Abstract
Introduction
Objectives were to analyze changes in fetal cephalic biometry and fetoplacental circulation throughout pregnancy in fetuses with congenital heart defects.
Material and methods
Prospective study conducted on three university tertiary referral hospitals. Fetuses with the diagnosis of isolated congenital heart defects attending between 2014 and 2018 were included. Congenital heart defects were divided into two groups according to the oxygen supply to the central nervous system: group I (expected low placental blood content and low oxygen delivery to the brain) and group II (expected intermediate and high placental blood content). Fetal biometry and Doppler parameters were collected between 25–30 weeks of gestation and 34–40 weeks of gestation and transformed into Z scores. The results were compared with healthy controls. Finally, general linear modeling was performed to analyze repeated measurements.
Results
In all, 71 fetuses met the inclusion criteria. Fetuses with congenital heart defects had significantly smaller head (biparietal diameter [p < 0.001], head circumference [p = 0.001]) and abdominal circumference (p < 0.001), and lower estimated fetal weight (p < 0.001) than controls. When analyzing according to congenital heart defects type, head size was significantly smaller in group I compared with group II (p = 0.04). Regarding Doppler parameters, fetuses with congenital heart defects showed higher umbilical artery pulsatility index (p < 0.001) and lower cerebroplacental ratio (p = 0.044) than controls. In group I, umbilical artery pulsatility index was above the 95th centile in 15.4% of fetuses compared with 6.7% in group II and 1.9% in controls (p < 0.001); moreover, middle cerebral artery pulsatility index was below the 5th centile in 5.4% of group I fetuses compared with 0% in group II and 1.2% in controls (p = 0.011). General linear model for two measurements showed significant longitudinal changes in biometric parameters. Growth rate of fetal head through pregnancy (head circumference Z score) was lower in fetuses with congenital heart defects compared with controls (p = 0.043). In group I, the head circumference Z score longitudinal decrease was significantly higher than in group II (p < 0.001).
Conclusions
Fetuses with congenital heart defects are at risk of smaller head size and Doppler changes. Growth rate of fetal head throughout pregnancy is also affected. These findings are mainly associated with cardiac defects with expected low oxygen supply to the central nervous system.
Keywords: congenital heart disease, Doppler flow, fetal biometry, head size, longitudinal changes
Abbreviations
- AC
abdominal circumference
- CHD
congenital heart defects
- CPR
cerebroplacental ratio
- EFW
estimated fetal weight
- GA
gestational age
- HC
head circumference
- MCA
middle cerebral artery
- PI
pulsatility index
- UA
umbilical artery
- UtA
uterine arteries
Key message.
Fetuses with congenital heart defects not only have smaller cephalic biometry but also less longitudinal cephalic growth throughout pregnancy. Placental resistance is higher and cerebroplacental ratio is lower than in controls. These changes are associated with any major cardiac defects.
1. INTRODUCTION
Congenital heart defects (CHD) are the most common congenital anomalies, affecting about of 0.8% of all live births. Despite all of the improvements in prenatal diagnosis, neonatal care, and surgical procedures, CHD remain as the leading cause of infant death secondary to birth defects. Half of them will require immediate neonatal surgery, and although currently the overall survival of children with CHD undergoing cardiac surgery is 80%–90%, 1 nearly 50% will show alterations in some neurodevelopmental areas. 1 , 2
Traditionally, neurodevelopmental delay in patients with CHD was attributed to brain injury during cardiac surgery. However, recent studies have reported neonatal/fetal brain lesions by neuroimaging, and reduced brain size in the newborn before cardiac surgery, and even in those patients who did not undergo such intervention. 3 , 4 The mechanism of brain injury in CHD is not fully understood, although there are several theories. One of them hypothesizes that the cardiac defect could lead to circulatory alterations, low cerebral blood flow, and subsequently low oxygen and nutrients supply, leading to a disruption in normal brain development. 4 Supporting this mechanism, several reports have described that fetuses with CHD show reduced fetal head circumference (HC), since the second trimester 5 and signs of cerebrovascular self‐regulation comparable to those presented by fetuses with intrauterine growth restriction, 6 that is a decrease in the pulsatility index (PI) of the middle cerebral artery (MCA), an increase in the PI of the umbilical artery (UA) and a decrease in the cerebroplacental ratio (CPR). 7
It is also known that the association between CHD and alterations in fetal growth (above all, head growth) and in Doppler flow patterns depends on the type of the heart defect. Since the demand of oxygen and nutrients increases with gestational age (GA), longitudinal analysis of these changes might reveal interesting information. 7 , 8
The aim of this study was first to analyze fetal biometry and Doppler blood flow patterns in fetuses with isolated CHD throughout gestation compared with healthy fetuses; second, to investigate these changes depending on the type of CHD classified according to the oxygen supply to the fetal brain.
2. MATERIAL AND METHODS
2.1. Study population
This was part of a multicenter prospective clinical study of cases of CHD prenatally diagnosed in three tertiary hospitals in Spain: La Paz University Hospital, Madrid; Vall d'Hebron University Hospital, Barcelona, and 12 Octubre University Hospital, Madrid. The complete protocol was previously described. 9 The study population included pregnant women referred to their respective fetal medicine units from January 2014 to January 2018. Local ethical approval to conduct the study was obtained from the ethics committees of the three hospitals. All patients gave written informed consent to participate.
Pregnant women at from 20 to 37 weeks of gestation with a fetus affected by a major CHD were included. Cases with associated arrhythmia, extracardiac malformation, chromosomal abnormalities (including microdeletion and microduplication syndromes), multiple pregnancies, fetal anemia, or maternal age under 18 years were excluded.
