Abstract
Introduction
Prevalence of endometriosis is commonly reported based on surgery findings and varies widely depending on study population and indication for surgery. Symptoms such as dysmenorrhea, pelvic pain, dyspareunia, dysuria, and dyschezia can be associated with endometriosis and adenomyosis. Transvaginal ultrasound examination is proposed to be the first‐line diagnostic method, nevertheless there are no published ultrasound‐based studies reporting prevalence of endometriosis and adenomyosis in symptomatic women other than those scheduled for surgery. The aim of this study was to determine the prevalence of endometriosis and adenomyosis as assessed by transvaginal ultrasound in women with symptoms suggestive of endometriosis and adenomyosis.
Material and methods
This is a retrospective cross‐sectional study performed at a tertiary‐care center including 373 symptomatic women who were systematically examined with transvaginal ultrasound by an experienced ultrasound examiner. Before ultrasound examination women filled in a questionnaire including self‐assessment of the severity of their symptoms (dysmenorrhea, chronic pelvic pain, dyspareunia, dysuria, dyschezia) using a visual analog scale. Abnormal findings in the uterus, ovaries, bowel, urinary bladder, uterosacral ligaments, and rectovaginal septum were noted, and their size and location were described. Prevalence of endometriosis, adenomyosis, endometrioma, and deep endometriosis in different anatomical locations was reported.
Results
Prevalence of ovarian endometrioma and/or deep endometriosis was 25% and of adenomyosis was 12%. Prevalence of endometrioma was 20% and of deep endometriosis was 9%, for each location being 8% in the bowel, 3% in the uterosacral ligaments, 3% in the rectovaginal septum and 0.5% in the urinary bladder.
Conclusions
In symptomatic women examined with transvaginal ultrasound by an experienced ultrasound examiner, ovarian endometrioma and/or deep endometriosis was found in one of four women and adenomyosis in one of nine women. Deep endometriosis was present in one of 11 women. Despite having symptoms, half of the women had no abnormal ultrasound findings.
Keywords: adenomyosis, deep endometriosis, dysmenorrhea, endometrioma, endometriosis, prevalence, ultrasonography
Abbreviation
- CI
confidence interval
Key message.
At transvaginal ultrasound examination performed by an experienced examiner in women referred because of symptoms suggestive of endometriosis the prevalence of ovarian endometrioma and/or deep endometriosis was 25%, adenomyosis 12%, and deep endometriosis 9%. Interestingly, 48% of women had no abnormal ultrasound findings.
1. INTRODUCTION
Women with symptoms suggestive of endometriosis are often referred to further investigation. Endometriosis is estimated to affect 5%–10% of women of reproductive age and early diagnosis is essential to initiate treatment and reduce risk for pain sensitization. 1 , 2 Association between different symptoms and endometriosis has been assessed, indicating high prevalence of endometriosis in women with dysmenorrhea. 3 , 4 Other symptoms associated with endometriosis are chronic abdominal or pelvic pain, dysuria, dyschezia, dyspareunia, and infertility. 4 , 5 However, the diagnostic delay of endometriosis is approximately 10 years despite clinical symptoms. 6 Women who present with these symptoms would benefit from early investigation, correct diagnosis, and treatment to improve quality of life. 7 Adenomyosis correlates with increasing parity, age, and severity of dysmenorrhea and can be found in women with deep endometriosis. 8
Until recently, the reference standard for the diagnosis of endometriosis has been visible endometriotic lesions during laparoscopy and histologically verified endometrial tissue outside the uterine cavity, which is no longer the case. 5 It is known that deep endometriosis, especially in the lower rectum, may be missed at laparoscopy. 9 , 10 Transvaginal ultrasound is considered an accurate method for the diagnosis of endometriosis in the pelvis and for evaluation of the severity of the disease. 10 , 11 , 12 , 13 , 14 Good intra‐observer and inter‐observer reproducibility in detection of endometriotic lesions has been reported. 15 Furthermore, transvaginal ultrasound is proposed as a first‐line diagnostic method and is cost‐effective. 4 , 16 , 17
Most studies report prevalence of endometriosis and adenomyosis in women scheduled for surgery. 8 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Other studies report prevalence of endometriosis based on questionnaires, self‐reported clinical symptoms, or registries. 4 During common gynecological practice the transvaginal ultrasound examination normally include the uterus and ovaries, and the presence of deep endometriosis is not investigated. To the best of our knowledge, there are no published studies aiming to report the prevalence of endometriosis, including deep endometriosis, at ultrasound examination in symptomatic non‐pregnant women other than those scheduled for surgery.
The aim of this study was to determine the prevalence of endometriosis and adenomyosis as assessed by transvaginal ultrasound in women with symptoms suggestive of endometriosis and adenomyosis.
