Abstract
In recent years, LASER has been introduced as a minimally invasive treatment for a broad range of vaginal and vulvar symptoms and diseases. However, the efficacy and safety of vaginal and vulvar LASER has continuously been questioned. The aim of this study is to create an overview of the current literature and discuss the controversies within the use of LASER for genitourinary syndrome of menopause, vulvovaginal atrophy, urinary incontinence and lichen sclerosus. A search string was built in PubMed. The search was commenced on August 25, 2021 and closed on October 27, 2021. Two authors screened the studies in Covidence for inclusion according to the eligibility criteria in the protocol. The data were extracted from the studies and are reported in both text and tables. This review included 114 papers, of which 15 were randomized controlled trials (RCTs). The effect of LASER as a vaginal treatment was investigated for genitourinary syndrome of menopause in 36 studies (six RCTs), vulvovaginal atrophy in 34 studies (four RCTs) and urinary incontinence in 30 studies (two RCTs). Ten studies (three RCTs) investigated the effect of vulvar treatment for lichen sclerosus. Half of the included RCTs, irrespective of indication, did not find a significant difference in improvement in women treated with vaginal CO2 or Er:YAG LASER compared with their respective controls. However, most non‐comparative studies reported significant improvement after exposure to vaginal or vulvar LASER across all indications. Included studies generally had a short follow‐up period and only a single RCT followed their participants for more than 6 months post treatment. Adverse events were reported as mild and transient and 99 studies including 51 094 patients provided information of no serious adverse events. In conclusion, this review found that the effect of vaginal and vulvar LASER decreases with higher study quality where potential biases have been eliminated. We therefore stress that all patients who are treated with vaginal or vulvar LASER should be carefully monitored and that LASER for those indications as a treatment should be kept on a research level until further high‐quality evidence is available.
Keywords: atrophy, CO2 LASER, genitourinary syndrome, incontinence lichen, vaginal LASER
Abbreviations
- AE
adverse event
- Er:YAG
erbium:yttrium‐aluminum‐garnet
- GSM
genitourinary syndrome of menopause
- ICIQ‐UI‐SF
International Consultation on Incontinence Questionnaire ‐ Urinary Incontinence—Short Form
- IQR
interquartile range
- LASER
light amplification by stimulated emission of radiation
- LS
lichen sclerosus
- Nd:YAG
neodymium‐doped:yttrium‐aluminum‐garnet
- RCT
randomized controlled trial
- SAE
severe adverse event
- UI
urinary incontinence
- VAS
Visual Analog Scale
- VVA
vulvovaginal atrophy
Key message.
LASER technology is not yet recommended for routine treatment of genitourinary syndrome of menopause, vulvovaginal atrophy, urinary incontinence or lichen sclerosus, as high‐quality studies, including RCTs, are missing within the field. However, in the more than 50 000 women having LASER in studies, no serious short‐term adverse events are described.
1. INTRODUCTION
Female urogenital disorders affect the quality of life in several ways, physically, socially, emotionally and sexually, as detected in a study which found that more than 45% of postmenopausal women experience bothersome symptoms related to genitourinary syndrome of menopause (GSM), possibly having a negative impact on quality of life. This reflects the importance of an innovative approach within the therapeutic field of urogenital diseases. 1
The diagnostic term GSM was introduced in 2014 by North American Menopause Society and refers to vaginal, sexual and urinary symptoms caused by an estrogen deficiency in menopausal women and cancer survivors. This new diagnostic term has not replaced the diagnostic term vulvovaginal atrophy (VVA), which is characterized by vaginal dryness, burning, itching and pain. In many women, VVA and urinary incontinence (UI) occur at the same time. 2 , 3 Types of UI comprise stress UI, urge UI and mixed UI. UI may be associated with estrogen deficiency, which leads to a change in the metabolism of the connective tissue and pelvic floor dysfunction. 4 Treatment of symptoms related to the estrogen deficiency consist of hormonal treatment (estrogen, dehydroepiandrosterone (DHEA), etc.) and non‐hormonal treatment (lifestyle changes, moisturizers, etc.); however, women with relative contraindications to hormonal therapy are seeking non‐hormonal options such as light amplification by stimulated emission of radiation (LASER) technology. Studies have suggested that LASER technology may also help patients who suffer from vulvar lichen sclerosus (LS). 5 , 6
LASER has been used as a minimally invasive technology for a selection of diseases and symptoms within the gynecologic field for some years. Carbon dioxide (CO2) LASER was one of the earliest LASERs to appear in the 1960s, along with the erbium:yttrium‐aluminum‐garnet (Er:YAG) LASER and the neodymium‐doped:yttrium‐aluminum‐garnet (Nd:YAG) LASER. 7 In July 2018, the U.S. Food and Drug Administration released an alert about adverse events (AE) related to the vaginal LASER based on 14 cases of vaginal burns, scarring, acute and chronic pain. 8 , 9 In 2019, Preti et al. released a best practice document questioning the clinical trials and evidence behind the use of LASER in gynecology. Today, LASER is not recommended for general gynecologic use. 10
Vaginal and vulvar LASER are performed with a handpiece and each of the impulses is fired by the treating operator, who decides the number of impulses; the treatment takes only a few minutes. The LASER generates small impulses which exit through a small window affecting the mucosa of the tissue. 11 Previous cohort studies (Table 1) reported the histologic and immunologic effects of LASER, which encompass a change in epithelial proliferation and cellularity. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 Biopsies have shown that the lamina propria in the vaginal mucosa developed neo‐angiogenesis 12 , 14 , 15 , 16 , 17 , 20 and neo‐collagenesis, 14 , 15 representing a higher concentration of cytokines and fibroblasts. 12 , 14 , 15 , 16 , 17 , 18 , 19 Nevertheless, these studies do not differentiate between regeneration and healing from LASER, which questions the durability of the LASER effect. In a randomized controlled trial (RCT), Mackowa et al. investigated the histology in menopausal animals and concluded that Er:YAG LASER was not better than sham‐LASER and was inferior to estrogen replacement for increasing epithelial thickness. 21
TABLE 1.
Histologic and immunologic findings
| Indication | LASER | Author | Country | Design | Follow‐up a | Sample size, n | Age (years) b ; menopause status | Treatment settings | No. treatments, interval | Conclusion | Adverse events | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GSM | CO2 | Pagano et al. (2021) 12 | Italy | Cohort | N/A | 20 | 58.7 ± 6.6; Postmenopausal | Internal: 30 W, stack 1–3. External: 24 W, stack 1 | 3 sessions, 1 months | Remodeling of vulvar connective tissue, improvement in vulvar epithelium trophism, and neovascularization | N/A | |
| Athanasiou et al. (2016) 13 | Greece | Cohort | 3 months, first | 53 | 57.2 ± 5.4; Postmenopausal | 40 W, stack 1–3 | 3 sessions, 1 months | Significant reduction in vaginal pH, increase in Lactobacillus morphotypes and improvement in vaginal epithelia | No SAE. Transient: mild irritation of the introitus | |||
| UI | Er:YAG | Lapii et al. (2017) 14 | Russia | Cohort | 2 months, last | 98 | 49.0 ± 12.5; N/A | 2940 nm | 2 sessions, 1–1.5 months | Neo‐collagengenesis. Elastogenesis. Neo‐angiogenesis. Reduction of epithelial degeneration and atrophy. Improvement in fibroblast population | N/A | |
| Lapii et al. (2017) 15 | Russia | Cohort | 2 months, last | 18 | 49 ± 12.5; N/A | 2940 nm | N/A | Significant improvement in Ki‐67‐labeled nuclei. Epithelial proliferative activity. Neocollagenogenesis. Neoangiogenesis. High concentration of elastic fibers | N/A | |||
| VVA | CO2 | Salvatore et al. (2018) 16 | Italy | Cohort | N/A | 1 | 63; Postmenopausal | 30 W | 1 session | Thicker epithelium, and cells are larger. Connective tissue different; numerous papillae, richer in blood vessels, and many fibroblasts | N/A | |
| Zerbinati et al. (2014) 17 | Italy | Other | 2 months, last | 5 | 57 (54–63); Postmenopausal | 100 mJ | 1 session | Improvement in fibroblasts and rough endoplasmatic reticulum. Thicker epithelium. Large amount of glycogen. Improvement in capillaries | No SAE | |||
| Becorpi et al. (2018) 18 | Italy | Cohort | 1 months, last | 20 | 58.2; Postmenopausal | 30 W, stack 1 | N/A | High remodeling status in vaginal epithelium is demonstrated by the significant changes in inflammatory and modulatory cytokine patterns. No significant change in the bacteria | N/A | |||
| Salvatore et al. (2015) 19 | Italy | Cohort | N/A | 5 | 63 (57–71); Postmenopausal | 30 W | 1 session | Changes in the epithelium and lamina propria in relation to mild ablative effects, fibroblasts activation, modifications of collagen, elastic fibers, and mucopolysaccharides in the lamina propria | N/A | |||
| Er:YAG | Gaspar et al. (2020) 20 | Argentina | Cohort | 6 months, last | 10 | 60.6 ± 6.82; Postmenopausal | 6.0 J/cm2 | 2 sessions, 1 months | Improvement in epithelial thickness. Significant improvement in glycogen load, new papillae and neo‐angiogenesis in lamina propria with capillaries reaching the epithelium | No SAE |
General characteristics, findings, and adverse events in included studies. The table is sorted by (1) treatment indication, (2) LASER type, (3) year of publication and (4) author name.
Abbreviations: cm2, square centimeter(s); CO2, Carbon Dioxide LASER; Er:YAG, Erbium:Yttrium‐Aluminum‐Garnet LASER; GSM, genitourinary syndrome of menopause; J, joule; mJ, milijoule; N/A, not available or not applicable; SAE, severe adverse event(s); UI, urinary incontinence; VVA, vulvovaginal atrophy; W, watt.
Follow‐up is reported as time from initial treatment session (first) or final treatment session (last).
Age is reported in mean ± SD unless otherwise specified.
This review aimed to identify the evidence behind gynecologic LASER for the indications GSM, VVA, UI and LS.
2. MATERIAL AND METHODS
This review is an exploratory investigation of the evidence available on vaginal and vulvar LASER.
2.1. Eligibility criteria
The authors set up an internal protocol to use as a guideline for the review, listing the criteria and outcomes for this review. The eligibility criteria for this state‐of‐the‐art review adhered to the principals of PICO—participants, interventions, comparison and outcome. Studies that investigated the effect of any vaginal and vulvar LASER on women with symptoms of GSM, VVA, UI or LS were eligible for inclusion. No outcome restrictions were applied. Only original studies were included; unpublished work, editorials, conference abstracts, reviews and meta‐analysis were excluded. Likewise, in vivo studies on animals, histologic cohort studies, and studies of the effect of radiofrequency treatment were excluded. Language restrictions were applied and only studies in English were included.
2.2. Search strategy
The search string was generated in the PubMed database. The search terms were branched in treatment‐associated search terms and symptom‐ and disease‐associated search terms (Table 2). The PubMed search was commenced August 25 and closed October 27, 2021. Titles and abstracts and were screened by two authors (OEM and SEC) to meet the eligibility criteria listed above. Subsequently, the two authors performed a full‐text screening on the papers. The reference lists of systematic reviews and meta‐analyses identified through the initial database search were also screened to find additional studies. The authors used Covidence for the screening process. 22 If any discrepancies about the eligibility criteria occurred, the papers were re‐screened until consensus was reached. Two authors (OEM and SEC) performed the data extraction.
TABLE 2.
The search string in PubMed
| Treatment | Indication | |
|---|---|---|
|
Vaginal LASER OR CO2 LASER OR Energy based device OR Fractional CO2 LASER OR |
AND |
Atrophy PR Lichen OR Incontinence OR Genitourinary Syndrome |
(((((((Fractional CO2 LASER) OR (energy based devices)) OR (CO2 LASER)) OR (LASER therapy)) OR (Vaginal LASER)) AND (Incontinence)) OR ((((((Fractional CO2 LASER) OR (energy based devices)) OR (CO2 LASER)) OR (LASER therapy)) OR (Vaginal LASER)) AND (lichen sclerosus))) OR ((((((Fractional CO2 LASER) OR (energy based devices)) OR (CO2 LASER)) OR (LASER therapy)) OR (Vaginal LASER)) AND (Vaginal atrophy)) OR ((((((Fractional CO2 LASER) OR (energy based devices)) OR (CO2 LASER)) OR (LASER therapy)) OR (Vaginal LASER)) AND (genitourinary syndrome)).
3. RESULTS
A total of 114 papers were included according to the eligibility criteria listed above. Of these, 111 studies investigated GSM, VVA, UI, and LS symptoms as primary indication (Tables 3, 4, 5, 6); 15 RCT, 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 87 cohort studies, 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 eight case reports, 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 one case‐control study, 133 including a total of 9000 women, not accounting for overlap between the studies. Additionally, three cross‐sectional studies focused solely on the characteristics of AEs. 134 , 135 , 136 The full screening process is shown in Figure 1.
TABLE 3.
