Abstract
Tuberculosis continues to be a worldwide problem for both humans and animals. The development of tests to differentiate between infection with Mycobacterium tuberculosis or Mycobacterium bovis and vaccination with M. bovis BCG could greatly assist in the diagnosis of early infection as well as enhance the use of tuberculosis vaccines on a wider scale. Recombinant forms of four major secreted proteins of M. bovis—MPB59, MPB64, MPB70, and ESAT-6—were tested in a whole-blood gamma interferon (IFN-γ) assay for differentiation between cattle vaccinated with BCG and those experimentally infected with M. bovis. BCG vaccination induced minimal protection in the present study, with similar numbers of animals infected with M. bovis in BCG-vaccinated and nonvaccinated groups. Following vaccination with BCG, the animals produced moderate IFN-γ responses to bovine purified protein derivative (PPDB) but very weak responses to the recombinant antigens. Cattle from both the BCG-vaccinated and nonvaccinated groups which were M. bovis culture positive following challenge produced IFN-γ responses to PPDB and ESAT-6 which were significantly stronger than those observed in the corresponding M. bovis culture-negative animals. IFN-γ responses to MPB59, MPB64, and MPB70 were significantly weaker, and these antigens could not discriminate between vaccinated animals which develop disease and the culture-negative animals. The results of the study indicate that of the four antigens tested in the IFN-γ assay, only ESAT-6 would be suitable for differentiating BCG-vaccinated animals from those infected with bovine tuberculosis.
Tuberculosis in humans and animals continues to cause major health problems on a global scale. Human tuberculosis accounts for eight million cases of clinical disease and three million deaths annually (22) and is predominantly caused by Mycobacterium tuberculosis. Bovine tuberculosis is a major cause of economic loss and represents a significant zoonotic infection (8). Mycobacterium bovis is the etiological agent of bovine tuberculosis and is closely related to M. tuberculosis within the tuberculosis complex. An important control strategy for the prevention of these diseases is the use of effective vaccines. Vaccination of cattle against bovine tuberculosis has particular application in countries which cannot afford the traditional “test and slaughter” control approach and in those which have wildlife reservoirs of M. bovis infection.
The M. bovis bacillus Calmette-Guérin (BCG) vaccine, an attenuated strain of M. bovis, has been widely used for control of human tuberculosis despite controversy over its protective efficacy (2). In cattle, BCG has been used in a series of trials, with various degrees of protection against M. bovis challenge (4, 5, 19). However, a major constraint in the use of attenuated mycobacterial vaccines such as BCG is that vaccination of humans or cattle interferes with detection of tuberculosis by means of the tuberculin skin test. The development of tests which can distinguish between infection with M. tuberculosis or M. bovis and vaccination with BCG could greatly assist in the diagnosis of early infection as well as enhance the use of tuberculosis vaccines on a wider scale.
The sensitivity of diagnostic tests could be enhanced by using alternative blood tests rather than conventional skin tests. Blood tests based on in vitro detection of gamma interferon (IFN-γ) released in response to mycobacterial antigens have been suggested as an improved method for detecting human (7) and bovine (25) tuberculosis. The whole-blood IFN-γ assay for cattle has been shown to be a robust and effective assay for measuring cell-mediated immune responses to M. bovis antigens in cattle (25). The specificity of the tests could be improved by using selected secreted mycobacterial proteins which have a restricted genetic distribution or markedly different levels of expression between BCG and virulent strains of the tuberculosis complex. ESAT-6, a recently identified low-molecular-weight secreted antigen, has a restricted species distribution, being found in M. tuberculosis and M. bovis but not in BCG (12). This antigen is an early and dominant T-cell target during tuberculosis in experimental animals (1, 3) and during M. bovis infection in cattle (20). MPB64 is restricted to the tuberculosis complex of mycobacterial species and can be found in M. tuberculosis, M. bovis, and some, but not all, substrains of BCG (13). This protein elicits delayed-type hypersensitivity reactions in sensitized guinea pigs (9) and lymphocyte proliferation responses in patients with human tuberculosis (23). MPB70, originally identified as a unique product of M. bovis (16), also occurs in M. tuberculosis and BCG but is expressed at a low level in some BCG strains. MPB59 is highly conserved between pathogenic and environmental mycobacterial species and is a dominant product in mycobacterial culture filtrates. MPB59 has been shown to induce delayed-type hypersensitivity reactions in sensitized guinea pigs (17).
