RR‐20). The study is registered in the Indian clinical trials register (CTRI/2019/02/017872). All study activities were performed in compliance with Drugs and Cosmetic Rules 1945‐Schedule Y, Indian Council of Medical Research, and ISO14155 standards.
3. RESULTS
3.1. Patient disposition and baseline characteristics
Of the 154 consenting women who were screened, 145 were eligible for the study and 144 proceeded to robot‐assisted MAS (Figure 2). One patient underwent total hysterectomy and cholecystectomy in the same surgery. Although the two procedures were successfully completed using the device without complications or readmission, this patient was excluded from the analysis presented herein as only gynecology procedures were included. A total of 144 women underwent robot‐assisted total hysterectomy, of whom two also underwent robot‐assisted bilateral salpingo‐oophorectomies. The median age was 44 years (range: 28–78), and the median BMI was 25.8 kg/m2 (range: 14.3–47.8). The physical status of most patients was classified as ASA class I (114/144; 79.2%), and all others were considered ASA class II (30/144; 20.8%). Common indications included dysfunctional uterine bleeding (n = 46; 31.9%), uterine fibroids/leiomyoma (n = 30; 20.8%), adenomyosis (n = 13; 9.0%), and uterine prolapse (n = 9; 6.3%; Table S2). In total, 67 women (46.5%) had previously undergone abdominal/pelvic surgeries, one of which was in the previous 12 months. Median uterine weight (n = 41) was 170 g (range 50–600). The demographic and clinical characteristics of the cohort are summarized in Table 1.
FIGURE 2.
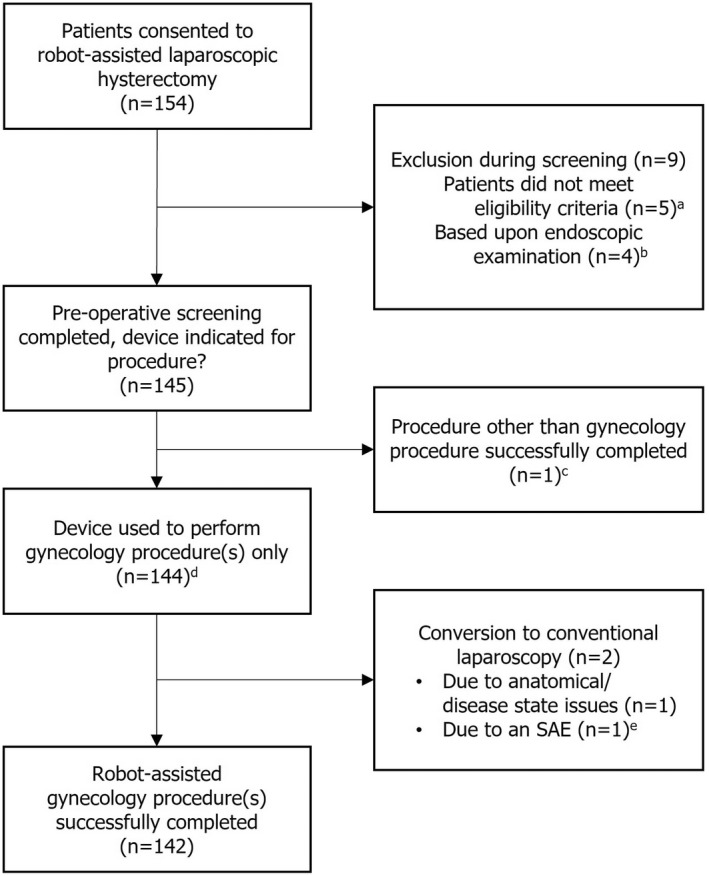
Study CONSORT diagram. aOne patient did not return to the site for screening. bBased on routine endoscopic examination, surgeons decided that alternative more familiar approaches (three conventional laparoscopic hysterectomies and one open surgery) were more appropriate for four women who had highly complex anatomies and/or disease states, such as an enlarged uterus and fundal fibroid, and multiple extensive adhesions involving the uterus, sigmoid colon, rectum, ovaries, and ureter. cOne patient underwent total hysterectomy and cholecystectomy in the same surgery, both of which were successfully completed using the device without complications or adverse events. dIncludes two patients who also underwent bilateral salpingo‐oophorectomies. eOne patient had a urinary bladder injury at the time of anterior colpotomy, which was sutured with conventional laparoscopy by a urologist. SAE, serious adverse event
TABLE 1.
