Abstract
Introduction
Little is known about the optimal simulation‐based team training in obstetric emergencies. We aimed to review how simulation‐based team training affects patient outcomes in obstetric emergencies.
Material and methods
Search Strategy: MEDLINE, Embase, Cochrane Library, and Cochrane Central Register of Controlled Trials were searched up to and including May 15, 2021. Selection criteria: randomized controlled trials (RCTs) and cohort studies on obstetric teams in high‐resource settings comparing the effect of simulation‐based obstetric emergency team training with no training on the risk of Apgar scores less than 7 at 5 min, neonatal hypoxic ischemic encephalopathy, severe postpartum hemorrhage, blood transfusion of four or more units, and delay of emergency cesarean section by more than 30 min. Data collection and analysis: The included studies were assessed using PRISMA, EPCO, and GRADE.
Results
We found 21 studies, four RCTs and 17 cohort studies, evaluating patient outcomes after obstetric team training compared with no training. Annual obstetric emergency team training may reduce brachial plexus injury (six cohort studies: odds ratio [OR] 0.47, 95% CI 0.33–0.68; one RCT: OR 1.30, 95 CI% 0.39–4.33, low certainty evidence) and suggest a positive effect; but it was not significant on Apgar score below 7 at 5 min (three cohort studies: OR 0.77, 95% CI 0.51–1.19; two RCT: OR 0.87, 95% CI 0.72–1.05, moderate certainty evidence). The effect was unclear for hypoxic ischemic encephalopathy, umbilical prolapse, decision to birth interval in emergency cesarean section, and for severe postpartum hemorrhage. Studies with in situ multi‐professional simulation‐based training demonstrated the best effect.
Conclusions
Emerging evidence suggests an effect of obstetric team training on obstetric outcomes, but conflicting results call for controlled trials targeted to identify the optimal methodology for effective team training.
Keywords: birth, brachial plexus, delivery, emergency teams, obstetric, postpartum hemorrhage, shoulder dystocia, simulation training, systematic review
Abbreviations
- OR
odds ratio
- RCT
randomized controlled trial
Key message.
Obstetric emergency simulation‐based team training may reduce brachial plexus injury with a low certainty level of evidence. Furthermore, our analysis suggests a positive effect on Apgar score less than 7 at 5 min, although not statistically significant.
1. INTRODUCTION
Every day around the world about 800 women die from preventable causes related to pregnancy and childbirth. 1 It is therefore an important development goal for the World Health Organization to improve maternal, fetal, and neonatal care in childbirth. 2 , 3 Obstetric emergencies can often be resolved by timely, competent multidisciplinary teamwork. 4 , 6 Obstetric emergencies do, however, occur infrequently for the individual healthcare provider and consequently it is a challenge for obstetric staff to become experienced in handling these situations on the Labor and Delivery Unit.
Reducing preventable harm to mothers and neonates is a universal goal. 6 Though only 10% of preventable maternal deaths occur in high‐resource settings, audits into perinatal and maternal care in high‐resource settings have shown that adverse outcomes in emergency obstetrics are frequent and often preventable. 2 , 5 , 7 It seems obvious that training maternity care staff in simulated obstetric scenarios in order to establish practiced routines in these clinical challenges would be beneficial and could improve maternal and neonatal outcomes. 8 In many settings, such intrapartum training is recommended or even mandatory. 5 Whereas most staff members appreciate participation in obstetric team training and state after the training that they feel more confident in managing such emergencies in real life, data regarding the actual effects on clinical outcomes are sparse and conflicting. 9
The objective of this review was to assess the effect of simulation‐based team training of healthcare providers in the Labor and Delivery Unit on the outcome of obstetric emergencies.
2. MATERIAL AND METHODS
2.1. Protocol and registration
The review was conducted following the protocol for systematic reviews by using the assessment tools PRISMA, EPCO, and GRADE (www.equator‐network.com). 10 The full study protocol was designed a priori and published on July 23, 2019 in PROSPERO (CRD42019136775).
2.2. Identification of studies
The eligibility criteria for included studies were as per protocol. 11 The Population was obstetric emergency teams in hospitals. We considered a team to be at least two healthcare providers working within a team. Teams of either a single professional group or a multi‐professional team were accepted. We included studies conducted in high‐income countries, defined by the World Bank classification system of 2019. 12 The healthcare providers could be at any stage of clinical experience. We excluded studies investigating students or non‐healthcare professionals. For the Intervention, we considered all types of simulation‐based obstetric team training and all types of educational intervention where simulation was used with the aim of improving care of patients in labor. The intervention could be delivered as simulation training alone or in combination with lectures, tutorials, online tests, or workshops. Comparators were teams not exposed to simulation training. All studies with an Outcome of any of the levels of Kirkpatrick 13 were selected for full‐text analysis, and studies where patient outcomes related to an obstetrical emergency were reported were selected for further core outcome analysis (see core outcome set below). Eligible study designs were randomized controlled trials (RCT), cluster‐randomized trials and cohort studies.
2.3. Core outcome set
All studies with an evaluation of a patient outcome were included. All predefined core outcomes were selected for the meta‐analysis, ie, neonatal asphyxia (defined as Apgar score <7 at 5 min and neonatal hypoxic ischemic encephalopathy), shoulder dystocia (brachial plexus injury at birth), umbilical cord prolapse (with an Apgar score <7 at 5 min), postpartum hemorrhage (blood loss >1500 ml, transfusion of four or more units of red blood cells), delay of birth at an emergency cesarean section (decision‐to‐delivery time excess of 30 min).
2.4. Study selection and data extraction
A first literature search was conducted May 23, 2020 and updated on May 15, 2021 (Appendix S1). The databases used were: (a) Ovid MEDLINE (year 1946 to present), (b) Embase (year 1947 to present), and (c) Cochrane Library, including the Cochrane Central Register of Controlled Trials. The literature review was supplemented with studies found by reviewing the reference list of the retrieved studies. We applied language restrictions to an abstract either in Danish, Swedish, Norwegian, or English. Two authors (LB and LH) independently reviewed all references, read all abstracts and reviewed all full‐text studies. Any disagreements between the two reviewers during screening or assessment were resolved in a discussion between the authors. We documented the process using a PRISMA flow chart and kept a record of each full‐text study and the reasons for exclusion of studies (Figure 1, Appendix S2).
