Abstract
Introduction
The aim of this study was to investigate long‐term outcomes in terms of pain, quality of life (QoL), and gastrointestinal symptoms in women following colorectal surgery for deep endometriosis.
Material and methods
In this historical cohort, women who underwent surgical treatment for deep endometriosis by either nerve‐sparing full‐thickness discoid resection (DR) or colorectal segmental resection (SR) between March 2011 and August 2016 were re‐evaluated through telephone interviews about their long‐term pain symptoms, subjective overall QoL as rated using a score from 0 (worst) to 10 (optimal), and gastrointestinal outcomes reflected by lower anterior resection syndrome (LARS) following a first postsurgical evaluation (visit 1) published previously and a long‐term follow‐up evaluation (visit 2).
Results
The median long‐term follow‐up time was 35.4 months at visit 1 and 86 months at visit 2. Of 134 patients, 77 were eligible for final analysis and 57 were lost to follow‐up. Compared with presurgical values, QoL scores were significantly increased at both postsurgical evaluation visits in both the SR cohort (scores of 3, 8.5, and 10 at the presurgical visit, visit 1, and visit 2, respectively; p < 0.001) and the DR cohort (scores of 3, 9, and 10, respectively; p < 0.001). Pain scores for dysmenorrhea (SR group scores of 8, 2, and 2, respectively; p < 0.001; DR group scores of 9, 2, and 1, respectively; p < 0.001), dyspareunia (SR group scores of 4, 0, and 0, respectively; p < 0.001; DR group scores of 5, 0, and 1, respectively; p = 0.003), and dyschezia (SR group scores of 8, 2, and 2, respectively; p < 0.001; DR group scores of 9, 2, and 1, respectively; p < 0.001) significantly decreased after surgery and remained stable in both cohorts over the follow‐up period. Minor and major LARS, reflecting gastrointestinal function, was observed in 6.5% and 8.1% of the SR group and in 13.3% and 6.7% of the DR group, respectively, at visit 1 and in 3.2% and 3.2% of the SR group and 0% and 0% of the DR group, respectively, at visit 2, without significant differences between the SR and DR groups.
Conclusions
Colorectal surgery for deep endometriosis, either by DR or SR, provides stable and long‐term pain relief with low rates of permanent gastrointestinal function impairment.
Keywords: endometriosis, endoscopic surgery, laparoscopy, surgical techniques
Abbreviations
- DE
deep endometriosis
- DR
discoid resection
- LARS
lower anterior resection syndrome
- SR
segmental resection
- QoL
quality of life
Key message.
Colorectal surgery for deep endometriosis, either by discoid or segmental resection, provides stable and long‐term outcomes in terms of pain, quality of life, and gastrointestinal symptoms.
1. INTRODUCTION
Endometriosis predominantly affects women in their reproductive years and may impair quality of life (QoL) and fertility. Deep lesions are usually associated with more adverse pain and gastrointestinal outcomes. 1 , 2 , 3 , 4 The optimal treatment modality for symptomatic deep endometriosis (DE) should improve QoL and preserve or improve fertility with low recurrence and complication rates. Several studies have demonstrated a significant decrease in pain symptoms in women following surgical treatment for colorectal DE. 5 , 6 , 7 , 8 However, debate is ongoing about how far surgical radicality – reflected by either segmental colorectal resection (SR) or discoid resection (DR), i.e. partial resection of the rectal wall, including the DE lesion or so‐called rectal shaving – is really warranted to achieve optimal surgical outcomes, including preservation of gastrointestinal function. 9
SR is usually performed in symptomatic patients with extensive, partially occluding, and/or multifocal disease, 10 , 11 which may render so‐called conservative approaches, including DR or shaving procedure, inappropriate. However, several lines of evidence indicate that SR may confer higher complication rates, including anastomotic leakage and fistula formation, compared with rectal shaving, 12 , 13 with grade III complication rates similar to those with DR. 13 , 14 Long‐term sequelae of full‐thickness colorectal resection also include lower anterior resection syndrome (LARS), which has been shown to occur following both techniques, without significant advantages of one method over the other. 14 , 15 Interestingly, SR may be associated with a higher incidence of de novo bowel symptoms such as constipation. 16 Nevertheless, data on long‐term pain and functional outcomes following colorectal surgery for DE are limited. To further elucidate the therapeutic effect of surgical treatment over time, we evaluated the long‐term results of our previously published data for SR and DR 17 in terms of pain symptoms, QoL, and LARS scores.
