Abstract
Outbred OF1 mice were immunized subcutaneously with flu vaccine, either in the neck or in the lumbar region (back), in combination with adjuvants inducing either a Th1- or a Th2-type response, referred to as adjuvants A1 and A2, respectively. After two parenteral immunizations, the mice were boosted intranasally with nonadjuvanted vaccine. The serum response was analyzed after each immunization by measuring specific immunoglobulin A (IgA), IgG1, and IgG2a antibody levels, while the local response (same isotypes) was measured in the salivary glands after the mucosal boost by ELISPOTs. We observed that systemic priming at any of the two sites with a Th2 rather than a Th1 adjuvant dramatically enhanced the mucosal IgG1 and IgA responses following a mucosal boost with unadjuvanted vaccine. In addition, as judged by the IgG2a/IgG1 ratios and serum IgA levels, immunization of mice in the back induced a rise in Th2 response compared to neck immunization with adjuvant A1. In contrast, such back immunization with adjuvant A2 reversed the Th1-Th2 balance in favor of the Th1 response compared to neck immunization. Similar differences were observed in mucosal antibody levels according to the site of priming with one given adjuvant; priming in the back with adjuvant A1 increased the mucosal IgA and IgG1 responses compared to neck priming, while the local IgG2a levels were decreased. The reverse was true for adjuvant A2. Back versus neck priming with this latter adjuvant decreased the mucosal IgG1 response, while local IgG2a levels were increased. The different lymphatic drainages of the two sites of parenteral immunization may explain these differences, due to the targeting of particular lymphoid inductive sites. Some of these sites may represent crossroads between systemic and mucosal immunity.
Most current vaccines are administered by parenteral routes, and humans are preferentially injected in the arm or in the scapular region (deltoid muscle). On the other hand, a large number of preclinical studies are performed in mice. The site of systemic immunization varies according to different studies and different researchers, although it may have a critical influence on the outcome of induced immune responses. For instance, a clear link has been established in mice between the site of inoculation and the outcome of the infection caused by Leishmania major (8, 14, 15). Differences in levels of infection were also correlated with different Th1-Th2 balances, and the role of the lymphatic drainages was questioned in that respect (16). Lymphatic routes have also been considered in studies initiated by T. Lehner and coworkers to target a systemic response to mucosal surfaces and further protect against a mucosal challenge in the simian immunodeficiency virus model (5, 9, 10). Other experiments have also evaluated the influence of parenteral priming in the induction of mucosal responses following mucosal boosts, with contradictory results (for a review, see reference 17), but neither the influence of the site of systemic immunization nor the nature of the adjuvant was investigated. In our own laboratory, we had noticed on several occasions that not only the adjuvant but also the site of systemic immunization could influence the qualitative serum and local responses against different antigens (data not shown). We have therefore readdressed the role of the site of parenteral immunization with regard to systemic and mucosal responses following a mucosal boost. The flu vaccine was used as an antigen, and the importance of the nature of the adjuvant used for parenteral priming was addressed within the same experiment, as it might dramatically influence the quality of the immune response, both locally and peripherally. Thus, we used a derivative of saponins inducing a predominant Th1 response (6), referred to as adjuvant A1, and a phosphopolymer inducing a predominant Th2 response, referred to as adjuvant A2. Both adjuvants were used for systemic priming by performing two successive subcutaneous immunizations in the neck or in the lumbar region (referred to as the back), while a mucosal boost was performed nasally with unadjuvanted vaccine in a third step. Finally, we used outbred mice in order to be closer to the natural human or animal heterogeneous situation.
MATERIALS AND METHODS
Antigens and adjuvants.
