Abstract
Human neutrophils by a chemiluminescence assay exhibit diminished phagocytic activity in the presence of abnormally high levels of the serum metabolite acetoacetate. These findings, along with our previous evidence demonstrating myeloperoxidase inhibition by acetoacetate, implicate metabolic ketosis in the inhibition of neutrophil microbicidal activity and thus in increased susceptibility to infections.
Neutrophils or polymorphonuclear cells are an important component of humans’ innate phagocytic defense against pathogens and usually arrive first at sites of infection and/or inflammation (6). Within neutrophils, the heme protein myeloperoxidase (MPO) mediates the production of hypochlorous acid (HOCl), a potent microbicidal compound (5). Our previous research has shown that purified MPO is inhibited by acetoacetate, an elevated serum metabolite in ketosis (2–4). Further, we have previously shown that Candida albicans, an opportunistic pathogen, survives in the presence of acetoacetate and the MPO-H2O2-Cl− system, while the organism is killed when exposed to this system in the absence of acetoacetate (10). The study reported herein was undertaken to determine the effect of acetoacetate on whole-neutrophil phagocytic activity by a chemiluminescence assay.
Early in the phagocytic process, neutrophils undergo a respiratory burst, generating oxygen-free radicals which, after their production, decay naturally and emit light (1). Thus, chemiluminescence enhanced by the presence of luminol (5-amino 2,3-dihydro-1,4-phthalazine-dione) may be used as a measure of phagocytic activity. Our method of evaluating the effect of acetoacetate on neutrophil activity relied on this characteristic of neutrophils.
Neutrophils were isolated from human blood collected in the anticoagulant EDTA and processed within 2 hours of collection for optimal neutrophil isolation. In a sterile centrifuge tube, 6 ml of blood was carefully layered onto 4 ml of a Ficoll-Hypaque density gradient, NIM (neutrophil isolation medium) (Cardinal Associates). Centrifugation without braking was conducted at 400 × g for 30 min at 21°C. Postcentrifugation fractions consisted of plasma, mononuclear cells, upper NIM, neutrophils, lower NIM, and an erythrocyte pellet. The neutrophil layer was collected and dispensed into a 15-ml sterile centrifuge tube filled with Hanks’ balanced salt solution (HBSS) with calcium and magnesium but without sodium bicarbonate and phenol red (Cardinal Associates). This preparation was centrifuged at 350 × g for 10 min. After the supernatant was discarded, residual erythrocytes in the pellet were lysed with 2 ml of erythrocyte lysing buffer (E-lyse; Cardinal Associates), followed by centrifugation at 250 × g for 5 min. After the supernatant was discarded, the previous step was repeated. The neutrophil pellet was resuspended in HBSS to 15 ml and centrifuged at 250 × g for 5 min. After the supernatant was discarded, neutrophils were resuspended in 1 ml of 5% fetal bovine serum (Cardinal Associates) and counted in a hemocytometer. Cells were diluted in 5% fetal bovine serum to obtain a cell count of 106/ml. Upon isolation, neutrophils were immediately utilized for experimentation.
Chemiluminescence studies were conducted with a Flyte 400 Luminometer (Cardinal Associates) with a 37°C circulating-water bath for temperature control of the measurement chamber. All measurements were taken at 60-s intervals. Assays were performed with the following components in a total reaction volume of 360 μl: 100 μl of neutrophils, 200 μl of a lyophilized suspension of opsonized Zymosan A particles containing luminol (ZAP; Cardinal Associates) or 200 μl of a lyophilized suspension of phorbol 12-myristate 13-acetate with luminol (PMA; Cardinal Associates), 50 μl of HBSS, and 0 to 2.8 mM (final concentration) acetoacetate (Sigma). Prior to the commencement of these studies, background chemiluminescence from neutrophils in the absence of ZAP was measured and found to be negligible. To verify that neutrophils were viable for the duration of our experiments, duplicate control assays (no acetoacetate) were conducted at the beginning as well as upon the completion of all experiments with each isolated preparation of neutrophils. In addition, the viability of neutrophils, both untreated and exposed to acetoacetate, was confirmed by trypan blue exclusion. Thus, loss of cell viability over time or resulting from exposure to acetoacetate were not factors in this study.
Normal human neutrophil phagocytic activity as revealed by chemiluminescence in the absence of acetoacetate is shown in Fig. 1. In the presence of 2.8 mM acetoacetate, however, neutrophil chemiluminescence dropped dramatically.
FIG. 1.
