Abstract
Introduction
Coagulopathy may be the result of hyperfibrinolysis and could exacerbate bleeding following childbirth. Timely recognition of hyperfibrinolysis during the earliest stages of postpartum hemorrhage could identify women at risk of more severe blood loss who may benefit from targeted anti‐fibrinolytic therapy. Rotational thromboelastometry (ROTEM®) is a point‐of‐care test that could detect hyperfibrinolysis. The aim of this study was to evaluate whether early assessment of hyperfibrinolysis by ROTEM during postpartum hemorrhage could predict progression to severe postpartum hemorrhage.
Material and methods
During a prospective cohort study in the Netherlands among women with postpartum hemorrhage (total blood loss at least 1000 ml within 24 h after childbirth) ROTEM measurements were performed following 800–1500 ml of blood loss. Hyperfibrinolysis was defined as an enzymatic fibrinolysis index (ROTEM EXTEM maximum clot lysis [ML] minus the ROTEM APTEM ML) above 15%. Severe postpartum hemorrhage was defined as a composite end point of total blood loss greater than 2000 ml, transfusion of four or more units of packed cells, and/or need for an invasive intervention. The predictive value of hyperfibrinolysis for progression to severe postpartum hemorrhage was assessed by area under the receiver operating curve (AUC) and positive and negative predictive values. Trial registration: ClinicalTrials.gov (NCT02149472).
Results
Of 390 women included, 82 (21%) had severe postpartum hemorrhage. Four (1%) women had thromboelastometric evidence of hyperfibrinolysis, of whom two developed severe postpartum hemorrhage. The AUC for enzymatic fibrinolysis index more than 15% for progression to severe postpartum hemorrhage was 0.47 (95% CI 0.40–0.54). Positive and negative predictive values for this index were 50.0% (95% CI 6.8–93.2) and 79.3% (95% CI 74.9–83.2), respectively.
Conclusions
Thromboelastometric evidence of hyperfibrinolysis was rare in women with postpartum hemorrhage when assessed between 800 and 1500 ml of blood loss. The clinical predictive value of viscoelastometric point‐of‐care testing for hyperfibrinolysis for progression to severe postpartum hemorrhage during early postpartum hemorrhage is limited.
Keywords: hyperfibrinolysis, postpartum hemorrhage, rotational thromboelastometry, ROTEM® , severity of bleeding
Abbreviations
- APTEM
ROTEM® test activated by tissue factor in the presence of the anti‐fibrinolytic agent aprotinin
- AUC
area under the receiver operating characteristics curve
- EFI
enzymatic fibrinolysis index
- EXTEM
ROTEM® test activated by tissue factor in the absence of the anti‐fibrinolytic agent aprotinin
- IQR
interquartile ranges
- ML
maximum clot lysis
- PPH
postpartum hemorrhage
- ROTEM®
rotational thromboelastometry
- TeMpOH‐2
Towards better prognostic and diagnostic strategies for hemostatic changes during Major Obstetric Hemorrhage study
- TXA
tranexamic acid
- WOMAN‐trial
World maternal antifibrinolytic trial
Key message.
Thromboelastometric evidence of hyperfibrinolysis during the earliest stage of postpartum hemorrhage is not predictive for progression to severe postpartum hemorrhage. Clinical value of viscoelastometric point‐of‐care testing for hyperfibrinolysis is limited.
1. INTRODUCTION
Postpartum hemorrhage (PPH) is the leading cause of maternal mortality and responsible for one in five maternal deaths worldwide. 1 Although the maternal mortality ratio due to PPH is low in high‐income countries, PPH remains a life‐threatening condition associated with substantial maternal morbidity and psychosocial sequelae. 2
PPH most commonly has a primary obstetric cause, but can be aggravated by hemostatic impairment. 3 Presence of coagulopathy in the course of PPH is associated with maternal morbidity and is an important predictor for massive transfusion. 3 Hyperfibrinolysis is a key component of coagulopathy and refers to a disproportionately increased breakdown of fibrin clots, associated with excessive bleeding. 4 Research into the maternal coagulation profile has illustrated increased fibrinolytic activity shortly after birth. 5 , 6 This suggests that hyperfibrinolysis may contribute to hemostatic impairment and excessive blood loss following childbirth. Clot breakdown can be countered by anti‐fibrinolytic therapy with tranexamic acid (TXA). Multiple national guidelines recommend administration of TXA to all women when blood loss exceeds 500 ml after childbirth. 7 , 8 , 9 However, it is not yet clear which women would benefit specifically from the administration of TXA.