Genitourinary syndrome of menopause
| LASER | Author | Country | Design | Follow‐up a | Sample size, n | Age (years); b menopause status | Treatment settings | No. treatments, interval | Comparison | Outcome | Conclusion | Adverse events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | Cruff & Khandwala (2021) 23 | USA | RCT | 6 months, first | 34 | Median (IQR): LASER = 61 (54−66), sham = 59 (56−65); Postmenopausal |
Internal: 30 W, stack 1–3. External: 26 W, stack 1 |
3 sessions, 6 weeks | Sham LASER | Primary: 2‐stage improvement in GSM symptoms. Secondary: VHIS, FSFI, DIVA, UDI‐6, modified PGI‐I and VAS for GSM | No significant difference between improvement in sham vs LASER at 6 months, but the study lacked power | No SAE |
| Li et al. (2021) 24 | Australia | RCT | 12 months, first | 85 | 57 ± 8; Postmenopausal | 40 W, stack 2 | 3 sessions, 1 months | Sham LASER (1:1) | Primary: VAS for symptom severity and VSQ. Secondary: QoL, SS, VHIS, vaginal histology, and cytology | No significant between‐group difference in change in overall VAS, VAS for most severe symptom or VSQ score, but scores improved in both groups at follow‐up | No SAE. AE: LASER (n = 16) vs sham (n = 17); vaginal pain/discomfort (44% vs 68%), spotting (30% vs 5%), lower urinary tract symptoms or confirmed UTI (15% vs 5%), and vaginal discharge (11% vs 11%). Upper UTI in LASER group (n = 1) | |
| Quick et al. (2021) 26 | USA | RCT | 4 weeks, last | 18 | 56.3 ± 8.98; N/A |
Internal: 30 W, stack 1–3 External: 26 W, stack 1 |
3 sessions, 1 months | Sham LASER (1:1) |
Primary: VAS*. Secondary: VuAS, FSFI, UDI‐6, objective vaginal symptoms |
No significant difference in overall VAS* from baseline to follow‐up between active vs sham group | No SAE. AE: discharge (n = 3), dryness (n = 3), pain (n = 1), inflammation (n = 2), flank pain (n = 1) (unrelated) | |
| Paraiso et al. (2020) 27 | USA | RCT | 6 months, last | 69 | 61 ± 7; Postmenopausal |
Internal: 30 W, stack 1–3 External: 26 W, stack 1 |
3 sessions, 6 weeks | Vaginal estrogen (1:1) |
Primary: VAS for GSM symptoms. Secondary: VHIS, VMI, Quality of Life FSFI, DIVA and UDI‐6. |
No significant difference in any VAS scores from baseline to follow‐up between treatment groups | No SAE. AE: Vaginal bleeding (n = 2), vaginal pain (n = 1), vaginal discharge (n = 1), UTI (n = 1) | |
| Salvatore et al. (2020) 25 | Italy | RCT | 1 months, last | 58 | LASER = 57.0 ± 6.9, sham = 58.4 ± 6.0; Postmenopausal |
Internal: 30 W, stack 1–3. External: 24 W, stack 1 |
3 sessions, 1 months | Sham LASER (1:1) |
Primary; VAS for dryness and dyspareunia. Secondary: FSFI, UDI‐6 |
Significantly lower VAS for dryness and dyspareunia in the LASER group compared with sham LASER | No SAE. Transient: mild irritation of the vulva (n = 28/28 active) | |
| Politano et al. (2019) 28 | Brazil | RCT | 14 weeks, last | 72 |
1: 57.83 ± 5.01. 2: 57.21 ± 5.26. 3: 56.79 ± 5.33; Postmenopausal |
40 W, stack 2 | 3 sessions, 1 months |
1) CO2 LASER, 2) intravaginal promestriene, 3) vaginal lubricant (1:1:1) |
Primary: VHIS and VMI Secondary: FSFI |
Significant difference in improvement in VHIS, with highest score in the LASER group, then promestriene and lastly lubricant | NO SAE or AE | |
| Bretas et al. (2021) 38 | Brazil | Cohort | 20 weeks, first | 14 | 54.4 ± 4.5; Postmenopausal | 60 mJ (1st), 75 mJ (2nd) and 90 mJ (3rd). | 3 sessions, 1 months | B&A treatment | Primary: VHIS, FSFI, ICIQ‐SF and histologic analyses of the vaginal wall | Significant improvement in VHIS, FSFI and ICIQ‐SF cores but not in vaginal pH at week 20 | No SAE. Transient: dysuria (n = 2), vaginosis (n = 2) | |
| Li et al. (2021) 39 | China | Cohort | 12 months, last | 162 | 56.56 ± 7.59; Postmenopausal | 35–40 W, stack 1 or 2 | 2–3 sessions, 4 ± 1 week | Topical estriol cream (n = 54) | Primary: VHIS and VAS for GSM symptoms | No significant between‐group difference in VAS and VHIS. VHIS were significantly better at 12 months than at baseline for both groups | No SAE | |
| Quick et al. (2021) 40 | USA | Cohort | 12 months, last | 67 | 57.4 ± 9.5; Postmenopausal |
Internal: 30 W, stack 1 and 3 External: 26 W, stack 1 |
3 sessions, 30–45 days | B&A treatment | Primary: FSFI and FSDS‐R | Significant improvement in FSFI and FSDS‐R scores was found at 12 months, but FSFI still indicated sexual problems | No SAE | |
| Ruffolo et al. (2021) 41 | Italy | Cohort | 16 weeks, first | 61 |
A: 57.18 ± 5.27 B: 58.07 ± 7.21 |
30 W, stack 1–3 | 3 sessions, 1 months | Symptoms before menopause (A) vs postmenopausal (B). |
Primary: UDI‐6 and ICIQ‐SF. Secondary: VAS for VVA symptoms |
Significant improvement in postmenopausal contra menopausal. Significant improvement in VVA symptoms | No SAE. Transient: vaginal burning (n = 3) | |
| Siliquini et al. (2021) 42 | Italy | Cohort | 12 months, last | 135 |
BC: 60.62 ± 8.18. No BC: 58.37 ± 8.40; Postmenopausal |
Internal: 40 W, stack 1–3. External: 15–35 W, stack 1–2 |
3 sessions, 1 months | BC and no BC | Primary: VHI, VVHI, VAS (dyspareunia and dryness), procedure‐related pain | Significant improvement in VHI and VAS in both groups | No SAE | |
| Sindou‐Faurie et al. (2021) 43 | France | Cohort | 3 months, last | 46 | 57.3 ± 11.1; Postmenopausal (n = 43) | 30–35 W, N/A | 3 sessions, 1 months | B&A treatment | Primary: QoL, VAS, and FSFI | Significant improvement in dryness and stress urinary incontinence | N/A | |
| Veron et al. (2021) 44 | France | Cohort | 18 months, last | 46 | Median (IQR): 56.6 (47–59.4); Postmenopausal | 26 to 40 W, stack 1–3 | 3 sessions, 1 months | B&A treatment |
Primary: SF12, FSFI, and Ditrovie score. Secondary: Vaginal pH and maturity pattern on SMEAR |
Significant improvement in FSFI. Improvement in Ditrovie scale | No SAE. Transient: vaginal bleeding (n = 3) | |
| Filippini et al. (2020) 45 | Italy | Cohort | Open, yearly follow‐up | 645 | Median: 56 ± 7.9; Postmenopausal |
Internal: 40 W, stack 1–2. External: 30 W, stack 1 |
3 sessions, N/A | B&A treatment | Primary: VAS | Significant improvement in VAS symptoms dryness, dyspareunia, burning, pain and itching | No SAE or AE | |
| Takacs et al. (2020) 46 | USA | Cohort | 6 weeks, last | 52 |
Premenopausal: 46 ± 6. Postmenopausal: 63 ± 6 |
30 W, stack 1 | 3 sessions, 1 months | B&A treatment | Primary: VAS and Vaginal Maturation Values | Significant improvement in VAS for both groups | N/A | |
| Athanasiou et al. (2019) 47 | Greece | Cohort | 12 months, last | 94 |
Median (IQR) 3: 57 (45–71), 4: 57 (44–71), 5: 57 (52–61); Postmenopausal |
Internal: 30–40 W, stack 1–3. External: 24 W, stack 1 | N/A | 3, 4 or 5 sessions | Primary: VAS, FSFI, ICIQ, and UDI‐6 | Significant improvement in all groups in VAS and FSFI. Differences between 4 and 5 sessions not found | No SAE | |
| Gittens et al. (2019) 48 | USA | Cohort | N/A | 25 | 55.2 ± 9.5; Postmenopausal | N/A | 3 sessions, N/A | B&A treatment | Primary: FSFI, WBFS, FSDS‐R | Significant improvement in every domain of FSFI, WBFS, and FSDS‐R | No SAE | |
| Murina et al. (2019) 49 | Italy | Cohort | 3 months, last | 72 |
1: 56 ± 6.1, 2: 55 ± 5.9; Postmenopausal |
30 W, stack 2 | 3 sessions, 1 months | 1) LASER + ospemifene and 2) LASER only | Primary: VHS and VAS | Significant overall within‐group improvement. Dryness and dyspareunia significant higher in LASER + ospemifene group vs LASER group | No SAE. Transient: mild to moderate pain and edema | |
| Quick et al. (2019) 50 | Germany | Cohort | 1 months, last | 64 | 57.4 ± 9.5; N/A | 30 W, stack 1–3 | 3 sessions, 1 months | B&A treatment | Primary: VAS and SAE. Secondary: FSFI, UDI | Improvement in VAS, FSFI, and UDI | No SAE. Transient: vaginal discharge (n = 69) and vaginal dryness (n = 30) | |
| Tovar‐Huamani et al. (2019) 51 | Perú | Cohort | 1 months, last | 60 | Median (IQR): 55 (49–69); Postmenopausal | 40 W, N/A | 3 sessions, 1 months | B&A treatment |
Primary: VAS. Secondary: FSFI, and VHI |
Improvement in VAS for GSM symptoms | N/A | |
| Athanasiou et al. (2017) 52 | Greece | Cohort | 1 months, last | 55 | 57 ± 14; Postmenopausal | N/A | 3–5 sessions, 1 months | 3, 4 or 5 sessions |
Primary: VAS. Secondary: VHIS and cytological evaluation |
Significant improvement after 3rd session. Significant improvement in VAS and FSFI after 4th session, no difference between 4th and 5th | No SAE. Transient: mild irritation at the introitus | |
| Behnia‐Willison et al. (2017) 53 | Australia | Cohort | 24 months, last | 102 | 61 ± 7; Postmenopausal | 30 W, stack 2 | 3 sessions, 6 weeks | B&A treatment | Primary: GSM symptoms frequency and severity. Secondary: APFQ | Significant improvement in GSM symptoms at 2–4‐month follow‐up and 12–24‐month follow‐up | No SAE. AE: UTI (n = 3), vaginal infection (n = 2), pain (n = 3), genital herpes breakout (n = 1), bleeding (n = 2) | |
| Lang et al. (2017) 54 | USA | Cohort | Mean of 31.7 ± 21 weeks, last | 368 | 62 ± 8; 90% postmenopausal | N/A | 3 sessions, N/A | B&A treatment | Primary: vaginal dryness, sexual function, and PGI | Significant improvement in vaginal dryness. 86% satisfied with the treatment | No SAE. AE: urinary tract symptoms (n = 5), vaginal pain/burning (n = 2), vaginal itching (n = 1), dyspareunia (n = 1) | |
| Sokol et al. (2017) 55 | USA | Cohort | 1 year, last | 30 | 58.6 ± 8.8; Postmenopausal | 30 W, stack 1–3 | 3 sessions, 6 weeks | B&A treatment | Primary: VAS. Secondary: FSFI, and VHI | Significant improvement in VAS the first year (except dysuria), VHIS and FSFI. | No SAE. Transient: pain (n = 2) and bleeding (n = 2) | |
| xx | ||||||||||||
| Murina et al. (2016) 56 | Italy | Cohort | 4 months, last | 70 | N/A; Menopausal (n = 33) | 30 W, stack 2 | N/A | B&A treatment | Primary: VAS, Marinoff score, and efficacy | Significant improvement in VAS, Marinoff and efficacy. Improvement gradually increased through 4 months of follow‐up | No SAE | |
| Pitsouni et al. (2016) 57 | Greece | Cohort | 4 weeks, last | 53 | 57.2 ± 5.4; Postmenopausal | 30 W, stack 1–3 | 3 sessions, 1 months | B&A treatment | Primary: VMV and VHIS. Secondary: FSFI, ICIQ‐FLUTS, ICIQ‐UI SF, UDI‐6, KHQ | Significant improvement in VMV and VHIS at follow‐up | No SAE. Transient: mild irritation at the introitus | |
| Sokol et al. (2016) 58 | USA | Cohort | 3 months, last | 30 | 58.6 ± 8.8; Postmenopausal | 30 W, stack 1–3 | 3 sessions, 6 weeks | B&A treatment |
Primary: VAS. Secondary: VHI, dilator size, FSFI, SF‐12, difficulty in performing treatment, PGI 5 scale |
Significant improvement in VAS for all categories of symptoms | No SAE. Transient: mild to moderate pain (n = 2), minor bleeding (n = 1) | |
| Pitsouni et al. (2017) 133 | Greece | Case‐Control | 1 months, last | 50 | 30W = 56.3 ± 5.1. 40W = 56.8 ± 3.6; Postmenopausal | 30 and 40 W, stack 1–3 | 3 sessions, 1 months | 30 W (n = 25) vs 40 W (n = 25) | Primary: VAS (dyspareunia + dryness). Secondary: VAS (other GSM symptoms) FSFI, ICIQ‐FLUTS, VMV and VHIS | No significant between‐group differences in VAS, but within‐group improvement was significant |
No SAE. Transient: mild irritation, burning sensation |
|
| Gordon et al. (2019) 125 | USA | Case Report | N/A | 4 | 58, 61, 65 and 68 y; Postmenopausal | N/A | 3 sessions, N/A | N/A | N/A | Case series of complications following treatment of GSM with CO2 LASER | Fibrosis, scarring, agglutination and penetration injury following CO2 LASER treatment | |
| Er:YAG | Gambacciani et al. (2020) 59 | Italy | Cohort | 24 weeks, last | 1081 | 54.3 ± 3; Postmenopausal | 6.0 J/cm2 | 2–3 sessions, 1 months | B&A treatment | Primary: FSFI and FSDS‐R. | Significant improvement in FSFI and FSDS‐R scores | No SAE |
| Gambacciani et al. (2018) 60 | Italy | Cohort | 24 months, last | 254 | LASER = 61.2 ± 7.2. LT = 62.0 ± 7.5; Postmenopausal | 6.0 J/cm2 | 3 sessions, 1 months | Local treatments (LT): hormonal or non‐hormonal (n = 49) | Primary: VAS and VHIS. Secondary: ICIQ‐UI SF | Significant improvement in VAS and VHIS until 12 and 18 months respectively. VAS was significantly improved in the LASER group compared with LT at 6 months | No SAE or AE | |
| Mothes et al. (2018) 63 | Germany | Cohort | 6 weeks, last | 16 | 71 ± 7; Postmenopausal | Phase 1: 15–35 J/cm2. Phase 2: 3–9 J/cm2 | N/A | B&A treatment | Primary: subjective satisfaction, vaginal pH, VHI | Significant improvement in VHI, but not in pH and 94% of patients were satisfied | No SAE | |
| Gambacciani & Levancini (2017) 61 | Italy | Cohort | 18 months, last | 43 | 50.8 ± 8.1; Postmenopausal | 6.0 J/cm2 | 3 sessions, 30 days | B&A treatment | Primary: VAS and VHIS. | Significant improvement in VAS and VHIS up to 12‐month follow‐up, but not after 18 months | No SAE or AE | |
| Gaspar et al. (2017) 62 | Argentina | Cohort | 18 months, first | 50 |
LASER = 55.0 ± 6.7. Estriol = 53.5 ± 5.7; Postmenopausal |
Total: 1000–1500 J | 3 sessions, 3 weeks + 2 weeks pretreatment with estriol | Topical estriol (1:1) | Primary: Biopsies, MV, Vaginal pH, VAS (dyspareunia, dryness, irritation, and leukorrhea) | Significant reduction in VAS at 18‐month follow‐up in the LASER group only. Overall bigger improvement in the LASER group on all outcomes | No SAE. Transient: mild to moderate pain (4%), edema, pain (n = 1), spotting (n = 1) | |
| Gambacciani & Levancini (2015) 64 | Italy | Cohort | 4 weeks, last | 65 | 62.9 ± 8.1; Postmenopausal | 3 and 8.5 J | 3 sessions, 30 days | B&A treatment | Primary: VAS and VHIS. Secondary: ICIQ‐UI SF | Significant improvement in VAS and VHIS | No SAE. Transient: “bad experience” at first application (n = 3) | |
| Gambacciani et al. (2015) 65 | Italy | Cohort | 24 weeks, last | 70 | LASER = 60.9 ± 8.1. Estriol = 63 ± 4.5; Postmenopausal | 6 J/cm2 | 3 sessions, 30 days | Topical estriol (n = 25) | Primary: VAS and VHIS. Secondary: ICIQ‐UI SF | Significant between‐group difference in VAS and VHIS after 24 months, with biggest improvement in the LASER group | No SAE. Transient: burning sensation (n = 1), “bad experience” (n = 2) |
Note: General characteristics, findings, and adverse events in included studies. The table is sorted by (1) LASER type, (2) study design, (3) year of publication and (4) author name.
Abbreviations: AE, adverse event(s); APFQ, Australian Pelvic Floor Questionnaire; BC, breast cancer; B&A treatment, before & after treatment; CO2, carbon dioxide LASER; DIVA, Day‐to‐day Impact of Vaginal Aging Questionnaire; Er:YAG, Erbium: Ytrium‐Aluminum‐Garnet LASER; FSDS‐R, The Female Sexual Distress Scale‐Revised Questionnaire; FSFI, Female Sexual Function Index; GSM, genitourinary syndrome of menopause; ICIQ‐FLUTS, International Consultation on Incontinence Questionnaire ‐ Female Lower Urinary Tract Symptoms; ICIQ‐SF or ICIQ‐UI SF, International Consultation on Incontinence Questionnaire ‐ Urinary Incontinence Short Form; IQR, interquartile range; J, joule; KHQ, King's Health Questionnaire; mJ, millijoule; MV, maturation value; N/A, not available or not applicable; PGI‐I, patient global impression of improvement; QoL, quality of life; SAE, serious adverse event(s); SF‐12, 12‐item short‐form health survey; UDI, Urinary Distress Inventory; UDI‐6, Urinary Distress Inventory, short form; UTI, urinary tract infection; VAS, Visual Analog Scale; VAS*, Vaginal Assessment Scale; VHI or VHIS, Vaginal Health Index or Vaginal Health Index Score; VMI, Vaginal Maturation Index; VuAS, Vulvar Assessment Scale; VVA, vulvovaginal atrophy; WBFS, Wong–Baker Faces Scale.
Follow‐up is reported as time from initial treatment session (first) or final treatment session (last).
Age is reported in mean ± SD unless otherwise specified.
TABLE 4.