The experimental model of tuberculosis established with cattle allows the study of immune responses following BCG vaccination and M. bovis infection in a natural host. Intratracheal challenge of cattle with a low dose of M. bovis has previously been found to result in the majority of nonvaccinated and a small number of BCG-vaccinated animals developing tuberculous lesions (4, 5). In this study, using a whole-blood IFN-γ assay, we evaluated recombinant mycobacterial antigens for their ability to distinguish between BCG-vaccinated and M. bovis-infected cattle.
MATERIALS AND METHODS
Animals.
Twenty-four Friesian cross 5- to 6-month-old calves were obtained from tuberculosis-free accredited herds from an area of New Zealand free of infection. The calves grazed on pasture in a high-security isolation unit. Some of the calves had transient reactions to avian purified protein derivative (PPD) in the IFN-γ test prior to vaccination. Twelve of the calves which were randomly selected were vaccinated subcutaneously in the left side of the neck with 5 × 105 CFU of BCG Pasteur 1173P2 and revaccinated in a similar manner 7 weeks later. A group of 12 nonvaccinated calves served as controls. Twelve weeks after the initial vaccination, blood samples were collected from vaccinated and nonvaccinated animals. Fifteen weeks after the initial vaccination, nine animals from each of the BCG-vaccinated and nonvaccinated groups were challenged intratracheally with a low dose (103 CFU) of virulent M. bovis. The three nonchallenged calves from each group were kept in a separate paddock within the high-security isolation unit. Sixteen weeks after challenge, blood samples were collected for the IFN-γ assay and a tuberculin skin test was carried out. Animals were skin tested by intradermal injection in the right side of the neck with 0.1 ml containing 0.1 mg of bovine PPD (PPDB; Ministry of Agriculture and Forestry, Central Animal Health Laboratory, Upper Hutt, New Zealand). The injection site was first clipped, and then the thickness of the skin fold was measured with calipers. The skin fold thickness before injection was compared with that 72 h later. A skin test response was considered positive when there was at least a 3-mm increase in skin fold thickness (26). Two to three weeks later, all animals were killed and examined for tuberculous lesions, and pulmonary lymph nodes were collected for mycobacterial culture. The methods of vaccination, M. bovis challenge, necropsy, and processing of tissues for bacteriology and histopathology have been described in detail previously (4).
Antigens.
(i) Recombinant MPB59, MPB64, and MPB70 were prepared as described previously (15). Briefly, the recombinant proteins were expressed in Escherichia coli by using the vector pRSET C (Invitrogen, Leek, The Netherlands) and purified by metal affinity column chromatography with Talon resin (Clontech, Cambridge, United Kingdom). (ii) Recombinant ESAT-6 was produced in E. coli XL1 blue by using a construct based on the vector pMCT6 and purified by metal affinity chromatography as described previously (11). (iii) PPDs used in the IFN-γ test were prepared from Mycobacterium avium (PPDA) and M. bovis (PPDB) and were obtained from CSL Limited (Melbourne, Australia). (iv) The nonmycobacterial antigens keyhole limpet hemocyanin and ovalbumin were obtained from Sigma Chemical Co. (St. Louis, Mo.).
IFN-γ assay.
The in vitro release of IFN-γ from whole-blood cultures was determined by a method described previously (4). Briefly, heparinized blood was dispensed in eight 1-ml aliquots and 70 μl of either phosphate-buffered saline, preservative-free PPDA, or PPDB (14-μg/ml final concentration); the four recombinant mycobacterial antigens separately (MPB59, MPB64, MPB70, and ESAT-6; 2-μg/ml final concentration); or a pool of these four antigens with each antigen at a final concentration of 2 μg/ml. Titration of these recombinant mycobacterial antigens at final concentrations of 2, 4, and 8 μg/ml in whole-blood cultures from M. bovis-infected cattle had previously shown that 2 μg/ml was the optimum concentration for obtaining IFN-γ responses. The blood cultures were incubated for 24 h at 37°C in a humidified atmosphere of 5% CO2 in air, after which the plasma supernatants were harvested from each well. The supernatants were assayed for IFN-γ with a sandwich enzyme-linked immunosorbent assay (ELISA) kit (CSL Limited). Positive and negative controls were included in all plates, and there was very little variation between plates. A titration of recombinant bovine IFN-γ showed a linear relationship between concentrations of IFN-γ and optical density (OD) units (450 nm). Samples in the IFN-γ ELISA were run in duplicate. IFN-γ responses to antigens were expressed as OD indices (ODI) and were calculated as follows: ODI = OD for the antigen sample/OD for the phosphate-buffered saline sample.