We hypothesized that recognition of hyperfibrinolysis by a point‐of‐care test early during the course of PPH could potentially identify women at risk of progression to severe PPH, and—in this manner—may help to identify women who will benefit from targeted anti‐fibrinolytic therapy with TXA. Detecting hyperfibrinolysis has traditionally been a clinical challenge, but viscoelastometric testing using dynamic rotational thromboelastometry (ROTEM®; Tem International GmbH, Munich, Germany) enables rapid identification of (ongoing) hyperfibrinolysis. 4 , 10 ROTEM is a point‐of‐care test using whole blood providing a qualitative assessment of a woman's coagulation status, which can be used to diagnose various forms of coagulopathy. The aim of this study was to evaluate the predictive value of hyperfibrinolysis for progression to severe PPH, when determined by a point‐of‐care test (ROTEM) after 800–1500 ml of blood loss following childbirth.
2. MATERIAL AND METHODS
2.1. Study aim, design and setting
This study was a secondary analysis of the Towards better prognostic and diagnostic strategies for hemostatic changes during Major Obstetric Hemorrhage (TeMpOH‐2) study. 11 , 12 The TeMpOH‐2 study was a multicenter prospective cohort study in the Netherlands between February 2015 and April 2018. Pregnant women were recruited at outpatient clinics and maternity wards of three participating hospitals: Leiden University Medical Center (university hospital), Erasmus Medical Centre (university hospital), and Isala Clinics Zwolle (non‐university teaching hospital). Women of at least 18 years old and with a gestational age of 24 weeks onwards were included. Women were monitored for the occurrence of PPH (in the Netherlands defined as a total blood loss of 1000 ml or more within 24 h after childbirth) and followed until discharge. The Royal College of Obstetricians and Gynaecologists in the United Kingdom and the Dutch Society of Obstetrics and Gynaecology (NVOG) in the Netherlands recommend routine coagulation testing when blood loss exceeds 1000 ml. 13 , 14 However, in anticipation of PPH, in some women intravenous access with simultaneous collection of blood for coagulation screening may already have been established before reaching this threshold. Therefore, when participants were likely to develop PPH, the clinician in charge was instructed to draw at least one blood sample at between 800 and 1500 ml of blood loss for full blood count, standard coagulation parameters, and a full ROTEM analysis on a ROTEM Delta device (Tem International GmbH, Munich, Germany). All measurements on the ROTEM Delta device were immediately conducted after blood sampling with a single‐use reagent and in conformity with the recommendations of the producer of the device. In one hospital, ROTEM analyses were performed by clinical midwives and well‐trained research nurses as the ROTEM device was located in a utility room equipped with laboratory supplies at the maternity ward. In the two other participating hospitals, the device was positioned in the laboratory and laboratory staff carried out ROTEM analyses. Test characteristics have been described previously. 11 , 12 ROTEM results were intended to be blinded to the clinicians and women were treated according to the Dutch national guideline for the management of PPH. 11 , 13 When bleeding ceased before reaching the threshold of 1000 ml blood loss, these specific samples were excluded from analysis. When multiple blood samples were drawn, the first sample above or closest to 1000 ml was selected for analysis, as advised by the Dutch and English national PPH management guidelines. 13 , 14 Participants with a known coagulation disorder or who used anticoagulants were excluded from the analysis.
2.2. Data collection
Maternal and obstetric characteristics were obtained by trained research nurses and collected from medical files available at the maternity ward and operating theater. The following data were collected: maternal age, maternal body mass index, ethnicity (Caucasian or non‐Caucasian), nulliparity or multiparity, multiple pregnancy, gestational age at time of birth, presence of HELLP (hemolysis elevated liver enzymes and low platelets) syndrome or pre‐eclampsia, mode of birth (spontaneous vaginal birth, instrumental vaginal birth, planned cesarean section, secondary cesarean section), cause of PPH (uterine atony, uterine rupture, placental pathology [placenta previa, retained placenta, placental remnants, abnormally invasive placenta, and placental abruption], surgical trauma, and laceration of the birth canal), total amount of blood loss (measured by weighing gauzes or other soaked materials and by use of a collector bag and suction system in the operating room), administration of clear fluids and blood products (crystalloids, colloids, packed red blood cells, fresh frozen plasma, and platelets), administration of uterotonics (oxytocin, sulprostone, ergometrine, misoprostol, and carbetocin), administration of non‐uterotonics (TXA, fibrinogen concentrate, and recombinant factor VIIa), and invasive procedures to control bleeding (uterine balloon tamponade, uterine artery ligation, uterine compression sutures, uterine artery embolization, and peripartum hysterectomy).