Vulvovaginal atrophy
| LASER | Author | Country | Design | Follow‐up a | Sample size, n | Age [years] b ; menopause status | Treatment settings | No. treatments, interval | Comparison | Outcome | Conclusion | Adverse events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | Dutra et al. (2021) 29 | Brazil | RCT | 4 months, first | 25 | 55.3 ± 4.3; Postmenopausal | 30 W, stack 2 | 3 sessions, 1 months | Topical estrogen | Primary: Frost Index, Meisel index, SQ‐F, histomorphometry of the vaginal mucosa and sexual function | Significant improvement in vaginal thickness and sexual function in both groups. No difference between the groups at baseline and after treatment | No SAE |
| Ruanphoo et al. (2020) 30 | Thailand | RCT | 12 weeks, last | 88 | 60.78 ± 7.77; Postmenopausal | 40 W, stack 1–3 | 4 sessions, 1 months | Sham LASER (1:1) | Primary: VHI. Secondary: VAS and ICIQ‐VS | Significant improvement in VHI, VAS and ICIQ‐VS in both groups. Significant difference between LASER group and sham group | No SAE | |
| Cruz et al. (2018) 31 | Brazil | RCT | 20 weeks, first | 45 |
LASER: 55.9 ± 5.2, Estriol: 56.9 ± 6.0, L+E: 55.7 ± 4.4; Postmenopausal |
30 W, stack 2 | 2 sessions, 1 months | Estriol vs LASER vs LASER+estriol (L+E) | Primary: VHI, VAS, FSFI, and MV | No significant between‐group difference at follow‐up. Significant improvement in VHI and FSFI for L+E and in dyspareunia, burning and dryness for LASER and L+E group. Significant improvement only in dryness for estriol group | No SAE | |
| Alexiades (2021) 66 | USA | Cohort | 12 months, last | 18 | 53 ± 7; Postmenopausal | 50 mJ | 3 sessions, N/A | B&A treatment | Primary: VHI, VAS, and FSFI | Significant improvement in VHI and FSFI | No SAE. Transient: mild erythema at the introitus and vulva | |
| Gardner & Aschkenazi (2021) 67 | USA | Cohort | 13 weeks, first | 139 | 62 ± 10; N/A | 30 W, stack 1–3 | 3 sessions, 6 weeks | B&A treatment | Primary: FSFI, VSQ, and VAS | Significant improvement in FSFI, VSQ (18/21 questions) and VAS for intercourse and vulvar dryness | No SAE | |
| Luvero et al. (2021) 68 | Italy | Cohort | 3 months, last | 44 | 34.5 ± 5.1; Premenopausal | Internal: 40 W, stack 1. External: 25 W, stack 1 | 3–4 sessions, 1 months | No treatment | Primary: VAS | Significant improvement in all symptoms compared with the control group | No SAE | |
| Rosner‐Tenerowicz et al. (2021) 69 | Poland | Cohort | 12 months, last | 205 | 58.45 ± 8.73; Perimenopausal | N/A | 3 sessions, 4–6 weeks | B&A treatment | Primary: VAS, VHIS, and ICIQ‐UI‐SF | Significant improvement in VAS, VHIS and ICIQ‐UI‐SF | No SAE | |
| Salvatore et al. (2021) 70 | Italy | Cohort | 20 weeks, first | 40 | 57.6 ± 7.2; N/A | 30 W, stack 1–3 | 5 sessions, 1 months | Past vs current use of endocrine therapies | Primary: Satisfaction. Secondary: VHI, VAS, QoL, SF‐12, PCS‐12, MCS‐12, FSFI | Significant improvement in VAS and VHI with no difference between the two groups | No SAE | |
| Adabi et al. (2020) 71 | Iran | Cohort | 3 months, last | 140 | 56.8 ± 9.3; Postmenopausal | 50 to 60 mJ. | 3 sessions, 1 months | B&A treatment | Primary: VHI, ICIQ, FSFI, and SF‐12 | Significant improvement in QoL, arousal and SS. Significant improvement in vaginal elasticity, fluid, epithelial integrity, wetness, urinary incontinence, enuresis, urgency and leaking | N/A | |
| Angioli et al. (2020) 72 | Italy | Cohort | 4 weeks, last | 165 | 53 (31–73); N/A | 40 W, N/A | 3–4 sessions, 1 months | B&A treatment | Primary: VAS | Improvement in VAS for VVA symptoms | No SAE | |
| Di Donato et al. (2020) 73 | Italy | Cohort | 3 months, last | 53 | 57.8 ± 10.4; Postmenopausal | 7.5–12.5 mJ | 3 sessions, 1 months | B&A treatment | Primary: Pain related to probe insertion | Significant improvement in pain related to probe insertion and rotation. The pain did not significantly change. High satisfaction in 89.7% | No SAE. Transient: dizziness (n = 1), dysuria (n = 2) | |
| Ghanbari et al. (2020) 74 | Iran | Cohort | 3 months, first | 47 | 57.2 ± 6.8; Postmenopausal | 40 W, stack 1 | 3 sessions, 1 months | B&A treatment | Primary: VAS for VVA symptoms severity | Significant improvement in VAS for VVA symptoms | No SAE | |
| Hersant et al. (2020) 75 | France | Cohort | 6 months, last | 20 | 56.1 ± 8.8; Menopause (n = 17) | 11.5 J/cm2, stack 3 | 2 sessions, N/A | B&A treatment | Primary: VHIS. Secondary: FSD and VAS | Significant improvement in VHIS for vaginal elasticity, fluid volume, epithelial integrity and moisture | No SAE. Transient: bleeding (n = 2) | |
| Marin et al. (2020) 76 | France | Cohort | 6 months, first | 50 | M: 44 (24–52). NM: 58 (52–73); Menopausal (n = 25), non‐menopausal (n = 25) | 18 W, N/A | 2 sessions, 6 weeks | Menopausal (M) vs non‐menopausal (NM) | Primary: FSFI. Secondary: QoL | Significant improvement in FSFI and QoL for both groups. No between‐group comparison available | AE: worsening of symptoms (n = 2) and UTI (n = 1) | |
| Mezzana (2020) 77 | Italy | Cohort | 12 weeks, first | 40 | N/A, Menopausal | 8 & 5 W | 3 sessions, 1 months | B&A treatment | Primary: FSFI and SUI scale | Significant improvement in both FSFI and SUI in all outcomes | No SAE | |
| Eder (2019) 78 | USA | Cohort | 18 months, last | 20 | 60.65 ± 6.34; Postmenopausal | 7.5–12.5 mJ | N/A | B&A treatment | Primary: VHI, VAS, FSFI, satisfaction with treatment | Significant improvement in VHI, VAS and FSFI at 12, 15 and 18 months | No SAE. Transient: mild to moderate severity | |
| Pearson et al. (2019) 79 | Australia | Cohort | 1 months, last | 29 | Median: 56 y; Postmenopausal | 40W, stack 2 | 3 sessions, 1 months | B&A treatment | Primary: VAS. Secondary: FSFI and QoL | Significant improvement in dryness, burning and dysuria | N/A | |
| Singh et al. (2019) 80 | Singapore | Cohort | 6 months, last | 45 | 59.7 ± 9.2; Premenopausal (n = 4), postmenopausal (n = 41) | 40 W, stack 2 | 5 sessions, N/A | B&A treatment | Primary: Severity of symptoms, VHI, SF‐2, FSFI, treatment satisfaction | General improvement: 90% of the patients improved in dryness, 89.5% of the patients improved in dyspareunia | No SAE | |
| Eder (2018) 81 | USA | Cohort | 6 months, last | 28 |
60.1 ± 5.55 Postmenopausal |
7.5–15.5 mJ | 3 sessions, 1 months | B&A treatment | Primary: VHI. Secondary: VAS and FSFI | Significant improvement in VHI the 1st mo. following the 1st treatment. Significant improvement in VHI from baseline to 6‐month follow‐up | No SAE. Transient: vaginal bleeding (n = 1) | |
| Samuels et al. (2018) 82 | USA | Cohort | 12 months, last | 40 | 56 ± 8; Postmenopausal | 45–60 mJ | 3 sessions, 1 months | B&A treatment | Primary: VHI. Secondary: VAS, FSFI, treatment satisfaction, histology, and ICIQ‐UI‐SF | Significant improvement in VHI after the 1st treatment. Improvement in VHI after 6 months. Significant improvement in all evaluations | No SAE. AE: mild itching (n = 2), mild itching and swelling (n = 1), moderate burning sensation with urination (n = 2), moderate soreness and spotting (n = 1), major itching (n = 1) | |
| Arroyo (2017) 83 | Spain | Cohort | 24 weeks, last | 21 | 45 ± 7; Perimenopausal | 40–55 mJ | 3 sessions, 3–4 weeks | B&A treatment | Primary: VHI at 12 weeks. Secondary: VHI at 24 weeks, sexual function, satisfaction and improvement | Significant improvement in VHI score 12 weeks after last treatment. The improvement was also significant at 24 weeks follow‐up | No SAE. AE: Mild urinary infection (n = 1). Transient: Burning sensation, itching, bruising, swelling, twinging sensation, numbness, and purpura | |
| Filippini et al. (2017) 84 | Italy | Cohort | 2 months, last | 386 | Range: 48‐>70; Postmenopausal | Internal: 40 W, stack 2. External: 30 W, stack 1 | 3 sessions, N/A | B&A treatment | Primary: VAS (laxity, dryness, irritation/burning, and dyspareunia) | Patients reported improvement in symptoms 2 months after last treatment | No SAE. Transient: Discomfort during insertion, blood–serum secretions (1–2 days), mild burning (1–2 hours) after treatment | |
| Pagano et al. (2017) 85 | Italy | Cohort | 1 months, last | 82 | Median: 44 y; Postmenopausal (n = 10) | 30 W, stack 1–3 | 3 sessions, 30–40 days | B&A treatment | Primary: VAS for VVA symptoms | Significant reduction in VAS for all VVA related symptoms except vaginal laxity | No SAE | |
| Pieralli et al. (2017) 86 | Italy | Cohort | 24 months, last | 184 | 56 y (range 38–72 y); Postmenopausal | 30 W, stack 1 | 3 sessions, 1 months | N/A | Primary: Patient satisfaction | Patient satisfaction declined over time, from 92% being satisfied after 6 months, to 25% at 24 months | N/A | |
| Siliquini et al. (2017) 87 | Italy | Cohort | 15 months, last | 91 | 58.6 ± 6.9; Postmenopausal | 40 W, stack 1–3 | 3 sessions, 1 months | B&A treatment | Primary: VAS (dryness and dyspareunia), DIVA, VHI, VVHI | Significant improvement in VAS, VHI and VVHI scores at 15‐month follow‐up | No SAE | |
| Lekskulchai et al. (2016) 88 | Thailand | Cohort | 3 months, last | 112 | 61.0+7.0; Postmenopausal | 30 W, stack 1–3 | 3 sessions, 1 months | B&A treatment | Primary: VVA symptom‐score, vaginal pH and VMI | Significant improvement in VVA symptom‐score, pH and VMI | No SAE | |
| Pagano et al. (2016) 89 | Italy | Cohort | 1 months, last | 26 | Median: 42 y; Postmenopausal (n = 1) | 30 W, stack 1–3 | 3 sessions, 30–40 days | B&A treatment | Primary: VAS for VVA symptoms | Significant improvement in all VAS scores except for vaginal laxity among BC survivors | No SAE | |
| Pieralli et al. (2016) 90 | Italy | Cohort | 4 weeks, last | 50 | 53.3 (range: 41–66); Postmenopausal | 30 W, stack 2 | 3 sessions, 1 months | B&A treatment | Primary: VHI and VAS | Significant improvement in VHI and VAS scores among BC survivors | No SAE | |
| Perino et al. (2014) 91 | Italy | Cohort | 1 months, last | 48 | Median (IQR): 56 (7.75); Postmenopausal | 40W, stack 2 | 3 sessions, 1 months | B&A treatment | Primary: VHI and VAS for VVA symptoms | Significant improvement in VHI and VAS scores | No SAE or AE | |
| Salvatore et al. (2014) 92 | Italy | Cohort | 4 weeks, last | 50 | 59.6 ± 5.8; Postmenopausal | 30 W, stack 1–3 | 3 sessions, 1 months | B&A treatment | Primary: VHIS, VAS for VVA symptoms, SF‐12 | Significant improvement in VHIS, SF‐12 and VVA scores, except for vaginal burning | No SAE or AE | |
| Salvatore et al. (2014) 93 | Italy | Cohort | 4 weeks, last | 77 | 60.6 ± 6.2; Postmenopausal | Internal: 30 W, stack 1–3. External: 20 W | 3 sessions, 1 months | B&A treatment | Primary: FSFI. Secondary: SF‐12, VAS (SS and VVA) | Significant improvement in FSFI and sexual activity | N/A | |
| CO2 & Er:YAG | Salcedo et al. (2020) 126 | Spain | Case Report | Case 1: N/A, Case 2: 24 weeks | 2 | 61 and 63 y; Postmenopausal | Case 1: 40 W, case 2: 5.5 + 10 J/cm2 | C1: 3+3 sessions, 4–6 weeks. C2: 3 sessions, 1 months | N/A | Case1: VAS, case 2: VAS, VHI | Combination of LASER and ospemifene showed improvement in VVA symptoms | N/A |
| Er:YAG | Lee (2014) 32 | South Korea | RCT | 2 months, last | 30 | 41.7 (33–56); Premenopausal (n = 23), perimenopausal (n = 2), postmenopausal (n = 5) | Group A:1.7 J. Group B: 1.7 J and 3.7 J | 4 sessions, 1–2 weeks | Group A: 2x360° & 2x90°. Group B: 2x90°; 2x90°+360° | Punch biopsies, perineometer, partner’s evaluation of vaginal tightening and patient’s SS | Thicker and more cellular epithelium. More compact lamina propria with more connective tissue. Significant between group difference in maximum pressure and SS in group A compared to B | No SAE. |
| Arêas et al. (2019) 94 | Brazil | Cohort | 1 months, last | 24 | 53.67 ± 9.66; Postmenopausal | 2.0 J/cm2(360°) and 35 mJ/MTZ (90°) | 3 sessions, 1 months | B&A treatment | Primary: VHIS and SPEQ | Significant improvement in VHIS and SPEQ at follow‐up | No SAE. AE: vaginal candidiasis (n = 1), acute cystitis (n = 1) |
Note: General characteristics, findings, and adverse events in included studies. The table is sorted by (1) LASER type, (2) study design, (3) year of publication and then (4) author name.
Abbreviations: AE, adverse event(s); B&A treatment, before and after treatment; CO2, carbon dioxide LASER; DIVA, Day‐to‐day Impact of Vaginal Aging Questionnaire; Er:YAG, Erbium: Ytrium‐Aluminum‐Garnet LASER; FSD, The Female Sexual Distress Scale; FSFI, Female Sexual Function Index; ICIQ, International Consultation on Incontinence Questionnaire; ICIQ‐SF or ICIQ‐UI SF, International Consultation on Incontinence Questionnaire – Urinary Incontinence Short Form; ICIQ‐VS, International Consultation on Incontinence Questionnaire – Vaginal Symptoms Module; IQR, inter quartile range; MCS‐12, 12‐item Short‐Form Health Survey's Mental health Component Scale ; MV, maturation value; N/A, not available or not applicable; PCS‐12, 12‐item Short‐Form Health Survey's Physical health Component Scale; QoL, quality of life; SAE, serious adverse event(s); SF‐12, 12‐item Short‐Form Health Survey; SPEQ, Short Personal Experiences Questionnaire; SQ‐F, female sexual quotient; SS, sexual satisfaction; SUI, stress urinary incontinence; UTI, urinary tract infection; VAS, Visual Analog Scale; VHI or VHIS, Vaginal Health Index or Vaginal Health Index Score; VMI, Vaginal Maturation Index; VSQ, Vulvovaginal Symptoms Questionnaire; VVA, vulvovaginal atrophy; W, watt.
Follow‐up is reported as time from initial treatment session (first) or final treatment session (last).
Age is reported in mean ± SD unless otherwise specified.
TABLE 5.
