Abstract
The purpose of this study was to evaluate immune function through the assessment of lymphocyte subpopulations (total T cells, major histocompatibility complex [MHC] I- and II-restricted T cells, B cells, NK cells, MHC II-restricted T-cell-derived naive and memory cells, and several MHC I-restricted T-cell activation markers) and the measurement of cytokine gene expression (interleukin 2 [IL-2], IL-4, IL-6, IL-10, IL-12, gamma interferon [IFN-γ], and tumor necrosis factor alpha [TNF-α]) from peripheral blood lymphocytes. Subjects included two groups of patients meeting published case definitions for chronic fatigue syndrome (CFS)—a group of veterans who developed their illness following their return home from participating in the Gulf War and a group of nonveterans who developed the illness sporadically. Case control comparison groups were comprised of healthy Gulf War veterans and nonveterans, respectively. We found no significant difference for any of the immune variables in the nonveteran population. In contrast, veterans with CFS had significantly more total T cells and MHC II+ T cells and a significantly higher percentage of these lymphocyte subpopulations, as well as a significantly lower percentage of NK cells, than the respective controls. In addition, veterans with CFS had significantly higher levels of IL-2, IL-10, IFN-γ, and TNF-α than the controls. These data do not support the hypothesis of immune dysfunction in the genesis of CFS for sporadic cases of CFS but do suggest that service in the Persian Gulf is associated with an altered immune status in veterans who returned with severe fatiguing illness.
One explanation for the lassitude, malaise, and flu-like symptoms seen in chronic fatigue syndrome (CFS) is the existence of immune dysregulation with increased levels of cytokines. Supporting this hypothesis are a number of reports of reduced NK cell activity (2, 13), upregulation of immune activation markers (19, 36), and increases in levels of cytokines in serum (4, 21, 28). However, these results are far from uniform. In contrast, some researchers have found no evidence of immune dysfunction (29), while others have found support for the immune dysfunction hypothesis only in a subset of CFS patients (25) or only when functional tests are performed (40).
We have had the unique opportunity to evaluate further the immune dysfunction hypothesis in two groups of patients with CFS—a group of civilians who developed the illness sporadically and a group of veterans who developed the illness following their service in the Gulf War. We have assessed three hypotheses in exploring the immunological data obtained: first, that CFS patients would have evidence of immune dysregulation compared to controls and that this would be more marked in the civilians because of the increased frequency of sudden onset in this group (32); second, that more severely ill patients, those with a sudden onset of their illness, and/or those free of concurrent psychiatric illness would exhibit the most marked immune dysregulation; and third, that CFS patients in general and Gulf War veterans in particular would show a type 2 cytokine profile according to the hypothesis of Rook and Zumla (33).
MATERIALS AND METHODS
Sample and data.
The patients were 43 Gulf War veterans on the Department of Veterans Affairs’ (DVA) Gulf War Registry and 68 nonveterans—all of whom initially completed health questionnaires indicating the symptom profile of CFS. These patients came to East Orange or Newark, N.J., respectively, where they provided a 3-h history and received a physical examination, a blood test, and a diagnostic psychiatric interview (24) by medical and psychological personnel to rule out medical and psychiatric (10) causes of chronic fatigue. Every CFS patient studied here was found to meet the 1994 case definition (10) of CFS (for details of the intake protocol, see reference 32). Based on the self-reported percentage of decrease in activity and the number and severity of their CFS minor symptoms, they were rated on a six-category CFS severity scale. The most severely affected patients, those with severe CFS, met the 1988 case definition (14) with the following modifications: seven symptoms were required to fulfill criteria, but symptoms were counted only if their severity was rated as 3 or higher on symptom intensity scales from 0 to 5 in the month prior to intake. In addition, patients were classified according to mode of illness onset, with “sudden” being defined as the development of the illness in 1 to 2 days and “gradual” being defined as a more delayed onset.
The healthy unmedicated controls were 34 Gulf War veterans on the Gulf War Registry and 53 civilians. We used a health survey to identify healthy Gulf War veterans and then recruited them for our studies. Healthy nonveterans were self-selected based on their response to newspaper advertisements and flyers placed throughout neighboring communities. Healthy nonveterans who exercised more than once a week or who had an Axis I psychiatric disorder were excluded. These criteria were not applied to healthy Gulf War veterans.
Veteran recruitment was limited to patients in the computer database of the DVA New Jersey Health Care System and individuals with major complaints of fatigue who were in the Gulf War Registry and resided in 10 states east of the Mississippi River. Civilian recruitment was of people residing within a 100-mile radius of our center. Because of their distance from our center, veterans were admitted to the VA Medical Center for their studies; nonveterans came to the center for limited times only.
After written informed consent was obtained, subjects underwent venipuncture, and blood was collected in EDTA anticoagulated tubes that were coded to disguise the identity of the subject group. Peripheral blood lymphocytes (PBLs) were labeled within 6 h of collection with commercially available combinations of monoclonal antibody to the following cell surface markers: CD45-CD14, CD3-CD8, CD3-CD4, CD3-CD19, CD3-CD(16+56) (Simulset Reagents, Becton Dickinson [BDIS], San Jose, Calif.), CD8-CD38, CD8-HLA-DR, CD8-CD11b, CD8-CD28, CD4-CD45RO, and CD4-CD45RA (antibodies to CD11b from DAKO, Carpinteria, Calif.; all other antibodies from BDIS). After the samples had been fixed in 0.5 ml of 1% formalin (methanol free) and refrigerated overnight, all flow cytometric analyses were performed in the same laboratory with a FACscan cytometer (BDIS) equipped with a 15-mW air-cooled 488-nm argon ion laser and with standard techniques (9). Thus, the following cell populations were quantified for each group of subjects: total leukocyte (WBC) count; number (and percentage of total WBC) of lymphocytes; number (and percentage of total lymphocyte count) of CD3+ (total T cells), CD3+ CD4+ (major histocompatibility complex [MHC] II-restricted T cells), CD3+ CD8+ (MHC I-restricted T cells), CD3− CD19+ (B cells), and CD3− CD(16+56)+ (NK cells); percentage of class II-restricted T cells that were CD45RO+ and CD45RA+; and percentage of class I-restricted T cells that were CD28+, HLA-DR+, CD38+, and CD11b−.