Patient characteristics and surgical history
| Total laparoscopic hysterectomy (N = 144) a | |
| Characteristic | |
| Sex, female | 144 (100) |
| Age (years) | 44 (28–78) |
| Height (cm) | 154.0 (138–171) |
| Weight (kg) | 61.5 (34.4–115.1) |
| BMI (kg/m2) | 25.8 (14.3–47.8) |
| <18.5 | 9 (6.3) |
| 18.5–<25 | 53 (36.8) |
| 25–<30 | 53 (36.8) |
| 30–<40 | 26 (18.1) |
| ≥40 | 3 (2.1) |
| ASA status | |
| Class I | 114 (79.2) |
| Class II | 30 (20.8) |
| Diagnoses b | |
| Abdominal abscess | 2 (1.4) |
| Adenomyosis | 13 (9.0) |
| Cervicitis | 7 (4.9) |
| Dysfunctional uterine bleeding | 46 (31.9) |
| Endometriosis | 5 (3.5) |
| Menorrhagia | 8 (5.6) |
| Ovarian cyst | 2 (1.4) |
| Uterine fibroids/leiomyoma | 30 (20.8) |
| Uterine prolapse | 9 (6.3) |
| Surgical history | |
| Previous abdominal/pelvic surgeries | |
| Yes | 67 (46.5) |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index.
Data are expressed as median (range) or n (%) unless specified otherwise.
Patients could have had more than one diagnosis; all diagnoses are listed in Table S2.
3.2. Intra‐operative endpoints
Overall, 142/144 women (98.6%) underwent a successful total laparoscopic hysterectomy using the device (Figure 2). No procedure was converted to open surgery. Two (1.4%) surgeries were completed using conventional laparoscopy. One of the conversions was secondary to a bladder injury, which occurred at the time of anterior colpotomy (Table 2). This was sutured by a urologist who had no training on the device and therefore used conventional laparoscopy. The CEC determined that this was a recognized (expected) complication of that stage of the procedure and was not related to the device. The second converted surgery was performed on an obese patient (BMI 47.8 kg/m2) who had multiple large uterine fibroids and had previously undergone cesarean section, resulting in an adherent bladder and dense omental adhesions. The combination of these findings led to a very difficult surgery and so, in line with the principles of the IDEAL‐D collaboration, 19 the surgeon decided that the safest option for the patient was to convert to their more familiar conventional laparoscopic approach. There were no AEs in this case.
TABLE 2.
Adverse events
| Surgery number | AE | Days after procedure | Seriousness (AE/SAE) | CDC grade | Converted | Relatedness to device | Expectedness |
| 41 | Acute urinary tract infection | 16 | SAE | II | No | Not related | Expected |
| 43 | Vaginal bleeding | 25 | SAE | IIIb | No | Related | Expected |
| Left ureteric duplication requiring later surgery | 74 | SAE | IIIb | Not related | Expected | ||
| 63 | Breathlessness | 1 | SAE | IVa | No | Not related | Expected |
| 88 | Urinary tract infection | 15 | AE | II | No | Not related | Expected |
| Tingling sensation in both lower limbs | 25 | AE | II | Not related | Unexpected | ||
| Lower backache radiating to both lower limbs | 25 | AE | II | Not related | Expected | ||
| 103 | Burning sensation in epigastric region | 5 | AE | I | No | Not related | Expected |
| Dysuria and increased frequency of micturition | 9 | AE | II | Not related | Expected | ||
| Pain in lower abdomen | 10 | AE | I | Not related | Expected | ||
| 107 | Breathlessness | <1 | SAE | IVa | No | Not related | Expected |
| Diabetic ketoacidosis | 3 | SAE | IVa | Not related | Expected | ||
| 117 | Urinary bladder injury | Intra‐operative | SAE | NA | Conventional laparoscopy | Not related | Expected |
Abbreviations: AE, adverse event; CDC, Clavien–Dindo classification 27 ; NA, not applicable; SAE, serious adverse event.