FIGURE 1.
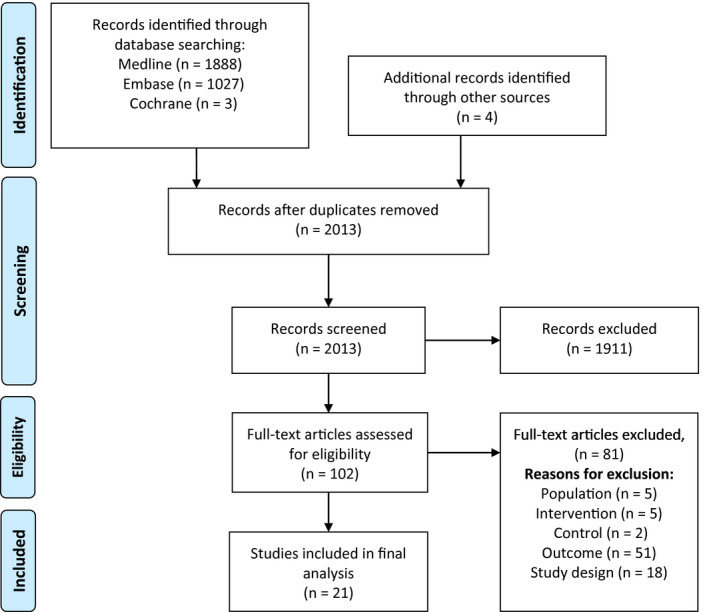
Flow diagram of literature selection
Three authors (LB, LH, and SB) independently extracted data from each trial included in the final analysis. Any disagreements between the reviewers were resolved in a discussion between the authors. Where multiple publications were identified from the same trial, presenting both the primary analysis and a secondary analysis of the same outcome, only the primary analysis was included in the meta‐analysis.
2.5. Assessment of study quality and bias
Risk of bias assessment was conducted by two authors (LB and LH) who independently assessed all the included studies using the Cochrane Collaboration's tool for assessing risk of bias. 10 As recommended in the Cochrane handbook, other bias tools can be included according to the study design. Therefore, we made a supplementary assessment with the tool MERSQI 14 designed to assess medical educational studies.
2.6. Statistical analyses
Statistical analysis was conducted using reviewmanager ® software 5.3. As all our outcomes were dichotomous data, we presented results as odds ratio (OR) with 95% CI. In studies where an adjusted analysis was presented, the adjusted result was included. In the meta‐analysis, two confidence intervals slightly differ from the authors’ reported values as reviewmanager automatically rounds off to two digits. Therefore, Lenguerrand et al 15 report an effect of OR 0.79 (95% CI 0.63–1.01) where we report the effect as OR 0.79 (95% CI 0.62–1.01) and Fransen et al 16 report an effect of OR 0.96 (95% CI 0.74–1.2) where we report the effect as OR 0.96 (95% CI 0.74–1.25). As a result of the nature of the intervention, there was a significant risk of heterogeneity in the intervention and the timeline. We assessed statistical heterogeneity using the chi‐squared test for heterogeneity and defined considerable heterogeneity if I 2 was more than 75%. We addressed heterogeneity in our analysis by using random‐effects assessment in our meta‐analysis and by downgrading the evidence. 10 , 17
2.7. Quality of evidence
Rating of evidence was done with the GRADE approach, where the initial level of quality was defined by the study design, and then reasons for downgrading or upgrading were assessed. Five factors for downgrading the evidence were assessed: (a) risk of bias by the study design and tools for bias evaluation, (b) inconsistency of results if there was unexplained heterogeneity in the results, (c) indirectness of evidence by whether the correct intervention, population, and outcomes were directly or indirectly compared, (d) imprecision by the width of the confidence intervals, and (e) publication bias evaluated by funnel plots. Three factors could increase the quality of evidence: (a) a large magnitude of effect, (b) plausible confounding that would reduce the demonstrated effect, or (c) a dose‐response gradient. 8 Two authors (LB and LH) assessed these factors independently and listed arguments for downgrading or upgrading the evidence.
2.8. Patient involvement
This systematic review was conducted without patient or public involvement.
3. RESULTS
3.1. Description of the studies
The literature search identified 2013 references, and after eligibility assessment 102 articles were analyzed in full‐text analysis. A total of 21 studies were included 15 , 16 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 and 81 studies were excluded. Study characteristics of excluded and included studies are available in Appendices S2, S3. The selection process is shown in a PRISMA flow‐diagram (Figure 1). The included studies consisted of four RCTs 15 , 16 , 35 , 36 and 17 observational cohort studies. 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 The settings were Labor and Delivery Units in the USA, 20 , 23 , 29 , 34 , 35 , 36 Australia, 21 , 25 , 33 and Europe. 15 , 16 , 18 , 19 , 22 , 24 , 26 , 27 , 28 , 30 , 31 , 32 The studies were published in 2006–2020. Details of the interventions are listed and compared in Table 1.
TABLE 1.
Comparison of studies in the meta‐analysis.