Statistical analyses.
The numbers of mycobacteria isolated were compared by analysis of variance. IFN-γ responses of different animal groups and with different antigens were analyzed by analysis of variance on loge-transformed data. Correlations between IFN-γ responses to PPDB and ESAT-6 and skin test responses were calculated by using Pearson’s correlation.
RESULTS
Protection against challenge with M. bovis.
The intratracheal inoculation of nonvaccinated cattle with a low dose of virulent M. bovis resulted in the development of macroscopic lesions in five of the nine animals. These lesions were observed in the pulmonary lymph nodes of the five animals and in the lungs of three of the animals. Lesions were usually numerous in affected lymph nodes and ranged from 1 to 10 mm in diameter, while lung lesions were found in low numbers (2 to 10 per affected lung) and were 2 to 3 mm in diameter. M. bovis was isolated from all of these lesions but not from any pulmonary nodes without lesions.
BCG vaccination induced little protection, and macroscopic lesions were observed in the pulmonary lymph nodes of three BCG-vaccinated animals which had been challenged with M. bovis and in the lungs of one of these animals (Table 1). The lesions were similar in appearance to those seen in the nonvaccinated animals. M. bovis was isolated from all of the lesions from the BCG-vaccinated animals as well as from the pulmonary lymph nodes of two animals from this group which had been challenged with M. bovis but had no macroscopic lesions. Microscopic tuberculous-like lesions were observed in a pulmonary lymph node of one of these two animals. No significant difference in the numbers of mycobacteria isolated from the lung and pulmonary lymph nodes of the nonvaccinated and BCG-vaccinated groups was observed. No mycobacteria were isolated from nonchallenged animals from the nonvaccinated and BCG-vaccinated groups.
TABLE 1.
Comparison of IFN-γ responses to PPDB and ESAT-6 with skin test responses to PPDB in cattle challenged with M. bovis 16 weeks previously
| Animala | Tuberculous lesionsb | IFN-γ response
(ODI)c
|
Skin test response (mm)d | |
|---|---|---|---|---|
| PPDB | ESAT-6 | |||
| Nonvaccinated | ||||
| Challenged, culture positive | ||||
| 1 | ++ | 100.0 | 100.0 | 17 |
| 2 | ++ | 80.6 | 55.9 | 20 |
| 3 | ++ | 80.4 | 56.7 | 24 |
| 4 | ++ | 64.1 | 35.2 | 16.5 |
| 5 | ++ | 54.7 | 13.1 | 24 |
| Challenged, culture negative | ||||
| 6 | − | 24.1 | 4.0 | 11 |
| 7 | − | 3.3 | 2.5 | 0 |
| 8 | − | 3.1 | 2.7 | 0 |
| 9 | 2.7 | 1.4 | 0 | |
| Nonchallenged | ||||
| 10 | − | 1.9 | 2.1 | 0 |
| 11 | − | 1.9 | 1.4 | 0 |
| 12 | − | 1.7 | 1.7 | 0 |
| BCG vaccinated | ||||
| Challenged, culture positive | ||||
| 13 | ++ | 113.6 | 30.9 | 19 |
| 14 | ++ | 95.1 | 28.6 | 20.5 |
| 15 | + | 80.6 | 22.5 | 13.5 |
| 16 | ++ | 62.5 | 52.7 | 14.5 |
| 17 | − | 46.0 | 17.3 | 7.5 |
| Challenged, culture negative | ||||
| 18 | − | 69.4 | 7.3 | 4 |
| 19 | − | 32.1 | 10.3 | 1.5 |
| 20 | − | 18.4 | 5.3 | 5.5 |
| 21 | − | 8.0 | 1.3 | 2 |
| Nonchallenged | ||||
| 22 | − | 31.8 | 7.0 | 8 |
| 23 | − | 12.7 | 3.0 | 8.5 |
| 24 | − | 9.9 | 2.8 | 11.5 |
Culture positive, M. bovis cultured from tissues at necropsy; culture negative, no M. bovis cultured.
