Abstract
MXenes encompass attractive properties such as a large surface area, unique chemical structures, stability, elastic mechanical strength, excellent electrical conductivity, hydrophilicity, and ease of surface functionalization/modifications, which make them one of the broadly explored two-dimensional materials in the world. MXene-based micro- and nanocomposites/systems with special optical, mechanical, electronic, and excellent targeting/selectivity features have been explored for cancer nanotheranostics. These materials exhibit great diagnostic and therapeutic potential and offer opportunities for cancer photoacoustic imaging along with photodynamic and photothermal therapy. They can be applied to targeted anticancer drug delivery while being deployed for the imaging/diagnosis of tumors/cancers and malignancies. MXene-based systems functionalized with suitable biocompatible or bioactive agents have suitable cellular uptake features with transferring potential from vascular endothelial cells and specific localization, high stability, and auto-fluorescence benefits at different emission–excitation wavelengths, permitting post-transport examination and tracking. The surface engineering of MXenes can improve their biocompatibility, targeting, bioavailability, and biodegradability along with their optical, mechanical, and electrochemical features to develop multifunctional systems with cancer theranostic applications. However, challenges still persist in terms of their environmentally benign fabrication, up-scalability, functionality improvement, optimization conditions, surface functionalization, biocompatibility, biodegradability, clinical translational studies, and pharmacokinetics. This manuscript delineates the recent advancements, opportunities, and important challenges pertaining to the cancer nanotheranostic potential of MXenes and their derivatives.
Keywords: MXenes, MXene-based composites, nanotheranostics, cancer diagnostics, cancer therapeutics
1. Introduction
Today, designing multifunctional systems with unique mechanical, photothermal, and optical characteristics for simultaneous treatment and diagnosis is very helpful for faster diagnosis and recovery as well as better monitoring of the therapeutic process of the patient [1,2,3]. Typically, chemotherapy, hormonal therapy, surgery, radiotherapy, and immunotherapy have been widely applied in the treatment of cancers or elimination of tumors [4,5]. However, these routes may suffer from some limitations/challenges such as low selectivity/sensitivity, poor drug bioavailability, low biocompatibility, high toxicity, off-target effects, high dose necessities, multidrug resistance, etc., which has prompted researchers to envision and explore effective alternative strategies or systems with high efficiency and specific targeting properties to reduce possible off-target effects and improve multifunctionality [6,7,8,9]. Designing two-dimensional (2D) nanostructures from graphene and its derivatives, black phosphorus, graphitic carbon nitride, MXenes, and transition metal dichalcogenides for simultaneous cancer therapy and diagnosis is one of them [10,11,12,13]. Overall, 2D nanostructures have shown the appealing advantages of more tumbling and rolling dynamics during flow in the blood compared to other nanomaterials, providing significant enhancement in the lateral accumulation via the vessels in tumors [14,15,16,17,18,19]. The optical and thermodynamic properties of 2D nanostructures can be organized by controlling the number of atomic layers, defect sites, dimensions, or decoration of other plasma materials; these materials with a large specific surface area can be applied to the loading and targeted delivery of chemotherapy drugs, photosensitizers, and immune adjuvants, among others [20,21].
MXenes with the general formula of Mn+1XnTx and abundance of functionalities (such as –O, –OH, –Cl, and –F) are ideally suited for surface functionalization or modification, thus paving the way for designing smart micro- and nanosystems with multifunctionality. In general, several strategies have been introduced for surface functionalization of MXenes based on covalent and noncovalent modification processes [22,23,24]. The noncovalent surface modification can be achieved via the combination of van der Waals forces, hydrogen bonding, and electrostatic attraction [25]. On the other hand, covalent surface functionalization techniques are based on the application of small molecules (e.g., epoxy compounds, organic amines, acid anhydrides, alkali metal hydroxides, and acid halides) and surface-initiated polymerization by macromolecules as well as single heteroatom methods [26]. MXenes have shown excellent capabilities for synergistic treatment, encompassing chemotherapeutic drugs, photosensitizers, and immune adjuvants [27]. Compared to other 2D structures such as graphene, transition metal dichalcogenides, black phosphorus nanosheets, metal–organic framework nanosheets, and hexagonal boron nitride, MXenes have a low density, excellent electrical conductivity, hydrophilicity, unique optical/thermal properties, and biocompatibility. They exhibit the advantages of magnetic properties and tunable electric features, making them promising candidates for cancer nanotheranostics [28]. Although graphene displays high electrical conductivity, it has low grade magnetic properties. Therefore, it cannot be applied to electromagnetic interference shielding applications as adequate magnetic dipoles where conductivity for interacting with electromagnetic waves is essential. However, several hydrogels combined with conductive functional materials were introduced with unique mechanical flexibility, fatigue durability, and suitable stretchability, which can be further utilized in designing flexible functional devices [29,30]. In addition, tunable conductivity by MXenes ranges from metallic to semiconductor; these structures can be fabricated using cost-effective and simple techniques. However, designing multifunctional MXene-based systems with controllable properties and high stability is an important challenging issue [31,32]. In this context, the long-term toxicity of pristine and surface-modified MXenes ought to be systematically evaluated on humans and the environment [23].
Recently, MXenes and their composites have garnered much attention in cancer nanotheranostics due to their fascinating mechanical, optical, electronic, and thermal features [27,33,34,35,36,37,38]; their hydrophilicity and high surface area for functionalization/modification make them promising candidates for targeted cancer nanotherapy along with specific imaging/diagnosis of cancer cells/tumor sites (Figure 1 and Table 1) [12,39,40,41,42]. Advanced MXene-based systems with the benefits of improved solubility, high targeting/selectivity properties, multifunctionality, biocompatibility, and low toxicity have shown suitability for targeted anticancer drug delivery, and photothermal, photodynamic, and chemodynamic therapy along with magnetic resonance and computed tomography imaging [27,43,44,45,46]. Zhu et al. [47] demonstrated that when MXenes (Ti3C2) nanosheets with superb near-infrared (NIR) responsiveness were combined with gold nanorods, nanohybrids with excellent photothermal conversion efficiency could be obtained for cancer therapy owing to the notable photothermal synergy between the gold nanorods and MXenes. Additionally, MXenes can be applied to load anticancer drugs (doxorubicin) with distinct pH/NIR responsive drug release behaviors upon NIR irradiation, owing to the strong π-π stacking interaction between the MXene-based composites and doxorubicin [47]. In addition, MXenes with a high drug-loading capacity and photothermal conversion capability exhibited pH-responsive and NIR laser-stimulated on-demand drug release behaviors, thus opening a new window for synergistic photothermal tumor ablation and chemotherapy (in vitro and in vivo) [48,49]. These materials can be employed as contrast agents for photoacoustic imaging, offering excellent potential for diagnostic imaging guidance and monitoring during the therapeutic process [50,51]. Herein, the most recent developments in cancer nanotheranostic applications of MXenes and their composites are deliberated.
