Abstract
Introduction
Epithelial ovarian cancer (EOC) patients undergoing splenectomy during cytoreductive surgery represent a small subgroup of patients. Splenic metastases or technical reasons due to extensive upper abdominal disease may require a splenectomy. It has been hypothesized that as the spleen’s antitumor immunologic functions may inhibit cancer growth, splenectomy may promote the growth of residual disease as observed in other cancer types of murine studies. The few studies assessing the impact of splenectomy on the oncologic outcomes of advanced stage EOC patients have reported inconsistent results. It remains unclear whether splenectomy during cytoreductive surgery is justified to achieve complete cytoreduction. The aim of this study was to assess the impact of a splenectomy on perioperative outcomes and survival of advanced stage EOC patients.
Material and methods
In this nationwide population‐based study, all consecutive patients diagnosed with FIGO stage IIIC and IV EOC between 1 January 2008 and 31 December 2015 were identified from the Netherlands Cancer Registry. Patients who underwent cytoreductive surgery combined with platinum‐based chemotherapy as primary treatment were selected. Differences in clinicopathologic characteristics between splenectomy and non‐splenectomy patients were assessed. Progression‐free survival (PFS) and overall survival (OS) were analyzed using Kaplan–Meier survival curves and log‐rank tests. Cox proportional hazards models were used to adjust for covariates that influence survival.
Results
A total of 3911 patients were identified: 99 splenectomy and 3812 non‐splenectomy patients. Splenectomy patients were more likely to undergo extensive surgery or surgical reintervention, to receive intraperitoneal chemotherapy, intraoperative and postoperative blood transfusion, to experience postoperative infections, and to be admitted to an intensive care unit (all p < 0.002). No significant differences in PFS or OS were observed between splenectomy vs non‐splenectomy patients after adjusting for covariates.
Conclusions
Although advanced stage EOC patients who undergo splenectomy during cytoreductive surgery have less favorable perioperative outcomes, no adverse impact of splenectomy on the survival of advanced stage EOC patients was observed. Splenectomy during cytoreductive surgery seems to be justified to achieve complete cytoreduction in advanced stage EOC patients.
Keywords: epithelial ovarian cancer, perioperative outcomes, population‐based study, splenectomy, survival analyses
Abbreviations
- EOC
epithelial ovarian cancer
- FIGO
International Federation of Gynecology and Obstetrics
- NACT‐ICS
neoadjuvant chemotherapy after interval cytoreductive surgery
- OS
overall survival
- PCS
primary cytoreductive surgery
- PFS
progression‐free survival
Key message.
Advanced stage epithelial ovarian cancer patients who underwent splenectomy during cytoreductive surgery experienced more perioperative complications. Splenectomy during cytoreductive surgery does not negatively impact survival of advanced stage epithelial ovarian cancer patient and may extend the progression‐free survival of patients.
1. INTRODUCTION
Epithelial ovarian cancer (EOC) is the leading cause of death of gynecologic malignancies in the western world. 1 It has generally been accepted that EOC patients who undergo complete cytoreduction have better survival than those who underwent an optimal or incomplete cytoreduction (i.e. residual disease ≤1 or >1 cm, respectively). 2 Accordingly, radical surgery has been increasingly adopted to achieve a “no macroscopic residual disease” status. In some cases, extensive upper abdominal disease (eg omental cake or metastatic splenic involvement) may even require a splenectomy to ensure complete cytoreduction. However, there is limited knowledge about the impact of splenectomy on the long‐term outcome of patients. It has been hypothesized that as the antitumor immunologic functions of the spleen may inhibit cancer growth, splenectomy may promote the growth of residual disease during the postoperative period as observed in murine models of other cancer types. 3 , 4 , 5 , 6 Nevertheless, the role of the spleen in the antitumor immune response remains only partly understood, due to the contradictory literature on the relation between the function of the spleen and cancer growth. 7
Similarly, studies on the impact of splenectomy on the perioperative and survival outcomes of advanced stage EOC patients have also reported inconsistent results. For instance, some have stated that splenectomy at the time of cytoreductive surgery may contribute to achieving complete cytoreduction with low perioperative complications, implying survival benefit. 8 , 9 , 10 , 11 Conversely, another study suggested that although splenectomy during up‐front cytoreductive surgery is associated with acceptable perioperative complications, the added procedure appears to be associated with a shortened survival that seems to be unrelated to perioperative morbidity. 12 Nonetheless, the impact of splenectomy on the progression‐free or overall survival, while adjusting for other prognostic factors, could often not reliably be demonstrated due to the low sample sizes of these studies. 8 , 9 , 10 , 12 Moreover, their outcomes were mostly based on institution‐based data (rather than population‐based data), so it remains unclear to what extent patient selection for (surgical) treatment approaches might have affected their study outcomes. These studies also did not report data on patients who underwent neo‐adjuvant chemotherapy followed by interval cytoreductive surgery (NACT‐ICS) (but solely on patients who underwent primary cytoreductive surgery [PCS]). It thus remains to be determined whether the impact of splenectomy on perioperative and survival outcomes differs per treatment approach.
Although the impact of splenectomy on the surgical outcome of cytoreductive surgery may improve survival, the increased complication rate and/or the suppressed immunological effect may negatively influence the prognosis of advanced stage EOC patients. Therefore, the aim of this study is to assess the impact of a splenectomy during initial cytoreductive surgery on perioperative outcomes and survival of International Federation of Gynecology and Obstetrics (FIGO) stage IIIC and IV EOC patients using nationwide data.
2. MATERIAL AND METHODS
2.1. Data collection
In a retrospective cohort study, all patients consecutively diagnosed with FIGO stage IIIC and IV EOC, including peritoneal, ovarian and fallopian tube cancers (International Classification of Disease for Oncology [ICD‐O] codes C48.1, C48.2, C56.9 and C57.0), between 1 January 2008 and 31 December 2015 were identified from the Netherlands Cancer Registry (NCR). The NCR is a population‐based registry which is notified weekly of all newly histologically confirmed malignancies in the Netherlands through an automated nationwide pathology archive (PALGA). The NCR was initiated in 1989 and contains extensive data on all newly diagnosed malignancies in the Netherlands. It covers more than 95% of all histologically confirmed malignancies. 13 Dedicated registrars have previously extracted data on patient, tumor and treatment characteristics from patients’ medical records. The data collected include information on surgical procedures and perioperative outcomes. Complementary patient (eg Charlson Comorbidity Index) and follow‐up data (eg date of recurrence) were recently collected for a Dutch Cancer Society project (IKNL2014‐6838). To obtain recent information on vital status and date of death, the NCR is annually linked to municipality registries (where citizens’ data on death are registered by government officials as mandated by Dutch law).
