Abstract
Dense granule protein 7 (GRA7) of Toxoplasma gondii was expressed in Escherichia coli as a fusion protein. The leader peptide contained a 25-amino-acid mouse tumor necrosis factor fragment and six histidyl residues. After purification by metal chelate affinity chromatography, the antigen was evaluated in an enzyme-linked immunosorbent assay for detection of immunoglobulin G (IgG). For two sets of IgG-positive human serum samples, obtained from routine screening, an overall sensitivity of 81% was obtained. For chronic-phase sera, the sensitivity of detection was 79%, but chronic-phase sera with low titers were more difficult to detect (65% sensitivity for sera with immunofluorescence titer of 1/64). When GRA7 was combined with Tg34AR (rhoptry protein 2 C-terminal fragment), the sensitivity rose to 96%. For a set of acute-phase serum samples tested on GRA7, the sensitivity of detection was 94%, and high-titer IgM-positive sera were detected at an especially high rate. In contrast, when Tg34AR was used, the sensitivity was only 85% for this latter set of serum samples. Three truncated GRA7 fragments containing the same leader peptide as that of recombinant GRA7 were produced. The shortest fragment (97 N-terminal amino acids) was not reactive with human sera or with a specific anti-GRA7 monoclonal antibody, while the two larger fragments were reactive. The most important antigenic domain of GRA7 for human sera was localized between residues 97 and 146. The epitope for the specific monoclonal antibody could be further narrowed down by the use of synthetic peptides, but this epitope is not recognized by sera from T. gondii-infected humans. These results indicate that GRA7 may be considered as an additional tool for studying the immune response to T. gondii.
Toxoplasmosis, caused by the apicomplexan parasite Toxoplasma gondii, is generally clinically asymptomatic in healthy individuals but may cause severe complications in pregnant women and immunocompromised patients (23). If infection occurs during pregnancy, the parasite can cross the placental barrier and cause severe damage to the fetus. In AIDS patients, toxoplasmic encephalitis can be life-threatening (12).
A diagnosis of toxoplasmosis is usually based on serological assays. Comparison of immunoglobulin G (IgG) levels with IgM and/or IgA levels is used to differentiate between chronic and acute infections. Most commercial serological assays detect antibodies by means of natural antigens originating from T. gondii grown on host cells or in the peritoneal cavity of mice. The production of these antigens is rather expensive, and the constant quality of the antigen preparations cannot be easily guaranteed. Such antigens can possibly be contaminated by host cell material. The use of recombinant antigens could overcome these drawbacks. Also, selected antigens that are characteristic for the acute or chronic stages of the infection could serve as a tool to discriminate between both stages.
In recent years, many toxoplasma genes have been cloned, and several genes or gene fragments have been expressed in heterologous systems. Until now, only a limited number of recombinant antigens have been tested in an enzyme-linked immunosorbent assay (ELISA) and/or their B-cell epitopes were analyzed. The first fragments to be expressed, as glutathione S-transferase (GST) fusions, were H4 and H11 (10, 26), of which H11 was later shown to be a GRA4 fragment (15). Recently, B-cell epitopes of this antigen were analyzed (16). GRA2 also was produced as a GST fusion, and the B-cell epitopes were studied (14, 17). Recombinant SAG2 was also tested for use in an ELISA, after removal of the GST fusion partner (21). SAG1 was extensively studied and was expressed in CHO cells and by use of the Sindbis virus expression system (11, 29). Recently, a SAG1 fragment missing the signal peptide and devoid of the C-terminal hydrophobic domain was produced as a histidine fusion protein in Escherichia coli (7). SAG1 B-cell epitopic regions were studied by several groups (18, 28).
Cloning of ROP2 (1, 25) allowed the expression of a ROP2 C-terminal fragment (Tg34AR) as a Cro-LacI fusion protein (27). In an ELISA detecting ROP2 IgG antibodies, a sensitivity of 89% relative to that of the Sabin-Feldman dye test was obtained. To find an antigen which could complement Tg34AR in serology, a targeted cDNA library screening was performed, yielding the GRA7 antigen as recently reported (9). Here we describe the performance of GRA7 for application in serology, alone and in combination with Tg34AR. B-cell epitope analysis was used to define the most important antigenic regions of this protein.
MATERIALS AND METHODS
Reagents, sera, and monoclonal antibodies (MAbs).
All reagents were of analytical grade and were obtained from Merck (Darmstadt, Germany), Sigma (St. Louis, Mo.), or Bio-Rad Laboratories (Richmond, Calif.). Restriction enzymes and DNA-modifying enzymes were purchased from Boehringer Mannheim (Brussels, Belgium) and were used in accordance with the manufacturer’s instructions. Protein concentrations were determined by the bicinchoninic acid method (Pierce, Rockford, Ill.).
Serum samples were obtained from patients during routine screening for toxoplasmosis. A first set of 95 positive and 48 negative serum samples was tested by immunofluorescence (IF) (Toxo-Spot IF; bioMérieux Benelux, Brussels, Belgium) as well as with an ELISA (Toxo IgG Micro EIA2; bioMérieux Benelux). Discrepant results were confirmed by the Sabin-Feldman dye test. A second set of 192 positive and 94 negative serum samples was tested only by IF. Results discrepant with those of the GRA7 ELISA were checked by the bioMérieux ELISA. A third set consisted of 67 serum samples determined to be IgM positive by IF (titers between 1/50 and 1/800).