Figure 1.
MXenes with cancer nanotheranostic applications.
Table 1.
Some selected examples of MXenes for cancer nanotheranostics.
| MXenes | Applications | Advantages/Benefits | Refs. |
|---|---|---|---|
| Ta4C3 | Dual-mode photoacoustic/computed tomography (CT) imaging along with effective photothermal ablation of tumors (in vivo) |
|
[52] |
| Ti3C2 | Cancer theranostics; photothermal elimination of cancerous cells and ablation of tumors; magnetic resonance imaging (MRI) of tumors |
|
[53] |
| Ti3C2 | Photothermal cancer nanotherapy with MR/CT imaging capabilities towards tumor cells or xenografts; applications of GdW10@Ti3C2 nanocomposites as CT contrast agents |
|
[54] |
| Ti3C2 | MR and photoacoustic dual-modality imaging-guided photothermal cancer therapy |
|
[55] |
| Ti3C2 | Dual-modal NIR-II/MRI-guided tumor hyperthermia |
|
[56] |
| Ti3C2 | Photothermal cancer therapy; photoacoustic imaging capabilities |
|
[57] |
| Mo2C | Phototherapy of tumor/cancer using multi-modal imaging-guided strategy |
|
[58] |
| Nb2C | Chemo/photothermal cancer therapy; diagnostic potential |
|
[59] |
| Ta4C3 | Photothermal therapy and photoacoustic imaging of cancers with contrast-enhanced properties |
|
[60] |
| Ta4C3 | MRI/CT imaging guided photothermal breast cancer therapy |
|
[61] |
| Ti3C2 | MRI/CT imaging guided photothermal cancer therapy |
|
[62] |
| V2C | MR/photoacoustic guided photothermal cancer treatment |
|
[63] |
| V2C | MR/photoacoustic guided photothermal cancer treatment |
|
[64] |
| Ti3C2 | Photoacoustic/CT guided photothermal cancer treatment |
|
[65] |
2. MXenes with Cancer Nanotheranostic Potential
Several studies have focused on the design of MXenes and their composites with diagnostic and therapeutic potential [65,66,67,68,69,70,71]. However, compared to other evaluated 2D structures such as graphene and its derivatives, limited studies have been devoted to the simultaneous therapeutic and diagnostic use of these structures thus far [59,72,73]. MXenes with their unique architectures and surface chemistry specifically for the in situ growth of superparamagnetic Fe3O4 nanocrystals were applied in the design of superparamagnetic 2D MXene (Ti3C2)-based structures for precise cancer theranostic applications [53]. These biocompatible composites exhibited a high photothermal conversion efficiency (~48.6%) for the photothermal elimination of cancer cells and ablation of tumor tissues with high efficiency (in vitro and in vivo) along with excellent T2 relaxivity (~394.2 mM−1 s−1) and efficient contrast-enhanced MRI of tumors, paving a new pathway for cancer theranostics [53]. Similarly, ultrathin Ta4C3 MXene nanosheets were synthesized and utilized for in situ growth of superparamagnetic iron oxide nanomaterials onto their surfaces [61]. These biocompatible composites were further functionalized with soybean phospholipid to improve their stability in physiological conditions. They can be employed for photothermal therapy (with the photothermal conversion efficiency of ~32.5%) as well as contrast-enhanced CT and T2-weighted MRI of breast tumors with a high performance, offering promising platforms for cancer theranostics [61].
The salient advantages of hydrophilicity and low cytotoxicity make MXene-based structures promising candidates for cancer therapy and diagnosis with biosafety and clinical translation potential [74,75,76]. In addition, these materials, with their broad and strong absorbance in the NIR region, along with their significant light-to-heat conversion efficiency, should be further explored for photoacoustic imaging and photothermal therapy [77,78]. Notably, MXenes exhibited great surface-engineering capabilities due to the abundant oxygen-containing groups on their surfaces, thus enhancing their colloidal stability and prolonging in vivo blood circulation [36]. Despite all these advantages, a large number of them have not been investigated for their biomedical applicability, and most explorations have centered around a few examples such as Ti3C2, Nb2C, Mo2C, V2C, and Ta3C4 [52,79]. Designing novel MXene-based structures with multiple theranostic applicability, high biocompatibility, and rapid biodegradation can almost guarantee their multipurpose biomedical application and clinical translation [80]. In addition, hyperthermia-amplified nanozyme catalytic therapy using MXenes can be considered as an alternative strategy for the treatment of cancers [57]. In this context, MXene nanosheets could be employed as substrates to anchor functional components such as nanozymes and nanodrugs. For instance, platinum (Pt) artificial nanozymes were decorated on the surface of MXene (Ti3C2) nanosheets to obtain nanocomposites with peroxidase-like performance, which could, in situ, catalyze hydrogen peroxide to form hydroxyl radicals (•OH) to stimulate cell apoptosis and necrosis. These composites exhibited suitable photothermal effects upon NIR-II light irradiation with a low power density, offering new opportunities for synergistic photothermal/enzyme cancer along with photoacoustic imaging capabilities to guide the therapeutic procedure [57].
Zong et al. [54] reported the utilization of GdW10 nanoclusters (as the contrast agents) for the surface engineering of MXene (Ti3C2) nanosheets with tumor photothermal therapy and dual MR/CT imaging capabilities (in vivo). These composites with high biocompatibility ought to be further explored for multipurpose nanotheranostics, especially for targeted cancer therapy and diagnosis [54]. In addition, biocompatible MXene (Ti3C2)-based composites (MnOx/Ti3C2) were introduced for cancer theranostics, providing efficient nanoplatforms for photothermal cancer/tumor nanotherapy with significant tumor ablation and tumor growth suppression effects guided by MR and photoacoustic imaging [55]. In this context, photoacoustic imaging with its advantages of high resolution and contrast in real time and at long penetration depths ought to be further explored using MXenes and their composites owing to their unique optical properties and their excellent potential in photoacoustic imaging for tissue visualization [81]. Dai et al. [60] modified the surface of MXenes (Ta4C3) utilizing manganese oxide nanoparticles (MnOx) for imaging-guided photothermal tumor ablation (Figure 2). Accordingly, the tantalum components of these composites could act as contrast agents with high performance or contrast-enhanced CT, while the incorporated MnOx component performed as the tumor microenvironment-responsive contrast agent for the T1-weighted MRI. These composites with high photothermal conversion efficiency could be employed for contrast-enhanced photoacoustic imaging along with the superb growth suppression of tumors via photothermal hyperthermia, providing a biocompatible MXene-based platform for cancer nanotheranostics [60].