++, macroscopic tuberculosis lesions; +, only microscopic tuberculous lesions; −, no tuberculous lesions.
Measured by ELISA.
Increase in skin thickness measured 72 h after intradermal injection of PPDB.
IFN-γ responses after BCG vaccination.
Levels of IFN-γ released from PPDB-stimulated blood cultures peaked at 4 weeks after the initial BCG vaccination (Fig. 1). Revaccination of calves with BCG resulted in a marked increase in the levels of IFN-γ released from stimulated cultures, and these high levels persisted for at least 8 weeks after revaccination. The testing of different recombinant mycobacterial antigens at 12 weeks after the initial BCG vaccination (5 weeks after revaccination) corresponded to a time point at which the highest levels of IFN-γ were released from PPDB-stimulated cultures.
FIG. 1.
Effect of BCG vaccination on the levels of IFN-γ released from cultures of whole blood of calves after stimulation with PPDB. ○, BCG-vaccinated calves; ⧫, nonvaccinated calves. Arrows indicate vaccinations with BCG. Data are means ± standard errors of the means (n = 12), in ODI units.
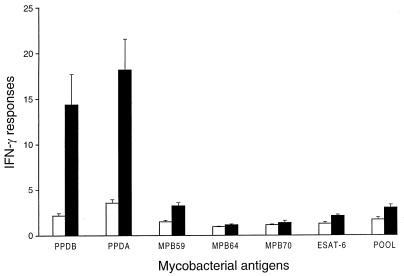
Blood cultures from BCG-vaccinated animals produced moderate IFN-γ responses to PPDA and PPDB but very weak responses to the recombinant mycobacterial antigens (Fig. 2). Although the mean IFN-γ responses to the recombinant antigens were all below an ODI of 3.5, the levels for the recombinant antigens except for MPB70 were significantly higher than those for the nonvaccinated animals (P < 0.05). However, IFN-γ responses to both PPDA and PPDB for the BCG-vaccinated animals were significantly stronger than those to the recombinant mycobacterial antigens (P < 0.05). The response to the pooled recombinant antigens was very similar to that to MPB59 alone.
FIG. 2.
Effect of BCG vaccination on the levels of IFN-γ released from cultures of whole blood of calves after stimulation with different mycobacterial antigens. Immune responses were measured at 12 weeks after the initial BCG vaccination. ■, BCG-vaccinated calves; □, nonvaccinated calves. Data are means ± standard errors of the means (n = 12), in ODI units.
To determine whether BCG-vaccinated cattle nonspecifically responded to antigens, blood cultures from six BCG-vaccinated and six nonvaccinated animals were set up 2 weeks later in wells containing 2 μg of keyhole limpet hemocyanin or ovalbumin per ml, and the levels of IFN-γ were measured. No significant differences in the IFN-γ responses to these two antigens between the BCG-vaccinated and nonvaccinated groups were observed.
IFN-γ responses after M. bovis infection.
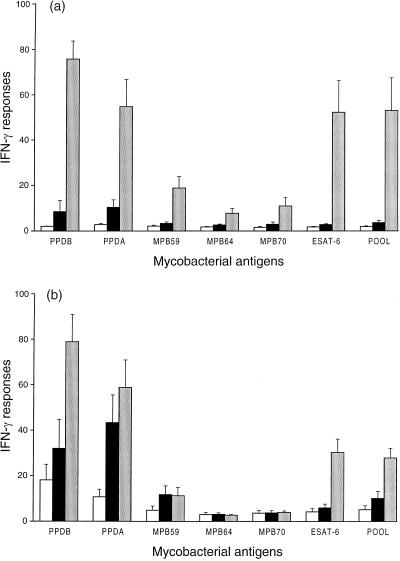
IFN-γ responses to recombinant mycobacterial antigens were measured at the final bleed (16 weeks after M. bovis challenge) to determine the relationship between these responses and active M. bovis infection. In the nonvaccinated group, IFN-γ responses to all antigen preparations for the five M. bovis-infected animals were all significantly stronger than for the seven M. bovis culture-negative animals from the same group (combined nonchallenged and M. bovis-challenged, culture-negative animals) (P < 0.05 [Fig. 3a]). However, the IFN-γ responses to PPDA, PPDB, ESAT-6, and the pooled recombinant antigens were all significantly stronger than the responses to MPB59, MPB64, and MPB70 (P < 0.05).