MAb BATO 214 directed to GRA7 (24) was obtained from ascites fluid. A MAb directed to an epitope in the mouse tumor necrosis factor (mTNF) leader peptide was also available and was purified from culture supernatant.
Plasmid constructions.
Molecular biology methods such as digestions with restriction enzymes, blunting with T4 DNA polymerase, ligations of DNA fragments, and transformation of E. coli with plasmids were all carried out as described previously (13). Purification of DNA fragments after agarose gel electrophoresis was performed with the Geneclean II kit (Bio 101, La Jolla, Calif.).
(i) Vectors pmTNFMPH and pIGFH111.
The vector pmTNFMPH enabled expression of recombinant proteins as N-terminal fusions with a short (25 residues) mTNF peptide followed by six consecutive histidine residues (6). The mTNF peptide contained an antigenic epitope for which a specific MAb is available. The polyhistidine allowed purification using immobilized metal affinity chromatography (8). Transcription of heterologous genes cloned in this vector was initiated by the early leftward lambda promoter (Pl), which was controlled by the C1 repressor. The host cell used for expression was E. coli MC1061(pAC1), containing a compatible plasmid which carries the C1-857 mutant gene, which encodes a temperature-sensitive variant of the C1 repressor (22). This allowed the initiation of expression of heterologous genes by shifting the temperature of the culture from 28 to 42°C.
Vector pIGFH111 was a derivative of pmTNFMPH containing the bacteriophage T7 gene 10 fragment (Ε-enhancer) that stimulated expression of genes due to enhanced translation efficiency (19, 20). A double-stranded synthetic oligonucleotide (CCCAATTTTGTTTAACTTTAA) was inserted into a KpnI-blunted site downstream from the lambda Pl promoter. Variations in expression levels of GRA7 fragments, caused by the Ε-enhancer, were not examined in the present study. Leader peptide sequences and polylinkers were identical in both vectors.
(ii) Construction of expression plasmids.
Plasmid pmTNFMPHTg20 expressing the gra7 gene has been described before (9). Three truncated fragments of Tg20 were obtained by digesting pmTNFMPHTg20 with BamHI and Asp700, BamHI and NaeI, or BamHI and StyI (StyI blunted). The BamHI site was part of the polylinker and situated just upstream from the N-terminal end of Tg20, while Asp700, NaeI, and StyI were internal sites of the Tg20 gene (see Fig. 1). Vector pIGFH111 was digested with BamHI and StuI, the latter generating blunt ends. This construction design enabled cloning of the BamHI-blunt fragments in the BamHI and StuI ends of the vector. In all four expression plasmids, the leader peptides were exactly the same length. By removing 3′ fragments from Tg20, the stop codon was also removed. However, vector pIGFH111 contains a stop codon in each reading frame downstream from the polylinker. The recombinant proteins GRA7BA, GRA7BN, and GRA7BS contain 1, 2, and 12 foreign amino acids respectively, at the C-terminal end, due to read-through into the vector sequence.
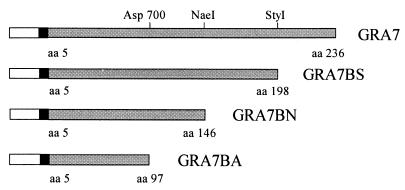
FIG. 1.
Schematic representation of recombinant GRA7 and GRA7 fragments produced in E. coli. The mTNF portion (open bar), the His6 tag (black bar), and the GRA7 portion (grey bar) are indicated. The amino acids present in the recombinant GRA7 fragments are indicated, relative to the initiator methionine.
(iii) Expression of recombinant protein.
Plasmids were transformed into strain MC1061(pAC1). A small-scale induction (shift from 28 to 42°C) was carried out on a 15-ml culture at a cell optical density at 600 nm (OD600) of 0.2. Samples were taken 1, 2, and 3 h after induction. Total cell lysates were loaded on sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to nitrocellulose membranes. When the optimal induction conditions were determined, a large-scale fermentation (15 liters) was carried out.
Purification of recombinant antigens.
Small-scale purifications were carried out with Ni-nitrilotriacetic acid spin columns (Qiagen, Hilden, Germany) by using a slightly modified method. Cells from a 25-ml culture that was induced for 3 h at 42°C were harvested by centrifugation. The cell pellet was lysed by shaking for 30 min in 50 mM phosphate buffer (pH 8.3) containing 6 M guanidine HCl. The lysate was cleared by centrifugation (10 min, 18,000 × g). The spin column was equilibrated with the same buffer, and the sample was loaded (600 μl). The column was sequentially washed two times with 6 M guanidine HCl–50 mM phosphate buffer (pH 6.3) and eluted with 6 M guanidine HCl–50 mM phosphate buffer at pH 4.3. Fifty microliters from the purified protein solution was supplemented with 3 volumes of methanol to precipitate the protein for analysis by polyacrylamide gel electrophoresis (PAGE) and Western blotting (WB).
For large-scale purifications, bacteria from an induced culture (3.75 liters from a 15-liter fermentor vessel) were harvested by centrifugation. The cell pellet was resuspended in lysis buffer (100 mM KCl, 10 mM Tris HCl [pH 6.8], 5 mM EDTA, 20 mM ɛ-aminocaproic acid, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride) and passed three times through a French press. The lysate was centrifuged to obtain a pellet containing the recombinant protein; this pellet was extracted with 8 M guanidine HCl–50 mM phosphate buffer (pH 7.2) and homogenized (Polytron PT1200; Kinematica AG, Litau, Switzerland). A column containing 10 ml of chelating Sepharose Fast Flow (Pharmacia, Uppsala, Sweden) was activated with NiCl2 and washed with 6 M guanidine HCl–50 mM phosphate buffer (pH 7.2) as described by the manufacturer. The extract was then loaded onto the column, and elution was carried out with an imidazole step gradient (35, 50, 200 mM imidazole) in the same buffer. By using this procedure, the fusion protein was purified to 98% homogeneity as determined by gel electrophoresis, silver staining, and WB. Antigen Tg34AR and fragment GRA7BN were purified by the same procedure.