The monovalent flu vaccine A/Texas, batch M1ATM02, was prepared in Pasteur Merieux Sérums et Vaccins (Marcy l’Etoile, France). Adjuvant 1 (A1) was a derivative of saponins (6); adjuvant 2 (A2) was a phosphopolymer (Virus Research Institute, Cambridge, Mass.). These adjuvants were chosen with regard to their immunological characteristics (Th2-Th1 profile). Based on the serum immunoglobulin G1 (IgG1)/IgG2a ratio, gamma interferon, and interleukin-4 levels obtained in our lab after immunization with different antigens, adjuvant A1 (saponin family) was classified as a predominant Th1 inducer, while adjuvant A2 (phosphopolymer) was classified as a predominant Th2 inducer (reference 4 and data not shown).
Mice and immunizations.
Outbred Swiss female mice 6 to 8 weeks old were purchased from Janvier (Le Genest Saint Isle, France). During the studies, cages were covered (with Isocaps) and mice were given filtered water. Irradiated food and autoclaved material were used.
Mice were immunized subcutaneously in the back or in the neck at days 0 and 21 with the equivalent of 5 μg of hemagglutinin (HA) per mouse with 15 μg of adjuvant A1 or 100 μg of adjuvant A2. Eight control mice (unimmunized) were analyzed in parallel (serum and local responses). At day 42, all mice except controls were boosted intranasally while awake with an equivalent of 10 μg of unadjuvanted HA (20 μl/nostril). Mice were sacrificed at day 56, and salivary glands were sampled for analysis of mucosal responses by enzyme-linked immunospot (ELISPOT) assay. Blood samples were taken at days 21, 42, and 56 for analysis of serum responses by enzyme-linked immunosorbent assay (ELISA).
ELISAs.
ELISAs were performed according to standard protocols (biotinylated conjugates and streptavidin-peroxidase complex were from Amersham (Buckinghamshire, United Kingdom), and ortho-phenylenediamine dihydrochloride substrate was from Sigma (St. Louis, Mo.). Plates (Maxisorb; Nunc) were coated overnight at 4°C with flu vaccine (5 μg/ml) in carbonate buffer. After saturation with bovine serum albumin (Sigma), plates were incubated with the sera (1.5 h), biotinylated conjugate (1.5 h), streptavidin peroxidase complex (1 h), and substrate (10 min). The titers were expressed as the inverse of the dilution giving 50% of the maximal absorbance value at 490 nm.
ELISPOT assay.
ELISPOT assays with salivary gland cells were performed as previously described (2) by adapting the technique of Mega et al. (12). Salivary glands were taken just after sacrifice and placed immediately in RPMI 1640 medium (Gibco, Paisley, United Kingdom). The organs were cut into small pieces (1 by 1 mm) with an automated tissue chopper (McIllwain tissue chopper; Mickle Laboratory Engineering, Gilford, United Kingdom) and then digested in 4 ml of RPMI 1640 medium containing 5% fetal calf serum (FCS) and 1 mg of collagenase type IV (Sigma) per ml for 30 min at 37°C under gentle agitation. The digested cells and fragments were passed through a 70-μm-pore-size filter (Falcon), and the digestion was repeated three more times. The digested cells were pooled and washed twice in a large volume of medium. The red cells were then lysed with Gey’s solution for 4 min on ice. After two more washes, the cells were resuspended in 4 ml of medium (plus 5% FCS) and counted. Cells were aliquoted in 96-well plates with nitrocellulose bottoms (Millipore, Bedford, Mass.) that had been coated overnight with a dilution of flu vaccine corresponding to 25 μg of HA per ml in phosphate-buffered saline (PBS) and then saturated with complete medium for 1 h at 37°C. Two fivefold dilutions of the cells were loaded into the wells (100 μl/well) in quadruplicate for each dilution and each isotype. After 16 h at 37°C under 5% CO2, the cells were lysed 3 times for 5 min in PBS-Tween 20 (0.005%), and biotinylated anti-isotype antibodies (Amersham) were added for 2 h at room temperature (dilution, 1/1,000). After three washes with PBS-Tween, biotinylated streptavidin-peroxidase complex (Amersham) was added for 1 h at a 1/500 dilution, and after three more washes with PBS, the spots were revealed with 1 mM 3-amino-9-ethylcarbazole (Sigma). Once the plates dried, the spots were counted under a dissecting microscope (magnification, ×16 or 40). Only dark-brown circular and regular spots, which clearly varied from the occasionally seen background, were counted. The values represent the means of eight wells for each individual mouse, expressed as spots per million cells.