Figure 2.
(A,B) The preparative process of MXene nanosheets including hydrogen fluoride (HF) etching and sonication and their surface functionalization/modification using MnOx and soybean phospholipid (SP). (C) MXene-based nanocomposites with photoacoustic (PA), MR, and CT imaging capabilities combined with photothermal effects for tumor ablation. Adapted from Reference [60] with permission. Copyright: 2017, American Chemical Society.
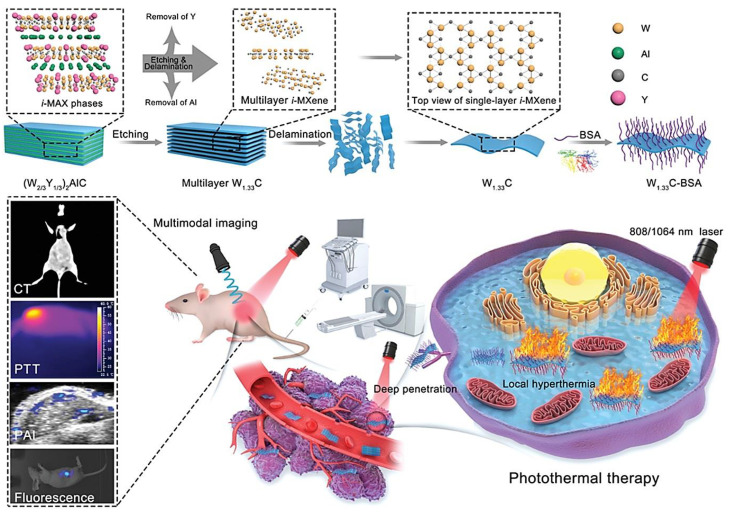
A distinct W1.33C i-MXene was developed for theranostic applications with the advantages of rapid biodegradation (in normal tissue rather than in tumors) and improved biocompatibility (Figure 3) [77]. These MXene nanosheets exhibited an excellent predominance of NIR absorbance along with high photothermal conversion effectiveness (~32.5% at NIR-I and ~49.3% at NIR-II); they could be applied as suitable platforms with multimodal-imaging features (suitable for CT and photoacoustic imaging) and photothermal-ablation effects against tumors (in vitro and in vivo). The underlying mechanisms ought to be comprehensively explored using genomics and proteomics [77]. In addition, functionalized MXene-based structures were constructed to obtain distinct tumor microenvironment-responsive T1 and T2 MRI-guided photothermal breast cancer hyperthermia in the NIR-II bio-window, providing nanoplatforms for the imaging-guided photonic hyperthermia of breast cancers [82]. Accordingly, superparamagnetic Fe3O4 and paramagnetic MnOx nanomaterials were grown onto the large surface of ultrathin MXene (Nb2C) nanosheets. These composites with significant photothermal conversion efficiency in the NIR-II bio-window and suitable biocompatibility could be applied for photothermal tumor suppression [82]. Several MXene-based systems were introduced for synergistic cancer therapy, and their capabilities can be extended with the addition of imaging/diagnostic ability. Liu et al. [83] introduced ultrathin MXene (Ti3C2) nanosheets (~100 nm) for targeted photothermal/photodynamic/chemo synergistic tumor therapy. These nanomaterials with good in vitro/in vivo biocompatibility demonstrated a superb mass extinction coefficient (~28.6 Lg−1 cm−1 at 808 nm), high photothermal conversion efficiency (~58.3%), and effective singlet oxygen generation (1O2) upon 808 nm laser irradiation. In addition, after layer-by-layer surface modification, these multifunctional nanoplatforms could be deployed for targeted delivery of the doxorubicin anticancer drug [83]. It appears that the next step ought to focus on improving their biocompatibility and theranostic application.
Figure 3.
The preparative process of bovine serum albumin (BSA)-modified W1.33C i-MXene with high photothermal conversion efficacy for theranostic applications (multimodal imaging and photothermal therapy). CT: computed tomography; PTT: photothermal therapy; and PAI: photoacoustic imaging. Adapted from Reference [77] with permission (CC BY). Copyright: 2021, Wiley-VCH GmbH.
Mo2C MXene nanospheres (~50 nm) were fabricated as theranostic agents, wherein their light harvesting covered the total NIR region. In addition, hyperthermia and reactive oxygen species (ROS) generation can be simultaneously triggered by NIR irradiation [58]. These nanospheres with excellent biocompatibility could be deployed for synergistic photothermal and photodynamic cancer therapy, thus eliminating cancer cells and removing solid tumors (by the typical liquefactive necrosis procedure). They additionally demonstrated suitable photoacoustic and CT imaging applicability (in vivo) [58]. In another study, nanosheets of MXene (Ti3C2) were functionalized with NaErF4 nanoparticles to develop multifunctional platforms for NIR-IIb (1530 nm) and MRI-guided photothermal cancer nanotherapy under 808 nm excitation, providing tumor ablation with an inhibition ratio of ~92.9% (Figure 4) [56]. These nanocomposites, with excellent photothermal conversion potential (43.62% at 808 nm irradiation) and photothermal stability, could be efficiently applied to T2-weighted MRI due to the inherent magnetic features of Er3+ ions; interestingly, no noticeable toxicity could be detected at the injected dose [56]. Zhang et al. [24] reported the construction of photo/sono-responsive antitumor theranostic nanoplatforms via the decoration of the TiO2−x nanoparticle (~10 nm) on the surface of MXenes (Ti3C2) for photoacoustic/photothermal bimodal imaging-guided NIR-II photothermal enhanced sonodynamic therapy of tumors. These nanocomposites unveiled enhanced sonodynamic ROS formation along with induced extensive localized hyperthermia, showing excellent tumor eradication (in vivo), with no tumor recurrence and systemic toxicity [24].
Figure 4.
(A) The preparative process of NaErF4@Ti3C2 MXene-based nanosystems for cancer theranostic applications. (B) Photothermal therapy and MR/NIR-II b imaging of cancer/tumor using the MXene nanocomposites. Adapted from Reference [56] with permission. Copyright: 2022, American Chemical Society.