Gel electrophoresis and WB.
The total E. coli extracts or purified recombinant proteins were analyzed by SDS–12% PAGE in the presence of β-mercaptoethanol. When necessary, proteins were transferred to nitrocellulose membranes by the wet WB technique in carbonate buffer (10 mM NaHCO3, 3 mM Na2CO3, 20% [vol/vol] methanol) (4).
The membrane was saturated with 5% fat-free milk in 10 mM Tris–150 mM NaCl–0.05% Tween 20 (TNT) for 1 h, followed by two washes in TNT. The membranes were incubated for 90 min with MAbs, appropriately diluted in TNT containing 1% bovine serum albumin. For screening purposes, sera were preadsorbed on ice for 30 min by using 10% E. coli lysate (protein concentration, 16 mg/ml) in the dilution buffer (TNT plus 1% bovine serum albumin). After three washes with TNT, the bands were revealed with rabbit anti-mouse IgG conjugate (Dako, Glostrup, Denmark) or rabbit anti-human IgG alkaline phosphatase-labelled conjugate (Dako). Conjugates were diluted 1/2,000. Alkaline phosphatase activity was detected by using the chromogenic substrate nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP) in 50 mM Tris HCl (pH 9.5)–150 mM NaCl–5 mM MgCl2 buffer (3).
Prestained protein markers (New England Biolabs, Beverly, Mass.) were used for both SDS-PAGE and WB (maltose-binding protein–β-galactosidase, 175 kDa; maltose-binding protein–paramyosin, 83 kDa; glutamic dehydrogenase, 62 kDa; aldolase, 47.5 kDa; triosephosphate isomerase, 32.5 kDa; β-lactoglobulin A, 25 kDa; lysozyme, 16.5 kDa; aprotinin, 6.5 kDa).
Synthesis of peptides.
The peptides were synthesized on Tentagel S resin (Rapp Polymere GmbH, Tubingen, Germany) by using a Rainin Symphony Multiplex synthesizer (Protein Technologies, Tucson, Ariz.) with standard 9-fluorenylmethoxycarbonyl (Fmoc) chemistry. Standard double couplings were performed by using a fourfold excess of Fmoc-protected amino acids activated in situ with equimolar amounts of N-hydroxybenzotriazole and 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate twice for 20 min each time. The Fmoc protecting group was removed by using a mild base treatment with 2% piperidine–2% 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in dimethyl formamide. N-terminal biotinylation was performed by dissolving biotin in 30% dimethyl sulfoxide–70% dimethyl formamide with in situ activation.
After completion of the peptide synthesis, the peptide was cleaved from the resin by incubation for 2.5 h with 90% trifluoroacetic acid–5% thioanisole–3% ethanedithiol–2% anisole. The peptide was precipitated from the mixture by using t-butyl methyl ether. After centrifugation, the pellet was washed three times with t-butyl methyl ether and dried overnight in a vacuum. The purity of the crude peptide was checked by reversed-phase high-performance liquid chromatography.
ELISA.
ELISA plates (Immuno Plate Maxisorp F96; Nunc, Roskilde, Denmark) were coated with 100-μl of 50 mM carbonate buffer (pH 9.6)/well, containing 2 μg of GRA7 per ml or 6 μg of Tg34AR per ml, by incubation at 37°C for 1 h. When GRA7 and Tg34AR were used in combination, 1 and 6 μg/ml, respectively, were used to coat the ELISA plates. Blocking of the solid phase was carried out by incubation at 37°C for 1 h with phosphate-buffered saline (PBS) containing 0.1% casein and 0.1% Kathon CG (Haas and Rohm Benelux, Antwerp, Belgium) as a preservative (300 μl/well). After the wells were emptied, 100 μl of human serum diluted at 1/100 was added to the wells (sample diluent was PBS, 0.1% casein, 0.1% Kathon CG, and 2.86 g of Triton X-705 per liter). Serum incubation was continued for 1 h at 37°C. After three washes with PBS plus 0.05% Tween 20, goat anti-human IgG-Fc conjugated to horseradish peroxidase (BRL, Gaithersburg, Md.) diluted 1/30,000 in blocking buffer was added and incubation was continued for 1 h at 37°C (100 μl/well). After three washes, the peroxidase activity was detected with H2O2 and 3,3′,5,5′-tetramethylbenzidine for 30 min at room temperature. The reaction was stopped with 1 N H2SO4, and the OD450 was read (Bio-Tek Instruments, Winooski, Vt.).
When the antigens used for coating were of lower purity (<96%), 5% E. coli lysate was added to the sample diluent. E. coli lysate was prepared from strain MC1061(pACI) (protein concentration, 16 mg/ml).