FIG. 3.
Effect of infection with M. bovis on levels of IFN-γ released from cultures of whole blood of nonvaccinated (a) and BCG-vaccinated (b) calves after stimulation with different mycobacterial antigens. Immune responses were measured at 16 weeks after challenge with M. bovis. □, nonchallenged calves (n = 3); ■, M. bovis-challenged calves which were culture negative (n = 4); ░⃞, M. bovis-challenged calves which were infected with M. bovis (n = 5). Data are means ± standard errors of the means, in ODI units.
In the BCG-vaccinated group, IFN-γ responses to PPDA, PPDB, ESAT-6, and pooled antigen preparations were all significantly stronger for the five M. bovis-infected animals than for the seven M. bovis culture-negative animals from the same group (P < 0.05 [Fig. 3b]). In contrast to the nonvaccinated group, IFN-γ responses to MPB59, MPB64, and MPB70 for the BCG-vaccinated group were not significantly different for the culture-positive and the culture-negative animals.
Following challenge with M. bovis, the IFN-γ response to the pooled antigen preparation was similar to that for the individual mycobacterial antigen with the greatest response, ESAT-6, for both the nonvaccinated and BCG-vaccinated animals.
A comparison of IFN-γ responses to PPDB and ESAT-6 with skin test responses to PPDB is shown in Table 1. Very strong positive correlations between the IFN-γ responses to PPDB and ESAT-6 and between the IFN-γ and the skin test responses to PPDB were observed in the nonvaccinated animals. The coefficients for correlation between IFN-γ responses to PPDB and ESAT-6, IFN-γ response to PPDB and skin test PPDB response, and IFN-γ response to ESAT-6 and skin test PPDB response were 0.924, 0.904, and 0.698, respectively, for the 12 nonvaccinated animals and 0.698, 0.719, and 0.690, respectively, for the 12 BCG-vaccinated animals. All of these correlations were positive and significant (P < 0.05).
Based on the Office International des Epizooties standard for a positive skin test (a ≥3-mm increase in skin thickness), positive responses were detected in five of five M. bovis culture-positive calves from both the nonvaccinated and BCG-vaccinated groups. For the culture-negative animals, positive responses were detected in one of four and two of four challenged calves from the nonvaccinated and BCG-vaccinated groups, respectively, and in zero of three and three of three nonchallenged calves from the nonvaccinated and BCG-vaccinated groups, respectively (Table 1).
DISCUSSION
Widespread use of tuberculosis vaccines in domestic animals depends on the development of diagnostic tests which can readily differentiate between vaccinated and tuberculosis-infected individuals. The present work used a whole-blood IFN-γ assay to detect immune responses to different mycobacterial antigens in BCG-vaccinated and M. bovis-infected cattle. Since vaccination of cattle with BCG induced little protection in this study, immune responses to selected mycobacterial antigens could be measured in BCG-vaccinated animals which subsequently developed an infection with M. bovis.
The majority of the vaccinated calves produced moderate responses to PPDB and PPDA in the IFN-γ assay but very weak responses to the recombinant mycobacterial antigens. Sixteen weeks after challenge with M. bovis, there were marked differences in IFN-γ responses to recombinant mycobacterial antigens in the nonvaccinated and BCG-vaccinated groups of animals that were culture positive. In the nonvaccinated group, an infection with M. bovis induced strong IFN-γ responses to ESAT-6 (mean, 52.2 ODI units) which were comparable to those for PPDB, while IFN-γ responses to MPB59, MPB64, and MPB70 were weak to moderate (means ranged from 7.8 to 18.8 ODI units). The responses to all of these mycobacterial antigens were significantly greater for the culture-positive animals than for culture-negative animals from the same group. In contrast, in the BCG-vaccinated group ESAT-6 was the only recombinant mycobacterial antigen for which the IFN-γ responses were significantly greater for the culture-positive animals than for the culture-negative animals. This interesting finding indicates that a reagent based on ESAT-6 may discriminate active disease from exposure to M. bovis and no infection.