2. MATERIAL AND METHODS
We conducted a retrospective comparative follow‐up study on pain outcomes, QoL, and LARS scores among women who underwent surgery for colorectal DE by either nerve‐sparing, full‐thickness DR or SR at the Clinic Ottakring and Hospital of St John of God, Vienna, Austria, from March 2011 to August 2016. All patients enrolled in a previous analysis were eligible for follow‐up. All surgical procedures were performed by one main gynecological surgeon (GH) in a multidisciplinary team setting consisting of four colorectal surgeons (BD, TB, FB, MD) and two urological surgeons. All procedures were initially performed laparoscopically under general anesthesia using four ports with single‐shot intravenous antibiotic treatment 1 h before surgery. The surgical steps of each technique were previously defined in detail. 17
2.1. Patients and outcomes
Postoperative data were obtained via a telephone survey. The interviews were performed by one co‐author (DD). Pain symptoms such as dysmenorrhea, dyspareunia, dyschezia, and dysuria were evaluated using a numerical 10‐point analogue rating scale as used in the primary work. Patients rated QoL on a scale of 0–10, with 0 being the lowest and 10 the highest score possible. LARS scores were used to evaluate gastrointestinal function. This is a widely applied tool designed to establish a scoring system for postoperative bowel dysfunction in patients who have undergone a low anterior resection. It asks about five important items: incontinence for flatus, incontinence for liquid stools, frequency, clustering, and urgency. 18 Scores range from 0 to 42, where 0–20 is interpreted as no LARS, 20–30 is minor LARS, and 30–42 is major LARS.
2.2. Statistical analyses
No parameters except for age were distributed normally. In descriptive statistics, age was given as mean ± standard deviation, and the other parameters were given as median (25–75th percentile). For the quantitative data, we used Student’s t test to compare normally distributed parameters and the Mann–Whitney U test to compare non‐normally distributed parameters. The chi‐squared test was used to compare qualitative data between surgery groups. We used Friedman’s test and a post‐hoc Wilcoxon signed‐rank test to evaluate the differences in QoL and symptom scores for each surgery group during the follow‐up periods. All statistical analyses were performed using SPSS 17 (SPSS Inc.) software. A p‐value of <0.05 was considered statistically significant.
2.3. Ethical approval
The study was approved by the local institutional review board at Hospital St John of God (Reference number 2102/2018) on January 12, 2021.
3. RESULTS
Of 134 patients, 77 (57.5%) were included in the final analysis and 57 (42.5%) were lost to follow‐up. In total, 15 of the 77 (19%) patients underwent DR and 62 (81%) underwent SR. The median follow‐up interval at visit 1 was 35.4–36.5 (±21.9) months in the SR group and 34.3 (±24.3) months in the DR group 17 as published in our first work. The median follow‐up interval at long‐term visit 2 was 86 (68–104) months for all patients, with a median follow‐up period of 90 months (range 67–104) in the DR group and 79 months (range 70–100) in the SR group. Patient characteristics, demographic data, and severity of DE are depicted in Table 1. Severity of DE was evaluated with the revised American Society for Reproductive Medicine score and the ENZIAN classification. 19 , 20 Further data on intra‐ and perioperative outcomes are depicted in Table 1. Of the 15 patients receiving DR, three (20%) needed repeat surgery for possible disease recurrence. Three (8%) patients underwent two subsequent surgical procedures for endometriosis in the SR cohort (p = 0.17). Two patients who primarily underwent DR underwent secondary SR resection because of recurrent rectal DE. Three of the 15 patients in the DR group and 10 of the 62 patients in the SR group used oral contraceptives or levonorgestrel‐releasing intrauterine devices, primarily for contraceptive reasons.
TABLE 1.