2.2. Study population
Only FIGO stage IIIC and IV EOC patients who have undergone cytoreductive surgery combined with platinum‐based chemotherapy were selected. Surgical care for EOC patients is publicly available to all citizens owing to the Dutch healthcare system and is centralized in the Netherlands, where cytoreductive surgery is only performed in 16 high‐volume hospitals (i.e. secondary and tertiary centers) by experienced gynecologic‐oncologists. Splenectomy procedures were performed by surgical oncologists from the Departments of General Surgery who joined gynecologic‐oncologists during cytoreductive surgery. Patients who underwent a partial splenectomy were excluded from this study, since it is unclear to what extent this procedure affects the function of the spleen. Patients with missing information on the execution of a splenectomy during cytoreductive surgery were also excluded from this study. Finally, patients with unknown survival or recurrence data were excluded from the survival analyses.
2.3. Definitions
Residual disease after debulking was defined as the maximum diameter of the largest tumor nodule remaining after cytoreductive surgery: classified as no macroscopic (complete), ≤1 cm (optimal) or >1 cm (incomplete) residual disease. Progressive or recurrent disease has previously been defined as clinical signs of tumor growth, i.e. increase in CA‐125 serum levels (greater than or equal to twice the upper limit of CA‐125 serum level on two separate occasions at least one week apart) or the appearance of tumor lesions on imaging, either (re)growth of preexisting lesions or new lesions, combined with the clinical judgement of the treating medical oncologist or gynecologic‐oncologist. 14 Progression‐free survival (PFS) was defined as the time between the date of diagnosis and the date of progressive or recurrent disease, or death (whichever occurred first). Patients who were alive without a record of progressive or recurrent disease were censored at the date of their last hospital visit. Overall survival (OS) was defined as the time between the date of diagnosis and the date of death, or last follow‐up for patients who were still alive (31 January 2020). Postoperative complications were recorded if they occurred within 30 days after cytoreductive surgery and included complications such as infectious complications, surgical complications, thromboembolic events, reinterventions and intensive care unit (ICU) admissions.
2.4. Statistical analyses
Patients’ characteristics were summarized using descriptive statistics. Patients were divided into a splenectomy (i.e. exposed) and a non‐splenectomy (i.e. unexposed) group. Pearson χ2 test or Fisher’s exact test was used for categorical variables and two‐sample Wilcoxon rank‐sum test for continuous variables to compare the two groups. Kaplan–Meier survival curves, log‐rank tests and Cox proportional hazards models were used to analyze the PFS and OS. To assess whether splenectomy was independently associated with PFS or OS, the following established prognostic factors were included in the multivariable model: age, FIGO stage (FIGO stage IIB‐IIC vs. IIIA‐IIB vs. IIIC vs. IV), tumor grade (grade 1 vs. grade 2 vs. grade 3), treatment approach (NACT‐ICS vs. PCS) and residual disease after debulking (macroscopic free vs. ≤1 cm vs. >1 cm residual disease). All of the covariables were treated as categorical variables except for age at diagnosis, which was treated as a continuous variable. Proportional hazards assumption was tested for both survival analyses using the Schoenfeld residual test. If the assumption was violated, time‐varying covariates were included in the Cox proportional hazards models. Interaction effects were assessed using interaction terms and interaction plots were generated using margin plots to obtain the predicted hazard ratios of PFS or OS at each combination of the two variables which demonstrated interaction effects. If an interaction effect was statistically significant, that interaction term was included in the multivariable model. No sample size estimation were performed for this study. All statistical analyses were performed using STATA/SE, version 14.1 (StataCorp, College Station, TX, USA) and a p value of < 0.05 was considered statistically significant.
2.5. Study outcomes
Primary outcomes of our study were the perioperative and survival outcomes (PFS and OS). Secondarily, survival of the two groups was assessed through stratification by treatment approach (NACT‐ICS and PCS) and by the presence of solid splenic metastases (these included both clinically [CT‐imaging] and pathologically [splenic tissue] confirmed splenic metastases).
2.6. Ethical approval
Ethical approval for this study has been granted by the NCR Committee of Privacy (K20.157) on 12 August 2020.
3. RESULTS
3.1. Study population
A total of 6502 patients were diagnosed with FIGO stage IIB‐IV EOC between 1 January 2008 and 31 December 2015 in the Netherlands (~810 patients annually). Specifically, 5443 patients were diagnosed with FIGO stage IIIC and IV EOC. Of these patients, 3997 patients underwent cytoreductive surgery combined with platinum‐based chemotherapy. The 19 patients who underwent a partial splenectomy were excluded from this study. Data on the splenectomy procedure was unavailable for 67 patients and these were also excluded from this study. Finally, 99 patients were classified as splenectomy patients and 3812 patients as non‐splenectomy patients (see Figure 1).
FIGURE 1.
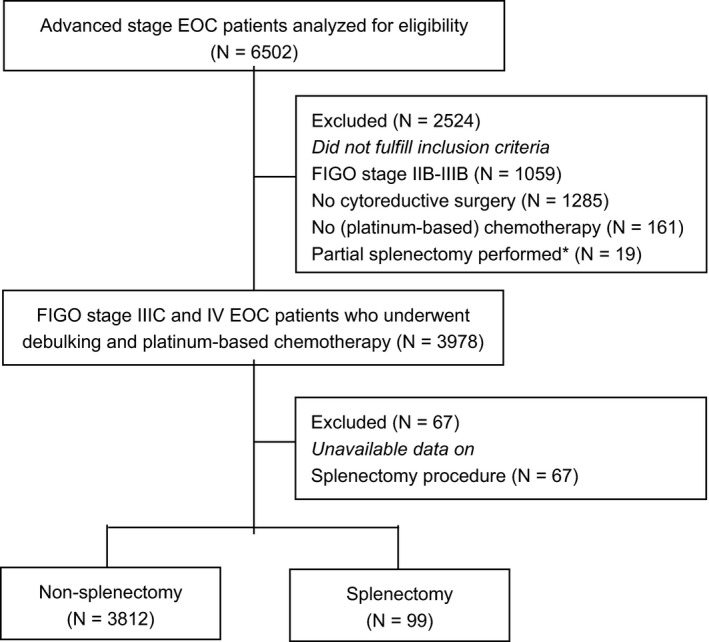
Flow chart of the study population. *A partial splenectomy refers to a procedure where only a part of the spleen is removed to preserve spleen and its functions. EOC, epithelial ovarian cancer
Patient, tumor and treatment characteristics are summarized in Table 1. For the splenectomy group, the median age at diagnosis was 63 years (range 24–79) compared with 65 years (range 20–91) for the non‐splenectomy group. Splenectomy patients comprised a similar percentage of patients with omental cake as the non‐splenectomy group (100% vs. 99.1%, p = 0.352). Splenectomy patients more often underwent extensive surgical procedures (ie bowel resection [56.7% vs. 20.8], diaphragmatic stripping [38.4% vs. 12.0%] or distal pancreatectomy [18.2% vs. 0.1%]), and relatively more often received intraperitoneal chemotherapy compared with the non‐splenectomy patients (11.1% vs. 4.0%) (all p < 0.002).