Statistical analysis.
Comparison between geometric means of antibody titers and spot numbers was evaluated by analysis of variance. Differences were considered significant when P was <0.05. The numbers of mice that responded by the ELISPOT assay were compared by Fisher’s exact test.
RESULTS
Serum response.
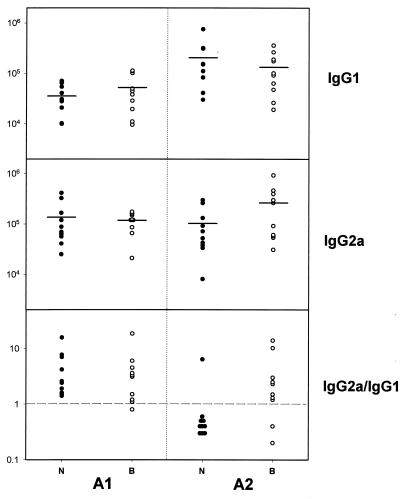
The serum responses differed between mice immunized at the same site depending upon which one of the two adjuvants was used. The observed differences between groups were of similar amplitude for each time point considered. The parenteral boost induced in all mice an equivalent increase in IgG serum titers that were unaffected by the mucosal boost (data not shown). Therefore, only results corresponding to day 42 are presented in Fig. 1. When used in the neck, adjuvant A2 induced stronger IgG1 titers and lower IgG2a/IgG1 ratios than A1 (P < 0.001 in both cases), in agreement with our previous data showing that A1 was a Th1-type adjuvant, while A2 was a Th2-type adjuvant (4). All control mice presented negative responses (data not shown).
FIG. 1.
Serum IgG1 and IgG2a responses and IgG2a/IgG1 ratios for the different groups as measured after two systemic immunizations. Titers are expressed as the inverse of the dilution giving 50% of the maximal absorbance value at 492 nm (log scale); IgG2a/IgG1 ratio represents the ratio between the corresponding ELISA titers in each individual mouse. A1, mice primed in the presence of adjuvant A1; A2, mice primed in the presence of adjuvant A2; B, mice primed in the back (open circles); N, mice primed in the neck (closed circles). Means are represented by horizontal bars. Negative controls (eight mice) were negative for all isotypes considered (data not shown).
However, further differences between serum levels in mice immunized in the neck and back were observed for each adjuvant considered. Back versus neck immunization with adjuvant A2 resulted in higher IgG2a titers and in an inversion of the IgG2a/IgG1 ratios; 8 of 10 mice immunized in the back had a ratio superior to 1, compared to only 1 of 10 mice immunized in the neck (P < 0.001). In contrast, back versus neck immunization with adjuvant A1 induced no significant changes in the IgG2a/IgG1 ratios, although back immunization favored high IgG1 levels, while neck immunization favored high IgG2a responses (Fig. 1).
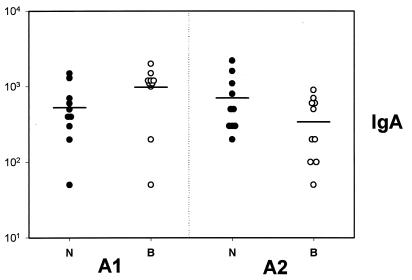
Specific serum IgA levels were detectable only 2 weeks after the mucosal boost (day 56); at this time point, significant interactions were observed between neck and back immunization and adjuvant. Back versus neck priming with A1 increased serum IgA levels, while the opposite was true for adjuvant A2; in addition, neck priming was more effective for A2 than A1, while back priming was more effective for A1 than for A2 (P < 0.05) (Fig. 2).
FIG. 2.