MXene-based quantum dots exhibited unique optical features including light absorption, photoluminescence, and electrochemiluminescence, which need to be systematically studied in the field of biomedical engineering, optoelectronic catalysis, and optoelectronics [84]. Among reported MXene-based quantum dots, Ti2N quantum dots (~5 nm) with unique photophysical properties displayed high photothermal conversion efficiency under laser irradiation in NIR-I, 808 nm (~48.62%) and NIR-II, 1064 nm (~45.51%) [85]. These quantum dots with high biocompatibility, sufficient stability in circulation, appropriate excretion rate from the body, photoacoustic effects, and photothermal therapy efficiency showed detectable aggregations in tumors after 4 h post-injection and could be deployed for photoacoustic imaging-guided photothermal therapy in NIR-I/II bio-windows with no noticeable toxic effects (in vitro/in vivo) [85]. Such quantum dots can be considered for cancer theranostic applications; future explorations ought to focus on their degradability, biocompatibility, and multifunctionality.
3. Toxicity and Biosafety Issues
Although numerous studies have focused on the applications and synthesis of MXenes and their composites, limited studies have comprehensively explored their toxicity and biosafety aspects, especially in biomedical sciences [86]. Notably, clinical translation studies, along with the industrialization of assigned synthesis techniques, are very important challenges. The crucial factors affecting the toxicity of MXenes ought to be focused on by researchers, including their chemical nature, solubility, size, surface, morphology, aggregation, and their structure [86]. One of most important challenging issues in the translation of theranostic nanomedicines from in vitro to in vivo and then to clinical studies is finding the association between the physicochemical nature of the designed micro- and nanosystems along with their interactions with biological systems [87]. Thus, clinical translational studies are warranted to evaluate the efficiency, off-target toxic effects, and their physicochemical features while they are in circulation. Comprehensive preclinical studies as well as the evaluation of the nanobiological interactions of MXenes and their composites in in vivo systems can help to better identify the limitations and challenges that lie ahead [87]. Another parameter is biodegradability, which is a very important index for analyzing the biosafety of MXenes before their practical application. For instance, it was revealed that MXenes (Nb2C) underwent rapid decomposition within 24 h in the presence of human myeloperoxidase and hydrogen peroxide [88]. In addition, ultrathin MXenes (Ti3C2) exhibited enzymatic and ROS-stimulated biodegradability [89], and MXenes (Mo2C) displayed pH-dependent degradation behavior [49]. In contrast, MXenes demonstrated suitable stability in tumor tissues with relatively longer degradation times, offering opportunities for the photothermal ablation of tumors. As the underlying mechanisms of the biodegradability of MXenes have rarely been evaluated, future explorations need to focus on this critical aspect [33,90]. By optimizing the synthesis/reaction and functionalization conditions and avoiding harsh etching/delamination processes, MXenes with good stability can be obtained; ultrathin MXenes with poor chemical stability underwent decomposition through the oxidation reactions. On the other hand, the biodegradation and in vivo bioclearance of MXene-based structures can be accelerated due to the presence of high-strength ionic conditions and abundant enzymes in the physiological environments [33,77].
Several studies have concentrated on the toxicity and biocompatibility of MXenes and their derivatives. In one study, the acute toxicity and histocompatibility evaluations of intravenously administered Ti3C2-soybean phospholipid nanocomposites exhibited no evidence of pathologies and histomorphological alterations in the examined organs compared to control samples, showing no acute toxicity and adverse effects of these MXenes. In addition, the excretion with urine and feces was ~18.70% and 10.35%, respectively, after 48 h [50]. Furthermore, the biocompatibility and biosafety analyses (in vivo) of the nanocomposites of MnOx/Ti3C2-soybean phospholipid after a single-dose intravenous administration demonstrated that all the major vital signs were normal, indicating no signs of toxic action [55]. Lin et al. [88] studied the toxicity of polyvinyl pyrrolidone-modified MXene (Nb2C) nanocomposites with biodegradability attributes as they exhibited no adverse effects on the blood chemistry values. The histological assessments of the heart, liver, spleen, lung and kidney illustrated no pathological alterations in the tissues; the excretion from the body rate and clearance routes indicated that 20% of the niobium was excreted with urine and feces within 48 h, exposing their high biocompatibility [88].
4. Conclusions and Perspectives
Although the emergence of MXenes has significantly expanded the family of 2D materials and their versatile applications, the rational design of MXenes and their composites for cancer nanotheranostics with photothermal/photodynamic therapy, radiotherapy, catalytic therapy, and imaging features still remains an important challenge in biomedicine. Hybridization and surface functionalization/modification can help to improve the mechanical, electronic, thermal, and optical properties of these materials for application in cancer therapy and diagnosis. Since clinical translation studies on MXenes and their applications in bio- and nanomedicine are still in their infancy, additional explorations need to focus on the main aspects including optimization conditions, facile and environmentally benign synthesis techniques, clinical translational studies, long-term toxicity/biosafety issues, pharmacokinetics, targeting properties, and their stimuli-responsive manner. Future biosafety investigations are required to comprehensively address the critical issues, including biocompatibility, biodegradability (the degradation rate and degree), blood circulation, and excretion behaviors; the long-term existence of nanomaterials is a serious problem, as it may lead to inflammation, oxidative damage, and fibrosis. On the other hand, limited types of MXenes (mostly Ti3C2) have recently garnered major interest in biomedical applications owing to their remarkable chemical and physical features. Thus, future cancer theranostic explorations ought to explore other types of MXenes in addition to Ti3C2 with the careful consideration of optimal conditions/synthesis and functionalization techniques. Notably, MXenes have been applied to photothermal cancer nanotherapy, but their cellular internalization needs to be improved by coating their surfaces with ligands with high specificity towards cancer cells. MXene-based structures with responsiveness to biological triggers (such as pH value, temperature, and enzymes) ought to be innovatively designed for superior therapeutic outcomes.