TABLE 1.
Characteristics of study population (n = 3911)
| Non‐splenectomy group [n = 3812, 97.5%] | Splenectomy group [ n = 99, 2.5%] | ||||
|---|---|---|---|---|---|
| Characteristic | No. of patients | (%) | No. of patients | (%) | p value |
| Age at diagnosis (in yrs) | <0.140* | ||||
| Median (range) | 65 | (20–91) | 63 | (24–79) | |
| FIGO stage | 0.211 † | ||||
| Stage IIIC | 2762 | (72.5) | 66 | (66.7) | |
| Stage IV | 1050 | (27.5) | 33 | (33.3) | |
| Tumor grade | 0.036 † | ||||
| Grade 1 | 135 | (3.5) | 8 | (8.1) | |
| Grade 2 | 341 | (9.0) | 6 | (6.1) | |
| Grade 3 | 1987 | (52.1) | 43 | (43.4) | |
| Missing (n = 1391) | 1349 | (35.4) | 42 | (42.4) | |
| Histologic subtype | 0.317 † | ||||
| Serous | 3071 | (80.6) | 90 | (90.9) | |
| Mucinous | 68 | (1.8) | 0 | (0) | |
| Endometrioid | 118 | (3.1) | 1 | (1.0) | |
| Clear cell | 100 | (2.6) | 1 | (1.0) | |
| Adenocarcinoma NOS a | 407 | (10.7) | 7 | (7.1) | |
| Other a | 48 | (1.2) | 0 | (0) | |
| Karnofsky score (PS) | 0.447 † | ||||
| 10–50 | 24 | (0.6) | 0 | (0) | |
| 60–100 | 1865 | (48.9) | 45 | (45.5) | |
| Missing (n = 1977) | 1923 | (50.5) | 54 | (54.5) | |
| Pretreatment CA−125 level (in kU/L) | <0.049 * | ||||
| Median (range) | 665 | (3–60 000) | 809 | (18–22 300) | |
| Missing (n = 147) | 142 | (3.7) | 5 | (5.1) | |
| BRCA status | 0.946 † | ||||
| Negative | 977 | (25.6) | 28 | (28.3) | |
| BRCA 1 | 204 | (5.3) | 5 | (5.1) | |
| BRCA 2 | 101 | (2.7) | 3 | (3.0) | |
| Missing (n = 2593) | 2530 | (66.4) | 63 | (63.6) | |
| Presence of ascites | 0.354 † | ||||
| No | 1619 | (42.5) | 37 | (37.4) | |
| Yes | 2192 | (52.5) | 62 | (62.6) | |
| Missing (n = 1) | 1 | (0) | 0 | (0) | |
| CCI (in points) b | 0.255 † | ||||
| 0 | 280 | (7.3) | 11 | (11.1) | |
| 1–2 | 1730 | (45.4) | 47 | (47.5) | |
| ≥3 | 1802 | (47.3) | 41 | (41.4) | |
| Solid splenic metastasis c | <0.001 † | ||||
| No | 3787 | (99.3) | 81 | (81.8) | |
| Yes | 25 | (0.7) | 18 | (18.2) | |
| Presence of omental cake | 0.352 † | ||||
| No | 33 | (0.9) | 0 | (0) | |
| Yes | 3777 | (99.1) | 99 | (100) | |
| Missing (n = 2) | 2 | (0) | 0 | (0) | |
| Treatment approach | 0.501 † | ||||
| PCS | 1091 | (28.6) | 25 | (25.3) | |
| NACT‐ICS | 2721 | (71.4) | 74 | (74.7) | |
| Bowel resection | <0.001 * | ||||
| No | 3017 | (79.1) | 43 | (43.4) | |
| Yes | 791 | (20.8) | 56 | (56.6) | |
| Missing (n = 4) | 4 | (0.1) | 0 | (0) | |
| Diaphragmatic stripping | <0.001 * | ||||
| No | 3348 | (87.8) | 61 | (61.6) | |
| Yes | 457 | (12.0) | 38 | (38.4) | |
| Missing (n = 7) | 7 | (0.2) | 0 | (0) | |
| Lymphadenectomy | 0.760* | ||||
| No | 3322 | (87.2) | 88 | (88.9) | |
| Yes | 484 | (12.7) | 11 | (11.1) | |
| Missing (n = 6) | 6 | (0.1) | 0 | (0) | |
| Distal pancreatectomy | <0.001 * | ||||
| No | 3806 | (99.8) | 81 | (81.8) | |
| Yes | 4 | (0.1) | 18 | (18.2) | |
| Missing (n = 2) | 2 | (0.1) | 0 | (0) | |
| Intraperitoneal chemotherapy d | <0.002 * | ||||
| No | 3661 | (96.0) | 88 | (88.9) | |
| Yes | 151 | (4.0) | 11 | (11.1) | |
Abbreviations: BRCA, breast cancer gene; CA‐125, cancer antigen 125; CCI, Charlson Comorbidity Index; FIGO, International Federation of Gynecology and Obstetrics; NACT‐ICS, neoadjuvant chemotherapy followed by interval cytoreductive surgery; NOS, not otherwise specified; PCS, primary cytoreductive surgery; PS, performance score.
Statistically significant values (p<0.05) are in bold.
