Abstract
Introduction
The incidence of ectopic pregnancy is up to four times higher after in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) than in spontaneous pregnancies, and the risk of ectopic pregnancy is increased by tubal factor infertility and the transfer of multiple embryos. However, the effect of embryo quality on the probability of ectopic pregnancy has not been investigated until now and it is not clear whether ovarian stimulation parameters affect the incidence of ectopic pregnancy.
Material and Methods
An historical cohort study of 15 006 clinical pregnancies (diagnosed by ultrasound at 6–8 gestational weeks) after non‐donor IVF/ICSI with fresh embryo transfer (n = 8952) or frozen–thawed embryo transfer (n = 6054). Treatments were performed during 2000–2017 in Finland. A total of 9207 (61.4%) single and 5799 (38.6%) double embryo transfers of no more than one top‐quality embryo were evaluated. We analyzed the effects of multiple factors on ectopic pregnancy by logistic regression, including type of cycle (fresh vs frozen embryo transfer), female age, number and quality of embryos transferred, tubal factor infertility and factors of ovarian response to gonadotropin stimulation.
Results
Ectopic pregnancy was observed in 2.3% of cycles. There was no significant difference in ectopic pregnancy rate after fresh embryo transfer and frozen embryo transfer (2.2% vs 2.4%, p = 0.3). The ectopic pregnancy rate was lower in cycles with top‐quality embryo transfer (1.9%) than of those where only non‐top quality embryos were transferred (2.7%, p < 0.0001). Tubal factor infertility was diagnosed more often in ectopic pregnancy than in intrauterine pregnancies (21.2% vs 11.0%, p < 0.0001). Logistic regression revealed lower odds for ectopic pregnancy after a top‐quality embryo transfer than after transfer of a non‐top quality embryo (odds ratio [OR] 0.72, 95% confidence interval [CI] 0.56–0.92, p = 0.007). Transfer of two vs one embryo (OR 1.35, 95% CI 1.05–1.70, p = 0.02) and tubal factor infertility (OR 2.21, 95% CI 1.68–2.91, p < 0.0001) significantly increased the risk of ectopic pregnancy.
Conclusions
Transfer of non‐top quality embryos is associated with a higher rate of ectopic pregnancy. This is particularly important to keep in mind in treatments with only non‐top embryos available even in the absence of tubal factor infertility. To minimize the risk of ectopic pregnancy, the number of embryos transferred should be as low as possible.
Keywords: ectopic pregnancy, embryo quality, frozen embryo transfer, IVF/ICSI, single embryo transfer
Abbreviations
- CAIC
consistent Akaike information criterion
- CI
confidence interval
- EP
ectopic pregnancy
- FET
frozen embryo transfer
- ICSI
intracytoplasmic sperm injection
- IVF
in vitro fertilization
- OR
odds ratio
Key message.
The odds for ectopic pregnancy are reduced when transferring a single, top‐quality embryo. Transfer of several non‐top quality embryos should be avoided in patients with tubal factor infertility whenever possible.
1. INTRODUCTION
Ectopic pregnancy (EP) is a potentially life‐threatening condition. Despite significant improvement in assisted reproduction techniques over the last decades, the rate of EP in assisted reproduction techniques varies from 1.4% to 8.6%. 1 , 2 , 3 , 4 This is up to four times higher than the prevalence after spontaneous conception. 1
The risk of EP is increased in women with tubal infertility, 4 , 5 , 6 and only a few studies have evaluated other in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) factors such as female age and type of embryo transfer (fresh or FET), with conflicting results. 1 , 4 , 7 , 8 The transfer of more than two embryos has been shown to increase the possibility of EP by 1.6%–2.2% for each additional embryo transferred. 4 However, the impact of embryo quality on EP has not previously been analyzed.
The associations between factors related to ovarian response to gonadotropin stimulation and EP rate is also largely unknown. Low ovarian reserve (anti‐Müllerian hormone <1.0 ng/dL or basal follicle‐stimulating hormone >10 mIU/ml) may be related to an increased risk of EP in IVF cycles,9 but the impact of the number of oocytes retrieved relative to gonadotropin dose used for stimulation has not been evaluated before.
The aim of the present study was to find out which patient and treatment characteristics are associated with EP. We were particularly interested in the effects of factors related to ovarian response and embryo quality.