The disparity in responses to the different mycobacterial antigens between culture-positive and culture-negative animals from the two groups could be related to the stage of the M. bovis infection. Pollock and Andersen (20) recently showed that cattle in the early stages of experimental infection were characterized by strong IFN-γ responses directed predominantly against the low-molecular-mass ESAT-6. Cattle in later stages of experimental infection (16 weeks postinfection) exhibited a broader recognition of antigens of various molecular masses. Cattle with field cases of bovine tuberculosis preferentially recognized low-molecular-mass antigens, which is characteristic of animals in the early stages of infection. There is a suggestion from the present study that the M. bovis infections in the BCG-vaccinated animals were at an early stage, since two of the M. bovis-infected animals which had been vaccinated had no macroscopic tuberculous lesions and one of these had microscopic tuberculous lesions. Secondly, in a previous study (4), the IFN-γ responses to PDDB in M. bovis-infected cattle which had previously been vaccinated with BCG peaked 2 months after corresponding responses observed in M. bovis-infected animals which were not vaccinated.
It is unlikely that the pulmonary isolates from M. bovis-infected animals were BCG, since no M. bovis isolates were recovered from pulmonary lymph nodes of BCG-vaccinated cattle which had not been challenged. Furthermore, in three vaccination-challenge cattle studies (4–6) in which M. bovis isolates were typed by molecular techniques, all of the pulmonary isolates corresponded to the challenge strain and not to BCG. No tuberculous lesions were seen in any of the studies in which BCG-vaccinated animals had not been challenged.
The reason that BCG induced no or little protection in the present trial is not clearly understood. However, some of the calves had transient reactions to PPDA in the IFN-γ assay prior to vaccination, suggesting exposure to environmental mycobacteria (data not shown). Prior exposure to environmental mycobacteria has been proposed as an explanation for the failure of BCG to induce protection in a number of human tuberculosis vaccine trials (2). In two earlier cattle trials where animals had no sensitization to PPDA prior to BCG vaccination, significantly fewer BCG-vaccinated animals than nonvaccinated animals developed tuberculous lesions following challenge (4, 5). In these two trials, in which BCG vaccination induced protection, the mean IFN-γ responses to PPDB following initial vaccination were stronger than in the present trial and in one other trial where there was no protection (6). However, for individual animals, there has been no correlation between the strength of the IFN-γ response to PPDB postvaccination and subsequent protection.
Although IFN-γ responses to the recombinant mycobacterial antigens in the present study were very weak following vaccination with BCG, the responses of the BCG-vaccinated animals were still significantly stronger than those of the nonvaccinated animals. The result for MPB59 could be expected since the gene for MPB59 is found in BCG strains and in one study 78% of human BCG vaccinees recognized MPB59 at the cellular level (23). In addition, T-cell responses to MPB59 in cattle could be stimulated by environmental mycobacteria as well as by M. bovis (14). In contrast, the genes for MPB64 and ESAT-6 are not present in BCG Pasteur (12, 18), and MPB70 is expressed only at low levels by this strain of BCG (10). The most likely explanation is that there is a low level of cross-reactivity between these antigens and those from BCG Pasteur, since it did not appear that the BCG-vaccinated animals nonspecifically responded to other antigens. There have been reports of cross-reactivity between mycobacterial antigens. MPT64 has been shown to have regions of some sequence similarity to the antigen 85 family members (24). A second possibility is that since the recombinant antigens were expressed in E. coli, very low concentrations of E. coli antigens or lipopolysaccharide may be present in the antigen preparations, and these may cross-react with antigens from BCG. However, it is important to note that the IFN-γ responses to the recombinant antigens in the BCG-vaccinated animals were very weak.