Patient characteristics, demographic data, and intraoperative findings of women undergoing segmental and discoid resection for colorectal deep infiltrating endometriosis
| Segmental resection (n = 62) | Discoid resection (n = 15) | p‐value | |
|---|---|---|---|
| Age (years) | 34.3 ± 5.5 | 35.7 ± 7.1 | 0.39 |
| Gravidity | 1 (0.75–2) | 0 (0–1) | 0.002 |
| Parity | 1 (0–2) | 0 (0–0) | 0.002 |
| Previous pelvic surgery | 0.55 | ||
| 1 | 14 (22.5) | 2 (13.3) | |
| ≥2 | 7 (11.3) | 3 (20.0) | |
| Laparoscopy | 61 (98.4) | 15 (100) | 0.62 |
| Conversion to laparotomy | 1 (1.6) | 0 (0) | 0.62 |
| Duration of surgery (h) | 3.36 (2.54–4.30) | 3.30 (2.33–4.82) | 0.72 |
| Hemoglobin level (g/dl) difference | 1.7 (1.1–2.2) | 1.1 (0.8–2.4) | 0.34 |
| Hospital stay (days) | 7 (6–8) | 7 (6–8) | 0.75 |
| Protective stoma | 5 (8.1) | 1 (6.7) | 0.86 |
| Presence of adenomyosis | 26 (41.9) | 6 (40.0) | 0.89 |
| AFSr stage | |||
| Stage I | 3 (4.8) | 1 (6.7) | |
| Stage II | 9 (14.5) | 3 (20) | |
| Stage III | 13 (21) | 1 (6.7) | |
| Stage IV | 37 (59.7) | 10 (66.6) | |
| ENZIAN A (vagina/RVS) | 30 (48.4) | 7 (46.7) | 0.92 |
| ENZIAN B (USL, parametrium) | 11 (17.7) | 3 (20.0) | 0.15 |
| ENZIAN C (rectum/sigmoid) | 62 (100) | 15 (100.0) | 1 |
| C1 (<1 cm) | 12 (19.4) | 1 (6.7) | 0.44 |
| C2 (1–3 cm) | 16 (25.8) | 3 (20.0) | 0.75 |
| C3 (>3 cm) | 34 (54.8) | 11 (73.3) | 0.25 |
| FA, n (%) | 34 (54.8) | 6 (40.0) | 0.30 |
| FB, n (%) | 4 (6.5) | 0 (0) | 0.58 |
| FU, n (%) | 3 (4.8) | 5 (33.3) | 0.006 |
| Surgery for endometriosis during postoperative follow‐up | |||
| 0 | 48 (91.9) | 12 (80) | |
| 1 | 3 (4.8) | 2 (13.3) | |
| 2 | 2 (3.2) | 1(6.6) | |
Note: Data are presented as n (%) unless otherwise indicated.
Abbreviations: AFSr, revised American Fertility Society; FA, adenomyosis; FB, bladder endometriosis, FU, ureteral endometriosis; RVS, rectovaginal space; USL, uterosacral ligaments.
As depicted in Table 2, QoL scores significantly increased and the symptom scores for dysmenorrhea, dyspareunia, and dyschezia significantly decreased at the first postsurgical visit (visit 1) and remained stable over the follow‐up period at visit 2 in both the SR and DR groups (Figure 1). Interestingly, dysuria had decreased after surgery at the first postsurgical visit and had increased at the second postsurgical visit in the SR group but did not significantly decrease after surgery at the first and second postsurgical visits in the DR group. Nine of the 62 (14.5%) patients in the SR group and three of the 15 (20%) patients in the DR group underwent bladder resection for endometriosis. In the subgroup analysis of the patients who underwent bladder resection, there were no significant changes in dysuria scores between preoperative and postsurgical visit 1, between postsurgical visits 1 and 2, and between preoperative and postsurgical visit 2 in both groups.
TABLE 2.