Urinary incontinence
| LASER | Author | Country | Design | Follow‐up a | Sample size, n | Age (years); b menopause status | Treatment settings | No. treatment, interval | Comparison | Outcome | Conclusion | Adverse events | UI type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | Aguiar et al. (2020) 33 | Brazil | RCT | 2 weeks, last | 72 | 57.28 ± 5.15; Postmenopausal | 40 W, stack 2–3 | 3 sessions, 30–45 days |
1) CO2 LASER, 2) intravaginal promestriene, 3) vaginal lubricant (1:1) |
Primary: ICIQ‐UI SF and ICIQ‐OAB. Secondary: Urinary symptoms related to GSM | No significant between‐group difference in ICIQ‐UI scores, but significant in‐group change in the LASER arm only. Significant improvement in ICIQ‐OAB between LASER vs lubricant, but not promestriene | No SAE | UI |
| Alcalay et al. (2021) 95 | Israel | Cohort | 12 months, first | 42 | 49 (32–73); N/A | 40–120 mJ | 3 sessions, 1 months | B&A treatment | Primary: 1‐hour pad test, PFDI‐20, PFIQ, PGI‐I, and VHI | Significant improvement in 1‐h pad test, PFDI and PFIQ. Improvement in PGI‐I | No SAE. Transient: vaginal secretion and irritation, fever, and UTI | SUI | |
| Franić et al. (2021) 96 | Slovenia | Cohort | 6 months, last | 85 | 47(42–56); N/A | Menopause > 10 y: 60–70 mJ/px, <50 y old: 80–90 mJ/px. Thereafter + 10 mJ/px | 2 sessions, 1 months | B&A treatment | Primary: VAS, and ICIQ‐UI SF | Significant improvement in ICIQ‐UI‐SF for women (BMI >30). No significant results in VAS | No SAE | SUI | |
| Nalewczynska et al. (2021) 97 | Poland | Cohort | 12 months, last | 59 | 51.0 ± 1.4; N/A | 70–120 mJ/pixel | 3 sessions, 1 months | B&A treatment | Primary: Sandvik score, 1‐h pad test, VHIS, FSFI, PGI‐S, PGI‐I, and PFIQ‐7 | Gradual improvement of symptoms and the best outcome was observed between 3 and 6 months | No SAE | SUI | |
| Toplu et al. (2021) 98 | Turkey | Cohort | 6 months, last | 30 | 48.3 ± 7; Premenopausal (n = 3), perimenopause (n = 22), postmenopausal (n = 5) | 30–45 mJ | 1–3 sessions, 1 months | B&A treatment | Primary: Discomfort during and satisfaction with the procedure. Secondary: QUID, PISQ‐12 | A general high level of patient comfort and satisfaction related to the procedure was found | NO SAE | SUI | |
| Zhang et al. (2021) 99 | China | Cohort | 6 months, last | 33 | 43.15 ± 6.49; Premenopausal | 30 W, 60–100 mJ/ppxl. | 3 sessions, 1 months | B&A treatment | Primary: ICIQ‐SF, and 1‐h pad test | Significant improvement in ICIQ‐SF. Improvement in 1‐h pad test for all patients | No SAE | UI | |
| Dabaja et al. (2020) 100 | Israel | Cohort | 6 months, last | 33 | 43 (32–51); N/A | N/A | 3 sessions, 1 months | B&A treatment | Primary: UDI‐6 and ICIQ‐UI |
Significant improvement in UDI‐6 and ICIQ‐UI at 3‐month follow‐up. Both returned to baseline at 6‐month follow‐up |
No SAE. Transient: stinging sensation (70%), vulvar sensitivity (30%), untimely menstrual pain (10%) | SUI | |
| Palacios et al. (2020) 101 | Spain | Cohort | 6 weeks, last | 25 | 54.4 ± 9.9; N/A | 70 mJ, 396 J/cm2 | 3 sessions, 4–6 weeks | B&A treatment | Primary: ICIQ‐UI, Sandvik Index, and FSFI | Significant improvement in ICIQ‐UI and Sandvik Index after 2nd and 3rd treatment. Significant improvement after 1st treatment in UI severity | No SAE | SUI + MUI | |
| Behnia‐Willison et al. (2019) 102 | Australia | Cohort | 12–24 months, last | 58 | 57.4 ± 11.4; Postmenopausal (n = 45) | 40 W, stack 3 | 3 sessions, 4–6 weeks | B&A treatment | Primary: APFQ | Improvement in 82% after treatment. Improvement in 71% at 12–24‐month follow‐up | No SAE. Transient: thrush (n = 3), UTI (n = 2) and genital herpes (n = 1) | SUI | |
| González Isaza et al. (2018) 103 | Colombia | Cohort | 36 months, last | 161 | 53 ± 5.1; Postmenopausal | N/A | 4 sessions, 30–45 days | B&A treatment | Primary: ICIQ‐UI SF, 1‐h pad test, and punch biopsies | Significant improvement in ICIQ‐UI‐SF and 1‐h pad test at 36‐month follow‐up | No SAE | SUI | |
| Perino et al. (2016) 104 | Italy | Cohort | 1 months, last | 30 | 56(8.5); Menopausal | 40 W, stack 2 | 3 sessions, 1 months | B&A treatment | Primary: VHI, VAS, and micturition diary | Significant improvement in VHI, micturition diary in number of urge episodes and VAS; dryness, burning, itching and dyspareunia | No SAE | OAB | |
| CO2 & Er:YAG | Lin et al. (2018) 105 | Taiwan | Cohort | 2 months, last | 31 | 48.43 ± 12.75; Menopause (44.8%) | CO2: Internal: 30 W, external: 20 W. Er:YAG: 3, 6 and 10 J/cm2 | N/A | CO2 (n = 10) and Er:YAG (n = 21) | Primary: ICIQ‐ SF, 1‐h pad test, and FSFI | Significant improvement in ICIQ‐SF, but not in 1‐h pad test or FSFI. No between‐group analysis available. | No SAE. Transient: mild irritation | SUI |
| CO2+ other | Behnia‐Willison et al. (2020) 106 | Australia | Cohort | 24 months, last | 62 | 55.98 ± 11.27; Postmenopausal (n = 48). | 40 W, stack 3 | 3 sessions, 4–6 weeks + platelet rich plasma | B&A treatment | Primary: APFQ | Significant improvement in bladder function at 12‐month follow‐up except pad use | No SAE | SUI |
| Er:YAG | Blaganje et al. (2018) 34 | Slovenia | RCT | 3 months, last | 114 | LASER: 39.95 ± 6.36. Sham: 41.84 ± 5.67; Premenopausal | 2940 nm, 10 J/cm2 | 1 session | Sham LASER (1:1) | Primary: ICIQ‐UI SF. Secondary: PISQ‐12, FSFI, and perineometry | Significant superiority of the LASER vs sham group in ICIQ‐SF | No SAE | SUI |
| Okui et al. (2021) 107 | Japan | Cohort | 12 months, last | 327 | TVT = 42.5 (35–48), VEL = 42.7 (37–49); Postmenopausal (TVT = 11.8%; VEL = 11.5%) | 1st step: 6 J/cm2, 2nd step: 3 J/cm2 and 3rd step: 10 J/cm2 | 3 sessions, 1 months | TVT | Primary: 1‐h pad test. Secondary: ICIQ‐SF, OABSS | No significant between‐group differences in 1‐h pad test, but significant within‐group improvement in both groups. | N/A | SUI | |
| Erel et al. (2020b) 108 | Turkey | Cohort | Open (6–48 months) | 82 | 53.72 (29–78); Premenopausal (n = 28), postmenopausal (n = 54) | 2940 nm, 10.0 J/cm2 | 1–4 sessions, N/A | B&A treatment | Primary: ICIQ‐SF and KHQ | Significant improvement in ICIQ‐SF and KHQ. Significant better results in the premenopausal group | No SAE | SUI + MUI | |
| Erel et al. (2020a) 109 | Turkey, Croatia and Italy | Cohort | Open (6–24 months) | 69 | Hysterectomized 62 (53–66) and non‐hysterectomized 50 (45–55) | 2940 nm, 10.0 J/cm2 | 1–4 sessions, 1 months | Hysterectomized vs non‐hysterectomized. | Primary: ICIQ‐SF. Secondary: ‘Maximum improvement time’ and ‘total improvement time’ | Significant improvement in ICIQ‐SF in both hysterectomized and non‐hysterectomized patients | N/A | SUI | |
| Kuszka et al. (2020) 110 | Germany | Cohort | 2 years, last | 59 | 49 ± 11, postmenopausal (n = 25) | 2940 nm, 3J/cm2, 6 J/cm2, and 10 J/cm2 | 5 sessions, N/A | B&A treatment | Primary: 1‐h pad test, ICIQ‐UI SF, and PISQ‐12 | Significant improvement in mild and moderate UI after 2 treatments. Improvement sustained at 1‐year follow‐up. Minor effect on severe UI | No SAE. AE: vaginal discharge (n = 1). Transient: Pain (n = 6) | SUI | |
| Lin et al. (2019) 111 | Taiwan | Cohort | 6 months, last | 41 | 45.9 ± 7.2; menopausal (n = 33). | 2940 nm, 10 J/cm2 | 3 sessions, 1 months | B&A treatment | Primary: ICIQ‐SF, UDI‐6, IIQ‐7, OABSS, and POPDI‐6 | Significant improvement in ICIQ‐SF, UDI‐6, IIQ‐7, OABSS, and POPDI‐6 | No SAE. Transient: Burning sensation and vaginal bleeding | SUI | |
| Okui et al. (2019) 112 | Japan | Cohort | 12 months, first | 50 | LASER: 63.8 ± 2.56, anticholinerg: 63.9 ± 2.76, and beta3: 65.32 ± 2.28; N/A | 2940 nm | 3 sessions, 1 months | Anticholinergic agent vs beta3‐adrenoreceptor agonist vs LASER | Primary: OABSS and VHIS | Significant improvement for all groups in OABSS. Significant improvement for LASER group in VHIS. After LASER, negative correlation between urinary urgency and UI | No SAE | OAB | |
| Reisenauer et al. (2019) 113 | Germany | Cohort | 5 months, last | 33 | 51.9 ± 9.8; N/A | Phase 1: 25 J/cm2 + 300 μs. Phase 2: 9 J/cm2 + 1000 μs. | 2 sessions, 1 months | B&A treatment | Primary: ICIQ‐SF and QoL | Significant improvement in ICIQ‐SF and QoL 5 months after treatment. | No SAE. Transient: Vaginal discharge, spotting and burning/irritation (n = 10) | SUI (70%) + MUI (30%) | |
| Su et al. (2019) 114 | Taiwan | Cohort | 3 months, last | 20 | SUI = 46.5 (36–59) MUI = 45.5 (34–54); N/A | 10 J/cm2 | 2 sessions, 1 months | MUI and SUI | Primary: ICIQ‐SF | No significant between‐group difference in change in ICIQ‐SF scores | No SAE or AE. | SUI (50%) + MUI (50%) | |
| Okui et al. (2018) 115 | Japan | Cohort | 12 months, last | 150 | TVT = 48.7 ± 13.9; TOT = 47.8 ± 13.9; LASER = 50.3 ± 13.2; N/A | N/A | 3 sessions, 1 months | TVT and TOT | Primary: 1‐h pad test. Secondary: ICIQ‐SF and OABSS | No significant between‐group differences in 1‐h pad test, but significant within‐group improvement for 1‐h pad test and ICIQ‐SF in all groups | No SAE or AE in the LASER group | SUI | |
| Lin et al. (2017) 116 | Taiwan | Cohort | 12 months, last | 30 | 52.6 ± 8.8, N/A | 2940 nm | 2 sessions, 1 months | B&A treatment | Primary: OABSS, ICIQ‐SF, UDI‐6, IIQ‐7, POPDI‐6, PISQ‐12, 1‐h pad test, urodynamic testing, and vaginal pressure | Significant improvement in OABSS, ICIQ‐SF, UDI‐6, IIQ‐7, POPDI‐6, PISQ‐12, 1‐h pad test, and vaginal pressure at 3‐month follow‐up. Significant improvement in POPDI‐6 at 12‐month follow‐up | No SAE. | SUI | |
| Fistonić et al. (2016) 117 | Croatia | Cohort | 6 months, last | 31 | 46.6 ± 9.1; N/A | 3 and 10 J/cm2 | 1 session | B&A treatment | Primary: ICIQ‐UI‐SF and mucosa surface temperatures. Secondary: Perineometry and residual urine volume | Significant improvement in ICIQ‐UI‐SF after all follow‐ups | No SAE. Transient: vaginal discharge and slight vulvar edema | SUI | |
| Pardo et al. (2016) 118 | Chile | Cohort | 3–6 months, first | 42 | Median (IQR): 46.5 y (42–57); N/A | 1st step: 3 J/cm2, 2nd step: 6 J/cm2 and 3rd step: 10 J/cm2 | 2 sessions, 3–4 weeks | B&A treatment | Primary: ICIQ‐SF | Significant improvement in ICIQ‐SF | No SAE. Transient: mild pain during treatment | SUI | |
| Tien et al. (2016) 119 | Taiwan | Cohort | 6 months, first | 35 | 43.3 ± 7.2; Postmenopausal (n = 7) | N/A | 1 session | B&A treatment | Primary: Pad test. Secondary; Urodynamic assessment, PPBC, USS, OABSS, UDI‐6, IIQ‐7, KHQ and FSFI. | Significant improvement in pad weights at follow‐up. | NO SAE or AE. | SUI | |
| Fistonić et al. (2015) 120 | Croatia | Cohort | 6 months, last | 73 | Median (IQR): 47 y (41–54); Premenopausal (n = 51), postmenopausal (n = 22) | Total: 2500–3000 J | 1 session | B&A treatment | Primary: ICIQ‐UI SF. Secondary: PISQ‐12 | Significant improvement in ICIQ‐SF scores at follow‐up. | No SAE. Transient: irritation, vaginal discharge, slight vulvar edema, de novo urgency (n = 1). | SUI | |
| Ogrinc et al. (2015) 121 | Slovenia | Cohort | 12 months, last | 175 | 49.7 ± 10; N/A | 10.0 J/cm2 | 3 sessions, 4–6 weeks | B&A treatment | Primary: ICIQ‐SF and ISI | Significant improvement at follow‐up and patients with SUI improved significantly more than MUI patients. | No SAE. Transient: mild discomfort. | SUI (66%) and MUI (34%) | |
| Cañadas Molina & Baro (2021) 127 | Spain | Case Report | 3months, last | 1 | 48 y | N/A | 2 sessions, N/A | N/A | AE | A case of complete transverse vaginal septum and shortening of vaginal length after two sessions of vaginal Er:YAG LASER treatment for SUI. | SAE | SUI |
Note: General characteristics, findings, and adverse events in included studies. The table is sorted by (1) LASER type, (2) treatment indication, (3) study design, (4) year of publication and (5) author name.
Abbreviations: AE, adverse event(s); APFQ, Australian Pelvic Floor Questionnaire; B&A treatment, before and after treatment; CO2, carbon dioxide LASER; Er:YAG, Erbium: Ytrium‐Aluminum‐Garnet LASER; FSFI, Female Sexual Function Index; GSM, Genitourinary syndrome of menopause; ICIQ, International Consultation on Incontinence Questionnaire; ICIQ‐OAB, International Consultation on Incontinence Questionnaire—Overactive Bladder Module; ICIQ‐SF or ICIQ‐UI SF, International Consultation on Incontinence Questionnaire – Urinary Incontinence Short Form; IIQ‐7, Incontinence Impact Questionnaire; IQR, interquartile range; KHQ, King’s Health Questionnaire; MUI, mixed urinary incontinence; N/A, not available or not applicable; OAB, overactive bladder; OABSS, Over‐Active Bladder Symptom Score; PFDI‐20, pelvic floor distress inventory 20; PFIQ, Pelvic Floor Impact Questionnaire; PFIQ‐7, Pelvic Floor Impact Questionnaire—short form 7; PGI‐I, Patient Global Impression of Improvement; PGI‐S, patient global impression of severity; PISQ‐12, The Pelvic Organ Prolapse Urinary Incontinence Sexual Questionnaire with 12 questions; POPDI‐6, pelvic organ prolapse distress inventory 6; PPBC, patient perception of bladder condition; QoL, quality of life; QUID, Questionnaire for Urinary Incontinence Diagnosis; SAE, serious adverse event(s); SUI, stress urinary incontinence; TOT, transoburator tape; TVT, tension‐free vaginal tape; UDI‐6, urinary distress inventory, short form; USS, Urgency Severity Scale questionnaire; UTI, urinary tract infection; VAS, Visual Analog Scale; VEL, vaginal Erbium:YAG LASER; VHI or VHIS, Vaginal Health Index or Vaginal Health Index Score.
Follow‐up is reported as time from initial treatment session (first) or final treatment session (last).
Age is reported in mean ± SD unless otherwise specified.
TABLE 6.
Lichen sclerosus
| LASER | Author | Country | Design | Follow‐up a | Sample size [n] | Age [years] b ; menopause status | Treatment settings | No. treatments, interval | Comparison | Outcome | Conclusion | Adverse events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | Burkett et al. (2021) 35 | USA | RCT | 6 months, first | 52 | 64.5 ± 10.4; Postmenopausal (n = 52) | 26 W (1st) and 30 W (2nd and 3rd) | 3 sessions, 4–6 weeks | Topical clobetasol propionate steroid (1:1) | Primary: mean Skindex‐29. Secondary: VAS, VSQ, Skindex‐29 sub‐scores, PGI‐S and PGI‐I | Skindex‐29 scores were significantly improved in the LASER group compared with the steroid group | No SAE. Transient: burning, irritation and poor healing (n = 1) |
| Mitchell et al. (2021) 36 | USA | RCT | 8 weeks, last | 40 | Median (IQR): 59 (51–64); N/A | 18–26 W, stack 1 | 5 sessions, 1 months | Sham LASER (1:1) |
Primary: histopathologic change on biopsy on a 0–6 point scale. Secondary: CSS |
No significant difference in improvement in histopathologic changes between CO2 and sham group | No SAE. Transient: mild discomfort | |
| Stewart et al. (2021) 122 | USA | Cohort | 12 months, last | 12 | 57 ± 10; Postmenopausal (n = 11) | Deep: 50–65 mJ, Fusion: 50–70 mJ, Ring: 78.5–94.4 mJ | 3–5 sessions, 1 months | B&A treatment | Primary: Investigator assessed severity, clinical signs. Secondary: VLS symptoms, QoL, sexual function, FSFI, biopsies (n = 4) | Significant improvement in severity of clinical signs and architectural changes at 12‐month follow‐up | No SAE. Transient: Severe erythema (n = 1) and mild pinpoint bleeding (n = 1) | |
| Balchander & Nyirjesy (2020) 123 | USA | Cohort | 6 months, last | 40 | 59.3 ± 9; N/A | 24 W, stack 1 | ≥2 sessions, 1 months | B&A treatment | Primary: NRS of symptoms. Secondary: Physical examination, reported events and patient self‐assessment. | Significant improvement in all symptoms except from dryness | No SAE. Transient: mild or moderate pain (n = 12), burning pain lasting longer than 7 days (n = 2) | |
| Pagano et al. (2020) 124 | Italy | Cohort | 3 month, last | 40 | 57.9 ± 11.1; Menopausal (n = 37) | External: 25 W, stack 1–3. Internal: 30 W, stack 1–3. | 2 sessions, 30–40 days | B&A treatment | Primary: VAS for vulvar itching. Secondary: VAS for other lichen‐related symptoms and treatment | Significant improvement in vulvar itching before and after treatment | No SAE | |
| Mendieta‐ Eckert et al. (2021) 128 | Spain | Case Report | 4–16 weeks, last | 4 | 53–62 years; N/A | 15–17.5 mJ | 5–7, 1 months | N/A | N/A | General improvement. | No SAE. Transient: superficial ulcer (n = 1), allergic contact dermatitis (n = 1) | |
| Lee et al. (2016) 129 | Australia | Case Report | 6–48 months, N/A | 5 | 56 (39–65); Postmenopausal (n = 3) | 40 W and 140–170 mJ | 1–3, N/A | N/A | N/A | General improvement. | No SAE. Transient: discomfort posttreatment (n = 2) | |
| Kroft & Shier (2012) 130 | Canada | Case Report | 11–120 months, last | 20 | 47 ± 14; Postmenopausal (n = 9) | 6 W and 200 mJ pr. pulse | 1 | N/A | N/A | General improvement. | No SAE. Transient: wound infection (n = 1) | |
| Kartamaa & Reitamo (1997) 131 | Finland | Case Report | 1 and 6 y | 2 | 47 and 56; N/A | 20 W | 1 | N/A | N/A | General improvement. | No SAE | |
| Er:YAG | Hobson et al. (2019) 132 | USA | Case Report | >1 year, last | 2 | 58 and 73; Postmenopausal | C1: Depth 750 μm. C2: Depth 550–750 μm | 1 and 3, N/A | N/A | N/A | General improvement. | N/A |
| Nd:YAG | Bizjak Ogrinc et al. (2019) 37 | Slovenia | RCT | 6 months, last | 38 | LASER: 59 ± 10. Corticosteroids: 57 ± 14; N/A | 90 J/cm2 + corticosteroid | 3 sessions, 2 weeks | Topical corticosteroids only (1:1) | Primary: VAS for symptoms. Secondary: sexual activity, treatment satisfaction, histologic and clinical evaluation | VAS scores were significantly lower in the LASER group at 1 and 3 months compared with the corticosteroids group | No SAE |
Note: General characteristics, findings, and adverse events in included studies. The table is sorted by (1) LASER type, (2) study design, (3) year of publication and (4) author name.