2. MATERIAL AND METHODS
This is a retrospective cross‐sectional study performed at a tertiary care center with systematic documentation of patient symptoms and ultrasound findings during 2014–2017. Women with symptoms suggestive of endometriosis referred to advanced ultrasound examination were eligible for the study. Exclusion criteria were: previously known endometriosis, substantial clinical data missing, non‐experienced examiner, and if complete examination was not possible. Women were examined by an ultrasound examiner experienced with transvaginal ultrasound. Before ultrasound examination, demographic data including age, parity, history of infertility, duration of infertility, current and previous hormonal treatment, and duration of hormonal treatment, were collected from the patient and documented. Women were asked to fill in a questionnaire regarding their symptoms (dysmenorrhea, chronic pelvic pain, dyspareunia, dyschezia, and dysuria) and to assess the severity of symptoms by using a visual analog scale ranging from 0 to 100 arbitrary units according to clinical routine for women with suspected endometriosis. The symptom was considered not present at 0–5, mild at 6–30, moderate at 31–70, and severe at 71–100.
An experienced ultrasound examiner performed systematic transvaginal ultrasound examination in women in the lithotomy position using either GE E8 or E10 ultrasound equipment (GE, Milwaukee, WI, USA) with a vaginal transducer of 5–9 MHz. An experienced ultrasound examiner was defined as an examiner who had performed at least 10 000 gynecological ultrasound examinations, including women with deep endometriosis, and who was continuously examining at least 1000 women per year. Any abnormal findings in the uterus, ovaries, bowel, urinary bladder, uterosacral ligaments, and rectovaginal septum were noted, and their size and location were described. Size of the lesions was measured in three diameters perpendicular to each other. If endometriotic lesions were found at transvaginal ultrasound examination, transabdominal ultrasound examination was performed to assess kidneys regarding hydronephrosis. Prevalence of endometriosis, endometrioma, deep endometriosis in different anatomical locations, and adenomyosis were reported.
Adenomyosis was judged to be present if at least one of the following features described by the Morphological Uterus Sonographic Assessment group was present: enlargement of the uterus, asymmetry of the uterine walls, fan‐shaped shadowing, myometrial cysts, and heterogeneous myometrium. 25
Presence of ovarian cysts was noted and cyst size was measured in three diameters perpendicular to each other. The cysts were classified and described according to the terms and definitions proposed by the International Ovarian Tumor Analysis group. 26 The type of the cyst was described as unilocular, unilocular‐solid, multilocular, multilocular‐solid, and solid. The cyst content was classified as anechoic, low‐level echogenicity, ground glass, mixed, or hemorrhagic. Typical endometrioma (Figure 1A) was described as a unilocular cyst, with content of ground‐glass echogenicity, with no to moderate vascularization. 27 Presence of kissing ovaries, described as ovaries adherent to each other, was noted. 28
FIGURE 1.
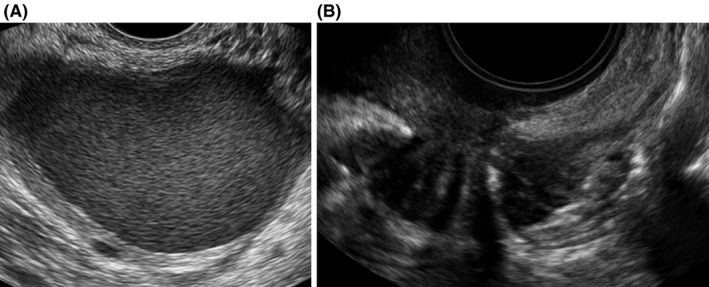
Transvaginal ultrasound images of (A) endometrioma, round unilocular cyst with ground glass echogenicity and (B) deep endometriosis in the bowel, hypoechoic solid mass distorting muscular layer in anterior wall
Presence of polycystic ovaries was noted; a definition of at least 25 antral follicles per ovary was used. 29 , 30
Bowel (rectum, rectosigmoid junction, sigmoid), urinary bladder, uterosacral ligaments, and rectovaginal septum were examined and assessed for deep endometriosis. Deep endometriosis in the bowel (Figure 1B) was described as a hypoechoic mass, which distorts and replaces the normal appearance of the muscularis propria layer in the bowel. 31 Deep endometriosis in the urinary bladder was described as a spherical or comma‐shaped solid hypoechoic lesion with regular borders and infiltration of at least the muscularis propria of the bladder. 32 , 33 Deep endometriosis in uterosacral ligaments was described as hypoechoic homogeneous or heterogeneous thickening with regular or irregular margins within the hyperechoic adipose tissue surrounding the uterosacral ligaments. 32 Deep endometriosis in the rectovaginal septum was described as a hypoechoic nodule between the rectum and the posterior vaginal wall from introitus to the lower border of the posterior cervix lip. 32 All lesions were measured in three diameters perpendicular to each other and for the uterosacral ligaments the side was noted.