PBLs, harvested from additional aliquots of blood, were homogenized in RNA-zol (Cine/Biotech, Friendswood, Tex.) at 50 mg per 0.2 ml/106 cells. The quantitative reverse transcriptase PCR (RT-PCR) cytokine assay was used as previously described (11, 38, 39). RNA samples were reverse transcribed with Superscript RT (Bethesda Research Labs, Rockville, Md.), and cytokine-specific primers were used to amplify the following cytokines (38): gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin 2 (IL-2), IL-4, IL-6, IL-10, and IL-12. Amplified PCR product was detected by Southern blot analysis (38, 39), and the resultant signal was quantified as the relative differences between samples with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) (38, 39). Subjects had no missing data for any of the cell surface markers or cytokines in the final analysis.
Analysis.
Our goal was to determine if immune alteration or dysregulation existed in CFS patients, relative to the healthy controls (comprised of both Gulf War veterans and civilians) and to compare veteran and civilian data when possible. A problem in prior reports of immunological abnormalities in CFS lies in their use of multiple, separate, univariate inferential tests, even in the cases in which multivariate analysis of variance (MANOVA) techniques are used. An additional problem lies in the relative inability of classical statistical methods to capture theoretical integrated immunological patterns thought to exist, such as type 1 versus type 2 cytokine profiles. These methods do not allow the simultaneous assessment of such patterns when one increases and the other decreases at the same time. Further, MANOVA or multivariate regression guards only against a type I error of overall group differences for all the immune indicators. However, no standard statistical method exists in these classical tests to determine appropriate procedures of protecting against type 1 errors when multiple contrasts are made (5).
Traditional repeated-measures analysis is not appropriate for hypothesis testing in this report, because the data showed marked heterogeneity of both variances and covariances (7, 22). To overcome these problems, we used mixed-effects, repeated-measures models which allowed the simultaneous assessment of group differences in the level of activation and differentiation markers and in the level of cytokines (6, 18, 42). This approach allowed us not only to explicitly estimate the extent of heterogeneity of variances and covariances of the multiple immunological measures in each model but also to test group differences in the multiple immunological measures as interaction terms (5, 41). With this format of analysis, we increased the efficiency and precision of our statistical comparisons and controlled for the risk of inflating the frequency of type I errors.
Differences in the percentages and actual numbers of cell surface markers were tested in CFS patients and the respective controls for Gulf War veterans and civilians and between these two samples simultaneously. Comparisons based on cytokines were performed only within the veteran and civilian sample groups because the assays were run separately for the veterans and civilians. We systematically controlled for potential confounding by the subject’s demographic characteristics, including age, race, sex, and Axis I diagnostic status in every model. Subsequently, we also evaluated CFS groups based on illness severity and mode of illness onset (i.e., sudden versus gradual); since these analyses were independent of the major statistical model described above, individual comparisons did not correct for the possibility of the inflated frequency of type 1 errors.
Several steps were taken to test our hypotheses about group differences in the immune status indicators. First, a general model was constructed to test a three-way interaction between CFS status (Yes = 1 and No = 0), veteran membership (Yes = 1 and No = 0), and the percentages of cell surface markers, while incorporating the main effects and interaction effects between those covariates and the percentages of cell surface markers. Special attention was paid to the testing of interaction effects because of the possibility that some immune variables in the patient groups would show increases while others showed decreases relative to controls or that differences from controls might occur preponderantly in only one of the CFS groups. Likewise, the effects of the covariates may have differed for the various immunological measures. For example, men may have lower levels than women of one cell surface marker but have higher levels than women of another. These specific differences must be accounted for in order to determine if a true difference exists between CFS patients and the controls. The model testing group differences in the percentages of cell surface markers was specified as follows:
percentij = b0 + b1(sex)i + b2(race)i + b3(age)i + b4 (Axis I)i + b5 (CFS)i + b6(veteran)i + b7(cell surface marker)ij + b8(CFS × veteran)i + b9(sex × cell surface marker)ij + b10(race × cell surface marker)ij + b11(age × cell surface marker)ij + b12(CFS × cell surface marker)ij + b13(veteran × cell surface marker)ij + b14(CFS × veteran × cell surface marker)ij + rij (i = 1,2, … , n, number of subjects; j = 1, 2, … , k, number of cell surface markers)
The same specifications were used for the model testing group differences in the numbers of cell surface markers, except that the response function was log transformed. The model testing group differences in cytokine levels was specified as follows:
log(cytokine)ij = b0 + b1(assay)i + b2(sex)i + b3(race)i + b4(age)i + b5(Axis I)i + b6(CFS)i + b7(cytokine)ij + b8(sex × cytokine)ij + b9(race × cytokine)ij + b10(age × cytokine)ij + b11(CFS × cytokine)ij + rij (i = 1,2, … , n, number of subjects; j = 1, 2, … , k, number of cytokines)
This approach differed from a traditional repeated-measures analysis in that the residual terms rij in the equations were not assumed to be homogeneous within subject i. Instead, error variances and covariances among subjects were fully estimated in every model by using the SAS mixed procedure (22) with an unconstrained error structure to reflect the interrelations among cell surface markers and among cytokines.
Next, we did a stepwise elimination of nonsignificant interaction terms between the covariates and the immune status indicators from the models specified above. A nonsignificant interaction term indicates that the group differences of interest were not explained by a given covariate. Thus, for example, the interaction terms of Axis I versus surface markers and cytokines did not show statistical significance, and so they were not included in the specified models to be evaluated.
Finally, we systematically evaluated higher-order interactions testing group differences of interest. A higher-order interaction was eliminated from a model if it failed to reach statistical significance (P < 0.05), and then the model with only lower-order terms was evaluated. The final models retained are reported in the next section. The numbers of cell surface markers and cytokine measurements were log transformed before analyses to achieve normality and reduce undue influences from outlying measurements.
RESULTS
Table 1 gives descriptive statistics about the sample. The veteran sample consists of predominantly white men, below age 50, with a high school or college education, while the civilian sample is predominantly white women of whom a higher percentage received postgraduate education than the veterans. For the healthy veterans, the chance of having an education higher than high school was three times that for the CFS veterans. The chance of having an Axis I diagnosis among the CFS veterans was over 2.5 times that among civilian CFS patients (odds ratio = 2.78, P < 0.05) and 12 times that of the healthy veterans (odds ratio = 12, P < 0.05). Sixteen percent of veteran patients were diagnosed in the most severe CFS category, while 87% of civilian patients were in that category (odds ratio = 0.11, P < 0.05). Sixteen percent of veteran patients reported a sudden onset of symptoms in comparison to 63% of civilians with CFS (odds ratio = 0.03, P < 0.05).
TABLE 1.