2. MATERIAL AND METHODS
We analyzed data on 15 006 pregnancies after IVF/ICSI with 8952 fresh or 6054 FET cycles during 2000–2017 in Finland. Data were extracted from the LUMI database, a collaborative research database of 10 Finnish IVF clinics. All cycles had IVF/ICSI with the couple's own gametes and transfer of ≤2 embryos, of which no more than one with top‐quality morphology. None of the treatments analyzed had preimplantation genetic diagnosis, assisted hatching or oocyte in vitro maturation. FET cycles were matched with the stimulation cycles from which the embryo(s) originated, after which the number of oocytes retrieved, total gonadotropin dose and total number of top‐quality embryos were recorded into FET cycle data. After this, cycle matching identifiers were destroyed to have a fully anonymized dataset. We assumed that ovarian response factors should be evaluated simultaneously in fresh and FET cycles in order to separate ovarian function from endometrial receptivity in fresh and in FET cycles. Details of ovarian stimulation and FET have been described previously. 10 , 11 Ovarian response was defined as total gonadotropin dose per oocyte retrieved (dose/oocyte) because of the existing ambiguity in the definition of ovarian sensitivity index (calculated as number of oocytes × 1000/total gonadotropin dose in its initial study). 12 , 13 Embryo quality was graded as reported previously. 14 , 15 , 16 , 17 Briefly, a top‐quality cleavage‐stage embryo was defined as having four to five cells and <20% fragmentation when cultured for 2 days, or ≥8 cells and <20% fragmentation when cultured for 3 days, equal‐sized blastomeres and no multinuclearity. Blastocysts were graded according to Gardner's criteria. 15 The presence of tubal factor infertility before IVF/ICSI, a well‐known risk factor for EP1, 5, 6 was also extracted for analysis from the database. During the study period, tubal factor infertility was most frequently diagnosed by sonohysterography, which is the method of choice in Finland as well as elsewhere in the Nordic countries. Other diagnostic criteria were also applied: tubal occlusion at laparoscopic chromopertubation or a history of pelvic inflammatory disease, previous EP and/or salpingectomy.
Patients with a positive pregnancy test (urinary or serum hCG test according to local clinic protocol) were examined by transvaginal ultrasound at 6–8 gestational weeks. The present study analyzed only clinical pregnancies in which transvaginal ultrasound revealed an intrauterine gestational sac with positive heartbeat (intrauterine pregnancies) 10 or a gestational sac in an ectopic location. In cases of unclear pregnancy location during the first ultrasound examination, the patient was followed up for an additional 1–2 weeks, after which an additional ultrasound examination was performed using the same clinical pregnancy criteria. Cycles without a confirmed clinical pregnancy diagnosis were not considered clinical pregnancies and therefore were not included when extracting data. Heterotopic pregnancies were classified as ectopic at the time of data extraction.
2.1. Statistical analyses
Stimulation and embryo characteristics of cycles with EP were compared with those of the women with an intrauterine clinical pregnancy after IVF/ICSI. Presence of tubal factor infertility was analyzed in addition to number of oocytes retrieved, total gonadotropin dose used for stimulation, dose/oocyte, female age as well as body mass index during treatment. We also analyzed the number and quality of embryos transferred, as well as the day of transfer. Data were analyzed with unpaired t‐test (continuous variables) or chi‐square analysis (categorical variables). Univariate logistic regression for factors associated with EP at ultrasonographic diagnosis of pregnancy was performed for each of the tested variables. After this, the factors having a significant association with EP were included in a multivariate logistic regression model. As the dataset was completely anonymized, we could not access how many patients had had more than one pregnancy. The fit of the logistic regression model was evaluated with the Hosmer–Lemeshow test. As means of a sensitivity analysis, we investigated the interaction between the number and the quality of embryos transferred. The fits of the final model and of an “interaction model” featuring the interaction term were compared using the consistent Akaike information criterion (CAIC). The model with the better fit has lower CAIC. We also performed a subanalysis for the associations between ovarian stimulation factors and embryo quality specifically in EP and live birth cycles. Analyses were performed with IBM SPSS (Statistical Package for the Social Sciences) software, version 25. A value of p < 0.05 was taken as the limit of significance.
2.2. Ethical approval
An Institutional Review Board approval was not required in Finland for this retrospective study.
3. RESULTS
Of the 15 006 pregnancies analyzed, 8952 (59.7%) were detected after a fresh embryo transfer and 6054 (40.3%) after FET (Figure 1). EP was diagnosed in 343 (2.3%) cycles. In all other cases, an intrauterine pregnancy was verified. The outcome of intrauterine pregnancies was a live birth in 10 757 (68.0%) cycles. There were 3906 (24.7%) spontaneous abortions before the 12th gestational week, 335 (2.2%) pregnancies that ended with either spontaneous intrauterine death at a higher gestational age or with an induced abortion, and 354 (2.3%) ongoing intrauterine pregnancies beyond the 12th gestational week at the time of data extraction. No cycles had missing data.
FIGURE 1.