Obliteration of the pouch of Douglas was determined using the sliding sign and classified as obliterated (negative sliding sign) or not (positive sliding sign). 34
2.1. Statistical analyses
Statistical calculations were performed using the Statistical Package for Social Sciences version 25 (IBM, Armonk, NY, USA). Continuous variables were described as median and range. Difference in continuous variables between three or more independent groups using Kruskal–Wallis test. Categorical variables were analyzed using chi‐squared test or Fisher's exact test. Statistical significance was considered at p values less than 0.05. Demographic data and severity of symptoms in women with endometriosis were compared with those of women without abnormal ultrasound findings. Prevalence was reported as percentage with 95% confidence interval (CI).
2.2. Ethical approval
This study was approved by the Regional Ethical Review Board of Lund University, Lund, Sweden on September 11, 2018 with a reference number 555/2018.
3. RESULTS
Out of 513 eligible women, 140 women were excluded: 105 because of previously known endometriosis, 15 because of missing substantial clinical data, 10 because the ultrasound examination was performed by a non‐experienced examiner, and 10 because it was not possible to perform a complete examination. Hence, 373 women were included in this study.
Demographic data of all the included women and separately for those with endometriosis, with no abnormal ultrasound findings and with other abnormal ultrasound findings are shown in Table 1. Median age of women with endometriosis at ultrasound was higher compared with women without abnormal ultrasound findings and with other abnormal findings (p < 0.0001) and history of infertility was more common in women with endometriosis at ultrasound than in women without abnormal ultrasound findings and with other abnormal findings (p < 0.0001). Women with no abnormal ultrasound findings were more likely to be on hormonal treatment at the time of ultrasound examination and median length of use of hormonal treatment was longer than in women with endometriosis at ultrasound and women with other abnormal ultrasound findings (p = 0.040 and p = 0.027, respectively).
TABLE 1.
Demographic data of study population
| Variable | All women (n = 373) | Endometriosis (n = 94) | No abnormal ultrasound findings (n = 178) | Other abnormal ultrasound findings (n = 81) | p value |
|---|---|---|---|---|---|
| Age (years) | 30 (16–51) | 33 (22–50) | 27 (16–49) | 30 (16–50) | <0.0001 |
| Parity | 0 (0–7) | 0 (0–4) | 0 (0–7) | 0 (0–3) | 0.202 |
| Number of deliveries | |||||
| Nullipara | 258 (69) | 60 (64) | 126 (71) | 63 (78) | |
| 1 | 42 (11) | 17 (18) | 14 (8) | 7 (9) | |
| 2 | 56 (15) | 14 (15) | 31 (17) | 8 (10) | |
| 3 | 11 (3) | 1 (1) | 4 (2) | 3 (4) | |
| ≥4 | 6 (2) | 2 (2) | 3 (2) | 0 (0) | |
| Infertility | 64 (17) | 31 (33) | 13 (7) | 16 (20) | <0.0001 |
| Current hormonal treatment | 183 (49) | 39 (42) | 100 (56) | 36 (44) | 0.040 |
| Systemic progestins | 48 (26) | 7 (18) | 34 (34) | 6 (17) | |
| COC or vaginal contraceptive ring | 83 (45) | 19 (49) | 46 (46) | 12 (33) | |
| Hormonal intrauterine device | 32 (18) | 6 (15) | 13 (13) | 12 (33) | |
| GnRH analogs | 4 (2) | 2 (5) | 0 (0) | 2 (6) | |
| Combination of treatments a | 13 (7) | 4 (10) | 7 (7) | 2 (6) | |
| Other b | 3 (2) | 1 (3) | 0 (0) | 2 (6) | |
| Median length of use of hormonal treatment (months) | 8 (1–240) | 5 (1–48) | 11 (1–240) | 9 (1–180) | 0.027 |
| Dysmenorrhea | 373 (100) | 94 (100) | 178 (100) | 81 (100) | 0.008 |
| Severity of dysmenorrhea c | n = 352 | n = 86 | n = 169 | n = 79 | |
| VAS | 76 (7–100) | 67 (8–100) | 76 (7–100) | 79 (14–100) | 0.030 |
| Severe (VAS 71–100) | 197 (56) | 38 (44) | 94 (56) | 51 (65) | |
| Chronic pelvic pain | 289 (78) | 68 (72) | 144 (81) | 61 (75) |
0.244 |
| Dyspareunia | 302 (81) | 69 (73) | 152 (85) | 66 (82) |
0.024 |
| Dysuria | 208 (56) | 42 (45) | 108 (61) | 47 (58) |
0.032 |
| Dyschezia | 286 (77) | 66 (70) | 141 (79) | 63 (78) | 0.194 |
Data are given as n (%) or as median (range). p values are given from comparison between women with endometriosis, no abnormal ultrasound findings and other abnormal ultrasound findings. Women with isolated adenomyosis at ultrasound (n = 20) are not shown in this table.