2.3. Assessment of hyperfibrinolysis
Different ways of measuring hyperfibrinolysis on the ROTEM device have been proposed. 4 , 15 However, fibrinolysis is frequently determined by comparing the maximum clot lysis (ML) of the ROTEM APTEM ML and EXTEM ML tests. 14 Both tests are activated by tissue factor in the presence (APTEM) and absence (EXTEM) of the anti‐fibrinolytic agent aprotinin. 16 , 17 Hyperfibrinolysis is often defined as EXTEM ML greater than 15%. 15 To correct EXTEM ML for mechanical clot retraction, the enzymatic fibrinolysis index (EFI) can be used. EFI is calculated by subtracting APTEM ML (%) from EXTEM ML (%). 4 , 10 Therefore, in this study, hyperfibrinolysis was defined as an EFI greater than 15%. 14
2.4. Main outcome measure
Severe PPH was defined as blood loss exceeding 2000 ml and/or need for transfusion of at least four units of packed red blood cells and/or need for an invasive procedure to control bleeding (i.e., uterine balloon tamponade, uterine artery ligation, uterine compression sutures, uterine artery embolization, and peripartum hysterectomy) following childbirth. This definition was based on severe PPH‐related core outcome sets of two Delphi studies. 18 , 19
2.5. Statistical analyses
Statistical analyses were performed in SPSS Statistics 25 and statase 16 software. Categorical data are presented as frequencies with percentages, and continuous data are presented as medians with interquartile ranges (IQR). Values of p were calculated by independent sample t test or chi‐squared test. The predictive value of hyperfibrinolysis detected by an EFI greater than 15% was evaluated by calculating the positive and negative predictive value with 95% CI. The ability of the EFI to predict progression to severe PPH was studied by the area under the receiver operating characteristics curve (AUC) with 95% CI. To determine the optimal threshold for EFI to predict progression towards severe PPH, the maximum Youden's index was calculated. The Youden's index displays the performance of a diagnostic test and can be used to select the optimum cut‐off point of a numeric result such as EFI.
2.6. Ethical approval
The study was approved by the ethics committee of the Leiden University Medical Center (P13.246; February 5, 2014) and by the institutional review board of each participating hospital. Written informed consent was obtained from all women included in the study. The TeMpOH‐2 study was registered at ClinicalTrials.gov (NCT02149472).
3. RESULTS
During the 3‐year inclusion period, 17 203 women of at least 18 years old and with a gestational age of 24 weeks onwards gave birth in one of the three participating hospitals. Of these women, 390 had lost at least 1000 ml of blood following birth and had blood samples taken between 800 and 1500 ml of blood loss with valid APTEM (ML) and EXTEM (ML) measurements (Figure 1). Of all the 390 women included in this study, 82 (21%) women experienced severe PPH as defined by our composite primary outcome.
FIGURE 1.
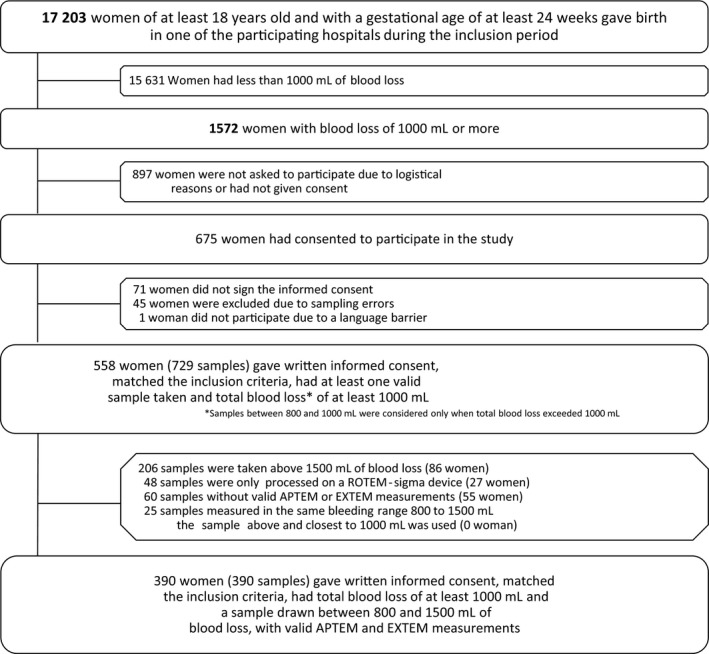
Flowchart of study enrollment
3.1. Baseline characteristics
Characteristics of women are presented in Table 1. Median age was 32 years (IQR 29–35 years) and median body mass index was 24 kg/m2 (IQR 22–28 kg/m2). The majority (78.2%) had given birth vaginally and had a median gestational age of 39 weeks (IQR 38–40 weeks) at time of birth. The commonest causes of PPH were uterine atony and retained placental tissue in, respectively, 144 of 390 (36.9%) and 152 of 390 (39.0%) women.