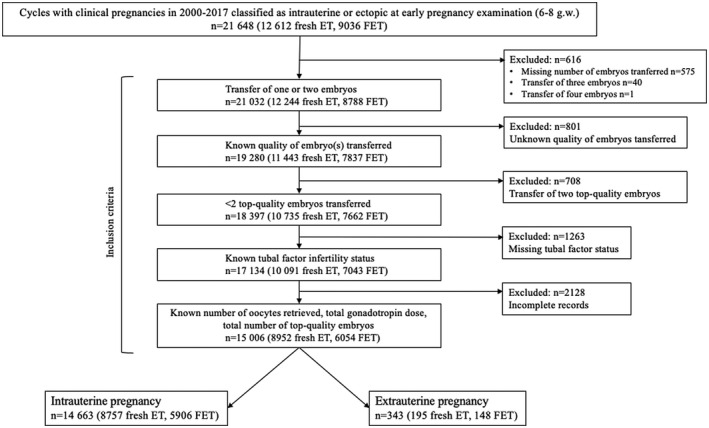
Study design. ET, embryo transfer; FET, frozen–thawed embryo transfer
There was no significant difference in EP rate after the transfer of fresh or cryopreserved embryos (195/8952, 2.2% vs 148/6054, 2.4%, p = 0.3) (Table 1). Cycles with and without EP were characterized by comparable age and similar stimulation gonadotropin doses. The incidence of tubal factor infertility in cases with EP was two times higher in fresh (42/195, 21.5% vs 922/8757, 10.5%, p < 0.0001) and FET cycles (31/148, 20.9% vs 687/5906, 11.6%, p < 0.0001). Women with EP in both fresh and FET cycles also had a higher number of oocytes collected after stimulation. However, there was no significant difference in gonadotropin dose/oocyte between EP and control groups.
TABLE 1.
Background and treatment characteristics of cycles with and without ectopic pregnancy after IVF/ICSI with fresh or frozen–thawed embryo transfer (FET)
| Fresh cycles | FET cycles | |||||
|---|---|---|---|---|---|---|
| Intrauterine pregnancy | Extrauterine pregnancy | p‐value | Intrauterine pregnancy | Extrauterine pregnancy | p‐value | |
| Number of cases | 8757 (97.8%) | 195 (2.2%) | – | 5906 (97.6%) | 148 (2.4%) | – |
| Age, years | 33.1 ± 4.4 | 33.5 ± 3.4 | 0.3 | 33.6 ± 4.3 | 33.7 ± 4.2 | 0.7 |
| BMI, kg/m2 | 23.8 ± 4.0 | 23.7 ± 3.7 | 0.8 | 23.6 ± 3.9 | 24.4 ± 3.7 | 0.03 |
| Presence of tubal factor infertility a | 922 (10.5%) | 42 (21.5%) | <0.0001 | 687 (11.6%) | 31 (20.9%) | <0.0001 |
| Total gonadotropin dose, IU | 2003.3 ± 988.4 | 1982.3 ± 864.9 | 0.8 | 1936.3 ± 974.5 | 1995.9 ± 868.0 | 1.0 |
| Number of oocytes retrieved | 11.5 ± 6.6 | 12.5 ± 6.6 | 0.03 | 11.7 ± 6.9 | 12.5 ± 6.6 | 0.02 |
| Total gonadotropin dose/oocyte, IU/oocyte | 286.4 ± 373.5 | 241.5 ± 253.9 | 0.1 | 272.2 ± 363.5 | 241.5 ± 253.9 | 0.1 |
| Total number of top‐quality embryos | 0.7 ± 0.6 | 0.6 ± 0.7 | 0.1 | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.4 |
| Number of embryos transferred | 1.3 ± 0.5 | 1.5 ± 0.5 | <0.0001 | 1.4 ± 0.5 | 1.5 ± 0.5 | 0.1 |
| Number of top‐quality embryos transferred | 0.7 ± 0.6 | 0.6 ± 0.7 | 0.1 | 0.4 ± 0.5 | 0.3 ± 0.5 | 0.4 |
| Number of cycles with a | ||||||
| Single embryo transfer | 5662 (64.7%) | 102 (52.3%) | <0.0001 | 3366 (59.7%) | 77 (52.0%) | 0.3 |
| Double embryo transfer | 3095 (35.3%) | 93 (47.6%) | 2540 (40.3%) | 71 (48.0%) | ||
| Day of transfer a | ||||||
| Day 2/3 | 8230 (93.9%) | 187 (95.9%) | 0.5 | 4917 (83.3%) | 133 (89.9%) | 0.09 |
| Day 4 | 181 (2.1%) | 2 (1.0%) | 532 (9.0%) | 7 (4.7%) | ||
| Day 5/6 | 346 (4.0%) | 6 (3.1%) | 457 (7.7%) | 8 (5.4%) | ||
Note: Unless otherwise indicated, data are presented as mean + SD, and p‐values are calculated using an unpaired t‐test.
p‐values were calculated using chi‐square analysis.
The percentage of double embryo transfer was higher in cycles with EP, compared with cycles with an intrauterine pregnancy after a fresh transfer (93/195, 47.7% vs 3095/8757, 35.3%, p < 0.0001). However, fewer top‐quality embryos were transferred in cycles with EP (0.6 ± 0.7 vs 0.7 ± 0.6, p = 0.04). Such differences were not observed in the FET cycles but overall, compared with treatments in which a top‐quality embryo was transferred (132/7566, 1.7%), The EP rate in cycles with only non‐top quality embryos was higher (206/7440, 2.8%, p < 0.0001). Neither in the fresh nor FET cycles was a significant difference in EP observed in the groups with cleavage‐stage, day 4 and blastocyst transfers. When comparing fresh and FET groups by day of transfer, there was no significant difference in cleavage‐stage (p = 0.1), day 4 (p = 0.8) or blastocyst transfer (p = 0.9).