Abbreviations: COC, combined oral contraceptives; GnRH, gonadotropin‐releasing hormone; VAS, visual analog scale.
Combination of hormonal intrauterine device with systemic hormonal treatment or combination of two systemic treatments.
Esmya or progestin cream.
Given as number of women who reported severity of dysmenorrhea according to visual analog scale.
Severe dysmenorrhea was more common in women with other abnormal ultrasound findings and with no abnormal ultrasound findings than in women with endometriosis at ultrasound (p = 0.030). In women with endometriosis at ultrasound, dyspareunia and dysuria were less common than in women without abnormal ultrasound findings and other abnormal ultrasound findings (p = 0.024 and p = 0.032, respectively), while no difference was observed in chronic pelvic pain and dyschezia (p = 0.244 and p = 0.194, respectively).
Prevalence and description of ultrasound findings are shown in Tables 2 and 3. Out of 373 women included, 94 women (25%, 95% CI 21%–30%) had ovarian endometrioma and/or deep endometriosis, 43 women (12%, 95% CI 9%–15%) had adenomyosis, 81 women (22%, 95% CI 18%–26%) had other abnormal findings and 178 women (48%, 95% CI 43%–53%) had no abnormal ultrasound findings. Prevalence of endometrioma was 20% (95% CI 16%–24%), detected in 73 women. Endometrioma was the most common ultrasound finding, present as the only finding in 39 women (10%, 95% CI 8%–14%). Deep endometriosis was found in 35 women (9%, 95% CI 7%–13%). Bowel was the most common location of deep endometriosis with a prevalence of 8% (95% CI 6%–11%). Prevalence of deep endometriosis in the uterosacral ligaments was 3% (95% CI 2%–5%) and in the rectovaginal septum was 3% (95% CI 1%–5%). Deep endometriosis in the bladder was present in two women (0.5%, 95% CI 0%–2%) and both lesions were located in the bladder base.
TABLE 2.
Prevalence of ultrasound findings in study population
| Variable | N (%) (n = 373) | 95% CI |
|---|---|---|
| No abnormal ultrasound findings | 178 (48) | 43–53 |
| Endometriosis | 94 (25) | 21–30 |
| Endometrioma | 73 (20) | 16–24 |
| Deep endometriosis | 35 (9) | 7–13 |
| Adenomyosis | 43 (12) | 9–15 |
| Only endometrioma | 39 (10) | 8–14 |
| Only deep endometriosis | 11 (3) | 2–5 |
| Only adenomyosis | 20 (5) | 3–8 |
| Endometrioma and deep endometriosis | 15 (4) | 2–7 |
| Endometrioma and adenomyosis | 12 (3) | 2–6 |
| Adenomyosis and deep endometriosis | 2 (1) | 0–2 |
| Endometrioma, deep endometriosis, and adenomyosis | 7 (2) | 1–4 |
| Other abnormal findings | 81 (22) | 18–26 |
| Polycystic ovaries | 34 (9) | 6–13 |
| Uterine fibroid | 16 (4) | 3–7 |
| Adhesions only | 9 (2) | 1–5 |
| Ovarian cyst a | 6 (2) | 1–4 |
| Paraovarian/peritoneal cyst | 7 (2) | 1–4 |
| Uterine malformation | 6 (2) | 1–4 |
| Hemato/pyometra | 2 (0.5) | 0–2 |
| Uterine polyp | 1 (0.2) | 0–2 |
| Obliteration of pouch of Douglas | 46 (12) | 9–16 |
| Hydronephrosis in women with ultrasound findings of endometriosis | n = 94 | |
| Right | 3 (3) | 1–9 |
| Left | 1 (1) | 0–6 |
| Bilateral | 0 (0) | 0–4 |
Abbreviation: CI, confidence interval.
Three dermoid, one mucinous, one serous, and one other ovarian cyst.
TABLE 3.