| Study | Setting | Design | Time | Intervention | Subgroups by outcome | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Who? | Where? | How? | Duration | When? | Apgar | HIE | BPI | PPH | DD | CP | AOI | ||||
| Fransen 2017 | 24 units, Netherlands 27 509 births/year | Open cluster RCT |
2 years: 1 year = pre 1 year = post |
Delivery ward all staff | Sim center | CRM training, MOET program | 1 day | Only once | x | x | x | x | |||
| Lenguerrand 2019 | 12 units, Scotland 34 881 births/year | Stepped‐wedge cluster RCT | 2.5 years | Delivery ward all staff | In situ | PROMPT training | 1 day | Only once | x | ||||||
| Draycott 2006 | 1 unit, England 6000 births/year | Retrospective cohort |
6 years: 2 years = pre 1 years = train 3 years = post |
Delivery ward all staff | In situ | NA | 1 day | Annually | x | x | |||||
| Weiner 2016 | 1 unit, USA 6000 births/year | Retrospective cohort |
9 years: 2 year = pre 7 year = post |
Delivery ward all staff | In situ | PROMPT training | 1 day | Annually | x | x | x | ||||
| Shoushtharian 2014 | 8 units, Australia 12 402 births/year | Retrospective cohort |
3.5 years: 18 months = pre 12 months = train 12 months = pos |
Delivery ward 50% off staff | NA | PROMPT training | 1 day | NA | x | x | |||||
| Van de ven 2016 | 1 unit, Netherlands 1800 births/year | Retrospective cohort |
100 months: 38 months pre 24 months train 38 months post |
Delivery ward all staff | In situ | 2 scenarios | NA | NA | x | ||||||
| Inglis 2011 | 1 unit, USA, 3800 births/year | Retrospective cohort |
5 years: 2.5 years = pre 2 months = train 2.5 years = post |
Delivery ward all staff | NA | NA | NA | NA | x | ||||||
| Dahlberg 2018 | 1 unit, Sweden, 3000 births/year | Retrospective cohort |
12 years: 4 years = pre, 4 years = train 4 years= post |
Delivery ward all staff | Sim center | PROBE training | 3 h | Every 1.5 year | x | ||||||
| Kumar 2018 | 3 units, Australia 9000 birth/year | Retrospective cohort |
5 years: 2 years = pre 1 year = train 2 years = post |
Delivery ward all staff | In situ | NA | 0.5 day | Every 2. Year | x | x | |||||
| Croft 2016 | 1 unit, England 6000 births/year | Retrospective cohort |
12 years: 4 years = pre 4 years = train 4 years = post |
Delivery ward all staff | In situ | PROMPT training | 1 day | Annually | x | ||||||
| Baldvinsdottir 2018 | 1unit, Sweden 3000 births/year | Retrospective cohort |
8 years: 4 years = pre 4 years = post |
Delivery ward all staff | In situ | NA | 3 h | Every 1.5 year | x | ||||||
| Egenberg 2015 | 1 unit, Norway 4800 births/year | Retrospective cohort |
3 years: 1 year = pre 1 year = train 1 year = post |
Delivery ward all staff | Sim center | NA | 6 h | Annually | x | ||||||
| Egenberg 2017 | 1 unit, Norway 4800 births/year | Retrospective cohort |
4.5 years: 2 years = pre 0.5 year = train 2 years = post |
Delivery ward 80% of staff | Off‐site | NA | NA | Annually | x | ||||||
| Lutgendorf 2017 | 1 unit, California NA births/year | Retrospective cohort |
10 months 6 months = pre 4 months = post |
NA | In situ | NA | 2 day | Only once | x | ||||||
| Markova 2012 | 1 unit, Denmark 3500 birth/year | Retrospective cohort |
5 years 2 years = pre 1 year = train 2 years = post |
NA | NA | Include lecture, multi‐professional skills training in PPH, debrief | 2.5 h | NA | x | ||||||
| Fuhrmann 2015 | 1 unit, Denmark, 4500 births/year | Retrospective cohort |
1 year: 5 months = pre 2 months = train 5 months = post |
Delivery ward 95% of staff | Sim center | NA | NA | Only once | x | ||||||
| Siassakos 2009 | 1 unit, UK 5500 births/year | Retrospective cohort | 16 years: 7 years = pre 2 years = train 7 years: = post | Delivery ward 95% of staff | In situ | PROMPT training | 1 day | Annually | x | x | |||||
| Copson 2017 | 1 unit, West Australia 6000 births/year | Retrospective cohort | 11 years: 3 years = pre, 5 years = train 3 years = post | NA | NA | NA | 1 day | Annually | x | ||||||
| Phipps 2012 | 1 unit, USA 9200 births/year | Retrospective cohort |
4 years 24 months = pre 6 months = train 18 months = post |
Delivery ward 72% of staff | NA | 4 h CRM, 4 h Multi professional simulation training | 8 h | Only once | x | ||||||
| Nielsen 2007 | 15 units, USA, 27 509 births/year | Open cluster RCT |
1 year: 2 months = prem 4 months = train 5 months = post |
Delivery ward all staff | In situ | CRM training, (MedTeams Labor & De‐livery Team Coordination Course) | NA | Only once | x | ||||||
| Riley 2011 | 3 units, USA, 1800/year | Open cluster RCT | 3 years: 1 year = pre 1 year = train 1 year = post | NA | In situ | Webinars and simulation Team‐STEPPS | 2 h 30 month | 11 simulations | x | ||||||
X = mark the outcome/outcomes reported.
AOI, adverse outcome index; Apgar, Apgar score less than 7 at 5 min; BPI, brachial plexus injury at birth; CP, cord prolapse; DD, decision to delivery interval in emergency cesarean >30 min; HIE, hypoxic ischemic encephalopathy; PPH, severe blood loss and/or transfusion four or more units of red blood cells.
3.2. Risk of bias
Studies with the highest quality design were three open‐cluster RCTs 16 , 35 , 36 followed by a stepped‐wedge RCT. 15 The observational cohort studies had a low to moderate risk of bias. Detailed assessments of each study and the arguments for assessment are described in Appendix S3 and in the risk of bias figure (Figure 2).
FIGURE 2.
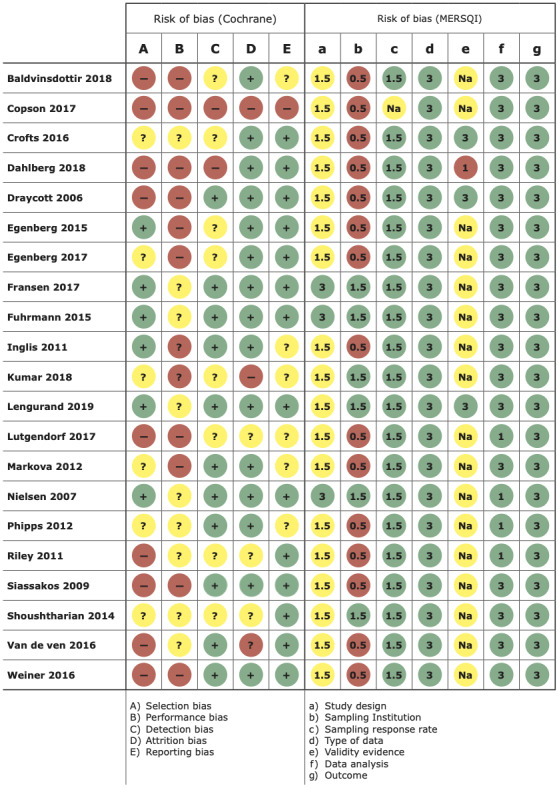
Risk of bias
3.3. Effect of intervention
Three studies were excluded from the meta‐analysis, as they only reported an adverse outcome index, 34 , 35 , 36 defined by a summative effect measure including maternal and perinatal mortality, transfer to a neonatal intensive care unit, low Apgar scores, uterine rupture, anal sphincter rupture, and blood transfusion.