Prenatal diagnosis of CHDs was ascertained by postnatal echocardiography or at autopsy in the case of intrauterine or neonatal death. Cases with postnatally confirmed genetic disease were excluded from the analysis.
Maternal characteristics (age, ethnicity, body mass index, comorbidities, parity, method of conception), gestational age at delivery, type of delivery, sex, birthweight, birthweight centile, birth length, Apgar scores, and UA pH were recorded.
In order to assess the effect on brain development according to the expected main patterns of placental (oxygenated and nutrient‐rich) vs systemic (deoxygenated and nutrient‐poor) mix of blood supply to the brain in different types of CHD, we stratified our fetuses into two groups 10 (Table 1):
Group I: CHD fetuses with low placental blood content and low oxygen delivery to the the brain, due to severe left outflow tract obstruction (hypoplastic left heart syndrome; critical aortic stenosis; aortic arch defects, including cases of aortic hypoplasia and aortic coarctation with reverse flow through the aortic isthmus) and cases with the aorta blood flow coming from the right ventricle with a high proportion of systemic blood (such as transposition of the great arteries).
Group II: CHD fetuses with mixed placental and systemic blood delivered to the brain, due to the presence of the intracardiac shunts, including in this group the remaining CHD (septal defects, right CHD, conotruncal defects other than transposition of the great arteries, and complex CHD).
TABLE 1.
CHD subgroups
| Subgroup I: expected low placenta blood content | 39 (54.9) |
| TGA | 27 (38) |
| HLHS | 10 (14.1) |
| AoAD | 2 (2.8) |
| Subgroup II: expected intermediate and high placenta blood content | 32 (45.1) |
| TOF | 11 (15.5) |
| VSD | 5 (7) |
| DORV | 4 (5.6) |
| AVSD | 4 (5.6) |
| TA | 3 (4.2) |
| TRUN | 3 (4.2) |
| PS | 1 (1.4) |
| L‐TGA | 1 (4.2) |
Note: Values are n (%).Abbreviations: AoAD, aortic arch defects (including coarctation of the aorta and aortic hypoplasia); AVSD, atrioventricular septal defects; CHD, congenital heart defects; DORV, double output right ventricle; HLHS, hypoplastic left heart syndrome; L‐TGA, congenitally corrected transposition of great arteries; PS, pulmonary stenosis; TA, tricuspid atresia; TGA, transposition of great arteries; TOF, tetralogy of Fallot; TRUN, truncus arteriosus; VSD, ventriculoseptal defect.
The control group comprised 3546 normal singleton pregnancies without chromosomal or congenital abnormalities with known outcome obtained from our database.
2.2. Ultrasound assessment
Fetal anatomic scan, including echocardiography, was performed in late second/early third trimester (25–30 weeks of gestation) and in late third trimester (34–40 weeks of gestation); GA was assessed according to crown–rump length measurement at the first trimester scan (11–13.6 weeks of pregnancy). Voluson E8 or E6 (GE Healthcare Technologies, Milwaukee, WI, USA), equipped with a 4D convex curved transducer were used.
Fetal biometric parameters included as routine and measured according to the guidelines of the International Society of Ultrasound in Obstetrics and Gynecology, were biparietal diameter (BPD), HC, abdominal circumference (AC) and femur length; estimated fetal weight (EFW) was calculated using the method of Hadlock et at. 11 Doppler flow parameters included: UA‐PI, MCA‐PI, CPR, PI of ductus venous (DV), flow through the aortic isthmus (anterograde vs retrograde), and mean PI of the uterine arteries (UtA‐PI). Doppler recordings were made in the absence of fetal movements and voluntarily suspended maternal breathing, and at least three consecutive waveforms were analyzed. The UA was evaluated in a free loop of the umbilical cord. 12 The fetal MCA was measured in a transverse view of the skull at the level of its origin (proximal third) from the circle of Willis. 13 CPR was calculated by dividing MCA by UA‐PI. 13 The DV was evaluated in a midsagittal view of the fetus, peak systolic and diastolic velocities and velocity during atrial contraction were measured, and DV‐PI was automatically calculated [(peak systolic velocity – atrial contraction velocity)/ average mean velocity]. 14 UtA was evaluated with the probe placed on the lower quadrant of the abdomen, angled medially, with identification by color Doppler imaging of the apparent crossover with the external iliac artery; mean UtA‐PI was calculated as the average PI of right and left arteries. 15 Values of MCA‐PI and/or CPR below the 5th centile were considered as a sign of brain vasodilatation or redistribution respectively; 13 values of UA‐PI above the 95th centile, 12 DV‐PI above the 95th centile, 14 and mean UtA‐PI above the 95th centile 15 were considered abnormal.
Furthermore, fetal echocardiographic examination was performed by a fetal medicine expert together with an experienced pediatric cardiologist.
2.3. Statistical analyses
Statistical analysis was performed using IBM SPSS version 26.0 for Windows21 (IBMCorp.). All parameters were converted to Z scores using previously published nomograms. 12 , 13 , 14 , 15 , 16 , 17 , 18 Continuous variables were expressed as mean ± standard deviation and categorical variables as absolute number (percentage). Biometric and Doppler parameters were compared using Student's t test to determine differences between two groups (CHD vs healthy ones). For multiple comparisons, one‐way analysis of variance was used. Dichotomous variables were analyzed using the chi‐squared test and Fisher's exact test. To analyze the longitudinal changes throughout gestation, a general linear model between–within subject analysis of variance test was performed. Significance was previously set at 95% level (p < 0.05).