Description of endometriosis lesions at transvaginal ultrasound examination
| Location | N (%) (n = 373) | 95% CI |
|---|---|---|
| Endometrioma | 73 (20) | 16–24 |
| Right only | 18 (5) | 3–8 |
| Left only | 31 (8) | 6–12 |
| Bilateral | 24 (6) | 4–9 |
| Kissing ovaries | 7 (2) | 1–4 |
| Maximal diameter of endometrioma (mm), (n = 73) | 33 (10–100) | |
| Right (n = 42) | 33 (12–95) | |
| Left (n = 55) | 30 (10–100) | |
| Deep endometriosis | 35 (9) | 7–13 |
| Bowel | 30 (8) | 6–11 |
| Rectum | 16 (4) | 3–7 |
| Maximal diameter (mm) | 24 (10–36) | |
| Recto‐sigmoid | 16 (4) | 3–7 |
| Maximal diameter (mm) | 36 (10–67) | |
| Sigmoid | 3 (1) | 0–3 |
| Maximal diameter (mm) | 18 (14–21) | |
| Number of bowel lesions per woman with deep endometriosis | n = 35 | |
| One | 25 (83) | |
| Two | 4 (13) | |
| Three | 1 (3) | |
| Uterosacral ligaments | 11 (3) | 2–5 |
| Right only | 1 (0.3) | |
| Left only | 6 (2) | |
| Bilateral | 3 (0.8) | |
| Side not specified | 1 (0.3) | |
| Rectovaginal septum | 10 (3) | 1–5 |
| Urinary bladder (bladder base) | 2 (0.5) | 0–2 |
| Other a | 4 (1) |
Data are given as n (%) or as median (range).
Abbreviation: CI, confidence interval.
Vaginal lesion or lesion in pouch of Douglas.
In women with adenomyosis, concomitant ovarian endometrioma and/or deep endometriosis were found in 21 women (49%, 95% CI 33%–65%), distributed as endometrioma in 12 women (28%), deep endometriosis in 2 women (5%), and with endometrioma and deep endometriosis in 7 women (16%). Isolated adenomyosis was found in 20 women (5%, 95% CI 3%–8%).
In women with ovarian endometrioma and/or deep endometriosis, concomitant adenomyosis was found in 21 women (22%, 95% CI 14%–32%). In women with deep endometriosis, concomitant adenomyosis was found in nine women (26%, 95% CI 12%–43%). The pouch of Douglas was obliterated in 46 women (12%, 95% CI 9%–16%).
4. DISCUSSION
We have found that in women undergoing transvaginal ultrasound examination because of symptoms suggestive of endometriosis the prevalence of ovarian endometrioma and/or deep endometriosis and adenomyosis was 25% and 12%, respectively. In 48% of women, the ultrasound examination was without abnormal findings and 22% had abnormal findings other than endometriosis. Most common location of endometriosis was ovaries, with a prevalence of 20%. The prevalence of deep endometriosis was 9%.
To the best of our knowledge there are no published studies aiming to report prevalence of endometriosis and adenomyosis at ultrasound examination in non‐pregnant women with symptoms suggestive of endometriosis. Most studies report the prevalence of endometriosis and adenomyosis based on findings at surgery with or without histological specimens, surgery being performed for different indications and in different populations. 8 , 18 , 19 , 20 , 21 , 22 , 23 , 24 A recent review showed that the reported prevalence of endometriosis in 69 studies was exceedingly variable, ranging from 0.2% to 71% depending on study setting, diagnostic method, and population. 35 Prevalence of endometriosis is expected to be higher in women undergoing surgery, because of suspected endometriosis or purpose of surgical treatment, than in those undergoing ultrasound examination as part of the investigation. Patients with advanced disease or more severe symptoms might be more likely to be selected for surgery. Moreover, surgery makes the diagnosis of superficial peritoneal endometriosis possible where ultrasound still has a limited value.
In a study with the primary aim to estimate the prevalence of adenomyosis at ultrasound in women attending a general gynecological clinic, the reported prevalence of endometriosis was 6.4%. 36 However, no detailed information on locations of the lesions is available. A publication on 1341 pregnant women reported ultrasound‐based prevalence of endometriosis during early pregnancy being 4.9%, endometrioma being found in 2.5% and deep endometriosis in 4.3% of women. 37 Prevalence of endometriosis is much lower in this study than in ours and population selection and indication for ultrasound might explain the discrepancy. Moreover, women with endometriosis might have lower pregnancy rates, therefore women who conceive would have lower prevalence of endometriosis. Prevalence of endometrioma in our study was in agreement with other studies reporting findings at transvaginal ultrasound before scheduled surgery 31 and surgery‐based findings. 8 , 20 , 24 Other studies reported much higher prevalence of deep endometriosis compared with our study. 20 , 21 , 24 , 31 Women selected for surgery are more likely to have more advanced disease with higher prevalence of deep endometriotic lesions than those undergoing ultrasound in diagnostic purpose, as the majority of women are treated conservatively.