Median time from incision to skin closure was 158.5 minutes (range: 50–385) (Figure 3A). Technical issues with the device (including an instrument not performing and an alarm sounding during surgery) in one surgery led to the longest recorded operative time; this procedure was not converted, and there were no complications or AEs in this patient at any time during the surgery or follow‐up period. Median operative time was 191.5 minutes (range: 67–280) for the first 20 cases and 147 minutes (range: 90–273) for the last 20 cases. No patient lost more than 500 ml of blood during their surgery, and blood loss of less than 5 ml was noted in 18 patients (12.5%; Figure 3B). No patient required a blood transfusion during or after surgery.
FIGURE 3.
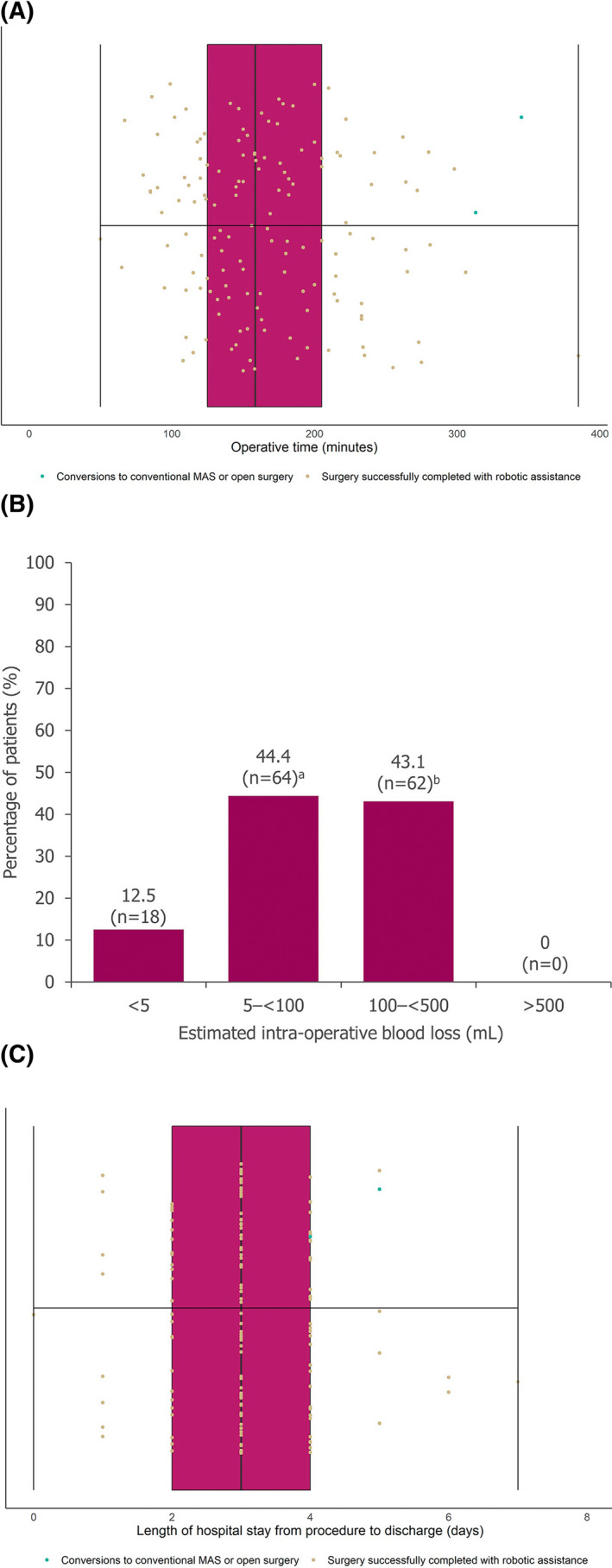
Operative time, intra‐operative blood loss, and length of hospital stay. Operative time from first incision to skin closure (A), estimated intra‐operative blood loss (B), length of hospital stay from day procedure performed to patient discharge from hospital (C). For A and C, middle vertical lines represent the medians, left and right box edges represent the first and third quartiles, and lower and upper whiskers extend to the respective lowest and highest values. aIncludes patients with estimated blood loss recorded as <100 ml. bIncludes patients with estimated blood loss recorded as <500 ml
3.3. Postoperative endpoints
The median length of hospital stay, from admission to discharge, was 3 days (range: 1–9), and the median length of postoperative stay was 3 days (range: 0–7; Figure 3C); 72.2% of patients were discharged within 3 days of their surgery (104/144). The postoperative stay of 7 days for one patient was due to transportation limitations, as the patient lived a long distance from the hospital. This patient had no complications or AEs at any time in the study. No patient returned to the OR within 24 h of their surgery. There were no deaths within the 90‐day follow‐up period.