TABLE 1.
Baseline characteristics
| Severe PPH (n = 82) | Non‐severe PPH (n = 308) | Total (n = 390) | p value | |
|---|---|---|---|---|
| Maternal age (years) a | 32 (28–36) | 32 (29–35) | 32 (29–35) | 0.98 |
| Gestational age (weeks) a | 39 (38–40) | 40 (38–41) | 39 (38–40) | 0.32 |
| Body mass index (kg/m2) a | 25 (22–29) | 24 (22–28) | 24 (22–28) | 0.27 |
| Ethnicity, Caucasian, n (%) | 67 (81.7) | 270 (87.7) | 337 (86.4) | 0.16 |
| Multiparity, n (%) | 44 (53.7) | 152 (49.4) | 196 (50.3) | 0.49 |
| Multiple pregnancy, n (%) | 13 (15.9) | 22 (7.1) | 35 (9.0) | 0.01 |
| Pregnancy‐related hypertensive disease, n (%) | 10 (12.2) | 26 (8.4) | 36 (9.2) | 0.30 |
| Mode of birth, n (%) | 0.83 | |||
| Spontaneous vaginal birth | 56 (68.3) | 206 (66.9) | 262 (67.2) | |
| Instrumental vaginal birth | 7 (8.5) | 36 (11.7) | 43 (11.0) | |
| Planned cesarean section | 9 (11) | 40 (13.0) | 49 (12.6) | |
| Secondary cesarean section | 10 (12.2) | 26 (8.4) | 36 (9.2) | |
| Primary cause of PPH, n (%) | 0.02 | |||
| Uterine atony | 30 (36.6) | 114 (37.0) | 144 (36.9) | |
| Retained placenta or placental tissue | 37 (45.1) | 115 (37.3) | 152 (39.0) | |
| Abnormally invasive placenta | 5 (6.1) | 2 (0.6) | 7 (1.8) | |
| Placenta previa | 1 (1.2) | 5 (1.6) | 6 (1.5) | |
| Placental abruption | 1 (1.2) | 4 (1.3) | 5 (1.3) | |
| Laceration of the birth canal | 6 (7.3) | 32 (10.4) | 38 (9.7) | |
| Uterine rupture | 0 (0) | 4 (1.3) | 4 (1.0) | |
| Pre‐existent clotting disorder | 0 (0) | 1 (0.3) | 1 (0.3) | |
| Surgical trauma | 2 (2.4) | 31 (10.1) | 33 (8.5) | |
| Total amount of blood loss (ml) a | 2500 (2200–3000) | 1300 (1200–1600) | 1500 (1200–2000) | <0.001 |
| Hemoglobin level (mmol/L) a | 5.9 (5.0–6.9) | 6.4 (5.8–7.0) | 6.4 (5.6–7.0) | <0.001 |
| ICU admission because of PPH, n (%) | 4 (4.9) | 0 (0) | 4 (1.0) | <0.001 |
| Maternal mortality, n (%) | 0 (0) | 0 (0) | 0 (0) | – |
Abbreviations: ICU, intensive care unit; PPH, postpartum hemorrhage.
Reported as median (interquartile ranges).
3.2. ROTEM measurements
The results of the ROTEM APTEM and EXTEM measurements of women with severe PPH and non‐severe PPH are presented in Table 2. Of all 390 women, four (1%) were identified as having hyperfibrinolysis (EFI >15%), of whom two (50%) developed severe PPH. No differences were seen between women with and without hyperfibrinolysis. Median EXTEM ML was 3% (IQR 1%–5%), similar for severe and non‐severe PPH. Six women (1.5%) had an EXTEM ML greater than 15%, of whom three (50%) experienced severe PPH. Median APTEM ML were 3% (IQR 1%–5%) and 3% (IQR 1%–4%) for women with severe and non‐severe PPH, respectively. Median EFI was 0% (IQR −1% to 0%) for women with severe PPH and 0% (IQR −0.5% to 1%) for women with non‐severe PPH.