Chemiluminescence from neutrophils engaged in phagocytic activity. Neutrophils at equivalent concentrations were exposed to Zymosan A particles containing luminol (ZAP) in the presence and absence of 2.8 mM acetoacetate. As shown, normal neutrophil phagocytic activity was accompanied by a high level of chemiluminescence, which was nearly abolished in the presence of acetoacetate.
Figure 2 shows a comparison of neutrophil chemiluminescence in the presence of varying concentrations of acetoacetate. Normal serum acetoacetate levels range from 28 to 280 μM (9). Therefore, a wide range of acetoacetate concentrations was tested for effects on chemiluminescence. Final acetoacetate concentrations in the reaction mixtures ranged from 28 μM to 2.8 mM, reflecting the normal serum range to 10 times the upper normal serum level. Neutrophils in the presence of normal serum levels of acetoacetate exhibited greater chemiluminescence than neutrophils in the presence of concentrations representing excess serum levels. Both a decline in chemiluminescence and a longer lag period until peak chemiluminescence was reached were observed in preparations with abnormally high levels of acetoacetate. Interestingly, within the accepted normal serum acetoacetate range, phagocytic activity as demonstrated by chemiluminescence declined as acetoacetate levels increased from the lower-normal to upper-normal serum range. By trypan blue exclusion, neutrophil viability in the presence of 0 to 2.8 mM acetoacetate remained at approximately 92%. Thus, diminished chemiluminescence in the presence of increasing levels of acetoacetate cannot be attributed to decreased viability of neutrophils.
FIG. 2.
Inhibition of neutrophil phagocytic activity as demonstrated by diminished chemiluminescence in the presence of increasing levels of acetoacetate. Chemiluminescence of ZAP-treated neutrophils in the presence of acetoacetate levels representing normal serum range (28 μM to 0.28 mM) was monitored. As shown, neutrophils exposed to acetoacetate concentrations within the normal serum range were actively phagocytic, albeit as the acetoacetate level reached a concentration representing the upper limit of normal, chemiluminescence declined. As the acetoacetate concentration was increased above normal serum range, a greater lag period in attaining peak chemiluminescence and diminished levels of chemiluminescence were observed.
The effect of acetoacetate on neutrophils exposed to PMA, a nonphagocytic stimulus of neutrophils which activates protein kinase C and causes degranulation without phagocytosis (5a, 8a, 11), is shown in Fig. 3. Chemiluminescence of PMA-stimulated neutrophils in the presence of 2.8 mM acetoacetate was approximately equivalent to that obtained from untreated cells. This finding implies that the inhibitory effect of acetoacetate occurs early in the phagocytic process. Additional studies are ongoing, with chemotactic stimuli and chemiluminescence enhancers other than luminol, which may permit further elucidation of the underlying inhibitory mechanism of acetoacetate.
FIG. 3.
Effect of acetoacetate on chemiluminescence from PMA-stimulated neutrophils. Chemiluminescence was measured from PMA-stimulated neutrophils alone as well as those exposed to 2.8 mM acetoacetate, the highest level of acetoacetate evaluated in this study. As shown, there was no evident effect of acetoacetate on chemiluminescence from PMA-stimulated neutrophils.
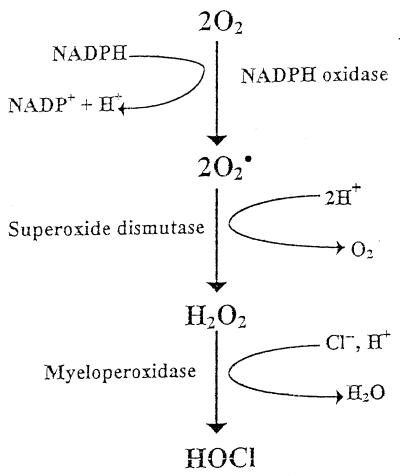
MPO plays an important role in the microbicidal action of neutrophils (Fig. 4). F. A. Saeed and J. E. Harrison (2–4) have previously demonstrated in work with MPO that acetoacetate is an electron donor to MPO and promotes formation of compound 2, thus inactivating the enzyme. Here we demonstrate the adverse effect of acetoacetate, if the range is above normal physiologic levels, on whole neutrophils in an assay of chemiluminescence attendant to phagocytosis.
FIG. 4.
The phagocytic process, in which perturbation of the polymorphonuclear membrane results in a respiratory burst, activating membrane-bound NADPH oxidase to yield O2−. Superoxide dismutase catalyzes the rapid generation of H2O2, at whose expense MPO catalyzes oxidation of Cl− to yield HOCl.