In univariate logistic regression, presence of tubal factor infertility (odds ratio [OR] 2.54, 95% confidence interval [CI] 1.77–3.64, p < 0.0001) and transfer of two embryos compared with one (OR 1.52, 95% CI 1.21–1.91, p < 0.0001) increased the odds for EP (Table 2). The transfer of a top‐quality embryo was associated with a lower risk of EP (OR 0.70, 95% CI 0.53–0.84, p = 0.001). Age, ovarian response, the day of transfer and whether the transfer was fresh or FET did not have independent associations with EP.
TABLE 2.
Univariate logistic regression of factors affecting ectopic pregnancy after IVF/ICSI with fresh or frozen–thawed embryo transfer (FET)
|
Study population: 14 663 intrauterine pregnancies 343 ectopic pregnancies |
p‐value | OR | 95% CI |
|---|---|---|---|
| FET vs fresh transfer | 0.3 | 1.16 | 0.87–1.56 |
| Age, years | 0.8 | 1.01 | 0.97–1.04 |
| BMI, kg/m2 | 0.2 | 1.03 | 0.99–1.07 |
| Tubal factor infertility vs no tubal factor | <0.0001 | 2.54 | 1.77–3.64 |
| Total gonadotropin dose, per 100 IU | <0.0001 | 0.989 | 0.974–0.999 |
| Number of oocytes retrieved | 0.3 | 1.01 | 0.94–1.02 |
| Total gonadotropin dose per oocyte retrieved, IU/oocyte (continuous variable) | 0.2 | 1.00 | 0.99–1.00 |
| Total gonadotropin dose per oocyte retrieved | |||
| <100 IU/oocyte | Ref. | 1 | |
| 100–200 IU/oocyte | 0.7 | 0.95 | 0.70–1.29 |
| 200–300 IU/oocyte | 0.4 | 0.85 | 0.57–1.28 |
| 300–400 IU/oocyte | 0.4 | 0.75 | 0.42–1.32 |
| >400 IU/oocyte | 0.4 | 0.84 | 0.56–1.26 |
| Total number of top‐quality embryos | 0.4 | 0.97 | 0.90–1.03 |
| Number of embryos transferred | |||
| 2 vs 1 | <0.0001 | 1.52 | 1.21–1.91 |
| Transfer of a single top‐quality embryo vs non‐top quality embryo transfer | 0.001 | 0.70 | 0.53–0.84 |
| Day of transfer | |||
| Day 4 vs Day 2/3 | 0.07 | 0.54 | 0.28–1.06 |
| Day 5/6 vs Day 2/3 | 0.3 | 0.75 | 0.44–1.29 |
In multivariate analysis, tubal factor infertility (OR 2.21, 95% CI 1.68–2.91, p < 0.0001) and transfer of two embryos (OR 1.35, 95% CI 1.05–1.70, p = 0.02) were associated with higher odds of EP (Table 3). The transfer of a top‐quality embryo decreased the risk of EP (OR 0.72, 95% CI 0.56–0.92, p = 0.007). There was only a weak correlation between the number of embryos transferred and the transfer of a top‐quality embryo (correlation coefficient −0.30). Analysis for interactions also revealed a lack of interaction between these two variables (p = 0.1). Results from the multivariate subanalysis of EP and intrauterine pregnancies resulting in live birth are shown in Table 4. The number of embryos transferred, embryo quality and presence of tubal factor infertility were the only factors with independent effects on the odds for EP compared with live birth, and odds ratios were similar to those in the main analysis. None of the characteristics of ovarian stimulation examined, ie number of oocytes retrieved, total gonadotropin dose, dose/oocyte or type of cycle (fresh vs FET), was associated with the type of outcome.
TABLE 3.
Multivariate logistic regression analysis of factors associated with ectopic pregnancy at ultrasonographic diagnosis of pregnancy after IVF/ICSI with fresh or frozen–thawed embryo transfer
|
Study population: 15 006 pregnancies total 14 663 intrauterine pregnancies 343 ectopic pregnancies Tubal factor infertility: 1682 pregnancies in total, 73 ectopic pregnancies No tubal factor infertility: 13 324 pregnancies in total, 270 ectopic pregnancies Transfer of 1 embryo: 9207 pregnancies in total, 179 ectopic pregnancies Transfer of 2 embryos: 5799 pregnancies in total, 164 ectopic pregnancies Transfer of 1 top‐quality embryo: 7566 pregnancies in total, 132 ectopic pregnancies Transfer of non‐top quality embryo(s): 7440 pregnancies in total, 206 ectopic pregnancies |
p‐value | OR | 95% CI |
|---|---|---|---|
| Tubal factor vs no tubal factor infertility | <0.0001 | 2.21 | 1.68–2.91 |
| 2 vs 1 embryos transferred | 0.02 | 1.35 | 1.05–1.70 |
| Transfer of a top‐quality embryo vs non‐top quality embryo(s) | 0.007 | 0.72 | 0.56–0.92 |
Note: A model with an interaction variable (number of embryos transferred × number of top‐quality embryos transferred) was associated with poorer fit, as determined by the consistent Akaike information criterion (CAIC): CAIC interaction 96.74 vs CAIC main effects 89.66. The interaction variable had no significant effect on ectopic pregnancy (OR 0.71, 95% CI 0.47–1.07, p‐value 0.1).