IFN-γ responses to the pooled recombinant mycobacterial antigen preparation were very similar to the strongest response to an individual mycobacterial antigen. Following BCG vaccination, the responses to the pooled antigens were similar to those to MPB59, and in the M. bovis-infected animals, they were similar to the responses to ESAT-6. Hence, the response to the pooled antigen preparation was not additive, and use of a selected pool of mycobacterial antigens may be helpful in the diagnosis of field cases of bovine tuberculosis in which all of the infected animals may not respond to a single mycobacterial antigen.
Based on the Office International des Epizooties standard, the skin test was effective in identifying all of the M. bovis culture-positive cattle; however, five of the seven BCG-vaccinated cattle which were culture negative also showed positive responses. In contrast, when a positive cutoff ODI of ≥11 was used for the ESAT-6 IFN-γ response, all of the M. bovis culture-positive animals (n = 10) were correctly identified and the responses of all of the culture-negative animals (n = 14) were below the positive cutoff. Larger numbers of naturally infected and BCG-vaccinated animals need to be tested to confirm these results.
In conclusion, the strong correlation between the IFN-γ responses to ESAT-6 and those to PPDB in M. bovis-infected and noninfected cattle confirmed previous findings (20, 21) that ESAT-6 would be a very useful reagent for specific diagnosis of bovine tuberculosis. IFN-γ responses to ESAT-6 were very weak in calves following vaccination with BCG, contrasting with consistently strong IFN-γ responses to ESAT-6 in M. bovis-infected animals for both nonvaccinated and BCG-vaccinated groups. These findings indicate that ESAT-6 should be a suitable antigen for use in diagnostic tests for differentiating between BCG-vaccinated and M. bovis-infected cattle.
ACKNOWLEDGMENTS
We thank Gary Yates and Carol Wilson for the bacteriology, Geoff de Lisle for pathological examinations, and Lilian Morrison for statistical analyses.
We are grateful to Ministry of Agriculture and Forestry Policy and the British Council for financial assistance.
REFERENCES
- 1.Andersen P, Andersen A B, Sørensen A L, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosisinfection in mice. J Immunol. 1995;154:3359–3372. [PubMed] [Google Scholar]
- 2.Bloom B R, Fine P E M. The BCG experience: implications for future vaccines against tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: American Society for Microbiology; 1994. pp. 531–557. [Google Scholar]
- 3.Brandt L, Oettinger T, Holm A, Andersen A B, Andersen P. Key epitopes on ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J Immunol. 1996;157:3527–3533. [PubMed] [Google Scholar]
- 4.Buddle B M, de Lisle G W, Pfeffer A, Aldwell F E. Immunological responses and protection against Mycobacterium bovisin calves vaccinated with a low dose of BCG. Vaccine. 1995;13:1123–1130. doi: 10.1016/0264-410x(94)00055-r. [DOI] [PubMed] [Google Scholar]
- 5.Buddle B M, Keen D, Thomson A, Jowett G, McCarthy A R, Heslop J, de Lisle G W, Stanford J L, Aldwell F E. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res Vet Sci. 1995;59:10–16. doi: 10.1016/0034-5288(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 6.Buddle B M, Aldwell F E, Wedlock D N. Vaccination of cattle and possums against bovine tuberculosis. In: Griffin F, de Lisle G, editors. Tuberculosis in wildlife and domestic animals, Otago Conference Series no. 3. Dunedin, New Zealand: University of Otago Press; 1995. pp. 111–115. [Google Scholar]
- 7.Converse P J, Jones S L, Astemborski J, Vlahov D, Graham N M H. Comparison of a tuberculin interferon-γ assay with the tuberculin skin test in high-risk adults: effect of human immunodeficiency virus infection. J Infect Dis. 1997;176:144–150. doi: 10.1086/514016. [DOI] [PubMed] [Google Scholar]
- 8.Daborn C J, Grange J M. HIV/AIDS and its implications for control of animal tuberculosis. Br Vet J. 1993;149:405–417. doi: 10.1016/S0007-1935(05)80107-1. [DOI] [PubMed] [Google Scholar]