Frequency distributions of demographic and disease status variables
| Characteristic | No. of subjects (%)
|
||||
|---|---|---|---|---|---|
| Veterans
|
Civilian
|
Total | |||
| CFS | Control | CFS | Control | ||
| Total | 43 | 34 | 68 | 53 | 198 |
| Sex | |||||
| Male | 32 | 30 | 14 | 7 | 83 |
| Female | 11 | 4 | 54 | 46 | 115 |
| Race | |||||
| White | 33 | 29 | 68 | 52 | 182 |
| Other | 10 | 5 | 0 | 1 | 16 |
| Age | |||||
| 20–29 yr | 11 | 9 | 10 | 9 | 39 |
| 30–39 yr | 18 | 13 | 25 | 20 | 76 |
| 40–49 yr | 11 | 11 | 26 | 19 | 67 |
| 50 or above | 3 | 1 | 7 | 5 | 16 |
| Education level | |||||
| High school or below | 19 | 7 | 21 | 9 | 56 |
| College or equivalent | 21 | 19 | 33 | 31 | 104 |
| Graduate or equivalent | 3 | 8 | 14 | 13 | 38 |
| Marital status | |||||
| Divorced or separated | 16 | 2 | 11 | 8 | 37 |
| Married or living together | 15 | 17 | 34 | 28 | 94 |
| Single or widowed | 10 | 14 | 22 | 17 | 63 |
| Axis I diagnosis | |||||
| Yes | 31 | 6 | 33 | 0 | 70 |
| No | 12 | 28 | 35 | 53 | 128 |
| Onset of disease | |||||
| Gradual | 36 | 0 | 25 | 0 | 61 |
| Sudden | 7 | 0 | 43 | 0 | 50 |
| No. (%) with severe CFSa | 7 (16) | 59 (87) | |||
“Severe CFS” means 50% or greater reduction in activity and seven or more severe symptoms (14).
Eleven cell surface markers, the percentage and number of lymphocytes, WBC counts, and levels of seven cytokines were examined in the present study. We found no systematic group differences between patient groups and the respective controls for any of the following markers phenotypes: CD4+ CD45RO+, CD4+ CD45RA+, CD8+ CD28+, CD8+ HLA-DR+, CD8+ CD38+, or CD8+ CD11b−. Therefore, our report focuses on the group differences in the five parent cell surface markers and the seven cytokines.
Table 2 shows the means and standard errors of cell surface markers in percentages and numbers and of cytokine levels for the four comparison groups. No significant differences were found with mixed-effects models for any of these variables between the civilian CFS group and the controls. Subsequent analysis did not reveal an effect based on CFS illness severity, but differences from controls for several cell markers were seen following the stratification by mode of illness onset. Patients with a gradual onset (n = 25) showed a decrease in the percent total lymphocytes (mean = 26.6, standard error [SE] = 1.8, P = 0.022) and an increase in the numbers of total WBC (mean = 7,200, SE = 472.7, P = 0.026). However, these differences were not mutually independent, in that the decrease in the percent lymphocytes was a result of an increased number of WBC. Indeed, we found very similar numbers of lymphocytes in the gradual-onset group (mean = 1,846) and the control group (mean = 1,874). An increase in the percent activated T suppressor cells (CD8+ CD38+) (mean = 58.9, SE = 2.3, P = 0.023), compared to the control (mean = 51.1 and SE = 1.8 [Table 2]), was also found in the gradual onset group. Patients with a sudden onset of CFS (n = 18) showed a decreased percentage of CD8+ CD11b− cells (P = 0.056). No differences were found for cytokines after these stratifications. There were not enough veterans with a sudden onset of CFS (n = 7) to allow a similar stratified test.
TABLE 2.
Means and standard errors for cytokines and CD cell surface markers
| Cell type, phenotype, or cytokine | Gulf War veterans
|
Civilians
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nf | CFS
|
n | Control
|
n | CFS
|
n | Control
|
|||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |||||
| No. ofa: | ||||||||||||
| WBC | 42 | 6,564.29 | 262.54 | 33 | 6,233.33 | 253.67 | 43 | 6,516.28 | 284.89 | 39 | 6,064.1 | 213.08 |
| Lymphocytes | 42 | 2,119.76 | 103.81 | 33 | 1,918.33 | 96.03 | 43 | 1,826.63 | 75.93 | 39 | 1,874.36 | 68.69 |
| CD(16+56)+a | 42 | 261.10 | 28.72 | 33 | 290.70 | 26.13 | 43 | 180.74 | 13.69 | 39 | 196.08 | 15.11 |
| CD19+ | 42 | 248.07 | 19.63 | 33 | 262.18 | 28.33 | 43 | 256.86 | 18.37 | 39 | 252.46 | 16.23 |
| CD3+ | 42 | 1,613.62 | 78.65 | 33 | 1,373.58 | 72.47 | 43 | 1,385.86 | 63.84 | 39 | 1,425.95 | 57.89 |
| CD3+ CD4+ | 42 | 1,014.69 | 51.62 | 33 | 809.15 | 45.34 | 43 | 889.93 | 41.29 | 39 | 927.26 | 40.77 |
| CD3+ CD8+ | 42 | 567.62 | 34.95 | 33 | 515.33 | 42.20 | 43 | 458.37 | 28.99 | 39 | 476.85 | 32.11 |
| % Lymphocytesb | 42 | 32.67 | 1.22 | 33 | 31.73 | 1.55 | 43 | 29.51 | 1.48 | 39 | 31.31 | 0.97 |
| CD(16+56)+c | 42 | 12.24 | 0.94 | 33 | 15.52 | 1.12 | 43 | 10.19 | 0.73 | 39 | 10.62 | 0.76 |
| CD19+c | 42 | 11.55 | 0.67 | 33 | 13.03 | 1.02 | 43 | 14.23 | 0.80 | 39 | 13.38 | 0.66 |
| CD3+c | 42 | 76.36 | 0.93 | 33 | 71.85 | 1.23 | 43 | 75.53 | 1.21 | 39 | 75.87 | 0.97 |
| CD3+ CD4+c | 42 | 48.31 | 1.01 | 33 | 42.45 | 1.39 | 43 | 48.60 | 1.03 | 39 | 49.59 | 1.18 |
| CD3+ CD8+c | 42 | 26.62 | 1.11 | 33 | 26.73 | 1.39 | 43 | 24.81 | 0.97 | 39 | 25.72 | 1.20 |
| CD4+ CD45RO+d | 40 | 71.40 | 1.85 | 33 | 72.18 | 2.12 | 43 | 67.83 | 2.26 | 39 | 70.51 | 2.44 |
| CD4+ CD45RA+d | 40 | 42.80 | 1.73 | 33 | 40.76 | 2.18 | 40 | 46.23 | 1.70 | 39 | 43.18 | 1.79 |