For use in the ELISA, MAb BATO 214 ascites fluid was diluted 1/100,000 and the anti-mTNF MAb was diluted 1/5,000. The conjugate used was labelled with rabbit anti-mouse immunoglobulin–horseradish peroxidase (Dako), diluted 1/2,000. In ELISAs using biotinylated peptides as antigen, the plates were coated with 5 μg of streptavidin (Boehringer Mannheim) per ml in carbonate buffer (1 h, 37°C) and subsequently incubated with the peptides at 1 μg/ml (1 h, 37°C). Further processing was as described above. Peptides 1, 4, and 5 were dissolved in 0.1% trifluoroacetic acid in water at a concentration of 5 mg/ml; peptides 2 and 3 were dissolved in 0.1% TFA–20% acetonitrile in water at 4 mg/ml.
RESULTS
Recombinant expression of GRA7 and GRA7 fragments.
The gra7 insert (Tg20) was transferred from pBKS(+)Tg20 to the expression vector pmTNFMPH as described previously (9). The fusion protein resulting from expression plasmid pmTNFMPHTg20 contained 272 amino acids, 37 residues of which were provided by the leader peptide. The calculated molecular mass of this recombinant GRA7 protein was 29,846 Da.
Three truncated fragments of gra7 were generated by using internal restriction sites: Asp700 at position 290, NaeI at position 437, and StyI at position 588 (Fig. 1). All three fragments were cloned into expression vector pIGFH111 to give rise to plasmids pIGFH111GRA7BA, pIGFH111GRA7BN, and pIGFH111GRA7BS. Exactly the same leader peptide is encoded by the four plasmids.
The four expression constructs were each transformed into E. coli MC1061(pAC1) and induced. The highest expression level was obtained after 3 h of induction for all four expression products (data not shown). The expression level was highest for GRA7 and GRA7BA and lower for GRA7BN and for GRA7BS, as determined on WB with anti-mTNF MAb detection (Fig. 2A). For GRA7BN, the expression level at 28°C was nearly equal to that of the induced culture. The apparent sizes of GRA7 and the truncated fragments as determined on WB—18 kDa for GRA7BA, 25 kDa for GRA7BN, 31 kDa for GRA7BS, and 36 kDa for GRA7—are larger than the calculated sizes of 14.5, 20, 25, and 30 kDa, respectively.
FIG. 2.
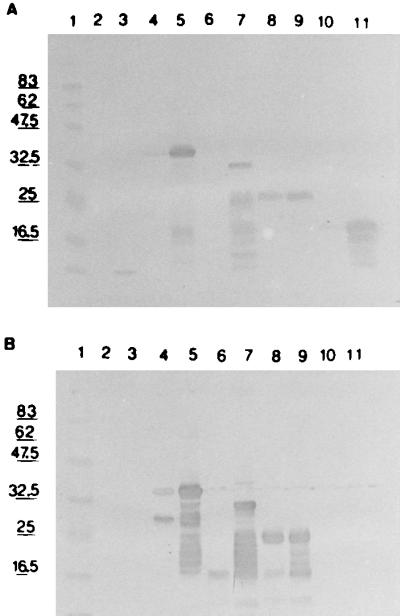
WB of E. coli lysate expressing recombinant GRA7 or recombinant GRA7 fragments. Lanes 2, 4, 6, 8, and 10, lysates of noninduced cultures; lanes 3, 5, 7, 9, and 11, lysates of induced cultures. Lane 1, protein marker; lanes 2 and 3, leader peptider only; lanes 4 and 5, GRA7; lanes 6 and 7, GRA7BS; lanes 8 and 9, GRA7BN; lanes 10 and 11, GRA7BA. (A) Reaction with anti-mTNF MAb; (B) reaction with MAb BATO 214.
Purification of recombinant proteins.
Recombinant proteins GRA7BN and GRA7BA were purified by using the Ni-nitrilotriacetic acid spin columns. The protein concentration for purified GRA7BN was 180 μg/ml, and that for GRA7BA was 265 μg/ml. The purified proteins still contained some E. coli contaminants, based on WB developed with anti-E. coli serum, but were considered to be of sufficient purity for a first evaluation. Antigen fragment GRA7BS was not purified due to its lower expression level.
From a larger-scale purification of fragment GRA7BN, 0.46 mg/liter of fermentor broth was obtained at 95% purity. The large-scale purification of GRA7 and Tg34AR yielded 7.5 and 13.5 mg per liter of fermentor broth, respectively. In both cases, a purity of at least 98% was obtained.
IgG ELISA with recombinant GRA7.
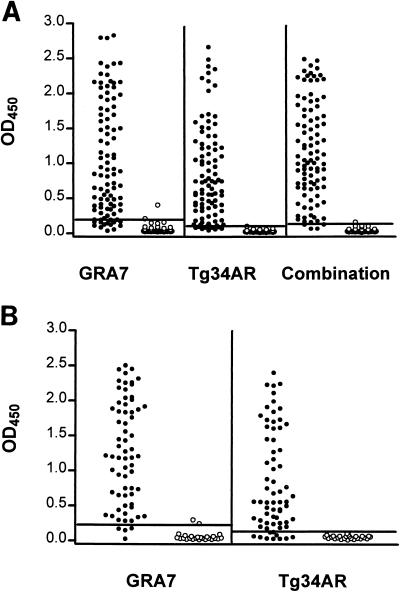
To determine the optimal coating conditions for the GRA7 antigen, different coating concentrations and coating buffers were tested. The GRA7 antigen at a concentration of 2 μg/ml in 50 mM carbonate buffer (pH 9.6) was found to be optimal for coating. The first set of samples, composed of 95 IgG-positive and 48 IgG-negative serum samples, was tested as described in Materials and Methods (Fig. 3A). The cut-off was determined by calculating the mean value for the 48 negative serum samples and adding three standard deviations. One negative serum sample scored above the cut-off (no. 244, OD 0.309), resulting in a specificity of 98%. Of 95 positive serum samples, 77 were detected, resulting in a sensitivity of 81%. The 10 serum samples included in the 95 positive serum samples that were both IgM and IgG positive were all detected. For the chronic-phase serum samples (IgG positive only), the sensitivity was 79% (67 of 85 serum samples). When ranked by IF titer, high-titer serum samples (titer of 1/1,024 or higher) were all detected (sensitivity, 100%), whereas serum samples with IF titers of 1/256 or 1/64 were less reactive (83 and 65% sensitivities, respectively) (Table 1). Results obtained with the second set of serum samples confirmed the data from the first evaluation with a sensitivity of 80% (154 of 192 serum samples detected) and a specificity of 98% (2 false positives out of 94 serum samples).