Serum IgA responses as measured 2 weeks after the nasal boost. A1, mice primed in presence of adjuvant A1; A2, mice primed in presence of adjuvant A2; B, mice primed in the back (open circles); N, mice primed in the neck (closed circles). Means are represented by horizontal bars. Negative controls (eight mice) were negative (data not shown).
Mucosal responses.
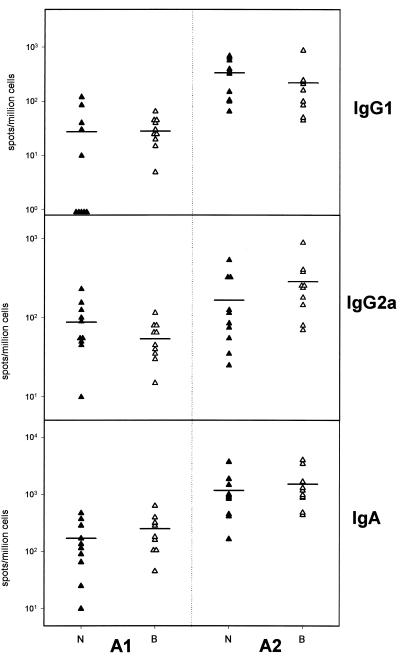
The mucosal responses induced by nasal boost after priming at the same site with A1 or A2 were analyzed at day 56. Figure 3 shows that dramatically higher local IgG1 and IgA responses (average, 10-fold; P < 0.0001 and P < 0.001, respectively) were observed in mice primed with A2 rather than A1; these higher levels were more pronounced in mice immunized in the neck. Local IgG2a levels were also increased by A2 priming, but only in mice immunized in the back (P < 0.001). Negative controls presented negative responses (data not shown).
FIG. 3.
Values for local IgA, IgG1, and IgG2a measured by ELISPOTs in the salivary glands 2 weeks after the nasal boost and expressed as number of spots per million cells. A1, mice primed in presence of adjuvant A1; A2, mice primed in the presence of adjuvant A2; B, mice primed in the back (open triangles); N, mice primed in the neck (closed triangles). Means are represented by horizontal bars. Negative controls (eight mice) were negative for all isotypes considered (data not shown).
Additional differences were observed depending upon the immunization site. In mice immunized with adjuvant A1, the local IgG1 and IgA responses were increased in mice immunized in the back versus mice immunized in the neck (about twofold in mean values; P<0.01 for IgA), while IgG2a levels were decreased (about twofold in mean values). Immunization in the back with adjuvant A2 induced the opposite effect of immunization in the neck on mucosal responses in the salivary glands. A stronger median local IgG2a response was observed in the former group, while IgG1 responses were decreased. Except for IgA with A1, analysis of variance showed no significant differences between local levels according to immunization site. However, a higher number of nonresponders for the IgG1 isotype with adjuvant A1 were observed in mice immunized in the neck versus mice immunized in the back (5 of 10 versus 1 of 10; P < 0.065). In addition, for the same adjuvants at the same sites, IgG1 and IgA responses and IgG2a responses evolved in opposite directions, consistent with the evolution toward a Th1- or Th2-type response.
DISCUSSION
Our work with outbred mice illustrates the complexity of the interactions between systemic and mucosal immunity and points out the roles of adjuvant and immunization site.
First of all, the nature of the two adjuvants used for priming dramatically modified the outcome of the local response induced by a mucosal boost with unadjuvanted vaccine. Regardless of the immunization site, mucosal IgA, IgG1, and, to a lesser extent, IgG2a responses were dramatically enhanced in mice primed with A2 rather than A1. The need for a Th2 environment for the induction of mucosal responses (11, 17) may explain these observations. Although cells mobilized at the periphery or in mucosal inductive sites present different homing receptors and follow different routes (11, 17), this is not an all-or-none phenomenon, and interactions between mucosal and systemic immunity are clearly exemplified by our present findings.