3.4. Meta‐analysis
Seven studies 16 , 20 , 22 , 23 , 24 , 25 , 26 reported the occurrence of brachial plexus injury at birth before and after training. In one RCT 16 an OR of 1.3 (95% CI 0.39–4.33) was found, whereas a combined OR of 0.47 (95% CI 0.33–0.68) was found in six observational cohort studies 20 , 22 , 23 , 24 , 25 , 26 with low heterogeneity (I 2 = 0%). The certainty of evidence was low because the level of certainty was downgraded one level because of risk of bias and one level for imprecision, but upgraded one level for a large magnitude of effect.
Apgar scores less than 7 at 5 min were reported in two RCTs 15 , 16 with a combined OR of 0.87 (95% CI 0.72–1.05) (I 2 = 13%) and in three observational trials 19 , 20 , 21 with a combined OR of 0.77 (95% CI 0.51–1.19). These observational trials involved considerable heterogeneity (I 2 = 83%). The grade of evidence for Apgar scores less than 7 at 5 min was moderate as the level of certainty was downgraded one level because of inconsistency in the studies.
Neonatal hypoxic ischemic encephalopathy was reported in two studies. In the one RCT 16 an OR of 3.20 (95% CI 0.77–13.30) was found, whereas in the one observational study 19 an OR of 0.50 (95% CI 0.26–0.96) was reported. The certainty of evidence was downgraded to very low because of imprecision and inconsistency.
The effect of training in umbilical cord prolapse was evaluated by Apgar scores less than 7 at 5 min in two observational studies. 32 , 33 The studies had a combined OR of 1.31 (95% CI 0.11–15.96) with a substantial risk of heterogeneity (I 2 = 62%). The certainty of evidence became very low, because it was downgraded because of risk of bias, inconsistency, and imprecision.
In eight studies, the effect of training on postpartum hemorrhage was evaluated. 16 , 18 , 21 , 25 , 27 , 28 , 29 , 30 Severe blood loss was reported in one RCT 16 with an OR of 2.20 (95% CI 1.24–3.90) and in two observational studies 21 , 25 there was a combined OR of 1.08 (95% CI 0.96–1.23) (I 2 = 0%). The certainty of evidence was categorized as very low. Transfusion of four or more units of red blood cells was reported from one RCT 16 with an OR of 2.10 (95% CI 1.10–4.01) and in two observational studies, 28 , 30 with an OR of 0.63 (95% Cl 0.38–1.04) (I 2 = 0%). The certainty of evidence was considered very low.
The delay of birth at an emergency cesarean section (decision‐to‐delivery time excess of 30 min) was evaluated in two observational cohort studies 31 , 32 and the combined OR was 0.35 (95% CI 0.18–0.71) (I 2 = 0%). The certainty of evidence level was very low.
The effect of simulation‐based training is presented by forest plots (Figure 3) with effect stacked by decreasing order of study quality. All studies except the RCT of Fransen et al 16 report a positive effect of simulation‐based team training. Studies with in situ multiprofessional simulation‐based training demonstrated the best effect.
FIGURE 3.
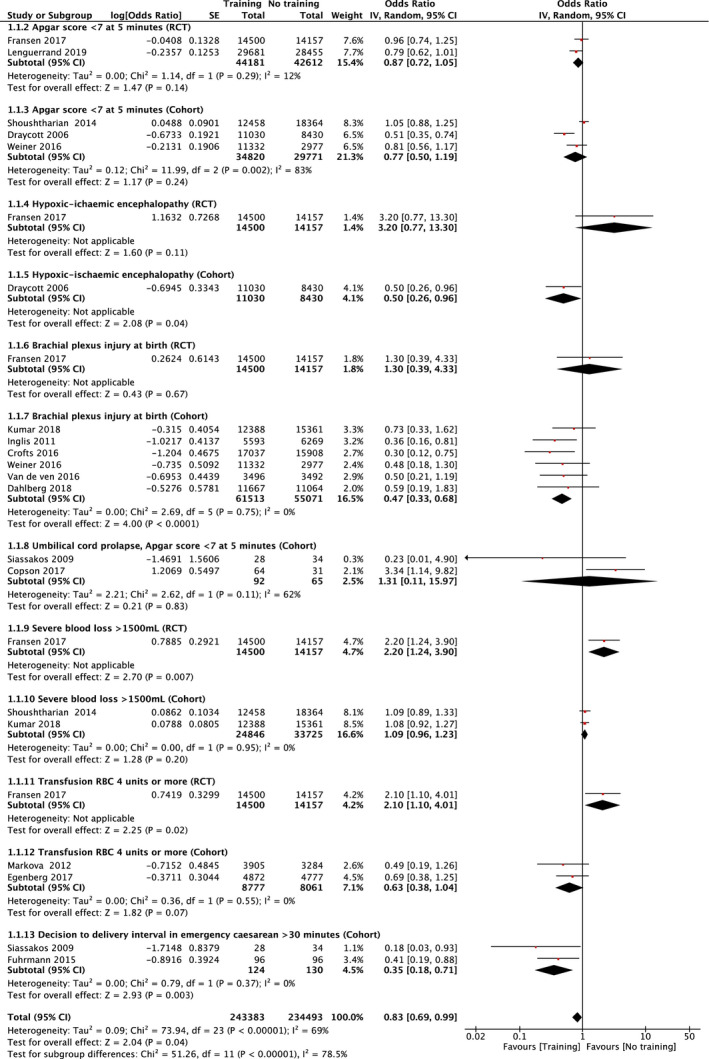
Forest plot of studies investigating the risk of: Neonatal outcomes: Apgar score <7 at 5 min (1.1.1‐.1.1.2), hypoxic ischemic encephalopathy (1.1.3–1.1.4), brachial plexus injury at birth (1.1.5–1.1.6), cord prolapse (1.1.7). Maternal outcomes: severe blood loss (1.1.8–1.1.9), transfusion red blood cells (RBC) 4 units or more (1.1.10–1.1.11), decision to delivery interval in emergency cesarean >30 min (1.1.12)
Detailed assessments for each outcome are shown in Appendix S4, arguments for decision on quality of evidence are listed in the evidence profile in Appendix S5, and main findings in Appendix S6.