Prevalence of adenomyosis at ultrasound was reported to be 21%–22% in non‐pregnant women attending a general gynecological clinic for any reason 36 or scheduled for surgery because of endometriosis, 8 which is higher than our value of 12%. The younger population in our study compared with theirs might explain the difference, as adenomyosis has been shown to correlate with increasing age. 8 Lower prevalence of adenomyosis than in our study was reported in pregnant women during early pregnancy at ultrasound examination, 37 which could be explained by a possible adenomyosis effect on fertility. Adenomyosis has been shown to be associated with endometriosis 36 and deep endometriosis. 8 , 38 We have found that 22% of women with ovarian endometrioma and/or deep endometriosis had concomitant adenomyosis, which is in line with a study including women scheduled for surgery because of endometriosis. 8 In women with deep endometriosis scheduled for surgery, the reported prevalence of adenomyosis was 49% 39 , which is much higher than in our study. However, the included women had more advanced disease.
Of 43 women with adenomyosis in our study, concomitant ovarian endometrioma and/or deep endometriosis were observed in half of them. This was much higher than the 4.9% observed in a general gynecological population. 36 All women in our study were symptomatic and all had dysmenorrhea, which might be the main reason for this difference.
No abnormal ultrasound findings were observed in 48% women in our study. Peritoneal endometriosis might be missed at transvaginal ultrasound examination because the value of unenhanced transvaginal ultrasound in diagnosing peritoneal endometriosis is limited, leading to underestimation of the prevalence of endometriosis in women with only peritoneal disease. 14 , 40 In our study, median age of women with ovarian endometrioma and/or deep endometriosis was higher compared with women without abnormal ultrasound findings, this may make us speculate that it takes time to develop endometriotic lesions detectable at transvaginal ultrasound.
An important strength of our study is study design, because all women were systematically examined by an experienced ultrasound examiner and ultrasound findings, demographic data and symptoms were systematically documented in line with clinical routine. Another strength is that this study includes women referred for ultrasound examination because of suspected endometriosis, which corresponds to a patient population being investigated because of symptoms suggestive of endometriosis and not to women selected for surgery for diagnostic or treatment purposes.
Limitations of unenhanced transvaginal ultrasound in detecting peritoneal endometriosis might be considered a weakness of this study. Assessment of the posterior compartment without intravaginal gel might also be considered a weakness, as it has been shown to have higher accuracy in evaluating posterior compartment of the pelvis when assessing endometriosis in the vagina and rectovaginal septum than unenhanced transvaginal ultrasound. 41 Use of intravaginal gel, however, was not part of our clinical routine during the study. It is also possible that women referred for ultrasound examination were those that had more severe symptoms compared with the general gynecological population.
Further investigation and follow up of women with no abnormal ultrasound findings are of great interest and may help us to understand the development of endometriosis disease. Despite symptoms, ultrasound findings of endometriosis might not be visible at an early stage of the disease or there might be other reasons for symptoms requiring further investigation in case of failed conservative treatment. Further studies are needed to elucidate the development of endometriosis.
5. CONCLUSION
In symptomatic women examined with transvaginal ultrasound by an experienced ultrasound examiner, ovarian endometrioma and/or deep endometriosis were found in one of four women and adenomyosis in one of nine women. Deep endometriosis was present in one of 11 women. As other studies report endometriosis in patients already scheduled for surgery with much higher prevalence of deep endometriosis, our study shows that most women will have no abnormal ultrasound findings despite symptoms suggestive of endometriosis and adenomyosis when ultrasound examination is performed by an experienced ultrasound examiner. The results of this study are important because transvaginal ultrasound is proposed as a first‐line diagnostic method.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
AUTHOR CONTRIBUTIONS
Both authors participated in the study design. LJ performed the ultrasound examinations. SO collected data and prepared it for statistical analysis. Both authors interpreted and performed the data analysis. SO drafted the manuscript under supervision from LJ. Both authors approved the final submitted version of the manuscript.
Orlov S, Jokubkiene L. Prevalence of endometriosis and adenomyosis at transvaginal ultrasound examination in symptomatic women. Acta Obstet Gynecol Scand. 2022;101:524–531. doi: 10.1111/aogs.14337
REFERENCES
- 1. Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018;4:9. [DOI] [PubMed] [Google Scholar]
- 2. Li T, Mamillapalli R, Ding S, et al. Endometriosis alters brain electrophysiology, gene expression and increases pain sensitization, anxiety, and depression in female mice. Biol Reprod. 2018;99:349‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vigano P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 2004;18:177‐200. [DOI] [PubMed] [Google Scholar]
- 4. Agarwal SK, Chapron C, Giudice LC, et al. Clinical diagnosis of endometriosis: a call to action. Am J Obstet Gynecol. 2019;220:354.e1‐e12. [DOI] [PubMed] [Google Scholar]
- 5. ESHRE . Guideline Endometriosis 2022. Available at: https://www.eshre.eu/guidelines‐and‐legal/guidelines/endometriosis‐guideline.aspx (Accessed February 2, 2022).