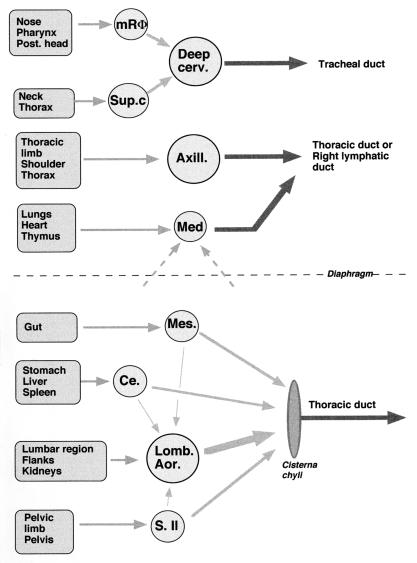
We also observed, as judged by IgG2a/IgG1 ratios and serum IgA levels, that back versus neck immunization with flu vaccine induced opposite Th1/Th2 shifts in the systemic responses depending upon which adjuvant was used. Our data are in agreement with the results obtained by other researchers (8, 14–16) in a murine model of leishmaniasis indicating the importance of the inoculation site with respect to rate of infection and to qualitative immune response against Leishmania. In our study, back priming with adjuvant A1 favored a Th2 shift, while the opposite was true with adjuvant A2. Different hypotheses can be made, first taking into consideration lymphatic drainages (1). As shown in Fig. 4, immunization in the lumbar region would have indirectly targeted the abdominal lymph nodes, including the celiac nodes draining the spleen. In contrast, neck immunization would have mainly targeted the deep cervical lymph nodes. While the qualitative and/or quantitative immune responses developing in different inductive sites may vary (12), amplification of the responses with specific antigenic formulations would have different consequences. This would explain why A2 was a stronger Th2 adjuvant than A1 in mice immunized in the neck but not in mice immunized in the back. We surmise that responses induced in the spleen and/or abdominal lymph nodes with the A2-flu formulation were shifted toward a Th1 type more than responses induced in the peripheral lymph node and that the opposite was true for the A1-flu formulation. Additional work measuring responses in the spleen or in the draining lymph nodes is required to confirm this hypothesis.
FIG. 4.
Simplified representation of the lymphatic drainages of the infra- and supradiaphragmatic regions of the body in mammals, obtained from the book of R. Barone (1). Arrows represent afferent and efferent lymphatic vessels. Circles correspond to lymph nodes. Abbreviations correspond to the following lymph nodes: mRΦ, median retropharyngeal; Deep cerv., deep cervical; Sup.c, superficial cervical; Axill., axillary; Med, mediastinal; Mes., mesenteric; Ce., celiac; Lomb. Aor., lumbo-aortic; S.Il, sacroiliac.
In addition to the adjuvant, the antigen itself likely plays a critical role. Indeed, it is the combination of antigen and adjuvant that may finally determine the quality of the induced response. For instance, we have observed, using Helicobacter pylori urease in naive mice, that a dramatic shift (10- to 50-fold) toward a Th2 response was induced when adjuvant A1 or A2 was used in the back, the opposite of what we observed with flu antigen in this study (data not shown). This may be due to different effects on antigen presenting cells or to more- or less-efficient immune targeting, as the efficacy of lymphatic uptake may be dramatically modified by the physical nature of the vaccinal preparation (13).
Moreover, inducing an immune response in specific lymph nodes by systemic immunization should further modulate a subsequent mucosal response developing through the same crossroad lymph nodes (5, 9, 10). In the case of neck immunization (Fig. 4), nasal boost would have targeted the same deep cervical lymph nodes targeted by parenteral priming, which would then represent a crossroads between mucosal and systemic immune responses. In addition, regardless of which lymph node is targeted, the nature of the adjuvant should play a critical role, as observed here. Compared with neck immunization, priming in the back with a Th1 adjuvant (A1) resulted, after the nasal boost, in an enhanced local IgG1 and decreased IgG2a local response, while priming with a Th2 adjuvant (A2) resulted in the reverse situation. These local differences were in agreement with the systemic differences but do not reflect simply the peripheral situation. There was no correlation between the overall levels of systemic and local IgA responses (Fig. 2 and 3) or between the levels of systemic and local responses for each isotype in individual mice (data not shown). In addition, the difference between local IgG1 levels in mice immunized in the back versus mice immunized in the neck with A1 adjuvant almost reached significance only in the case of local responses, indicating that local mechanisms as well as systemic mechanisms were involved. All the experiments were performed in parallel, with the same materials and reagents. The existence of nonresponding mice for local response (IgG1 and A1) thus reflects the heterogeneity linked to outbred mice and is not due to technical problems.