Abbreviations: AE, adverse event(s); B&A treatment, before and after treatment; CO2, carbon dioxide LASER; CSS, Clinical Scoring System for Vulvar Lichen Sclerosus; Er:YAG, Erbium: Ytrium‐Aluminum‐Garnet LASER; FSFI, Female Sexual Function Index; IQR, interquartile range; N/A, not available or not applicable; Nd:YAG, Neodymium‐doped yttrium aluminum garnet; NRS, Numeric Rating Scale; PGI‐I, Patient Global Impression of Improvement; PGI‐S, Patient Global Impression of Severity; QoL, quality of life; SAE, serious adverse event(s); VAS, Visual Analog Scale; VLS, vulvar lichen slerosus; VSQ, Vulvovaginal Symptoms Questionnaire.
Follow‐up is reported as time from initial treatment session (first) or final treatment session (last).
Age is reported in mean ± SD unless otherwise specified.
FIGURE 1.
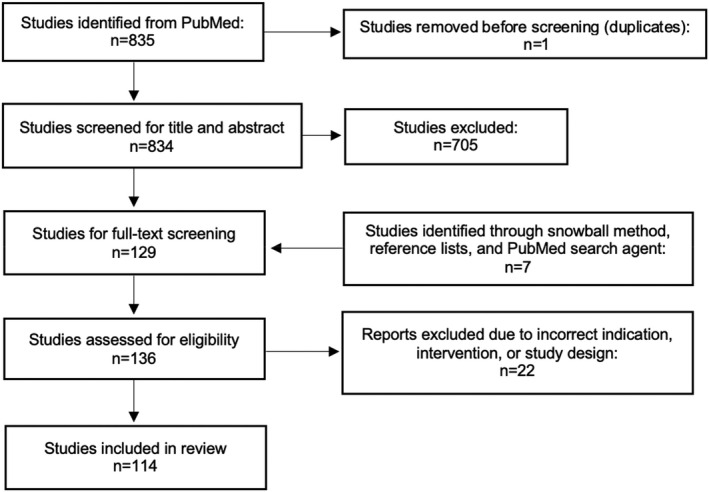
Flow diagram for the screening process for the review
Of the included studies, 81 studies investigated CO2 ‐LASER from different manufacturers. 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 33 , 35 , 36 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 122 , 123 , 124 , 125 , 128 , 129 , 130 , 131 , 133 , 136 Twenty‐eight studies investigated Er:YAG LASER from different manufacturers. 32 , 34 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 94 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 127 , 132 , 135 Three studies reported on CO2 or Er:YAG simultaneously. 105 , 126 , 134 One study investigated the effect of CO2 ‐LASER in combination with a platelet‐rich plasma injection. 106 A single study investigated the effect of a Nd:YAG LASER. 37 The most common energy setting reported for internal CO2 LASER application is 30–40 W and for the Er.YAG LASER 3–10 J/cm2. Year of publication ranged from 1997 to 2021, with a median (interquartile range [IQR]) of articles published in 2019 (2017–2020).
3.1. Genitourinary syndrome of menopause
Thirty‐six studies on the effect of vaginal LASER on GSM were identified through this review (Table 3). 23 , 24 , 26 , 27 , 28 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 70 , 125 , 133 The studies included 4220 women with study sizes ranging from 4 to 1081 women with a median (IQR) of 60.5 (42.25–75.25) women. Among these studies, 29 studies investigated the effect of CO2 LASER, 23 , 24 , 25 , 26 , 27 , 28 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 125 , 133 counting six RCTs including 336 women 23 , 24 , 25 , 26 , 27 , 28 , and 21 cohort studies including 2251 women. 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 Seven cohort studies including 1579 women investigated the effect of Er:YAG. 59 , 60 , 61 , 62 , 63 , 64 , 65
Three RCTs with a total of 137 women who received either CO2 LASER or sham LASER reported no significant between‐group difference in subjective and objective measures at a follow‐up of 1–12 months. 23 , 24 , 26 In contrast, Salvatore et al. used CO2 LASER or sham LASER on 58 women and found a significantly higher improvement in visual analog scale (VAS) at the 1‐month follow‐up in the CO2 group compared with sham LASER. 25 Two RCTs of 141 women compared LASER with estrogen treatment using the Vaginal Health Index Score, Vaginal Maturation Index (VMI), and Female Sexual Function Index (FSFI); Politano et al. found a significant between‐group improvement at a 14‐week follow‐up favoring the LASER group, 28 whereas Paraiso et al. 27 found no significant difference in improvement at a 6‐month follow‐up.
In observational studies, data from 2089 women exposed to CO2 LASER 38 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 and 1579 women exposed to Er:YAG 59 , 60 , 61 , 64 , 65 , 137 , 138 showed improvement across outcome measures of subjective and objective symptom severity, sexual function and UI symptoms. Of 3880 women exposed to either CO2 or Er:YAG in observational studies, 940 were followed for 12 months or more. 39 , 40 , 42 , 44 , 47 , 53 , 55 , 60 , 61 , 62
3.2. Vulvovaginal atrophy
Thirty‐four studies examining the effect of vaginal LASER on VVA were identified through this review (Table 4). 29 , 30 , 31 , 32 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 126 The studies include 2464 women with study sizes ranging from 2 to 386 women with a median (IQR) of 46 (28.25–86.5) women. Among these studies, 31 studies investigated the effect of CO2 LASER 29 , 30 , 31 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 and two studies the effect of Er:YAG. 32 , 94 Four RCTs included 188 women 29 , 30 , 31 , 32 and 29 cohort studies included 2274 women; 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 a case report of two cases included one case treated with CO2 and one with Er:YAG for VVA. 126
Two RCTs randomized 70 women to topical hormone treatment, CO2 LASER or a combination of these; no significant histologic 29 or clinical 31 difference in VVA symptoms was found between groups at respectively 4 and 5 months after the first session.
Ruanphoo et al. studied 88 women exposed to either CO2 LASER or sham LASER and found significant improvement in Vaginal Health Index Score at 3 months post treatment in both groups, with a significantly higher improvement in the LASER group. 30 Two different treatment regimens for the Er:YAG LASER were examined in an RCT with 30 women. At a 2‐month follow‐up after the last session, they found a significant difference in improvement in sexual satisfaction and maximum pressure measured by a perineometer between the two treatment regimens of Er:YAG LASER favoring group A (sessions 1 and 2 with a 360° scope at 1.7 J/shot, and sessions 3 and 4 with a 90° scope at 1.7 J/shot). 32
Across different subjective and objective outcome measurements, observational studies found a significant improvement in vaginal atrophic symptoms after application of CO2 LASER. 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 87 , 88 , 89 , 90 , 91 , 92 , 93 Of women exposed to either CO2 or Er:YAG in observational studies, 558 of 2274 women were followed for 12 months or more. 66 , 69 , 78 , 82 , 86 , 87
3.3. LASER application for GSM and VVA symptoms among cancer survivors
Twenty‐four of the studies identified in this review provided information on including patients with a history of breast cancer or other gynecologic cancers, 24 , 26 , 39 , 40 , 42 , 43 , 44 , 48 , 50 , 54 , 61 , 63 , 67 , 70 , 72 , 75 , 79 , 80 , 85 , 86 , 87 , 89 , 90 , 94 two of which were RCT (Table 7). 24 , 40 All of the women studied had either GSM or VVA primary indication for LASER application. Across these studies, 959 women with current or previous breast cancer or gynecologic cancers were included. The review identified a single study with the aim of comparing the effect in women with and without breast cancer. In a controlled cohort of 45 women with breast cancer and 90 healthy women, Siliquini et al. found significant improvement in Vaginal Health Index Score and VAS for GSM symptoms in both groups 12 months after application of CO2 LASER. The authors did not, however, report on the statistical or clinical significance of between‐group differences. 42 All observational studies which either partly or solely included women with a history of breast cancer or gynecologic cancer found significant improvement at follow‐up across outcomes. 39 , 40 , 42 , 43 , 44 , 48 , 50 , 54 , 61 , 63 , 67 , 70 , 72 , 75 , 79 , 80 , 85 , 86 , 87 , 89 , 90 , 94 Nevertheless, in a pilot randomized study among 18 women with gynecologic cancer, Quick et al. did not find any difference in VAS at follow‐up for CO2 compared with sham LASER. 26
TABLE 7.
General characteristics, findings and adverse events in included studies that provide information of inclusion of patients with breast cancer (BC) or other gynecologic cancers
| LASER | Author (year) | Design | Sample size [n] | Cancer (n or %) | Indication | Conclusion | Adverse events |
|---|---|---|---|---|---|---|---|
| CO2 | Li et al. (2021) 24 | RCT | 85 | BC (50%) | GSM | No significant between‐group difference in change in overall VAS, VAS for most severe symptom or VSQ score for LASER vs sham, but scores improved in both groups at follow‐up | No SAE. AE: LASER (n = 16) vs sham (n = 17); vaginal pain/ discomfort (44% vs 68%), spotting (30% vs 5%), fewer UTI symptoms or confirmed UTI (15% vs 5%), and vaginal discharge (11% vs 11%). Upper UTI in LASER group (n = 1) |
| Quick et al. (2021) 26 | RCT | 18 | Gynecologic cancer (n = 18) | GSM | No significant difference in overall VAS* from baseline to follow‐up between active vs sham group | No SAE. AE: Vaginal discharge (n = 3), vaginal dryness (n = 3), vaginal pain (n = 1), vaginal inflammation (n = 2), flank pain (n = 1) (unrelated) | |
| Li et al. (2021) 39 | Cohort | 162 | BC (n = 3), gynecologic (n = 3), other (n = 2) | GSM | No significant difference was found for VAS and VHIS between CO2 and topical estriol. VHIS was significantly better at 12 months than at baseline for both groups | No SAE | |
| Quick et al. (2021) 40 | Cohort | 67 | BC (n = 67) | GSM | Significant improvement in FSFI and FSDS‐R scores was found at 12 months, but FSFI still indicated sexual problems | No SAE | |
| Siliquini et al. (2021) 42 | Cohort | 135 | BC (n = 45) | GSM | Significant improvement in VHI and VAS in both groups | No SAE | |
| Sindou‐Faurie et al. (2021) 43 | Cohort | 46 | BC (n = 13) and gynecologic (n = 5) | GSM | Significant improvement in dryness and SUI | N/A | |
| Veron et al. (2021) 44 | Cohort | 46 | BC (n = 46) | GSM | Significant improvement in FSFI. Improvement in Ditrovie | No SAE. Transient: vaginal bleeding (n = 3) | |
| Gittens et al. (2019) 48 | Cohort | 25 | BC (n = N/A) | GSM | Significant improvement in every domain of FSFI, WBFS and FSDS‐R | No SAE | |
| Quick et al. (2019) 50 | Cohort | 64 | BC (n = 64) | GSM | Improvement in VAS, FSFI and UDI | No SAE. Transient: vaginal discharge (n = 69) and vaginal dryness (n = 30) | |
| Lang et al. (2017) 54 | Cohort | 368 | BC (10%) | GSM | Significant improvement in vaginal dryness. 86% satisfied with the treatment | No SAE. AE: UTI symptoms (n = 5), vaginal pain/burning (n = 2), vaginal itching (n = 1) and dyspareunia (n = 1) | |
| Gardner & Aschkenazi (2021) 67 | Cohort | 139 | BC (n = 38) | VVA | Significant improvement in FSFI, VSQ (18/21 questions) and VAS for intercourse and vulvar dryness. BC cohort had same improvement as general cohort | No SAE | |
| Salvatore et al. (2021) 70 | Cohort | 40 | BC (n = 40) | VVA | Significant improvement in VAS and VHI, but no difference between patients with past vs current use of endocrine therapies | No SAE | |
| Angioli et al. (2020) 72 | Cohort | 165 | BC and gynecologic (n = 165) | VVA | Improvement in VAS for VVA symptoms | No SAE | |
| Hersant et al. (2020) 75 | Cohort | 20 | BC (n = 20) | VVA | Significant improvement in VHIS for vaginal elasticity, fluid volume, epithelial integrity and moisture | No SAE. Transient: bleeding (n = 2) | |
| Pearson et al. (2019) 79 | Cohort | 29 | BC (n = 29) | VVA | Significant improvement in dryness, burning and dysuria | N/A | |
| Singh et al. (2019) 80 | Cohort | 45 | BC (n = 8) and gynecologic (n = 5) | VVA | General improvement: 90% of the patients improved in dryness,. 89.5% of the patients improved in dyspareunia | No SAE | |
| Pagano et al. (2017) 85 | Cohort | 82 | BC (n = 82) | VVA | Significant reduction in VAS for all VVA‐related symptoms except vaginal laxity | No SAE | |
| Pieralli et al. (2017) 86 | Cohort | 184 | BC (n = 56) | VVA | Patient satisfaction declined over time, from 92% being satisfied after 6 month(s), to 25% at 24 months | N/A | |
| Siliquini et al. (2017) 87 | Cohort | 91 | BC (n = 13) | VVA | Significant improvement in VAS, VHI and VVHI scores at 15‐month follow‐up | No SAE | |
| Pagano et al. (2016) 89 | Cohort | 26 | BC (n = 26) | VVA | Significant improvement in all VAS scores except for vaginal laxity among BC survivors | No SAE | |
| Pieralli et al. (2016) 90 | Cohort | 50 | BC (n = 50) | VVA | Significant improvement in VHI and VAS scores among BC survivors | No SAE | |
| Er:YAG | Mothes et al. (2018) 63 | Cohort | 16 | BC (n = 16) | GSM | Significant improvement in VHI, but not in pH; 94% of patients were satisfied | No SAE |
| Gambacciani & Levancini (2017) 61 | Cohort | 43 | BC (n = 43) | GSM | Significant improvement in VAS and VHIS up to 1 ‐month follow‐up, but not after 1 month | No SAE or AE | |
| Arêas et al. (2019) 94 | Cohort | 24 | BC (n = 24) | VVA | Significant improvement in VHIS and SPEQ at follow‐up | No SAE. AE: Vaginal candidiasis (n = 1), acute cystitis (n = 1) |
Note: The table is sorted by (1) LASER type, (2) treatment indication, (3) study design, (4) year of publication and (5) author name.