The subcategory “other” in the category “histologic subtype” comprises the patients with other histologic subtypes than noted such as Brenner, undifferentiated, mixed or other carcinoma. The subcategory “adenocarcinoma NOS” may consist of a large part of “serous” type of EOC.
In accordance with the National Cancer Institute’s Charlson Comorbidity index, patients only received points for solid or metastatic tumors if other cancer types (with an incidence date of 5 years prior to or 30 days after the diagnosis date of advanced EOC) were present.
Solid splenic metastasis is based on information of clinically (CT‐imaging) and pathologically (splenic tissue) confirmed metastases.
This variable includes both intraperitoneal chemotherapy and hyperthermic intraperitoneal chemotherapy. Inconsistent data on chemotherapy regimen did not allow for these two regimens to be analyzed separately.
Wilcoxon rank‐sum test.
Fisher’s exact or Pearson χ2 test.
3.2. Perioperative outcomes
The perioperative outcomes are shown in Table 2. A slightly higher percentage of patients with complete cytoreduction (56.6% vs. 48.4%) and a lower percentage of patients with incomplete cytoreduction (7.1% vs. 12.6%) were observed in the splenectomy group than in the non‐splenectomy group. However, these discrepancies were not statistically significant (p = 0.156). Splenectomy patients had relatively more intraoperative blood loss compared with the non‐splenectomy patients (median 1545 vs. 600 ml). Accordingly, the splenectomy group received more intraoperative (44.4% vs. 21.6%) and postoperative blood transfusions (44.4% vs. 21.1%) compared with the non‐splenectomy group. Splenectomy patients were also more likely to experience postoperative infections (15.2% vs. 4.3%), to undergo surgical reintervention (12.0% vs. 3.0%) and to be admitted to an intensive care unit (ICU) (28.3% vs. 8.5%). The median hospital length‐of‐stay (10 vs. 7 days) and time to start adjuvant chemotherapy after cytoreductive surgery (35.5 vs. 29 days) were prolonged for the splenectomy patients when compared with the non‐splenectomy patients (all p < 0.001).
TABLE 2.
Perioperative outcomes (n = 3911)
| Non‐splenectomy group [n = 3812, 97.5%] | Splenectomy group [n = 99, 2.5%] | ||||
|---|---|---|---|---|---|
| Characteristic | No. of patients | (%) | No. of patients | (%) | p value |
| Residual disease after debulking | 0.156 † | ||||
| No macroscopic disease | 1846 | (48.4) | 56 | (56.6) | |
| ≤1 cm | 1433 | (37.6) | 35 | (35.3) | |
| >1 cm | 481 | (12.6) | 7 | (7.1) | |
| Missing (n = 53) | 52 | (1.4) | 1 | (1.0) | |
| Intraoperative estimated blood loss (mL) | <0.001 * | ||||
| Median (range) | 600 | (50–4600) | 1545 | (400–6900) | |
| Missing (n = 270) | 265 | (7.0) | 5 | (5.1) | |
| Intraoperative blood transfusion | <0.001 † | ||||
| No | 2550 | (66.9) | 43 | (43.4) | |
| Yes | 824 | (21.6) | 44 | (44.5) | |
| Missing (n = 450) | 438 | (11.5) | 12 | (12.1) | |
| Intraoperative blood transfusion (mL) | <0.001 * | ||||
| Median (range) | 600 | (100–6000) | 1150 | (300–5100) | |
| Not applicable (n = 2593) | 2550 | (66.9) | 43 | (43.4) | |
| Missing (n = 450) | 438 | (11.5) | 12 | (12.1) | |
| Postoperative blood transfusion | <0.001 † | ||||
| No | 2538 | (66.6) | 43 | (43.4) | |
| Yes | 805 | (21.1) | 44 | (44.5) | |
| Missing (n = 481) | 469 | (12.3) | 12 | (12.1) | |
| Postoperative infection a | <0.001 † | ||||
| No | 3650 | (95.8) | 84 | (84.8) | |
| Yes | 162 | (4.2) | 15 | (15.2) | |
| Thromboembolic events b | 0.318 † | ||||
| No | 3774 | (99.0) | 99 | (100) | |
| Yes | 38 | (1.0) | 0 | (0) | |
| Surgical reintervention | <0.001 † | ||||
| No | 3696 | (97.0) | 87 | (87.9) | |
| Yes | 114 | (3.0) | 12 | (12.1) | |
| Missing (n = 2) | 2 | (0) | 0 | (0) | |
| Postoperative ICU stay | <0.001 † | ||||
| No | 3389 | (88.9) | 68 | (68.7) | |
| Yes | 325 | (8.5) | 28 | (28.3) | |
| Missing (n = 101) | 98 | (2.6) | 3 | (3.0) | |
| Length‐of‐stay at ICU (days) | 0.193* | ||||
| Median (range) | 2 | (2–30) | 3 | (1–15) | |
| Not applicable (n = 3457) | 3389 | (88.9) | 68 | (68.7) | |
| Missing (n = 101) | 98 | (2.6) | 3 | (3.0) | |
| Length‐of‐stay at hospital (days) | <0.001 † | ||||
| Median (range) | 7 | (1–123) | 10 | (4–55) | |
| Missing (n = 2) | 2 | (0) | 0 | (0) | |
| TTC (days) c | <0.001 * | ||||
| Median (range) | 29 | (0–307) | 35.5 | (20–136) | |
| Missing (n = 161) | 156 | (4.1) | 5 | (5.1) | |
| 30‐day mortality | 0.703 † | ||||
| No | 3785 | (99.3) | 99 | (100) | |
| Yes | 23 | (0.6) | 0 | (0) | |
| Missing (n = 4) | 4 | (0.1) | 0 | (0) | |
| Recurrent or progressive disease | 0.906 † | ||||
| No | 738 | (19.4) | 20 | (20.2) | |
| Yes | 3068 | (80.5) | 79 | (79.8) | |
| Missing (n = 6) | 6 | (0.1) | 0 | (0) | |
Abbreviations: ICU, intensive care unit; TTC, time to start adjuvant chemotherapy.
Statistically significant values (p<0.05) are in bold.
The variable “postoperative infection” includes postoperative infections ranging from surgical site infections to systemic infections.
The variable “thromboembolic events” includes both deep venous thrombosis and pulmonary embolism.
The variable “Time To start Adjuvant Chemotherapy” comprises the time interval between cytoreductive surgery and the start of adjuvant chemotherapy.
Wilcoxon rank‐sum test.
Fisher’s exact or Pearson’s χ2 test.