TABLE 2.
Hemostatic parameters of women with PPH
| Severe PPH (n = 82) | Non‐severe PPH (n = 308) | Total (n = 390) | ||||
|---|---|---|---|---|---|---|
| Median (IQR) | >15% | Median (IQR) | >15% | Median (IQR) | >15% | |
| APTEM ML | 3% (1–5) | 3% (1–4) | 3% (1–4) | |||
| EXTEM ML | 3% (1–5) | 3 (3.7) a | 3% (1–5) | 3 (1.0) a | 3% (1–5) | 6 (1.5) a |
| EFI c | 0% (−1 to 0) | 2 (2.4) b | 0% (−0.5 to 1) | 2 (0.6) b | 0% (−1 to 1) | 4 (1.0) b |
Abbreviations: EFI =EXTEM (ML) – APTEM (ML). EFI, enzymatic fibrinolysis index; APTEM (ML), ROTEM® test activated by tissue factor in the presence of the anti‐fibrinolytic agent aprotinin; EXTEM (ML), ROTEM® test activated by tissue factor in the absence of the anti‐fibrinolytic agent aprotinin.
Number of participants with EXTEM ML >15%, reported as number of women with (%).
Number of participants with EFI >15%, reported as number of women with (%).
Hyperfibrinolysis is defined as EFI >15%.
3.3. Management of hemorrhage
All 390 women received a uterotonic agent. Thirty‐four (8.7%) participants received TXA before blood sampling, while 70 (17.9%) women eventually received TXA during the course of PPH. Among these 70 women, two (2.9%) were found to have had hyperfibrinolysis at the time of blood sampling. Among the 34 women who received TXA before sampling, zero (0.0%) women were found to have had hyperfibrinolysis.
Colloids and crystalloids were infused in, respectively, 158 (40.5%) and 339 (86.9%) participants; the median accumulated infused volume of colloids and crystalloids at time of sampling was 500 ml (IQR 0–1000 ml). There were 24 women (6.2%) in need of an invasive intervention to control bleeding. In total, invasive interventions were performed in 27 women, including 16 (59%) uterine balloon tamponades, five (19%) uterine compression sutures, four (15%) uterine artery embolizations, and two (7%) peripartum hysterectomies.
3.4. Outcomes
Eighty‐two (21%) women developed severe PPH, of whom 74 (90%) had blood loss over 2000 ml, 16 (20%) were in need of transfusion of at least four units of packed red blood cells, and 24 (29%) needed an invasive intervention to manage bleeding. Median total blood loss was 1500 mL (IQR 1200–2000 ml). Maternal deaths did not occur in this study.
3.5. Predictive value
The AUC for the EFI to predict progression to severe PPH was 0.47 (95% CI 0.40–0.54) with a maximum Youden's index of 0.02, corresponding to an EFI of 3%. The positive and negative predictive values of an EFI greater than 15% for progression to severe PPH were 50.0% (95% CI 6.8%–93.2%) and 79.3% (95% CI 74.9%–83.2%), respectively. After exclusion of the 34 women receiving TXA before sampling, the AUC for progression to severe PPH was 0.46 (95% CI 0.38–0.53), with a maximum Youden's index of 0.03, corresponding to an EFI of 19%. The positive and negative predictive values of hyperfibrinolysis for progression to severe PPH were 50% (95% CI 6.76%–93.2%) and 83% (95% CI 78.6%–86.7%), respectively.
4. DISCUSSION
This multicenter prospective cohort study found that hyperfibrinolysis was rare during the early stages of PPH when detected by rotational thromboelastometry. Hyperfibrinolysis did not discriminate women who developed severe PPH from those who did not, when routinely measured between 800 and 1500 ml of blood loss following childbirth. Therefore, it remains questionable whether assessment of hyperfibrinolysis by rotational thromboelastometry has the potential to guide individualized treatment with TXA.