Abbreviations: AE, adverse event(s); CO2, carbon dioxide LASER; Er:YAG, Erbium: Ytrium‐Aluminum‐Garnet LASER; FSDS‐R, The Female Sexual Distress Scale‐Revised Questionnaire; FSFI, Female Sexual Function Index; GSM, genitourinary syndrome of menopause; N/A, not available or not applicable; SAE, serious adverse event(s); SPEQ, Short Personal Experiences Questionnaire; SUI, stress urinary incontinence; UDI, urinary distress inventory; UTI, urinary tract infection; VAS, Visual Analog Scale; VAS*, Vaginal Assessment Scale; VHI or VHIS, Vaginal Health Index or Vaginal Health Index Score; VSQ, Vulvovaginal Symptoms Questionnaire; VVA, vulvovaginal atrophy; WBFS, Wong–Baker Faces Scale.
3.4. Urinary incontinence
Thirty studies on the effect of vaginal LASER on UI were identified through this review (Table 5). 33 , 34 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 127 The studies include 2053 women with study sizes ranging from 1 to 327 women with a median (IQR) of 46 (31.5–72.75) women. Of these studies, 17 studies investigated the effect of Er:YAG, 34 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 127 and 11 studies the effect of CO2 LASER. 33 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 We identified two RCTs including 186 women, 33 , 34 27 cohort studies including 1866 women 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 and one case‐report with one woman. 127
One RCT of 72 women found no significant between‐group differences between CO2 laser and intravaginal promestriene measured by the International Consultation on Incontinence Questionnaire – Urinary Incontinence—Short Form (ICIQ‐UI‐SF) and International Consultation on Incontinence Questionnaire—Over‐Active Bladder (ICIQ‐OAB) 2 weeks after the last session; however, they found a significant within‐group improvement at follow‐up in the LASER group only. 33 One RCT of 114 women showed a significantly higher improvement in ICIQ‐UI‐SF in the Er:YAG LASER group compared with sham LASER 3 months after the last session. 34
Four observational studies on CO2 LASER with 320 women had a follow‐up of 12 months or longer, 95 , 97 , 102 , 103 of whom 262 women did a 1‐h pad test which showed a significant improvement of UI symptoms. 95 , 97 , 103 Thirteen observational studies with 1132 women investigated the ICIQ‐SF for Er:YAG. The follow‐up period was 3 months to 2 years after the last session, and the findings generally show an improvement in ICIQ‐SF score at follow‐up. 107 , 108 , 109 , 110 , 111 , 113 , 114 , 115 , 116 , 117 , 118 , 120 , 121 Of these 1132 women, 741 were followed for more than 12 months. 107 , 110 , 115 , 116 , 121
3.5. Lichen sclerosus
Eleven studies examining the effect of vulvar LASER on LS were identified (Table 6). 35 , 36 , 37 , 122 , 123 , 124 , 128 , 129 , 130 , 131 , 132 The studies include 263 women with study sizes ranging from two to 52 women with a median (IQR) of 20 (7.5–40) women. Among these studies, nine studies investigated the effect of CO2 LASER, 35 , 36 , 122 , 123 , 124 , 128 , 129 , 130 , 131 counting two RCTs including 92 women 35 , 36 and three cohort studies including 92 women. 122 , 123 , 124 One study investigated the effect of Er:YAG 32 and one RCT with 38 women investigated the effect of Nd:YAG. 37
One RCT of 40 women treated with either CO2 or sham LASER showed no significant between‐group difference in histopathologic change 8 weeks after the last session. 36 Two RCTs consisting of 90 women comparing respectively CO2 and Nd:YAG with steroid treatment reported significant between‐group and in‐group improvement favoring the LASER groups. 35 , 37 Women in RCTs were followed for a maximum of 6 months after the last session. 35 , 36 , 37
Across different outcome measures, observational studies including 92 women found a significant improvement in vulvar LS symptoms 3–12 months after application of CO2 LASER. 122 , 123 , 124 The short follow‐up meant that no follow‐up concerning malignant transition was possible.
3.6. Adverse events
In this review, 99 studies including 51 094 patients provided no information on severe adverse events (SAE) related to using LASER as a vaginal or vulvar treatment. 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 44 , 45 , 47 , 48 , 49 , 50 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 80 , 81 , 82 , 83 , 84 , 85 , 87 , 88 , 89 , 90 , 91 , 92 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 108 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 128 , 129 , 130 , 131 , 133 , 135 Eleven studies gave no information on SAE or AEs. 43 , 46 , 51 , 71 , 79 , 86 , 93 , 107 , 109 , 126 , 132 Two studies reported a total of five cases of SAE with fibrosis, scarring, agglutination, penetration injury, vaginal shortening, and complete transvaginal septum (Tables 3, 4, 5, 6). 125 , 127 Of the 99 studies without SAEs, 47 studies reported mild to moderate AEs, eg pain and burning; most AEs were transient. 24 , 25 , 26 , 27 , 35 , 36 , 38 , 41 , 44 , 49 , 50 , 52 , 53 , 54 , 55 , 57 , 58 , 62 , 64 , 65 , 66 , 73 , 75 , 76 , 78 , 81 , 82 , 83 , 84 , 94 , 95 , 100 , 102 , 105 , 110 , 111 , 113 , 117 , 118 , 120 , 121 , 122 , 123 , 128 , 129 , 130 , 133 Three cross‐sectional studies investigated the prevalence of AEs associated with vaginal LASER. 134 , 135 , 136 Ahluwalia et al. reported pain as the most common AE among 46 patients with AEs reported between October 2015 and January 2019. Of these patients, 33 reported chronicity of the AE. 134 In the review by Gambacciani et al., 188 practitioners reported that all observed AEs in 43 095 patients treated with vaginal erbium LASER were mild to moderate, transient and with a low prevalence. 135 Wallace et al. reported CO2 LASER as the LASER with the highest prevalence of AE in the Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) database. Two‐thirds of AE in the MAUDE database were related to pain, and SAEs were rare. 136
4. DISCUSSION
In a best practice review from 2019, Preti et al. stated that vaginal laser could not be recommended as routine treatment for the indications VVA, UI, vulvodynia and LS unless high‐quality clinical trials were done. 10 Since then, multiple papers have been published on the subject, including sham‐controlled RCTs. As LASER technology is still a contentious topic in gynecology, this review provides an updated summary of the evidence within this field.
We identified 114 studies meeting our eligibility criteria. Across all indications, most observational studies show a significant improvement in urogenital symptoms after LASER application. The with‐in group effects found in observational studies are reproducible in RCTs; however, the effect of neither CO2 or Er:YAG LASER differs consistently from that of sham LASER or selected steroid treatments. To our knowledge, an RCT on vaginal histology in humans to prove the effect of LASER have not been conducted, signifying that LASER technology to this day remains controversial.
Studies on GSM suggest that 137 women in sham‐controlled RCTs show a similar improvement 4 weeks after the last session, and 6 and 12 months after the first session when treated with either sham or CO2 LASER. However, one RCT from Salvatore et al. with 58 women randomized to either CO2 LASER or sham LASER showed a difference in improvement 1 month after the last session, favoring the LASER group. 25 In RCTs comparing CO2 LASER and hormonal treatment, findings are likewise heterogeneous; one study found bigger improvement in the LASER group 14 weeks after the last session, 28 and another study found that vaginal estrogen and CO2 had a similar effect on VAS score 6 months after the last session. 27 Both RCTs and observational studies are characterized by a short follow‐up period. In observational studies on GSM, only 940 of 3880 women were followed for more than a year.
Studies with VVA as indication are also characterized by a short follow‐up; in two RCTs, 70 women showed similar improvement in CO2 and estrogen groups at 4 and 5 months of follow‐up; 29 , 31 however, Ruanphoo et al. found a significant improvement with CO2 LASER compared with sham LASER at a 3‐month follow‐up. 30 In observational studies on VVA, only 558 of 2274 women were followed for more than a year. Adelman et al. released an editorial in 2021 that discussed the optimistic short‐term studies, thereby highlighting the need for long‐term evidence to illuminate the durability of LASER, since women can suffer from urogenital symptoms for several years. 2
There is less high‐quality evidence of the effect of vaginal LASER on UI symptoms compared with GSM and VVA, as we could only identify two RCTs on this topic. 33 , 34 The most recent RCT shows similar improvement at a 2‐week follow‐up for CO2 and intravaginal promestriene 33 and an RCT from 2018 find more explicit improvement in the Er:YAG group than in the sham group among 114 women at a 3‐month follow‐up. 34 However, the heterogeneity of the trials complicates further comparisons. In accordance with the current literature, the identified cohort studies in the current review suggest improvement in stress UI and mixed UI symptoms after LASER application. Wang et al. conducted a meta‐analysis on clinical studies on Er:YAG and CO2 LASER and found a positive impact for stress UI patients measured by ICIQ‐SF score and 1‐h pad test. However, those authors highlight the same limitations as found in this review, namely, a lack of randomized controlled trials, small sample sizes and short‐term follow‐up. 139
Vulvar LASER for LS patients is less documented than for the above‐mentioned indications, as only 222 patients were distributed across six clinical studies investigating LS. 35 , 36 , 37 , 122 , 123 , 124 Data from RCTs on 90 women showed greater improvement in LASER groups than in topical steroid groups. One RCT did find similar improvement after CO2 compared with sham LASER, 36 but the women were only followed for a maximum of 6 months in the RCTs, which is not long enough to illuminate the cancer‐preventive effect. Tasker et al. investigated the use of CO2 LASER for LS in a systematic review; a meta‐analysis could not be done, as the studies were too heterogenous. They rated all included RCTS as ‘high risk of bias’, including two RCTs from the present review. 36 , 37 , 140
Vaginal LASER therapy is often highlighted as a potential treatment alternative for women with hormone‐sensitive diseases in the literature on vaginal LASER. 6 All observational studies on CO2 and Er.YAG LASER, which include women with BC or gynecologic cancer, show significant improvement in GSM and VVA symptom severity. However, evidence from RCTs including women with BC or gynecologic cancer does not show a significant effect on primary outcomes after CO2 LASER compared with sham LASER. In a pilot study, Quick et al. (2020) randomized 18 women (all with gynecologic cancer) to LASER (n = 10) or sham treatment (n = 8); they concluded that vaginal LASER was safe for cancer patients suffering from GSM. 26 However, we did not identify any large RCT studies comparing the effect and safety in women with a history of cancer.
This review illustrates how the evidence in the field of vaginal and vulvar LASER has developed over time. Although the most studied LASER systems have been allowed for medical use on soft tissue since 2014 (DEKA SmartXide2 Laser System) and 2010 (Fotona LightWalker Laser System Family), 75% of studies, identified in current review, were published in the last 5 years. 141 , 142 They demonstrate substantial marketing prior to a surge in studies investigating the effect and safety of vaginal and vulvar LASER. The initial evidence that has led to a widespread clinical use is based primarily on short‐term observational or uncontrolled studies showing promising improvement in GSM, VVA, UI and LS symptom severity. The limited use of control groups in current vaginal and vulvar LASER literature is problematic, considering potential treatment biases and the rapid uptake of the treatment among practitioners. 2 However, in recent years there has been an increase in RCTs, possibly as a result of the U.S. Food and Drug Administration alert on SAEs in 2018 yielding high‐quality evidence in the area of LASER technology. 9 Most recently, Li et al. published the largest and longest term double‐blinded randomized sham‐controlled trial of whether CO2 LASER reduces GSM symptoms. Even though VAS and Vaginal Health Index scores were improved 12 months after treatment, there was no statistically significant difference between the active and sham groups. 24 The study has been highlighted by editors as financially independent of the industry and as overcoming methodologic limitations in previous studies. 2 One limitation, however, is that it appears the study was powered to detect a with‐in group improvement of 50% in the LASER group, and it is unclear whether it was powered to detect a statistical difference between groups. 24
In accordance with previous reviews on the field, we identified several weaknesses in the current literature. 10 , 143 , 144 , 145 The relative shortness of follow‐up is a challenge, as the majority of studies do not follow their participants more than 6 months post treatment, and in only one high‐quality study a follow‐up of 1 year after the first treatment. 24 A longer follow‐up period after treatment is needed to establish the long‐term effect of vaginal LASER. Comparison of the studies is made difficult by heterogeneity in reporting practices related to LASER settings and intensity. To heighten the comparability between studies, reporting practices need to be standardized, eg energy setting, total number of shots emitted, and stack used. If the total amount of energy delivered per session is not reported systematically, it is difficult to establish when and whether vaginal LASERs are safe and effective.
Current literature lacks reporting of adherence to the international guidelines of good clinical practice. Good clinical practice is important to secure standardization, improve data, and eliminate bias within the trials. Li et al. carried out a review using the QUADAS‐2 tool and Cochrane REVIEW MANAGER version 5.4 to assess the risk of bias; they found that most of the studies on women with postmenopausal genital symptomswere at high risk of bias. The types of bias included reporting bias and industry involvement, as some of the studies are industry‐financed, and some of the authors are consultants for the LASER firms. 145 A cost‐effective analysis estimated an out‐of‐pocket cost at US$2733 for three sessions of LASER. 146 The ethics of increased uptake and high out‐of‐pocket spending should be carefully considered, as RCTs and histologic studies cast doubt on the evidence of the effect and durability related to LASER in gynecology. 2 The U.S. Food and Drug Administration and several studies have flagged up the problem that some manufacturers marketing “vaginal rejuvenation” devices, profit from women suffering from vaginal symptoms, without sufficient evidence of treatment effect. The possibility that LASER is driven by a commercial interest rather than well‐founded evidence should be considered. 9
4.1.
This review covers the quantity and variety of evidence, providing an overview of the field to highlight gaps in the current literature. As a result of the broad scope we did not estimate the quality and risk of bias for all included studies according to PRISMA best practice. A limitation to this study is that the search string specifically includes search terms for CO2 LASER but not for other LASERs, favoring this type of LASER in the search, as we hypnotized that CO2 LASERs were the most commonly used LASER for female urogenital diseases. Broad terms such as “vaginal LASER” and “energy‐based device” were used to allow studies on other LASER types to be included. PubMed was the only database used for this state‐of‐the‐art review, which could result in the authors missing relevant articles. However, after the initial database‐search, the authors screened references in systematic reviews on vaginal LASER in order to confirm that all relevant studies were included.
5. CONCLUSION
Observational studies identified in this review found a positive amendment in GSM, VVA, UI and LS symptoms over time; however, this association is not as noticed in RCTs, as the effect of LASER does not deviate considerably from steroid treatment and sham LASER. Hence LASER technology continues to be highly controversial, as there is no consistency in the existing evidence. Reporting practices for gynecologic LASER need standardization in the treatment protocols and homogeneity within the literature. The current literature is dominated by short‐term cohort studies; larger long‐term and high‐quality RCTs are needed within this field before LASER can be considered a routine treatment for GSM, VVA, UI and LS.
CONFLICT OF INTEREST
All authors state that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
EL contributed to the idea. OEM, SEC and EL conceptualized and designed the review. OEM and SEC carried out the screening process and data extraction. OEM, OE and SEC drafted the initial manuscript. All authors reviewed and approved the final manuscript as submitted.
ACKNOWLEDGMENTS
Jette Meelby, librarian at North Zealand Hospital, helped with the search string in PubMed.