3.3. Survival outcomes
No significant differences in PFS and OS were observed between the splenectomy and non‐splenectomy patients. When adjusted for FIGO stage, tumor grade, treatment approach and residual disease after debulking, splenectomy was not independently associated with PFS (hazard ratio [HR] 0.60, 95% confidence interval [CI] 0.36–1.02]). Multivariable analysis demonstrated that the effect of splenectomy on the PFS was dependent on treatment approach. The joint effect of splenectomy and the treatment approach on the hazard estimates of PFS is demonstrated on the interaction plot (Figure S1). Moreover, when adjusted for age, FIGO stage, tumor grade, treatment approach and residual disease after debulking, splenectomy was also not independently associated with OS (HR 0.97, 95% CI 0.77–1.22). The Kaplan–Meier estimates of the progression‐free survival and overall survival are demonstrated in Figures 2 and 3, respectively. The Cox proportional hazards models for PFS and OS with their crude and adjusted hazard ratios are listed in Tables 3 and 4, respectively.
FIGURE 2.
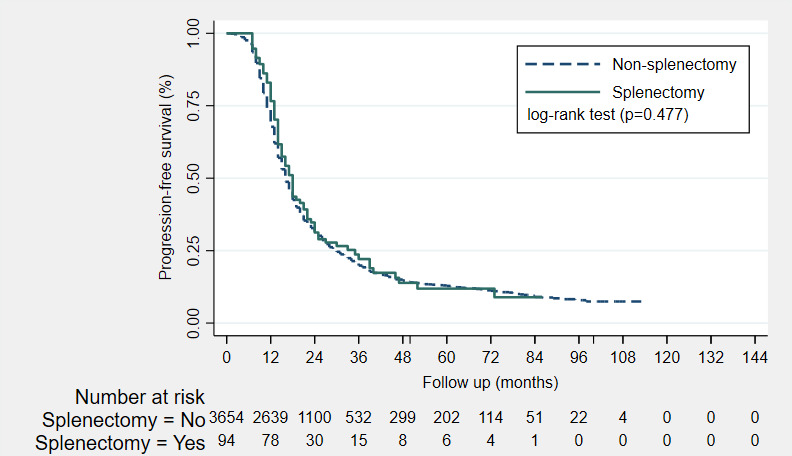
Kaplan–Meier estimates of the progression‐free survival (PFS) of non‐splenectomy patients (n = 3654, dash line) and splenectomy patients (n = 94, solid line). The median PFS in months were 16 and 18 months for the non‐splenectomy and splenectomy patients, respectively. No significant difference in PFS was observed with the log‐rank test (p = 0.477). *An additional 163 patients were excluded from the survival analysis with reference to Figure 1, since these patients had unknown follow‐up or survival data
FIGURE 3.
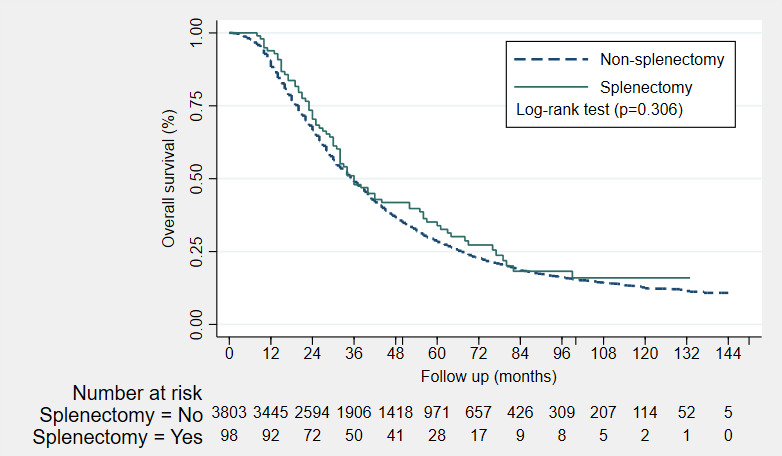
Kaplan–Meier estimates of the overall survival (OS) of non‐splenectomy patients (n = 3803, dash line) and splenectomy patients (n = 98, solid line). The median OS in months was 36 months for both the non‐splenectomy and splenectomy patients. No significant difference in OS was observed with the log‐rank test (p = 0.306). *An additional 10 patients were excluded from the survival analysis with reference to Figure 1, since these patients had unknown follow‐up or survival data
TABLE 3.
Cox proportion hazards model reporting crude hazard ratios and adjusted hazards ratios of progression‐free survival (n = 3748) a
| Characteristic | Crude HR | (95% CI) | Adjusted HR | (95% CI) |
|---|---|---|---|---|
| FIGO stage b | ||||
| Stage IIIC | Reference | Reference | ||
| Stage IV | 1.29 | (1.19 – 1.40) | 1.37 | (1.18–1.59) |
| Tumor grade | ||||
| Grade 1 | Reference | Reference | ||
| Grade 2 | 1.46 | (1.17–1.84) | 1.38 | (1.10–1.74) |
| Grade 3 | 1.33 | (1.09–1.63) | 1.27 | (1.04–1.56) |
| Unknown | 1.50 | (1.22–1.84) | 1.28 | (1.04–1.58) |
| Treatment approach c | ||||
| PCS | Reference | Reference | ||
| NACT‐ICS | 1.52 | (1.40–1.65) | 3.02 | (1.69–5.39) |
| Residual disease after debulking b | ||||
| No macroscopic disease | Reference | Reference | ||
| >1 cm | 1.69 | (1.56–1.82) | 0.71 | (0.58–0.83) |
| ≤1 cm | 2.60 | (2.32–2.90) | 1.24 | (1.05–1.44) |
| Splenectomy c | ||||
| No | Reference | Reference | ||
| Yes | 0.92 | (0.74–1.16) | 0.60 | (0.36–1.02) |
| Treatment approach # Splenectomy | ||||
| NACT‐ICS & Splenectomy | NA | NA | 1.95 | (1.08–3.49) |
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not applicable; NACT‐ICS, neoadjuvant chemotherapy followed by interval cytoreductive surgery; PCS, primary cytoreductive surgery.
An additional 163 and 209 patients were excluded respectively from the univariable and multivariable Cox regression analysis with reference to Figure 1, since these patients had unknown data on recurrence data or one of the other variables included in the multivariable model.
These variables (FIGO stage and residual disease after debulking) are time‐varying covariates and were included in the multivariable model as such.