4. DISCUSSION
In this meta‐analysis, we found that obstetric emergency simulation‐based team training may reduce brachial plexus injury with a low certainty level of evidence. Furthermore, our analysis suggests a positive effect on Apgar score less than 7 at 5 min, although this was not statistically significant. The effect was unclear for hypoxic ischemic encephalopathy, umbilical prolapse, emergency cesarean section, and severe postpartum hemorrhage, because of few and conflicting studies. Studies with in situ multiprofessional simulation‐based training demonstrated the best effect.
The strength of this systematic review and meta‐analysis is the systematic approach by applying the PRISMA, EPCO, and GRADE guidelines in a comprehensive way. We searched multiple databases without date restrictions in four languages to limit bias by identifying all relevant studies. Two authors independently assessed all the published studies and selected the studies for inclusion in order to minimize bias. Three authors performed the data extraction, data synthesis, and quality of evidence assessment. The entire author group discussed the results and the interpretation.
This systematic review included studies with low quality of evidence for several of our core outcomes. In this review, one of the reasons for downgrading the evidence was limitations in study design or execution, also described as risk of bias and inconsistency of results. Further investigation into study design and execution of the intervention revealed differences in the included studies. For instance, only half of the studies encompassed all of the professional staff in the Labor and Delivery Unit, 15 , 16 , 18 , 19 , 20 , 22 , 23 , 24 , 25 , 26 , 27 , 31 whereas in 15% of the studies around half of the staff took part in the training, 21 , 28 , 34 and in 25% there was no information of how many participated. 29 , 30 , 32 , 33 , 36 It was reassuring, however, that the plausible confounding would reduce the demonstrated effect.
Further heterogeneity was found in the context of training, where only 50% used pre‐defined guidelines or a previously described program. 15 , 16 , 20 , 21 , 24 , 26 , 35 , 36 The studies were better aligned with respect to duration of training, with the majority describing a one‐day program 15 , 16 , 19 , 20 , 21 , 26 , 27 , 32 , 33 , 34 and others using a half‐day program (Table 1). The majority trained staff in the Labor and Delivery Unit (in situ training) annually and only a few described training in simulation centers. 16 , 24 , 27 , 31 Hence, the differences in interventions used also limit the conclusions that can be drawn from the meta‐analysis.
A further limitation was that only two RCTs could be included in the meta‐analysis. One of these impacted heavily in our meta‐analysis, as this study 16 was large and evaluated training for all outcomes, but with little or no effect being shown. It is important to evaluate all the included studied effects with regard to the intervention and type of training and the forest plots and not just to consider the summative result of the meta‐analysis.
The management of shoulder dystocia was evaluated in six cohort studies and one RCT. The RCT by Fransen et al 16 reported 1 year after training an OR of 1.30 (95% CI 0.39–4.33). In a secondary analysis of the same trial, 37 a significant decrease in brachial plexus injury was reported in the first quarter (0.06% vs. 0.26%, OR 0.19, 95% CI 0.03–0.98), but in the subsequent quarters, no significant reductions were observed. One could argue that the lack of effect was due to loss of obtained skills after 3 months, as reported in other research. 38 , 39 However, in six observational cohort studies 20 , 22 , 23 , 24 , 25 , 26 annual training was also evaluated and a beneficial effect regarding brachial plexus injuries (OR 0.47; 95% CI 0.33–0.68) was suggested. It is difficult to discount this clear result. Training may therefore reduce the risk of brachial plexus injury; however, certainty of evidence is low.
Regarding Apgar scores less than 7 at 5 min; the two RCTs 15 , 16 had an OR of 0.87 (95% CI 0.72–1.05) (I 2 = 13%). Although they have a low heterogeneity, the studies differed substantially from each other. The THISTLE trial by Lenguerrand et al 15 used a step‐wedge RCT design with in situ training where the PROMPT 40 course methodology was used, whereas the TOSTI trial by Fransen et al 16 was an open‐cluster RCT with simulation center training using the MOET 41 program, which has a focus on crisis resource management. The study of Fransen et al 16 is large and of high quality; however, an effect after 1 year was not demonstrated. It has been argued that the intervention is based on off‐site training and CRM training, and as a result lacks the introduction to checklists and procedures at the residing hospital. 42 Furthermore, training was not blinded and hospitals not selected for training may have been motivated to improve treatment by other initiatives. The three observational cohort studies 19 , 20 , 21 carried a substantial heterogeneity (I 2 = 83%) and differed in the proportion of the staff that were trained. Shoushtarian et al, 21 who trained 50% of the staff, did not find a reduced effect on the risk of low Apgar scores; however, Draycott et al 19 who trained all the staff in the Labor and Delivery Unit, showed a significant risk reduction of OR 0.51 (95% CI 0.35–0.74). The meta‐analysis suggests a positive effect on Apgar score less than 7 at 5 min, although not statistically significant.
Hypoxic ischemic encephalopathy is a rare complication with high morbidity. This outcome was only reported in two studies. Fransen et al 16 reported an OR of 3.20 (95% CI 0.77–13.30). Intuitively, it seems unlikely that training would increase the risk of ischemic encephalopathy and a large degree of uncertainty is also evident from the wide confidence interval. Their findings contradict the observational study of Draycott et al, 19 where a beneficial effect was indicated with an OR of 0.50 (95% CI 0.26–0.96), but the two studies differed with regard to study design and intervention. Overall it must be considered uncertain whether the training efforts have an effect on neonatal hypoxic ischemic encephalopathy.