2.4. Ethical approval
Ethical approval to conduct the study was obtained from every participant hospital ethics committee. Ethical approval in La Paz University Hospital was obtained from the Investigation Ethics Committee on June 25, 2013 (internal code, PI‐1499). All patients included gave written informed consent.
3. RESULTS
During the study period, 152 fetuses diagnosed with CHD fulfilled the inclusion criteria. Of these, data on ultrasound biometry and Doppler parameters in late second/early third trimester (25–30 weeks of gestation) and in late third trimester (34–40 weeks of gestation), and perinatal outcome were available for 79 patients. Eight cases, five fetuses and three neonates, were excluded because of the presence of chromosomal abnormalities (fetuses: two cases of 22q11.2 deletion syndrome, two cases of 15q11.2 microdeletion, one case of Klipple–Feil syndrome; neonates: one case of Kabuky syndrome, one case of Rubistein–Taybi syndrome, one case of KBG syndrome), leaving 71 fetuses for analysis.
Distribution of CHD types according to oxygen delivery and blood flow to the brain is shown in Table 1: there were 39 (54.9%) fetuses with an expected low placental blood content and low oxygen delivery to the brain (group I) and 32 (45.1%) with expected intermediate and high placental blood content (group II). The main maternal characteristics and perinatal outcomes are summarized in Table 2. Birthweight was below the 10th centile in 22.1% of the newborns and 22.1% had a HC below the 10th centile without significant differences between groups I and II. Microcephaly (HC < 3 standard deviations) was diagnosed in 2.9% of newborns. Five‐minute Apgar score was significantly lower in group I compared with group II (8.79 ± 1.031 vs 9.25 ± 0.718, p = 0.032), although only 7% of CHD newborns required advanced fetal resuscitation (10.8% in group I and 2.9% in group II, p = 0.195). There were no cases of intrauterine fetal death and all deliveries ended in live births.
TABLE 2.
Maternal characteristics and perinatal outcomes
| Group I (n = 39) | Group II (n = 32) | Total (n = 71) | p value | |
|---|---|---|---|---|
| Age (years) | 34 ± 4.779 | 32.38 ± 5.512 | 33.27 ± 5.512 | 0.195 |
| BMI (kg/m2) | 25.27 ± 5.074 | 25.49 ± 4.471 | 28.38 ± 4.775 | 0.847 |
| Ethnic group | 0.085 | |||
| Caucasian | 34 (87.2) | 30 (93.8) | 64 (90.1) | |
| Asian | 3 (7.7) | ‐ | 3 (4.2) | |
| Latin | – | 2 (9.3) | 2 (2.8) | |
| African | 2 (5.1) | – | 2 (2.8) | |
| Comorbidity | 8 (20.5) | 11 (34.4) | 19 (26.8) | 0.189 |
| Diabetes | 2 (5.2) | 1 (3.1) | 3 (4.2) | |
| Hypothyroidism | 1 (2.6) | 3 (9.4) | 4 (5.6) | |
| Hypertension | 1 (2.6) | – | 1 (1.4) | |
| Obesity | 1 (2.6) | 2 (6.2) | 3 (4.2) | |
| Cancer | 1 (2.6) | 1 (3.1) | 2 (2.8) | |
| Others | 2 (5.2) | 4 (12.4) | 6 (8.4) | |
| Smoker | 5 (12.8) | 2 (6.3) | 7 (9.9) | 0.355 |
| Parity | 0.747 | |||
| Nulliparous | 18 (46.2) | 16 (50) | 34 (47.9) | |
| Multiparous | 21 (53.8) | 16 (50) | 37 (52.1) | |
| Method of conception | 0.184 | |||
| Spontaneous | 33 (84.6) | 30 (93.8) | 63 (88.7) | |
| AI | – | 1 (3.1%) | 1 (1.4) | |
| IVF | 4 (10.3) | – | 4 (5.6) | |
| IVF with oocyte donor | 2 (5.1) | 1 (3.1) | 3 (4.2) | |
| GA at delivery | 38.73 (±1.340) | 39.01 (±1.399) | 38.88 (±1.363) | 0.440 |
| Type of delivery | 0.092 | |||
| Vaginal delivery | 23 (58.9) | 25 (78.1) | 48 (67.6) | |
| Instrumental delivery | 1 (2.6) | 2 (6.3) | 3 (4.2) | |
| Cesarean delivery | 15 (38.5) | 5 (15.6) | 20 (28.2) | |
| Sex | 0.914 | |||
| Male | 20 (51.3) | 16 (50) | 36 (50.7) | |
| Female | 19 (48.7) | 16 (50) | 35 (49.3) | |
| Birthweight (g) | 3039 ± 457 | 3190 ± 478 | 3107 ± 469 | 0.178 |
| Birthweight‐centile | 38 ± 29.822 | 47.84 ± 32.057 | 42.44 ± 31.02 | 0.185 |
| Birthweight < 10 centile | 8 (22.2) | 7 (21.9) | 15 (22.1) | 0.973 |
| Birth HC (cm) | 33.50 ± 1.390 | 33.78 ± 1.375 | 33.63 ± 1.381 | 0.405 |
| Birth HC centile | 35.05 ± 26.189 | 39.93 ± 21.789 | 37.21 ± 24.295 | 0.415 |
| Birth HC <10th centile | 9 (23.7) | 6 (20) | 15 (22.1) | 0.716 |
| pH UA | 7.26 ± 0.061 | 7.26 ± 0.054 | 7.27 ± 0.058 | 0.857 |
| 5‐minute Apgar score | 8.79 ± 1.031 | 9.25 ± 0.718 | 9 ± 0.926 | 0.032 |
| Respiratory support | 12 (32.4) | 11 (32.4) | 23 (32.4) | 0.994 |
| AFR | 4 (10.8) | 1 (2.9) | 5 (7) | 0.195 |
Note: Values are n (%) or mean ± standard deviation.Abbreviations: AFR, advanced fetal resuscitation; AI, artificial insemination; BMI, body mass index; GA, gestational age; Group I, expected low placenta blood content; Group II, expected intermediate and high placenta blood content; HC, head circumference; IVF, in vitro fertilization; UA, umbilical artery.