These variables (treatment approach and splenectomy) demonstrated interaction effects. Therefore the interaction term “treatment approach#splenectomy” was included in the model.
The # demonstrates that this was an interaction effect.
TABLE 4.
Cox proportional hazards model reporting crude hazard ratios and adjusted hazards ratios of overall survival (n = 3848) a
| Characteristic | Crude HR | (95% CI) | Adjusted HR | (95% CI) |
|---|---|---|---|---|
| Age at diagnosis (in years) b | ||||
| 1.02 | (1.01–1.02) | 1.02 | (1.01–1.03) | |
| FIGO stage b | ||||
| Stage IIIC | Reference | Reference | ||
| Stage IV | 1.33 | (1.23–1.44) | 1.35 | (1.17–1.56) |
| Tumor grade | ||||
| Grade 1 | Reference | Reference | ||
| Grade 2 | 1.51 | (1.21–1.89) | 1.33 | (1.06–1.67) |
| Grade 3 | 1.36 | (1.11–1.66) | 1.21 | (0.99–1.48) |
| Unknown | 1.57 | (1.28–1.92) | 1.25 | (1.02–1.54) |
| Treatment approach | ||||
| PCS | Reference | Reference | ||
| NACT‐ICS | 1.56 | (1.44–1.69) | 1.57 | (1.44–1.71) |
| Residual disease after debulking b | ||||
| No macroscopic disease | Reference | Reference | ||
| ≤1 cm | 1.65 | (1.53–1.78) | 2.15 | (1.90–2.42) |
| >1 cm | 2.86 | (2.58–3.19) | 4.24 | (3.53–5.10) |
| Splenectomy | ||||
| No | Reference | Reference | ||
| Yes | 0.89 | (0.71–1.12) | 0.97 | (0.77–1.22) |
Abbreviations: CI, confidence interval; HR, hazard ratio; NACT‐ICS, neoadjuvant chemotherapy followed by interval cytoreductive surgery; PCS, primary cytoreductive surgery.
An additional 63 patients were excluded respectively from multivariable Cox regression analysis with reference to Figure 1, since these patients had unknown data on recurrence data or one of the other variables included in the multivariable model.
These variables (age at diagnosis, FIGO stage and residual disease after debulking) are time‐varying covariates and were included in the multivariable model as such.
3.4. Survival outcomes stratified by treatment approach
Stratification by treatment approach demonstrated a prolonged median PFS of patients who underwent PCS with splenectomy (n = 23, median OS of 32 months) compared with patients who underwent PCS without splenectomy (n = 1038, median OS of 20 months) (p = 0.043). However, no increase in median PFS was observed for patients who underwent NACT‐ICS with splenectomy (n = 71, median OS of 16 months) compared with patients who underwent NACT‐ICS without splenectomy (n = 2616, median OS of 15 months) (p = 0.614). No statistically significant difference in median OS was demonstrated between patients who underwent PCS with splenectomy (n = 25, median OS of 63 months) and patients who underwent PCS without splenectomy (n = 1091, median OS of 48 months) (p = 0.134). Consistently, no difference in median OS was found between patients who underwent NACT‐ICS with splenectomy (n = 73, median OS of 32 months) and patients who underwent NACT‐ICS without splenectomy (n = 2714, median OS of 32 months) (Figures S2 and S3).
3.5. Survival outcomes stratified by splenic metastases
Stratification by solid splenic metastases did not demonstrate a statistically significant difference in median OS between patients with splenic metastasis who underwent splenectomy (n = 18, median OS of 52 months) and those who did not undergo splenectomy (n = 25, median OS of 33 months) (p = 0.162). Similarly, no statistically significant difference in median OS between patients without splenic metastasis who underwent splenectomy (n = 80, median OS of 34 months) those who did not undergo splenectomy (n = 3778, median OS of 36 months) was found (p = 0.759) (Figure S4).
4. DISCUSSION
In this nationwide study, the prognostic impact of splenectomy during cytoreductive surgery on perioperative outcomes and survival of FIGO stage IIIC and IV EOC patients was assessed. Splenectomy patients underwent significantly more extensive surgical procedures, probably due to their widespread (upper) abdominal disease and, consequently, experienced more perioperative complications compared with non‐splenectomy patients. Survival analyses suggested that patients who underwent PCS with splenectomy had an extended median PFS compared with patients who underwent PCS without splenectomy. No other significant differences in median PFS and OS were observed between splenectomy and non‐splenectomy patients after correction for other prognostic factors.
