Abstract
Objective
To explore risk profiles of the different types of postpartum hemorrhage (PPH >500ml or severe PPH >1500ml) and their recurrence risks in a subsequent delivery.
Methods
With data from The Medical Birth Registry of Norway and Statistics Norway we performed a population-based cohort study including all singleton deliveries in Norway from 1967–2017. Multilevel logistic regression was used to calculate odds ratio (OR), with 95% confidence interval (CI), with different PPH types (PPH >500ml or PPH >1500ml (severe PPH) combined with retained placenta, uterine atony, obstetric trauma, dystocia, or undefined cause) as outcomes.
Result
We identified 277 746 PPH cases of a total of 3 003 025 births (9.3%) from 1967 to 2017. Retained placenta (and/or membranes) was most often registered as severe PPH (29.3%). Maternal, fetal, and obstetric characteristics showed different associations with the PPH types. Male sex of the neonate was associated with reduced risk of PPH. This effect was strongest on PPH due to retained placenta (adjusted OR, (aOR): 0.80, 95% CI 0.78–0.82), atony (aOR 0.92, 95% CI: 0.90–0.93) and PPH with undefined cause (aOR 0.96, 95% CI: 0.95–0.97). Previous cesarean section showed a strong association with PPH due to dystocia (aOR of 13.2, 95% CI: 12.5–13.9). Recurrence risks were highest for the same type: PPH associated with dystocia (aOR: 6.8, 95% CI: 6.3–7.4), retained placenta and/or membranes (aOR: 5.9, 95% CI: 5.5–6.4), atony (aOR: 4.0, 95% CI: 3.8–4.2), obstetric trauma (aOR: 3.9, 95% CI: 3.5–4.3) and PPH of undefined cause (aOR: 2.2, 95% CI: 2.1–2.3).
Conclusion
Maternal, fetal and obstetric characteristics had differential effects on types of PPH. Recurrence differed considerably between PPH types. Retained placenta was most frequently registered with severe PPH, and showed strongest effect of sex; delivery of a boy was associated with lower risk of PPH. Previous cesarean increased the risk of PPH due to dystocia.
Introduction
Postpartum hemorrhage (PPH) is the leading direct cause of maternal mortality worldwide [1]. Main types of PPH described in literature are PPH associated with uterine atony and retention of the placenta [2–5]. It is important to disentangle the different types of PPH, in order to gain insight into the pathophysiological mechanisms, and to find potential clinical interventions that may reduce occurrence and severity of PPH.
In studies on risk factors of types of PPH, emphasis has usually been placed on two main causes of PPH; uterine atony [4,6,7] or retained placenta [3,8–11], while important types, like PPH caused by obstetric trauma or dystocia, are widely ignored. Further, the considerable variation in estimated occurrence rates between populations [2,3,12], exceeds what could be expected to be caused by environmental and genetic variations.
Studies have reported associations of PPH (in general) with demographic [3,10,13–17], and pregnancy-related factors [3,10], induction of labor [11], obstetric history, including recurrence risk [9–11], and complications related to the fetus, placenta, membranes and umbilical cord [9,10,18–21], while studies on risk factors of type specific PPH are scarce. Thus, we aimed to explore risk profiles of different PPH types through our specific objectives: to calculate the effects of demographic and pregnancy-related factors, obstetric history and complications related to the fetus, placenta, membranes and umbilical cord, and to investigate the recurrence risk of the different types of PPH in the Norwegian population.
Material and methods
Data sources
The Medical Birth Registry of Norway (MBRN), established in 1967, is a mandatory register containing information of all births in Norway [22]. For our main analyses we identified singleton births in the MBRN from 1967 to 2017 with gestational age at birth of ≥22 weeks and spontaneous onset or induction of labor. This selection excluded planned cesarean sections, but included cesareans after onset or induction of labor. Gestational age was estimated from the last menstrual period and based on ultrasonography when data for the last menstrual period were lacking. Information on the parental education level and country of birth was provided by Statistics Norway and linked with the birth registry using the unique national identification number of each parent.
Record linkage
During the period from 1967 to 2017, 3 003 025 births were registered. Using the national identification number, we linked the first two births in women who gave their first birth in 1967 or later, to assess the risk of PPH types according to pregnancy- and birth-related factors and obstetric history, including recurrence risk.
Ethics statement/approval
The study was approved by the Regional Committee for Medical and Health Research Ethics (2013/1484) and the registry owners (the Medical Birth Registry of Norway, the Norwegian Institute of Public Health, Statistics Norway and the Norwegian Tax Administration).
Outcome variables
The main outcome variables were PPH defined as the loss of more than 500ml of blood during labor or within 24 hours postpartum (hereafter referred to as PPH) in combination with one of seven predefined types of PPH described below. The PPH types were not mutually exclusive as more than one PPH type could be recorded in the same delivery.
Before 1999 bleeding volume during labor was registered to MBRN as free text and categorized as PPH if the volume was more than 500ml. In 1999, the notification form was upgraded with new, predominantly categorical, variables: PPH 500ml to 1500ml: Blood loss from 500ml to 1500ml during labor or within the first 24 hours after labor; and PPH >1500 ml: blood loss of more than 1500ml during or within 24 hours after labor or the need for blood transfusion (regardless of bleeding volume) (hereafter referred to as severe PPH) [22–24].
PPH types were defined as PPH combined with each of the following complications:
1 Retained placenta and/or membranes
Defined as lack of expulsion of the placenta within 30 minutes of delivery [25], or retention of membranes. This was notified to the MBRN by plain text before 1999 and by check box from 1999, or by plain text as manual removal of the placenta, postpartum uterine curettage or abnormally invasive placenta from 1967 to 2017.
2 Uterine atony
Failure of the uterus to contract adequately following delivery [26], notified in the MBRN by plain text before 1999 and by check box from 1999.
3 Obstetric trauma
Notified in the MBRN as perineal laceration (1st to 4th degree) (by plain text before 1999 and by check boxes from 1999) or notified by plain text as other obstetric trauma (e.g., cervical or vaginal trauma) or inversio uteri from 1967.
4 Dystocia
Duration of labor with spontaneous onset extends beyond the normal duration defined by the World Health Organization, (based on observational studies from 1973–2018) [27]. First stage (time from five centimeters to full cervical dilatation) 12 and 10 hours in first and subsequent labors, respectively. Second stage (time from full cervical dilatation to birth) three and two hours in first and subsequent labors, respectively. Protracted labor or cephalopelvic disproportion has been notified in the MBRN by plain text before 1999 and from 1999 by check box.
5 Undefined PPH cause
PPH without recorded cause.
6 Placental abruption
Notified in the MBRN before 1999 by plain text, and from 1999 by check box.
7 Placenta previa
Notified in the MBRN before 1999 by plain text, and from 1999 by check box.
Independent variables
Independent variables were demographic characteristics (maternal age, country of origin, marital status, education), obstetric history, pregnancy and fetal complications, and characteristics of the placenta, membranes, or umbilical cord. Independent variables also included a history of PPH (including the type of PPH) in the first delivery, inter-delivery interval, change of father between pregnancies, and previous cesarean section. Our analyses included possible confounding factors: maternal age (in five categories), parity, marital status, inter-delivery interval, mother’s country of birth, level of education, and the period of birth divided into five groups of approximately equal length (1967–1977, 1978–1987, 1988–1997, 1998–2007 and 2008–2017). (S1 File) includes additional details.
Statistical analysis
We used multilevel logistic regression analyses to calculate odds ratios (ORs) with 95% confidence intervals (CIs) for PPH types as outcomes, and variables related to demographic characteristics, obstetric history, pregnancy, and fetal complications, and characteristics of the placenta, membranes, and umbilical cord as exposures. We also calculated ORs for PPH types in the actual birth as the outcomes and previous PPH types as exposure variables.
We used sensitivity analyses to assess if the associations studied persisted after adjusting for unmeasured confounders and to indicate potentially false positive associations by chance conducting multiple analyses. (S1 File) includes additional details.
The statistical analyses were performed using SPSS (version 25) and MLwiN (version 3.05).
Results
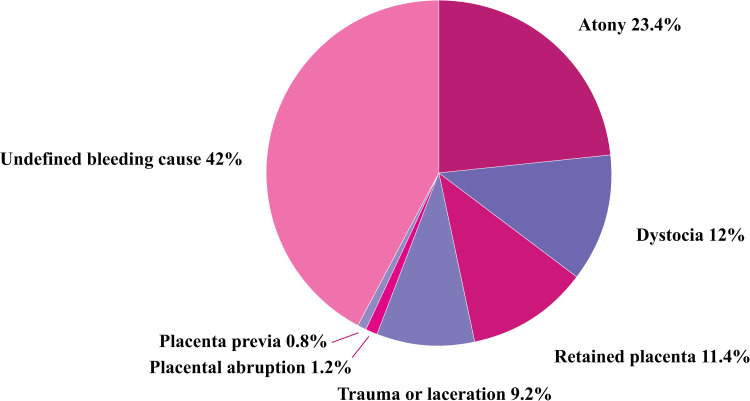
Table 1 and Fig 1 shows occurrence of type specific PPH among singleton pregnancies with gestational age at birth of ≥22 weeks of gestation. The distribution of PPH types, in decreasing order of group size, included 42.0% (n = 131 170) without specified cause of PPH, 23.4% (n = 73 284) due to atony, 12.0% (n = 37 597) dystocia, 11.4% (n = 35 664) retained placenta and/or membranes, and 9.2% (n = 28 673) obstetric trauma. Placental abruption and placenta previa were registered as cause of PPH in 1.2% (n = 3598) and 0.8% (n = 2542), respectively. The total number of PPH registrations (n = 312 528) exceeded the total number of births with PPH (n = 277 746), since more than one PPH type could be recorded in the same birth.
Table 1. Occurrence of type specific postpartum hemorrhage (PPH); singleton births ≥22 weeks of gestation.
| All types | Retained placenta | Atony | Trauma or laceration | Placental abruption | Placenta previa | Dystocia | Undefined bleeding cause | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n) | %a | %b | (n) | %a | %b | (n) | %a | %b | (n) | %a | %b | (n) | %a | %b | (n) | %a | %b | (n) | %a | %b | (n) | %a | %b | ||
| All PPH (>500ml) | 1967–2017 | 312528 | 100.0 | 35664 | 11.4 | 73284 | 23.4 | 28673 | 9.2 | 3598 | 1.2 | 2542 | 0.8 | 37597 | 12.0 | 131170 | 42.0 | ||||||||
| Mild PPH (500–1500ml) | 1999–2017 | 191730 | 100.0 | 87.2 | 17455 | 9.1 | 70.7 | 39048 | 20.4 | 84.3 | 16760 | 8.7 | 86.6 | 1785 | 0.9 | 77.9 | 1647 | 0.9 | 76.1 | 26644 | 13.9 | 87.0 | 88391 | 46.1 | 93.6 |
| Severe PPH (>1500ml) | 1999–2017 | 28149 | 100.0 | 12.8 | 7229 | 25.7 | 29.3 | 7276 | 25.8 | 15.7 | 2586 | 9.2 | 13.4 | 506 | 1.8 | 22.1 | 517 | 1.8 | 23.9 | 3980 | 14.1 | 13.0 | 6055 | 21.5 | 6.4 |
a Distribution between types in percent (row percent)
b Proportions according to severity of PPH types (column percent) (since 1999, when severe PPH was specified).