A total of 142 observations on 71 fetuses with CHD were recorded: two examinations per fetus, the first one in late second/early third trimester (25–30 weeks GA) and the second one in late third trimester (34–40 weeks GA).
The results of comparing biometric and Doppler parameters between fetuses with CHD and controls are summarized in Table 3. Biometric variables showed significant differences between the two groups in terms of smaller BPD (Z score, −0.342 ± 1.139 vs 0.037 ± 1.093, p < 0.001), HC (Z score, −0.138 ± 1.159 vs 0.200 ± 0.961, p = 0.001), and AC (Z score, −0.377 ± 1.137 vs 0.330 ± 1.055, p < 0.001), and lower EFW centile (37.386 ± 27.429 vs 49.329 ± 25.099, p < 0.001), but with no significant differences in femur length (Z score, −0.057 ± 1.169 vs 0.016 ± 0.924, p = 0.680). When we analyzed these parameters according to the type of CHD (Table 4), AC was lower in groups I and II compared with controls (p = 0.002 and p = 0.048, respectively) as well as EFW (p < 0.001 and p = 0.04, respectively), BPD was smaller only in group I compared with controls (p < 0.001), and HC was smaller in group I compared with controls and group II (p < 0.001 and 0 = 0.04, respectively). Regarding Doppler parameters (Table 3), fetuses with CHD showed higher UA‐PI (Z score, 0.321 ± 1.126 vs 0.113 ± 1.032, p < 0.001) and lower CPR (Z score, −0.265 ± 1.604 vs 0.007 ± 1.392, p = 0.044) than controls. No significant differences were found in MCA‐PI (Z score, 0.130 ± 1.210 vs 0.136 ± 1.045, p = 0.271) or UtA‐PI (Z score, 0.224 ± 1.451 vs 0.021 ± 1.125, p = 0.210). Besides, 11.6% of CHD fetuses showed UA‐PI values above the 95th centile (compared with 1.9% in controls, p < 0.001) Nevertheless, Doppler variables showed no significant differences among the three groups (Table 4), except for UA‐PI Z score, which was higher in group I compared with controls (p = 0.043), and it was also reflected in terms of centile, as 15.4% of fetuses in group I showed a UA‐PI above the 95th centile (compared with 6.7% in group II and 1.9% in controls, p < 0.001). Moreover, 5.4% fetuses in group I showed an MCA‐PI below the 5th centile (compared with 0% in group II and 1.2% in controls, p = 0.011).
TABLE 3.
Comparison of biometric and Doppler parameters between CHD and controls
| Group I and II (n = 142) | Controls (n = 3546) | p value | |
|---|---|---|---|
| Biometric parameters | |||
| BPD | 78.5 ± 10.182 | 81.6 ± 9.680 | 0.001 |
| BPD Z score | −0.342 ± 1.139 | 0.037 ± 1.093 | <0.001 |
| HC | 286.1 ± 34.14 | 294.6 ± 33.11 | 0.004 |
| HC Z score | −0.138 ± 1.159 | 0.200 ± 0.961 | 0.001 |
| AC | 276.46 ± 0.061 | 286.9 ± 0.061 | 0.008 |
| AC Z core | −0.377 ± 1.137 | 0.330 ± 1.055 | <0.001 |
| FL | 60.27 ± 8.942 | 61.60 ± 8.605 | 0.071 |
| FL Z score | −0.057 ± 1.169 | −0.016 ± 0.924 | 0.680 |
| EFW | 1932.04 ± 830.948 | 2118.26 ± 841.321 | 0.01 |
| cEFW | 37.386 ± 27.429 | 49.329 ± 25.099 | <0.001 |
| Doppler assessment | |||
| UA‐PI | 1.017 ± 0.211 | 0.934 ± 0.183 | <0.001 |
| UA‐PI Z‐Score | 0.321 ± 1.126 | 0.113 ± 1.032 | 0.024 |
| MCA‐PI | 1.916 ± 0.409 | 1.863 ± 0.394 | 0.159 |
| MCA‐PI Z‐Score | 0.130 ± 1.210 | 0.136 ± 1.045 | 0.271 |
| CPR | 1.942 ± 0.534 | 2.058 ± 0.477 | 0.012 |
| CPR Z‐Score | −0.265 ± 1.604 | 0.007 ± 1.392 | 0.044 |
| UAt‐PI | 0.842 ± 0.330 | 0.776 ± 0.216 | 0.049 |
| UAt‐PI Z‐Score | 0.224 ± 1.451 | 0.021 ± 1.125 | 0.210 |
Note: Values are n (%), mean ± standard deviation.Abbreviations: AC, abdominal circumference; BPD, biparietal diameter; CPR, cerebro‐placental ratio; EFW, estimated fetal weight; FL, femur length; HC, head circumference; MCA‐PI, middle cerebral artery pulsatility index; UA‐PI, umbilical artery pulsatility index; UAt‐PI, uterine artery pulsatility index.