FIG. 3.
ELISA reactivity of two different sets of serum samples. The cut-off value is indicated for each experiment by a horizontal line. (A) Detection of a set of 95 IgG-positive (•) and 48 IgG-negative (○) human serum samples with the recombinant antigens GRA7, Tg34AR, and a combination of both; (B) detection of a set of 67 IgM-positive (•) and 25 IgM- and IgG-negative (○) human serum samples with the recombinant antigens GRA7 and Tg34AR.
TABLE 1.
Relationship between IF assay titer and ELISA sensitivity
| IF titer | No. of serum samples tested | Sensitivity (%)
|
||
|---|---|---|---|---|
| GRA7 | Tg34AR | GRA7-Tg34ARa | ||
| 1/1,024 | 19 | 100 | 100 | 100 |
| 1/256 | 47 | 83 | 91 | 96 |
| 1/64 | 29 | 65 | 76 | 93 |
Combination of GRA7 and Tg34AR as described in Materials and Methods.
To further substantiate the results with IgM-positive sera (acute-phase sera), the GRA7 ELISA was carried out with an additional set of 67 IgM-positive serum samples, resulting in a sensitivity of 94% (cut-off calculated with 25 IgM- and IgG-negative serum samples) (Fig. 3B). The OD values of serum samples with IF IgM titers of 1/400 and 1/800 were very high in the latter experiment.
IgG ELISA with Tg34AR and with combined antigens.
When Tg34AR was tested for the first set of serum samples as described above, a sensitivity of 88% and a specificity of 100% were demonstrated. Some serum samples that were nonreactive with Tg34AR were reactive with GRA7 and vice versa (Table 2), indicating that the two antigens in combination could increase the sensitivity of the test.
TABLE 2.
Reactivity in ELISA of selected serum samples with GRA7, Tg34AR, or a combination of both
| Serum sample no. | Reactivity (OD value)
|
||
|---|---|---|---|
| GRA7 | Tg34AR | GRA7-Tg34AR | |
| 8 | 0.194 | 0.626 | 0.706 |
| 17 | 0.396 | 0.073 | 0.425 |
| 32 | 0.517 | 0.086 | 0.401 |
| 34 | 0.684 | 0.099 | 0.716 |
| 46 | 0.195 | 0.766 | 0.783 |
| 85 | 0.126 | 1.392 | 1.260 |
| 86 | 0.493 | 0.115 | 0.546 |
When both GRA7 and Tg34AR were coated onto the ELISA plates simultaneously, the optimal sensitivity was obtained when GRA7 was used at 1 μg/ml and Tg34AR was used at 6 μg/ml in carbonate buffer (pH 9.6).
Under these conditions, one negative serum sample scored positive (specificity, 98%) with a cut-off value of 0.134. Of the positive serum samples, 91 of 95 reacted positively, bringing the sensitivity to 96% (Fig. 3A). Four serum samples continued to score close to the cut-off, which adversely affected the clear separation between positive and negative serum samples. Just as for GRA7, the relationship between the IF titer and the sensitivity was analyzed for Tg34AR and for the antigen combination (Table 1). Serum samples with an IF titer of 1/1,024 or higher were all detected by both ELISAs. Serum samples with an IF titer of 1/256 were detected with a sensitivity of 96%, whereas serum samples with an IF titer of 1/64 were detected with 93% sensitivity when the antigen combination was used.
In contrast to the ELISA with GRA7, the ELISA with Tg34AR with the set of IgM-positive sera detected only 85% of the serum samples (Fig. 3B). Of 10 serum samples not detected by the Tg34AR ELISA, 8 were positive for GRA7. Most serum samples scored higher with GRA7. In particular, those serum samples with high IgM titers, indicative of an early infectious stage, were more easily detected with GRA7 (Table 3). This suggests an earlier IgG antibody response to GRA7 than to Tg34AR.
TABLE 3.
Reactivity with GRA7 and Tg34AR in ELISA of serum samples IgM positive by IF assay
| Serum sample no. | IgM titer in IF assay | Reactivity (OD value)
|
|
|---|---|---|---|
| GRA7 | Tg34AR | ||
| M2 | 1/800 | 2.457 | 2.233 |
| M6 | 1/400 | 2.265 | 1.915 |
| M14 | 1/800 | 2.320 | 0.038 |
| M15 | 1/400 | 2.451 | 0.206 |
| M23 | 1/400 | 1.176 | 0.128 |
| M47 | 1/400 | 1.949 | 0.084 |
| M52 | 1/400 | 1.920 | 0.244 |
| M66 | 1/800 | 1.974 | 0.450 |
| M68 | 1/400 | 1.837 | 1.456 |
Localization of the epitope for BATO 214.