3.4. AEs
Of the 144 women who underwent total hysterectomy, there were 13 AEs in seven patients (4.9%), comprising seven SAEs in five patients and six non‐serious AEs in two patients (Table 2). One SAE was determined by the CEC to be related to the device; the surgeon experienced difficulty when suturing, and the patient had a vaginal bleed 25 days later. Consensus was that postoperative vaginal bleeding is a well‐described complication of hysterectomy and may have been due to other complications such as infection. This patient was readmitted to hospital within the 30‐day follow‐up period. All of the other AEs and SAEs were judged as unrelated to the device. The other SAEs included acute urinary tract infection, breathlessness, and diabetic ketoacidosis. The patient with an acute urinary tract infection was the only other to be readmitted to hospital within the 30‐day follow‐up period, and this patient was readmitted for a second time with the same SAE within the 90‐day follow‐up period. Left ureteral duplication was found incidentally in one patient and required a separate surgery at a later date (which was classified as an SAE). The SAEs were judged as expected based on the comorbidities for each patient and in line with complications commonly observed following conventional laparoscopic total hysterectomy (which were listed on the patient consent form). The AEs included urinary tract infection, dysuria and increased frequency of micturition, burning sensation in the epigastric region, and lower abdominal pain, none of which were unexpected. The only unexpected AE was tingling sensation in both lower limbs in one patient.
As described, two patients were readmitted to hospital within 30 days of their surgery because of postoperative complications; one of these patients was readmitted for a second time in the 90‐day follow‐up period. Both patients made a full recovery within the 90‐day period.
4. DISCUSSION
This early clinical study demonstrated the safe and effective use of the device for major gynecology surgery in women with varied diagnoses and a range of BMIs and uterine weights. The rate of unplanned conversion to conventional MAS was very low at 1.4%, and no surgery was converted to open; surgeons and their teams were able to complete 98.6% of procedures as planned using the device. Very few patients had AEs. Estimated intra‐operative blood loss, if any, was minimal. Comparable to previously reported postoperative lengths of hospital stay following robot‐assisted and conventional laparoscopic hysterectomy, 25 , 26 all patients in this study were discharged within 1 week of their surgery, with the majority discharged within 3 days. No patient returned to the OR within 24 h of surgery, and the 90‐day mortality rate was 0.0%. The rate of hospital readmittance within 30 days was 1.4%. The postoperative vaginal bleed that led to readmission in one patient was determined as related to the device. However, relatedness did not necessarily indicate causation, and the bleed occurred more than 3 weeks after the surgery, possibly due to an infection.