TABLE 4.
Comparison of biometrical and Doppler parameters between CHD groups and control
| Group I (n = 78) | Group II (n = 64) | Controls (n = 3546) | p value | |
|---|---|---|---|---|
| Biometric parameters | ||||
| BPD Z score | −0.500 ± 1.201 | −0.150 ± 1.037 | 0.037 ± 1.093 | <0.001 |
| HC Z score | −0.317 ± 1.255 | 0.080 ± 0.966 | 0.200 ± 0.961 | <0.001 |
| AC Z score | −0.081 ± 1.059 | 0.015 ± 1.231 | 0.330 ± 1.055 | <0.001 |
| cEFW | 34.735 ± 26.45 | 41.616 ± 28.325 | 49.329 ± 25.099 | <0.001 |
| Doppler assessment | ||||
| UA‐PI Z score | 0.41 ± 1.216 | 0.215 ± 1.001 | 0.11 ± 1.032 | <0.001 |
| MCA‐PI Z score | 0.039 ± 1.223 | −0.019 ± 1.204 | 0.136 ± 1.045 | 0.450 |
| PSV MCA Z score | −0.059 ± 1.493 | −0.331 ± 0.812 | 0.275 | |
| CPR Z score | −0.297 ± 1.532 | −0.225 ± 1.704 | 0.007 ± 1.392 | 0.126 |
| UAt‐PI Z score | 0.289 ± 1.695 | 0.150 ± 1.124 | 0.021 ± 1.125 | 0.398 |
| DV‐PI Z score | 0.421 ± 1.257 | 0.434 ± 1.301 | 0.967 |
Note: Values are n (%), mean ± standard deviation. Abbreviations: AC, abdominal circumference; BPD, biparietal diameter; CPR, cerebroplacental ratio; DV‐PI, ductus venous pulsatility index; EFW, estimated fetal weight; HC, head circumference; MCA‐PI, middle cerebral artery pulsatility index; PSV MCA, peak systolic velocity middle cerebral artery; UA‐PI, umbilical artery pulsatility index; UAt‐PI, uterine artery pulsatility index.
There were significant longitudinal changes in the studied variables among the three groups. In terms of biometrical changes, HC Z score decreased with GA in fetuses with CHD (p = 0.043) (Figure 1) and this decrease was affected by the type of CHD. In line with this, when HC Z scores were compared among the three groups throughout gestation, those CHD fetuses at higher risk of severe impairment of blood supply to the brain (group I) showed the highest decrease of this parameter throughout pregnancy (p < 0.001). In contrast, the decrease with GA of the EFW centile in fetuses with CHD was not significant (p = 0.330), but it was affected by the type of cardiac defect (p < 0.001) (Figure 2). BPD and AC Z scores tended to decrease with GA but this was not statistically significant.
FIGURE 1.
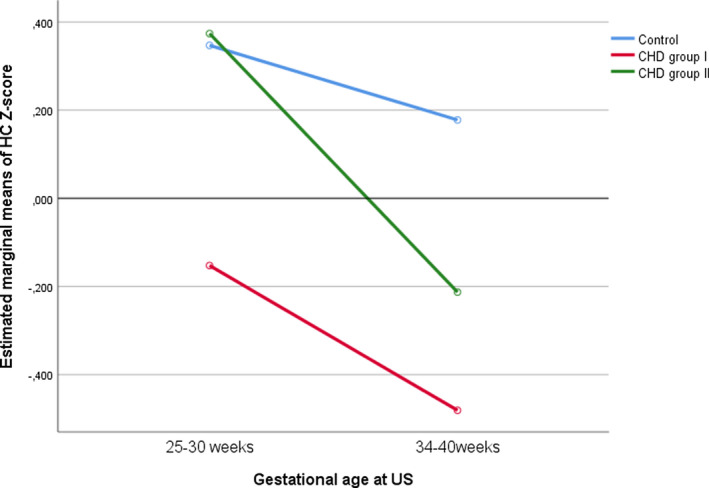
Longitudinal changes in head circumference (HC) Z score. CHD, congenital heart defects.
FIGURE 2.
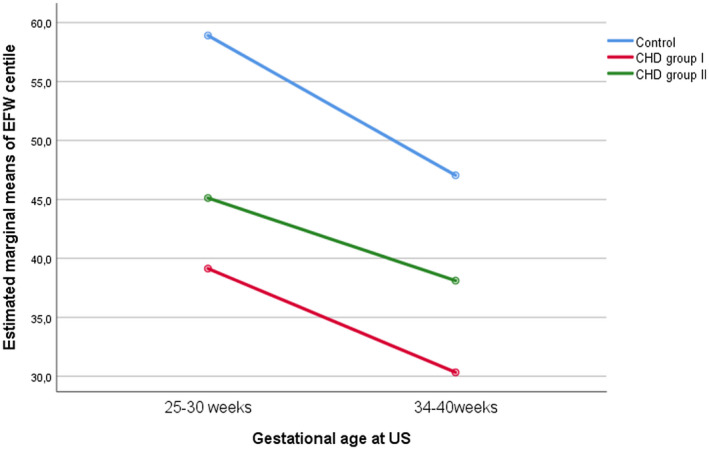
Longitudinal changes in estimated fetal weight (EFW) centile. CHD, congenital heart defects.