Eight studies addressed the value of training in the management of postpartum hemorrhage. 16 , 18 , 21 , 25 , 27 , 28 , 29 , 30 Fransen et al 16 reported an increase of hemorrhage cases (OR 2.20, 95% CI 1.24–3.90), and Kumar et al 25 and Shoushtarian et al 21 reported an inconclusive result (OR 1.08, 95% CI 0.96–1.23). Underestimation of blood loss is well described, 43 and learning to more accurately assess this will likely raise these rates. Therefore, it seems unlikely that there was a real increase in severe postpartum hemorrhage. The effect on transfusion rates (four or more units) was varied with one RCT 16 showing higher rates and two observational cohort studies 28 , 30 having lower rates. The inconsistency of these results also leads to uncertainty. We conclude that the effect of training on reducing postpartum hemorrhage and the need for subsequent blood transfusion remains unclear.
Two observational studies 32 , 33 evaluated the effect of training on the rare event of umbilical cord prolapse using the end‐point low Apgar scores at 5 min. The studies had a similar design and were both limited by the inclusion of only a few events. Siassakos et al 32 reported OR of 0.23 (95% CI 0.01–4.90), whereas the study from Copson et al 33 reported a marginally significant increased OR of 2.42 (95% CI 1.03–5.72). This meant that the meta‐analysis result was inconclusive, so the effect of training on umbilical cord prolapse remains unclear.
The delay of birth at an emergency cesarean birth (decision‐to‐delivery time excess of 30 min) was dealt with in two observational cohort studies. Fuhrmann et al 31 evaluated the effect of one training session 1 year later, whereas Siassakos et al 32 trained their staff annually over 16 years. Both studies suggested that training would reduce the proportion of delayed emergency cesarean sections, but because of the design and different observation times, the certainty of the evidence was very low and a conclusion that there is an effect of training cannot be drawn.
Simulation‐based team training is defined by teams applying principles or guidelines in a scenario using a mannequin. 44 Several types of simulation‐based training were used in this review.
Based on our review, it seems that local (in situ) multiprofessional training for all staff members is the most beneficial with regards to improving patient outcomes. Not all training is equally effective, and it is noteworthy that none of the two RCTs demonstrates the same effect as cohort studies. It has been speculated whether the lack of blinding may play a role, as the non‐training hospitals may train anyhow. Furthermore, a national or regional simulation program may be difficult to implement locally, wherefore training is offered in a simulation center.
Studies included in this review described staff being trained annually for 1 day. Research on resuscitation has suggested that shorter training sessions with shorter intervals can be more efficient. 45 , 46 , 47 , 48 However, little is known on how often obstetric training should take place. We anticipated that this review could provide more information on this matter; however, analysis on the frequency was not possible because of the lack of studies using interventions more frequently than yearly. 49
In this review, we selected patient outcome measures to evaluate the effect of simulation‐based training in obstetrical emergencies. We included outcomes that are widely accepted as obstetric quality indicators to cover management, trauma, and injury with regard to both the women and neonates. 50 The strength of the selected outcomes is that they are internationally defined and reported. However, the weakness in several of these outcomes is that multifactorial events can evolve even when the team provides optimal care. Furthermore, some of the included outcomes are considered to be pseudo‐outcomes and therefore constitute only an indirect measure. The number of administered blood transfusions is an example of this. A more direct approach would involve auditing the direct performance of the emergency team, such as by live recordings. This is, however, rarely described and not easily used. 51
In the last decade, use of obstetric simulation training has been increasing as healthcare providers, insurance companies, and hospitals request this provision. Staff use simulation training for improved personal confidence and preparedness. 9 , 29 , 52 , 53 The insurance companies strive for a reduction in malpractice claims 54 and hospitals aim for reduced sick leave among healthcare providers, 55 for higher patient satisfaction, 56 and better obstetric patient safety indicator measures. Training of an entire department is costly, though studies have reported it to be cost‐effective. 54 , 57 Research is therefore needed to ensure effective training in the future and to improve levels of evidence.
5. CONCLUSION
Emerging evidence suggest an effect of simulation‐based obstetric team training for multiprofessional teams trained locally/in situ, but conflicting results call for future controlled trials targeting the methodology for effective team training.
CONFLICT OF INTEREST
A contribution towards this work was received from the Department of Education in the Central Region of Denmark (MidtSim).
AUTHOR CONTRIBUTIONS
LB, LH, and SB are responsible for acquisition of data and all authors are responsible for the interpretation of data and writing of the article.
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Brogaard L, Glerup Lauridsen K, Løfgren B, et al. The effects of obstetric emergency team training on patient outcome: A systematic review and meta‐analysis. Acta Obstet Gynecol Scand.2022;101:25–36. 10.1111/aogs.14263
REFERENCES
- 1. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323‐333. http://dx.doi.org/10.1016/S2214‐109X(14)70227‐X See [DOI] [PubMed] [Google Scholar]
- 2. WHO . Every newborn: An action plan to end Preventable deaths: Executive summary Geneva: World Health Organization. Who, Unicef. 2014; p. 12.
- 3. Khan I, Wojdyla D, Say L, Gülmezoglu A, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066‐1074. [DOI] [PubMed] [Google Scholar]
- 4. Brogaard L, Kierkegaard O, Hvidman L, et al. The importance of non‐technical performance for teams managing postpartum haemorrhage: video review of 99 obstetric teams. BJOG. 2019;126:1015‐1023. [DOI] [PubMed] [Google Scholar]
- 5. Cantwell R, ed, Clutton‐Brock T, Cooper G G & et al. Saving Mothers' Lives: Reviewing maternal deaths to make motherhood safer: 2006‐2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1‐203. [DOI] [PubMed] [Google Scholar]
- 6. Baskett TF, Calder AA, Arulkumaran S. Munro Kerr´s Operative obstetrics, 12th ed. Saunders elsevier; 2019:28–35. [Google Scholar]
- 7. Johansen LT, Braut GS, Acharya G, Andresen JF, Øian P. How common is substandard obstetric care in adverse events of birth asphyxia, shoulder dystocia and postpartum hemorrhage? Findings from an external inspection of Norwegian maternity units. Acta Obstet Gynecol Scand. 2021;100:139‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Draycott TJ, Collins KJ, Crofts JF, et al. Myths and realities of training in obstetric emergencies. Best Pract Res Clin Obstet Gynaecol. 2015;29:1067‐1076. [DOI] [PubMed] [Google Scholar]
- 9. Bergh A‐M, Baloyi S, Pattinson RC. What is the impact of multi‐professional emergency obstetric and neonatal care training? Best Pract Res Clin Obstet Gynaecol. 2015;29:1028‐1043. [DOI] [PubMed] [Google Scholar]
- 10. Higgens J & Green S, eds. Cochrane Handbook for systematic review of interventions Version 5.1.0. The cochrane collaboration; 2011. https://handbook‐5‐1.cochrane.org/
- 11. Stone PW. Popping the (PICO) question in research and evidence‐based practice. Appl Nurs Res. 2002;15:197‐198. [DOI] [PubMed] [Google Scholar]
- 12. The World Bank . https://data.worldbank.org/income‐level/high‐income?view=chart.