Mortensen OE, Christensen SE, Løkkegaard E. The evidence behind the use of LASER for genitourinary syndrome of menopause, vulvovaginal atrophy, urinary incontinence and lichen sclerosus: A state‐of‐the‐art review. Acta Obstet Gynecol Scand. 2022;101:657‐692. doi: 10.1111/aogs.14353
REFERENCES
- 1. Nappi RE, Kokot‐Kierepa M. Vaginal Health: Insights, Views & Attitudes (VIVA) ‐ results from an international survey. Climacteric. 2012;15:36‐44. [DOI] [PubMed] [Google Scholar]
- 2. Adelman M, Nygaard IE. Time for a “Pause” on the use of vaginal laser. JAMA. 2021;326:1378‐1380. [DOI] [PubMed] [Google Scholar]
- 3. Jin J. Vaginal and Urinary Symptoms of Menopause. JAMA. 2017;317:1388‐. [DOI] [PubMed]
- 4. Franić D, Fistonić I. Laser therapy in the treatment of female urinary incontinence and genitourinary syndrome of menopause: an update. Biomed Res Int. 2019;2019:1576359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akel R, Fuller C. Updates in lichen sclerosis: British Association of Dermatologists guidelines for the management of lichen sclerosus 2018. Br J Dermatol. 2018;178:823‐824. [DOI] [PubMed] [Google Scholar]
- 6. Angelou K, Grigoriadis T, Diakosavvas M, Zacharakis D, Athanasiou S. The genitourinary syndrome of menopause: an overview of the recent data. Cureus. 2020;12:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Omi T, Numano K. The role of the CO2 laser and fractional CO2 laser in dermatology. Laser Therapy. 2014;23:49‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaunitz AM, Pinkerton JV, Manson JE. Women harmed by vaginal laser for treatment of GSM‐the latest casualties of fear and confusion surrounding hormone therapy. Menopause. 2019;26:338‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. FDA . Gottlieb, M.D., on efforts to safeguard women’s health from deceptive health claims and significant risks related to devices marketed for use in medical procedures for “vaginal rejuvenation” FDA website July 30, 2018 [October 26, 2021]. Available from: https://www.fda.gov/news‐events/press‐announcements/statement‐fda‐commissioner‐scott‐gottlieb‐md‐efforts‐safeguard‐womens‐health‐deceptive‐health‐claims
- 10. Preti M, Vieira‐Baptista P, Digesu GA, et al. The clinical role of LASER for vulvar and vaginal treatments in gynecology and female urology: an ICS/ISSVD best practice consensus document. J Low Genit Tract Dis. 2019;23:151‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adelman MR, Tsai LJ, Tangchitnob EP, Kahn BS. Laser technology and applications in gynaecology. J Obstet Gynaecol. 2013;33:225‐231. [DOI] [PubMed] [Google Scholar]
- 12. Pagano T, Travaglino A, Raffone A, et al. Fractional microablative CO(2) laser‐related histological changes on vulvar tissue in patients with genitourinary syndrome of menopause. Lasers Surg Med. 2021;53:521‐527. [DOI] [PubMed] [Google Scholar]
- 13. Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19:512‐518. [DOI] [PubMed] [Google Scholar]
- 14. Lapii GA, Yakovleva AY, Neimark AI. Structural reorganization of the vaginal mucosa in stress urinary incontinence under conditions of Er:YAG laser treatment. Bull Exp Biol Med. 2017;162:510‐514. [DOI] [PubMed] [Google Scholar]
- 15. Lapii GA, Yakovleva AY, Neimark AI, Lushnikova EL. Study of proliferative activity of vaginal epithelium in women with stress urinary incontinence treated by Er:YAG laser. Bull Exp Biol Med. 2017;163:280‐283. [DOI] [PubMed] [Google Scholar]
- 16. Salvatore S, França K, Lotti T, et al. Early regenerative modifications of human postmenopausal atrophic vaginal mucosa following fractional CO(2) laser treatment. Open Access Maced J Med Sci. 2018;6:6‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30:429‐436. [DOI] [PubMed] [Google Scholar]
- 18. Becorpi A, Campisciano G, Zanotta N, et al. Fractional CO(2) laser for genitourinary syndrome of menopause in breast cancer survivors: clinical, immunological, and microbiological aspects. Lasers Med Sci. 2018;33:1047‐1054. [DOI] [PubMed] [Google Scholar]
- 19. Salvatore S, Leone Roberti Maggiore U, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: an ex vivo study. Menopause 2015;22:845‐9. [DOI] [PubMed] [Google Scholar]
- 20. Gaspar A, Silva J, Calderon A, Di Placido V, Vizintin Z. Histological findings after non‐ablative Er:YAG laser therapy in women with severe vaginal atrophy. Climacteric. 2020;23:S11‐s3. [DOI] [PubMed] [Google Scholar]
- 21. Mackova K, Mazzer AM, Mori Da Cunha M, et al. Vaginal Er:YAG laser application in the menopausal ewe model: a randomised estrogen and sham‐controlled trial. BJOG 2021;128:1087‐96. [DOI] [PubMed] [Google Scholar]
- 22. Covidence systematic review software VHI, Melbourne, Australia. Available at www.covidence.org
- 23. Cruff J, Khandwala S. A double‐blind randomized sham‐controlled trial to evaluate the efficacy of fractional carbon dioxide laser therapy on genitourinary syndrome of menopause. J Sex Med. 2021;18:761‐769. [DOI] [PubMed] [Google Scholar]
- 24. Li FG, Maheux‐Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381‐1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salvatore S, Pitsouni E, Grigoriadis T, et al. CO(2) laser and the genitourinary syndrome of menopause: a randomized sham‐controlled trial. Climacteric. 2020;24:187‐193. [DOI] [PubMed] [Google Scholar]
- 26. Quick AM, Dockter T, Le‐Rademacher J, et al. Pilot study of fractional CO(2) laser therapy for genitourinary syndrome of menopause in gynecologic cancer survivors. Maturitas. 2020;144:37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paraiso MFR, Ferrando CA, Sokol ER, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET trial. Menopause. 2020;27:50‐56. [DOI] [PubMed] [Google Scholar]
- 28. Politano CA, Costa‐Paiva L, Aguiar LB, Machado HC, Baccaro LF. Fractional CO2 laser vs promestriene and lubricant in genitourinary syndrome of menopause: a randomized clinical trial. Menopause. 2019;26:833‐840. [DOI] [PubMed] [Google Scholar]
- 29. Dutra P, Heinke T, Pinho SC, et al. Comparison of topical fractional CO2 laser and vaginal estrogen for the treatment of genitourinary syndrome in postmenopausal women: a randomized controlled trial. Menopause. 2021;28:756‐763. [DOI] [PubMed] [Google Scholar]
- 30. Ruanphoo P, Bunyavejchevin S. Treatment for vaginal atrophy using microablative fractional CO2 laser: a randomized double‐blinded sham‐controlled trial. Menopause. 2020;27:858‐863. [DOI] [PubMed] [Google Scholar]
- 31. Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double‐blind, placebo‐controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21‐28. [DOI] [PubMed] [Google Scholar]
- 32. Lee MS. Treatment of vaginal relaxation syndrome with an Erbium:YAG laser using 90° and 360° scanning scopes: a pilot study & short‐term results. Laser Ther. 2014;23:129‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aguiar LB, Politano CA, Costa‐Paiva L, Juliato CRT. Efficacy of fractional CO(2) laser, promestriene, and vaginal lubricant in the treatment of urinary symptoms in postmenopausal women: a randomized clinical trial. Lasers Surg Med. 2020;52:713‐720. [DOI] [PubMed] [Google Scholar]
- 34. Blaganje M, Šćepanović D, Žgur L, Verdenik I, Pajk F, Lukanović A. Non‐ablative Er:YAG laser therapy effect on stress urinary incontinence related to quality of life and sexual function: A randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2018;224:153‐158. [DOI] [PubMed] [Google Scholar]
- 35. Burkett LS, Siddique M, Zeymo A, et al. Clobetasol compared with fractionated carbon dioxide laser for lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:968‐978. [DOI] [PubMed] [Google Scholar]
- 36. Mitchell L, Goldstein AT, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bizjak Ogrinc U, Senčar S, Luzar B, Lukanović A. Efficacy of non‐ablative laser therapy for lichen sclerosus: a randomized controlled trial. J Obstet Gynaecol Can. 2019;41:1717‐1725. [DOI] [PubMed] [Google Scholar]
- 38. Bretas TLB, Issa MCA, Fialho S, Villar EAG, Velarde LGC, Pérez‐López FR. Vaginal collagen I and III changes after carbon dioxide laser application in postmenopausal women with the genitourinary syndrome: a pilot study. Climacteric. 2022;25:186‐194. [DOI] [PubMed] [Google Scholar]
- 39. Li J, Li H, Zhou Y, et al. The fractional CO(2) laser for the treatment of genitourinary syndrome of menopause: a prospective multicenter cohort study. Lasers Surg Med. 2021;53:647‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Quick AM, Zvinovski F, Hudson C, et al. Patient‐reported sexual function of breast cancer survivors with genitourinary syndrome of menopause after fractional CO2 laser therapy. Menopause. 2021;28:642‐649. [DOI] [PubMed] [Google Scholar]
- 41. Ruffolo AF, Casiraghi A, Marotta E, et al. Does the time of onset of urinary symptoms affect microablative fractional CO(2) laser efficacy in postmenopausal women? Lasers Surg Med. 2021;53:953‐959. [DOI] [PubMed] [Google Scholar]
- 42. Siliquini GP, Bounous VE, Novara L, Giorgi M, Bert F, Biglia N. Fractional CO₂ vaginal laser for the genitourinary syndrome of menopause in breast cancer survivors. Breast J. 2021;27:448‐455. [DOI] [PubMed] [Google Scholar]
- 43. Sindou‐Faurie T, Louis‐Vahdat C, Oueld Es Cheikh E, et al. Evaluation of the efficacy of fractional CO(2) laser in the treatment of vulvar and vaginal menopausal symptoms. Arch Gynecol Obstet 2021;303:955‐63. [DOI] [PubMed] [Google Scholar]
- 44. Veron L, Wehrer D, Annerose‐Zéphir G, et al. Effects of local laser treatment on vulvovaginal atrophy among women with breast cancer: a prospective study with long‐term follow‐up. Breast Cancer Res Treat. 2021;188:501‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Filippini M, Luvero D, Salvatore S, et al. Efficacy of fractional CO2 laser treatment in postmenopausal women with genitourinary syndrome: a multicenter study. Menopause. 2020;27:43‐49. [DOI] [PubMed] [Google Scholar]
- 46. Takacs P, Sipos AG, Kozma B, et al. The effect of vaginal microablative fractional CO(2) laser treatment on vaginal cytology. Lasers Surg Med. 2020;52:708‐712. [DOI] [PubMed] [Google Scholar]
- 47. Athanasiou S, Pitsouni E, Grigoriadis T, et al. Microablative fractional CO2 laser for the genitourinary syndrome of menopause: up to 12‐month results. Menopause. 2019;26:248‐255. [DOI] [PubMed] [Google Scholar]
- 48. Gittens P, Mullen G. The effects of fractional microablative CO(2) laser therapy on sexual function in postmenopausal women and women with a history of breast cancer treated with endocrine therapy. J Cosmet Laser Ther. 2019;21:127‐131. [DOI] [PubMed] [Google Scholar]
- 49. Murina F, Felice R, Di Francesco S, Nelvastellio L, Cetin I. Ospemifene plus fractional CO(2) laser: a powerful strategy to treat postmenopausal vulvar pain. Gynecol Endocrinol. 2019;36:431‐435. [DOI] [PubMed] [Google Scholar]
- 50. Quick AM, Zvinovski F, Hudson C, et al. Fractional CO2 laser therapy for genitourinary syndrome of menopause for breast cancer survivors. Support Care Cancer. 2019;28:3669‐3677. [DOI] [PubMed] [Google Scholar]
- 51. Tovar‐Huamani J, Mercado‐Olivares F, Grandez‐Urbina JA, Pichardo‐Rodriguez R, Tovar‐Huamani M, García‐Perdomo H. Efficacy of fractional CO(2) laser in the treatment of genitourinary syndrome of menopause in Latin‐American Population: first Peruvian experience. Lasers Surg Med. 2019;51:509‐515. [DOI] [PubMed] [Google Scholar]
- 52. Athanasiou S, Pitsouni E, Falagas ME, Salvatore S, Grigoriadis T. CO(2)‐laser for the genitourinary syndrome of menopause. How many laser sessions? Maturitas. 2017;104:24‐28. [DOI] [PubMed] [Google Scholar]
- 53. Behnia‐Willison F, Sarraf S, Miller J, et al. Safety and long‐term efficacy of fractional CO(2) laser treatment in women suffering from genitourinary syndrome of menopause. Eur J Obstet Gynecol Reprod Biol. 2017;213:39‐44. [DOI] [PubMed] [Google Scholar]
- 54. Lang P, Dell JR, Rosen L, Weiss P, Karram M. Fractional CO(2) laser of the vagina for genitourinary syndrome of menopause: is the out‐of‐pocket cost worth the outcome of treatment? Lasers Surg Med. 2017;49:882‐885. [DOI] [PubMed] [Google Scholar]
- 55. Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1‐year outcomes. Menopause. 2017;24:810‐814. [DOI] [PubMed] [Google Scholar]
- 56. Murina F, Karram M, Salvatore S, Felice R. Fractional CO(2) laser treatment of the vestibule for patients with vestibulodynia and genitourinary syndrome of menopause: a pilot study. J Sex Med. 2016;13:1915‐1917. [DOI] [PubMed] [Google Scholar]
- 57. Pitsouni E, Grigoriadis T, Tsiveleka A, Zacharakis D, Salvatore S, Athanasiou S. Microablative fractional CO(2)‐laser therapy and the genitourinary syndrome of menopause: An observational study. Maturitas. 2016;94:131‐136. [DOI] [PubMed] [Google Scholar]
- 58. Sokol ER, Karram MM. An assessment of the safety and efficacy of a fractional CO2 laser system for the treatment of vulvovaginal atrophy. Menopause. 2016;23:1102‐1107. [DOI] [PubMed] [Google Scholar]
- 59. Gambacciani M, Albertin E, Torelli MG, et al. Sexual function after vaginal erbium laser: the results of a large, multicentric, prospective study. Climacteric. 2020;23:S24‐s7. [DOI] [PubMed] [Google Scholar]
- 60. Gambacciani M, Levancini M, Russo E, Vacca L, Simoncini T, Cervigni M. Long‐term effects of vaginal erbium laser in the treatment of genitourinary syndrome of menopause. Climacteric. 2018;21:148‐152. [DOI] [PubMed] [Google Scholar]
- 61. Gambacciani M, Levancini M. Vaginal erbium laser as second‐generation thermotherapy for the genitourinary syndrome of menopause: a pilot study in breast cancer survivors. Menopause. 2017;24:316‐319. [DOI] [PubMed] [Google Scholar]
- 62. Gaspar A, Brandi H, Gomez V, Luque D. Efficacy of Erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mothes AR, Runnebaum M, Runnebaum IB. Ablative dual‐phase Erbium:YAG laser treatment of atrophy‐related vaginal symptoms in post‐menopausal breast cancer survivors omitting hormonal treatment. J Cancer Res Clin Oncol. 2018;144:955‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gambacciani M, Levancini M. Short‐term effect of vaginal erbium laser on the genitourinary syndrome of menopause. Minerva Ginecol. 2015;67:97‐102. [PubMed] [Google Scholar]
- 65. Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second‐generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Alexiades MR. Fractional Co(2) laser treatment of the vulva and vagina and the effect of postmenopausal duration on efficacy. Lasers Surg Med. 2021;53:185‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gardner AN, Aschkenazi SO. The short‐term efficacy and safety of fractional CO2 laser therapy for vulvovaginal symptoms in menopause, breast cancer, and lichen sclerosus. Menopause. 2021;28:511‐516. [DOI] [PubMed] [Google Scholar]
- 68. Luvero D, Filippini M, Salvatore S, Pieralli A, Farinelli M, Angioli R. The beneficial effects of fractional CO(2) laser treatment on perineal changes during puerperium and breastfeeding period: a multicentric study. Lasers Med Sci. 2021;36:1837‐1843. [DOI] [PubMed] [Google Scholar]
- 69. Rosner‐Tenerowicz A, Zimmer‐Stelmach A, Zimmer M. The CO2 ablative laser treatment in perimenopausal patients with vulvovaginal atrophy. Ginekol Pol 2021. doi: 10.5603/GP.a2021.0140. Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 70. Salvatore S, Nappi RE, Casiraghi A, et al. Microablative fractional CO(2) laser for vulvovaginal atrophy in women with a history of breast cancer: a pilot study at 4‐week follow‐up. Clin Breast Cancer. 2021;21:e539‐e546. [DOI] [PubMed] [Google Scholar]
- 71. Adabi K, Golshahi F, Niroomansh S, Razzaghi Z, Ghaemi M. Effect of the fractional CO(2) laser on the quality of life, general health, and genitourinary symptoms in postmenopausal women with vaginal atrophy: a prospective cohort. J Lasers Med Sci. 2020;11:65‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Angioli R, Stefano S, Filippini M, et al. Effectiveness of CO(2) laser on urogenital syndrome in women with a previous gynecological neoplasia: a multicentric study. Int J Gynecol Cancer. 2020;30:590‐595. [DOI] [PubMed] [Google Scholar]
- 73. Di Donato V, D'Oria O, Scudo M, et al. Safety evaluation of fractional CO(2) laser treatment in post‐menopausal women with vaginal atrophy: A prospective observational study. Maturitas. 2020;135:34‐39. [DOI] [PubMed] [Google Scholar]