In accordance with the literature, our data show that splenectomy during cytoreductive surgery is indeed rarely performed in patients with advanced stage EOC disease. In this study, ~2.5% of patients with advanced EOC underwent splenectomy during initial cytoreductive surgery, similar to the previously reported incidences of 1.3%–13.8% of other population‐based studies. 9 , 12 , 15 Conversely, recent studies in which patients were selected based on their feasibility of attaining complete cytoreduction reported higher percentages of patients who underwent splenectomy during cytoreductive surgery, probably due to the low proportion of NACT‐ICS patients or time period of more radical surgery. 16 Furthermore, ~1.1% of the study population had isolated splenic metastases (n = 43), confirming that this occurrence is uncommon in EOC. Although the nationwide registry did not provide data on the exact indication of splenectomy among the patients, information on which patients had splenic metastases and which patients had omental cake was available. Most patients probably underwent a splenectomy due to technical reasons relating to perisplenic disease (82%) instead of direct metastatic involvement of the spleen (18%). Consistently, Magtibay et al. also reported that patients were more likely to undergo a splenectomy due to technical reasons (42 of 66 patients, 63.6%) rather than splenic metastases (24 of 66 patients, 36.4%) in primary treatment. 10 Other studies did not disclose the percentage of patients who underwent a splenectomy due to splenic metastases or due to technical reasons. 8 , 9 , 11 , 12 , 17
Splenectomy patients were more likely to undergo other extensive upper abdominal surgical procedures (eg bowel resection or diaphragmatic stripping) in addition to splenectomy. Accordingly, relatively fewer patients with incomplete cytoreduction and slightly more patients with complete cytoreduction were observed in the splenectomy group than in the non‐splenectomy group (p = 0.156). Although no data were available to quantify the extent of disease prior to surgery (eg the Sugarbaker peritoneal cancer index [PCI]), the aforementioned finding suggests that splenectomy is mainly performed during cytoreductive surgery if this additional procedure increases the probability of successful complete cytoreduction. 10 , 18 , 19 Similarly, Zapardiel et al. did not find significant differences in residual disease after cytoreduction between splenectomy and non‐splenectomy patients (which may also be due to their matching of cases). 8 Other studies did not report the rates of complete cytoreduction among splenectomy patients compared with non‐splenectomy patients. 10 , 12
On account of the more radical surgical procedures being performed, higher rates of perioperative complications were observed in the splenectomy group. In particular, the rate of postoperative infections varying from surgical site infections to sepsis was higher among splenectomy patients. Specifically, a total of six splenectomy patients developed sepsis (6.1%) compared with 47 non‐splenectomy patients (1.2%) (p < 0.001). Nevertheless, it was unclear to what extent sepsis was a direct result of splenectomy or because of other surgical procedures (eg bowel resection). Other reports also found slightly higher percentages of patients developing sepsis in the splenectomy group compared to the non‐splenectomy group (3%–12.2% vs. 1%–9%, respectively). 8 , 12 , 17 Magtibay et al. reported that five of the 112 patients who underwent splenectomy during primary or secondary debulking (4.5%) developed sepsis. Of these five patients, three patients died from septic shock. 10 However, no cases of sepsis could be directly attributed to the splenectomy. Despite a relatively more complicated postoperative recovery period (i.e. a prolonged hospital length‐of‐stay) among splenectomy patients, no differences in the 30‐day mortality were found between the groups in our study. These findings are in line with the results of other reports. 8 , 12 , 17
Joneborg et al. found that upper abdominal surgery does not prolong time to adjuvant chemotherapy despite an increased rate of postoperative complications and longer length of hospital stay. 20 Our results suggest, in contrast, that splenectomy patients have a longer time to adjuvant chemotherapy compared with non‐splenectomy patients owing to the relatively longer recovery period due to a higher rate of postoperative complications (eg more postoperative infections, more surgical reinterventions, more postoperative ICU admissions, and longer hospital length‐of‐stay), which is a result of the more extensive surgical procedures. Delayed initiation of adjuvant chemotherapy after complete cytoreductive surgery has been found to be an independent prognostic factor for shortened overall survival. 21 Nevertheless, the median time to adjuvant chemotherapy of splenectomy patients is within the advised 5–6 weeks after cytoreductive surgery. 21
McCann et al. demonstrated that patients who underwent PCS with splenectomy resulting in maximum ≤1 cm residual disease had shortened OS compared with patients who underwent PCS without splenectomy (median OS 30 vs. 45 months, p < 0.045). 12 Our results suggested a favorable median PFS for patients who underwent PCS with splenectomy as opposed to patients who underwent PCS without splenectomy. Patients who underwent PCS with splenectomy comprised a larger proportion of patients younger than 64 years and a smaller proportion of patients older than 75 years compared with patients who underwent PCS without splenectomy. In addition, patients who underwent PCS with splenectomy more often underwent aggressive tumor‐reductive abdominal procedures (eg bowel resection and diaphragmatic stripping). It might therefore be possible that the patients who underwent PCS with splenectomy underwent more radical procedures where the gynecologic‐oncologists endeavored to attain the maximum surgical effort, which could explain the extended progression‐free survival. Another, albeit speculative, explanation might be that splenectomy may even have inhibited tumor growth or the development of metastases of EOC by modulating anti‐tumor adaptive and innate immune responses as observed in murine models of other cancer types (eg lung cancer, mammary cancer or hepatocellular cancers). 7 , 22 , 23 Nevertheless, this finding is based on small number of patients and no significant differences in median OS were observed between splenectomy and non‐splenectomy patients (even after stratification by treatment approach). Other studies did not observe an independent association of splenectomy on both PFS or OS of advanced stage EOC patients. 8 , 9 , 17
Our results suggested that patients who underwent NACT‐ICS had worsened PFS (adjusted HR 3.02, 95% CI 1.69–5.39) and OS (adjusted HR 1.57, 95% CI 1.44–1.71) compared with patients who underwent PCS. However, it remains unclear whether it is due to the NACT‐ICS approach itself or other reasons (eg aggressive tumor biology). Ongoing trials such as the “Trial of Radical Upfront Surgical Therapy” (TRUST) trial may further determine this issue. 24
Two studies have reported that the presence of a solitary splenic metastasis is associated with shortened OS in advanced stage EOC patients. 15 , 25 Stratifying survival outcomes by splenic metastasis in our data, did not demonstrate a decreased OS of patients with splenic metastasis compared with patients without splenic metastasis. Herewith, it can possibly be concluded that the amount of residual disease at the end of cytoreductive surgery rather than the initial bulk of tumor seems to be of significant importance for the oncologic outcomes of this group of patients. 8
This population‐based study reports the largest cohort of patients undergoing splenectomy as part of primary treatment of advanced stage EOC patients. Additionally, our data demonstrated the impact of splenic metastasis and treatment approach on the survival of EOC patients. Nonetheless, several limitations apply to our study. Despite the robust population‐based registry with a large sample size, our study is also based on a small number of patients who underwent splenectomy as part of initial cytoreductive surgery. Additionally, the lack of data on surgical complexity scores (eg Sugarbaker’s PCI or Mayo surgical complexity scores) prevented adjustment of the extent of disease prior to surgery in our analysis, which might have impaired the external validity of our study. Similarly, no data on chemotherapy regimens other than the primary chemotherapy regimens were available for survival analyses. Insufficient data on thromboembolic events also limited the assessment of the risk of thromboembolic events in advanced stage EOC patients undergoing splenectomy. In addition, unfortunately no data on the occurrence of certain infections (eg pneumonia) or other long‐term effects of splenectomy were available.
5. CONCLUSION
Despite the small number of patients who underwent splenectomy as part of initial cytoreductive surgery and the increased rate of perioperative complications, splenectomy at the time of cytoreductive surgery does not seem to negatively affect oncologic outcomes of advanced stage EOC patients and seems to be justified to achieve complete cytoreduction.
AUTHOR CONTRIBUTIONS
All authors: Conceptualization, methodology, visualization, writing ‐ review & editing. Additionally, SAS carried out formal analysis, investigation and writing original draft; MAvdA carried out formal analysis and supervision; GV carried out formal analysis and investigation; JAdH and AMvA carried out supervision.
CONFLICT OF INTEREST
None.