TABLE 4.
Multivariate logistic regression of factors associated with ectopic pregnancy and live birth after IVF/ICSI with fresh or frozen–thawed embryo transfer
|
Study population: 10 757 live births 343 ectopic pregnancies Tubal factor infertility: 1146 pregnancies in total, 310 ectopic pregnancies No tubal factor infertility: 9954 pregnancies in total, 246 ectopic pregnancies Transfer of 1 embryo: 7569 pregnancies in total, 182 ectopic pregnancies Transfer of 2 embryos: 3531 pregnancies in total, 129 ectopic pregnancies Transfer of 1 top‐quality embryo: 5728 pregnancies in total, 121 ectopic pregnancies Transfer of no top‐quality embryo(s): 5372 pregnancies in total, 189 ectopic pregnancies |
Ectopic pregnancy compared with live birth | ||
|---|---|---|---|
| p‐value | OR a | 95% CI a | |
| Tubal factor vs no tubal factor infertility | <0.0001 | 2.19 | 1.66–2.89 |
| 2 vs 1 embryos transferred | 0.04 | 1.29 | 1.02–1.64 |
| Transfer of a top‐quality embryo vs non‐top quality embryo(s) | <0.0001 | 0.67 | 0.53–0.86 |
OR and 95% CI for ectopic pregnancy.
4. DISCUSSION
This study showed that in IVF/ICSI, the transfer of a top‐quality embryo is associated with the lowest risk of EP after transfer of fresh or frozen embryos, compared with the transfer of a non‐top quality embryo. Transfer of two embryos regardless of embryo morphology, and presence of tubal factor infertility are associated with increased odds for EP.
The overall EP rate in our study was 2.3%, which is in agreement with prevalences reported earlier in IVF/ICSI pregnancies 1 , 7 , 8 and is similar to that in spontaneous pregnancies (2%). 18 Due to the fact that previous studies have investigated a variety of settings and study populations, EP rates after IVF/ICSI have varied significantly, from 1.6% in a study of 550 000 fresh non‐donor cycles 4 to 5.4% in IVF pregnancies of poor prognosis patients. 19
The present study found an association between top‐quality embryo morphology and a decreased risk of EP. Previously, a large register study evaluated embryo implantation potential, defined as blastocyst culture and presence of extra embryos for cryopreservation, as part of several embryo transfer alternatives. 7 The analysis showed that transfer of ≤2 embryos with high implantation potential was associated with lower odds of EP (OR 0.55) compared with the transfer of >2 cleavage‐stage embryos with no additional frozen embryos. 7 It has also been shown that transfer of >1 embryo increases the odds of EP to 1.30 after double embryo transfer and to 1.70 when >3 embryos are transferred. 4 , 20 In the present study, embryo quality was evaluated independently of the number of embryos transferred in both fresh and FET cycles. These observations indicate that the risk of EP can be minimized by transferring only one top‐quality embryo and that transfer of several non‐top quality embryos compromises the outcome. In cases with only non‐top quality embryos available, patients and providers need to be aware of the higher risk for EP after transfer of more than one embryo.
The present analysis aimed to examine not only embryo quality but also factors of ovarian stimulation in cycles with EP. However, the main analysis did not find any independent effect of ovarian stimulation factors on EP. A strength of the present study is the evaluation of oocyte‐related factors simultaneously in the fresh and in the FET cycles. This separates the possible effect of oocyte‐related factors from the effect of the endometrial milieu, which is believed to be different in fresh and in FET cycles. 21 Previously, the association between the number of oocytes retrieved and the odds for EP has not been clear. A retrospective analysis of 109 000 clinical pregnancies from the Society for Assisted Reproductive Technology registry observed a weak positive correlation between the frequency of EP and the number of oocytes retrieved in autologous fresh cycles,22 but another analysis of 69 000 clinical pregnancies after autologous IVF/ICSI with fresh transfer in Japan found no such association. 23 Embryo quality was, however, not evaluated in these studies.