Fig 1. Occurrence of type specific postpartum hemorrhage (>500ml) (1967–2017); singleton births, ≥22 weeks of gestation.
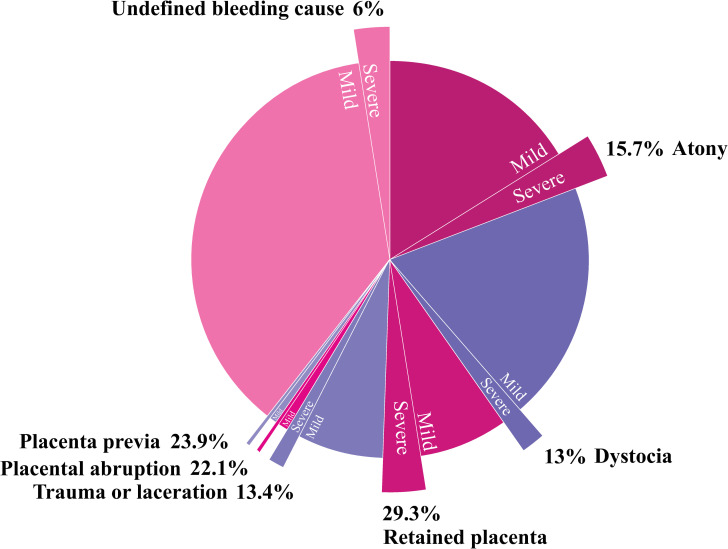
Severe PPH (registered after 1999, 28 149 type specific cases) showed a different distribution with 25.8% (n = 7276) caused by atony and 25.7% (n = 7229) by retained placenta, followed in decreasing order: undefined bleeding cause 21.5% (n = 6055), dystocia 14.1% (n = 3980), obstetric trauma 9.2% (n = 2586), placenta previa 1.8% (n = 517) and placental abruption 1.8% (n = 503) (Table 1).
Women who had PPH caused by retained placenta were more often registered with severe PPH (29.3%) compared with other categories of PPH (Table 1, Fig 2), while only 6.4% of those with undefined cause of PPH were severe PPH cases.
Fig 2. Proportions of severe postpartum hemorrhage (>1500ml) within type specific postpartum hemorrhage (1999–2017); singleton births, ≥22 weeks of gestation.
Table 2 shows the distribution of maternal, pregnancy and birth characteristics in types of PPH.
Table 2. Distribution of maternal, pregnancy and birth characteristics in types of postpartum hemorrhage (PPH >500ml); singleton births, ≥22 weeks of gestation.
| All types | Retained placenta | Atony | Trauma or laceration | Placental abruption | Placenta previa | Dystocia | Undefined bleeding cause | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n) | % | (n) | % | (n) | % | (n) | % | (n) | % | (n) | % | (n) | % | (n) | % | ||
| Maternal age (years) | <20 | 9332 | 3.0 | 863 | 2.4 | 2523 | 3.4 | 1054 | 3.7 | 106 | 2.9 | 13 | 0.5 | 801 | 2.1 | 3972 | 3.0 |
| 20–24 | 55452 | 17.7 | 5711 | 16.0 | 14388 | 19.6 | 6045 | 21.1 | 576 | 16.0 | 136 | 5.4 | 5829 | 15.5 | 22767 | 17.4 | |
| 25–29 | 103710 | 33.2 | 11198 | 31.4 | 25115 | 34.3 | 10641 | 37.1 | 1098 | 30.5 | 513 | 20.2 | 13315 | 35.4 | 41830 | 31.9 | |
| 30–34 | 92251 | 29.5 | 11223 | 31.5 | 20744 | 28.3 | 7685 | 26.8 | 1086 | 30.2 | 922 | 36.4 | 11806 | 31.4 | 38785 | 29.6 | |
| 35–39 | 42678 | 13.7 | 5494 | 15.4 | 8802 | 12.0 | 2807 | 9.8 | 578 | 16.1 | 746 | 29.3 | 4914 | 13.1 | 19337 | 14.7 | |
| 40–44 | 8595 | 2.8 | 1112 | 3.1 | 1621 | 2.2 | 414 | 1.4 | 148 | 4.1 | 200 | 7.9 | 884 | 2.4 | 4216 | 3.2 | |
| ≥45 | 510 | 0.2 | 63 | 0.2 | 91 | 0.1 | 27 | 0.1 | 6 | 0.2 | 12 | 0.5 | 48 | 0.1 | 263 | 0.2 | |
| Parity | 0 | 157617 | 50.4 | 16033 | 45.0 | 34849 | 47.6 | 18661 | 65.1 | 1290 | 35.9 | 739 | 29.1 | 28690 | 76.3 | 57355 | 43.7 |
| 1 | 99371 | 31.8 | 12316 | 34.5 | 24554 | 33.5 | 7485 | 26.1 | 1217 | 33.8 | 1032 | 40.6 | 6838 | 18.2 | 45929 | 35.0 | |
| 2 | 38856 | 12.4 | 5204 | 14.6 | 9792 | 13.4 | 1931 | 6.7 | 668 | 18.6 | 469 | 18.5 | 1511 | 4.0 | 19281 | 14.7 | |
| 3 | 10994 | 3.5 | 1421 | 4.0 | 2731 | 3.7 | 416 | 1.5 | 240 | 6.7 | 203 | 8.0 | 372 | 1.0 | 5611 | 4.3 | |
| 4 | 3983 | 1.3 | 463 | 1.3 | 928 | 1.3 | 140 | 0.5 | 128 | 3.6 | 71 | 2.8 | 135 | 0.4 | 2118 | 1.6 | |
| ≥5 | 1707 | 0.5 | 227 | 0.6 | 430 | 0.6 | 40 | 0.1 | 55 | 1.5 | 28 | 1.1 | 51 | 0.1 | 876 | 0.7 | |
| Year of delivery | 1967–1969 | 9042 | 2.9 | 779 | 2.2 | 2268 | 3.1 | 430 | 1.5 | 155 | 4.3 | 36 | 1.4 | 182 | 0.5 | 5192 | 4.0 |
| 1970–1979 | 24384 | 7.8 | 2718 | 7.6 | 6836 | 9.3 | 1701 | 5.9 | 318 | 8.8 | 61 | 2.4 | 853 | 2.3 | 11897 | 9.1 | |
| 1980–1989 | 25760 | 8.2 | 3510 | 9.8 | 8007 | 10.9 | 3108 | 10.8 | 384 | 10.7 | 99 | 3.9 | 2157 | 5.7 | 8495 | 6.5 | |
| 1990–1999 | 40825 | 13.1 | 4990 | 14.0 | 11430 | 15.6 | 4559 | 15.9 | 543 | 15.1 | 230 | 9.0 | 4669 | 12.4 | 14404 | 11.0 | |
| 2000–2009 | 96120 | 30.8 | 12294 | 34.5 | 22990 | 31.4 | 7858 | 27.4 | 1179 | 32.8 | 1003 | 39.5 | 12572 | 33.4 | 38224 | 29.1 | |
| 2010–2017 | 116397 | 37.2 | 11373 | 31.9 | 21753 | 29.7 | 11017 | 38.4 | 1019 | 28.3 | 1113 | 43.8 | 17164 | 45.7 | 52958 | 40.4 | |
| Previous 1st trimester spontaneous abortion a | No | 177174 | 80.4 | 19011 | 76.8 | 37367 | 80.5 | 16185 | 83.6 | 1759 | 76.5 | 1570 | 72.5 | 25314 | 82.5 | 75968 | 80.3 |
| Yes | 43142 | 19.6 | 5737 | 23.2 | 9046 | 19.5 | 3181 | 16.4 | 539 | 23.5 | 597 | 27.5 | 5362 | 17.5 | 18680 | 19.7 | |
| 1st trimester bleeding | No | 303194 | 97.0 | 33940 | 95.2 | 71180 | 97.1 | 28130 | 98.1 | 3473 | 96.5 | 2386 | 93.9 | 36477 | 97.0 | 127608 | 97.3 |
| Yes | 9334 | 3.0 | 1724 | 4.8 | 2104 | 2.9 | 543 | 1.9 | 125 | 3.5 | 156 | 6.1 | 1120 | 3.0 | 3562 | 2.7 | |
| Previous cesarean section | No | 279188 | 89.3 | 32597 | 91.4 | 68194 | 93.1 | 25881 | 90.3 | 3119 | 86.7 | 2050 | 80.6 | 33216 | 88.3 | 114131 | 87.0 |
| Yes | 33340 | 10.7 | 3067 | 8.6 | 5090 | 6.9 | 2792 | 9.7 | 479 | 13.3 | 492 | 19.4 | 4381 | 11.7 | 17039 | 13.0 | |
| Preeclampsia | No | 299179 | 95.7 | 34182 | 95.8 | 70433 | 96.1 | 27615 | 96.3 | 3310 | 92.0 | 2502 | 98.4 | 35943 | 95.6 | 125194 | 95.4 |
| Yes | 13349 | 4.3 | 1482 | 4.2 | 2851 | 3.9 | 1058 | 3.7 | 288 | 8.0 | 40 | 1.6 | 1654 | 4.4 | 5976 | 4.6 | |
| Smoking in start of pregnancy a | No | 165227 | 89.1 | 18531 | 86.7 | 36750 | 89.6 | 15846 | 91.1 | 1491 | 79.1 | 158 | 43.3 | 23531 | 90.3 | 68920 | 89.2 |
| Occasionally | 2880 | 1.6 | 333 | 1.6 | 604 | 1.5 | 258 | 1.5 | 52 | 2.8 | 28 | 7.7 | 405 | 1.6 | 1200 | 1.6 | |
| Yes | 17275 | 9.3 | 2509 | 11.7 | 3680 | 9.0 | 1288 | 7.4 | 341 | 18.1 | 179 | 49.0 | 2117 | 8.1 | 7161 | 9.3 | |
| Gestational or pregestational diabetes mellitus | No | 302981 | 96.9 | 34768 | 97.5 | 71523 | 97.6 | 28001 | 97.7 | 3510 | 97.6 | 2459 | 96.7 | 36002 | 95.8 | 126718 | 96.6 |
| Yes | 9547 | 3.1 | 896 | 2.5 | 1761 | 2.4 | 672 | 2.3 | 88 | 2.4 | 83 | 3.3 | 1595 | 4.2 | 4452 | 3.4 | |
| Start of delivery | Spontaneous | 216838 | 69.4 | 26006 | 72.9 | 54963 | 75.0 | 22129 | 77.2 | 1946 | 54.1 | 624 | 24.5 | 26318 | 70.0 | 84852 | 64.7 |
| Induction | 66721 | 21.3 | 7342 | 20.6 | 15513 | 21.2 | 6337 | 22.1 | 815 | 22.7 | 148 | 5.8 | 11279 | 30.0 | 25287 | 19.3 | |