Author Contributions
S.I. and R.S.V.: conceptualization; and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Selestin Raja I., Kang M.S., Kim K.S., Jung Y.J., Han D.-W. Two-Dimensional Theranostic Nanomaterials in Cancer Treatment: State of the Art and Perspectives. Cancers. 2020;12:1657. doi: 10.3390/cancers12061657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang W., Lyu Q., Zhao J., Cao L., Hao Y., Zhang H. Recent advance in near-infrared/ultrasound-sensitive 2D-nanomaterials for cancer therapeutics. Sci. China Mater. 2020;63:2397–2428. doi: 10.1007/s40843-020-1387-7. [DOI] [Google Scholar]
- 3.Huang M., Gu Z., Zhang J., Zhang D., Zhang H., Yang Z., Qu J. MXene and black phosphorus based 2D nanomaterials in bioimaging and biosensing: Progress and perspectives. J. Mater. Chem. B. 2021;9:5195–5220. doi: 10.1039/D1TB00410G. [DOI] [PubMed] [Google Scholar]
- 4.Wan L., Zhao Q., Zhao P., He B., Jiang T., Zhang Q., Wang S. Versatile hybrid polyethyleneimine–mesoporous carbon nanoparticles for targeted delivery. Carbon. 2014;79:123–134. doi: 10.1016/j.carbon.2014.07.050. [DOI] [Google Scholar]
- 5.Senapati S., Mahanta A.K., Kumar S., Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018;3:7. doi: 10.1038/s41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chari R.V.J. Targeted cancer therapy: Conferring specificity to cytotoxic drugs. Acc. Chem. Res. 2007;41:98–107. doi: 10.1021/ar700108g. [DOI] [PubMed] [Google Scholar]
- 7.Murugan C., Sharma V., Murugan R.K., Malaimegu G., Sundaramurthy A. Two-dimensional cancer theranostic nanomaterials: Synthesis, surface functionalization and applications in photothermal therapy. J. Control. Release. 2019;299:1–20. doi: 10.1016/j.jconrel.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Shi Z., Zhou Y., Fan T., Lin Y., Zhang H., Mei L. Inorganic nano-carriers based smart drug delivery systems for tumor therapy. Smart Mater. Med. 2020;1:32–47. doi: 10.1016/j.smaim.2020.05.002. [DOI] [Google Scholar]
- 9.Fusco L., Gazzi A., Peng G., Shin Y., Vranic S., Bedognetti D., Vitale F., Yilmazer A., Feng X., Fadeel B., et al. Graphene and other 2D materials: A multidisciplinary analysis to uncover the hidden potential as cancer theranostics. Theranostics. 2020;10:5435–5488. doi: 10.7150/thno.40068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain V., Jain S., Mahajan S.C. Nanomedicines based drug delivery systems for anti-cancer targeting and treatment. Curr. Drug Deliv. 2015;12:177–191. doi: 10.2174/1567201811666140822112516. [DOI] [PubMed] [Google Scholar]
- 11.Kumar P., Srivastava R. Nanomedicine for Cancer Therapy: From Chemotherapeutic to Hyperthermia-Based Therapy. Springer; Berlin/Heidelberg, Germany: 2016. [Google Scholar]
- 12.Hiremath N., Kumar R., Hwang K.C., Banerjee I., Thangudu S., Vankayala R. Near-Infrared Light Activatable Two-Dimensional Nanomaterials for Theranostic Applications: A Comprehensive Review. ACS Appl. Nano Mater. 2022;5:1719–1733. doi: 10.1021/acsanm.2c00170. [DOI] [Google Scholar]
- 13.Korupalli C., You K.-L., Getachew G., Rasal A.S., Dirersa W.B., Fahmi M.Z., Chang J.-Y. Engineering the Surface of Ti3C2 MXene Nanosheets for High Stability and Multimodal Anticancer Therapy. Pharmaceutics. 2022;14:304. doi: 10.3390/pharmaceutics14020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Nanotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbasi Z., Feizi S., Taghipour E., Ghadam P. Green synthesis of silver nanoparticles using aqueous extract of dried Juglans regia green husk and examination of its biological properties. Green Process. Synth. 2017;6:477–485. doi: 10.1515/gps-2016-0108. [DOI] [Google Scholar]
- 16.Iravani S. MXenes and MXene-based (nano)structures: A perspective on greener synthesis and biomedical prospects. Ceram. Int. 2022;48:24144–24156. doi: 10.1016/j.ceramint.2022.05.137. [DOI] [Google Scholar]
- 17.Iravani S., Varma R.S. MXenes and MXene-based materials for tissue engineering and regenerative medicine: Recent advances. Mater. Adv. 2021;2:2906–2917. doi: 10.1039/D1MA00189B. [DOI] [Google Scholar]
- 18.Iravani S., Varma R.S. MXenes for Cancer Therapy and Diagnosis: Recent Advances and Current Challenges. ACS Biomater. Sci. Eng. 2021;7:1900–1913. doi: 10.1021/acsbiomaterials.0c01763. [DOI] [PubMed] [Google Scholar]
- 19.Iravani S., Varma R.S. Bioinspired and biomimetic MXene-based structures with fascinating properties: Recent advances. Mater. Adv. 2022;3:4783–4796. doi: 10.1039/D2MA00151A. [DOI] [Google Scholar]
- 20.Ni N., Zhang X., Ma Y., Yuan J., Wang D., Ma G., Dong J., Sun X. Biodegradable two-dimensional nanomaterials for cancer theranostics. Coord. Chem. Rev. 2022;458:214415. doi: 10.1016/j.ccr.2022.214415. [DOI] [Google Scholar]
- 21.Yang B., Chen Y., Shi J. Material Chemistry of Two-Dimensional Inorganic Nanosheets in Cancer Theranostics. Chem. 2018;4:1284–1313. doi: 10.1016/j.chempr.2018.02.012. [DOI] [Google Scholar]
- 22.Ibragimova R., Erhart P., Rinke P., Komsa H.-P. Surface Functionalization of 2D MXenes: Trends in Distribution, Composition, and Electronic Properties. J. Phys. Chem. Lett. 2021;12:2377–2384. doi: 10.1021/acs.jpclett.0c03710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mozafari M., Soroush M. Surface functionalization of MXenes. Mater. Adv. 2021;2:7277–7307. doi: 10.1039/D1MA00625H. [DOI] [Google Scholar]
- 24.Zhang D.-Y., Liu H., Younis M.R., Lei S., Chen Y., Huang P., Lin J. In-situ TiO2−x decoration of titanium carbide MXene for photo/sono-responsive antitumor theranostics. J. Nanobiotechnol. 2022;20:53. doi: 10.1186/s12951-022-01253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chitteth Rajan A., Mishra A., Satsangi S., Vaish R., Mizuseki H., Lee K.-R., Singh A.K. Machine-Learning-Assisted Accurate Band Gap Predictions of Functionalized MXene. Chem. Mater. 2018;30:4031–4038. doi: 10.1021/acs.chemmater.8b00686. [DOI] [Google Scholar]