On WB, MAb BATO 214 reacted with GRA7, GRA7BS, and GRA7BN but not with GRA7BA, the shortest fragment (Fig. 2B). This result was confirmed with an ELISA in which GRA7, GRA7BA, or GRA7BN was coated onto plates and detected with MAb BATO 214 (Table 4). GRA7BA did not react, while full-size GRA7 and GRA7BN were recognized. This indicated that the shortest GRA7 fragment lacked the epitope for BATO 214 while GRA7BN still contained it. The protein fragment containing the epitope was thus expected to be situated between residue 97 and residue 146, in case a linear epitope was involved. Five biotinylated synthetic peptides of 20 residues and one peptide of 16 residues, each with an overlap of 10 residues, were synthesized starting with residue 91 and ending with residue 146. All peptides were tested in an ELISA as described in Materials and Methods. The first two peptides reacted with the MAb (Table 4), narrowing down the epitopic determinant to the overlap region of both peptides (RKRGVRSDAE).
TABLE 4.
Reactivity in ELISA of GRA7, GRA7 fragments, and synthetic peptides with MAbs
| Antigena | Reactivity (OD value)
|
|
|---|---|---|
| MAb BATO 214 | Anti-TNF MAb | |
| GRA7 | 2.291 | 1.709 |
| GRA7BN | 2.333 | 1.621 |
| GRA7BA | 0.010 | 1.213 |
| pep 90-110 | 1.350 | NAb |
| pep 100-120 | 2.169 | NA |
| pep 110-130 | 0.007 | NA |
| pep 120-140 | 0.009 | NA |
| pep 130-146 | 0.007 | NA |
For the peptides (pep), beginning and ending amino acids are given, with numbering starting from the initiation methionine.
NA, not applicable.
Determination of the epitopic region of GRA7 for human IgG.
Recombinant purified antigen fragments GRA7BA and GRA7BN and full-size GRA7 were evaluated in an ELISA with 32 GRA7-positive human serum samples.
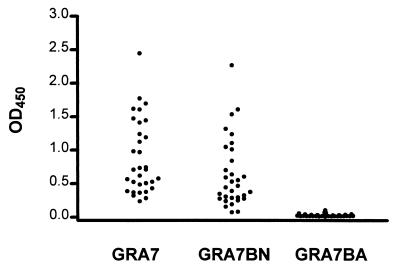
Of all the GRA7-positive serum samples tested, none was reactive with fragment GRA7BA, indicating that this fragment probably contains no dominant human B-cell epitopes. When compared to complete GRA7, GRA7BN displayed an equal or slightly reduced reactivity of up to 20% in 12 of the 32 serum samples tested (Fig. 4). A 30 to 50% reduction of reactivity was observed in 17 of 32 serum samples, while for 3 of 32 serum samples the reduction was >50% (two of which showed no reactivity with GRA7BN). This finding indicates that an important antigenic region of GRA7 is situated between residues 97 and 146. It seems that for the serum samples that were unreactive with GRA7BN, an epitope is present in the deleted part of GRA7 or that this fragment is at least needed to preserve the structure of the epitope. In contrast to GRA2 and GRA4 (16, 17), the major hydrophilic domain in the C-terminal region of GRA7 (results not shown) seems not to contain an important epitope.
FIG. 4.
ELISA reactivity of 32 GRA7-positive serum samples with complete GRA7, with GRA7BN, and with GRA7BA.
The sera were also subsequently tested with the five synthetic peptides mentioned above. Most sera showed a weak reaction with several peptides, while others failed to react at all (data not shown). The analysis of the peptide reactivity did not allow further elucidation of the epitope recognized by the human sera.
DISCUSSION
Recently, a new dense granule protein (GRA7) from T. gondii was described, and the corresponding gene was characterized (9). To study the potential of this protein for serological assays, the gra7 gene was expressed in E. coli with a short mTNF fragment and hexahistidine as a fusion partner. The subsequent purification of the recombinant protein allowed evaluation of this antigen as a diagnostic tool. In an indirect IgG ELISA, a sensitivity of 81% and a specificity of 98% were reached. High-titer IgG-positive sera (IF titer, >1/1,024) were readily detected (100%). IgM-positive sera were detected in 94% of cases, and for chronic-phase sera (IgM negative) the sensitivity was 79%. In general, chronic-stage sera with low IgG titers by IF were more difficult to detect with GRA7. These results were in the same range as those obtained for a recombinant fragment of GRA2 (75% for chronic-phase sera, 82.6% for acute-phase sera) (17) and for Tg34AR (ROP2 fragment; 89% overall sensitivity) (27). The combination of H4 and H11 detected 64% of acute-phase sera and 14% of chronic-phase sera (26).
The facts that IgM-positive sera were detected with high sensitivity and that especially high-titer IgM-positive sera (IgM titers by IF, 1/400 and 1/800) reached high OD values seem to contradict the findings of Fisher et al. (5), who found that GRA7 is released from bradyzoite-infected host cells (chronic phase) and not from tachyzoite-infected host cells (acute phase). If GRA7 is secreted from bradyzoites, a strong antibody response to this antigen would be expected in sera from chronic patients. Unexpectedly, we observed a low antibody response in these patients. On the other hand, in sera from patients with acute infections, a strong antibody response was found. This contradiction between in vitro and in vivo results needs further investigation. It is possible that bradyzoites studied in vitro behave differently from those found in cysts upon encapsidation of the parasite in the organs of infected patients. The fact that GRA7 was found in the parasitophorous vacuole (PV), the PV membrane, and the cytoplasm of the host cell infected with the tachyzoite stage (9) is in agreement with these ELISA results, where high reactivity with sera from acute-stage infections is found. In the acute stage, constant rupture of infected cells releases the contents of the PV and the cytoplasm and thus brings GRA7 in contact with the immune system. It would be interesting to compare the GRA7 response to that of other GRA antigens, especially GRA3, which has also been shown to be present in the host cell cytoplasm (2).