There are indications that ovarian stimulation itself might be associated with a higher frequency of EP. In the Japanese analysis, 23 EP rate after ovarian stimulation was compared with the rate after spontaneous cycle IVF/ICSI and was found to increase the odds of EP by 3.5–5.2. Recently, several studies have shown that Fallopian tube epithelium is sensitive to circulating hormonal levels through changes in the expression of steroid receptors 24 that affect Fallopian epithelial cilia movements. 25 , 26 In light of these findings, Jwa and colleagues have speculated that the higher hormonal levels associated with ovarian stimulation for IVF/ICSI are disruptive for the function of the Fallopian tube. 23 However, evidence from the present analysis reveals that there is no gonadotropin dose‐dependent effect on EP after adjusting for embryo quality and after inclusion of FET cycles into the analysis. This suggests that ovarian stimulation might have a threshold effect in the fresh cycles.
The high proportion of EP in cycles with tubal factor infertility in the present study highlights the well‐known fact that women with a history of Fallopian tube damage (pelvic inflammatory disease, tubal surgery or previous EP) are at increased risk for EP following both spontaneous conception and IVF/ICSI. 6 , 20 Moreover, our analysis on embryo quality also suggests that tubal epithelium, particularly a damaged one, might not be as sensitive to embryo quality factors as endometrium and could thus be more receptive to non‐top quality embryos. An investigation of implantation rates would be better suited to test this theory.
Our study showed that the type of transfer, fresh or FET, had no effect on EP. This finding is consistent with the results from previous, smaller analyses 2 , 3 but contradicts the conclusions of larger studies, which found decreased odds for EP after FET. 8 That may be due to the fact that in those studies, embryo quality was not taken into consideration and tubal factor was also not always evaluated. The present results of similar odds for EP after fresh transfer and FET also support recent findings that a freeze‐all strategy is not beneficial for patients at risk for EP. 27 Freezing and thawing can decrease embryo quality and can therefore constitute an additional risk for a couple with already existing risks for EP.
We did not observe an association between day of transfer and EP. Previously, a meta‐analysis of 22 studies showed lower odds for EP after blastocyst transfer in a variety of settings. 28 The same conclusion was also reached by a more recent analysis; 22 however, in those studies the numbers and/or quality of embryos transferred were not taken into account.
One limitation of the present study is that the analysis of several factors which are associated with the risk of EP could not be performed because relevant data such as smoking, adnexal surgery or history of Chlamydia trachomatis infection were unavailable. Tubal factor infertility evaluated in the analyses represents the risk factors for EP observed in the Finnish IVF patient population. The incidence of risk factors has varied according to country and practice; for example, an infection with C. trachomatis has been detected in 1.1% of subfertile patients in Brazil 29 and in 2.7% in France. 30 Accordingly, the associations of individual factors with EP have not been consistent. ORs of a previous EP have ranged from 2.72 to 6.34, 31 , 32 a previous pelvic inflammatory disease has been shown to have an OR of 2.5–3.02, 31 , 33 a history of infection with C. trachomatis has been implicated with an OR of 1.41–3.18, 2 , 34 and smoking with an OR of 1.7–3.9. 35 , 36
Because of the anonymization of the dataset, a history of EP or number of previous pregnancies could also not be examined separately. It was decided, however, that gerenalized estimation equation analyses for repeated cycles were not necessary for this study because EPs after IVF/ICSI or FET are rare events and even if all EP patients had another pregnancy included in the dataset, the effect of these cycles on overall conclusions would be negligible. A new meta‐analysis has shown possible association between endometriosis and EP because of ovarian factors and also because of associated inflammation and adhesions; 37 however, endometriosis was outside the scope of the present investigation, which focused on ovarian response and embryo quality.
5. CONCLUSION
The present analyses demonstrated that the transfer of a top‐quality embryo reduces the risk of EP after IVF/ICSI in both fresh and FET cycles. Tubal factor and the transfer of two embryos increase the odds of EP even if one of the embryos has top‐quality morphology. This means that especially in women with tubal factor infertility, the transfer of two embryos should be avoided, even if only non‐top quality embryos are available. Transfer of a single embryo should always be considered to avoid this iatrogenic complication.
ACKNOWLEGEMENTS
This study was funded by grants from the Academy of Finland, the Sigrid Jusélius Foundation, the Helsinki University Hospital Research Fund (to J.S.T.) and the Finnish Medical Foundation (to Z.V.). The funding organizations had no involvement in the study design, in the collection, analysis and interpretation of data,writing the report or in the decision to submit the manuscript.
AUTHOR CONTRIBUTIONS
ZV, HM, CT, AP, AH, TM, HT, SM contributed to data acquisition. SA and ZV contributed to data analysis and drafting of the manuscript. ZV, JST, AT, HM, CT, AP, AH, TM, HT, SM contributed to interpretation of data. ZV, JST, AT, HM, CT, AP, AH, TM, HT and SM revised the article critically for important intellectual content. All authors approved of the final version to be submitted.
CONFLICT OF INTEREST
None.
Anzhel S, Mäkinen S, Tinkanen H, et al. Top‐quality embryo transfer is associated with lower odds of ectopic pregnancy. Acta Obstet Gynecol Scand. 2022;101:779‐786. doi: 10.1111/aogs.14375
Funding information
This study was funded by grants from the Academy of Finland, the Sigrid Jusélius Foundation, the Helsinki University Hospital Research Fund (to JST) and the Finnish Medical Foundation (to ZV).