- 26.Qin L., Tao Q., Liu X., Fahlman M., Halim J., Persson P.O.Å., Rosen J., Zhang F. Polymer-MXene composite films formed by MXene-facilitated electrochemical polymerization for flexible solid-state microsupercapacitors. Nano Energy. 2019;60:734–742. doi: 10.1016/j.nanoen.2019.04.002. [DOI] [Google Scholar]
- 27.Dong L.M., Ye C., Zheng L.L., Gao Z.F., Xia F. Two-dimensional metal carbides and nitrides (MXenes): Preparation, property, and applications in cancer therapy. Nanophotonics. 2020;9:2125–2145. doi: 10.1515/nanoph-2019-0550. [DOI] [Google Scholar]
- 28.Shukla V. The tunable electric and magnetic properties of 2D MXenes and their potential applications. Mater. Adv. 2020;1:3104–3121. doi: 10.1039/D0MA00548G. [DOI] [Google Scholar]
- 29.Zeng Z.-H., Wu N., Wei J.-J., Yang Y.-F., Wu T.-T., Li B., Hauser S.B., Yang W.-D., Liu J.-R., Zhao S.-Y. Porous and Ultra-Flexible Crosslinked MXene/Polyimide Composites for Multifunctional Electromagnetic Interference Shielding. Nano-Micro Lett. 2022;14:59. doi: 10.1007/s40820-022-00800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y., Han M., Liu W., Wu N., Liu J. Hydrogel-based composites beyond the porous architectures for electromagnetic interference shielding. Nano Res. 2022 doi: 10.1007/s12274-022-4817-1. in press . [DOI] [Google Scholar]
- 31.Shukla V. Observation of critical magnetic behavior in 2D carbon based composites. Nanoscale Adv. 2020;2:962–990. doi: 10.1039/C9NA00663J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shukla V. Review of electromagnetic interference shielding materials fabricated by iron ingredients. Nanoscale Adv. 2019;1:1640–1671. doi: 10.1039/C9NA00108E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L., Dai X., Feng W., Chen Y. Biomedical Applications of MXenes: From Nanomedicine to Biomaterials. Acc. Mater. Res. 2022;3:785–798. doi: 10.1021/accountsmr.2c00025. [DOI] [Google Scholar]
- 34.Sadiq M., Pang L., Johnson M., Sathish V., Zhang Q., Wang D. 2D Nanomaterial, Ti3C2 MXene-Based Sensor to Guide Lung Cancer Therapy and Management. Biosensors. 2021;11:40. doi: 10.3390/bios11020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharifuzzaman M., Barman S.C., Zahed M.A., Sharma S., Yoon H., Nah J.S., Kim H., Park J.Y. An Electrodeposited MXene-Ti3C2Tx Nanosheets Functionalized by Task-Specific Ionic Liquid for Simultaneous and Multiplexed Detection of Bladder Cancer Biomarkers. Small. 2020;16:2002517. doi: 10.1002/smll.202002517. [DOI] [PubMed] [Google Scholar]
- 36.Sundaram A., Ponraj J.S., Wang C., Peng W.K., Manavalan R.K., Dhanabalan S.C., Zhang H., Gaspar J. Engineering of 2D transition metal carbides and nitrides MXenes for cancer therapeutics and diagnostics. J. Mater. Chem. B. 2020;8:4990–5013. doi: 10.1039/D0TB00251H. [DOI] [PubMed] [Google Scholar]
- 37.Xing C., Chen S., Liang X., Liu Q., Qu M., Zou Q., Li J., Tan H., Liu L., Fan D., et al. Two-Dimensional MXene (Ti3C2)-Integrated Cellulose Hydrogels: Toward Smart Three-Dimensional Network Nanoplatforms Exhibiting Light-Induced Swelling and Bimodal Photothermal/Chemotherapy Anticancer Activity. ACS Appl. Mater. Interfaces. 2018;10:27631–27643. doi: 10.1021/acsami.8b08314. [DOI] [PubMed] [Google Scholar]
- 38.Zamhuri A., Lim G.P., Ma N.L., Tee K.S., Soon C.F. MXene in the lens of biomedical engineering: Synthesis, applications and future outlook. BioMed Eng. OnLine. 2021;20:33. doi: 10.1186/s12938-021-00873-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hendijani F. Human mesenchymal stromal cell therapy for prevention and recovery of chemo/radiotherapy adverse reactions. Cytotherapy. 2015;17:509–525. doi: 10.1016/j.jcyt.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jamalipour Soufi G., Iravani S. Eco-friendly and sustainable synthesis of biocompatible nanomaterials for diagnostic imaging: Current challenges and future perspectives. Green Chem. 2020;22:2662–2687. doi: 10.1039/D0GC00734J. [DOI] [Google Scholar]
- 42.Zhu W., Li H., Luo P. Emerging 2D Nanomaterials for Multimodel Theranostics of Cancer. Front. Bioeng. Biotechnol. 2021;9:769178. doi: 10.3389/fbioe.2021.769178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong X., Mumper R.J. Nanomedicinal strategies to treat multidrug-resistant tumors: Current progress. Nanomedicine. 2010;5:597–615. doi: 10.2217/nnm.10.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nasrollahzadeh M., Sajjadi M., Iravani S., Varma R.S. Trimetallic Nanoparticles: Greener Synthesis and Their Applications. Nanomaterials. 2020;10:1784. doi: 10.3390/nano10091784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iravani S., Varma R.S. Plants and plant-based polymers as scaffolds for tissue engineering. Green Chem. 2019;21:4839–4867. doi: 10.1039/C9GC02391G. [DOI] [Google Scholar]
- 46.Iravani S., Varma R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020;18:703–727. doi: 10.1007/s10311-020-00984-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu B., Shi J., Liu C., Li J., Cao S. In-situ self-assembly of sandwich-like Ti3C2 MXene/gold nanorods nanosheets for synergistically enhanced near-infrared responsive drug delivery. Ceram. Int. 2021;47:24252–24261. doi: 10.1016/j.ceramint.2021.05.136. [DOI] [Google Scholar]
- 48.Shurbaji S., Abdul Manaph N.P., Ltaief S.M., Al-Shammari A.R., Elzatahry A., Yalcin H.C. Characterization of MXene as a Cancer Photothermal Agent Under Physiological Conditions. Front. Nanotechnol. 2021;3:689718. doi: 10.3389/fnano.2021.689718. [DOI] [Google Scholar]