| Cesarean section | 28969 | 9.3 | 2316 | 6.5 | 2808 | 3.8 | 207 | 0.7 | 837 | 23.3 | 1770 | 69.6 | 0 | 0.0 | 21031 | 16.0 | |
| Birthweight (grams) | <4000 | 222509 | 71.2 | 26665 | 74.8 | 49843 | 68.0 | 19593 | 68.3 | 3327 | 92.5 | 2404 | 94.6 | 23258 | 61.9 | 97419 | 74.3 |
| 4000–4499 | 68414 | 21.9 | 6928 | 19.4 | 17788 | 24.3 | 6956 | 24.3 | 222 | 6.2 | 118 | 4.6 | 10539 | 28.0 | 25863 | 19.7 | |
| 4500–4999 | 18621 | 6.0 | 1803 | 5.1 | 4911 | 6.7 | 1851 | 6.5 | 41 | 1.1 | 18 | 0.7 | 3245 | 8.6 | 6752 | 5.1 | |
| ≥5000 | 2984 | 1.0 | 268 | 0.8 | 742 | 1.0 | 273 | 1.0 | 8 | 0.2 | 2 | 0.1 | 555 | 1.5 | 1136 | 0.9 | |
| Fetal sex b | Girl | 154394 | 49.4 | 19241 | 54.0 | 37118 | 50.7 | 14021 | 48.9 | 1601 | 44.5 | 1184 | 46.6 | 16634 | 44.2 | 64595 | 49.2 |
| Boy | 158114 | 50.6 | 16414 | 46.0 | 36163 | 49.3 | 14652 | 51.1 | 1997 | 55.5 | 1358 | 53.4 | 20962 | 55.8 | 66568 | 50.8 | |
| Cesarean section (irrespective of start) | No | 237468 | 76.0 | 30622 | 85.9 | 65379 | 89.2 | 27751 | 96.8 | 1176 | 32.7 | 119 | 4.7 | 20995 | 55.8 | 91426 | 69.7 |
| Yes | 75060 | 24.0 | 5042 | 14.1 | 7905 | 10.8 | 922 | 3.2 | 2422 | 67.3 | 2423 | 95.3 | 16602 | 44.2 | 39744 | 30.3 | |
| Vacuum delivery | No | 275186 | 88.1 | 32333 | 90.7 | 66115 | 90.2 | 23458 | 81.8 | 3494 | 97.1 | 2537 | 99.8 | 22347 | 59.4 | 124902 | 95.2 |
| Yes | 37342 | 11.9 | 3331 | 9.3 | 7169 | 9.8 | 5215 | 18.2 | 104 | 2.9 | 5 | 0.2 | 15250 | 40.6 | 6268 | 4.8 | |
| Forceps delivery | No | 302559 | 96.8 | 34869 | 97.8 | 71598 | 97.7 | 26663 | 93.0 | 3547 | 98.6 | 2536 | 99.8 | 33700 | 89.6 | 129646 | 98.8 |
| Yes | 9969 | 3.2 | 795 | 2.2 | 1686 | 2.3 | 2010 | 7.0 | 51 | 1.4 | 6 | 0.2 | 3897 | 10.4 | 1524 | 1.2 | |
| Shoulder dystocia | No | 307711 | 98.5 | 35177 | 98.6 | 72058 | 98.3 | 27989 | 97.6 | 3586 | 99.7 | 2540 | 99.9 | 36558 | 97.2 | 129803 | 99.0 |
| Yes | 4817 | 1.5 | 487 | 1.4 | 1226 | 1.7 | 684 | 2.4 | 12 | 0.3 | 2 | 0.1 | 1039 | 2.8 | 1367 | 1.0 | |
| Episiotomy c | No | 171611 | 77.9 | 19634 | 79.3 | 35045 | 75.5 | 13164 | 68.0 | 2218 | 96.5 | 2149 | 99.2 | 19842 | 64.7 | 79559 | 84.1 |
| Yes | 48705 | 22.1 | 5114 | 20.7 | 11368 | 24.5 | 6202 | 32.0 | 80 | 3.5 | 18 | 0.8 | 10834 | 35.3 | 15089 | 15.9 | |
| Epidural anasthesia | No | 217383 | 69.6 | 26376 | 74.0 | 54188 | 73.9 | 19013 | 66.3 | 3101 | 86.2 | 2268 | 89.2 | 13166 | 35.0 | 99271 | 75.7 |
| Yes | 95145 | 30.4 | 9288 | 26.0 | 19096 | 26.1 | 9660 | 33.7 | 497 | 13.8 | 274 | 10.8 | 24431 | 65.0 | 31899 | 24.3 | |
| Placenta defined as normal c | No | 54383 | 24.7 | 18208 | 73.6 | 10490 | 22.6 | 3003 | 15.5 | 1050 | 45.7 | 714 | 32.9 | 6677 | 21.8 | 14241 | 15.0 |
| Yes | 165933 | 75.3 | 6540 | 26.4 | 35923 | 77.4 | 16363 | 84.5 | 1248 | 54.3 | 1453 | 67.1 | 23999 | 78.2 | 80407 | 85.0 | |
| Velamentous umbilical cord incertion c | No | 215432 | 97.8 | 23625 | 95.5 | 45476 | 98.0 | 19002 | 98.1 | 2205 | 96.0 | 2052 | 94.7 | 30159 | 98.3 | 92913 | 98.2 |
| Yes | 4884 | 2.2 | 1123 | 4.5 | 937 | 2.0 | 364 | 1.9 | 93 | 4.0 | 115 | 5.3 | 517 | 1.7 | 1735 | 1.8 | |
| Marginal umbilical cord incertion c | No | 206851 | 93.9 | 23027 | 93.0 | 43267 | 93.2 | 18090 | 93.4 | 2093 | 91.1 | 1949 | 89.9 | 28818 | 93.9 | 89607 | 94.7 |
| Yes | 13465 | 6.1 | 1721 | 7.0 | 3146 | 6.8 | 1276 | 6.6 | 205 | 8.9 | 218 | 10.1 | 1858 | 6.1 | 5041 | 5.3 | |
Incidences according to maternal, pregnancy and birth characteristics are given in Table 3.
a 1999–2017, smoking status available in 163731 deliveries with PPH.
b 18 newborns with unknown sex.
c 1999–2017.
Young women were more often registered with PPH due to obstetric trauma, and women with PPH caused by dystocia and obstetric trauma were more often nulliparous.
Smoking was more common in PPH associated with placenta previa and placental abruption.
Diabetes mellitus was more common in PPH associated with dystocia (4.2%), while preeclampsia was more common in PPH associated with placental abruption (8.0%).
High birthweight was commonly found in PPH caused by dystocia, atony and obstetric trauma.
A history of first trimester bleeding was more common in women with PPH due to placenta previa (6.1%) and retained placenta (4.8%), while the opposite was the case for PPH caused by obstetric trauma (1.9%).
Women who experienced PPH due to retained placenta or atony were more likely delivering girls than boys, while those with PPH caused by dystocia, obstetric trauma and undefined bleeding cause were more often delivering boys.
Placenta was defined as “normal” (tic box) in most deliveries with PPH without defined cause, in PPH due to obstetric trauma, and due to dystocia (75–85%). The opposite was found for PPH caused by retained placenta, where 26% of the placentas were defined as normal.
Table 3 shows risks of PPH types according to maternal, pregnancy and birth characteristics. We selected deliveries that were induced or had spontaneous onset, and found that PPH due to placental abruption and placenta previa represented a small proportion (2%) of all PPH registrations, and these were therefore not included in Tables 3–5. The risk of PPH increased with maternal age, and the association was strongest for PPH due to dystocia, followed by retained placenta, undefined bleeding cause and obstetric trauma. The effects were attenuated by adjustment for year of birth, while including parity in the model strengthened the associations. The risk of PPH was highest in primiparas, regardless of PPH type, especially with PPH caused by dystocia and obstetric trauma. By including maternal age to the models these associations were strengthened.
Table 3. Risk of type specific postpartum hemorrhage (PPH >500ml) according to maternal, pregnancy and birth characteristics; singleton births, ≥22 weeks of gestation and spontaneous onset or induction of labor.