- 13. Kierkpatrick DL, Evaluating KJD. Evaluating training programs, the four levels. 3rd ed. Berrett‐Koehler Publishers, Inc.; 2006:21–81. [Google Scholar]
- 14. Cook DA, Reed DA. Appraising the quality of medical education research methods: the medical education research study quality instrument and the newcastle‐ottawa scale‐education. Acad Med. 2015;90:1067‐1076. [DOI] [PubMed] [Google Scholar]
- 15. Lenguerrand E, Winter C, Siassakos D, et al. Effect of hands‐on interprofessional simulation training for local emergencies in Scotland: the THISTLE stepped‐wedge design randomised controlled trial. BMJ Qual Saf. 2020;29:122‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fransen AF, van de Ven J, Schuit E, van Tetering A, Mol BW, Oei SG. Simulation‐based team training for multi‐professional obstetric care teams to improve patient outcome: a multicentre, cluster randomised controlled trial. BJOG. 2017;124:641‐650. [DOI] [PubMed] [Google Scholar]
- 17. Khan K, Knuz R, Kleijnen J & Antes G Systematic reviews to support evidencebased medicine. 2nd ed. Boca Raton, FL: CRC Press; 2011. [Google Scholar]
- 18. Baldvinsdóttir T, Blomberg M, Lilliecreutz C. Improved clinical management but not patient outcome in women with postpartum haemorrhage—An observational study of practical obstetric team training. PLOS ONE. 2018;13:e0203806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Draycott T, Sibanda T, Owen L, et al. Does training in obstetric emergencies improve neonatal outcome? BJOG. 2006;113:177‐182. [DOI] [PubMed] [Google Scholar]
- 20. Weiner CP, Collins L, Bentley S, Dong Y, Satterwhite CL. Multi‐professional training for obstetric emergencies in a U.S. hospital over a 7‐year interval: an observational study. J Perinatol. 2016;36:19‐24. [DOI] [PubMed] [Google Scholar]
- 21. Shoushtarian M, Barnett M, McMahon F, Ferris J. Impact of introducing Practical Obstetric Multi‐Professional Training (PROMPT) into maternity units in Victoria, Australia. BJOG. 2014;121:1710–1718. [DOI] [PubMed] [Google Scholar]
- 22. van de Ven J, van Deursen FJHM, van Runnard Heimel PJ, Mol BWJ, Oei SG. Effectiveness of team training in managing shoulder dystocia: a retrospective study. J Matern Fetal Neonatal Med. 2016;29:3167‐3171. [DOI] [PubMed] [Google Scholar]
- 23. Inglis SR, Feier N, Chetiyaar JB, et al. Effects of shoulder dystocia training on the incidence of brachial plexus injury. Am J Obstet Gynecol. 2011;204:322.e1‐322.e6. [DOI] [PubMed] [Google Scholar]
- 24. Dahlberg J, Nelson M, Dahlgren MA, Blomberg M. Ten years of simulation‐based shoulder dystocia training‐ impact on obstetric outcome, clinical management, staff confidence, and the pedagogical practice ‐ a time series study. BMC Pregnancy Childbirth. 2018;18:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar A, Sturrock S, Wallace EM, et al. evaluation of learning from practical obstetric multi‐professional training and its impact on patient outcomes in Australia using Kirkpatrick’s framework: a mixed methods study. BMJ Open. 2018;8:e017451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crofts JF, Lenguerrand E, Bentham GL, et al. Prevention of brachial plexus injury‐12 years of shoulder dystocia training: an interrupted time‐series study. BJOG. 2016;123:111–118. [DOI] [PubMed] [Google Scholar]
- 27. Egenberg S, Øian P, Bru LE, Sautter M, Kristoffersen G, Eggebø TM. Can inter‐professional simulation training influence the frequency of blood transfusions after birth?. Acta Obstet Gynecol Scand. 2015;94:316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Egenberg S, Øian P, Eggebø TM, Arsenovic MG, Bru LE. Changes in self‐efficacy, collective efficacy and patient outcome following interprofessional simulation training on postpartum haemorrhage. J Clin Nurs. 2017;26:3174‐3187. [DOI] [PubMed] [Google Scholar]
- 29. Lutgendorf MA, Spalding C, Drake E, Spence D, Heaton JO, Morocco KV. Multidisciplinary in situ simulation‐based training as a postpartum hemorrhage quality improvement project. Mil Med. 2017;182:e1762‐e1766. [DOI] [PubMed] [Google Scholar]
- 30. Markova V, Sørensen JL, Holm C, Nørgaard A, Langhoff‐Roos J. Evaluation of multi‐professional obstetric skills training for postpartum hemorrhage. Acta Obstetricia et Gynecologica Scandinavica. 2012;91:346–352. [DOI] [PubMed] [Google Scholar]
- 31. Fuhrmann L, Pedersen TH, Atke A, Møller AM, Østergaard D. Multidisciplinary team training reduces the decision‐to‐delivery interval for emergency Caesarean section. Acta Anaesthesiologica Scandinavica. 2015;59:1287–1295. [DOI] [PubMed] [Google Scholar]
- 32. Siassakos D, Hasafa Z, Sibanda T, et al. Retrospective cohort study of diagnosis‐delivery interval with umbilical cord prolapse: the effect of team training. BJOG. 2009;116:1089‐1096. [DOI] [PubMed] [Google Scholar]
- 33. Copson S, Calvert K, Raman P, Nathan E, Epee M. The effect of a multidisciplinary obstetric emergency team training program, the In Time course, on diagnosis to delivery interval following umbilical cord prolapse – A retrospective cohort study. Aust New Zeal J Obstet Gynaecol. 2017;57:327‐333. [DOI] [PubMed] [Google Scholar]
- 34. Phipps MG, Lindquist DG, McConaughey E, O’Brien JA, Raker CA, Paglia MJ. Outcomes from a labor and delivery team training program with simulation component. Am J Obstet Gynecol. 2012;206:3‐9. [DOI] [PubMed] [Google Scholar]