The main strength of this study was the prospective design and data collection of APTEM and EXTEM measurements in a large cohort of women within a specific range of blood loss during the onset of PPH. By using both EXTEM and APTEM measurements, the influence of clot retraction on assessment of hyperfibrinolysis has been limited. EXTEM ML reflects the degree of fibrinolysis, but can also be affected by mechanical clot retraction. 17 Therefore, in this study the APTEM test is used to calculate EFI and correct EXTEM ML for clot retraction. Considering that the APTEM test includes an anti‐fibrinolytic agent, APTEM ML illustrates mainly clot retraction and exposes how EXTEM would appear without hyperfibrinolysis. 17
Despite the large number of women in our cohort, it was challenging to obtain consent and execute trial procedures in women with acute, severe blood loss. This may have contributed to fewer women with blood loss of 1000 ml or more being enrolled in our study than expected. Additionally, fewer women with more severe blood loss may have been included, potentially causing underestimation of women with possible hyperfibrinolysis and severe PPH. Furthermore, managing clinicians were intended to be blinded for APTEM and EXTEM measurements. However, it appeared that clinicians from the Erasmus Medical Center Rotterdam may occasionally have seen APTEM and EXTEM results. It remains unknown to what extent this has affected management of PPH. Also, administration of TXA before blood testing in 34 women may have contributed to an underestimation of the occurrence of hyperfibrinolysis. As there is no reference standard for the assessment of hyperfibrinolysis at this time, comparison with our ROTEM measurements is difficult and its incidence remains uncertain. 15
This was the first study to assess the predictive value of hyperfibrinolysis detected on a ROTEM device between 800 and 1500 ml blood loss, for progression towards severe PPH. However, there have been previous attempts to determine the incidence of hyperfibrinolysis following childbirth using a ROTEM point‐of‐care test. 20 , 21 A Dutch multicenter trial aimed to establish reference ranges for thromboelastometric coagulation parameters in women within 1 hour following childbirth. 20 All women were included with unknown outcome with regard to development of PPH. 20 They found that hyperfibrinolysis (defined as only an EXTEM ML greater than 15%) occurred in 8.6% of women. 20 However, none of the women with thromboelastometric evidence of hyperfibrinolysis had blood loss exceeding 1000 ml, or needed blood transfusion, suggesting that hyperfibrinolysis did not lead to increased risk of more severe bleeding. 20 Another study assessed ROTEM parameters of 167 Nigerian women suffering from PPH. 21 Hyperfibrinolysis (defined as only a EXTEM ML greater than 15%) occurred in 23.3% of women. 21 ROTEM tests were performed before women were randomized to receive TXA or placebo, meaning that ROTEM measurements were not affected by anti‐fibrinolytic therapy. The median total blood loss was 1200 ml (IQR 1000–2000 ml), similar to the 1500 ml (IQR 1200–2000 ml) blood loss in our cohort. In accordance with our findings, this study did not find a correlation between hyperfibrinolysis and estimated total blood loss (r = 0.01, p = 0.86), which the authors attributed to inaccuracies in measuring blood loss. Also, administration of TXA after sampling may have obscured a possible relation between hyperfibrinolysis and total blood loss.
Furthermore, it is noteworthy that the incidence of women with EXTEM ML greater than 15% following childbirth varies widely in the literature. 20 , 21 These different incidences could reflect the heterogeneity among studies but may also be the result of inaccurate measurements of hyperfibrinolysis by viscoelastometric point of care testing. Therefore, further validation of hyperfibrinolysis measurement on ROTEM devices in women suffering from PPH might be needed before ROTEM point‐of‐care testing for assessment of hyperfibrinolysis can be established in practice.
It can be debated whether the routine administration of TXA when blood loss exceeds 500 ml is appropriate, as it is not yet clear which specific women benefit from TXA. 8 , 9 This policy was introduced in response to the results of the World Maternal Antifibrinolytic Trial (WOMAN‐trial) and was implemented in the Netherlands after the TeMpOH‐2 study was conducted. The WOMAN‐trial revealed that administration of TXA during the early stages of PPH reduced maternal death due to bleeding from 1.9% to 1.5% and reduced the need for laparotomy to control bleeding in low‐ and middle‐income countries. 22 However, as maternal deaths rarely occur in high‐income countries, it is not yet clear to what extent women in high‐resource settings may benefit from TXA. The efficacy of TXA in reducing blood loss was not studied in the WOMAN‐trial, and no differences were found regarding the occurrence of maternal morbidity, the number of blood products transfused, and the need for uterine artery ligation and embolization. 6 Moreover, a nationwide retrospective cohort study in the Netherlands found that early administration of TXA had no significant effect on blood loss, when compared with no/late administration of TXA. 23 Also, no significant difference on a composite end point of maternal morbidity and mortality was found between early and no/late administration of TXA. 23 Hence, the clinical relevance of administering TXA in high‐income countries is questionable. However, considering its good safety profile and the low costs of TXA, low‐threshold administration of TXA should be considered. 6 , 21
5. CONCLUSION
Rotational thromboelastometric evidence of hyperfibrinolysis was found to be rare when evaluated after 800–1500 ml of blood loss following childbirth. The assessment of hyperfibrinolysis by rotational thromboelastometry during the onset of PPH lacks the ability to discriminate between women with and without progression to severe PPH.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
This study was conceived by MT, PR, TvdA and JvdB. MT and PR conducted the statistical analysis and wrote the first draft of the manuscript. MT, PR, TvdA JvdB, AG, CCD, DH, MdM, JD, JE and KB contributed significantly to data analysis, interpretation and writing of the manuscript. All authors have read and approved the final version of this manuscript.