| Total | Retained placenta | Atony | Trauma or laceration | Dystocia | Undefined bleeding cause | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n) | (n) | % | aOR | 95% CI | (n) | % | aOR | 95% CI | (n) | % | aOR | 95% CI | (n) | % | aOR | 95% CI | (n) | % | aOR | 95% CI | |||||||
| Maternal age (Years) | <20 | 136032 | 841 | 0.6 | 0.55 | 0.51 | 0.59 | 2507 | 1.8 | 0.82 | 0.79 | 0.86 | 1049 | 0.8 | 0.55 | 0.51 | 0.59 | 801 | 0.6 | 0.33 | 0.31 | 0.36 | 3815 | 2.8 | 0.85 | 0.83 | 0.89 |
| 20–24 | 652556 | 5540 | 0.8 | 0.74 | 0.72 | 0.77 | 14209 | 2.2 | 0.92 | 0.90 | 0.94 | 6035 | 0.9 | 0.73 | 0.70 | 0.75 | 5829 | 0.9 | 0.56 | 0.54 | 0.58 | 21328 | 3.3 | 0.93 | 0.91 | 0.94 | |
| 25–29 | 916125 | 10647 | 1.2 | 1 | Ref | 24536 | 2.7 | 1 | Ref | 10600 | 1.2 | 1 | Ref | 13315 | 1.5 | 1 | Ref | 36781 | 4.0 | 1 | Ref | ||||||
| 30–34 | 662257 | 10324 | 1.6 | 1.32 | 1.28 | 1.34 | 19722 | 3.0 | 1.03 | 1.01 | 1.05 | 7593 | 1.1 | 1.09 | 1.06 | 1.13 | 11806 | 1.8 | 1.42 | 1.38 | 1.46 | 31205 | 4.7 | 1.07 | 1.05 | 1.09 | |
| 35–39 | 260686 | 4932 | 1.9 | 1.62 | 1.56 | 1.68 | 8012 | 3.1 | 1.06 | 1.03 | 1.09 | 2751 | 1.1 | 1.16 | 1.10 | 1.22 | 4914 | 1.9 | 1.79 | 1.73 | 1.86 | 13969 | 5.4 | 1.17 | 1.14 | 1.20 | |
| 40–44 | 46563 | 929 | 2.0 | 1.75 | 1.62 | 1.89 | 1395 | 3.0 | 1.09 | 1.03 | 1.15 | 410 | 0.9 | 1.14 | 1.02 | 1.28 | 884 | 1.9 | 2.04 | 1.90 | 2.20 | 2819 | 6.1 | 1.34 | 1.29 | 1.40 | |
| ≥45 | 2198 | 43 | 2.0 | 1.97 | 1.43 | 2.70 | 76 | 3.5 | 1.40 | 1.11 | 1.76 | 26 | 1.2 | 1.80 | 1.18 | 2.74 | 48 | 2.2 | 2.50 | 1.83 | 3.41 | 164 | 7.5 | 1.79 | 1.53 | 2.09 | |
| Parity | 0 | 1124388 | 15284 | 1.4 | 1 | Ref | 33966 | 3.0 | 1 | Ref | 18609 | 1.7 | 1 | Ref | 28690 | 2.6 | 1 | Ref | 50806 | 4.5 | 1 | Ref | |||||
| 1 | 935991 | 11389 | 1.2 | 0.77 | 0.75 | 0.79 | 23415 | 2.5 | 0.78 | 0.77 | 0.79 | 7409 | 0.8 | 0.42 | 0.41 | 0.44 | 6838 | 0.7 | 0.22 | 0.22 | 0.23 | 37766 | 4.0 | 0.86 | 0.85 | 0.88 | |
| 2 | 423535 | 4700 | 1.1 | 0.64 | 0.62 | 0.67 | 9225 | 2.2 | 0.66 | 0.65 | 0.68 | 1872 | 0.4 | 0.22 | 0.21 | 0.24 | 1511 | 0.4 | 0.10 | 0.09 | 0.11 | 14758 | 3.5 | 0.74 | 0.73 | 0.76 | |
| 3 | 128264 | 1273 | 1.0 | 0.55 | 0.51 | 0.58 | 2564 | 2.0 | 0.59 | 0.57 | 0.62 | 407 | 0.3 | 0.16 | 0.15 | 0.18 | 372 | 0.3 | 0.08 | 0.07 | 0.09 | 4305 | 3.4 | 0.71 | 0.69 | 0.74 | |
| 4 | 46957 | 412 | 0.9 | 0.48 | 0.43 | 0.53 | 868 | 1.8 | 0.52 | 0.57 | 0.62 | 131 | 0.3 | 0.14 | 0.11 | 0.16 | 135 | 0.3 | 0.08 | 0.06 | 0.09 | 1721 | 3.7 | 0.77 | 0.73 | 0.81 | |
| ≥5 | 17282 | 198 | 1.1 | 0.56 | 0.48 | 0.66 | 419 | 2.4 | 0.66 | 0.59 | 0.73 | 36 | 0.2 | 0.09 | 0.06 | 0.13 | 51 | 0.3 | 0.07 | 0.05 | 0.09 | 725 | 4.2 | 0.82 | 0.75 | 0.89 | |
| Previous 1st trimester spontaneous abortion a | No | 818952 | 17401 | 2.1 | 1 | Ref | 35221 | 4.3 | 1 | Ref | 16047 | 2.0 | 1 | Ref | 25314 | 3.1 | 1 | Ref | 60431 | 7.4 | 1 | Ref | |||||
| Yes | 188824 | 5032 | 2.7 | 1.24 | 1.19 | 1.28 | 8437 | 4.5 | 1.09 | 1.07 | 1.12 | 3118 | 1.7 | 0.97 | 0.93 | 1.02 | 5362 | 2.8 | 1.07 | 1.04 | 1.11 | 13960 | 7.4 | 0.99 | 0.97 | 1.01 | |
| 1st trimester bleeding | No | 2628264 | 31692 | 1.2 | 1 | Ref | 68462 | 2.6 | 1 | Ref | 27927 | 1.1 | 1 | Ref | 36477 | 1.4 | 1 | Ref | 107109 | 4.1 | 1 | Ref | |||||
| Yes | 48153 | 1564 | 3.2 | 2.10 | 1.99 | 2.22 | 1995 | 4.1 | 1.28 | 1.22 | 1.34 | 537 | 1.1 | 0.83 | 0.75 | 0.91 | 1120 | 2.3 | 1.10 | 1.03 | 1.17 | 2972 | 6.2 | 1.16 | 1.12 | 1.21 | |
| Previous cesarean section | No | 2560531 | 31040 | 1.2 | 1 | Ref | 66385 | 2.6 | 1 | Ref | 25783 | 1.0 | 1 | Ref | 33216 | 1.3 | 1 | Ref | 101631 | 4.0 | 1 | Ref | |||||
| Yes | 115886 | 2216 | 1.9 | 1.39 | 1.33 | 1.46 | 4072 | 3.5 | 1.27 | 1.22 | 1.31 | 2681 | 2.3 | 3.41 | 3.25 | 3.58 | 4381 | 3.8 | 6.08 | 5.82 | 6.35 | 8450 | 7.3 | 1.55 | 1.52 | 1.59 | |
| Start of labor | Spontaneous | 2247799 | 25951 | 1.2 | 1 | Ref | 54953 | 2.4 | 1 | Ref | 22128 | 1.0 | 1 | Ref | 26318 | 1.2 | 1 | Ref | 84815 | 3.8 | 1 | Ref | |||||
| Induction | 428618 | 7305 | 1.7 | 1.36 | 1.32 | 1.39 | 15504 | 3.6 | 1.41 | 1.38 | 1.43 | 6336 | 1.5 | 1.31 | 1.27 | 1.35 | 11279 | 2.6 | 1.87 | 1.82 | 1.91 | 25266 | 5.9 | 1.45 | 1.43 | 1.47 | |
| Birthweight (grams) | <2500 | 91074 | 1443 | 1.6 | 1.39 | 1.32 | 1.47 | 1073 | 1.2 | 0.42 | 0.39 | 0.45 | 245 | 0.3 | 0.22 | 0.19 | 0.25 | 191 | 0.2 | 0.12 | 0.11 | 0.14 | 3302 | 3.6 | 0.84 | 0.81 | 0.87 |
| 2500–2999 | 271661 | 2829 | 1.0 | 0.85 | 0.82 | 0.89 | 3579 | 1.3 | 0.45 | 0.44 | 0.47 | 1343 | 0.5 | 0.38 | 0.36 | 0.41 | 1178 | 0.4 | 0.23 | 0.22 | 0.25 | 7915 | 2.9 | 0.64 | 0.63 | 0.66 | |
| 3000–3499 | 845937 | 8638 | 1.0 | 0.83 | 0.81 | 0.86 | 16106 | 1.9 | 0.66 | 0.65 | 0.68 | 6710 | 0.8 | 0.64 | 0.62 | 0.67 | 7351 | 0.9 | 0.50 | 0.49 | 0.52 | 27869 | 3.3 | 0.75 | 0.73 | 0.76 | |
| 3500–3999 | 957654 | 11706 | 1.2 | 1 | Ref | 26825 | 2.8 | 1 | Ref | 11114 | 1.2 | 1 | Ref | 14538 | 1.5 | 1 | Ref | 41202 | 4.3 | 1 | Ref | ||||||
| 4000–4499 | 415535 | 6669 | 1.6 | 1.32 | 1.28 | 1.36 | 17389 | 4.2 | 1.56 | 1.52 | 1.59 | 6934 | 1.7 | 1.69 | 1.63 | 1.75 | 10539 | 2.5 | 1.98 | 1.92 | 2.03 | 22974 | 5.5 | 1.32 | 1.29 | 1.34 | |
| 4500–4999 | 83839 | 1727 | 2.1 | 1.71 | 1.62 | 1.80 | 4784 | 5.7 | 2.22 | 2.15 | 2.29 | 1845 | 2.2 | 2.59 | 2.46 | 2.73 | 3245 | 3.9 | 3.61 | 3.46 | 3.76 | 5886 | 7.0 | 1.75 | 1.70 | 1.80 | |
| ≥5000 | 10717 | 244 | 2.3 | 1.93 | 1.70 | 2.20 | 701 | 6.5 | 2.73 | 2.53 | 2.94 | 273 | 2.5 | 3.43 | 3.03 | 3.89 | 555 | 5.2 | 5.78 | 5.26 | 6.35 | 933 | 8.7 | 2.29 | 2.14 | 2.45 | |
| Fetal sex a | Girl | 1300887 | 18026 | 1.4 | 1 | Ref | 35733 | 2.7 | 1 | Ref | 13901 | 1.1 | 1 | Ref | 16634 | 1.3 | 1 | Ref | 54477 | 4.2 | 1 | Ref | |||||
| Boy | 1375459 | 15225 | 1.1 | 0.80 | 0.78 | 0.82 | 34722 | 2.5 | 0.92 | 0.90 | 0.93 | 14563 | 1.1 | 0.99 | 0.97 | 1.01 | 20962 | 1.5 | 1.18 | 1.16 | 1.21 | 55601 | 4.0 | 0.96 | 0.95 | 0.97 | |
| Placental weight (grams) b | <500 | 92475 | 3759 | 4.1 | 2.03 | 1.95 | 2.11 | 2361 | 2.6 | 0.72 | 0.68 | 0.75 | 1223 | 1.3 | 0.74 | 0.80 | 0.75 | 1175 | 1.3 | 0.52 | 0.48 | 0.55 | 5623 | 6.1 | 0.89 | 0.87 | 0.92 |
| 500–699 | 488935 | 9892 | 2.0 | 1 | Ref | 17118 | 3.5 | 1 | Ref | 8319 | 1.7 | 1 | Ref | 10732 | 2.2 | 1 | Ref | 31685 | 6.5 | 1 | Ref | ||||||
| 700–899 | 328966 | 6216 | 1.9 | 0.93 | 0.90 | 0.96 | 17552 | 5.3 | 1.55 | 1.51 | 1.59 | 7201 | 2.2 | 1.42 | 1.37 | 1.48 | 13355 | 4.1 | 2.07 | 2.02 | 2.12 | 27784 | 8.4 | 1.37 | 1.34 | 1.39 | |
| 900–1099 | 64314 | 1451 | 2.3 | 1.12 | 1.06 | 1.19 | 4995 | 7.8 | 2.32 | 2.24 | 2.40 | 1837 | 2.9 | 2.01 | 1.89 | 2.13 | 4175 | 6.5 | 3.74 | 3.60 | 3.89 | 6751 | 10.5 | 1.78 | 1.73 | 1.83 | |
| ≥1100 | 9934 | 270 | 2.7 | 1.37 | 1.21 | 1.54 | 933 | 9.4 | 2.85 | 2.65 | 3.06 | 336 | 3.4 | 2.59 | 2.28 | 2.94 | 746 | 7.5 | 4.62 | 4.26 | 5.00 | 1245 | 12.5 | 2.20 | 2.07 | 2.34 | |
| Velamentous cord insertion c | No | 992869 | 21427 | 2.2 | 1 | Ref | 42830 | 4.3 | 1 | Ref | 18809 | 1.9 | 1 | Ref | 30159 | 3.0 | 1 | Ref | 73055 | 7.4 | 1 | Ref | |||||
| Yes | 14907 | 1006 | 6.7 | 3.00 | 2.79 | 3.21 | 828 | 5.6 | 1.26 | 1.17 | 1.36 | 356 | 2.4 | 1.21 | 1.07 | 1.37 | 517 | 3.5 | 0.99 | 0.90 | 1.09 | 1336 | 9.0 | 1.24 | 1.17 | 1.31 | |
| Marginal umbilical cord insertion c | No | 953165 | 20948 | 2.2 | 1 | Ref | 40821 | 4.3 | 1 | Ref | 17907 | 1.9 | 1 | Ref | 28818 | 3.0 | 1 | Ref | 70568 | 7.4 | 1 | Ref | |||||
| Yes | 54611 | 1485 | 2.7 | 1.26 | 1.19 | 1.33 | 2837 | 5.2 | 1.24 | 1.19 | 1.29 | 1258 | 2.3 | 1.25 | 1.17 | 1.33 | 1858 | 3.4 | 1.12 | 1.06 | 1.18 | 3823 | 7.0 | 1.03 | 0.90 | 1.06 | |
CI confidence interval, aOR: OR adjusted for maternal age, parity and period (1967–1977, 1978–1987, 1988–1997, 1998–2007 and 2008–2017).