- 49.Feng W., Wang R., Zhou Y., Ding L., Gao X., Zhou B., Hu P., Chen Y. Ultrathin Molybdenum Carbide MXene with Fast Biodegradability for Highly Efficient Theory-Oriented Photonic Tumor Hyperthermia. Adv. Funct. Mater. 2019;29:1901942. doi: 10.1002/adfm.201901942. [DOI] [Google Scholar]
- 50.Han X., Huang J., Lin H., Wang Z., Li P., Chen Y. 2D Ultrathin MXene-Based Drug-Delivery Nanoplatform for Synergistic Photothermal Ablation and Chemotherapy of Cancer. Adv. Healthc. Mater. 2018;7:1701394. doi: 10.1002/adhm.201701394. [DOI] [PubMed] [Google Scholar]
- 51.Mohammadpour Z., Majidzadeh-A K. Applications of Two-Dimensional Nanomaterials in Breast Cancer Theranostics. ACS Biomater. Sci. Eng. 2020;6:1852–1873. doi: 10.1021/acsbiomaterials.9b01894. [DOI] [PubMed] [Google Scholar]
- 52.Lin H., Wang Y., Gao S., Chen Y., Shi J. Theranostic 2D Tantalum Carbide (MXene) Adv. Mater. 2018;30:1703284. doi: 10.1002/adma.201703284. [DOI] [PubMed] [Google Scholar]
- 53.Liu Z., Zhao M., Lin H., Dai C., Ren C., Zhang S., Peng W., Chen Y. 2D magnetic titanium carbide MXene for cancer theranostics. J. Mater. Chem. B. 2018;6:3541–3548. doi: 10.1039/C8TB00754C. [DOI] [PubMed] [Google Scholar]
- 54.Zong L., Wu H., Lin H., Chen Y. A polyoxometalate-functionalized two-dimensional titanium carbide composite MXene for effective cancer theranostics. Nano Res. 2018;11:4149–4168. doi: 10.1007/s12274-018-2002-3. [DOI] [Google Scholar]
- 55.Dai C., Lin H., Xu G., Liu Z., Wu R., Chen Y. Biocompatible 2D Titanium Carbide (MXenes) Composite Nanosheets for pH-Responsive MRI-Guided Tumor Hyperthermia. Chem. Mater. 2017;29:8637–8652. doi: 10.1021/acs.chemmater.7b02441. [DOI] [Google Scholar]
- 56.Pan J., Zhang M., Fu G., Zhang L., Yu H., Yan X., Liu F., Sun P., Jia X., Liu X., et al. Ti3C2 MXene Nanosheets Functionalized with NaErF4:0.5%Tm@NaLuF4 Nanoparticles for Dual-Modal Near-Infrared IIb/Magnetic Resonance Imaging-Guided Tumor Hyperthermia. ACS Appl. Nano Mater. 2022;5:8142–8153. doi: 10.1021/acsanm.2c01251. [DOI] [Google Scholar]
- 57.Zhu Y., Wang Z., Zhao R., Zhou Y., Feng L., Gai S., Yang P. Pt Decorated Ti3C2Tx MXene with NIR-II Light Amplified Nanozyme Catalytic Activity for Efficient Phototheranostics. ACS Nano. 2022;16:3105–3118. doi: 10.1021/acsnano.1c10732. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Q., Huang W., Yang C., Wang F., Song C., Gao Y., Qiu Y., Yan M., Yang B., Guo C. The theranostic nanoagent Mo2C for multi-modal imaging-guided cancer synergistic phototherapy. Biomater. Sci. 2019;7:2729–2739. doi: 10.1039/C9BM00239A. [DOI] [PubMed] [Google Scholar]
- 59.Han X., Jing X., Yang D., Lin H., Wang Z., Ran H., Li P., Chen Y. Therapeutic mesopore construction on 2D Nb2C MXenes for targeted and enhanced chemo-photothermal cancer therapy in NIR-II biowindow. Theranostics. 2018;8:4491–4508. doi: 10.7150/thno.26291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dai C., Chen Y., Jing X., Xiang L., Yang D., Lin H., Liu Z., Han X., Wu R. Two-Dimensional Tantalum Carbide (MXenes) Composite Nanosheets for Multiple Imaging-Guided Photothermal Tumor Ablation. ACS Nano. 2017;11:12696–12712. doi: 10.1021/acsnano.7b07241. [DOI] [PubMed] [Google Scholar]
- 61.Liu Z., Lin H., Zhao M., Dai C., Zhang S., Peng W., Chen Y. 2D Superparamagnetic Tantalum Carbide Composite MXenes for Efficient Breast-Cancer Theranostics. Theranostics. 2018;8:1648–1664. doi: 10.7150/thno.23369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu X., Cai X., Cui H., Lee S.-W., Yu X.-F., Liu B. Fluorine-free preparation of titanium carbide MXene quantum dots with high near-infrared photothermal performances for cancer therapy. Nanoscale. 2017;9:17859–17864. doi: 10.1039/C7NR05997C. [DOI] [PubMed] [Google Scholar]
- 63.Cao Y., Wu T., Zhang K., Meng X., Dai W., Wang D., Dong H., Zhang X. Engineered Exosome-Mediated Near-Infrared-II Region V2C Quantum Dot Delivery for Nucleus-Target Low-Temperature Photothermal Therapy. ACS Nano. 2019;13:1499–1510. doi: 10.1021/acsnano.8b07224. [DOI] [PubMed] [Google Scholar]
- 64.Zada S., Dai W., Kai Z., Lu H., Meng X., Zhang Y., Cheng Y., Yan F., Fu P., Zhang X., et al. Algae Extraction Controllable Delamination of Vanadium Carbide Nanosheets with Enhanced Near-Infrared Photothermal Performance. Angew. Chem. Int. Ed. 2020;59:6601–6606. doi: 10.1002/anie.201916748. [DOI] [PubMed] [Google Scholar]
- 65.Tang W., Dong Z., Zhang R., Yi X., Yang K., Jin M., Yuan C., Xiao Z., Liu Z., Cheng L. Multifunctional Two-Dimensional Core–Shell MXene@Gold Nanocomposites for Enhanced Photo–Radio Combined Therapy in the Second Biological Window. ACS Nano. 2019;13:284–294. doi: 10.1021/acsnano.8b05982. [DOI] [PubMed] [Google Scholar]
- 66.Anasori B., Lukatskaya M.R., Gogotsi Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017;2:16098. doi: 10.1038/natrevmats.2016.98. [DOI] [Google Scholar]
- 67.Assad H., Fatma I., Kumar A., Kaya S., Vo D.-V.N., Al-Gheethi A., Sharma A. An overview of MXene-Based nanomaterials and their potential applications towards hazardous pollutant adsorption. Chemosphere. 2022;298:134221. doi: 10.1016/j.chemosphere.2022.134221. [DOI] [PubMed] [Google Scholar]
- 68.Awasthi G.P., Maharjan B., Shrestha S., Bhattarai D.P., Yoon D., Park C.H., Kim C.S. Synthesis, characterizations, and biocompatibility evaluation of polycaprolactone–MXene electrospun fibers. Colloids Surf. A Physicochem. Eng. Asp. 2020;586:124282. doi: 10.1016/j.colsurfa.2019.124282. [DOI] [Google Scholar]