Regarding Doppler parameters throughout the pregnancy, MCA‐PI Z score showed a significant increase with GA in fetuses with CHD (p = 0.03) and this increase was affected by the type of CHD; fetuses with CHD with expected normal oxygen delivery to the brain (group II) showed the highest increase with GA. Considering placental circulation, UA‐PI Z score trends to increase through gestation, but without being influenced by the presence of CHD (p = 0.145) (Figure 3). CPR and UtA‐PI Z scores showed no significant changes throughout the gestation. When we analyzed DV‐PI Z score in fetuses with CHD (group I and II), it tended to increase throughout gestation, but these changes were not statistically significant (p = 0.072). On the other hand, MCA peak systolic velocity Z score did not show significant changes over time (p = 0.320)
FIGURE 3.
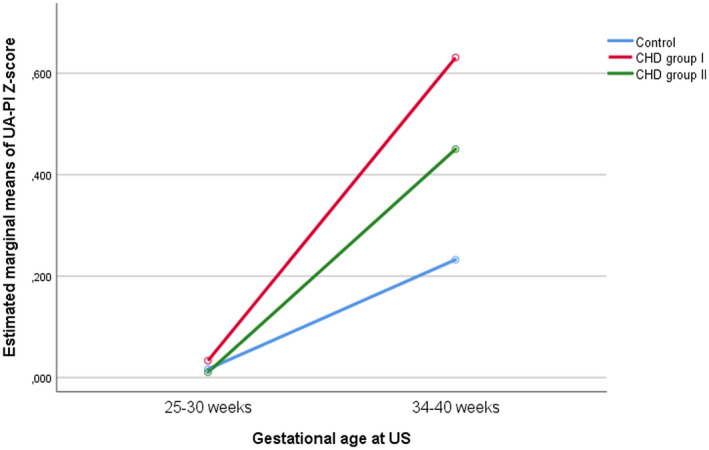
Longitudinal changes in umbilical artery pulsatility index (UA‐PI) Z score. CHD, congenital heart defects.
4. DISCUSSION
There are significant differences in terms of fetal biometry and Doppler parameters in CHD fetuses compared with controls. These changes are mainly associated with CHD with expected low oxygen supply to the brain. Moreover, when performing fetal growth assessment through time, we also found that CHD fetuses had lower head growth rates compared with controls. This flattening of the growth curve begins in the second trimester of pregnancy and continues through the third trimester, affecting fetuses with most types of major isolated CHD.
Our results show that fetuses with CHD have smaller HC, AC, and EFW by centile position compared with controls. When we classified them into subgroups according to the expected pattern of brain blood supply, there were no differences between the CHD groups in these biometrics values, except for HC, which was smaller in group I compared with group II. Previous studies described similar results. Donofrio et al 19 were the first to report smaller HC in CHD fetuses in the third trimester; Masoller et al 5 described similar changes after the second trimester of pregnancy. We found that these growth disturbances were more pronounced in some types of CHD, mainly in those with expected low placental blood content and low oxygen delivery to the brain (group I). However, recent studies showed that there were no significant differences in fetal biometry between CHD subgroups and even those classically considered as low risk had smaller head biometry, irrespective of the type of defect and the expected pattern of brain blood supply. 5 , 20
Describing brain circulation abnormalities in CHD, Donofrio et al 19 were the first to use CPR as a marker of cerebral auto‐regulation, and reported a decreased CPR in fetuses with CHD. Since then, multiple studies have reported similar results: fetuses with CHD showed lower MCA‐PI and lower CPR compared with controls. 5 , 6 , 8 , 21 We confirmed a lower CPR in CHD fetuses, but it was because od a higher UA‐PI; MCA‐PI was not decreased in the study group. However, when differences between groups were analyzed, more fetuses in group I showed MCA‐PI below the fifth centile compared with group II. Respecting the umbilical flow, our results showed a higher UA‐PI in the CHD group, being more remarkable in group I. This finding has also been described in previous studies, 8 , 22 , 23 and could suggest some grade of placental impairment, although in our cohort the UtA‐PI was within normal ranges. Recently a relation between placental dysfunction and CHD has been proposed, 24 , 25 an abnormal placental angiogenesis due to an excessive anti‐angiogenic pathway signaling in the embryogenic period, which may or may not lead to an alteration of placental perfusion and function, does seem to be related to fetal cardiac development defects. 26 , 27 In our cohort, only two patients (2.8%) developed pre‐eclampsia. Further studies are needed to assess this relation, but a screening for pre‐eclampsia, universally accepted nowadays, is mandatory in these patients.
Regarding longitudinal growth through gestation, the slope of the curve of fetal biometry and EFW usually decreases with GA, but in individuals with CHD this decrease could be more pronounced. 20 , 28 , 29 , 30 Our results confirm that on the one hand, head growth (HC Z score) but not EFW centile decreases with GA in fetuses with CHD, mainly in group I. On the other hand, UA‐PI tends to increase at the end of the pregnancy but without being influenced by the presence of CHD. Surprisingly, and the opposite of what we expected, MCA‐PI increases with GA in the presence of CHD, especially in group II. It is difficult to explain this finding; we could hypothesize that it could be related to the type of CHD included in this group, most of them associated with large ventricular or atrioventricular defects that might cause volume overload and secondary vasoconstriction in the cerebral territory.