Remarkably, Tg34AR failed to detect several high-titer IgM-positive sera while they clearly scored positive when GRA7 was used. It is expected that both antigens become exposed to the immune system at the moment of cell rupture and, hence, provoke an immune response from the same moment on. However, this seems not to occur, and the immune response towards these two antigens varies considerably. In part, these differences may be attributed to the intrinsic antigenic properties of the proteins. Further research is needed to clarify these findings.
The combination of GRA7 with Tg34AR could detect 96% of IgG-positive sera. Both antigens complement each other in the detection of some sera, but both fail to detect some chronic-phase sera with low titers. Possibly, a bradyzoite-specific antigen could be used to detect those sera and, hence, complement GRA7 and/or Tg34AR.
The study of GRA7 fragments and peptides allowed the localization of the epitope for the anti-GRA7 MAb BATO 214. However, this epitope was not found to be immunodominant when human sera were studied. In humans, the fragment comprising amino acids 97 to 146 is the most important antigenic region of GRA7. The major epitope recognized by human sera could not be further mapped by synthetic peptides, in contrast to the mouse monoclonal epitope. This may partly be attributed to the polyclonal nature of the antibody response generated upon infection. However, the fact that no clear reactivity with any of the peptides from the antigenic region is defined may indicate that peptides of 20 amino acids as used here are unable to form the relevant epitope. This observation leads us to assume that the major epitope recognized by human sera in this region has an important structural component. Only some sera recognize an epitope(s) beyond amino acid 146, which is not unexpected since this is the region downstream from the putative membrane anchor. However, this finding contrasts with the observations made for GRA2 and GRA4, where a major antigenic domain was found in the region comprising the C-terminal 50 amino acids (16, 17). Despite the more pronounced hydrophilic nature of the C terminus of GRA7 as compared to that of GRA2 and GRA4, this region is less antigenic in the GRA7 protein.
In conclusion, we have shown that the newly discovered GRA7 protein may be considered as an additional tool for studying the immune response of humans to T. gondii. On the other hand, additional questions have been raised concerning the role of this antigen in the infectious process. The tools which are now available as a consequence of the work described here will allow further extension of these studies.
ACKNOWLEDGMENTS
We thank I. Rockelé and G. Clemminck for protein purification, B. Van Der Perre for peptide synthesis, and F. Shapiro for editorial comments.
This work was partially funded by the Flemish IWT, grant no. 970013.
REFERENCES
- 1.Beckers C J, Dubremetz J-F, Mercereau-Puijalon O, Joiner K A. The Toxoplasma gondii rhoptry protein ROP 2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J Cell Biol. 1994;127:947–961. doi: 10.1083/jcb.127.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermudes D, Dubremetz J-F, Achbarou A, Joiner K A. Cloning of a cDNA encoding the dense granule protein GRA3 from Toxoplasma gondii. Mol Biochem Parasitol. 1994;68:247–257. doi: 10.1016/0166-6851(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 3.Blake M S, Johnson K H, Russell-Jones G J, Gotschlich E C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984;136:175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- 4.Dunn S D. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal Biochem. 1986;157:144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- 5.Fisher H-G, Stachelhaus S, Sahm M, Meyer H E, Reichman G. GRA7, an excretory 29 kDa Toxoplasma gondii dense granule antigen released by infected host cells. Mol Biochem Parasitol. 1998;91:251–262. doi: 10.1016/s0166-6851(97)00227-2. [DOI] [PubMed] [Google Scholar]
- 6.Gilot P, De Kesel M, Machtelinckx L, Coene M, Cocito C. Isolation and sequencing of the gene coding for an antigenic 34-kilodalton protein of Mycobacterium paratuberculosis. J Bacteriol. 1993;175:4930–4935. doi: 10.1128/jb.175.15.4930-4935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harning D, Spenter J, Metsis A, Vuust J, Petersen E. Recombinant Toxoplasma gondii surface antigen 1 (P30) expressed in Escherichia coli is recognized by human Toxoplasma-specific immunoglobulin M (IgM) and IgG antibodies. Clin Diagn Lab Immunol. 1996;3:355–357. doi: 10.1128/cdli.3.3.355-357.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hochuli E, Bannwarth H, Doebeli R, Gentz R, Stueber D. Genetic approach to facilitate purification of recombinant proteins with a novel metal chelate adsorbent. Biotechnology. 1988;6:1321–1325. [Google Scholar]