REFERENCES
- 1. Smith LP, Oskowitz SP, Dodge LE, Hacker MR. Risk of ectopic pregnancy following day‐5 embryo transfer compared with day‐3 transfer. Reprod Biomed Online. 2013;27:407‐413. [DOI] [PubMed] [Google Scholar]
- 2. Decleer W, Osmanagaoglu K, Meganck G, Devroey P. Slightly lower incidence of ectopic pregnancies in frozen embryo transfer cycles versus fresh in vitro fertilization–embryo transfer cycles: a retrospective cohort study. Fertil Steril. 2014;101:162‐165. [DOI] [PubMed] [Google Scholar]
- 3. Cheng LY, Lin PY, Huang FJ, et al. Ectopic pregnancy following in vitro fertilization with embryo transfer: a single‐center experience during 15 years. Taiwan J Obstet Gynecol. 2015;54:541‐545. [DOI] [PubMed] [Google Scholar]
- 4. Perkins KM, Boulet SL, Kissin DM, Jamieson DJ. National ART surveillance group. Risk of ectopic pregnancy associated with assisted reproductive technology in the United States 2001–2011. Obstet Gynecol. 2015;125:70‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rombauts L, McMaster R, Motteram C, Fernando S. Risk of ectopic pregnancy is linked to endometrial thickness in a retrospective cohort study of 8120 assisted reproduction technology cycles. Hum Reprod. 2015;30:2846‐2852. [DOI] [PubMed] [Google Scholar]
- 6. Bu Z, Xiong Y, Wang K, Sun Y. Risk factors for ectopic pregnancy in assisted reproductive technology: a 6‐year, single‐center study. Fertil Steril. 2016;106:90‐94. [DOI] [PubMed] [Google Scholar]
- 7. Clayton HB, Schieve LA, Peterson HB, Jamieson DJ, Reynolds MA, Wright VC. Ectopic pregnancy risk with assisted reproductive technology procedures. Obstet Gynecol. 2006;107:595‐604. [DOI] [PubMed] [Google Scholar]
- 8. Muller V, Savicheva A, Kogan I, et al. Association between anti‐chlamydial immunity and IVF outcome. Gynecol Endocrinol. 2015;31:69‐73. [Google Scholar]
- 9. Kim S, Kim Y, Shin J, et al. Correlation between ovarian reserve and incidence of ectopic pregnancy after in vitro fertilization and embryo transfer. Yonsei Med J. 2019;60:285‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Veleva Z, Tiitinen A, Vilska S, et al. High and low BMI increase the risk of miscarriage after IVF/ICSI and FET. Hum Reprod. 2008;23:878‐884. [DOI] [PubMed] [Google Scholar]
- 11. Veleva Z, Karinen P, Tomas C, Tapanainen JS, Martikainen H. Elective single embryo transfer with cryopreservation improves the outcome and diminishes the costs of IVF/ICSI. Hum Reprod. 2009;24:1632‐1639. [DOI] [PubMed] [Google Scholar]
- 12. Huber M, Hadziosmanovic N, Berglund L, Holte J. Using the ovarian sensitivity index to define poor, normal, and high response after controlled ovarian hyperstimulation in the long gonadotropin‐releasing hormone‐agonist protocol: suggestions for a new principle to solve an old problem. Fertil Steril. 2013;100:1270‐1276. [DOI] [PubMed] [Google Scholar]
- 13. Yadav V, Malhotra N, Mahey R, Singh N, Kriplani A. Ovarian sensitivity index (OSI): validating the use of a marker for ovarian responsiveness in IVF. J Reprod Infertil. 2019;20:83‐88. [PMC free article] [PubMed] [Google Scholar]
- 14. Van Royen E, Mangelschots K, Neubourg D, et al. Characterization of a top quality embryo, a step towards single‐embryo transfer. Hum Reprod. 1999;14:2345‐2349. [DOI] [PubMed] [Google Scholar]
- 15. Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft W. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155‐1158. [DOI] [PubMed] [Google Scholar]
- 16. Veleva Z, Orava M, Nuojua‐Huttunen S, Tapanainen JS, Martikainen H. Factors affecting the outcome of frozen–thawed embryo transfer. Hum Reprod. 2013;28:2425‐2431. [DOI] [PubMed] [Google Scholar]
- 17. Kaartinen N, Das P, Kananen K, Huhtala H, Tinkanen H. Can repeated IVF‐ICSI‐cycles be avoided by using blastocysts developing from poor‐quality cleavage stage embryos? Reprod Biomed Online. 2015;30:241‐247. [DOI] [PubMed] [Google Scholar]
- 18. Marion LL, Meeks GR. Ectopic pregnancy: history, incidence, epidemiology, and risk factors. Clin Obstet Gynecol. 2012;55:376‐386. [DOI] [PubMed] [Google Scholar]
- 19. Jun S, Milki A. Assisted hatching is associated with a higher ectopic pregnancy rate. Fertil Steril. 2004;81:1701‐1703. [DOI] [PubMed] [Google Scholar]