This study provides a full safety and performance evaluation of a novel robotic surgical device in the early clinical setting. All AEs were thoroughly reviewed by the CEC using surgery endoscope video recordings and patient hospital records to ensure independent and consensus‐based adjudication with respect to seriousness of events and their relatedness to the device. Independent video analysis allows for complete transparency when evaluating device safety. It is anticipated that post‐surgery video analysis or implementation of a “surgical black box” in the OR may become standard practice in the future to objectively assess surgical team performance and identify ways to further improve patient safety. 27
The rate of operative complications/AEs was comparatively low; in the eVALuate and VALUE studies, the two largest studies to date in women undergoing a conventional laparoscopic hysterectomy, operative complications were observed in 11.1% and 6.1% of patients, respectively. 28 , 29 Perioperative morbidity outcomes observed in this early clinical study were comparable to those in data reported in other robot‐assisted hysterectomy studies. 30 , 31 , 32 The median operative time was longer than expected from the published literature, 28 at approximately 2 h 40 min. However, as surgeons and surgical teams had limited prior experience using the device, it took time for them to become familiar with the device and operating setup. Median operative times decreased from the first 20 cases to the last 20. As such, with further surgeon experience and familiarity using the device, it is anticipated that operative times will become shorter. In any case, patient safety is of paramount importance and was the key outcome of this current study.
Alongside its sister general surgery cohort study, 33 this study supports the implementation of the device in more patients and in a greater range of abdominal and pelvic surgeries. Such expansion will continue to follow the IDEAL‐D recommendations, proceeding to stage 3 (Assessment), with the explicit aims of demonstrating middle‐ and long‐term clinical outcomes and cost effectiveness. We envisage that continued use and expansion of robot‐assisted MAS will improve surgical outcomes for patients, with fewer intra‐ and postoperative complications than with conventional MAS and open surgery. Robotic assistance in major surgeries may also reduce the overall length of hospital stay, leading to higher case throughput and surgeon availability. We hope that the evolution of robotic surgical system designs will facilitate wider access of MAS to surgeons, with a shorter learning curve and less challenging operating techniques than conventional instruments. Further, by removing the need for awkward and static positioning while operating, robotic devices may play an important part in alleviating the physical burden on surgeons, potentially extending surgical careers.
Results pertaining to length of surgery are important, and further clinical series will demonstrate mature use of the device in relation to these perioperative outcomes. The data collected in this clinical study have been entered into a Versius surgical registry created in alignment with IDEAL‐D stage 4 (Long‐term study) to enable monitoring of rare events, longer‐term outcomes, and quality assurance. The registry will enable continual collection of real‐world data to evaluate ongoing patient safety, a crucial aspect for medical device vigilance and postmarket surveillance.
5. CONCLUSION
This early clinical study demonstrates the safe and effective performance of the device in assisting total laparoscopic hysterectomy. The results support its continued implementation in larger patient cohorts in a wider range of major procedures, in line with IDEAL‐D stage 3 (Assessment).
AUTHOR CONTRIBUTIONS
Substantial contributions to study conception and design, substantial contributions to analysis and interpretation of the data, drafting the article or revising it critically for important intellectual content, final approval of the version of the article to be published: MBo, GG, DK, MBa, ED, MS.
FUNDING INFORMATION
This study was sponsored by CMR Surgical. Support for third‐party writing assistance for this article was funded by CMR Surgical in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
CONFLICT OF INTEREST
ED is a paid consultant for CMR Surgical, and MS is Chief Medical Officer and founder of CMR Surgical. The remaining authors have no conflicts of interest to declare.
Supporting information
Figure S1
Table S1
Table S2
ACKNOWLEDGMENTS
The authors thank the investigators, and their teams who took part in this study. The authors thank the members of the clinical events committee for their independent adjudication of all adverse events: Miss Elly Brockbank, Barts Health NHS Trust, London, UK; Prof. James Fleshman, Baylor University Medical Center, Dallas, TX, USA; Mr Richard Hardwick, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK; and Prof. Catherine Matthews, Wake Forest Baptist Health, Winston‐Salem, NC, USA. The authors also acknowledge Oliver Palmer, BSc (Hons), Marc Lynch, PhD, and Emma Phillips, PhD, from Costello Medical, UK, for medical writing and editorial assistance based on the authors' input and direction.