a 71 newborns with unknown sex. The negative association with PPH due to trauma or laceration disappeared after adjusting for unmeasured confounder or stratification by preterm/term delivery.
b 1999–2017, 23152 without placental weight.
c 1999–2017.
Table 5. Risk of postpartum hemorrhage (PPH>500ml) due to dystocia in second deliveries without previous cesarean section (CS) (reference), second deliveries with previous CS, and first deliveries; singleton births, ≥22 weeks of gestation and spontaneous onset or induction of labor.
| Total | Dystocia related PPH | |||||
|---|---|---|---|---|---|---|
| Groups | (n) | (n) | % | aOR | 95% CI | |
| 2nd delivery without previous CS | 864751 | 3146 | 0.4 | 1 | Ref | |
| 2nd delivery with previous CS | 71240 | 3692 | 5.2 | 18.85 | 17.84 | 19.92 |
| 1st delivery | 1124388 | 28690 | 2.6 | 9.10 | 8.72 | 9.48 |
CI confidence interval, aOR OR adjusted for maternal age and period (1967–1977, 1978–1987, 1988–1997, 1998–2007 and 2008–2017).
First trimester bleeding was associated with a doubled risk of PPH due to retained placenta and had weaker association with PPH due to atony and without defined cause.
The risks of PPH types included in Table 3 increased with birthweight, especially PPH due to dystocia, obstetric trauma and atony. Including parity, maternal age and year of delivery in the models strengthened the associations, mainly for PPH due to dystocia and obstetric trauma. In term but not preterm deliveries, low birthweight (<2500g) was associated with PPH due to retained placenta and/or membranes.
Exploring the effect of fetal sex on the PPH types, we found that the risk of PPH was lower if the newborn was a boy. This association was strongest for PPH due to retained placenta (aOR: 0.80, 95% CI 0.78–0.82), followed by atony (aOR 0.92, 95% CI: 0.90–0.93) and undefined cause of PPH (aOR 0.96, 95% CI: 0.95–0.97). These associations were similar in strata of birthweight (<2500g, 2500–2999g, 3000–3499g, 3500–4000g, 4000–4499g, 4500–4999 g, ≥5000g). Adjusted OR for PPH due to obstetric trauma was also lower for deliveries of a boy, but this effect was only significant in weight groups between 3000 and 4499g. However, if the newborn was a boy, there was increased risk of PPH due to dystocia, but this association disappeared after stratification according to birthweight.
The association between placenta weight categories and the specific causes of PPH generally showed a pattern like that of birthweight.
Velamentous and marginal umbilical placental cord insertion were strongest associated with PPH due to retained placenta. This effect was significantly stronger for velamentous- (aOR: 3.1, 95% CI: 2.9–3.4) than marginal cord insertion (aOR: 1.3, 95% CI: 1.2–1.3).
Table 4 shows the risk of PPH types in the second delivery (except for PPH caused by placental abruption and placenta previa) according to PPH types in the first delivery and pregnancy and birth related factors.
Table 4. Risk of type specific postpartum hemorrhage (PPH>500ml) in the second delivery according to PPH types in the first delivery and pregnancy- and birth characteristic; singleton births, ≥22 weeks of gestation and spontaneous onset or induction of labor.
| Type of PPH in second delivery | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of PPH in first delivery and pregnancy/birth related exposures | Total | Retained placenta | Atony | Trauma or laceration | Dystocia | Undefined bleeding cause | |||||||||||||||||||||
| (n) | (n) | % | aOR | 95% CI | (n) | % | aOR | 95% CI | (n) | % | aOR | 95% CI | (n) | % | aOR | 95% CI | (n) | % | aOR | 95% CI | |||||||
| Retained placenta | No | 785902 | 9107 | 1.2 | 1 | Ref | 19178 | 2.4 | 1 | Ref | 5795 | 0.7 | 1 | Ref | 4811 | 0.6 | 1 | Ref | 30674 | 3.9 | 1 | Ref | |||||
| Yes | 8788 | 734 | 8.4 | 5.90 | 5.45 | 6.39 | 729 | 8.3 | 2.93 | 2.71 | 3.17 | 155 | 1.8 | 1.77 | 1.50 | 2.08 | 132 | 1.5 | 1.56 | 1.31 | 1.86 | 841 | 9.6 | 1.94 | 1.80 | 2.09 | |
| Atony | No | 773349 | 8950 | 1.2 | 1 | Ref | 17745 | 2.3 | 1 | Ref | 5517 | 0.7 | 1 | Ref | 4612 | 0.6 | 1 | Ref | 29601 | 3.8 | 1 | Ref | |||||
| Yes | 21341 | 891 | 4.2 | 2.91 | 2.71 | 3.13 | 2162 | 10.1 | 4.00 | 3.82 | 4.20 | 433 | 2.0 | 2.15 | 1.95 | 2.37 | 331 | 1.6 | 1.77 | 1.58 | 1.98 | 1914 | 9.0 | 1.90 | 1.81 | 1.99 | |
| Trauma or laceration | No | 783198 | 9475 | 1.2 | 1 | Ref | 19031 | 2.4 | 1 | Ref | 5513 | 0.7 | 1 | Ref | 4855 | 0.6 | 1 | Ref | 30415 | 3.9 | 1 | Ref | |||||
| Yes | 11492 | 366 | 3.2 | 2.01 | 1.81 | 2.24 | 876 | 7.6 | 2.65 | 2.47 | 2.85 | 437 | 3.8 | 3.86 | 3.49 | 4.27 | 88 | 0.8 | 0.77 | 0.63 | 0.96 | 1100 | 9.6 | 1.89 | 1.77 | 2.02 | |
| Dystocia | No | 780869 | 9251 | 1.2 | 1 | Ref | 18800 | 2.4 | 1 | Ref | 5498 | 0.7 | 1 | Ref | 4080 | 0.5 | 1 | Ref | 29915 | 3.8 | 1 | Ref | |||||
| Yes | 13821 | 590 | 4.3 | 2.44 | 2.24 | 2.66 | 1107 | 8.0 | 2.58 | 2.42 | 2.75 | 452 | 3.3 | 2.98 | 2.70 | 3.29 | 863 | 6.2 | 6.81 | 6.31 | 7.36 | 1600 | 11.6 | 2.09 | 1.98 | 2.21 | |
| Undefined bleeding cause | No | 764798 | 9082 | 1.2 | 1 | Ref | 18281 | 2.4 | 1 | Ref | 5419 | 0.7 | 1 | Ref | 4501 | 0.6 | 1 | Ref | 28367 | 3.7 | 1 | Ref | |||||
| Yes | 29892 | 759 | 2.5 | 1.64 | 1.52 | 1.77 | 1626 | 5.4 | 1.90 | 1.80 | 2.00 | 531 | 1.8 | 1.80 | 1.64 | 1.97 | 442 | 1.5 | 1.62 | 1.46 | 1.79 | 3148 | 10.5 | 2.21 | 2.12 | 2.30 | |
| Inter-delivery interval (year) | <1 | 3433 | 56 | 1.6 | 2.00 | 1.53 | 2.62 | 64 | 1.9 | 0.90 | 0.70 | 1.15 | 10 | 0.3 | 0.57 | 0.31 | 1.07 | 12 | 0.3 | 1.14 | 0.64 | 2.02 | 118 | 3.4 | 1.01 | 0.84 | 1.22 |
| 1 to <2 | 151853 | 1815 | 1.2 | 1.08 | 1.01 | 1.14 | 3723 | 2.5 | 0.99 | 0.95 | 1.03 | 1001 | 0.7 | 0.92 | 0.86 | 1.00 | 809 | 0.5 | 1.00 | 0.91 | 1.09 | 5905 | 3.9 | 0.98 | 0.85 | 1.02 | |
| 2 to <3 | 239848 | 2924 | 1.2 | 1 | Ref | 6255 | 2.6 | 1 | Ref | 1906 | 0.8 | 1 | Ref | 1507 | 0.6 | 1 | Ref | 9898 | 4.1 | 1 | Ref | ||||||
| 3 to <4 | 166293 | 2118 | 1.3 | 1.08 | 1.02 | 1.15 | 4208 | 2.5 | 1.01 | 0.97 | 1.05 | 1319 | 0.8 | 1.05 | 0.98 | 1.12 | 968 | 0.6 | 0.98 | 0.91 | 1.07 | 6578 | 4.0 | 1.01 | 0.98 | 1.05 | |
| 4 to <5 | 88863 | 1046 | 1.2 | 1.02 | 0.95 | 1.10 | 2128 | 2.4 | 0.97 | 0.92 | 1.02 | 627 | 0.7 | 0.96 | 0.88 | 1.05 | 557 | 0.6 | 1.10 | 1.00 | 1.21 | 3364 | 3.8 | 1.01 | 0.97 | 1.05 | |
| ≥5 | 144400 | 1882 | 1.3 | 1.00 | 0.94 | 1.06 | 3529 | 2.4 | 0.93 | 0.89 | 0.97 | 1087 | 0.8 | 0.95 | 0.88 | 1.02 | 1090 | 0.8 | 1.10 | 1.02 | 1.19 | 5652 | 3.9 | 1.00 | 0.96 | 1.03 | |
| Change of father a | No | 713133 | 8704 | 1.2 | 1 | Ref | 17912 | 2.5 | 1 | Ref | 5351 | 0.8 | 1 | Ref | 4303 | 0.6 | 1 | Ref | 28160 | 3.9 | 1 | Ref | |||||
| Yes | 69371 | 981 | 1.4 | 1.00 | 0.94 | 1.07 | 1676 | 2.4 | 0.86 | 0.81 | 0.90 | 515 | 0.7 | 0.88 | 0.80 | 0.96 | 516 | 0.7 | 1.02 | 0.93 | 1.12 | 2808 | 4.0 | 0.93 | 0.90 | 0.97 | |
| Previous cesarean b | No | 743587 | 8893 | 1.2 | 1 | Ref | 18127 | 2.4 | 1 | Ref | 4518 | 0.6 | 1 | Ref | 2270 | 0.3 | 1 | Ref | 27723 | 3.7 | 1 | Ref | |||||
| Yes | 65619 | 1312 | 2.0 | 1.35 | 1.28 | 1.44 | 2368 | 3.6 | 1.28 | 1.23 | 1.34 | 1929 | 2.9 | 4.00 | 3.79 | 4.23 | 3368 | 5.1 | 13.16 | 12.46 | 13.89 | 5125 | 7.8 | 1.84 | 1.78 | 1.90 | |
CI confidence interval, aOR OR adjusted for maternal age, parity and period (1967–1977, 1978–1987, 1988–1997, 1998–2007 and 2008–2017).