- 6. Arruda MS, Petta CA, Abrão MS, Benetti‐Pinto CL. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003;18:756‐759. [DOI] [PubMed] [Google Scholar]
- 7. Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96:366‐73.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Donato N, Montanari G, Benfenati A, et al. Prevalence of adenomyosis in women undergoing surgery for endometriosis. Eur J Obstet Gynecol Reprod Biol. 2014;181:289‐293. [DOI] [PubMed] [Google Scholar]
- 9. Griffiths AN, Koutsouridou RN, Penketh RJ. Rectovaginal endometriosis ‐‐ a frequently missed diagnosis. J Obstet Gynaecol. 2007;27:605‐607. [DOI] [PubMed] [Google Scholar]
- 10. Hudelist G, English J, Thomas AE, Tinelli A, Singer CF, Keckstein J. Diagnostic accuracy of transvaginal ultrasound for non‐invasive diagnosis of bowel endometriosis: systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2011;37:257‐263. [DOI] [PubMed] [Google Scholar]
- 11. Holland TK, Yazbek J, Cutner A, Saridogan E, Hoo WL, Jurkovic D. Value of transvaginal ultrasound in assessing severity of pelvic endometriosis. Ultrasound Obstet Gynecol. 2010;36:241‐248. [DOI] [PubMed] [Google Scholar]
- 12. Reid S, Espada M, Lu C, Condous G. To determine the optimal ultrasonographic screening method for rectal/rectosigmoid deep endometriosis: ultrasound "sliding sign," transvaginal ultrasound direct visualization or both? Acta Obstet Gynecol Scand. 2018;97:1287‐1292. [DOI] [PubMed] [Google Scholar]
- 13. Mais V, Guerriero S, Ajossa S, Angiolucci M, Paoletti AM, Melis GB. The efficiency of transvaginal ultrasonography in the diagnosis of endometrioma. Fertil Steril. 1993;60:776‐780. [DOI] [PubMed] [Google Scholar]
- 14. Nisenblat V, Bossuyt P, Farquhar C, Johnson N, Hull M. Imaging modalities for the non‐invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bean E, Chaggar P, Thanatsis N, Dooley W, Bottomley C, Jurkovic D. Intra‐ and interobserver reproducibility of pelvic ultrasound for the detection and measurement of endometriotic lesions. Hum Reprod Open. 2020;2020:hoaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Piketty M, Chopin N, Dousset B, et al. Preoperative work‐up for patients with deeply infiltrating endometriosis: transvaginal ultrasonography must definitely be the first‐line imaging examination. Hum Reprod. 2009;24:602‐607. [DOI] [PubMed] [Google Scholar]
- 17. Leonardi M, Martin E, Reid S, Blanchette G, Condous G. Deep endometriosis transvaginal ultrasound in the workup of patients with signs and symptoms of endometriosis: a cost analysis. BJOG. 2019;126:1499‐1506. [DOI] [PubMed] [Google Scholar]
- 18. Reese K, Reddy S, Rock J. Endometriosis in an adolescent population: the Emory experience. J Pediatr Adolesc Gynecol. 1996;9:125‐128. [DOI] [PubMed] [Google Scholar]
- 19. Laufer MR, Goitein L, Bush M, Cramer DW, Emans SJ. Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding to conventional therapy. J Pediatr Adolesc Gynecol. 1997;10:199‐202. [DOI] [PubMed] [Google Scholar]
- 20. Leonardi M, Reid S, Lu C, Condous G. Prevalence of deep endometriosis and rectouterine pouch obliteration in the presence of normal ovaries. J Obstet Gynaecol Can. 2020;42:1211‐1216. [DOI] [PubMed] [Google Scholar]
- 21. Gabriel B, Nassif J, Trompoukis P, Barata S, Wattiez A. Prevalence and management of urinary tract endometriosis: a clinical case series. Urology. 2011;78:1269‐1274. [DOI] [PubMed] [Google Scholar]
- 22. Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, D'Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril. 2009;92:68‐74. [DOI] [PubMed] [Google Scholar]
- 23. Ferrero S, Arena E, Morando A, Remorgida V. Prevalence of newly diagnosed endometriosis in women attending the general practitioner. Int J Gynaecol Obstet. 2010;110:203‐207. [DOI] [PubMed] [Google Scholar]
- 24. Hudelist G, Oberwinkler KH, Singer CF, et al. Combination of transvaginal sonography and clinical examination for preoperative diagnosis of pelvic endometriosis. Hum Reprod. 2009;24:1018‐1024. [DOI] [PubMed] [Google Scholar]