- 20. Santos‐Ribeiro S, Tournaye H, Polyzos NP. Trends in ectopic pregnancy rates following assisted reproductive technologies in the UK: a 12‐year nationwide analysis including 160 000 pregnancies. Hum Reprod. 2016;31:393‐402. [DOI] [PubMed] [Google Scholar]
- 21. Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril. 2014;102:3‐9. [DOI] [PubMed] [Google Scholar]
- 22. Acharya KS, Acharya CR, Provost MP, et al. Ectopic pregnancy rate increases with the number of retrieved oocytes in autologous in vitro fertilization with non‐tubal infertility but not donor/recipient cycles: an analysis of 109 140 clinical pregnancies from the Society for Assisted Reproductive Technology registry. Fertil Steril. 2015;104:873‐878. [DOI] [PubMed] [Google Scholar]
- 23. Jwa S, Seto S, Takamura M, Kuwahara A, Kajihara T, Ishihara O. Ovarian stimulation increases the risk of ectopic pregnancy for fresh embryo transfers: an analysis of 68 851 clinical pregnancies from the Japanese Assisted Reproductive Technology Registry. Fertil Steril. 2020;114:1198‐1206. [DOI] [PubMed] [Google Scholar]
- 24. Maclean A, Bunni E, Makrydima S, et al. Fallopian tube epithelial cells express androgen receptor and have a distinct hormonal responsiveness when compared with endometrial epithelium. Hum Reprod. 2020;35:2097‐2106. [DOI] [PubMed] [Google Scholar]
- 25. Paltieli Y, Eibschitz I, Ziskind G, Ohel G, Silbermann M, Weichselbaum A. High progesterone levels and ciliary dysfunction–a possible cause of ectopic pregnancy. J Assist Reprod Genet. 2000;17:103‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jackson‐Bey T, Colina J, Isenberg B, et al. Exposure of human fallopian tube epithelium to elevated testosterone results in alteration of cilia gene expression and beating. Hum Reprod. 2020;35:2086‐2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ishihara O, Kuwahara A, Saitoh H. Frozen–thawed blastocyst transfer reduces ectopic pregnancy risk: an analysis of single embryo transfer cycles in Japan. Fertil Steril. 2011;95:1966‐1969. [DOI] [PubMed] [Google Scholar]
- 28. Zhang B, Linlin C, Tang R, Ding L, Yan L, Chen Z. Reduced ectopic pregnancy rate on day 5 embryo transfer compared with day 3: a meta‐analysis. PLoS One. 2017;12:e0169837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pantoja M, Campos EA, Da Rocha PD, Gabiatti JE, Bahamondes MV, dos Santos Fernandes AM. Prevalence of Chlamydia trachomatis infection among women candidates for in vitro fertilization at a public institution of the state of São Paulo, Brazil. Rev Bras Ginecol Obstet. 2012;34:425‐431. [DOI] [PubMed] [Google Scholar]
- 30. De Barbeyrac B, Papaxanthos‐Roche A, Mathieu C, et al. Chlamydia trachomatis in subfertile couples undergoing an in vitro fertilization program: a prospective study. Eur J Obstet Gynecol Reprod Biol. 2006;129:46‐53. [DOI] [PubMed] [Google Scholar]
- 31. Mahajan N, Raina R, Sharma P. Risk factors for ectopic pregnancy: a case–control study in tertiary care hospitals of Jammu and Kashmir. Iberoam J Med. 2021;3:293‐299. [Google Scholar]
- 32. Li C, Zhao W‐H, Zhu Q, et al. Risk factors for ectopic pregnancy: a multi‐center case–control study. BMC Pregnancy Childbirth. 2015;15:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ankum WM, Mol BWJ, Van der Veen F, Bossuyt PMM. Risk factors for ectopic pregnancy: a meta‐analysis. Fertil Steril. 1996;65:1093‐1099. [PubMed] [Google Scholar]
- 34. Reekie J, Donovan B, Guy R, et al. Chlamydia and reproductive health outcome investigators, risk of ectopic pregnancy and tubal infertility following gonorrhea and chlamydia infections. Clin Infect Dis. 2019;69:1621‐1623. [DOI] [PubMed] [Google Scholar]
- 35. Farquhar CM. Ectopic pregnancy. Lancet. 2005;366:583‐591. [DOI] [PubMed] [Google Scholar]
- 36. Gaskins AJ, Missmer SA, Rich‐Edwards JW, Williams PL, Souter I, Chavarro JE. Demographic, lifestyle, and reproductive risk factors for ectopic pregnancy. Fertil Steril. 2018;110:1328‐1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yong P, Matwani S, Brace C, et al. Endometriosis and ectopic pregnancy: a meta‐analysis. J Minim Invasive Gynecol. 2020;27:352‐361.e2. [DOI] [PubMed] [Google Scholar]