Long‐term outcomes regarding pain symptoms, quality‐of‐life scores, and lower anterior resection syndrome (LARS) scores following segmental and discoid resection for colorectal deep endometriosis
| Visit | Segmental resection (n = 62) | Discoid resection (n = 15) | ||||||
|---|---|---|---|---|---|---|---|---|
| Presurgical | Postsurgical‐1 | Postsurgical‐2 | p‐value | Presurgical | Postsurgical‐1 | Postsurgical‐2 | p‐value | |
| Follow‐up (months) | — | 37 (16–59) | 90 (67–104) | — | — | 27 (18–55) | 79 (70–100) | — |
| Symptom score (NAS) | ||||||||
| Dysmenorrhea | 8 (7–9) | 2 (0–4)* | 2 (0–4)* | <0.001 | 9 (8–10) | 2 (0–3)* | 1 (0–2)* | <0.001 |
| Dyspareunia | 4 (0–6) | 0 (0–1)* | 0 (0–2)* | <0.001 | 5 (0–7) | 0 (0–4)* | 1 (0–2)* | 0.003 |
| Dyschezia | 4 (0–8) | 0 (0–0)* | 0 (0–0)* | <0.001 | 3 (0–8) | 0 (0–1)* | 0 (0–1)* | 0.008 |
| Dysuria | 0 (0–0) | 0 (0–0)* | 0 (0–0) ** | 0.002 | 0 (0–3) | 0 (0–0) | 0 (0–0) | 0.09 |
| QoL score | 3 (2–4) | 8.5 (8–9)* | 10 (8–10)* | <0.001 | 3 (2–4) | 9 (8–10)* | 10 (9–10)* | <0.001 |
| LARS | ||||||||
| No LARS | — | 56 (90.3) | 55 (88.7) | — | 13 (86.7) | 14 (93.3) | ||
| Minor LARS | — | 4 (6.5) | 5 (8.1) | — | 2 (13.3) | 1 (6.7) | ||
| Major LARS | — | 2 (3.2) | 2 (3.2) | — | 0 (0) | 0 (0) | ||
Note: Data are presented as n (%) unless otherwise indicated.
Abbreviations: LARS, lower anterior resection syndrome; NAS, Numeric Analog Scale; QoL, quality of life.
p < 0.05 vs presurgical
p < 0.05 vs postsurgical‐1.
FIGURE 1.
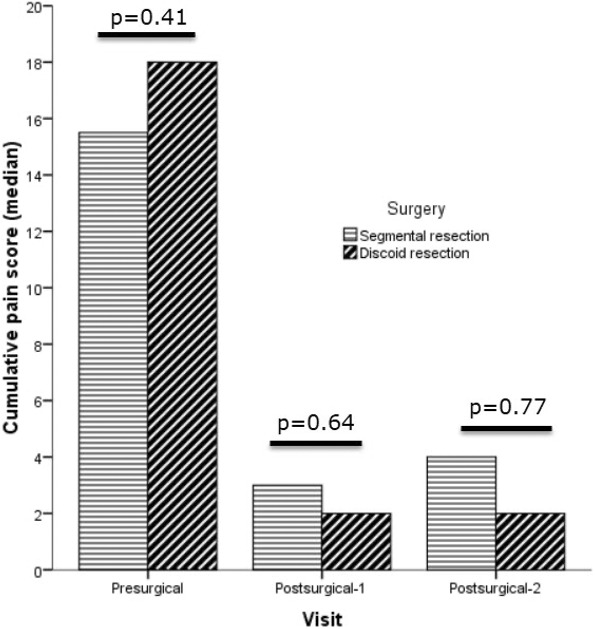
Semi‐quantitative data on pain symptoms before and at visit 1 and 2 after surgery for colorectal endometriosis.
We observed no differences between LARS scores over the follow‐up period at visit 2 in either surgery group (p = 0.45 and p = 0.79, respectively) (Table 2). All patients who were eligible for long‐term follow‐up were asked whether they would repeat the surgery with all its consequences; 80% of those in the DR cohort and 96% of those in the SR group were in favor of surgery.