- 25. Van den Bosch T, Dueholm M, Leone FP, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the morphological uterus sonographic assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015;46:284‐298. [DOI] [PubMed] [Google Scholar]
- 26. Timmerman D, Valentin L, Bourne TH, Collins WP, Verrelst H, Vergote I. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the international ovarian tumor analysis (IOTA) group. Ultrasound Obstet Gynecol. 2000;16:500‐505. [DOI] [PubMed] [Google Scholar]
- 27. Van Holsbeke C, Van Calster B, Guerriero S, et al. Endometriomas: their ultrasound characteristics. Ultrasound Obstet Gynecol. 2010;35:730‐740. [DOI] [PubMed] [Google Scholar]
- 28. Ghezzi F, Raio L, Cromi A, et al. "Kissing ovaries": a sonographic sign of moderate to severe endometriosis. Fertil Steril. 2005;83:143‐147. [DOI] [PubMed] [Google Scholar]
- 29. Lujan ME, Jarrett BY, Brooks ED, et al. Updated ultrasound criteria for polycystic ovary syndrome: reliable thresholds for elevated follicle population and ovarian volume. Hum Reprod. 2013;28:1361‐1368. [DOI] [PubMed] [Google Scholar]
- 30. Dewailly D, Lujan ME, Carmina E, et al. Definition and significance of polycystic ovarian morphology: a task force report from the androgen excess and polycystic ovary syndrome society. Hum Reprod Update. 2014;20:334‐352. [DOI] [PubMed] [Google Scholar]
- 31. Hudelist G, Ballard K, English J, et al. Transvaginal sonography vs. clinical examination in the preoperative diagnosis of deep infiltrating endometriosis. Ultrasound Obstet Gynecol. 2011;37:480‐487. [DOI] [PubMed] [Google Scholar]
- 32. Bazot M, Malzy P, Cortez A, Roseau G, Amouyal P, Darai E. Accuracy of transvaginal sonography and rectal endoscopic sonography in the diagnosis of deep infiltrating endometriosis. Ultrasound Obstet Gynecol. 2007;30:994‐1001. [DOI] [PubMed] [Google Scholar]
- 33. Savelli L, Manuzzi L, Pollastri P, Mabrouk M, Seracchioli R, Venturoli S. Diagnostic accuracy and potential limitations of transvaginal sonography for bladder endometriosis. Ultrasound Obstet Gynecol. 2009;34:595‐600. [DOI] [PubMed] [Google Scholar]
- 34. Reid S, Lu C, Casikar I, et al. Prediction of pouch of Douglas obliteration in women with suspected endometriosis using a new real‐time dynamic transvaginal ultrasound technique: the sliding sign. Ultrasound Obstet Gynecol. 2013;41:685‐691. [DOI] [PubMed] [Google Scholar]
- 35. Ghiasi M, Kulkarni MT, Missmer SA. Is endometriosis more common and more severe than it was 30 years ago? J Minim Invasive Gynecol. 2020;27:452‐461. [DOI] [PubMed] [Google Scholar]
- 36. Naftalin J, Hoo W, Pateman K, Mavrelos D, Holland T, Jurkovic D. How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum Reprod. 2012;27:3432‐3439. [DOI] [PubMed] [Google Scholar]
- 37. Bean E, Naftalin J, Horne A, Saridogan E, Cutner A, Jurkovic D. Prevalence of deep and ovarian endometriosis in early pregnancy: an ultrasound diagnostic study. Ultrasound Obstet Gynecol. 2022;59:107‐113. [DOI] [PubMed] [Google Scholar]
- 38. Marcellin L, Santulli P, Bourdon M, et al. Focal adenomyosis of the outer myometrium and deep infiltrating endometriosis severity. Fertil Steril. 2020;114:818‐827. [DOI] [PubMed] [Google Scholar]
- 39. Lazzeri L, Di Giovanni A, Exacoustos C, et al. Preoperative and postoperative clinical and transvaginal ultrasound findings of adenomyosis in patients with deep infiltrating endometriosis. Reprod Sci. 2014;21:1027‐1033. [DOI] [PubMed] [Google Scholar]
- 40. Brosens I, Puttemans P, Campo R, Gordts S, Kinkel K. Diagnosis of endometriosis: pelvic endoscopy and imaging techniques. Best Pract Res Clin Obstet Gynaecol. 2004;18:285‐303. [DOI] [PubMed] [Google Scholar]
- 41. Reid S, Lu C, Hardy N, et al. Office gel sonovaginography for the prediction of posterior deep infiltrating endometriosis: a multicenter prospective observational study. Ultrasound Obstet Gynecol. 2014;44:710‐718. [DOI] [PubMed] [Google Scholar]