| CD8+ CD28+d | 41 | 58.17 | 1.96 | 33 | 59.45 | 2.25 | 40 | 67.79 | 1.98 | 39 | 65.05 | 2.23 |
| CD8+ CD38+d | 41 | 51.56 | 2.35 | 33 | 53.12 | 2.74 | 40 | 58.08 | 1.92 | 38 | 51.10 | 1.77 |
| CD8+ HLA-DR+d | 41 | 20.90 | 1.69 | 33 | 20.73 | 2.23 | 40 | 19.85 | 2.14 | 39 | 22.82 | 2.18 |
| CD8+ CD11b+d | 40 | 56.20 | 2.49 | 32 | 53.44 | 2.95 | 40 | 69.58 | 2.24 | 39 | 61.66 | 3.64 |
| Cytokinesc | ||||||||||||
| IL-2 | 43 | 430.95 | 140.23 | 34 | 251.97 | 61.17 | 68 | 77.93 | 13.02 | 53 | 95.48 | 17.24 |
| IL-4 | 43 | 256.33 | 58.06 | 34 | 134.11 | 18.79 | 68 | 16.90 | 2.58 | 53 | 18.74 | 2.61 |
| IL-6 | 43 | 2,882.11 | 505.21 | 34 | 1,710.95 | 337.08 | 68 | 98.21 | 32.49 | 53 | 281.42 | 112.87 |
| IL-10 | 43 | 603.84 | 136.88 | 34 | 495.95 | 265.11 | 68 | 333.42 | 48.06 | 53 | 532.42 | 170.98 |
| IL-12 | 43 | 299.55 | 84.64 | 34 | 136.37 | 38.89 | 68 | 463.22 | 66.90 | 53 | 656.63 | 159.95 |
| TNF-α | 43 | 288.62 | 48.37 | 34 | 166.35 | 27.28 | 68 | 140.84 | 16.80 | 53 | 182.93 | 19.90 |
| IFN-γ | 43 | 1,002.06 | 163.52 | 34 | 632.74 | 146.11 | 68 | 736.37 | 113.41 | 53 | 986.20 | 246.56 |
Number of cells per cubic millimeter.
Percentage of WBC.
Percentage of lymphocytes.
Percentage of parent cells.
The units used are relative units indicating differences between samples obtained from quantifying the signal from a Southern blot of the PCR products with a PhosphorImager.
n, no. of subjects tested.
Turning to the Gulf War veterans, we systematically tested the three hypotheses stated in the introduction by utilizing mixed-effects models. The veterans with CFS were found to have a significantly lower percentage of NK cells (P = 0.011) and a significantly higher percentage of CD3+ cells (P = 0.007) and CD3+ CD4+ cells (P < 0.0003) than the healthy veteran controls (Table 3). These differences were tested simultaneously and confirmed by a significant three-way interaction (P = 0.0019) between CFS status, veteran status, and the five cell surface marker phenotypes (CD3− CD(16+56)+, CD3− CD19+, CD3+, CD3+ CD4+, and CD3+ CD8+) while controlling for sex, race, age, and Axis I diagnosis. When the results were averaged across veterans and civilians, subjects who were age 40 or older had significantly more CD3+ CD4+ cells (P = 0.006) and significantly fewer CD3+ CD8+ cells (P = 0.0006) than younger subjects. These significant age differences could have confounded our results had we not controlled for this variable in our analysis.
TABLE 3.
Linear contrasts of CD cell surface markers between Gulf War veteran and civilian groups from a mixed-effects model with three-way interaction between factors of veteran status (yes or no), CFS status (yes or no), and cell surface markersa
| Variable | Parameter estimatesb | t | P > t |
|---|---|---|---|
| Veteran with CFS versus veteran control | |||
| CD(16+56) | −3.32* | −2.57 | 0.011 |
| CD19 | −1.56 | −1.36 | 0.176 |
| CD3 | 4.35* | 2.74 | 0.007 |
| CD3 CD4 | 5.93* | 3.66 | 0.000 |
| CD3 CD8 | −0.42 | −0.26 | 0.797 |
| Veteran with CFS versus civilian with CFS | |||
| CD(16+56) | 2.22 | 1.84 | 0.068 |
| CD19 | −2.62* | −2.42 | 0.016 |
| CD3 | 0.65 | 0.44 | 0.662 |
| CD3 CD4 | 0.23 | 0.15 | 0.882 |
| CD3 CD8 | 1.21 | 0.79 | 0.428 |
Surface marker percentages were compared to determine the statistical values shown.
Parameter estimates are predicted differences in each of the cell surface markers between groups indicated by the subheadings.
∗, P < 0.05.
Veterans with CFS had significantly more CD3+ cells (P = 0.021) and CD3+ CD4+ cells (P = 0.002) than veteran controls, but no significant difference in the number of NK cells was found between the groups (Table 4). The veterans with CFS had significantly more NK cells, total T cells, CD3+ CD4+, and CD3+ CD8+ cells, but not more B cells, than both civilian groups. This general elevation in the numbers of cell surface markers in veteran patients was tested again and confirmed by a significant three-way interaction (P = 0.048) between CFS status, veteran status, and the five cell surface markers.
TABLE 4.
Linear contrasts of CD cell surface markers between Gulf War veteran groups and civilian groups from a mixed-effects model with three-way interaction between factors of veteran status (yes or no), CFS status (yes or no), and cell surfacer markersa
| Variable | Parameter estimateb | t | P > t |
|---|---|---|---|
| Veteran with CFS versus veteran control | |||
| CD(16+56) | −0.12 | −0.97 | 0.334 |
| CD19 | 0.06 | 0.44 | 0.661 |
| CD13 | 0.19* | 2.33 | 0.021 |
| CD3 CD4 | 0.27* | 3.21 | 0.002 |
| CD3 CD8 | 0.13 | 1.14 | 0.256 |
| Veteran with CFS versus civilian with CFS | |||
| CD(16+56) | 0.38* | 3.21 | 0.002 |
| CD19 | 0.00 | 0.02 | 0.981 |
| CD3 | 0.20* | 2.54 | 0.012 |
| CD3 CD4 | 0.20* | 2.41 | 0.17 |
| CD3 CD8 | 0.24* | 2.12 | 0.035 |
| Veterans with CFS versus civilian controls | |||
| CD(16+56) | 0.35* | 2.78 | 0.006 |
| CD19 | 0.04 | 0.32 | 0.747 |
| CD3 | 0.21* | 2.38 | 0.018 |
| CD3 CD4 | 0.19* | 2.12 | 0.036 |
| CD3 CD8 | 0.30* | 2.45 | 0.015 |
Surface marker counts were compared to determine the statistical values shown.