4. DISCUSSION
The present study demonstrates that colorectal surgery for DE, either by SR or DR, not only provides significant pain relief for all reported symptoms and increases QoL over 2 years after surgery but also confers stable improvements over a long‐term period of up to 7 years. In addition, the prevalence of digestive complaints following either SR or DR, as reflected by LARS scores, was very low, with long‐term minor and major LARS of 8.1% and 3.2% in the SR cohort and 6.7% and 0% in the DR cohort, respectively. Based on these observations, colorectal surgery for DE, either by DR or SR, appears to be an efficient and long‐term effective treatment for symptomatic patients.
This is in line with the sparse literature regarding the long‐term effects of colorectal surgery for DE. 21 , 22 Conducting a long‐term assessment of patient wellbeing allowed us to exclude unspecific negative post‐surgical effects on patients’ health status and support the positive pain effects and QoL of these patients. Another endpoint of our study was the long‐term effectiveness of SR compared with DR. We observed no differences in QoL scores, dysmenorrhea, dyspareunia, dyschezia, and LARS scores between the DR and SR groups, indicating no potential benefit of one technique over the other. Changes in dysuria scores indicating a moderate increase of symptoms in patients undergoing SR may be attributable to factors unrelated to surgery or endometriosis.
Altogether, these findings are in line with the literature offering equally effective surgical treatment modalities in terms of symptom relief. 23 , 24 Data regarding recurrence rates after surgical treatment of DE are scarce. Meuleman et al. reported a recurrence rate of 4%–25% at 2‐year follow‐up. 25 A recent review showed a complete improvement of symptoms in 85% of women, with recurrence rates lower than 5% with a correlation to surgical experience. 26 , 27 In our study, 8% and 19.9% of the patients in the SR and DR groups underwent repeated surgery for possible recurrent endometriosis over the long‐term follow‐up period, with no significant differences among the groups (p = 0.89). As published previously, 17 two patients in the DR group exhibited recurrent colorectal DE and finally underwent SR, which supports the observation of higher recurrence rates following DR techniques. It should therefore be questioned whether less invasive surgery for bowel DE may confer lower efficacy and higher re‐intervention rates. We observed no further cases of bowel stenosis in patients undergoing DR or SR.
An important point to highlight is the occurrence of postoperative LARS. It has been shown that increased LARS scores have a negative impact on QoL scores. 28 In a retrospective multicenter study, Bokor et al. found that LARS was not more frequent after SR than after DR in patients undergoing rectal surgery for low colorectal DE. 15 Our findings of no difference among LARS scores when comparing SR and DR also mirror those of other studies. However, our study does have some limitations. First and foremost, the high rate of patients lost to follow‐up needs to be taken into account and may be explained by the migrant and multiethnic background of our patients. As a consequence, a selection bias cannot be excluded. Second, other factors, such as concomitant adenomyosis and intake of combined oral contraceptives, could not be evaluated and may influence pain symptoms. However, this may be negligible because only three of the 15 patients in the DR group and ten of the 62 patients in the SR group used oral contraceptives or levonorgestrel intrauterine devices, primarily for contraceptive reasons. Furthermore, LARS symptoms must be interpreted with caution because LARS‐like symptoms may be prevalent in up to 10% of the healthy general population. 29 Finally, the retrospective nature of our study may cause a potential bias, and further prospective studies are needed in this field, including control groups. The strengths of our work include the long observation period; very few other publications have evaluated the long‐term effects of colorectal surgery for DE. Furthermore, the final argument in favor of surgery in symptomatic patients, despite the risk of complications and sequelae, such as LARS or bowel stenosis, can be observed in our patients’ responses to the question as to whether they would choose to undergo their surgery again. In total, 80% in the DR cohort and 96% in the SR cohort confirmed that they would.
5. CONCLUSION
Surgical treatment of DE confers improvement that later plateaus and remains stable, increasing QoL, with low rates of severe complications and sequelae such as LARS. Future prospective studies that include control groups are necessary to support these assumptions. Colorectal surgery for DE, either by DR or SR, provides stable and long‐term pain relief with low rates of permanent gastrointestinal function impairment.
AUTHOR CONTRIBUTIONS
ED: Project development, data collection and management, manuscript writing/editing, data analysis. DD: Project development, data collection and management. GH, DP, BD, TB: Project development, manuscript writing/editing, data analysis.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
This work and associated APCs were supported by the European Endometriosis League.