a 12186 births with unknown father in 1st or 2nd delivery.
b First deliveries starting with cesarean section included.
The risk of recurrent PPH was strongest for the same type. PPH associated with dystocia had highest risk of recurrence (aOR: 6.8, 95% CI: 6.3–7.4), followed by PPH due to retained placenta and/or membranes (aOR: 5.9, 95% CI: 5.5–6.4), atony (aOR: 4.0, 95% CI: 3.8–4.2) and obstetric trauma (aOR: 3.9, 95% CI: 3.5–4.3), while PPH of undefined cause had lowest risk of recurrence (aOR: 2.2, 95% CI: 2.1–2.3) (Table 4).
Exploring effects of pregnancy related factors on the PPH types in the second delivery, we found that inter-delivery interval had no significant effect on the PPH risk in second delivery, except for PPH due to retained placenta where a short inter-delivery interval (less than one year) was associated with a doubled risk (aOR: 2.0, 95% CI: 1.5–2.6).
Change of father slightly decreased ORs of PPH due to obstetric trauma, atony and undefined bleeding cause. Additional adjustment for inter-delivery interval did not influence the associations.
A previous cesarean delivery was associated with a marked increased risk of PPH due to dystocia, (aOR of 13.2, 95% CI: 12.5–13.9), and a weaker association with PPH caused by obstetric trauma (aOR: 4.0, 95% CI: 3.8–4.2), undefined PPH, retained placenta and atony (aORs between 1.3 and 1.8). In additional analyses we compared risks of PPH associated with dystocia in three groups: second deliveries without previous cesarean section (reference), second deliveries with previous cesarean section, and first deliveries (Table 5). We found that the risk of PPH due to dystocia was higher in women with a previous cesarean (vaginal primiparas) than in primiparas.
Our sensitivity analyses (S1 File) indicated that the associations described in Tables 3 and 4 persisted after adjusting for potential unmeasured confounders, and that false positive associations due to multiple testing were not present.
Discussion
Main findings
We found that maternal, fetal and obstetric characteristics had differential effects on the types of PPH. The risk of recurrence differed considerably between the PPH types; the strongest recurrence risks were found for PPH caused by dystocia, retained placenta and atony. PPH due to retained placenta was most prone to develop into severe PPH.
Strengths and limitations
A main strength of the study was the long study period with mandatory registration of all births in the country, and with almost complete record linkage, which made it possible to do comprehensive sub-analyses. We also consider it a strength that it has been possible to classify clinically relevant causes of PPH since the inception of the registry. The population-based design and prospective collection of data attenuate selection and recall bias. Ethically, this is the study design of choice as we investigate a potentially life-threatening outcome [28]. Furthermore, the PPH-variable has been validated and found to be of adequate quality for epidemiological studies [29]. The robustness of our results for potentially unknown confounding variables, assessed in the sensitivity analyses, is reassuring.
The introduction of activity-based financing and update of the MBRN registration form in 1999 may have improved the registration and contributed to the increased occurrence of PPH without specified cause after 1999, representing 29.2 percent of all registered PPH cases in the total study period.
It is possible that misclassification between types of PPH occurs, for example between retained placenta and atony. We expect that such misclassification to be non-differential and would therefore not affect the ORs. Coexistence of more than one PPH type in a delivery, for example atony and obstetric trauma caused by macrosomia is plausible, and there was no upper limit for registration of types of PPH in each delivery.
Previous studies
International variation and demographic factors
In contrast to the situation worldwide, the maternal mortality rate of PPH in Norway is low [1,30], which may limit the generalizability of our results. However, in other settings we assume that proportions of severe bleeding in the different types of PPH may show similar pattern.
There are considerable differences in the reported proportions of PPH types in the literature, especially for PPH caused by atony and retained placenta. Bateman et al. [12] and Widmer et al. [2] reported that 79% and 62% of all registered PPH cases (>500ml and refractory PPH, respectively) were accounted for by atony, which is in contrast with our findings (23% PPH due to atony) (Table 1). Our result is more in line with the 41% due to atony in a Swedish study (>1000ml) [3]. The proportion of PPH due to retained placenta in our study (11.4%) is in line with other studies [5,12]. Oberg et al. reported that 33.5% of PPH cases were due to retained placenta, which is comparable to our results in severe PPH (25.7% due to retained placenta) [3]. Additionally, a Danish study found higher proportions of retained placenta in severe PPH, compared to milder PPH [5], and a Turkish study found retained placenta to be associated with severe PPH [31]. These results agree with our results that PPH due to retained placenta most often caused severe PPH (Table 1).
These inter-study variations may be caused by differences in code availability or definitions of excessive bleeding, although it cannot be ruled out that variations of population genetic and/or environmental properties, or medical culture, may also play a role.
Our results confirm that maternal age was associated with all types of PPH (with the strongest association for PPH caused by dystocia). The effect of maternal age on PPH caused by atony are in line with existing knowledge [2,4,6,7,32]. Studies on associations between maternal age and other types of PPH are scarce, but an association with retained placenta in general has been reported [32]. Parity had strongest effect on PPH due to dystocia; 76% of the cases were primiparas, which agrees with the higher risk of dystocia in nulliparas [33].
As dystocia may result in uterine fatigue and atony, PPH due to dystocia may have been classified as atony in studies where dystocia is not recorded in the databases. This may, at least in part, explain the very high proportion of PPH due to atony found in some studies [12].
Pregnancy-related factors
We found a slightly reduced risk of recurrent PPH (caused by obstetric trauma and atony and undefined bleeding cause) in mothers who had changed partner, also after adjusting for inter-delivery interval. This fits with our previous findings of a weak but significant paternal effect on recurrent PPH [10]. In the present study there was a significantly increased risk of PPH due to retained placenta when the inter-delivery interval was short (less than one year). This contrast findings regarding PPH in general, where inter-delivery interval had a negligible effect on recurrence [10].
The association of first trimester bleeding and PPH caused by retained placenta is consistent with results from previous studies that retained placenta [34] and PPH in general [35] are associated with threatened abortion.
Obstetric history (including recurrence)
Recurrence risk of PPH due to retained placenta [3,5,8,11], atony and laceration [3], and increased duration and pushing time of the second stage of labor have been associated with PPH [36], which is in line with our results. However, we found that PPH caused by dystocia was the PPH type most prone to recur, which to our knowledge has not been reported before.
A history of cesarean section has been linked to risk of retained placenta in general [6,13,16] and atonic PPH [12], but not consistently [8,37]. In our population women with a previous cesarean carried increased risk of all causes of PPH, but the strongest association was found with dystocia PPH (Table 4).
Complications related to the fetus, placenta, membranes and umbilical cord
The finding that birthweight has a strong association to PPH (Table 3) is in line with previous findings [10,38,39]. However, a new finding was that the strength of associations markedly varied with type of PPH, and that birthweight had the strongest association with PPH due to dystocia.
Sex differences in properties of placenta, umbilical cord and birthweight are well known [40–44]. We found a strong effect of fetal sex on most types of PPH and especially for PPH caused by retained placenta. This was a new finding and is consistent with previous findings that delivery of girls carries higher risk of retained placenta in general [8,13] and PPH due to atony [45].
As expected, PPH without specific cause was dominated by mild cases. Its low risk of recurrence is in line with the concept that a mild phenotype of a polygenic trait or disease is generally less prone to recur than a severe phenotype [46]. This suggests that most of these cases were correctly assigned to the group.
Interpretation
Risk factors for dystocia, with and without PPH, have previously been reported [36,47–49], but the strong recurrence risk of PPH due to dystocia has to our knowledge not been studied before. As dystocia may be an indication for operative delivery, this may result in PPH due to trauma to the birth canal. The recurrence risk of PPH due to dystocia may be caused by sustained or recurrent factors associated with PPH or indicative of operative delivery, such as tendency to deliver large babies and fetopelvic disproportion. Further, dystocia may lead to atonic PPH through exhausting workload on the uterus without adequate progression of labor.