Parameter estimates are predicted differences in each of the cell surface markers between groups indicated by the subheadings. For activation markers, CFS veterans were found to have lower levels of several activation markers measured in percentages than CFS civilians: CD28 (−9.79 [P = 0.0009]), CD38 (−6.52 [P = 0.0325), and CD11bmn (−0.25 [P = 0.0168]). In addition, CFS veterans had lower levels of CD28 than the civilian controls (−7.01 [P = 0.0169]). ∗, P < 0.05.
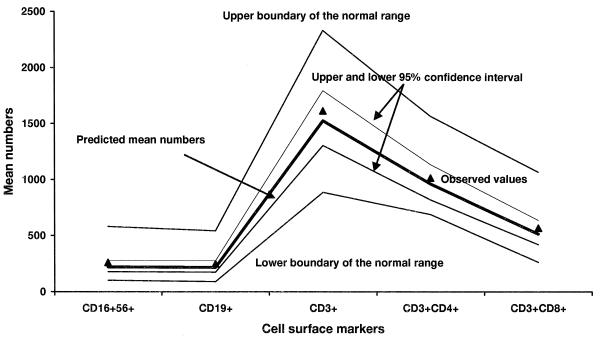
Figures 1 and 2 show the observed and predicted numbers of cell surface markers, confidence intervals for the predicted cell numbers, and the normative ranges of the five cell surface markers in the general population for the samples of veterans with CFS and of healthy civilians. Both the healthy civilian group and the CFS veteran group had cell number values in the normative range; the confidence intervals of all four subject groups studied here (only two are depicted in the figures) were within the boundaries of normative ranges. However, Fig. 1 and 2 show that the confidence intervals were broader in the healthy civilian group than those in the CFS veteran group, suggesting greater heterogeneity in the former. Not depicted are the broad confidence intervals for CFS civilians and the narrow confidence intervals for the veteran controls—suggesting that all civilians are more heterogeneous than Gulf War veterans in this regard.
FIG. 1.
Estimated and observed mean numbers of five cell surface markers in Gulf War veterans with CFS. Lines are drawn to connect the measurement points simply to help the reader see the pattern of the immunological variables. The predicted mean numbers of cell surface markers were derived from a three-way interaction model between CFS status (yes or no), veteran status (yes or no), and cell surface markers. The model was analyzed by a univariate, mixed-effects repeated-measures analysis that allowed more efficient, simultaneous, cross-group comparisons between sick and healthy veterans and sick and healthy civilians than regular multivariate analysis. All predicted values fell within the laboratory’s normative range. Note that the 95% confidence interval is narrower for all five markers relative to that of civilian controls.
FIG. 2.
Estimated and observed mean numbers of five cell surface markers in healthy veterans.
The levels of seven cytokines were examined in the veteran sample (Table 5). Consistent with the cell surface marker data, veterans with CFS showed a general tendency toward upregulation relative to the controls across all cytokines in this study, indicated by the nonsignificant two-way interaction between CFS status and cytokines (P = 0.642) and a highly significant main effect of CFS (P = 0.006). Linear contrasts indicated that the group differences were statistically significant for IL-2 (P = 0.021), IL-10 (P = 0.01), IFN-γ (P = 0.014), and TNF-α (P = 0.002). Since the veterans with CFS had uniformly higher levels of all the cytokines (Table 2) than the controls, the two-way interaction of CFS status versus cytokines was not expected to be significant.
TABLE 5.
Linear contrasts of cytokine levels between Gulf War veterans with CFS and healthy controls from a mixed-effects model with interaction terms between group membership and cytokine measurementsa
| Variable | Parameter estimateb | t | P > t |
|---|---|---|---|
| IL-2 | 0.504* | 2.34 | 0.021 |
| IL-4 | 0.428 | 1.61 | 0.111 |
| IL-6 | 0.598 | 1.73 | 0.086 |
| IL-10 | 0.754* | 2.61 | 0.010 |
| IL-12 | 0.362 | 0.92 | 0.359 |
| IFN-γ | 0.539* | 2.51 | 0.014 |
| TFN-α | 0.659* | 3.07 | 0.002 |
Cytokine levels (in units) were compared to determine the statistical values shown.
Parameter estimates are predicted differences in each of the cytokines between groups. ∗, P < 0.05.
We next evaluated the hypothesis of a shift from type I to type II cytokine responses in the Gulf War veterans, especially in those with CFS (33). This shift was not observed. Instead, the data indicated a type I response with significant upregulation in IL-2 and IFN-γ and not IL-4 and IL-6 in CFS veterans. In fact, instead of finding negative correlations between IFN-γ and IL-4 or IL-6, we found significant positive correlations. Similar positive correlations were found between IL-2, another type 1 cytokine, and IL-4 and IL-6 in both the veteran and civilian samples (Table 6). Significant increases in IL-10 were also detected; IL-10 elevations are characteristic of either a type 1 or type 2 response, at least partly because IL-12 induces IL-10 and IFN-γ production (26, 31).
TABLE 6.