The reasons why fetal growth is impaired in the setting of CHD remain speculative. Multiple hypotheses have been suggested but the most widespread theorizes that in CHD fetuses there is diminished oxygen and nutrients distribution and blood flow to the brain caused by abnormal heart morphology, leading to an inability to meet metabolic demands to the developing brain.. 7 , 8 The po 2 in the ascending aorta elicits brain vessel dilatation mediated by aortic chemoreceptors to maintain adequate brain perfusion, as happens in fetuses with intrauterine growth restriction. Haveman et al 30 hypothesized that oxygen saturation in fetuses with CHD might not be low enough to trigger cerebral vasodilatation, so the growth of fetal cephalic biometry was affected. Our results agree with this theory; we did not find a decrease in MCA‐PI with gestational age and the absence of a compensatory cerebral vasodilatation might explain the decrease of the fetal head growth. On the other hand, the increase of UA‐PI suggests some degree of placental impairment. A misbalance in angiogenesis factors, a plausible mechanism for these changes, might be implicated in the abnormal formation of the heart and brain during the fetal stage. What seems clear is that there are more mechanisms involved in these changes than fetal heart malfunction, and that they are still unknown.
In addition, and confirming previous results, 25 we found these head growth changes were more evident in fetuses with CHD with expected low placental blood content and low oxygen delivery to the brain (group I) than in group II. Nevertheless, other studies did not show significant differences in terms of fetal biometry and Doppler patterns among the subgroups of CHD. 5 , 8 Then, it seems reasonable to conclude that all types of major CHD are at risk of abnormal head biometry and Doppler changes, irrespective of the type of defect and the expected pattern of brain blood supply.
Concerning perinatal outcomes, in our cohort birthweight was below the 10th centile in 22.1% of the CHD newborns, which is similar to the 17%–18% reported elsewhere. 30 It is well known that low birthweight is associated with an increase of complications after corrective cardiac surgery, mortality, and higher risk of long‐term adverse neurodevelopmental outcome. HC was below the 10th centile in 22%, with an incidence of microcephaly of 2.9%, whereas other series report higher incidences, from 12% to 14%. 5 Group I newborns had significantly lower 5‐minute Apgar scores and nearly 11% of them required advanced fetal resuscitation. Then, as a whole, CHD fetuses have worse perinatal outcomes, most likely due to an underlying compromised circulation, especially those in group I.
5. CONCLUSION
Fetuses with CHD are at risk of presenting smaller head biometry, even without a compensatory decrease of MCA‐PI, compared with healthy fetuses. It starts as early as 25 weeks GA and remains smaller throughout gestation with a high incidence of newborns showing a HC below the 10th centile. Regarding Doppler parameters, an increased resistance in UA‐PI has been observed; a possible relation between placental impairment and the development of heart disease deserves future studies. EFW in these fetuses, as well as birthweight, is lower than in healthy fetuses, which is associated with an increase in morbidity and mortality. All these biometric and Doppler alterations seem to be more severe in fetuses with those types of CHD in which placental blood content and oxygen delivery to the brain are expected to be low, but we must highlight that as a whole, fetuses and newborns affected by major CHD are at risk of abnormal brain findings.
AUTHOR CONTRIBUTIONS
All the authors contributed to the design, drafting and revision of the manuscript and approved the final submitted version.
FUNDING INFORMATION
Instituto de Salud Carlos III (PI 13/02364, PI 19/00904).
CONFLICT OF INTEREST
None.
Ordás P, Rodríguez R, Herrero B, et al.. Longitudinal changes in fetal head biometry and fetoplacental circulation in fetuses with congenital heart defects. Acta Obstet Gynecol Scand. 2022;101:987‐995. doi: 10.1111/aogs.14401
REFERENCES
- 1. Latal B. Neurodevelopmental outcomes of the child with congenital heart disease. Clin Perinatol. 2016;43:173‐185. [DOI] [PubMed] [Google Scholar]
- 2. Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126:1143‐1172. [DOI] [PubMed] [Google Scholar]
- 3. Owen M, Shevell M, Majnemer A, Limperopoulos C. Abnormal brain structure and function in newborns with complex congenital heart defects before open heart surgery: a review of the evidence. J Child Neurol. 2011;26:743‐755. [DOI] [PubMed] [Google Scholar]
- 4. Mebius MJ, Kooi EMW, Bilardo CM, Bos AF. Brain injury and neurodevelopmental outcome in congenital heart disease: a systematic review. Pediatrics. 2017;140:e20164055. [DOI] [PubMed] [Google Scholar]
- 5. Masoller N, Martínez JM, Gómez O, et al. Evidence of second‐trimester changes in head biometry and brain perfusion in fetuses with congenital heart disease. Ultrasound Obstet Gynecol. 2014;44:182‐187. [DOI] [PubMed] [Google Scholar]
- 6. Berg C, Gembruch O, Gembruch U, Geipel A. Doppler indices of the middle cerebral artery in fetuses with cardiac defects theoretically associated with impaired cerebral oxygen delivery in utero: is there a brain‐sparing effect? Ultrasound Obstet Gynecol. 2009;34:666‐672. [DOI] [PubMed] [Google Scholar]