We did not measure in the present study the intestinal response occurring after the nasal boost, as such a boost would not have been an optimal way to target abdominal nodes. However, due to the existence of a common mucosal system (11, 17) and according to our hypothesis, back immunization should have been able to further modulate the mucosal intestinal response so that its effect was symmetric to that of neck immunization on the mucosal response in salivary glands (Fig. 4). In that case the lumbar-aortic lymph nodes would play the same role as deep cervical lymph nodes do in neck immunization. Supporting this hypothesis, we have recently observed in a murine model of H. pylori infection that protection was better after parenteral immunization with adjuvanted urease was performed in the back rather than in the neck (4a). We surmised in these studies that indirectly targeting celiac and lumbo-aortic lymph nodes draining both spleen and stomach would, in turn, have enhanced gastric immune response and protection. Indeed, we used the same strategy in monkeys to perform therapeutic immunization against Helicobacter infection and reduced infection by targeting parenteral immunization in the lumbar region or by combining it with oronasal immunization (3). Other researchers have observed that parenteral priming in the hips enhanced a subsequent mucosal intestinal response against Shigella flexneri (7), and this may also support our hypothesis (Fig. 4).
In conclusion, our results raised with flu antigen, together with our unpublished observations obtained with other antigens, demonstrate that parenteral immunization at one given site with different adjuvants determines the outcome of systemic as well as mucosal responses after a mucosal boost with unadjuvanted antigen. Our results may explain some discrepancies that have been observed between different studies with different adjuvants and different routes. The most striking evidence in the present study is the importance of systemic priming with a Th2 adjuvant to enhance a subsequent Th2 mucosal response. However, due to the unique nature of each combination of adjuvant and antigen, our work does not allow the proposal of a general rule to orientate systemic and local responses in one desired direction by immunization at different sites. Each situation should be tested and compared in this respect, particularly if the aim is to induce a protective response against a mucosal pathogen.
ACKNOWLEDGMENTS
We acknowledge P. Meulien for constant support and E. Trannoy for critical reading of the manuscript.
REFERENCES
- 1.Barone R. Anatomie comparée des mammifères domestiques. 5. Angiologie. Paris, France: Vigot; 1996. pp. 667–864. [Google Scholar]
- 2.Erdile L, Guy B. OspA lipoprotein of Borrelia burdogferi is a mucosal immunogen and adjuvant. Vaccine. 1997;15:988–996. doi: 10.1016/s0264-410x(96)00295-2. [DOI] [PubMed] [Google Scholar]
- 3.Guy B, Hessler C, Fourage S, Lecoindre P, Chevalier M, Peyrol S, Boude M, Haensler J, Rokbi B, Quentin-Millet M J. Mucosal, systemic or combined therapeutic immunizations in cynomolgus monkeys naturally infected with Gastrospirillum hominis like organisms. Vaccine Res. 1998;6:141–150. [Google Scholar]
- 4.Guy B, Hessler C, Fourage S, Haensler J, Vialon-Lafay E, Rokbi B, Quentin Millet M J. Systemic immunization with urease protects mice against Helicobacter pylori infection. Vaccine. 1998;16:850–856. doi: 10.1016/s0264-410x(97)00258-2. [DOI] [PubMed] [Google Scholar]