Borse M, Godbole G, Kelkar D, Bahulikar M, Dinneen E, Slack M. Early evaluation of a next‐generation surgical system in robot‐assisted total laparoscopic hysterectomy: A prospective clinical cohort study. Acta Obstet Gynecol Scand. 2022;101:978‐986. doi: 10.1111/aogs.14407
REFERENCES
- 1. He H, Zeng D, Ou H, Tang YZ, Li JJ, Zhong H. Laparoscopic treatment of endometrial cancer: systematic review. J Minim Invasive Gynecol. 2013;20:413‐423. [DOI] [PubMed] [Google Scholar]
- 2. Wang Y‐Z, Deng L, Xu H‐C, Zhang Y, Liang Z‐Q. Laparoscopy vs laparotomy for the management of early stage cervical cancer. BMC Cancer. 2015;15:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu T, Hutfless SM, Cooper MA, Zhou M, Massie AB, Makary MA. Hospital cost implications of increased use of minimally invasive surgery. JAMA Surg. 2015;150:489‐490. [DOI] [PubMed] [Google Scholar]
- 4. Cooper MA, Hutfless S, Segev DL, Ibrahim A, Lyu H, Makary MA. Hospital level under‐utilization of minimally invasive surgery in the United States: retrospective review. BMJ. 2014;349:g4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garcia‐Ruiz A, Gagner M, Miller JH, Steiner CP, Hahn JF. Manual vs robotically assisted laparoscopic surgery in the performance of basic manipulation and suturing tasks. Archives Surg. 1998;133:957‐961. [DOI] [PubMed] [Google Scholar]
- 6. Hawkins AT, Ford MM, Benjamin Hopkins M, et al. Barriers to laparoscopic colon resection for cancer: a national analysis. Surgical Endosc. 2018;32:1035‐1042. [DOI] [PubMed] [Google Scholar]
- 7. Morton J, Stewart GD. The burden of performing minimal access surgery: ergonomics survey results from 462 surgeons across Germany, the UK and the USA. J Robot Surg 2022. doi: 10.1007/s11701-021-01358-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park A, Lee G, Seagull FJ, Meenaghan N, Dexter D. Patients benefit while surgeons suffer: an impending epidemic. J Am Coll Surg. 2010;210:306‐313. [DOI] [PubMed] [Google Scholar]
- 9. Fuchs Weizman N, Maurer R, Einarsson JI, Vitonis AF, Cohen SL. Survey on barriers to adoption of laparoscopic surgery. J Surg Educ. 2015;72:985‐994. [DOI] [PubMed] [Google Scholar]
- 10. Nieboer TE, Spaanderman ME, Bongers MY, Vierhout ME, Kluivers KB. Gynaecologists estimate and experience laparoscopic hysterectomy as more difficult compared with abdominal hysterectomy. Gynecol Surg. 2010;7:359‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coronado PJ, Herraiz MA, Magrina JF, Fasero M, Vidart JA. Comparison of perioperative outcomes and cost of robotic‐assisted laparoscopy, laparoscopy and laparotomy for endometrial cancer. Eur J Obstet Gynecol Reprod Biol. 2012;165:289‐294. [DOI] [PubMed] [Google Scholar]
- 12. Leijte E, de Blaauw I, Van Workum F, Rosman C, Botden S. Robot assisted vs laparoscopic suturing learning curve in a simulated setting. Surgical Endosc. 2020;34:3679‐3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corrado G, Mereu L, Bogliolo S, et al. Comparison between single‐site and multiport robot‐assisted hysterectomy in obese patients with endometrial cancer: an italian multi‐institutional study. Int J Med Robot. 2020;16:e2066. [DOI] [PubMed] [Google Scholar]
- 14. Carey M, Bali A, Pandeva I, Pradhan A, Slack M. Preclinical evaluation of a new robot‐assisted surgical system for use in gynecology minimal access surgery. Gynecol Surg. 2020;17:2. [Google Scholar]