ACKNOWLEDGMENTS
We would like to express our appreciation to research nurses C. Kolster‐Bijdevaate, MS Bourgonje‐Verhart, CE Bleeker‐Taborh, E Roos‐van Milligen, RJM Berkhout, E Sucu, EC Willems of Brilman‐Tuinhof de Mode, M Stigter‐Dekker, NCW van Rijn, and J van Rhee, medical students M van de Sande, RH Wouters, and LS Smits, and clinical midwifes of the participating hospitals for their contributions to the TeMpOH‐2 study.
Tahitu M, Ramler PI, Gillissen A, et al. Clinical value of early assessment of hyperfibrinolysis by rotational thromboelastometry during postpartum hemorrhage for the prediction of severity of bleeding: A multicenter prospective cohort study in the Netherlands. Acta Obstet Gynecol Scand.2022;101:145–152. doi: 10.1111/aogs.14279
Funding information
The TeMpOH‐2 study was supported by an internal grant from Sanquin Research (PPOC 13‐029). The ROTEM device that was used by the Leiden University Medical Center was provided by Tem International GmbH (Munich, Germany) on the basis of a loan agreement without additional charge for the duration of the study. Reagents used in the ROTEM devices were at the expense of the study without discount. The funding body and Tem International GmbH had no control over the study design, conduct or decision to publish this study.
Data Availability Statement
The data set that was generated and analyzed for this study is not publicly available because of the General Data Protection Regulation. A pseudonymized data set can be obtained via a repository from the corresponding author (j.g.van_der_bom@lumc.nl) on reasonable request only.
REFERENCES
- 1. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health. 2014;2:323‐333. [DOI] [PubMed] [Google Scholar]
- 2. Zwart JJ, Richters JM, Öry F, de Vries JIP, Bloemenkamp KWM, van Roosmalen J. Severe maternal morbidity during pregnancy, delivery and puerperium in the Netherlands: a nationwide population‐based study of 371 000 pregnancies. BJOG. 2008;115:842‐850. [DOI] [PubMed] [Google Scholar]
- 3. Oh KJ, Hong JS, Youm J, Cho SH, Jung EY. Can coagulopathy in post‐partum hemorrhage predict maternal morbidity? J Obstet Gynaecol Res. 2016;42:1509‐1518. [DOI] [PubMed] [Google Scholar]
- 4. Gall LS, Brohi K, Davenport RA. Diagnosis and treatment of hyperfibrinolysis in trauma (A European Perspective). Semin Thromb Hemost. 2017;43:224‐234. [DOI] [PubMed] [Google Scholar]
- 5. Kruithof EK, Tran‐Thang C, Gudinchet A, et al. Fibrinolysis in pregnancy: a study of plasminogen activator inhibitors. Blood. 1987;69(2):460‐466. [PubMed] [Google Scholar]
- 6. Shreeve NE, Barry JA, Deutsch LR, Gomez K, Kadir RA. Changes in thromboelastography parameters in pregnancy, labor, and the immediate postpartum period. Int J Gynaecol Obstet. 2016;134:290‐293. [DOI] [PubMed] [Google Scholar]
- 7. Royal College of Obstetricians and Gynaecologists (RCOG) . Immediate management of major postpartum haemorrhage (PPH). Accessed January 17, 2021. https://www.rcog.org.uk/globalassets/documents/maternity‐emergencies/7‐major/postpartum‐haemorrhage‐algorithm‐‐checklists/postpartum‐haemorrhage‐pph‐algorithm‐‐‐june‐2019.pdf
- 8. Dutch Society of Obstatrics and Gynaecology (NVOG) . Wat is de rol van Tranexaminezuur. “Role of tranexamic acid in postpartum haemorrhage”. 2019. Accessed April 7, 2020. https://www.nvog.nl/wp‐content/uploads/2019/09/NVOG‐module‐Wat‐is‐de‐rol‐van‐tranexaminezuur‐2019‐.pdf
- 9. World Health Organization (WHO) . WHO recommendation on tranexamic acid for the treatment of postpartum haemorrhage. 2017. Accessed April 7, 2020. https://apps.who.int/iris/bitstream/handle/10665/259374/9789241550154‐eng.pdf;jsessionid=8CDD85DA0E8F1D51F1BCABA4350634FA?sequence=1 [PubMed]