- 74. Ghanbari Z, Sohbati S, Eftekhar T, et al. Fractional CO2 laser for treatment of vulvovaginal atrophy: a short time follow‐up. J Family Reprod Health. 2020;14:68‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hersant B, Werkoff G, Sawan D, et al. Carbon dioxide laser treatment for vulvovaginal atrophy in women treated for breast cancer: preliminary results of the feasibility EPIONE trial. Ann Chir Plast Esthet. 2020;65:e23‐e31. [DOI] [PubMed] [Google Scholar]
- 76. Marin J, Lipa G, Dunet E. The results of new low dose fractional CO2 Laser ‐ a prospective clinical study in France. J Gynecol Obstet Hum Reprod. 2020;49:101614. [DOI] [PubMed] [Google Scholar]
- 77. Mezzana P. "Two wavelengths endovaginal laser system": clinical evaluation of a new device for mild SUI and vaginal atrophy treatment. Dermatol Ther. 2020;33:e14445. [DOI] [PubMed] [Google Scholar]
- 78. Eder SE. Long‐term safety and efficacy of fractional CO(2) laser treatment in post‐menopausal women with vaginal atrophy. Laser Ther. 2019;28:103‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pearson A, Booker A, Tio M, Marx G. Vaginal CO(2) laser for the treatment of vulvovaginal atrophy in women with breast cancer: LAAVA pilot study. Breast Cancer Res Treat. 2019;178:135‐140. [DOI] [PubMed] [Google Scholar]
- 80. Singh P, Chong CYL, Han HC. Effects of vulvovaginal laser therapy on postmenopausal vaginal atrophy: a prospective study. J Gynecol Surg. 2019;35:99‐104. [Google Scholar]
- 81. Eder SE. Early effect of fractional CO(2) laser treatment in Post‐menopausal women with vaginal atrophy. Laser Ther. 2018;27:41‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Samuels JB, Garcia MA. Treatment to external labia and vaginal canal with CO2 laser for symptoms of vulvovaginal atrophy in postmenopausal women. Aesthet Surg J. 2019;39:83‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Arroyo C. Fractional CO(2) laser treatment for vulvovaginal atrophy symptoms and vaginal rejuvenation in perimenopausal women. Int J Womens Health. 2017;9:591‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Filippini M, Del Duca E, Negosanti F, et al. Fractional CO(2) laser: from skin rejuvenation to vulvo‐vaginal reshaping. Photomed Laser Surg. 2017;35:171‐175. [DOI] [PubMed] [Google Scholar]
- 85. Pagano T, De Rosa P, Vallone R, et al. Fractional microablative CO2 laser in breast cancer survivors affected by iatrogenic vulvovaginal atrophy after failure of nonestrogenic local treatments: a retrospective study. Menopause. 2017;25:657‐662. [DOI] [PubMed] [Google Scholar]
- 86. Pieralli A, Bianchi C, Longinotti M, et al. Long‐term reliability of fractioned CO(2) laser as a treatment for vulvovaginal atrophy (VVA) symptoms. Arch Gynecol Obstet. 2017;296:973‐978. [DOI] [PubMed] [Google Scholar]
- 87. Siliquini GP, Tuninetti V, Bounous VE, Bert F, Biglia N. Fractional CO(2) laser therapy: a new challenge for vulvovaginal atrophy in postmenopausal women. Climacteric. 2017;20:379‐384. [DOI] [PubMed] [Google Scholar]
- 88. Lekskulchai O, Mairaing K, Vinayanuvattikhun N. Fractional CO2 laser for vulvovaginal atrophy. J Med Assoc Thai. 2016;99(Suppl 4):S54‐S58. [PubMed] [Google Scholar]
- 89. Pagano T, De Rosa P, Vallone R, et al. Fractional microablative CO2 laser for vulvovaginal atrophy in women treated with chemotherapy and/or hormonal therapy for breast cancer: a retrospective study. Menopause. 2016;23:1108‐1113. [DOI] [PubMed] [Google Scholar]
- 90. Pieralli A, Fallani MG, Becorpi A, et al. Fractional CO2 laser for vulvovaginal atrophy (VVA) dyspareunia relief in breast cancer survivors. Arch Gynecol Obstet. 2016;294:841‐846. [DOI] [PubMed] [Google Scholar]
- 91. Perino A, Calligaro A, Forlani F, et al. Vulvo‐vaginal atrophy: a new treatment modality using thermo‐ablative fractional CO2 laser. Maturitas. 2014;80:296‐301. [DOI] [PubMed] [Google Scholar]
- 92. Salvatore S, Nappi RE, Zerbinati N, et al. A 12‐week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17:363‐369. [DOI] [PubMed] [Google Scholar]
- 93. Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO₂ laser in women with vulvovaginal atrophy. Climacteric. 2014;18:219‐225. [DOI] [PubMed] [Google Scholar]
- 94. Arêas F, Valadares ALR, Conde DM, Costa‐Paiva L. The effect of vaginal erbium laser treatment on sexual function and vaginal health in women with a history of breast cancer and symptoms of the genitourinary syndrome of menopause: a prospective study. Menopause. 2019;26:1052‐1058. [DOI] [PubMed] [Google Scholar]
- 95. Alcalay M, Ben Ami M, Greenshpun A, Hagay Z, Schiff E. Fractional‐pixel CO(2) laser treatment in patients with urodynamic stress urinary incontinence: 1‐year follow‐up. Lasers Surg Med. 2021;53:960‐967. [DOI] [PubMed] [Google Scholar]
- 96. Franić D, Fistonić I, Franić‐Ivanišević M, Perdija Ž, Križmarić M. Pixel CO(2) laser for the treatment of stress urinary incontinence: a prospective observational multicenter study. Lasers Surg Med. 2021;53:514‐520. [DOI] [PubMed] [Google Scholar]
- 97. Nalewczynska AA, Barwijuk M, Kolczewski P, Dmoch‐Gajzlerska E. Pixel‐CO(2) laser for the treatment of stress urinary incontinence. Lasers Med Sci. 2022;37:1061‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Toplu G, Serin M, Unveren T, Altinel D. Patient reported vaginal laxity, sexual function and stress incontinence improvement following vaginal rejuvenation with fractional carbon dioxide laser. J Plast Surg Hand Surg. 2021;55:25‐31. [DOI] [PubMed] [Google Scholar]
- 99. Zhang L, Lai Y, Pan W, et al. Application of ultra pulse CO(2) lattice laser in the treatment of female urinary incontinence. Transl Androl Urol. 2021;10:2471‐2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dabaja H, Lauterbach R, Matanes E, Gruenwald I, Lowenstein L. The safety and efficacy of CO(2) laser in the treatment of stress urinary incontinence. Int Urogynecol J. 2020;31:1691‐1696. [DOI] [PubMed] [Google Scholar]
- 101. Palacios S, Ramirez M. Efficacy of the use of fractional CO2RE intima laser treatment in stress and mixed urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 2020;244:95‐100. [DOI] [PubMed] [Google Scholar]
- 102. Behnia‐Willison F, Nguyen TTT, Mohamadi B, et al. Fractional CO(2) laser for treatment of stress urinary incontinence. Eur J Obstet Gynecol Reprod Biol X. 2019;1:100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. González Isaza P, Jaguszewska K, Cardona JL, Lukaszuk M. Long‐term effect of thermoablative fractional CO(2) laser treatment as a novel approach to urinary incontinence management in women with genitourinary syndrome of menopause. Int Urogynecol J. 2018;29:211‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Perino A, Cucinella G, Gugliotta G, et al. Is vaginal fractional CO2 laser treatment effective in improving overactive bladder symptoms in post‐menopausal patients? Preliminary results. Eur Rev Med Pharmacol Sci. 2016;20:2491‐2497. [PubMed] [Google Scholar]
- 105. Lin HY, Tsai HW, Tsui KH, et al. The short‐term outcome of laser in the management of female pelvic floor disorders: focus on stress urine incontinence and sexual dysfunction. Taiwan J Obstet Gynecol. 2018;57:825‐829. [DOI] [PubMed] [Google Scholar]
- 106. Behnia‐Willison F, Nguyen TTT, Norbury AJ, Mohamadi B, Salvatore S, Lam A. Promising impact of platelet rich plasma and carbon dioxide laser for stress urinary incontinence. Eur J Obstet Gynecol Reprod Biol X. 2020;5:100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Okui N, Miyazaki H, Takahashi W, et al. Comparison of urethral sling surgery and non‐ablative vaginal Erbium:YAG laser treatment in 327 patients with stress urinary incontinence: a case‐matching analysis. Lasers Med Sci. 2022;37:655‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Erel CT, Inan D, Mut A. Predictive factors for the efficacy of Er:YAG laser treatment of urinary incontinence. Maturitas. 2020;132:1‐6. [DOI] [PubMed] [Google Scholar]
- 109. Erel CT, Fistonić I, Gambacciani M, Oner Y, Fistonić N. Er:YAG laser in hysterectomized women with stress urinary incontinence: a VELA retrospective cohort, non‐inferiority study. Climacteric. 2020;23:S18‐s23. [DOI] [PubMed] [Google Scholar]
- 110. Kuszka A, Gamper M, Walser C, Kociszewski J, Viereck V. Erbium:YAG laser treatment of female stress urinary incontinence: midterm data. Int Urogynecol J. 2020;31:1859‐1866. [DOI] [PubMed] [Google Scholar]
- 111. Lin KL, Chou SH, Long CY. Effect of Er:YAG laser for women with stress urinary incontinence. Biomed Res Int. 2019;2019:7915813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Okui N. Efficacy and safety of non‐ablative vaginal erbium:YAG laser treatment as a novel surgical treatment for overactive bladder syndrome: comparison with anticholinergics and β3‐adrenoceptor agonists. World J Urol. 2019;37:2459‐2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Reisenauer C, Hartlieb S, Schoenfisch B, Brucker SY, Neis F. Vaginal therapy of mild and moderate stress urinary incontinence using Er:YAG laser: a real treatment option. Arch Gynecol Obstet. 2019;300:1645‐1650. [DOI] [PubMed] [Google Scholar]
- 114. Su CF, Chen GD, Tsai HJ. Preliminary outcome of non‐ablative vaginal Erbium laser treatment for female stress and mixed urinary incontinence. Taiwan J Obstet Gynecol. 2019;58:610‐613. [DOI] [PubMed] [Google Scholar]
- 115. Okui N. Comparison between erbium‐doped yttrium aluminum garnet laser therapy and sling procedures in the treatment of stress and mixed urinary incontinence. World J Urol. 2018;37:885‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lin YH, Hsieh WC, Huang L, Liang CC. Effect of non‐ablative laser treatment on overactive bladder symptoms, urinary incontinence and sexual function in women with urodynamic stress incontinence. Taiwan J Obstet Gynecol. 2017;56:815‐820. [DOI] [PubMed] [Google Scholar]
- 117. Fistonić N, Fistonić I, Guštek ŠF, et al. Minimally invasive, non‐ablative Er:YAG laser treatment of stress urinary incontinence in women‐‐a pilot study. Lasers Med Sci. 2016;31:635‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pardo JI, Solà VR, Morales AA. Treatment of female stress urinary incontinence with Erbium‐YAG laser in non‐ablative mode. Eur J Obstet Gynecol Reprod Biol. 2016;204:1‐4. [DOI] [PubMed] [Google Scholar]
- 119. Tien YW, Hsiao SM, Lee CN, Lin HH. Effects of laser procedure for female urodynamic stress incontinence on pad weight, urodynamics, and sexual function. Int Urogynecol J. 2017;28:469‐476. [DOI] [PubMed] [Google Scholar]
- 120. Fistonić N, Fistonić I, Lukanovič A, Findri Guštek Š, Sorta Bilajac Turina I, Franić D. First assessment of short‐term efficacy of Er:YAG laser treatment on stress urinary incontinence in women: prospective cohort study. Climacteric. 2015;18(Suppl 1):37‐42. [DOI] [PubMed] [Google Scholar]
- 121. Ogrinc UB, Senčar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Lasers Surg Med. 2015;47(9):689‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Stewart K, Javaid S, Schallen KP, Bartlett S, Carlson NA. Fractional CO(2) laser treatment as adjunctive therapy to topical steroids for managing vulvar lichen sclerosus. Lasers Surg Med. 2022;54:138‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Balchander D, Nyirjesy P. Fractionated CO2 laser as therapy in recalcitrant lichen sclerosus. J Low Genit Tract Dis. 2020;24:225‐228. [DOI] [PubMed] [Google Scholar]
- 124. Pagano T, Conforti A, Buonfantino C, et al. Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long‐term use of topic corticosteroid: a prospective longitudinal study. Menopause. 2020;27:418‐422. [DOI] [PubMed] [Google Scholar]
- 125. Gordon C, Gonzales S, Krychman ML. Rethinking the techno vagina: a case series of patient complications following vaginal laser treatment for atrophy. Menopause. 2019;26:423‐427. [DOI] [PubMed] [Google Scholar]
- 126. Salcedo FL, Blanco ZE. Experience with ospemifene in patients with vulvovaginal atrophy treated with laser therapy: case studies. Drugs Context. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cañadas Molina A, Sanz BR. The first major complication due to laser treatment for stress urinary incontinence: a short report. Climacteric. 2021;24:206‐209. [DOI] [PubMed] [Google Scholar]
- 128. Mendieta‐Eckert M, Torrontegui Bilbao J, Zabalza Estévez I, Landa GN. Treatment of vulvar lichen sclerosus et atrophicus with fractional carbon dioxide laser therapy: a report of 4 cases. Actas Dermosifiliogr (Engl Ed). 2021;112:85‐88. [DOI] [PubMed] [Google Scholar]
- 129. Lee A, Lim A, Fischer G. Fractional carbon dioxide laser in recalcitrant vulval lichen sclerosus. Australas J Dermatol. 2016;57:39‐43. [DOI] [PubMed] [Google Scholar]
- 130. Kroft J, Shier M. A novel approach to the surgical management of clitoral phimosis. J Obstet Gynaecol Can. 2012;34:465‐471. [DOI] [PubMed] [Google Scholar]
- 131. Kartamaa M, Reitamo S. Treatment of lichen sclerosus with carbon dioxide laser vaporization. Br J Dermatol. 1997;136:356‐359. [PubMed] [Google Scholar]
- 132. Hobson JG, Ibrahim SF, Mercurio MG. Recalcitrant vulvar lichen sclerosus treated with Erbium YAG laser. JAMA Dermatol. 2019;155:254‐256. [DOI] [PubMed] [Google Scholar]
- 133. Pitsouni E, Grigoriadis T, Falagas M, Tsiveleka A, Salvatore S, Athanasiou S. Microablative fractional CO(2) laser for the genitourinary syndrome of menopause: power of 30 or 40 W? Lasers Med Sci. 2017;32:1865‐1872. [DOI] [PubMed] [Google Scholar]
- 134. Ahluwalia J, Avram MM, Ortiz AE. Lasers and energy‐based devices marketed for vaginal rejuvenation: a cross‐sectional analysis of the MAUDE database. Lasers Surg Med. 2019;51:671‐677. [DOI] [PubMed] [Google Scholar]
- 135. Gambacciani M, Cervigni M, Gaspar A, et al. Safety of vaginal erbium laser: a review of 113,000 patients treated in the past 8 years. Climacteric. 2020;23:S28‐s32. [DOI] [PubMed] [Google Scholar]
- 136. Wallace SL, Sokol ER, Enemchukwu EA. Vaginal energy‐based devices: characterization of adverse events based on the last decade of MAUDE safety reports. Menopause. 2020;28:135‐141. [DOI] [PubMed] [Google Scholar]
- 137. Mothes AR, Runnebaum M, Runnebaum IB. An innovative dual‐phase protocol for pulsed ablative vaginal Erbium:YAG laser treatment of urogynecological symptoms. Eur J Obstet Gynecol Reprod Biol. 2018;229:167‐171. [DOI] [PubMed] [Google Scholar]
- 138. Gaspar A, Brandi H. Non‐ablative erbium YAG laser for the treatment of type III stress urinary incontinence (intrinsic sphincter deficiency). Lasers Med Sci. 2017;32:685‐691. [DOI] [PubMed] [Google Scholar]
- 139. Wang Y, Wang C, Song F, Zhou Y, Wang Y. Safety and efficacy of vaginal laser therapy for stress urinary incontinence: a meta‐analysis. Ann Palliat Med. 2021;10:2736‐2746. [DOI] [PubMed] [Google Scholar]
- 140. Tasker F, Kirby L, Grindlay DJC, Lewis F, Simpson RC. Laser therapy for genital lichen sclerosus: a systematic review of the current evidence base. Skin Health Dis. 2021;1:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. FDA . Laser surgical instrument for use in general and plastic surgery and in dermatology. September 5, 2014 [February 2, 2022]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf13/k133895.pdf
- 142. FDA . Fotona November 22, 2010 [February 2, 2022]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf10/K101817.pdf
- 143. Athanasiou S, Pitsouni E, Douskos A, Salvatore S, Loutradis D, Grigoriadis T. Intravaginal energy‐based devices and sexual health of female cancer survivors: a systematic review and meta‐analysis. Lasers Med Sci. 2020;35:1‐11. [DOI] [PubMed] [Google Scholar]
- 144. Khamis Y, Abdelhakim AM, Labib K, et al. Vaginal CO2 laser therapy vs sham for genitourinary syndrome of menopause management: a systematic review and meta‐analysis of randomized controlled trials. Menopause. 2021;28:1316‐1322. [DOI] [PubMed] [Google Scholar]
- 145. Li F, Picard‐Fortin V, Maheux‐Lacroix S, et al. The efficacy of vaginal laser and other energy‐based treatments on genital symptoms in postmenopausal women: a systematic review and meta‐analysis. J Minim Invasive Gynecol. 2021;28:668‐683. [DOI] [PubMed] [Google Scholar]
- 146. Wallace SL, St Martin B, Lee K, Sokol ER. A cost‐effectiveness analysis of vaginal carbon dioxide laser therapy compared with standard medical therapies for genitourinary syndrome of menopause‐associated dyspareunia. Am J Obstet Gynecol. 2020;223:890.e1‐890.e12. [DOI] [PubMed] [Google Scholar]