Considering that we show here via significantly diminished chemiluminescence that neutrophil phagocytic activity may be impaired by the presence of abnormally high levels of acetoacetate, the exact mechanism of this effect needs to be determined. Several possible explanations may be invoked, such as a direct effect on the neutrophil membrane and/or its ability to invaginate. Since chemiluminescence depends upon the production of oxygen radicals during the respiratory burst, effects on membrane-bound oxidase must also be considered.
Together with our previous findings on the inhibitory effect of acetoacetate on MPO, these results are very intriguing. That abnormally high levels of acetoacetate may undermine neutrophil function has significant implications for the effects of prolonged or chronic metabolic ketosis on an important first-line defense against infection. Apart from diabetes, ketosis is common to endocrine disorders involving glucocorticoid production by the adrenal glands, pregnancy (diabetogenic), alcoholism, stress, and malnutrition. An increased susceptibility to infections in general and to opportunistic pathogens in particular often accompanies all of these conditions. Also, it is well known that chronic infection with the opportunistic pathogen C. albicans is related to impaired neutrophil function (6–8) and is a common chronic infection observed in patients suffering from poorly controlled diabetes. Thus, the underlying basis for increased diabetic susceptibility to infections may be diminished neutrophil function. The implications of these findings are profound for the adverse role that metabolic ketosis may play in undermining a key first-line host defense against microbial infection.
REFERENCES
- 1.Babior B M, Kipnes R S, Curnutte J T. Biological defense mechanism: the production by leukocytes of superoxide, a potential bactericidal agent. J Clin Investig. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison J E, Saeed F A. Acetoacetate is an electron donor to myeloperoxidase and a promoter of myeloperoxidase-catalyzed fatty acid peroxidation. Biochem Med. 1981;26:339–355. doi: 10.1016/0006-2944(81)90010-7. [DOI] [PubMed] [Google Scholar]
- 3.Harrison J E, Saeed F A. ACS Annual Meeting Abstracts. 1981. Acetoacetate: an electron donor and halogen acceptor for neutrophil myeloperoxidase and a promoter of fatty acid peroxidation. [DOI] [PubMed] [Google Scholar]
- 4.Harrison J E, Saeed F A. Radical acetoacetate oxidation by myeloperoxidase, lactoperoxidase, prostaglandin synthetase, and prostacyclin synthetase: implications for atherosclerosis. Biochem Med. 1983;29:149–163. doi: 10.1016/0006-2944(83)90035-2. [DOI] [PubMed] [Google Scholar]
- 5.Harrison J E, Schultz J. Studies on the chlorinating mechanism of myeloperoxidase. J Biol Chem. 1976;251:1371–1374. [PubMed] [Google Scholar]
- 5a.Kankaanranta H, Moilanen E, Lindberg K, Vapaatalo H. Pharmacological control of human polymorphonuclear leukocyte degranulation by fenamates and inhibitors of receptor-mediated calcium entry and protein kinase C. Biochem Pharmacol. 1995;50(2):197–203. doi: 10.1016/0006-2952(95)00126-k. [DOI] [PubMed] [Google Scholar]
- 6.Klebanoff S J, Clark R A. The neutrophil: functional and clinical disorders. Amsterdam, The Netherlands: Elsevier Scientific Publications; 1978. [Google Scholar]
- 7.Lehrer R I, Cline M J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infections. J Clin Investig. 1969;48:1478. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehrer R I, Cline M J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969;98:996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Lopez I, Burns D, Lambeth J. Regulation of phospholipase D by protein kinase C in human neutrophils. J Biol Chem. 1995;270:19465–19472. doi: 10.1074/jbc.270.33.19465. [DOI] [PubMed] [Google Scholar]
- 9.Rhoades R, Pflanzer R. Human physiology. Orlando, Fla: Saunders College Publishing; 1996. [Google Scholar]
- 10.Saeed F A. 7th International Congress for Infectious Diseases Abstracts. 1996. Inactivation of myeloperoxidase (MPO) as a basis for diabetic susceptibility to Candidiasis, abstr. 110.020; p. 278. [Google Scholar]
- 11.Thelen M, Dewald B, Baggiolini M. Neutrophil signal transduction and activation of the respiratory burst. Physiol Rev. 1993;73:797–821. doi: 10.1152/physrev.1993.73.4.797. [DOI] [PubMed] [Google Scholar]