It is reasonable to assume that the placenta accreta spectrum constitutes some of the cases of severe PPH in the retained placenta group. However, we do not have exact information on the occurrence of placenta accreta spectrum in our population, and this was beyond the scope of our study. Another possible explanation for the higher occurrence of severe PPH among women with PPH due to retained placenta is the lack of effective initial medical treatment, along with the need of surgical intervention which may be delayed. In contrast, atony often is sufficiently treated with medications.
A previous cesarean was strongly associated with PPH due to dystocia in the second delivery, and we also found associations with PPH due to obstetric trauma, retained placenta and atony. The risk of PPH due to dystocia was higher than in nulliparas. A possible explanation for the association of previous cesarean section with PPH due to dystocia may be ineffective labor contractions due to the uterine scar, and that no previous vaginal delivery may mimic a primipara, with increased risk of delayed progression in labor and exhaustion of uterine contractility. One may speculate that the association of previous cesarean section with PPH due to retained placenta is associated with an early stage of abnormally invasive placenta, consistent with the increased risk of abnormally invasive placenta in women with previous cesarean section [50].
We found that birthweight was associated with all types of PPH, but especially PPH due to dystocia, birth canal lacerations and uterine atony. This was expected, as macrosomia is associated with PPH through distention of the uterus and large utero-placental wound surface [2,10,14,51]. In addition, macrosomia may increase tension on maternal tissue during labor leading to increased risk of obstetric trauma [52]. Another explanatory mechanism is that fetal macrosomia, dystocia and atony may be indications for operative vaginal delivery and result in surgical bleeding.
A possible explanation for the reduced risk of PPH due to retained placenta if the newborn was a boy (Table 3) may be the more inadequate transformation of the uterine spiral arteries in pregnancies with male fetus [53–55]. This agrees with the fetal sex preponderance in complications of the placenta, like placental abruption [45] and preeclampsia [56], although not consistently for the latter [45].
To increase the relevance for clinical practice we analyzed deliveries with spontaneous onset or induction of labor, thus excluding cesarean sections before the onset of labor. Deliveries with PPH due to placenta previa or placental abruption are underrepresented in our material (only 2% of PPH cases) since they primarily are delivered by cesarean section before labor and were therefore not included in the main analyses.
The substantial variation of reported incidence of causes of PPH among populations call for initiatives to unite the international definitions and improve the understanding of PPH pathophysiological mechanism.
We have already addressed the need of alertness when a delivering woman or her relatives has experienced PPH [9,10]. Based on our present results, we encourage special attention concerning PPH due to retention of placenta or membranes, as its recurrence risk is high, and that a retained placenta carried the highest risk of severe PPH.
PPH due to retention of placenta or membranes was related to velamentous and marginal umbilical cord insertion in a dose-response-pattern with strongest association to velamentous insertion. Both conditions are possible to diagnose by ultrasonography during pregnancy [57]. Thus, prenatal identification of an abnormal cord insertion may serve to alert clinicians and enhance their preparedness.
We found a strong association between previous cesarean section and PPH due to dystocia, and that it was likely to recur from the first to the second delivery. Dystocia is widely ignored as a cause of PPH in the literature, but our study indicates that a history of PPH due to dystocia should be included in risk assessment for PPH.
Conclusions
In this large population-based study we found that maternal, fetal and obstetric characteristics had differential effects on types of PPH. Recurrence differed considerably between PPH types. Retained placenta was most frequently registered with severe PPH, and showed strongest effect of sex; delivery of a boy was associated with lower risk of PPH. Previous cesarean increased the risk of PPH due to dystocia.
Our research adds to the understanding of recurrence risk of PPH and suggests that PPH can be inherited. In future studies genetic influence on specific types of PPH needs to be disentangled from environmental influence.
Supporting information
(DOCX)
Acknowledgments
A patient (Liv Kristin Heggheim) and a general practitioner (Stian Langeland Wesnes, MD, PhD) were involved from the planning stage of the project. The research group discussed the core research questions, outcome measures, design and results of the study with these two persons by correspondence and in meeting. We thank the user representatives for their effort and interest.
Abbreviations
- aOR
adjusted odds ratio
- CI
confidence interval
- MBRN
Medical Birth Registry of Norway
- OR
odds ratio
- PPH
postpartum hemorrhage
Data Availability
Availability of data and material: Legal restrictions do not permit the authors to provide the data that constitute the basis of this study. The main data utilized are available from the data owner, the Norwegian Institute of Public Health (https://www.fhi.no/en/more/research--access-to-data/), after obtaining approval from The Regional Committee for Medical Research Ethics (https://rekportalen.no/), for researchers who meet the criteria for access to confidential data. Contact information: The Medical Birth Registry of Norway, University of Bergen, P.O. Box 7804, 5020 Bergen, Norway. Code availability: The data are confidential and cannot be shared.
Funding Statement
L.E.L. is employed in a position at the University of Bergen: a 4-year Doctoral Research Fellowship. The research file was financed by a research grant from The Western Norway Regional Health Authority (project no. 990226). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. The Lancet Global Health. 2014;2(6):e323–e33. doi: 10.1016/S2214-109X(14)70227-X [DOI] [PubMed] [Google Scholar]
- 2.Widmer M, Piaggio G, Hofmeyr GJ, Carroli G, Coomarasamy A, Gallos I, et al. Maternal characteristics and causes associated with refractory postpartum haemorrhage after vaginal birth: a secondary analysis of the WHO CHAMPION trial data. BJOG. 2020;127(5):628–34. doi: 10.1111/1471-0528.16040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oberg AS, Hernandez-Diaz S, Palmsten K, Almqvist C, Bateman BT. Patterns of recurrence of postpartum hemorrhage in a large population-based cohort. Am J Obstet Gynecol. 2014;210(3):229 e1-8. doi: 10.1016/j.ajog.2013.10.872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lisonkova S, Mehrabadi A, Allen VM, Bujold E, Crane JM, Gaudet L, et al. Atonic Postpartum Hemorrhage: Blood Loss, Risk Factors, and Third Stage Management. J Obstet Gynaecol Can. 2016;38(12):1081–90 e2. doi: 10.1016/j.jogc.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 5.Edwards HM, Svare JA, Wikkelso AJ, Lauenborg J, Langhoff-Roos J. The increasing role of a retained placenta in postpartum blood loss: a cohort study. Arch Gynecol Obstet. 2019;299(3):733–40. doi: 10.1007/s00404-019-05066-3 [DOI] [PubMed] [Google Scholar]
- 6.Mehrabadi A, Hutcheon JA, Lee L, Kramer MS, Liston RM, Joseph KS. Epidemiological investigation of a temporal increase in atonic postpartum haemorrhage: a population-based retrospective cohort study. BJOG. 2013;120(7):853–62. doi: 10.1111/1471-0528.12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ende HB, Lozada MJ, Chestnut DH, Osmundson SS, Walden RL, Shotwell MS, et al. Risk Factors for Atonic Postpartum Hemorrhage: A Systematic Review and Meta-analysis. Obstet Gynecol. 2021;137(2):305–23. doi: 10.1097/AOG.0000000000004228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenbaum S, Wainstock T, Dukler D, Leron E, Erez O. Underlying mechanisms of retained placenta: Evidence from a population based cohort study. Eur J Obstet Gynecol Reprod Biol. 2017;216:12–7. doi: 10.1016/j.ejogrb.2017.06.035 [DOI] [PubMed] [Google Scholar]
- 9.Linde LE, Ebbing C, Moster D, Kessler J, Baghestan E, Gissler M, et al. Recurrence of postpartum hemorrhage in relatives: A population-based cohort study. Acta Obstet Gynecol Scand. 2021;100(12):2278–84. doi: 10.1111/aogs.14262 [DOI] [PubMed] [Google Scholar]
- 10.Linde LE, Ebbing C, Moster D, Kessler J, Baghestan E, Gissler M, et al. Recurrence of postpartum hemorrhage, maternal and paternal contribution, and the effect of offspring birthweight and sex: a population-based cohort study. Arch Gynecol Obstet. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thams AB, Larsen MH, Rasmussen SC, Jeppegaard M, Krebs L. Incidence of postpartum hemorrhage and risk factors for recurrence in the subsequent pregnancy. Arch Gynecol Obstet. 2022. doi: 10.1007/s00404-022-06591-4 [DOI] [PubMed] [Google Scholar]
- 12.Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110(5):1368–73. doi: 10.1213/ANE.0b013e3181d74898 [DOI] [PubMed] [Google Scholar]
- 13.Granfors M, Sandstrom A, Stephansson O, Belachew J, Axelsson O, Wikstrom AK. Placental location and risk of retained placenta in women with a previous cesarean section: A population-based cohort study. Acta Obstet Gynecol Scand. 2020;99(12):1666–73. doi: 10.1111/aogs.13943 [DOI] [PubMed] [Google Scholar]
- 14.Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115(10):1265–72. doi: 10.1111/j.1471-0528.2008.01859.x [DOI] [PubMed] [Google Scholar]
- 15.Nyflot LT, Sandven I, Stray-Pedersen B, Pettersen S, Al-Zirqi I, Rosenberg M, et al. Risk factors for severe postpartum hemorrhage: a case-control study. BMC Pregnancy Childbirth. 2017;17(1):17. doi: 10.1186/s12884-016-1217-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer R, Rottenstreich A, Tsur A, Cahan T, Levin G. Risk factors for third stage placental complications among primigravid women. Placenta. 2020;99:16–20. doi: 10.1016/j.placenta.2020.07.019 [DOI] [PubMed] [Google Scholar]
- 17.Humphrey MD. Is grand multiparity an independent predictor of pregnancy risk? A retrospective observational study. Med J Aust. 2003;179(6):294–6. [PubMed] [Google Scholar]