- 9.Haga S, Yamaguchi R, Nagai S, Matsuo K, Yamazaki A, Nakamura R M. Delayed type hypersensitivity to a recombinant mycobacterial antigen, MPB64, in guinea pigs sensitized to Mycobacterium tuberculosis or Mycobacterium bovisBCG. J Leukoc Biol. 1995;57:221–225. doi: 10.1002/jlb.57.2.221. [DOI] [PubMed] [Google Scholar]
- 10.Harboe M, Nagai G. MPB70, a unique antigen of Mycobacterium bovisBCG. Am Rev Respir Dis. 1984;129:444–452. doi: 10.1164/arrd.1984.129.3.444. [DOI] [PubMed] [Google Scholar]
- 11.Harboe M, Malin A S, Dockrell H S, Wiker H G, Ulvund G, Holm A, Jørgensen M C, Andersen P. B-cell epitopes and quantification of the ESAT-6 protein of Mycobacterium tuberculosis. Infect Immun. 1998;66:717–723. doi: 10.1128/iai.66.2.717-723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harboe M, Oettinger T, Wiker H G, Rosenkrands I, Andersen P. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovisBCG. Infect Immun. 1996;64:16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Ulstrup J C, Jonassen T Ø, Melby K, Nagai S, Harboe M. Evidence for absence of the MPB64 gene in some substrains of Mycobacterium bovisBCG. Infect Immun. 1993;61:1730–1734. doi: 10.1128/iai.61.5.1730-1734.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lightbody K A, Girvin R M, Pollock D A, Mackie D P, Neill S D, Pollock J M. Recognition of a common mycobacterial T-cell epitope in MPB59 of Mycobacterium bovis. Immunology. 1998;93:314–322. doi: 10.1046/j.1365-2567.1998.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lightbody K A, Skuce R A, Neill S D, Pollock J M. Mycobacterial antigen-specific antibody responses in bovine tuberculosis: an ELISA with potential to confirm disease status. Vet Rec. 1998;142:295–300. doi: 10.1136/vr.142.12.295. [DOI] [PubMed] [Google Scholar]
- 16.Nagai S, Matsumoto J, Nagasuga T. Specific skin-reactive protein from culture filtrate of Mycobacterium bovisBCG. Infect Immun. 1981;31:1152–1160. doi: 10.1128/iai.31.3.1152-1160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagai S, Wiker H G, Harboe M, Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991;59:372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oettinger T, Andersen Å B. Cloning and B-cell-epitope mapping of MPT64 from Mycobacterium tuberculosisH37Rv. Infect Immun. 1994;62:2058–2064. doi: 10.1128/iai.62.5.2058-2064.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Reilly, L. M., and C. J. Daborn. 1995. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tubercle Lung Dis. 76(Suppl. 1):1–46. [DOI] [PubMed]
- 20.Pollock J M, Andersen P. Predominant recognition of the ESAT-6 protein in the first phase of infection with Mycobacterium bovisin cattle. Infect Immun. 1997;65:2587–2592. doi: 10.1128/iai.65.7.2587-2592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollock J M, Andersen P. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J Infect Dis. 1997;175:1251–1254. doi: 10.1086/593686. [DOI] [PubMed] [Google Scholar]
- 22.Raviglione M C, Snider D E, Kochi A. Global epidemiology of tuberculosis: morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 23.Roche P W, Triccas J A, Avery D T, Fifis T, Billman-Jacobe H, Britton W J. Differential T cell responses to mycobacteria-secreted proteins distinguish vaccination with Bacille Calmette-Guerin from infection with Mycobacterium tuberculosis. J Infect Dis. 1994;170:1326–1330. doi: 10.1093/infdis/170.5.1326. [DOI] [PubMed] [Google Scholar]
- 24.Wiker H G, Nagai S, Harboe M, Ljungqvist L. A family of cross-reacting proteins secreted by Mycobacterium tuberculosis. Scand J Immunol. 1992;36:307–319. doi: 10.1111/j.1365-3083.1992.tb03104.x. [DOI] [PubMed] [Google Scholar]
- 25.Wood P R, Corner L A, Rothel J S, Baldock C, Jones S L, Cousins D B, McCormic B S, Francis B R, Creeper J, Tweedie N E. Field comparisons of the interferon-gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust Vet J. 1991;68:286–290. doi: 10.1111/j.1751-0813.1991.tb03254.x. [DOI] [PubMed] [Google Scholar]
- 26.Zorawski C. Manual of standards for diagnostics tests and vaccines. Paris, France: Office International des Epizooties; 1992. pp. 287–296. [Google Scholar]