- 15. Haig F, Medeiros ACB, Chitty K, Slack M. Usability assessment of versius, a new robot‐assisted surgical device for use in minimal access surgery. BMJ Surg Interv Health Technol. 2020;2:e000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hares L, Roberts P, Marshall K, Slack M. Using end‐user feedback to optimize the design of the versius surgical system, a new robot‐assisted device for use in minimal access surgery. BMJ Surg Interv Health Technol. 2019;1:e000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morton J, Hardwick RH, Tilney HS, et al. Preclinical evaluation of the versius surgical system, a new robot‐assisted surgical device for use in minimal access general and colorectal procedures. Surg Endosc. 2021;35:2169‐2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thomas BC, Slack M, Hussain M, et al. Preclinical evaluation of the versius surgical system, a new robot‐assisted surgical device for use in minimal access renal and prostate surgery. Eur Urol Focus. 2021;7:444‐452. [DOI] [PubMed] [Google Scholar]
- 19. McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the ideal recommendations. Lancet. 2009;374:1105‐1112. [DOI] [PubMed] [Google Scholar]
- 20. Sedrakyan A, Campbell B, Merino JG, Kuntz R, Hirst A, McCulloch P. Ideal‐d: a rational framework for evaluating and regulating the use of medical devices. BMJ. 2016;353:i2372. [DOI] [PubMed] [Google Scholar]
- 21. Butterworth J, Sadry M, Julian D, Haig F. Assessment of the training program for versius, a new innovative robotic system for use in minimal access surgery. BMJ Surg Interv Health Technol. 2021;3:e000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelkar D, Borse MA, Godbole GP, Kurlekar U, Slack M. Interim safety analysis of the first‐in‐human clinical trial of the versius surgical system, a new robot‐assisted device for use in minimal access surgery. Surgical Endosc. 2021;35:5193‐5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Daabiss M. American society of anaesthesiologists physical status classification. Indian J Anaesth. 2011;55:111‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Albright BB, Witte T, Tofte AN, et al. Robotic vs laparoscopic hysterectomy for benign disease: a systematic review and meta‐analysis of randomized trials. J Minim Invasive Gynecol. 2016;23:18‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gala RB, Margulies R, Steinberg A, et al. Systematic review of robotic surgery in gynecology: robotic techniques compared with laparoscopy and laparotomy. J Minim Invasive Gynecol. 2014;21:353‐361. [DOI] [PubMed] [Google Scholar]
- 27. Jung JJ, Jüni P, Lebovic G, Grantcharov T. First‐year analysis of the operating room black box study. Ann Surg. 2020;271:122‐127. [DOI] [PubMed] [Google Scholar]
- 28. Garry R, Fountain J, Mason S, et al. The evaluate study: two parallel randomised trials, one comparing laparoscopic with abdominal hysterectomy, the other comparing laparoscopic with vaginal hysterectomy. BMJ. 2004;328:129‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maresh MJ, Metcalfe MA, McPherson K, et al. The value national hysterectomy study: description of the patients and their surgery. BJOG. 2002;109:302‐312. [DOI] [PubMed] [Google Scholar]
- 30. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. Jama. 2013;309:689‐698. [DOI] [PubMed] [Google Scholar]
- 31. Payne TN, Dauterive FR. A comparison of total laparoscopic hysterectomy to robotically assisted hysterectomy: surgical outcomes in a community practice. J Minim Invas Gynecol. 2008;15:286‐291. [DOI] [PubMed] [Google Scholar]
- 32. Reynolds RK, Advincula AP. Robot‐assisted laparoscopic hysterectomy: technique and initial experience. Am J Surg. 2006;191:555‐560. [DOI] [PubMed] [Google Scholar]
- 33. Kelkar D, Kulekar U, Stevens L, Wagholikar G, Slack M. An early prospective clinical study to evaluate the safety and performance of the versius surgical system in robot‐assisted cholecystectomy. Ann Surg. 2022;Publish Ahead of Print. doi: 10.1097/SLA.0000000000005410. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1
Table S2