- 18.Kramer MS, Dahhou M, Vallerand D, Liston R, Joseph KS. Risk Factors for Postpartum Hemorrhage: Can We Explain the Recent Temporal Increase? Journal of Obstetrics and Gynaecology Canada. 2011;33(8):810–9. doi: 10.1016/S1701-2163(16)34984-2 [DOI] [PubMed] [Google Scholar]
- 19.Oberg AS, Hernandez-Diaz S, Frisell T, Greene MF, Almqvist C, Bateman BT. Genetic contribution to postpartum haemorrhage in Swedish population: cohort study of 466,686 births. BMJ. 2014;349:g4984. doi: 10.1136/bmj.g4984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebbing C, Kiserud T, Johnsen SL, Albrechtsen S, Rasmussen S. Third stage of labor risks in velamentous and marginal cord insertion: a population-based study. Acta Obstet Gynecol Scand. 2015;94(8):878–83. doi: 10.1111/aogs.12666 [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen S, Linde LE, Ebbing C. Recurrence of idiopathic polyhydramnios: A nationwide population study. Int J Gynaecol Obstet. 2022;157(1):198–9. doi: 10.1002/ijgo.14001 [DOI] [PubMed] [Google Scholar]
- 22.Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000;79(6):435–9. [PubMed] [Google Scholar]
- 23.Glover P. Blood loss at delivery: how accurate is your estimation? Aust J Midwifery. 2003;16(2):21–4. doi: 10.1016/s1031-170x(03)80005-3 [DOI] [PubMed] [Google Scholar]
- 24.Rath WH. Postpartum hemorrhage—update on problems of definitions and diagnosis. Acta Obstet Gynecol Scand. 2011;90(5):421–8. doi: 10.1111/j.1600-0412.2011.01107.x [DOI] [PubMed] [Google Scholar]
- 25.Deneux-Tharaux C, Macfarlane A, Winter C, Zhang WH, Alexander S, Bouvier-Colle MH, et al. Policies for manual removal of placenta at vaginal delivery: variations in timing within Europe. BJOG. 2009;116(1):119–24. doi: 10.1111/j.1471-0528.2008.01996.x [DOI] [PubMed] [Google Scholar]
- 26.Breathnach F, Geary M. Uterine atony: definition, prevention, nonsurgical management, and uterine tamponade. Semin Perinatol. 2009;33(2):82–7. doi: 10.1053/j.semperi.2008.12.001 [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization: Intrapartum care for a positive childbirth experience. Geneva: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO. Available in https://www.who.int/reproductivehealth/publications/intrapartum-care-guidelines/en/. [Google Scholar]
- 28.Booth CM, Tannock IF. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Cancer. 2014;110(3):551–5. doi: 10.1038/bjc.2013.725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engjom H, Klungsøyr K, Ebbing M. Alvorlige komplikasjoner hos kvinnen ved svangerskap og fødsel. Validering og rutiner for kobling mellom MFR og NPR. https://hrr.w.uib.no/hrr-reports/: Health Registries for Research, Norway; 2018 30.04.2018. [Google Scholar]
- 30.Bank TW. Maternal mortality ratio (model estimate, per 100,000 live births)—Norway data.worldbank.org2021 [cited 2021 october]. Available from: https://data.worldbank.org/indicator/SH.STA.MMRT?end=2017&locations=NO&start=2000. [Google Scholar]
- 31.Ekin A, Gezer C, Solmaz U, Taner CE, Dogan A, Ozeren M. Predictors of severity in primary postpartum hemorrhage. Arch Gynecol Obstet. 2015;292(6):1247–54. doi: 10.1007/s00404-015-3771-5 [DOI] [PubMed] [Google Scholar]
- 32.Favilli A, Tosto V, Ceccobelli M, Parazzini F, Franchi M, Bini V, et al. Risk factors for non-adherent retained placenta after vaginal delivery: a systematic review. BMC Pregnancy Childbirth. 2021;21(1):268. doi: 10.1186/s12884-021-03721-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selin L, Wallin G, Berg M. Dystocia in labour—risk factors, management and outcome: a retrospective observational study in a Swedish setting. Acta Obstet Gynecol Scand. 2008;87(2):216–21. doi: 10.1080/00016340701837744 [DOI] [PubMed] [Google Scholar]
- 34.Hertz JB, Heisterberg L. The Outcome of Pregnancy after Threatened-Abortion. Acta Obstet Gyn Scan. 1985;64(2):151–6. doi: 10.3109/00016348509154709 [DOI] [PubMed] [Google Scholar]
- 35.Wijesiriwardana A, Bhattacharya S, Shetty A, Smith N, Bhattacharya S. Obstetric outcome in women with threatened miscarriage in the first trimester. Obstetrics and Gynecology. 2006;107(3):557–62. doi: 10.1097/01.AOG.0000199952.82151.de [DOI] [PubMed] [Google Scholar]
- 36.Looft E, Simic M, Ahlberg M, Snowden JM, Cheng YW, Stephansson O. Duration of Second Stage of Labour at Term and Pushing Time: Risk Factors for Postpartum Haemorrhage. Paediatr Perinat Epidemiol. 2017;31(2):126–33. doi: 10.1111/ppe.12344 [DOI] [PubMed] [Google Scholar]
- 37.Belachew J, Cnattingius S, Mulic-Lutvica A, Eurenius K, Axelsson O, Wikstrom AK. Risk of retained placenta in women previously delivered by caesarean section: a population-based cohort study. BJOG. 2014;121(2):224–9. doi: 10.1111/1471-0528.12444 [DOI] [PubMed] [Google Scholar]
- 38.Ghosh RE, Berild JD, Sterrantino AF, Toledano MB, Hansell AL. Birth weight trends in England and Wales (1986–2012): babies are getting heavier. Arch Dis Child Fetal Neonatal Ed. 2018;103(3):F264–F70. doi: 10.1136/archdischild-2016-311790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ford JB, Roberts CL, Bell JC, Algert CS, Morris JM. Postpartum haemorrhage occurrence and recurrence: a population-based study. Med J Aust. 2007;187(7):391–3. doi: 10.5694/j.1326-5377.2007.tb01308.x [DOI] [PubMed] [Google Scholar]
- 40.Linde LE, Rasmussen S, Kessler J, Ebbing C. Extreme umbilical cord lengths, cord knot and entanglement: Risk factors and risk of adverse outcomes, a population-based study. PLoS One. 2018;13(3):e0194814. doi: 10.1371/journal.pone.0194814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acharya G, Ebbing C, Karlsen HO, Kiserud T, Rasmussen S. Sex-specific reference ranges of cerebroplacental and umbilicocerebral ratios: A longitudinal study. Ultrasound Obstet Gynecol. 2019. [DOI] [PubMed] [Google Scholar]
- 42.Ebbing C, Kiserud T, Johnsen SL, Albrechtsen S, Rasmussen S. Prevalence, risk factors and outcomes of velamentous and marginal cord insertions: a population-based study of 634,741 pregnancies. PLoS One. 2013;8(7):e70380. doi: 10.1371/journal.pone.0070380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Broere-Brown ZA, Adank MC, Benschop L, Tielemans M, Muka T, Goncalves R, et al. Fetal sex and maternal pregnancy outcomes: a systematic review and meta-analysis. Biol Sex Differ. 2020;11(1):26. doi: 10.1186/s13293-020-00299-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leon-Garcia SM, Roeder HA, Nelson KK, Liao X, Pizzo DP, Laurent LC, et al. Maternal obesity and sex-specific differences in placental pathology. Placenta. 2016;38:33–40. doi: 10.1016/j.placenta.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 45.Funaki S, Ogawa K, Ozawa N, Okamoto A, Morisaki N, Sago H. Differences in pregnancy complications and outcomes by fetal gender among Japanese women: a multicenter cross-sectional study. Sci Rep. 2020;10(1):18810. doi: 10.1038/s41598-020-75969-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fraser FC. The multifactorial/threshold concept—uses and misuses. Teratology. 1976;14(3):267–80. doi: 10.1002/tera.1420140302 [DOI] [PubMed] [Google Scholar]
- 47.Lu MC, Muthengi E, Wakeel F, Fridman M, Korst LM, Gregory KD. Prolonged second stage of labor and postpartum hemorrhage. J Matern Fetal Neonatal Med. 2009;22(3):227–32. doi: 10.1080/14767050802676709 [DOI] [PubMed] [Google Scholar]
- 48.Dionne MD, Deneux-Tharaux C, Dupont C, Basso O, Rudigoz RC, Bouvier-Colle MH, et al. Duration of Expulsive Efforts and Risk of Postpartum Hemorrhage in Nulliparous Women: A Population-Based Study. PLoS One. 2015;10(11):e0142171. doi: 10.1371/journal.pone.0142171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bais JM, Eskes M, Pel M, Bonsel GJ, Bleker OP. Postpartum haemorrhage in nulliparous women: incidence and risk factors in low and high risk women. A Dutch population-based cohort study on standard (> or = 500 ml) and severe (> or = 1000 ml) postpartum haemorrhage. Eur J Obstet Gynecol Reprod Biol. 2004;115(2):166–72. [DOI] [PubMed] [Google Scholar]
- 50.Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33(4):244–51. doi: 10.1016/j.placenta.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 51.Eskild A, Vatten LJ. Placental weight and excess postpartum haemorrhage: a population study of 308,717 pregnancies. BJOG. 2011;118(9):1120–5. doi: 10.1111/j.1471-0528.2011.02954.x [DOI] [PubMed] [Google Scholar]
- 52.Jansson MH, Franzen K, Hiyoshi A, Tegerstedt G, Dahlgren H, Nilsson K. Risk factors for perineal and vaginal tears in primiparous women—the prospective POPRACT-cohort study. BMC Pregnancy Childbirth. 2020;20(1):749. doi: 10.1186/s12884-020-03447-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brosens IA. Morphological changes in the utero-placental bed in pregnancy hypertension. Clin Obstet Gynaecol. 1977;4(3):573–93. [PubMed] [Google Scholar]
- 54.Hart B, Morgan E, Alejandro EU. Nutrient sensor signaling pathways and cellular stress in fetal growth restriction. J Mol Endocrinol. 2019;62(2):R155–R65. doi: 10.1530/JME-18-0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown ZA, Schalekamp-Timmermans S, Tiemeier HW, Hofman A, Jaddoe VW, Steegers EA. Fetal sex specific differences in human placentation: a prospective cohort study. Placenta. 2014;35(6):359–64. doi: 10.1016/j.placenta.2014.03.014 [DOI] [PubMed] [Google Scholar]
- 56.Verburg PE, Tucker G, Scheil W, Erwich JJ, Dekker GA, Roberts CT. Sexual Dimorphism in Adverse Pregnancy Outcomes—A Retrospective Australian Population Study 1981–2011. PLoS One. 2016;11(7):e0158807. doi: 10.1371/journal.pone.0158807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sepulveda W, Rojas I, Robert JA, Schnapp C, Alcalde JL. Prenatal detection of velamentous insertion of the umbilical cord: a prospective color Doppler ultrasound study. Ultrasound Obstet Gynecol. 2003;21(6):564–9. doi: 10.1002/uog.132 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Availability of data and material: Legal restrictions do not permit the authors to provide the data that constitute the basis of this study. The main data utilized are available from the data owner, the Norwegian Institute of Public Health (https://www.fhi.no/en/more/research--access-to-data/), after obtaining approval from The Regional Committee for Medical Research Ethics (https://rekportalen.no/), for researchers who meet the criteria for access to confidential data. Contact information: The Medical Birth Registry of Norway, University of Bergen, P.O. Box 7804, 5020 Bergen, Norway. Code availability: The data are confidential and cannot be shared.