- 10. Kutcher ME, Cripps MW, McCreery RC, et al. Criteria for empiric treatment of hyperfibrinolysis after trauma. J Trauma Acute Care Surg. 2012;73:87‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramler PI, Gillissen A, Henriquez DDCA, et al. Clinical value of early viscoelastometric point‐of‐care testing during postpartum hemorrhage for the prediction of severity of bleeding: a multicenter prospective cohort study in the Netherlands. Acta Obstet Gynecol Scand. 2021;100:1656‐1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gillissen A, van den Akker T, Caram‐Deelder C, et al. Comparison of thromboelastometry by ROTEM® Delta and ROTEM® Sigma in women with postpartum haemorrhage. Scand J Clin Lab Invest. 2019;79:32‐38. [DOI] [PubMed] [Google Scholar]
- 13. Dutch Society of Obstatrics and Gynaecology (NVOG) . Hemorrhagia postpartum “Postpartum haemorrhage”. 2015. Accessed April 7, 2020. https://www.nvog.nl/wp‐content/uploads/2018/02/Hemorrhagia‐postpartum‐HPP‐3.0‐14‐11‐2013.pdf
- 14. Mavrides E, Allard S, Chandraharan E, et al; On behalf of the Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum haemorrhage: green‐top guideline No. 52. BJOG. 2017;124:106‐149. [DOI] [PubMed] [Google Scholar]
- 15. Kim JS, Wang IJ, Yeom SR, et al. Usefulness of rotational thromboelastometry as a mortality predictor of hyperfibrinolysis in patients with severe trauma. Acute Crit Care. 2018;33:162‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lang T, Bauters A, Braun S, et al. Multi‐centre investigation on reference ranges for ROTEM. Blood Coagul Fibrinolysis. 2005;16:301‐310. [DOI] [PubMed] [Google Scholar]
- 17. Ilich A, Bokarev I, Key NS. Global assays of fibrinolysis. Int J Lab Hematol. 2017;39:441‐447. [DOI] [PubMed] [Google Scholar]
- 18. Schaap T, Bloemenkamp K, Deneux‐Tharaux C, et al. Defining definitions: a Delphi study to develop a core outcome set for conditions of severe maternal morbidity. BJOG. 2019;126:394‐401. [DOI] [PubMed] [Google Scholar]
- 19. Meher S, Cuthbert A, Kirkham JJ, et al. Core outcome sets for prevention and treatment of postpartum haemorrhage: an international Delphi consensus study. BJOG. 2019;126:83‐93. [DOI] [PubMed] [Google Scholar]
- 20. de Lange NM, van Rheenen‐Flach LE, Lancé MD, et al. Peri‐partum reference ranges for ROTEM® thromboelastometry. Br J Anaesth. 2014;112:852‐859. [DOI] [PubMed] [Google Scholar]
- 21. Roberts I, Shakur H, Fawole B, et al. Haematological and fibrinolytic status of Nigerian women with post‐partum haemorrhage. BMC Pregnancy Childbirth. 2018;18:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shakur H, Roberts I, Fawole B, et al. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post‐partum haemorrhage (WOMAN): an international, randomised, double‐blind, placebo‐controlled trial. Lancet. 2017;389:2105‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gillissen A, Henriquez DDCA, van den Akker T, et al. The effect of tranexamic acid on blood loss and maternal outcome in the treatment of persistent postpartum hemorrhage: a nationwide retrospective cohort study. PLoS One. 2017;12:e0187555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set that was generated and analyzed for this study is not publicly available because of the General Data Protection Regulation. A pseudonymized data set can be obtained via a repository from the corresponding author (j.g.van_der_bom@lumc.nl) on reasonable request only.