Correlations among cytokines and T-cell and T-helper-cell surface markersa
| Surface marker or cytokine | Correlation to:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % CD3 | %CD3 CD4 | No. of CD3 | No. of CD3 CD4 | IL-2 | IL-4 | IL-6 | IL-10 | IL-12 | IFN-γ | |
| Veterans | ||||||||||
| % CD3 | ||||||||||
| % CD3 CD4 | 0.33* | |||||||||
| No. of CD3 | 0.20 | −0.02 | ||||||||
| No. of CD3 CD4 | 0.10 | 0.36* | 0.89* | |||||||
| IL-2 | 0.01 | 0.18 | −0.19 | −0.10 | ||||||
| IL-4 | −0.01 | 0.34* | −0.05 | 0.11 | 0.51* | |||||
| IL-6 | 0.05 | 0.06 | 0.08 | 0.09 | 0.37* | 0.49* | ||||
| IL-10 | −0.01 | 0.23* | −0.09 | 0.03 | 0.55* | 0.33* | 0.48* | |||
| IL-12 | −0.07 | 0.06 | −0.08 | 0.04 | 0.14 | −0.10 | 0.06 | 0.35* | ||
| IFN-γ | 0.17 | 0.11 | 0.30* | 0.30* | 0.33* | 0.41 | 0.53* | 0.51* | 0.15 | |
| TNF-α | 0.05 | 0.27* | −0.15 | −0.03 | 0.75* | 0.70* | 0.62* | 0.61* | 0.11 | 0.41* |
| Civilians | ||||||||||
| % CD3 | ||||||||||
| % CD3 CD4 | 0.47* | |||||||||
| No. of CD3 | 0.44* | 0.21* | ||||||||
| No. of CD3 CD4 | 0.27* | 0.45* | 0.78* | |||||||
| IL-2 | 0.04 | −0.02 | −0.00 | −0.08 | ||||||
| IL-4 | 0.06 | 0.09 | −0.07 | −0.04 | 0.32* | |||||
| IL-6 | 0.00 | 0.05 | 0.00 | 0.01 | 0.43* | 0.16 | ||||
| IL-10 | 0.08 | −0.04 | 0.01 | −0.09 | 0.60* | 0.29* | 0.45* | |||
| IL-12 | −0.00 | −0.05 | 0.01 | −0.04 | 0.46* | 0.43* | 0.25* | 0.38* | ||
| IFN-γ | 0.26 | −0.02 | 0.24* | 0.12 | 0.33* | −0.01 | 0.22* | 0.19* | 0.09 | |
| TNF-α | 0.08 | −0.01* | 0.04 | 0.02 | 0.13 | 0.18* | 0.01 | 0.25* | 0.01 | 0.42* |
∗, P < 0.05.
DISCUSSION
This paper reports important negative and positive findings. The important negative finding is that we cannot confirm immune dysregulation in this carefully controlled study of nonveterans who developed CFS sporadically. When we began collecting these immunological samples, we had several a priori hypotheses. One was that we would find immune dysregulation in the entire group of CFS patients. A second related to our finding of decreased cognitive function and brain magnetic resonance imaging abnormalities in the group of CFS patients devoid of DSM-III-R psychopathology (8, 20). Those findings led us to hypothesize that we would find immunological abnormalities in this subgroup—especially in the most symptomatic of these patients. Again, those hypotheses were not supported. Even after stratifying the data for presence or absence of Axis I psychiatric disorder, there were no significant differences between patients and controls with regard to any of the immunological variables under study.
Because a Centers for Disease Control and Prevention (CDC) group also found negative results for their entire CFS patient pool but then did find some differences following stratification based on illness severity and mode of illness onset (25), we did a similar analysis. The CDC group reported differences based on severity, which we did not confirm. Concerning the differences based on mode of illness onset, we obtained results different from those of Mawle et al. The one similarity was the results for the phenotype CD8+ CD11b−. However, we found this cell population to be altered in the gradual-onset group, while Mawle et al. reported finding this in the sudden-onset group. These differences across studies show the problems associated with using statistical methods that do not guard against type 1 errors. In contrast to our use of the mixed-effects model detailed in Materials and Methods, this analysis (both for our data and for the CDC data) did not adjust the significance level for multiple comparisons. Since our results were not the same as those noted by the CDC group, our inference is that these results are probably statistical artifacts.
The question arises as to why we were unable to confirm immune dysregulation in the CFS patients studied. However, the literature makes it clear that inconsistency of results from laboratory to laboratory is the rule rather than the exception. Thus, some but not all laboratories find decreases in NK cell numbers (13, 29), T-cell populations (23, 34), and activation markers (19, 29, 40). Concerning cytokines, Strober’s 1994 conclusion seems applicable (37): “Studies of circulating levels of various lymphokines and cytokines in CFS have not yielded evidence of a clear-cut and reproducible abnormality of lymphokine/cytokine secretion in CFS.”
Cannon et al. have indicated that assay methods and type of tissue are crucial in evaluating cytokines (3). This is the first study of cytokine gene expression in CFS. Furthermore, we measured cytokine RNA from PBLs without in vitro restimulation in order to avoid artifacts resulting from mitogen activation of lymphocytes. Using RT-PCR, we are unable to confirm the prior reports of elevated IL-6 levels associated with CFS (1, 12); however, elevated IL-10 levels, seen in our veterans with CFS, has also been reported in a group of patients with a clinical picture resembling severe, chronic infectious mononucleosis (16).
Besides the difference in assay methodology, there is another major difference between our study and those of others. In our work, we excluded healthy controls who exercised regularly. It is known that some immune parameters are sensitive to state of fitness. For instance, NK cell numbers increase with fitness (30). Thus, reductions in NK cell numbers may reflect the inactivity inherent in CFS rather than the underlying illness itself. A rather different explanation is that our group is simply studying the wrong immunological markers. Consistent with this interpretation are unpublished results of a study on transforming growth factor B in serum in collaboration with Chun Chao, in which we found a small but significant increase in 20 subjects in the nonveteran CFS group compared to 19 of the sedentary controls (CFS = 187 ± 13 pg/ml; control = 141 ± 11 pg/ml [P < 0.01]). Thus, it is possible that the evaluation of other cytokines or the assessment of the function of stimulated lymphocytes might provide more consistent evidence of immune dysfunction in CFS patients than tests of lymphocytes in the static condition.
However, the argument that we have been studying the wrong immunological variables is unlikely given the results of our survey of immune parameters in Gulf War veterans with CFS. In contrast to the data for civilians with CFS, Gulf War veterans with CFS show definite evidence of immune status alteration in both lymphocyte subpopulations and in their cytokine message. Although we had hypothesized that any immunological activation found would occur preponderantly in specific subsets of CFS patients—those without concurrent major psychopathology—the data did not support this. The immunological upregulation, reflected by increased numbers of T cells and increases in PBL cytokine levels, was seen regardless of patient grouping.
A second reason for the study was to test the hypothesis of Rook and Zumla (33) that CFS patients in general and Gulf War veterans with CFS in particular would show a type 2 pattern of cytokine activation. Our data did not support that hypothesis for either patient group. In fact, the pattern of cytokine activation exhibited by the veterans with CFS was type 1, or inflammatory—with increases in IL-2 and IFN-γ possibly related to the concurrent increases in CD3+ CD4+-T-cell numbers. This type 1 shift was, if anything, suppressed, as it is known that IL-10, which was also present at elevated levels in the veterans with CFS, can suppress IFN-γ synthesis (27). Since both these cytokines are induced by IL-12 (26, 31), IL-10 is commonly associated with type 1 immune responses.
When we began these studies, we assumed that if we found any evidence of immune dysfunction in either patient group, it would be in the civilians. We based that line of thinking on the published data suggesting that sporadic CFS often involved a postinfectious process (17), while severe fatigue in Gulf War veterans usually did not have an infectious or sudden type of onset. In fact, our own data supported this belief, in that we found that civilians with CFS reported a significantly higher rate of sudden, rather than gradual, illness onset than Gulf War veterans (Table 1). However, we found evidence of altered immune status in Gulf War veterans with CFS and not in the civilian patients.