Darici E, Denkmayr D, Pashkunova D, Dauser B, Birsan T, Hudelist G. Long‐term surgical outcomes of nerve‐sparing discoid and segmental resection for deep endometriosis. Acta Obstet Gynecol Scand. 2022;101:972‐977. doi: 10.1111/aogs.14411
REFERENCES
- 1. Fourquet J, Gao X, Zavala D, et al. Patients' report on how endometriosis affects health, work, and daily life. Fertil Steril. 2010;93:2424‐2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turco LC, Scaldaferri F, Chiantera V, et al. Long‐term evaluation of quality of life and gastrointestinal well‐being after segmental Colo‐rectal resection for deep infiltrating endometriosis (ENDO‐RESECT QoL). Arch Gynecol Obstet. 2020;301:217‐228. [DOI] [PubMed] [Google Scholar]
- 3. Montanari E, Dauser B, Keckstein J, Kirchner E, Nemeth Z, Hudelist G. Association between disease extent and pain symptoms in patients with deep infiltrating endometriosis. Reprod Biomed Online. 2019;39:845‐851. [DOI] [PubMed] [Google Scholar]
- 4. Schliep KC, Mumford SL, Peterson CM, et al. Pain typology and incident endometriosis. Hum Reprod. 2015;30:2427‐2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iversen ML, Seyer‐Hansen M, Forman AJ, Aoeg S. Does surgery for deep infiltrating bowel endometriosis improve fertility? A systematic review. Acta Obstet Gynecol Scand. 2017;96:688‐693. [DOI] [PubMed] [Google Scholar]
- 6. De Cicco C, Corona R, Schonman R, Mailova K, Ussia A, Koninckx P. Bowel resection for deep endometriosis: a systematic review. BJOG. 2011;118:285‐291. [DOI] [PubMed] [Google Scholar]
- 7. Byrne D, Curnow T, Smith P, Cutner A, Saridogan E, Clark TJ. Laparoscopic excision of deep rectovaginal endometriosis in BSGE endometriosis centres: a multicentre prospective cohort study. BMJ Open. 2018;8:e018924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riiskjaer M, Forman A, Kesmodel US, Andersen LM, Ljungmann K, Seyer‐Hansen M. Pelvic pain and quality of life before and after laparoscopic bowel resection for rectosigmoid endometriosis: a prospective. Observ Study Dis Colon Rectum. 2018;61:221‐229. [DOI] [PubMed] [Google Scholar]
- 9. Donnez O, Roman H. Choosing the right surgical technique for deep endometriosis: shaving, disc excision, or bowel resection? Fertil Steril. 2017;108:931‐942. [DOI] [PubMed] [Google Scholar]
- 10. Abrão MS, Podgaec S, Dias JA, Averbach M, Silva LFF, de Carvalho FM. Endometriosis lesions that compromise the rectum deeper than the inner muscularis layer have more than 40% of the circumference of the rectum affected by the disease. J min Invasive Gynecol. 2008;15:280‐285. [DOI] [PubMed] [Google Scholar]
- 11. Abrao MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C. Deep endometriosis infiltrating the recto‐sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21:329‐339. [DOI] [PubMed] [Google Scholar]
- 12. Bafort C, van Elst B, Neutens S, Meuleman C, Laenen A, d’Hoore A, Wolthuis A, Tomassetti C Outcome after surgery for deep endometriosis infiltrating the rectum. Fertil Steril 2020;113:1319–27 e3, 1319, 1327.e3. [DOI] [PubMed] [Google Scholar]
- 13. Bendifallah S, Puchar A, Vesale E, Moawad G, Darai E, Roman H. Surgical outcomes after colorectal surgery for endometriosis: a systematic review and meta‐analysis. J Minim Invasive Gynecol. 2021;28:453‐466. [DOI] [PubMed] [Google Scholar]