- 7. Mebius MJ, Clur SAB, Vink AS, et al. Growth patterns and cerebroplacental hemodynamics in fetuses with congenital heart disease. Ultrasound Obstet Gynecol. 2019;53:769‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ruiz A, Cruz‐Lemini M, Masoller N, et al. Longitudinal changes in fetal biometry and cerebroplacental hemodynamics in fetuses with congenital heart disease. Ultrasound Obstet Gynecol. 2017;49:379‐386. [DOI] [PubMed] [Google Scholar]
- 9. Ribera I, Ruiz A, Sánchez O, et al. Multicenter prospective clinical study to evaluate children short‐term neurodevelopmental outcome in congenital heart disease (children NEURO‐HEART): study protocol. BMC Pediatr. 2019;19:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Masoller N, Sanz‐Cortés M, Crispi F, et al. Severity of fetal brain abnormalities in congenital heart disease in relation to the Main expected pattern of in utero brain blood supply. Fetal Diagn Ther. 2016;39:269‐278. [DOI] [PubMed] [Google Scholar]
- 11. Hadlock FP, Harrist RB, Shah YP, King DE, Park SK, Sharman RS. Estimating fetal age using multiple parameters: a prospective evaluation in a racially mixed population. Am J Obstet Gynecol. 1987;156:955‐957. [DOI] [PubMed] [Google Scholar]
- 12. Parra‐Cordero M, Lees C, Missfelder‐Lobos H, Seed P, Harris C. Fetal arterial and venous doppler pulsatility index and time averaged velocity ranges. Prenat Diagn. 2007;27:1251‐1257. [DOI] [PubMed] [Google Scholar]
- 13. Morales‐Roselló J, Khalil A, Morlando M, Hervás‐Marín D, Perales‐Marín A. Doppler reference values of the fetal vertebral and middle cerebral arteries, at 19–41 weeks gestation. J Matern Fetal Neonatal Med. 2015;28:338‐343. [DOI] [PubMed] [Google Scholar]
- 14. Hecher K, Campbell S, Snijders R, Nicolaides K. Reference ranges for fetal venous and atrioventricular blood flow parameters. Ultrasound Obstet Gynecol. 1994;4:381‐390. [DOI] [PubMed] [Google Scholar]
- 15. Gómez O, Figueras F, Fernández S, et al. Reference ranges for uterine artery mean pulsatility index at 11‐41 weeks of gestation. Ultrasound Obstet Gynecol off J Int Soc Ultrasound Obstet Gynecol. 2008;32:128‐132. [DOI] [PubMed] [Google Scholar]
- 16. Verburg BO, Steegers EAP, Ridder MD, et al. New charts for ultrasound dating of pregnancy and assessment of fetal growth: longitudinal data from a population‐based cohort study. Ultrasound Obstet Gynecol. 2008;31:388‐396. [DOI] [PubMed] [Google Scholar]
- 17. Hadlock FP, Harrist RB, Martinez‐Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181:129‐133. [DOI] [PubMed] [Google Scholar]
- 18. Mari G, Deter RL, Carpenter RL, et al. Noninvasive diagnosis by doppler ultrasonography of fetal anemia due to maternal red‐cell alloimmunization. N Engl J Med. 2000;342:9‐14. [DOI] [PubMed] [Google Scholar]
- 19. Donofrio MT, Bremer YA, Schieken RM, et al. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: the brain sparing effect. Pediatr Cardiol. 2003;24:436‐443. [DOI] [PubMed] [Google Scholar]
- 20. Puri K, Warshak CR, Habli MA, et al. Fetal somatic growth trajectory differs by type of congenital heart disease. Pediatr Res. 2018;83:669‐676. [DOI] [PubMed] [Google Scholar]
- 21. Masoller N, Sanz‐CortéS M, Crispi F, et al. Mid‐gestation brain doppler and head biometry in fetuses with congenital heart disease predict abnormal brain development at birth. Ultrasound Obstet Gynecol. 2016;47:65‐73. [DOI] [PubMed] [Google Scholar]
- 22. Arduini M, Rosati P, Caforio L, et al. Cerebral blood flow autoregulation and congenital heart disease: possible causes of abnormal prenatal neurologic development. J Matern‐Fetal Neonatal Med. 2011;24:1208‐1211. [DOI] [PubMed] [Google Scholar]
- 23. Yamamoto Y, Khoo NS, Brooks PA, Savard W, Hirose A, Hornberger LK. Severe left heart obstruction with retrograde arch flow influences fetal cerebral and placental blood flow. Ultrasound Obstet Gynecol. 2013;42:294‐299. [DOI] [PubMed] [Google Scholar]
- 24. Brodwall K, Leirgul E, Greve G, et al. Possible common Aetiology behind maternal preeclampsia and congenital heart defects in the child: a cardiovascular diseases in Norway project study. Paediatr Perinat Epidemiol. 2016;30:76‐85. [DOI] [PubMed] [Google Scholar]
- 25. Inversetti A, Fesslova V, Deprest J, Candiani M, Giorgione V, Cavoretto P. Prenatal growth in fetuses with isolated cyanotic and non‐cyanotic congenital heart defects. Fetal Diagn Ther. 2020;47:411‐419. [DOI] [PubMed] [Google Scholar]
- 26. Llurba E, Syngelaki A, Sánchez O, Carreras E, Cabero L, Nicolaides KH. Maternal serum placental growth factor at 11‐13 weeks' gestation and fetal cardiac defects. Ultrasound Obstet Gynecol off J Int Soc Ultrasound Obstet Gynecol. 2013;42:169‐174. [DOI] [PubMed] [Google Scholar]
- 27. Llurba E, Sánchez O, Ferrer Q, et al. Maternal and foetal angiogenic imbalance in congenital heart defects. Eur Heart J. 2014;35:701‐707. [DOI] [PubMed] [Google Scholar]
- 28. Hahn E, Szwast A, Cnota J, et al. Association between fetal growth, cerebral blood flow and neurodevelopmental outcome in univentricular fetuses. Ultrasound Obstet Gynecol. 2016;47:460‐465. [DOI] [PubMed] [Google Scholar]
- 29. Williams IA, Fifer WP, Andrews H. Fetal growth and neurodevelopmental outcome in congenital heart disease. Pediatr Cardiol. 2015;36:1135‐1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haveman I, Fleurke‐Rozema JH, Mulder EJH, et al. Growth patterns in fetuses with isolated cardiac defects. Prenat Diagn. 2018;38:328‐336. [DOI] [PubMed] [Google Scholar]