- 69.Carey M., Barsoum M.W. MXene polymer nanocomposites: A review. Mater. Today Adv. 2021;9:100120. doi: 10.1016/j.mtadv.2020.100120. [DOI] [Google Scholar]
- 70.Fu B., Sun J., Wang C., Shang C., Xu L., Li J., Zhang H. MXenes: Synthesis, Optical Properties, and Applications in Ultrafast Photonics. Small. 2021;17:2006054. doi: 10.1002/smll.202006054. [DOI] [PubMed] [Google Scholar]
- 71.Fu Y., Zhang J., Lin H., Mo A. 2D titanium carbide(MXene) nanosheets and 1D hydroxyapatite nanowires into free standing nanocomposite membrane: In vitro and in vivo evaluations for bone regeneration. Mater. Sci. Eng. C. 2021;118:111367. doi: 10.1016/j.msec.2020.111367. [DOI] [PubMed] [Google Scholar]
- 72.Soleymaniha M., Shahbazi M.-A., Rafieerad A.R., Maleki A., Amir A. Promoting Role of MXene Nanosheets in Biomedical Sciences: Therapeutic and Biosensing Innovations. Adv. Healthc. Mater. 2019;8:1801137. doi: 10.1002/adhm.201801137. [DOI] [PubMed] [Google Scholar]
- 73.Xie Z., Chen S., Duo Y., Zhu Y., Fan T., Zou Q., Qu M., Lin Z., Zhao J., Li Y., et al. Biocompatible Two-Dimensional Titanium Nanosheets for Multimodal Imaging-Guided Cancer Theranostics. ACS Appl. Mater. Interfaces. 2019;11:22129–22140. doi: 10.1021/acsami.9b04628. [DOI] [PubMed] [Google Scholar]
- 74.Iravani P., Iravani S., Varma R.S. MXene-Chitosan Composites and Their Biomedical Potentials. Micromachines. 2022;13:1383. doi: 10.3390/mi13091383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jamalipour Soufi G., Iravani P., Hekmatnia A., Mostafavi E., Khatami M., Iravani S. MXenes and MXene-based Materials with Cancer Diagnostic Applications: Challenges and Opportunities. Comments Inorg. Chem. 2022;42:174–207. doi: 10.1080/02603594.2021.1990890. [DOI] [Google Scholar]
- 76.Mostafavi E., Iravani S. MXene-Graphene Composites: A Perspective on Biomedical Potentials. Nano-Micro Lett. 2022;14:130. doi: 10.1007/s40820-022-00880-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou B., Yin H., Dong C., Sun L., Feng W., Pu Y., Han X., Li X., Du D., Xu H., et al. Biodegradable and Excretable 2D W1.33C i-MXene with Vacancy Ordering for Theory-Oriented Cancer Nanotheranostics in Near-Infrared Biowindow. Adv. Sci. 2021;8:2101043. doi: 10.1002/advs.202101043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gazzi A., Fusco L., Khan A., Bedognetti D., Zavan B., Vitale F., Yilmazer A., Delogu L.G. Photodynamic Therapy Based on Graphene and MXene in Cancer Theranostics. Front. Bioeng. Biotechnol. 2019;7:295. doi: 10.3389/fbioe.2019.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y., Feng W., Chen Y. Chemistry of two-dimensional MXene nanosheets in theranostic nanomedicine. Chin. Chem. Lett. 2020;31:937–946. doi: 10.1016/j.cclet.2019.11.016. [DOI] [Google Scholar]
- 80.Sivasankarapillai V.S., Somakumar A.K., Joseph J., Nikazar S., Rahdar A., Kyzas G. Cancer theranostic applications of MXene nanomaterials: Recent updates. Nano-Struct. Nano-Objects. 2020;22:100457. doi: 10.1016/j.nanoso.2020.100457. [DOI] [Google Scholar]
- 81.Iravani S., Varma R.S. MXenes in photomedicine: Advances and prospects. Chem. Commun. 2022;58:7336–7350. doi: 10.1039/D2CC01694J. [DOI] [PubMed] [Google Scholar]
- 82.Liu Z., Zhao M., Yu L., Peng W., Chen Y., Zhang S. Redox chemistry-enabled stepwise surface dual nanoparticle engineering of 2D MXenes for tumor-sensitive T1 and T2 MRI-guided photonic breast-cancer hyperthermia in the NIR-II biowindow. Biomater. Sci. 2022;10:1562–1574. doi: 10.1039/D1BM01957K. [DOI] [PubMed] [Google Scholar]
- 83.Liu G., Zou J., Tang Q., Yang X., Zhang Y.-W., Zhang Q., Huang W., Chen P., Shao J., Dong X. Surface Modified Ti3C2 MXene Nanosheets for Tumor Targeting Photothermal/Photodynamic/Chemo Synergistic Therapy. ACS Appl. Mater. Interfaces. 2017;9:40077–40086. doi: 10.1021/acsami.7b13421. [DOI] [PubMed] [Google Scholar]
- 84.Iravani S., Varma R.S. Smart MXene quantum dot-based nanosystems for biomedical applications. Nanomaterials. 2022;12:1200. doi: 10.3390/nano12071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shao J., Zhang J., Jiang C., Lin J., Huang P. Biodegradable titanium nitride MXene quantum dots for cancer phototheranostics in NIR-I/II biowindows. Chem. Eng. J. 2020;400:126009. doi: 10.1016/j.cej.2020.126009. [DOI] [Google Scholar]
- 86.Lim G.P., Soon C.F., Ma N.L., Morsin M., Nayan N., Ahmad M.K., Tee K.S. Cytotoxicity of MXene-based nanomaterials for biomedical applications: A mini review. Environ. Res. 2021;201:111592. doi: 10.1016/j.envres.2021.111592. [DOI] [PubMed] [Google Scholar]
- 87.Johnson K.K., Koshy P., Yang J.-L., Sorrell C.C. Preclinical Cancer Theranostics-From Nanomaterials to Clinic: The Missing Link. Adv. Funct. Mater. 2021;31:2104199. doi: 10.1002/adfm.202104199. [DOI] [Google Scholar]
- 88.Lin H., Gao S., Dai C., Chen Y., Shi J. A two-dimensional biodegradable niobium carbide (MXene) for photothermal tumor eradication in NIR-I and NIR-II biowindows. J. Am. Chem. Soc. 2017;139:16235–16247. doi: 10.1021/jacs.7b07818. [DOI] [PubMed] [Google Scholar]
- 89.Zhao X., Wang L.-Y., Li J.-M., Peng L.-M., Tang C.-Y., Zha X.-J., Ke K., Yang M.-B., Su B.-H., Yang W. Redox-Mediated Artificial Non-Enzymatic Antioxidant MXene Nanoplatforms for Acute Kidney Injury Alleviation. Adv. Sci. 2021;8:2101498. doi: 10.1002/advs.202101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liang R., Li Y., Huo M., Lin H., Chen Y. Triggering Sequential Catalytic Fenton Reaction on 2D MXenes for Hyperthermia-Augmented Synergistic Nanocatalytic Cancer Therapy. ACS Appl. Mater. Interfaces. 2019;11:42917–42931. doi: 10.1021/acsami.9b13598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.