- 4a.Guy, B., C. Hessler, S. Fourage, B. Rokbi, and M.-J. Quentin-Millet. Comparison between targeted and untargeted systemic immunization with adjuvanted urease to cure H. pylori infection in mice. Vaccine, in press. [DOI] [PubMed]
- 5.Kawabata S, Miller C J, Lehner T, Fujihashi K, Kubota M, McGhee J, Imaoka K, Hiroi T, Kyono H. Induction of Th2 cytokine expression for p27-specific IgA B cell responses after targeted lymph node immunization with simian immunodeficiency virus antigens in Rhesus macaques. J Infect Dis. 1998;177:26–33. doi: 10.1086/513811. [DOI] [PubMed] [Google Scholar]
- 6.Kensil C R, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol. 1991;146:431–439. [PubMed] [Google Scholar]
- 7.Keren D F, McDonald R A, Carey J L. Combined parenteral and oral immunization results in an enhanced mucosal IgA response to Shigella flexneri. Infect Immun. 1988;56:910–915. doi: 10.1128/iai.56.4.910-915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkpatrick C E, Nolan T J, Farrel J P. Rate of Leishmania-induced skin-lesion development in rodents depends on the site of inoculation. Parasitology. 1987;94:451–465. doi: 10.1017/s0031182000055803. [DOI] [PubMed] [Google Scholar]
- 9.Lehner T, Bergmaier L, Tao L, Panagiotidi C, Klavinskis L S, Hussain L, Ward R G, Meyers N, Adams S E, Gearing A J H, Brookes R. Targeted lymph node immunization with SIV p27 antigen to elicit genital, rectal and urinary immune responses in nonhuman primates. J Immunol. 1994;153:1858–1868. [PubMed] [Google Scholar]
- 10.Lehner T, Wang Y, Cranage M, Bergmaier L A, Mitchell E, Tao L, Hall G, Dennis M, Cook N, Brookes R, Klavinskins L, Jones I, Doyle C, Ward R. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat Med. 1996;2:767–775. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 11.McGhee J R, Kiyono H. Mucosal immunity to vaccines: current concepts for vaccine development and immune response analysis. In: Ciardi J E, et al., editors. Genetically engineered vaccines. New York, N.Y: Plenum Press; 1992. pp. 3–12. [DOI] [PubMed] [Google Scholar]
- 12.Mega J, McGhee J R, Kiyono H. Cytokine and Ig-producing cells in mucosal effector tissues: analysis of IL5 and IFNγ producing T cells, T cell receptor expression, and IgA plasma cells from mouse salivary gland associated tissues. J Immunol. 1992;148:2030–2038. [PubMed] [Google Scholar]
- 13.Moghimi S M, Rabaji-Siahboomi A R. Advanced colloid-based systems for efficient delivery of drugs and diagnostic agents to the lymphatic tissues. Prog Biophys Mol Biol. 1996;65:221–249. doi: 10.1016/s0079-6107(96)00012-0. [DOI] [PubMed] [Google Scholar]
- 14.Nabors G, Farrel J P. Site-specific immunity to Leishmania major in SWR mice: the site of infection influences susceptibility and expression of the antileishmanial immune response. Infect Immun. 1994;62:3655–3662. doi: 10.1128/iai.62.9.3655-3662.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nabors G, Nolan T, Croop W, Li J, Farrell J P. The influence of the site of parasite inoculation on the development of Th1 and Th2 type immune responses in (Balb/C × C57B1/6) F1 mice infected with Leishmania major. Parasite Immunol. 1995;17:569–579. doi: 10.1111/j.1365-3024.1995.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 16.Poulter L W, Pandolph C R. Mechanisms of immunity to leishmaniasis. IV. Significance of lymphatic drainage from the site of infection. Clin Exp Immunol. 1982;48:396–406. [PMC free article] [PubMed] [Google Scholar]
- 17.Walker R L. New strategies for using mucosal vaccination to achieve more effective immunization. Vaccine. 1994;12:387–400. doi: 10.1016/0264-410x(94)90112-0. [DOI] [PubMed] [Google Scholar]