Supporting information
Fig S1‐S4
ACKNOWLEDGMENTS
The authors thank the registration team of the Netherlands Comprehensive Cancer Organization (IKNL) for the collection of data for the Netherlands Cancer Registry.
Said SA, van der Aa MA, Veldmate G, de Hullu JA, van Altena AM. Oncologic outcomes after splenectomy during initial cytoreductive surgery in advanced epithelial ovarian cancer: a nationwide population‐based cohort study. Acta Obstet Gynecol Scand.2022;101:56–67. doi: 10.1111/aogs.14286
Funding information
This work was supported by the Dutch Cancer Society [IKNL2014‐6838].
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Chang SJ, Bristow RE. Evolution of surgical treatment paradigms for advanced‐stage ovarian cancer: redefining ‘optimal’ residual disease. Gynecol Oncol. 2012;125:483‐492. [DOI] [PubMed] [Google Scholar]
- 3. Cadili A, de Gara C. Complications of splenectomy. Am J Med. 2008;121:371‐375. [DOI] [PubMed] [Google Scholar]
- 4. Jeong O, Kim HG, Ryu SY, Park YK, Jung MR. Adverse prognostic impact of splenectomy on survival in gastric carcinoma patients: Regression and propensity score matching analysis of 1074 patients. PLoS One. 2018;13:e0203820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Higashijima J, Shimada M, Chikakiyo M, et al. Effect of splenectomy on antitumor immune system in mice. Anticancer Res. 2009;29:385‐393. [PubMed] [Google Scholar]
- 6. Ge YG, Gao H, Kong XT. Changes of peripheral T‐cell subsets in asplenic W256 tumor‐bearing rats. J Surg Oncol. 1989;42:60‐68. [DOI] [PubMed] [Google Scholar]
- 7. Prehn RT. The paradoxical effects of splenectomy on tumor growth. Theor Biol Med Model. 2006;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zapardiel I, Peiretti M, Zanagnolo V, et al. Splenectomy as part of primary cytoreductive surgery for advanced ovarian cancer: a retrospective cohort study. Int J Gynecol Cancer. 2012;22:968‐973. [DOI] [PubMed] [Google Scholar]
- 9. Sun H, Bi X, Cao D, et al. Splenectomy during cytoreductive surgery in epithelial ovarian cancer. Cancer Manag Res. 2018;10:3473‐3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Magtibay PM, Adams PB, Silverman MB, Cha SS, Podratz KC. Splenectomy as part of cytoreductive surgery in ovarian cancer. Gynecol Oncol. 2006;102:369‐374. [DOI] [PubMed] [Google Scholar]
- 11. Chen LM, Leuchter RS, Lagasse LD, Karlan BY. Splenectomy and surgical cytoreduction for ovarian cancer. Gynecol Oncol. 2000;77:362‐368. [DOI] [PubMed] [Google Scholar]
- 12. McCann CK, Growdon WB, Munro EG, et al. Prognostic significance of splenectomy as part of initial cytoreductive surgery in ovarian cancer. Ann Surg Oncol. 2011;18:2912‐2918. [DOI] [PubMed] [Google Scholar]
- 13. Kleppe M, van der Aa MA, Van Gorp T, Slangen BF, Kruitwagen RF. The impact of lymph node dissection and adjuvant chemotherapy on survival: A nationwide cohort study of patients with clinical early‐stage ovarian cancer. Eur J Cancer. 2016;66:83‐90. [DOI] [PubMed] [Google Scholar]
- 14. Timmermans M, Zwakman N, Sonke GS, et al. Perioperative change in CA125 is an independent prognostic factor for improved clinical outcome in advanced ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2019;240:364‐369. [DOI] [PubMed] [Google Scholar]
- 15. Hanprasertpong J, Fujiwara K. Splenectomy and surgical cytoreduction in epithelial ovarian cancer: a review. Eur J Cancer Care (Engl). 2011;20:287‐293. [DOI] [PubMed] [Google Scholar]
- 16. Harter P, Sehouli J, Lorusso D, et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N Engl J Med. 2019;380:822‐832. [DOI] [PubMed] [Google Scholar]
- 17. Eisenkop SM, Spirtos NM, Lin WC. Splenectomy in the context of primary cytoreductive operations for advanced epithelial ovarian cancer. Gynecol Oncol. 2006;100:344‐348. [DOI] [PubMed] [Google Scholar]
- 18. Jónsdóttir B, Lomnytska M, Poromaa IS, Silins I, Stålberg K. The Peritoneal Cancer Index is a strong predictor of incomplete cytoreductive surgery in ovarian cancer. Ann Surg Oncol. 2021;28:244‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359‐374. [DOI] [PubMed] [Google Scholar]
- 20. Joneborg U, Palsdottir K, Farm E, Johansson H, Salehi S. Time‐interval to adjuvant chemotherapy and postoperative management after upper abdominal surgical procedures in advanced ovarian cancer. Eur J Surg Oncol. 2021;47:353‐359. [DOI] [PubMed] [Google Scholar]
- 21. Timmermans M, van der Aa MA, Lalisang RI, et al. Interval between debulking surgery and adjuvant chemotherapy is associated with overall survival in patients with advanced ovarian cancer. Gynecol Oncol. 2018;150:446‐450. [DOI] [PubMed] [Google Scholar]
- 22. Levy L, Mishalian I, Bayuch R, Zolotarov L, Michaeli J, Fridlender ZG. Splenectomy inhibits non‐small cell lung cancer growth by modulating anti‐tumor adaptive and innate immune response. Oncoimmunology. 2015;4:e998469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Long X, Wang J, Zhao JP, et al. Splenectomy suppresses growth and metastasis of hepatocellular carcinoma through decreasing myeloid‐derived suppressor cells in vivo. J Huazhong Univ Sci Technolog Med Sci. 2016;36:667‐676. [DOI] [PubMed] [Google Scholar]
- 24. Reuss A, du Bois A, Harter P, et al. TRUST: trial of radical upfront surgical therapy in advanced ovarian cancer (ENGOT ov33/AGO‐OVAR OP7). Int J Gynecol Cancer. 2019;29:1327‐1331. [DOI] [PubMed] [Google Scholar]
- 25. Tanner EJ, Long KC, Feffer JB, et al. Parenchymal splenic metastasis is an independent negative predictor of overall survival in advanced ovarian, fallopian tube, and primary peritoneal cancer. Gynecol Oncol. 2013;128:28‐33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S4