- 14. Roman H, Tuech J‐J, Huet E, et al. Excision versus colorectal resection in deep endometriosis infiltrating the rectum: 5‐year follow‐up of patients enrolled in a randomized controlled trial. Hum Reprod. 2019;34:2362‐2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bokor A, Hudelist G, Dobó N, et al. Low anterior resection syndrome following different surgical approaches for low rectal endometriosis: a retrospective multicenter study. Acta Obstet Gynecol Scand. 2021;100:860‐867. [DOI] [PubMed] [Google Scholar]
- 16. Soto E, Catenacci M, Bedient C, Jelovsek JE, Falcone T. Assessment of long‐term bowel symptoms after segmental resection of deeply infiltrating endometriosis: a matched cohort study. J Minim Invasive Gynecol. 2016;23:753‐759. [DOI] [PubMed] [Google Scholar]
- 17. Hudelist G, Aas‐Eng MK, Birsan T, et al. Pain and fertility outcomes of nerve‐sparing, full‐thickness disk or segmental bowel resection for deep infiltrating endometriosis‐a prospective cohort study. Acta Obstet Gynecol Scand. 2018;97:1438‐1446. [DOI] [PubMed] [Google Scholar]
- 18. Ridolfi TJ, Berger N, Ludwig KA. Low anterior resection syndrome: current management and future directions. Clin Colon Rectal Surg. 2016;29:239‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson NP, Hummelshoj L, Adamson GD, et al. World endometriosis society consensus on the classification of endometriosis. Hum Reprod. 2017;32:315‐324. [DOI] [PubMed] [Google Scholar]
- 20. Haas D, Shebl O, Shamiyeh A, Oppelt P. The rASRM score and the Enzian classification for endometriosis: their strengths and weaknesses. Acta Obstet Gynecol Scand. 2013;92:3‐7. [DOI] [PubMed] [Google Scholar]
- 21. Roman H, Huet E, Bridoux V, et al. Long term outcomes following surgical management of rectal endometriosis: 7‐year follow‐up of patients enrolled in a randomized trial. J Minim Invasive Gynecol. 2022;29:767‐775. [DOI] [PubMed] [Google Scholar]
- 22. Turco LC, Tortorella L, Tuscano A, et al. Surgery‐related complications and long‐term functional morbidity after segmental Colo‐rectal resection for deep infiltrating endometriosis (ENDO‐RESECT morb). Arch Gynecol Obstet. 2020;302:983‐993. [DOI] [PubMed] [Google Scholar]
- 23. Fanfani F, Fagotti A, Gagliardi ML, et al. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case‐control study. Fertil Steril. 2010;94:444‐449. [DOI] [PubMed] [Google Scholar]
- 24. Acién P, Núñez C, Quereda F, Velasco I, Valiente M, Vidal V. Is a bowel resection necessary for deep endometriosis with rectovaginal or colorectal involvement? Int J Womens Health. 2013;5:449‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meuleman C, Tomassetti C, D'Hoore A, et al. Surgical treatment of deeply infiltrating endometriosis with colorectal involvement. Hum Reprod Update. 2011;17:311‐326. [DOI] [PubMed] [Google Scholar]
- 26. Vignali M, Bianchi S, Candiani M, Spadaccini G, Oggioni G, Busacca M. Surgical treatment of deep endometriosis and risk of recurrence. J Minim Invasive Gynecol. 2005;12:508‐513. [DOI] [PubMed] [Google Scholar]
- 27. Koninckx PR, Ussia A, Adamyan L, Wattiez A, Donnez J. Deep endometriosis: definition, diagnosis, and treatment. Fertil Steril. 2012;98:564‐571. [DOI] [PubMed] [Google Scholar]
- 28. Pape E, Pattyn P, Van Hecke A, et al. Impact of low anterior resection syndrome (LARS) on the quality of life and treatment options of LARS – a cross sectional study. Eur J Oncol Nurs. 2021;50:101878. [DOI] [PubMed] [Google Scholar]
- 29. Juul T, Elfeki H, Christensen P, Laurberg S, Emmertsen KJ, Bager P. Normative data for the low anterior resection syndrome score (LARS score). Ann Surg. 2019;269:1124‐1128. [DOI] [PubMed] [Google Scholar]