How do we interpret this result? There are two quite different answers to this question. One possibility is that something specific to service in the Gulf War altered the normal control of the immunological system in veterans with CFS. We have been careful up to this point not to use words such as abnormal or dysfunctional in describing these changes, because the standardized laboratory cell surface marker data for the veterans were within the normative range. However, the variability of each measurement for both sick and healthy veterans is substantially less than that exhibited by nonveterans (Fig. 1 and 2). This suggests that the veterans are immunologically more homogeneous than the nonveterans. As Rook and Zumla have hypothesized (33), a reduction in immunological heterogeneity may result from multiple vaccinations received by deployed troops (33), an interpretation supported by a recent study of children vaccinated with Mycobacterium bovis BCG (35). Finding such a degree of homogeneity would suggest that it is inappropriate to compare Gulf War veterans’ immunological data with those of a normative nonveteran group. If this view were taken, it would indicate that Gulf War veterans with CFS indeed do show evidence of immune dysfunction relative to the respective Gulf War veteran controls.
An alternative explanation is that the putative immune abnormalities reflect differences between patients and controls that are independent of illness. An example would focus again on NK cell numbers. Improved fitness can increase this lymphocyte population (30), while partially disturbed sleep can reduce it (15). Decreased fitness due to inactivity and disturbed sleep is common in CFS. However, there are some immunological data that contradict this explanation. Poorly conditioned subjects are reported to have no change in T-cell numbers (30), and individuals with disturbed sleep show reduced IL-2 production (15). In contrast, total T-cell counts and IL-2 levels were higher in Gulf War veterans with CFS than in Gulf War veteran controls.
We have presented two very different possible explanations for our finding of differences in immunological profiles between Gulf War veterans with CFS and healthy Gulf War veterans. Obviously the determination of which answer is correct will require further research. However, we believe that the data favor the first possibility—that something about serving in the Gulf War altered the immune status of that group of veterans who also have CFS. We conclude this because the veteran immunological data are relatively homogeneous but nonetheless show a clear difference between sick and healthy subjects and because veterans with CFS are less severely ill than civilians (32) and thus should have fewer problems with inactivity and poor sleep than nonveterans with CFS, whose immunological data are similar to those for carefully selected controls.
ACKNOWLEDGMENT
This study was supported by DVA medical research funds and by NIH Center grant AI-32247.
REFERENCES
- 1.Buchwald D, Wener M H, Pearlman T, Kith P. Markers of inflammation and immune activation in chronic fatigue and chronic fatigue syndrome. J Rheumatol. 1997;24:372–376. [PubMed] [Google Scholar]
- 2.Caliguri M, Murray C, Buchwald D, Levine H, Cheney P, Peterson D, Komaroff A L, Ritz J. Phenotypic and functional deficiency of natural killer cells in patients with chronic fatigue syndrome. J Immunol. 1987;139:3306–3313. [PubMed] [Google Scholar]
- 3.Cannon J G, Nerad J L, Poutsiaka D D, Dinarello C A. Measuring circulating cytokines. J Appl Physiol. 1993;75:1897–1902. doi: 10.1152/jappl.1993.75.4.1897. [DOI] [PubMed] [Google Scholar]
- 4.Chao C C, Janoff E N, Hu S, Thomas K, Gallagher M, Tsang M, Peterson P K. Altered cytokine release in peripheral blood monocyte cell cultures from patients with the chronic fatigue syndrome. Cytokine. 1991;3:292–298. doi: 10.1016/1043-4666(91)90497-2. [DOI] [PubMed] [Google Scholar]
- 5.Cliff N. Analyzing multivariate data. New York, N.Y: Harcourt Brace Jovanovich; 1987. [Google Scholar]
- 6.Cnaan A, Laird N M, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16:2349–2380. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Crowder M J, Hand D J. Analysis of repeated measures. London, England: Chapman and Hall; 1990. [Google Scholar]
- 8.DeLuca J, Johnson S K, Ellis S P, Natelson B H. Cognitive functioning is impaired in chronic fatigue syndrome patients devoid of psychiatric disease. J Neurol Neurosurg Psychiatry. 1997;62:151–155. doi: 10.1136/jnnp.62.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denny T, Yogev R, Gelman R, Skuza C, Oleske J, Chadwick E, Cheng S, Connor E. Lymphocyte subsets in healthy children during the first 5 years of life. JAMA. 1992;267:1484–1488. [PubMed] [Google Scholar]
- 10.Fukuda K, Straus S E, Hickie I, Sharpe M C, Komaroff A, Schluederberg A, Jones J F, Lloyd A R, Wessely S, Gantz N G, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 11.Gause W C, Adamovivz J. The use of the PCR to quantitate gene expression. PCR Methods Appl. 1994;3:S123–S135. doi: 10.1101/gr.3.6.s123. [DOI] [PubMed] [Google Scholar]
- 12.Gupta S, Aggarwal S, See D, Starr A. Cytokine production by adherent and non-adherent mononuclear cells in chronic fatigue syndrome. J Psychiatr Res. 1997;31:149–156. doi: 10.1016/s0022-3956(96)00063-5. [DOI] [PubMed] [Google Scholar]
- 13.Gupta S, Vayuvegula B. A comprehensive immunological analysis in chronic fatigue syndrome. Scand J Immunol. 1991;33:319–327. doi: 10.1111/j.1365-3083.1991.tb01777.x. [DOI] [PubMed] [Google Scholar]
- 14.Holmes G P, Kaplan J E, Gantz N M, Komaroff A L, Schonberger L B, Straus S E, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med. 1988;108:387–389. doi: 10.7326/0003-4819-108-3-387. [DOI] [PubMed] [Google Scholar]