- 9.Jacobs D, Dubremetz J-F, Loyens A, Bosman F, Saman E. Identification and heterologous expression of a new dense granule protein (GRA7) from Toxoplasma gondii. Mol Biochem Parasitol. 1998;91:237–249. doi: 10.1016/s0166-6851(97)00204-1. [DOI] [PubMed] [Google Scholar]
- 10.Johnson A M, Illana S. Cloning of Toxoplasma gondii gene fragments encoding diagnostic antigens. Gene. 1991;99:127–132. doi: 10.1016/0378-1119(91)90044-c. [DOI] [PubMed] [Google Scholar]
- 11.Kim K, Bulow R, Kampmeier J, Boothroyd J C. Conformationally appropriate expression of the toxoplasma antigen SAG1 (P30) in CHO cells. Infect Immun. 1994;62:203–209. doi: 10.1128/iai.62.1.203-209.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luft B J, Brooks R G, Conley F K, McCabe R E, Remington J S. Toxoplasma encephalitis in patients with AIDS. JAMA. 1984;252:913–917. [PubMed] [Google Scholar]
- 13.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 14.Mercier C, Lecordier L, Darcy F, Deslee D, Murray A, Tourvieille B, Maes P, Capron A, Cesbron-Delauw M-F. Molecular characterisation of a dense granule antigen (GRA 2) associated with the network of the parasitophorous vacuole in Toxoplasma gondii. Mol Biochem Parasitol. 1993;58:71–82. doi: 10.1016/0166-6851(93)90092-c. [DOI] [PubMed] [Google Scholar]
- 15.Mevelec M-N, Chardes T, Mercereau-Puijalon O, Bourgin I, Achbarou A, Dubremetz J-F, Bout D. Molecular cloning of GRA4, a Toxoplasma gondii dense granule protein, recognized by mucosal IgA antibodies. Mol Biochem Parasitol. 1992;56:227–238. doi: 10.1016/0166-6851(92)90172-g. [DOI] [PubMed] [Google Scholar]
- 16.Mevelec M-N, Mercereau-Puijalon O, Buzoni-Gatel D, Bourguin I, Chardès T, Dubremetz J-F, Bout D. Mapping of B epitopes in GRA4, a dense granule antigen of Toxoplasma gondii and protection studies using recombinant proteins administered by the oral route. Parasite Immunol. 1998;20:183–195. [PubMed] [Google Scholar]
- 17.Murray A, Mercier C, Decoster A, Lecordier L, Capron A, Cesbron-Delauw M-F. Multiple B-cell epitopes in a recombinant GRA2 secreted antigen of Toxoplasma gondii. Appl Parasitol. 1993;34:235–244. [PubMed] [Google Scholar]
- 18.Nam H-W, Im K-S, Baek E-J, Choi W-Y, Cho S-Y. Analysis of antigenic domain of GST fused major surface protein (P30) fragments of Toxoplasma gondii. Korean J Parasitol. 1996;34:135–141. doi: 10.3347/kjp.1996.34.2.135. [DOI] [PubMed] [Google Scholar]
- 19.Olins P O, Devine C S, Rangwala S H, Kavka K S. The T7 phage gene 10 leader RNA, a ribosome-binding site that dramatically enhances the expression of foreign genes in Escherichia coli. Gene. 1988;73:227–235. doi: 10.1016/0378-1119(88)90329-0. [DOI] [PubMed] [Google Scholar]
- 20.Olins P O, Rangwala S H. A novel sequence element derived from bacteriophage T7 mRNA acts as an enhancer of translation of the lacZ gene in Escherichia coli. J Biol Chem. 1989;264:16973–16976. [PubMed] [Google Scholar]
- 21.Parmley S F, Sgarlato G D, Mark J, Prince J B, Remington J S. Expression, characterisation, and serologic reactivity of recombinant surface antigen P22 of Toxoplasma gondii. J Clin Microbiol. 1992;30:1127–1133. doi: 10.1128/jcm.30.5.1127-1133.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remaut E, Tsao H, Fiers W. Improved plasmid vectors with a thermoinducible expression and temperature-regulated runaway replication. Gene. 1983;22:103–113. doi: 10.1016/0378-1119(83)90069-0. [DOI] [PubMed] [Google Scholar]
- 23.Remington J S, Desmonts G. Toxoplasmosis. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 2nd ed. Philadelphia, Pa: The W. B. Saunders Co.; 1990. pp. 89–195. [Google Scholar]
- 24.Saavedra R, De Meuter F, Hérion P. Monoclonal antigens identify new Toxoplasma gondii soluble antigens. Hybridoma. 1990;9:453–463. doi: 10.1089/hyb.1990.9.453. [DOI] [PubMed] [Google Scholar]
- 25.Saavedra R, De Meuter F, Decourt J L, Hérion P. T-cell clone identifies a potentially protective 54 kDa protein antigen of Toxoplasma gondii cloned and expressed in Escherichia coli. J Immunol. 1991;147:1975–1982. [PubMed] [Google Scholar]
- 26.Tenter A M, Johnson A M. Recognition of recombinant Toxoplasma gondii antigens by human sera in an ELISA. Parasitol Res. 1991;77:197–203. doi: 10.1007/BF00930858. [DOI] [PubMed] [Google Scholar]
- 27.Van Gelder P, Bosman F, De Meuter F, Van Heuverswyn H, Hérion P. Serodiagnosis of toxoplasmosis by using a recombinant form of the 54-kilodalton rhoptry antigen expressed in Escherichia coli. J Clin Microbiol. 1993;31:9–15. doi: 10.1128/jcm.31.1.9-15.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velge-Roussel F, Chardès T, Mévélec P, Brillard M, Hoebeke J, Bout D. Epitopic analysis of the Toxoplasma gondii major surface antigen SAG1. Mol Biochem Parasitol. 1994;66:31–38. doi: 10.1016/0166-6851(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 29.Xiong C, Grieve R B, Kim K, Boothroyd J C. Expression of Toxoplasma gondii P30 as fusions with glutathione S-transferase in animal cells by Sindbis recombinant virus. Mol Biochem Parasitol. 1993;61:143–148. doi: 10.1016/0166-6851(93)90167-v. [DOI] [PubMed] [Google Scholar]