- 35. Nielsen PE, Goldman MB, Mann S, et al. Effects of teamwork training on adverse outcomes and process of care in labor and delivery: A randomized controlled trial. Obstet Gynecol. 2007;109:48‐55. [DOI] [PubMed] [Google Scholar]
- 36. Riley W, Davis S, Miller K, Hansen H, Sainfort F, Sweet R. Didactic and Simulation Nontechnical Skills Team Training to Improve Perinatal Patient Outcomes in a Community Hospital. Jt Comm J Qual Patient Saf. 2011;37:357‐364. [DOI] [PubMed] [Google Scholar]
- 37. van de Ven J, Fransen AF, Schuit E, et al. Does the effect of one‐day simulation team training in obstetric emergencies decline within one year? A post‐hoc analysis of a multicentre cluster randomised controlled trial. Eur J Obstet Gynecol Reprod Biol. 2017;216:79‐84. [DOI] [PubMed] [Google Scholar]
- 38. Cheng A, Nadkarni VM, Mancini MB, et al. Resuscitation education science: educational strategies to improve outcomes from cardiac arrest: a scientific statement from the American Heart Association. Circulation. 2018;138:e82‐e122. [DOI] [PubMed] [Google Scholar]
- 39. Anderson R, Sebaldt A, Lin Y, Cheng A. Optimal training freqency for acquisition and retention of high‐quality CPR skills: a randomized trial. Resuscitation. 2019;135:153‐161. [DOI] [PubMed] [Google Scholar]
- 40. Crofts J, Draycott T, Muachatuta N, Winter C. PROMPT PRactiacl Obstetric Multi‐Professional Training Course Manual. 2nd Revise. ed. Cambridge University Press; 2017:1‐303. [Google Scholar]
- 41. Lawrence A, McLaren E. Managing obstetric emergencies and Trauma: The moet course manual. Acad Emerg Med. 2017;24:776. –777. [Google Scholar]
- 42. Draycott T. Not all training for obstetric emergencies is equal, or effective. BJOG. 2017;124:651. [DOI] [PubMed] [Google Scholar]
- 43. Bose P, Regan F, Paterson‐Brown S. Improving the accuracy of estimated blood loss at obstetric haemorrhage using clinical reconstructions. BJOG. 2006;113:919‐924. [DOI] [PubMed] [Google Scholar]
- 44. Salas E. Reporting guidelines for health care simulation research. Simul Healthc. 2016;11:249. [DOI] [PubMed] [Google Scholar]
- 45. Sutton RM, Niles D, Meaney PA, et al. Low‐dose, high‐frequency CPR training improves skill retention of in‐hospital pediatric providers. Pediatrics. 2011;128:e145‐e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Niles DE, Nishisaki A, Sutton RM, et al. Improved retention of chest compression psychomotor skills with brief “Rolling Refresher” training. Simul Healthc. 2017;12:213‐219. [DOI] [PubMed] [Google Scholar]
- 47. Patocka C, Cheng A, Sibbald M, et al. A randomized education trial of spaced versus massed instruction to improve acquisition and retention of paediatric resuscitation skills in emergency medical service (EMS) providers. Resuscitation. 2019;141:73‐80. [DOI] [PubMed] [Google Scholar]
- 48. Sullivan NJ, Duval‐Arnould J, Twilley M, et al. Simulation exercise to improve retention of cardiopulmonary resuscitation priorities for in‐hospital cardiac arrests: a randomized controlled trial. Resuscitation. 2015;86:6‐13. [DOI] [PubMed] [Google Scholar]
- 49. Cheng A, Eppich W, Grant V, Sherbino J, Zendejas B, Cook DA. Debriefing for technology‐enhanced simulation: a systematic review and meta‐analysis. Med Educ. 2014;48:657‐666. [DOI] [PubMed] [Google Scholar]
- 50. Pyykönen A, Gissler M, Jakobsson M, Petäjä J, Tapper AM. Determining obstetric patient safety indicators: The differences in neonatal outcome measures between different‐sized delivery units. BJOG. 2014;121:430‐436. [DOI] [PubMed] [Google Scholar]
- 51. Brogaard L, Uldbjerg N. Filming for auditing of real‐life emergency teams: a systematic review. BMJ Open Qual. 2019;8:e000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lavelle M, Abthorpe J, Simpson T, et al. MBRRACE in simulation: an evaluation of a multi‐disciplinary simulation training for medical emergencies in obstetrics (MEmO). J Obstet Gynaecol (Lahore). 2018;38:781‐788. [DOI] [PubMed] [Google Scholar]
- 53. Marzano D, Smith R, Mhyre J, et al. Evaluation of a simulation‐based curriculum for implementing a new clinical protocol. Int J Gynecol Obstet. 2016;135:333‐337. [DOI] [PubMed] [Google Scholar]
- 54. Geary M, Ruiter PJA, Yasseen AS 3rd. Examining the effects of an obstetrics interprofessional programme on reductions to reportable events and their related costs. J Interprof Care. 2018;8:1‐9. doi: 10.1080/13561820.2018.1543255. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 55. de Melo BCP, Van der Vleuten CPM, Muijtjens AMM, Rodrigues Falbo A, Katz L, Van Merriënboer JJG. Effects of an in situ instructional design based postpartum hemorrhage simulation training on patient outcomes: an uncontrolled before‐and‐after study. J Matern Fetal Neonatal Med. 2021;34:245‐252. [DOI] [PubMed] [Google Scholar]
- 56. Truijens SEM, Banga FR, Fransen AF, et al. The effect of multiprofessional simulation‐based obstetric team training on patient‐reported quality of care: a pilot study. Simul Healthc. 2015;10:210‐216. [DOI] [PubMed] [Google Scholar]
- 57. van de Ven J, van Baaren GJ, Fransen AF, van Runnard Heimel PJ, Mol BW, Oei SG. Cost‐effectiveness of simulation‐based team training in obstetric emergencies (TOSTI study). Eur J Obstet Gynecol Reprod Biol. 2017;216:130‐137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6