- 15.Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin J C. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996;10:643–653. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- 16.Kanegane H, Wakiguchi H, Kanegane C, Kurashige T, Tosato G. Viral interleukin-10 in chronic active Epstein-Barr virus infection. J Infect Dis. 1997;176:254–257. doi: 10.1086/517260. [DOI] [PubMed] [Google Scholar]
- 17.Komaroff A L. Chronic fatigue syndromes: relationship to chronic viral infections. J Virol Methods. 1988;21:3–10. doi: 10.1016/0166-0934(88)90047-x. [DOI] [PubMed] [Google Scholar]
- 18.Laird N M, Ware J H. Random effects models for longitudinal data: an overview of recent results. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 19.Landay A L, Jessop C, Lennette E T, Levy J A. Chronic fatigue syndrome: clinical condition associated with immune activation. Lancet. 1991;338:707–712. doi: 10.1016/0140-6736(91)91440-6. [DOI] [PubMed] [Google Scholar]
- 20.Lange G, DeLuca J, Lee H J, Maldjian J A, Natelson B H. Cerebral abnormalities in chronic fatigue syndrome. Abst Soc Neurosci. 1997;23:561. [Google Scholar]
- 21.Linde A, Andersson B, Svenson S B, Ahrne H, Carlsson M, Forsberg P, Hugo H, Karstorp A, Lenkei R, Lindwall A, Loftenius A, Säll C, Andersson J. Serum levels of lymphokines and soluble cellular receptors in primary Epstein-Barr virus infection and in patients with chronic fatigue syndrome. J Infect Dis. 1992;165:994–1000. doi: 10.1093/infdis/165.6.994. [DOI] [PubMed] [Google Scholar]
- 22.Little R C, Milliken G A, Stroup W W, Wolfinger R D. SAS system for mixed models. Cary, N.C: SAS Institute Inc.; 1996. [Google Scholar]
- 23.Lloyd A R, Wakefield D, Boughton C R, Dwyer J M. Immunological abnormalities in the chronic fatigue syndrome. Med J Aust. 1989;151:122–124. doi: 10.5694/j.1326-5377.1989.tb139594.x. [DOI] [PubMed] [Google Scholar]
- 24.Marcus S, Robins L N, Bucholz K. Quick diagnostic interview schedule 3R version 1. St. Louis, Mo: Washington University School of Medicine; 1990. [Google Scholar]
- 25.Mawle A C, Nisenbaum R, Dobbins J G, Gary H E, Jr, Stewart J A, Reyes M, Steele L, Schmid D S, Reeves W C. Immune responses associated with chronic fatigue syndrome: a case-control study. J Infect Dis. 1997;175:136–141. doi: 10.1093/infdis/175.1.136. [DOI] [PubMed] [Google Scholar]
- 26.Morris S C, Madden K B, Adamovicz J J, Gause W C, Hubbard B R, Gately M K, Finkelman F D. Effects of IL-12 on in vivo cytokine gene expression and Ig isotype selection. J Immunol. 1994;152:1047–1056. [PubMed] [Google Scholar]
- 27.O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 28.Patarca, R., N. G. Klimas, S. Lugtendorf, M. Antoni, and M. A. Fletcher. 1994. Dysregulated expression of tumor necrosis in chronic fatigue syndrome: interrelations with cellular sources and patterns of soluble immune mediator expression. Clin. Infect. Dis. 18(Suppl. 1):S147–S153. [DOI] [PubMed]
- 29.Peakman M, Deale A, Field R, Mahalingam M, Wessely S. Clinical improvement in chronic fatigue syndrome is not associated with lymphocyte subsets of function or activation. Clin Immunol Immunopathol. 1997;82:83–91. doi: 10.1006/clin.1996.4284. [DOI] [PubMed] [Google Scholar]
- 30.Pedersen B K. Influence of physical activity on the cellular immune system: mechanisms of action. Int J Sports Med. 1991;12:S23–S29. doi: 10.1055/s-2007-1024746. [DOI] [PubMed] [Google Scholar]
- 31.Peng X, Kasran A, Ceuppens J L. Interleukin 12 and B7/CD28 interaction synergistically upregulate interleukin 10 production by human T cells. Cytokine. 1997;9:639–649. doi: 10.1006/cyto.1997.0193. [DOI] [PubMed] [Google Scholar]
- 32.Pollet, C., B. H. Natelson, G. Lange, L. Tiersky, J. DeLuca, T. Policastro, P. Desai, J. E. Ottenweller, L. Korn, N. Fiedler, and H. Kipen. Medical evaluation of Persian Gulf veterans with fatigue and/or chemical sensitivity. J. Med., in press. [PubMed]
- 33.Rook G A W, Zumla A. Gulf War syndrome: is it due to a systemic shift in cytokine balance towards a Th2 profile? Lancet. 1997;349:1831–1833. doi: 10.1016/S0140-6736(97)01164-1. [DOI] [PubMed] [Google Scholar]
- 34.Sánchez-Franco F, Fernández L, Fernández G, Cacicedo L. Thyroid hormone action on ACTH secretion. Horm Metab Res. 1989;21:550–552. doi: 10.1055/s-2007-1009285. [DOI] [PubMed] [Google Scholar]
- 35.Shirakawa T, Enomoto T, Shimazu S, Hopkin J M. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275:77–79. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 36.Straus S E, Fritz S, Dale J K, Gould B, Strober W. Lymphocyte phenotype and function in the chronic fatigue syndrome. J Clin Immunol. 1993;13:30–40. doi: 10.1007/BF00920633. [DOI] [PubMed] [Google Scholar]
- 37.Strober W. Immunological function in chronic fatigue syndrome. In: Straus S, editor. Chronic fatigue syndrome. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 207–237. [Google Scholar]
- 38.Svetic A, Finkelman F D, Jian Y C, Dieffenbach C W, Scott D E, McCarthy K F, Steinberg A D, Gause W C. Cytokine gene expression after in vivo primary immunization with goat antibody to mouse IgD antibody. J Immunol. 1991;147:2391–2397. [PubMed] [Google Scholar]
- 39.Svetic A, Madden K B, Zhou X D, Lu P, Katona I M, Finkelman F D, Urban J F, Gause W C. A primary helminthic infection rapidly induces a gut-associated elevation of Th2-associated cytokines and IL-8. J Immunol. 1993;150:3434–3441. [PubMed] [Google Scholar]
- 40.Swanink C M A, Vercoulen J H M M, Galama J M D, Roos M T L, Meyaard L, Van der Ven-Jongekrijg J, De Nijs R, Bleijenberg G, Fennis J F M, Miedema F, Van der Meer J W M. Lymphocyte subsets, apoptosis, and cytokines in patients with chronic fatigue syndrome. J Infect Dis. 1996;173:460–463. doi: 10.1093/infdis/173.2.460. [DOI] [PubMed] [Google Scholar]
- 41.Thomas D R. Univariate repeated measures techniques applied to multivariate data. Psychometrika. 1993;48:451–464. [Google Scholar]
- 42.Timm N H, Mieczkowski T A. Univariate and multivariate general linear models: theory and applications using SAS software. Cary, N.C: SAS Institute Inc.; 1997. [Google Scholar]